1. Introduction
Chronic heart failure is a leading cause of hospitalization, especially among individuals aged 65 years and older.[
1,
2,
3] Heart failure is categorized according to left ventricular ejection fraction (LVEF) into three subtypes: heart failure with reduced ejection fraction (HFrEF; LVEF <40%), heart failure with mildly reduced ejection fraction (HFmrEF; LVEF 40–49%), and heart failure with preserved ejection fraction (HFpEF; LVEF ≥50%).[
4,
5,
6] Approximately half of all patients with heart failure present with HFmrEF or HFpEF, for which effective pharmacological therapies to improve clinical outcomes remain limited.[
6,
7]
Dapagliflozin is a selective oral sodium-glucose cotransporter 2 (SGLT2) inhibitor that acts by persistently, competitively, and reversibly inhibiting SGLT2, a key mediator of renal glucose reabsorption. Based on the results of the phase III DELIVER trial, dapagliflozin has been approved for the treatment of chronic heart failure irrespective of LVEF.[
8,
9] In addition to its osmotic diuretic and hemodynamic effects, emerging evidence suggests that dapagliflozin may exert secondary cardioprotective effects, including attenuation of myocardial fibrosis.[
10]
Moreover, dapagliflozin has been approved for the treatment of chronic kidney disease (CKD) based on findings from the phase III DAPA-CKD trial.[
11] SGLT2 inhibition increases distal tubular sodium delivery, enhancing tubuloglomerular feedback and consequently reducing intraglomerular pressure.[
12] The resulting hemodynamic benefits—such as correction of fluid overload, blood pressure reduction, and decreased cardiac preload and afterload—are believed to improve renal perfusion and confer renoprotective effects.[
13]
Long-term administration of SGLT2 inhibitors has been shown to slow the decline in renal function. However, a transient decrease in estimated glomerular filtration rate (eGFR), known as the initial dip, typically occurs within the first 1–2 weeks of therapy initiation.[
14,
15,
16] This phenomenon is thought to reflect hemodynamic changes within the glomerulus, particularly reductions in intraglomerular pressure. In patients with preexisting renal impairment, the initial dip may be associated with an increased risk of acute adverse events.
While several studies have investigated the determinants and prognostic implications of the initial dip in patients with chronic kidney disease or diabetes,[
16,
17] evidence remains limited in populations with chronic heart failure. Moreover, few studies have comprehensively assessed the clinical characteristics and contributing factors associated with the initial dip in this patient population. In our cardiovascular department, we have encountered patients with chronic heart failure who exhibited an initial dip in renal function following the initiation of dapagliflozin, often requiring clinical interventions such as discontinuation of concomitant medications or adjustments to fluid management. In contrast, other patients did not experience an initial dip; however, the comparative trajectory of renal function between these groups remains unclear. Previous studies[
16,
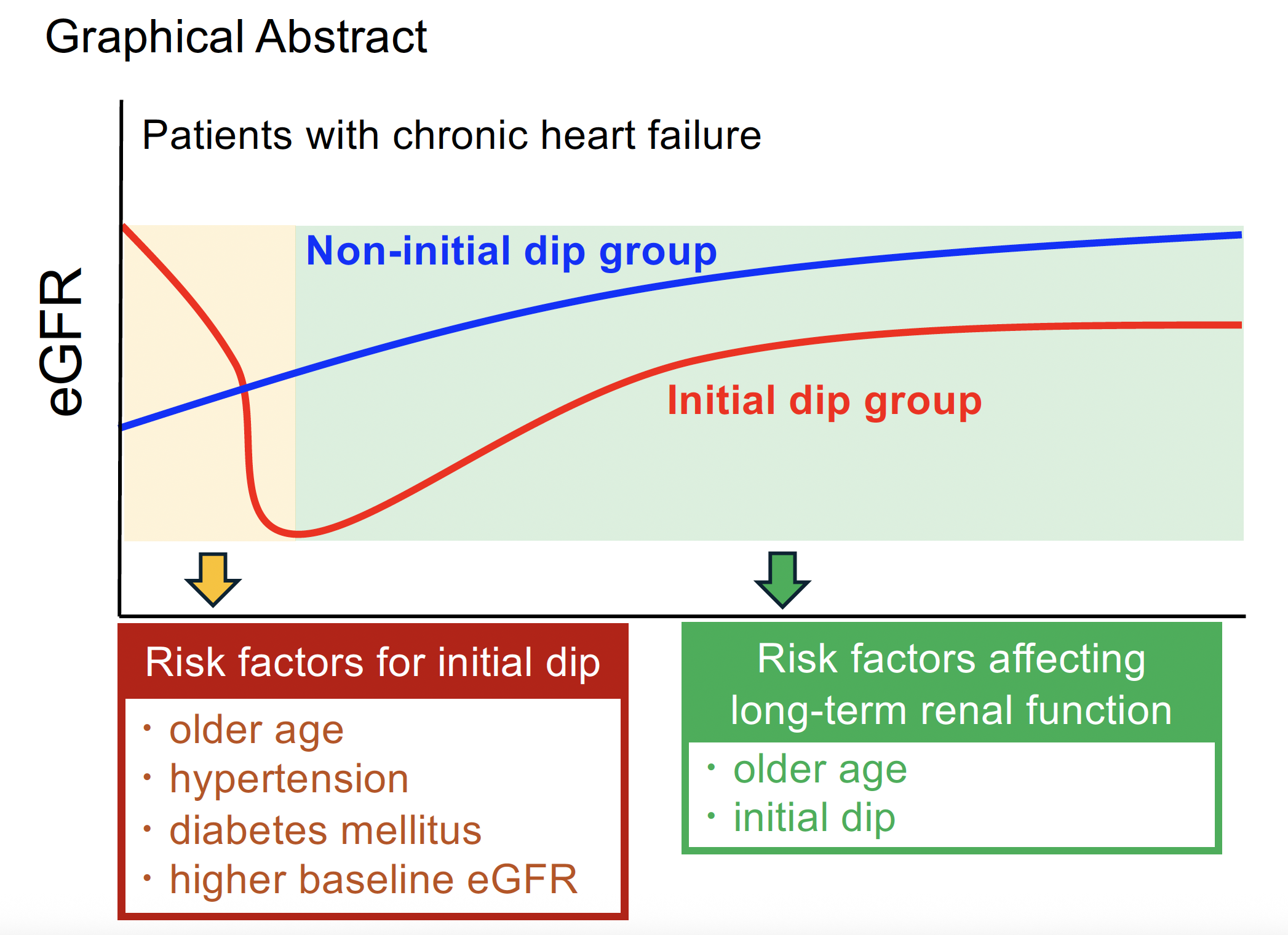
17] have evaluated long-term renal function using the post–initial dip values as the baseline. However, it is rare for renal function to return to its original baseline once it has declined due to the initial dip. We believe that an accurate assessment of the long-term impact of the initial dip on renal function requires using the pre–initial dip baseline as a reference. To our knowledge, no prior studies have taken this approach, and such a novel perspective may provide new insights into favorable renal trajectories following SGLT2 inhibitor initiation. Identifying factors associated with the initial dip may facilitate safer and more effective initiation of SGLT2 inhibitors. Furthermore, predicting long-term renal outcomes based on the presence or absence of the initial dip could support individualized treatment planning and enable timely clinical decision-making.
Therefore, this study aimed to investigate the trajectory of renal function following the initiation of dapagliflozin in patients with chronic heart failure. Specifically, we sought to identify factors associated with the occurrence of the initial dip, as well as those predictive of long-term changes in renal function. In addition, we evaluated the long-term impact of the initial dip on renal function using the pre–initial dip baseline as a reference point.
2. Materials and Methods
Study Design
This retrospective study included all patients with chronic heart failure who were hospitalized in the Department of Cardiovascular Medicine at our institution and initiated treatment with dapagliflozin 10 mg/day between November 27, 2020 (the date dapagliflozin received regulatory approval for the treatment of chronic heart failure in Japan), and May 1, 2023. Patients were excluded if they had a prior history of SGLT2 inhibitor use, including dapagliflozin, or if they received a reduced dose of dapagliflozin (<10 mg/day).
The present study received ethical approval from the Ethics Committee of Kurume University Hospital (approval no. 23156). The informed consent was waived due to the retrospective nature and opt-out was used in the study. The study was conducted following the ethical principles outlined in the Declaration of Helsinki.
Data Collection
Patient data were retrospectively extracted from electronic medical records. Demographic and clinical parameters at the time of dapagliflozin initiation were collected, including age, sex, height, and body weight. Body mass index was calculated as weight (kilograms) divided by the square of height (square meters). Blood samples were obtained from the antecubital vein and analyzed at a commercial laboratory within Kurume University Hospital to measure the following parameters: aspartate aminotransferase (AST), alanine aminotransferase (ALT), albumin, blood urea nitrogen (BUN), estimated glomerular filtration rate (eGFR), uric acid, blood glucose, sodium, potassium, C-reactive protein (CRP), comorbidities, and concomitant medications. Chronic kidney disease was defined as an eGFR of <60 mL/min/1.73 m².
In addition to the baseline eGFR (Before SGLT2 inhibitor), we also assessed the lowest eGFR value within two weeks of dapagliflozin initiation (Acute Phase), as well as the values at 6 months and 1 year after treatment initiation.
Definition of Initial Dip of eGFR
Based on previous reports,[
11,
17,
18] the initial dip in eGFR has been estimated to be approximately 3 to 8 mL/min/1.73 m². Therefore, we defined the initial dip as a decrease in eGFR of ≥5 mL/min/1.73 m² from baseline to the lowest value recorded within two weeks after initiating dapagliflozin. Patients were classified into the "initial dip group" (ΔeGFR ≥5 mL/min/1.73 m²) or the "non-initial dip group" (ΔeGFR <5 mL/min/1.73 m²).
Outcome
The primary endpoint was to explore factors influencing the initial dip of eGFR, along with baseline clinical characteristics. Secondary endpoints were to evaluate long-term renal function, changes in eGFR at 6 months and 1 year after dapagliflozin initiation, between the initial dip group and the non-initial dip group.
Statistical Analysis
Continuous variables were summarized as means with standard deviations (SDs), and categorical variables were expressed as counts and percentages. Group comparisons between patients with and without the initial dip were performed using Fisher’s exact test for categorical variables and Student’s t-test for continuous variables. To identify factors associated with the occurrence of the initial dip, univariate logistic regression analyses were first conducted. Variables found to be statistically significant in the univariate analysis were then included in a multivariate logistic regression model. To avoid multicollinearity, variables known to be correlated with eGFR—specifically uric acid and potassium levels—were excluded. Instead, clinically relevant factors presumed to influence changes in renal function were selected for inclusion in the multivariate model. To evaluate the impact of the initial dip on long-term renal function, multivariate linear regression analyses were performed using the change in eGFR at six months and one year as dependent variables. Independent variables included the presence or absence of the initial dip, as well as baseline characteristics found to be significant in univariate analyses. Changes in eGFR were calculated by subtracting the baseline eGFR from the eGFR measured at each respective follow-up time point.
Multiple imputation was used to handle missing data for baseline values for uric acid and electrolytes under the assumption of missing at random (MAR) in the multivariate analysis. All statistical tests were two-sided, with a significance level of 10% for univariate analyses and 5% for multivariate analyses.
All statistical analyses were performed using R Studio version 4.2.1 (Posit PBC, Boston, MA, United States).
3. Results
3.1. Patient Characteristics
Among the 123 patients who initiated dapagliflozin therapy, 65 patients (52.8%) were classified into the initial dip group, and 58 patients (47.2%) into the non-initial dip group. The mean age was higher in the initial dip group (70.5 years) compared to the non-initial dip group (65.3 years) (
p=0.07). Baseline eGFR was also higher in the initial dip group (59.4 mL/min/1.73 m²) than in the non-initial dip group (44.7 mL/min/1.73 m²) (
p<0.01). Patients in the initial dip group had a higher prevalence of comorbid hypertension and diabetes mellitus (
p=0.02 and 0.03, respectively). Although the use of medications potentially associated with the initial dip—such as angiotensin-converting enzyme (ACE) inhibitors, angiotensin II receptor blockers (ARB), and diuretics—was observed in both groups, no significant differences in concomitant medication use were found between the groups (
Table 1).
3.2. Risk Factors for Initial Dip
In the multivariate analysis of factors associated with the initial dip, both age and baseline eGFR at the time of dapagliflozin initiation were significantly associated with the development of an initial dip (
p<0.01 for both). Higher age and higher baseline eGFR were correlated with an increased risk of experiencing an initial dip. Additionally, the presence of hypertension (odds ratio [OR]: 3.25; 95% confidence interval [CI]: 1.19–9.63;
p=0.02) and diabetes mellitus (OR: 2.61; 95% CI: 1.10–6.42;
p=0.03) were identified as significant contributing factors to the occurrence of an initial dip (
Table 2).
3.3. Risk Factors Affecting Long-Term Renal Function
eGFR values were available for 77 patients at 6 months, and for 62 patients at 1 year; 56 patients had data available at both time points. In the analysis of factors associated with eGFR change at 6 months, both age and the presence of an initial dip were significantly associated with a decline in renal function (
p<0.01) (
Table 3). Similarly, at 1 year, both age and initial dip remained significant factors influencing eGFR change (
p<0.01) (
Table 4).
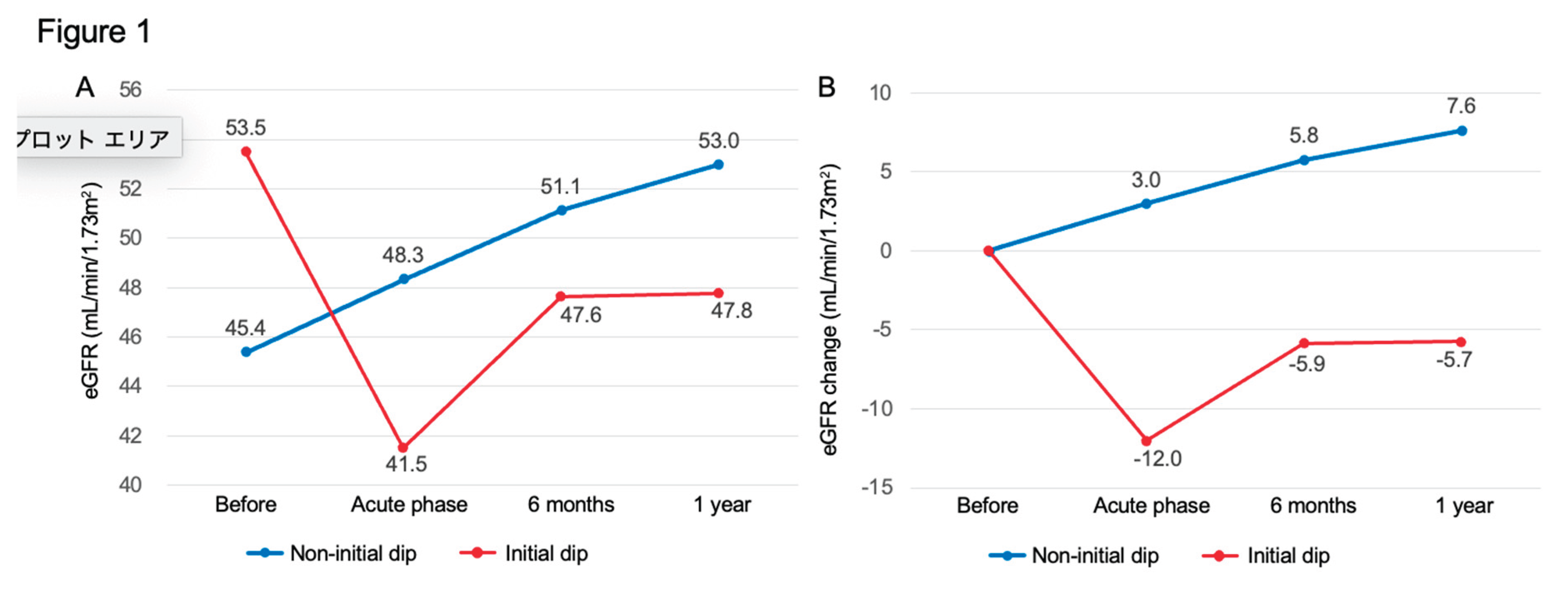
Figure 1 illustrates the trajectory of mean values of eGFR (panel A) and change in eGFR (panel B) at baseline, the initial dip timepoint, 6 months, and 1 year in the 56 patients with complete follow-up data. The non-initial dip group showed a trend toward increasing eGFR over time, whereas the initial dip group did not recover to baseline eGFR values at either 6 months or 1 year, with eGFR persistently declining.
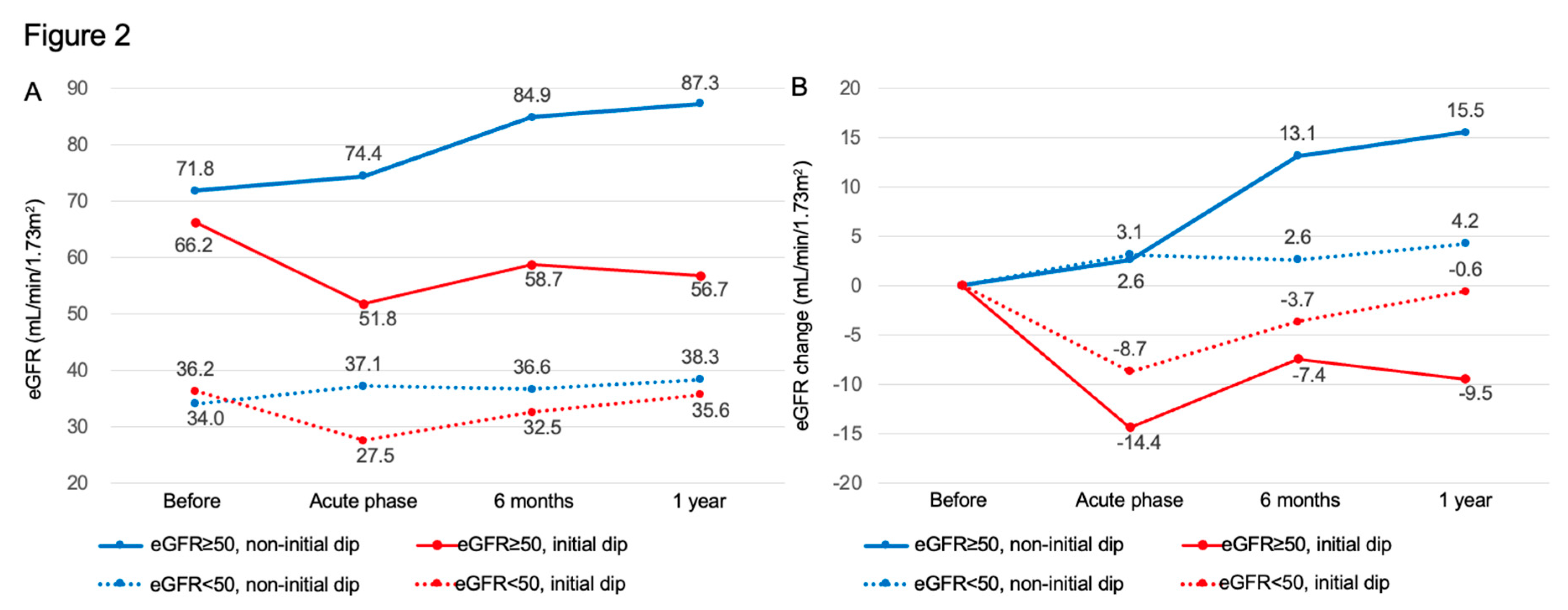
Figure 2 shows mean values of eGFR (panel A) and the corresponding changes (panel B) stratified by renal function status (normal renal function: eGFR ≥50 mL/min/1.73 m²; impaired renal function: eGFR <50 mL/min/1.73 m²) and by the presence or absence of an initial dip. Notably, in patients with eGFR ≥50 mL/min/1.73 m² who experienced an initial dip, the mean change in eGFR was −7.4 mL/min/1.73 m² at 6 months and −9.5 mL/min/1.73 m² at one year, indicating a sustained decline without recovery to baseline levels.
4. Discussion
To our knowledge, this is the first study to demonstrate that older age, presence of diabetes mellitus and/or hypertension, and a higher baseline eGFR prior to initiating dapagliflozin were associated with an increased likelihood of developing an initial dip in eGFR in chronic heart failure. Previous reports have identified several risk factors for the initial dip, including advanced age, elevated body mass index, higher systolic blood pressure, low hemoglobin levels, smoking, and the use of diuretics.[
17] Additionally, baseline use of diuretics and higher Kidney Disease: Improving Global Outcomes (KDIGO) risk categories have also been associated with the initial dip.[
19] Our findings are consistent with previous studies in that older age and comorbid hypertension were significant contributing factors.
In older individuals, sarcopenia and increased adiposity result in reduced lean body mass and total body water, which may contribute to latent renal dysfunction. Although individual variation exists, eGFR typically declines with age, independent of underlying kidney disease. Aging kidneys exhibit reductions in renal mass and cortical thickness, as well as nephron loss and vascular changes such as luminal narrowing of arterioles, all of which are exacerbated by hypertension or diabetes.[
20,
21] Given these physiological vulnerabilities, clinicians should consider cautious initiation of dapagliflozin in elderly patients, ensuring that blood pressure and glycemic control are well-optimized beforehand.
Furthermore, our study demonstrated that the presence of an initial dip was associated with greater declines in eGFR at both 6 months and 1 year. Among those who experienced an initial dip, higher baseline eGFR was associated with greater long-term decline. In the setting of chronic heart failure, acute or chronic dysfunction of the heart or kidneys can lead to deterioration of the other organ, a condition referred to as the cardiorenal syndrome.[
22] Renal function often deteriorates gradually over the course of heart failure. Sustained hypoperfusion may induce inflammatory cytokine production and profibrotic signaling, aggravating endothelial injury and promoting renal impairment. Additionally, activation of the sympathetic nervous system and renin–angiotensin system can further exacerbate glomerular dysfunction.[
23] Some reports have suggested that renal impairment during heart failure is more strongly influenced by elevated central venous pressure than by reductions in cardiac output.[
24] These findings highlight the need to consider the role of chronic heart failure–related renal dysfunction in the development of the initial dip and long-term renal trajectory following dapagliflozin initiation.
Multiple randomized controlled trials have demonstrated the cardiovascular benefits of SGLT2 inhibitors, including reductions in heart failure hospitalizations and renal composite outcomes, as well as a slowing of eGFR decline.[
25,
26] However, there is some evidence that the renal efficacy of SGLT2 inhibitors in preventing heart failure progression may diminish in patients with advanced renal dysfunction.[
26] Thus, minimizing the initial dip and preserving long-term renal function is crucial to achieving optimal disease control in chronic heart failure.
The DAPA-CKD trial and database studies have shown that SGLT2 inhibitors are not associated with an increased risk of acute kidney injury (AKI).[
11,
27] Moreover, several studies have suggested that the initial dip does not adversely affect long-term renal function.[
16,
17,
19] However, these studies typically evaluated renal outcomes using the post–initial dip eGFR as the new baseline. In contrast, our study assessed long-term eGFR changes relative to the original pre-treatment baseline. Since the eGFR in the initial dip group did not return to baseline levels after the early decline, this distinction may explain the greater cumulative eGFR loss compared to those without an initial dip. In clinical practice, a sustained decline in eGFR can lead to dose adjustments or discontinuation of renally excreted medications, potentially compromising heart failure management. In our study, patients with preserved renal function at baseline (eGFR ≥50 mL/min/1.73 m²) who developed an initial dip did not experience eGFR recovery at 6 months or 1 year. These findings suggest that avoiding the initial dip is important not only for preserving renal function from baseline but also for ensuring long-term renal stability. Moreover, the presence of an initial dip may serve as a clinical marker of the long-term renoprotective efficacy of SGLT2 inhibitors, indicating a need for further research into early therapeutic strategies for managing patients with initial dip.
Although concomitant medications were not associated with the initial dip in this study, previous reports have identified loop diuretics as a risk factor for AKI during SGLT2 inhibitor therapy.[
28] Patients with heart failure or advanced age are particularly prone to intravascular volume depletion, which can lead to renal hypoperfusion and AKI. The concurrent use of ACE inhibitors, ARBs, and NSAIDs may also increase the risk of volume depletion. In such situations, clinicians should consider withholding or adjusting concomitant medications or delaying dapagliflozin initiation to avoid worsening renal function.
Limitations
This study has several limitations. First, it was a retrospective analysis based on electronic medical records, and some data were missing. Moreover, due to the small sample size and the retrospective nature of the study, it was not feasible to account for cardiac function or heart failure events at each time point, which may have influenced the observed renal function trajectories. Although we employed multiple imputation under the assumption that data were missing at random, sensitivity analyses conducted without imputation yielded consistent results. Second, as a single-center study with a relatively small sample size, the generalizability of our findings may be limited. While the non–initial dip group showed an upward trend in eGFR over time, such improvement is rarely observed in patients with heart failure, regardless of SGLT2 inhibitor use. This unexpected finding may be attributable to the small sample size. Future prospective studies with larger cohorts and complete datasets are warranted to validate these observations.
5. Conclusions
This study suggested that older age, hypertension, and diabetes are risk factors for the development of an initial dip in eGFR following the initiation of dapagliflozin. In such patients, careful initiation of therapy with adequate control of blood pressure and glycemia, along with close monitoring of renal function during the early phase of treatment, is warranted. Notably, in patients with preserved renal function at baseline, the occurrence of an initial dip was associated with sustained long-term declines in eGFR. These findings indicated that the initial dip may serve as a prognostic indicator of long-term renoprotection, and strategies to mitigate its occurrence should be further investigated.
Author Contributions
Conceptualization, R.O. and Y.F.; methodology, R.O. and T.K.; formal analysis, N.K. and K.F.; investigation, K.T. and K.H.; data curation, R.O. and T.K.; writing—original draft preparation, R.O.; writing—review and editing, K.H., and Y.F.; visualization, R.O.; supervision, K.H., and Y.F.; project administration, R.O. and T.K.. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The present study received ethical approval from the Ethics Committee of Kurume University Hospital (approval no. 23156). The study was conducted following the ethical principles outlined in the Declaration of Helsinki.
Informed Consent Statement
The informed consent was waived due to the retrospective nature and opt-out was used in the study.
Data Availability Statement
The data underlying this article will be shared with others for the purpose of reproducing results or replicating procedures upon reasonable request to the corresponding author, subject to institutional and ethics committee approval.
Conflicts of Interest
Y.F. has received research honoraria (lecture fees) from AstraZeneca KK and Ono Pharmaceutical Co., Ltd. Other authors have no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Abbreviations
The following abbreviations are used in this manuscript:
| SGLT2 |
sodium-glucose cotransporter 2 |
| CHF |
chronic heart failure |
| eGFR |
estimated glomerular filtration rate |
| HFrEF |
heart failure with reduced ejection fraction |
| HFmrEF |
heart failure with mildly reduced ejection fraction |
| HFpEF |
heart failure with preserved ejection fraction |
| CKD |
chronic kidney disease |
| AST |
aspartate aminotransferase |
| ALT |
alanine aminotransferase |
| BUN |
blood urea nitrogen |
| CRP |
C-reactive protein |
| SDs |
standard deviations |
| MAR |
missing at random |
| ACE |
angiotensin-converting enzyme |
| ARB |
angiotensin II receptor blockers |
| OR |
odds ratio |
| CI |
confidence interval |
| AKI |
acute kidney injury |
References
- Azad, N.; Lemay, G. Management of chronic heart failure in the older population. J Geriatr Cardiol 2014, 11, 329-337. [CrossRef]
- Shibata, T.; Mawatari, K.; Nakashima, N.; Shimozono, K.; Ushijima, K.; Yamaji, Y.; Tetsuka, K.; Murakami, M.; Okabe, K.; Yanai, T.; et al. Multidisciplinary Team-Based Palliative Care for Heart Failure and Food Intake at the End of Life. Nutrients 2021, 13. [CrossRef]
- Fukumoto, Y.; Tada, T.; Suzuki, H.; Nishimoto, Y.; Moriuchi, K.; Arikawa, T.; Adachi, H.; Momomura, S.I.; Seino, Y.; Yasumura, Y.; et al. Chronic Effects of Adaptive Servo-Ventilation Therapy on Mortality and the Urgent Rehospitalization Rate in Patients Experiencing Recurrent Admissions for Heart Failure - A Multicenter Prospective Observational Study (SAVIOR-L). Circ J 2024, 88, 692-702. [CrossRef]
- Tsutsui, H.; Isobe, M.; Ito, H.; Ito, H.; Okumura, K.; Ono, M.; Kitakaze, M.; Kinugawa, K.; Kihara, Y.; Goto, Y.; et al. JCS 2017/JHFS 2017 Guideline on Diagnosis and Treatment of Acute and Chronic Heart Failure - Digest Version. Circ J 2019, 83, 2084-2184. [CrossRef]
- Tsutsui, H.; Ide, T.; Ito, H.; Kihara, Y.; Kinugawa, K.; Kinugawa, S.; Makaya, M.; Murohara, T.; Node, K.; Saito, Y.; et al. JCS/JHFS 2021 Guideline Focused Update on Diagnosis and Treatment of Acute and Chronic Heart Failure. Circ J 2021, 85, 2252-2291. [CrossRef]
- Heidenreich, P.A.; Bozkurt, B.; Aguilar, D.; Allen, L.A.; Byun, J.J.; Colvin, M.M.; Deswal, A.; Drazner, M.H.; Dunlay, S.M.; Evers, L.R.; et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol 2022, 79, e263-e421. [CrossRef]
- Dunlay, S.M.; Roger, V.L.; Redfield, M.M. Epidemiology of heart failure with preserved ejection fraction. Nat Rev Cardiol 2017, 14, 591-602. [CrossRef]
- Solomon, S.D.; Vaduganathan, M.; Claggett, B.L.; de Boer, R.A.; DeMets, D.; Hernandez, A.F.; Inzucchi, S.E.; Kosiborod, M.N.; Lam, C.S.P.; Martinez, F.; et al. Baseline Characteristics of Patients With HF With Mildly Reduced and Preserved Ejection Fraction: DELIVER Trial. JACC Heart Fail 2022, 10, 184-197. [CrossRef]
- Jhund, P.S.; Kondo, T.; Butt, J.H.; Docherty, K.F.; Claggett, B.L.; Desai, A.S.; Vaduganathan, M.; Gasparyan, S.B.; Bengtsson, O.; Lindholm, D.; et al. Dapagliflozin across the range of ejection fraction in patients with heart failure: a patient-level, pooled meta-analysis of DAPA-HF and DELIVER. Nat Med 2022, 28, 1956-1964. [CrossRef]
- Verma, S.; McMurray, J.J.V. SGLT2 inhibitors and mechanisms of cardiovascular benefit: a state-of-the-art review. Diabetologia 2018, 61, 2108-2117. [CrossRef]
- Heerspink, H.J.L.; Stefansson, B.V.; Correa-Rotter, R.; Chertow, G.M.; Greene, T.; Hou, F.F.; Mann, J.F.E.; McMurray, J.J.V.; Lindberg, M.; Rossing, P.; et al. Dapagliflozin in Patients with Chronic Kidney Disease. N Engl J Med 2020, 383, 1436-1446. [CrossRef]
- Cherney, D.Z.I.; Dekkers, C.C.J.; Barbour, S.J.; Cattran, D.; Abdul Gafor, A.H.; Greasley, P.J.; Laverman, G.D.; Lim, S.K.; Di Tanna, G.L.; Reich, H.N.; et al. Effects of the SGLT2 inhibitor dapagliflozin on proteinuria in non-diabetic patients with chronic kidney disease (DIAMOND): a randomised, double-blind, crossover trial. Lancet Diabetes Endocrinol 2020, 8, 582-593. [CrossRef]
- Cherney, D.Z.; Odutayo, A.; Aronson, R.; Ezekowitz, J.; Parker, J.D. Sodium Glucose Cotransporter-2 Inhibition and Cardiorenal Protection: JACC Review Topic of the Week. J Am Coll Cardiol 2019, 74, 2511-2524. [CrossRef]
- Bailey, C.J.; Day, C.; Bellary, S. Renal Protection with SGLT2 Inhibitors: Effects in Acute and Chronic Kidney Disease. Curr Diab Rep 2022, 22, 39-52. [CrossRef]
- Fioretto, P.; Zambon, A.; Rossato, M.; Busetto, L.; Vettor, R. SGLT2 Inhibitors and the Diabetic Kidney. Diabetes Care 2016, 39 Suppl 2, S165-171. [CrossRef]
- Oshima, M.; Jardine, M.J.; Agarwal, R.; Bakris, G.; Cannon, C.P.; Charytan, D.M.; de Zeeuw, D.; Edwards, R.; Greene, T.; Levin, A.; et al. Insights from CREDENCE trial indicate an acute drop in estimated glomerular filtration rate during treatment with canagliflozin with implications for clinical practice. Kidney Int 2021, 99, 999-1009. [CrossRef]
- Jongs, N.; Chertow, G.M.; Greene, T.; McMurray, J.J.V.; Langkilde, A.M.; Correa-Rotter, R.; Kashihara, N.; Rossing, P.; Sjostrom, C.D.; Stefansson, B.V.; et al. Correlates and Consequences of an Acute Change in eGFR in Response to the SGLT2 Inhibitor Dapagliflozin in Patients with CKD. J Am Soc Nephrol 2022, 33, 2094-2107. [CrossRef]
- Chertow, G.M.; Vart, P.; Jongs, N.; Toto, R.D.; Gorriz, J.L.; Hou, F.F.; McMurray, J.J.V.; Correa-Rotter, R.; Rossing, P.; Sjostrom, C.D.; et al. Effects of Dapagliflozin in Stage 4 Chronic Kidney Disease. J Am Soc Nephrol 2021, 32, 2352-2361. [CrossRef]
- Kraus, B.J.; Weir, M.R.; Bakris, G.L.; Mattheus, M.; Cherney, D.Z.I.; Sattar, N.; Heerspink, H.J.L.; Ritter, I.; von Eynatten, M.; Zinman, B.; et al. Characterization and implications of the initial estimated glomerular filtration rate 'dip' upon sodium-glucose cotransporter-2 inhibition with empagliflozin in the EMPA-REG OUTCOME trial. Kidney Int 2021, 99, 750-762. [CrossRef]
- Lindeman, R.D.; Tobin, J.; Shock, N.W. Longitudinal studies on the rate of decline in renal function with age. J Am Geriatr Soc 1985, 33, 278-285. [CrossRef]
- Denic, A.; Lieske, J.C.; Chakkera, H.A.; Poggio, E.D.; Alexander, M.P.; Singh, P.; Kremers, W.K.; Lerman, L.O.; Rule, A.D. The Substantial Loss of Nephrons in Healthy Human Kidneys with Aging. J Am Soc Nephrol 2017, 28, 313-320. [CrossRef]
- Shamseddin, M.K.; Parfrey, P.S. Mechanisms of the cardiorenal syndromes. Nat Rev Nephrol 2009, 5, 641-649. [CrossRef]
- Damman, K.; Testani, J.M. The kidney in heart failure: an update. Eur Heart J 2015, 36, 1437-1444. [CrossRef]
- Damman, K.; van Deursen, V.M.; Navis, G.; Voors, A.A.; van Veldhuisen, D.J.; Hillege, H.L. Increased central venous pressure is associated with impaired renal function and mortality in a broad spectrum of patients with cardiovascular disease. J Am Coll Cardiol 2009, 53, 582-588. [CrossRef]
- Packer, M.; Anker, S.D.; Butler, J.; Filippatos, G.; Pocock, S.J.; Carson, P.; Januzzi, J.; Verma, S.; Tsutsui, H.; Brueckmann, M.; et al. Cardiovascular and Renal Outcomes with Empagliflozin in Heart Failure. N Engl J Med 2020, 383, 1413-1424. [CrossRef]
- Zannad, F.; Ferreira, J.P.; Pocock, S.J.; Zeller, C.; Anker, S.D.; Butler, J.; Filippatos, G.; Hauske, S.J.; Brueckmann, M.; Pfarr, E.; et al. Cardiac and Kidney Benefits of Empagliflozin in Heart Failure Across the Spectrum of Kidney Function: Insights From EMPEROR-Reduced. Circulation 2021, 143, 310-321. [CrossRef]
- Cahn, A.; Melzer-Cohen, C.; Pollack, R.; Chodick, G.; Shalev, V. Acute renal outcomes with sodium-glucose co-transporter-2 inhibitors: Real-world data analysis. Diabetes Obes Metab 2019, 21, 340-348. [CrossRef]
- Yang, L.; Gabriel, N.; Hernandez, I.; Vouri, S.M.; Kimmel, S.E.; Bian, J.; Guo, J. Identifying Patients at Risk of Acute Kidney Injury Among Medicare Beneficiaries With Type 2 Diabetes Initiating SGLT2 Inhibitors: A Machine Learning Approach. Front Pharmacol 2022, 13, 834743. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).