Submitted:
20 June 2025
Posted:
23 June 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Animals
2.3. Mouse Model of Long-Term High-Fructose High-Fat (HFHF) Diet
2.4. Intraperitoneal Glucose Tolerance Test (IPGTT) and Biochemical Blood Analysis
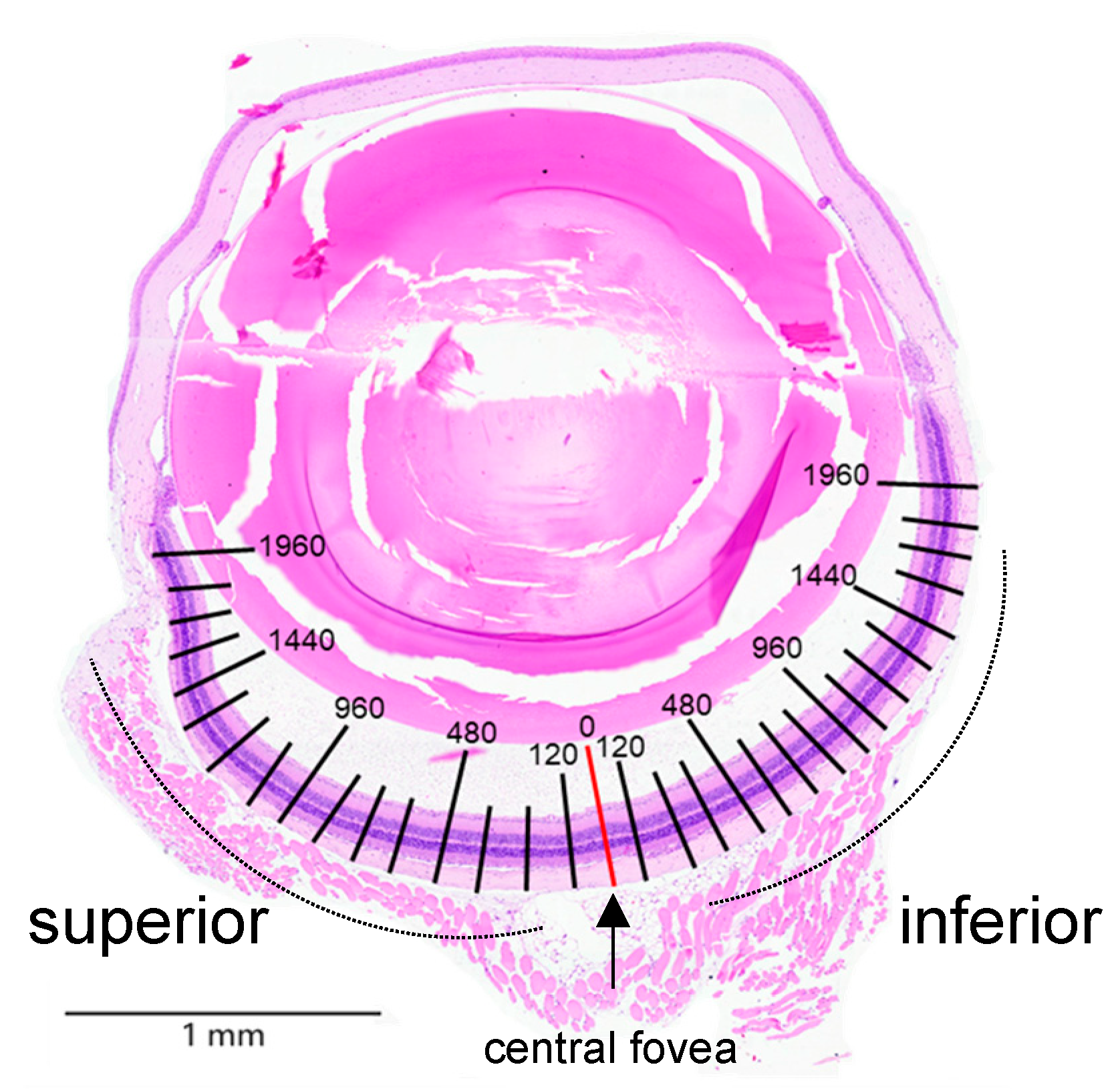
2.5. Procedures for Eye Fixation and Histopathological Analysis
2.6. Immunofluorescence Staining
2.7. Serum MDA and Fluorescent AGE Levels
2.8. TUNEL Assay
2.9. Statistical Analysis
3. Results
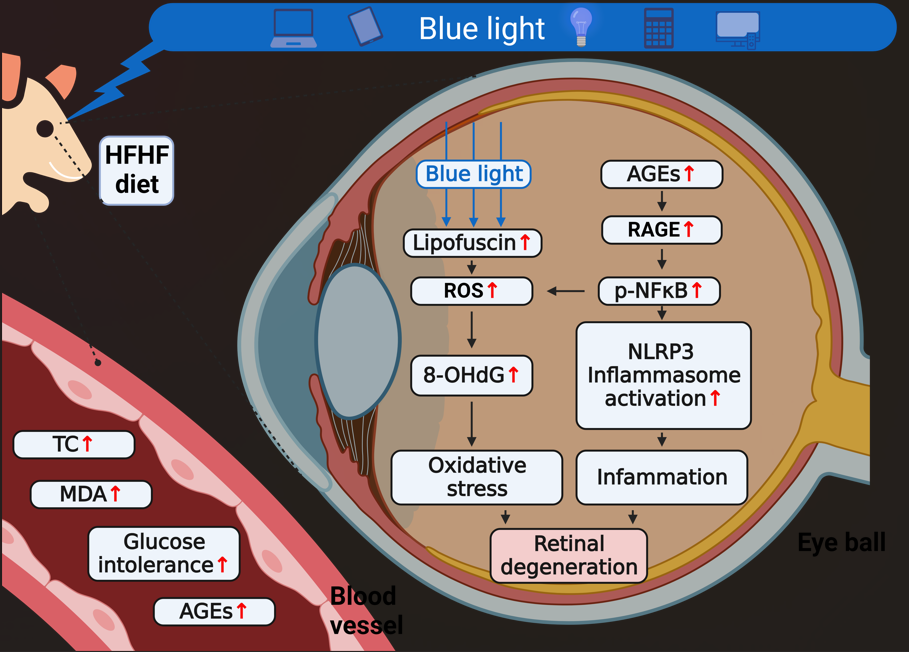
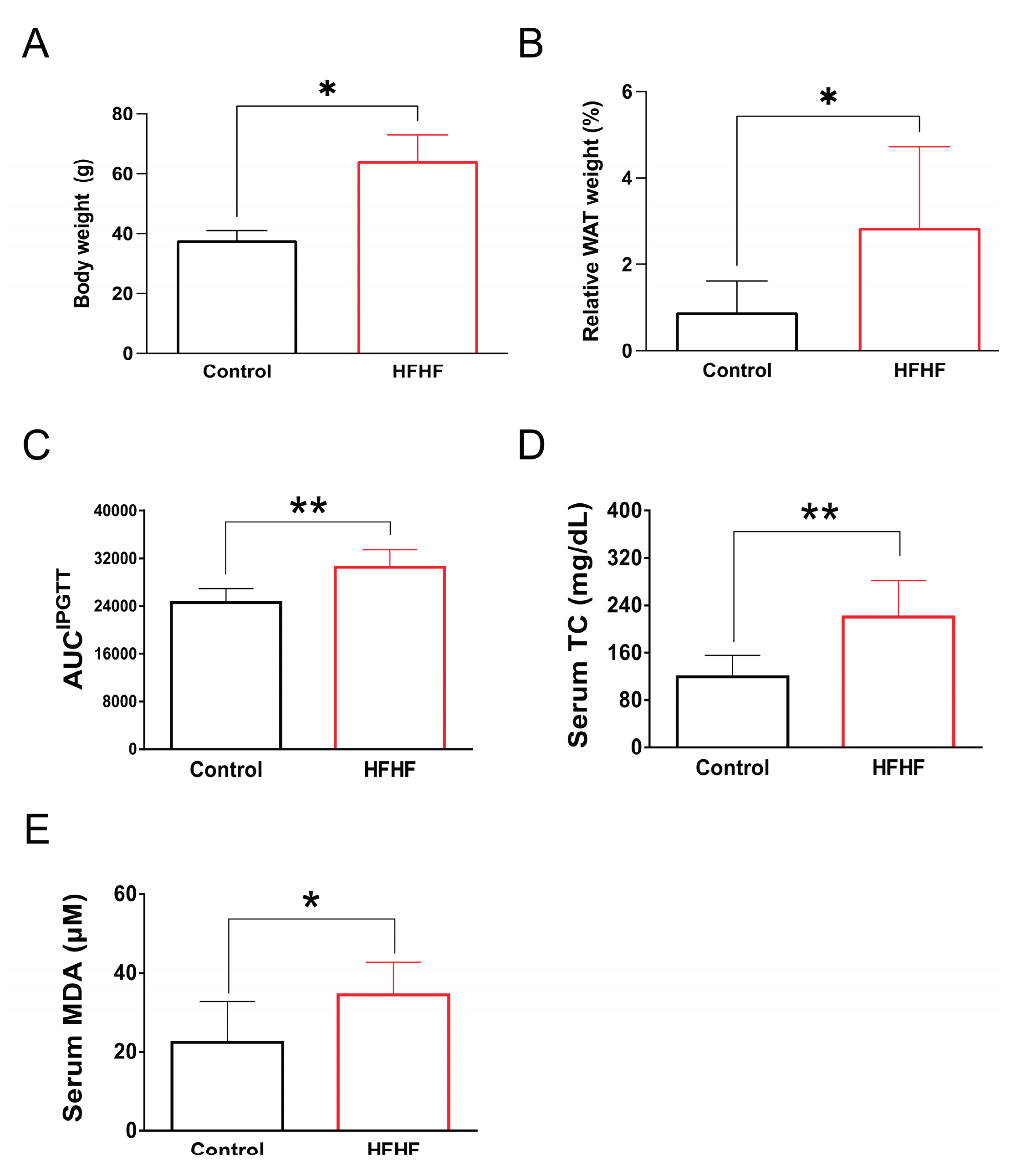
3.1. Physiological and Biochemical Alterations Induced by HFHF Diet
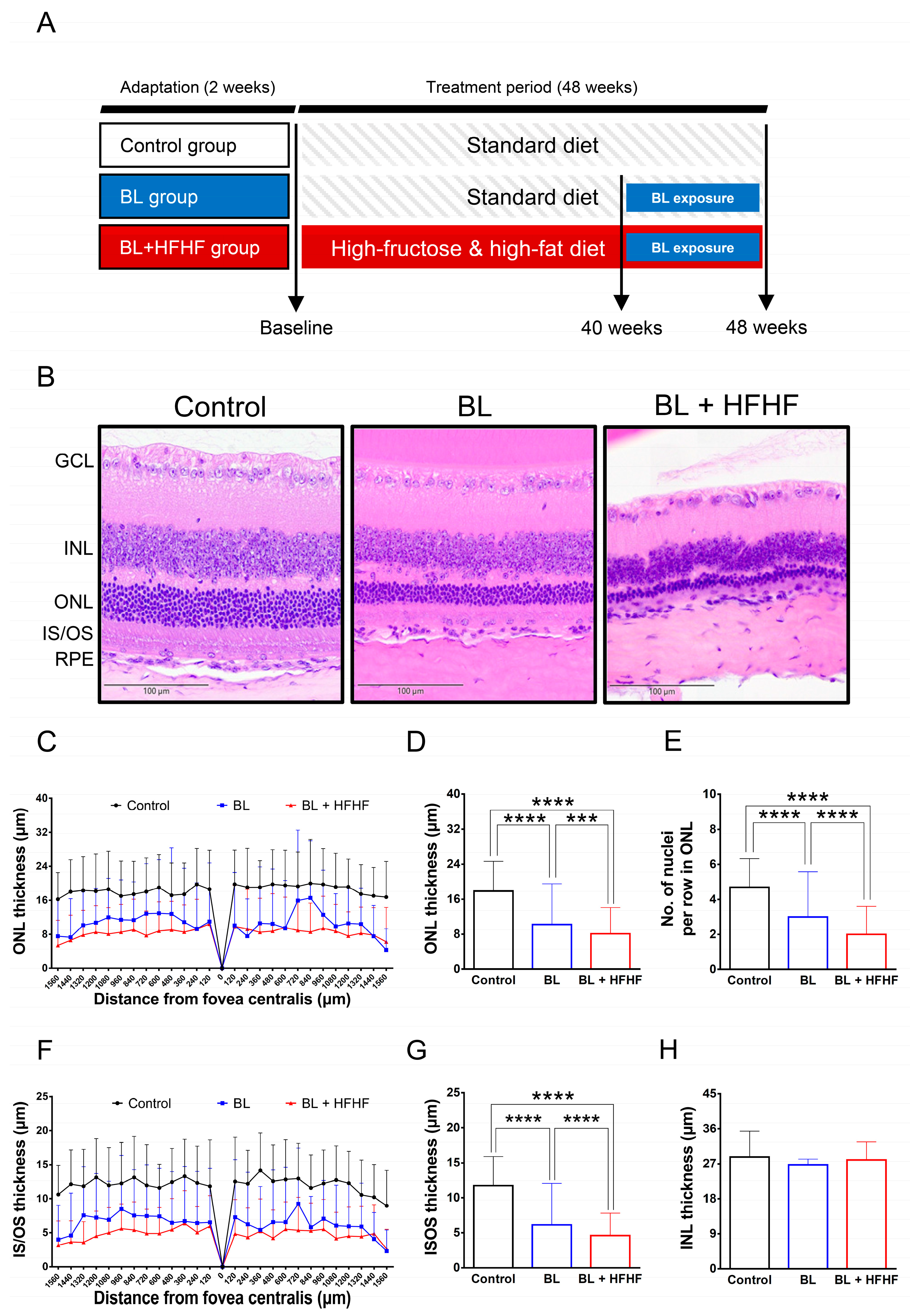
3.2. Potentiation of BL-Induced Photoreceptor Degeneration by HFHF Diet
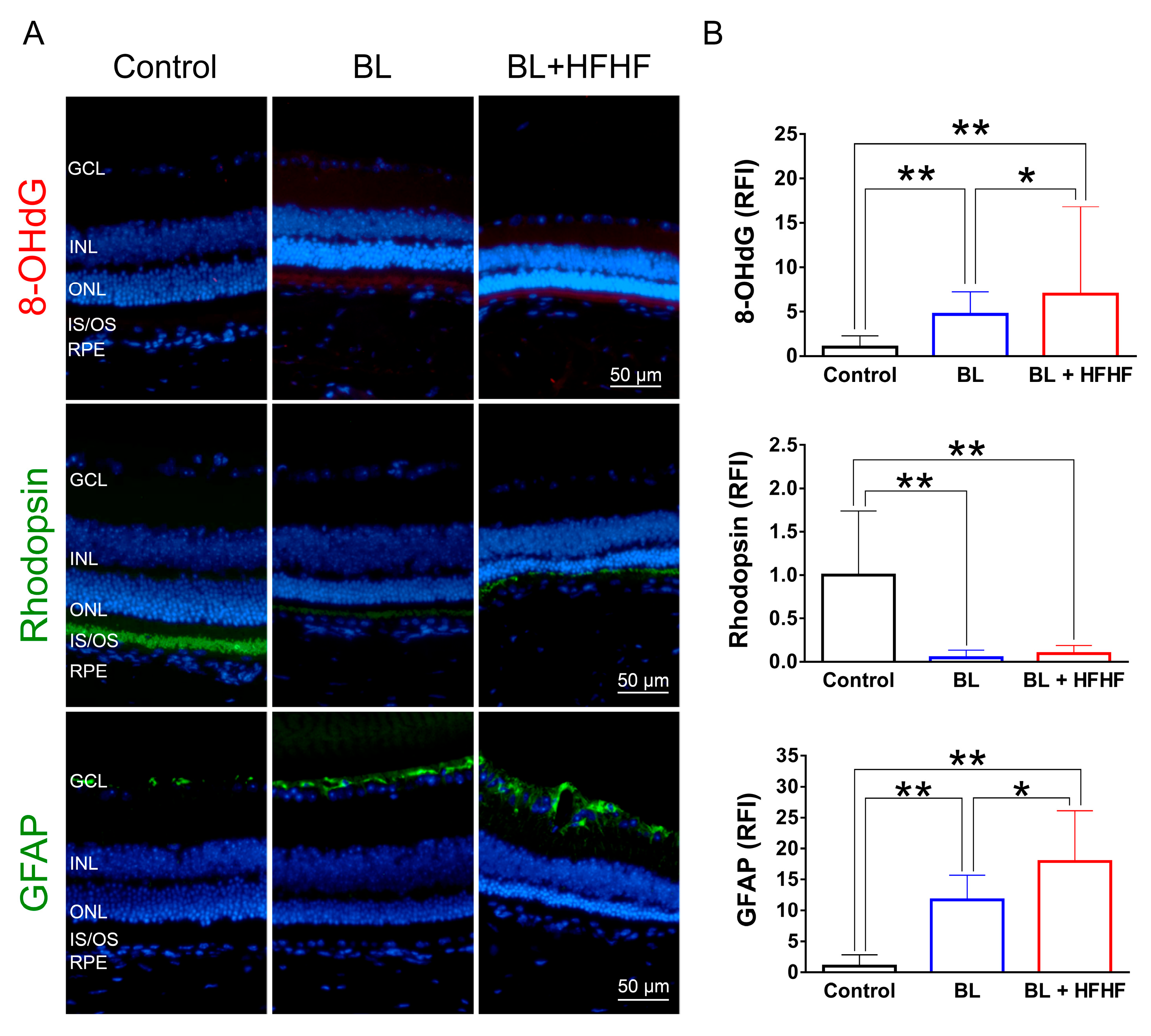
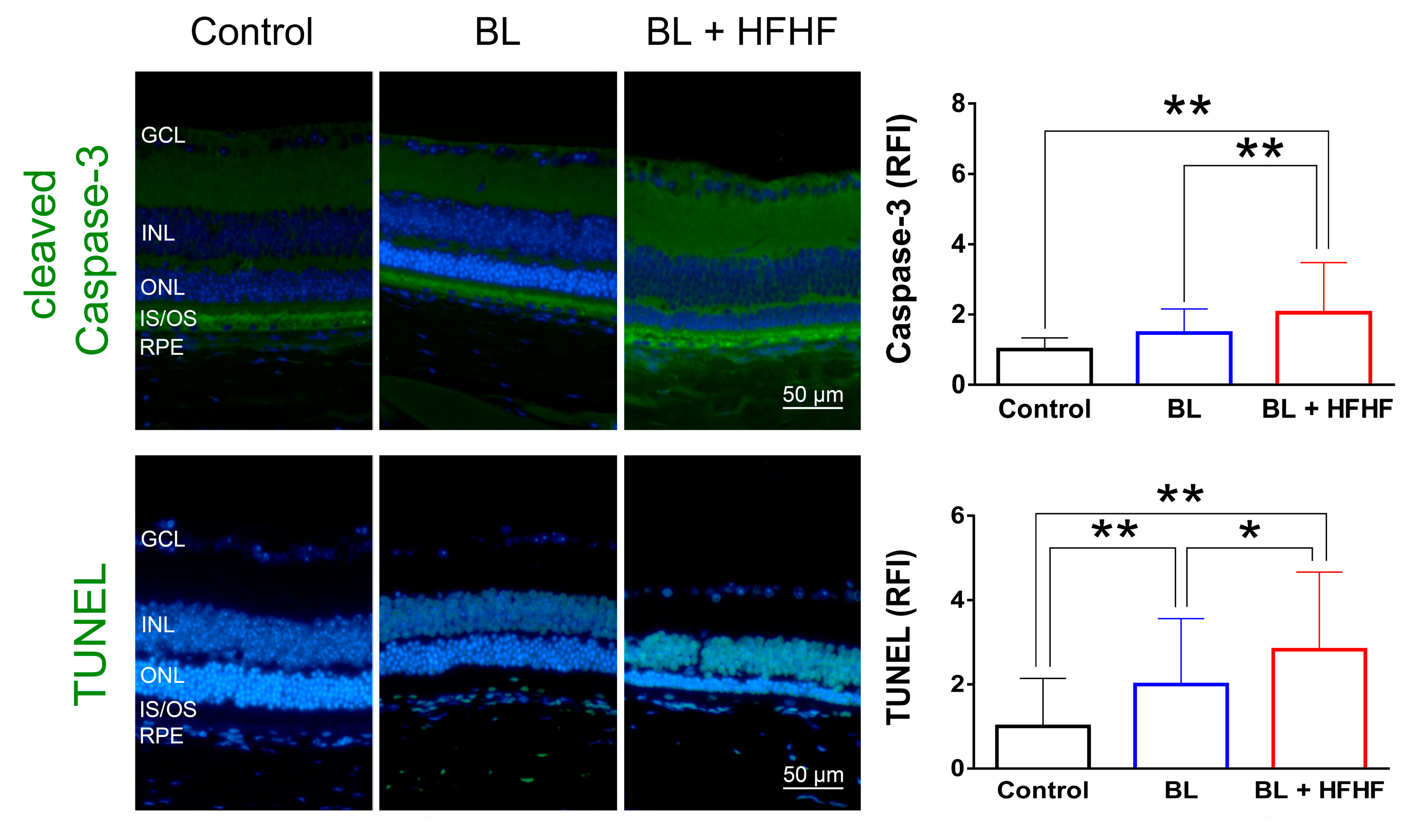
3.3. HFHF Diet Potentiates Oxidative Stress, Triggers Apoptosis, and Promotes the Activation of Müller cells Under BL Exposure
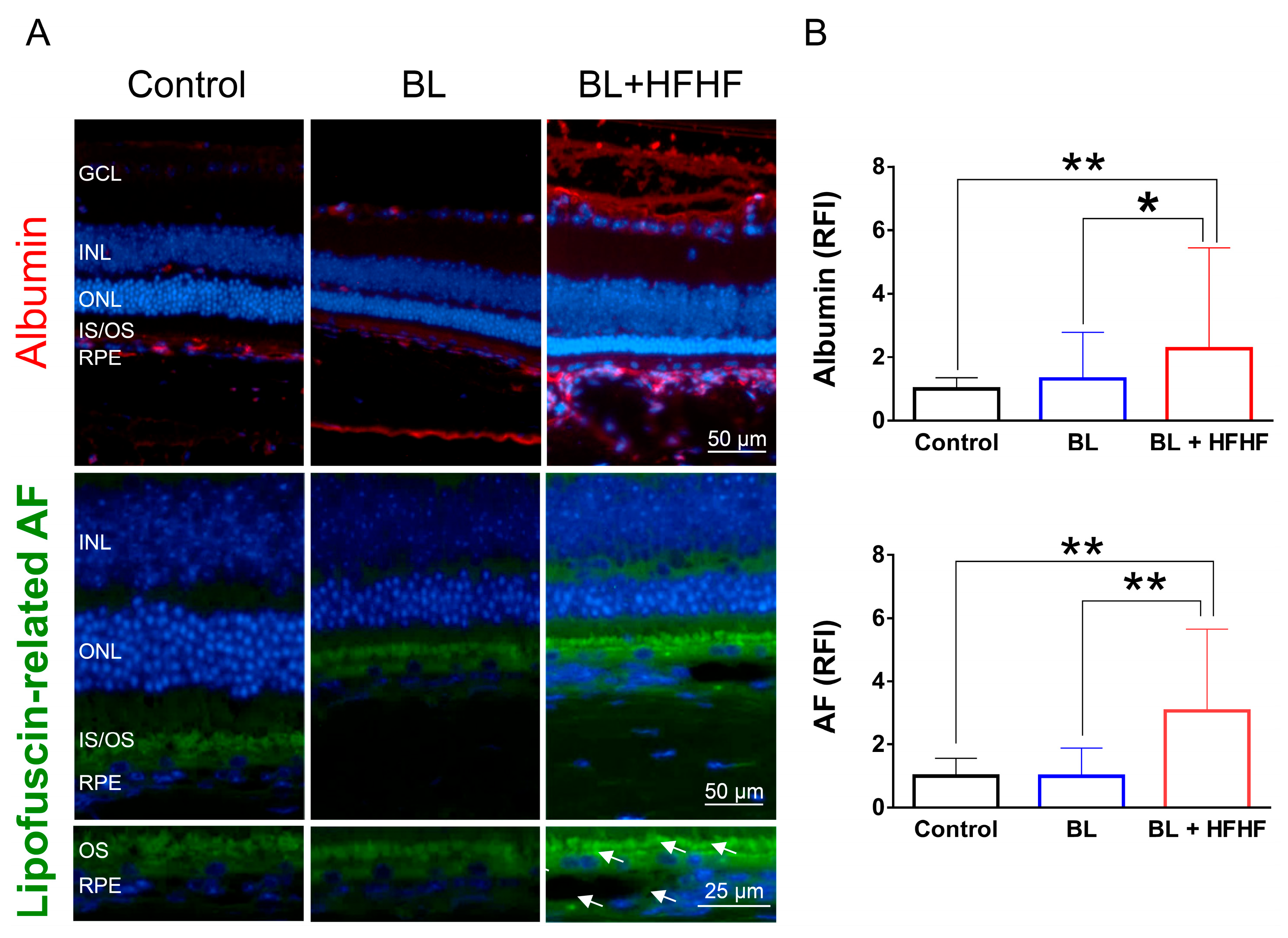
3.4. HFHF Diet Disrupts BRB Integrity and Promotes Lipofuscin Accumulation Under BL Exposure
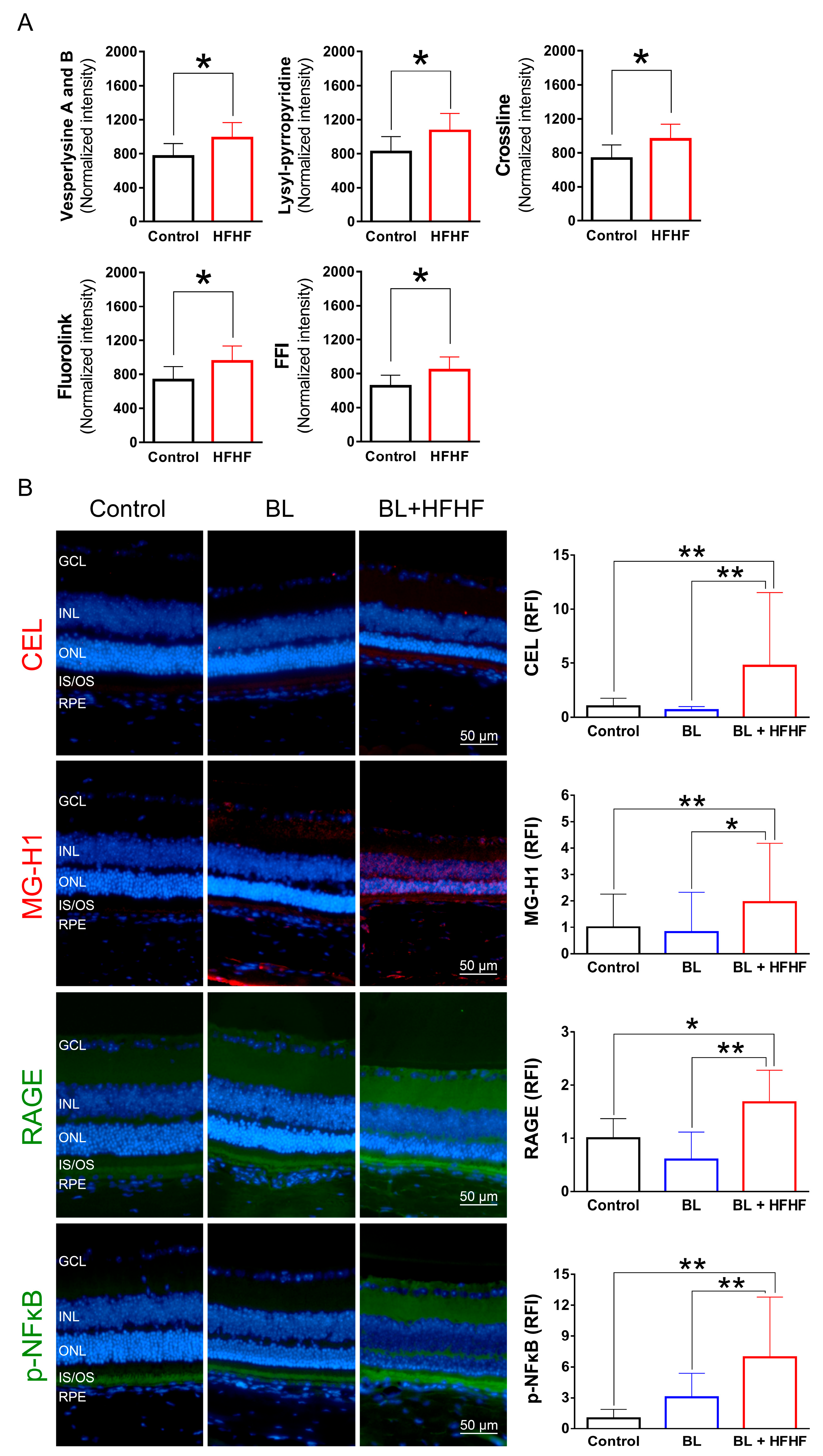
3.5. HFHF Diet Induces the Formation of AGEs and Triggers the Activation of RAGE and NFκB in the Retina
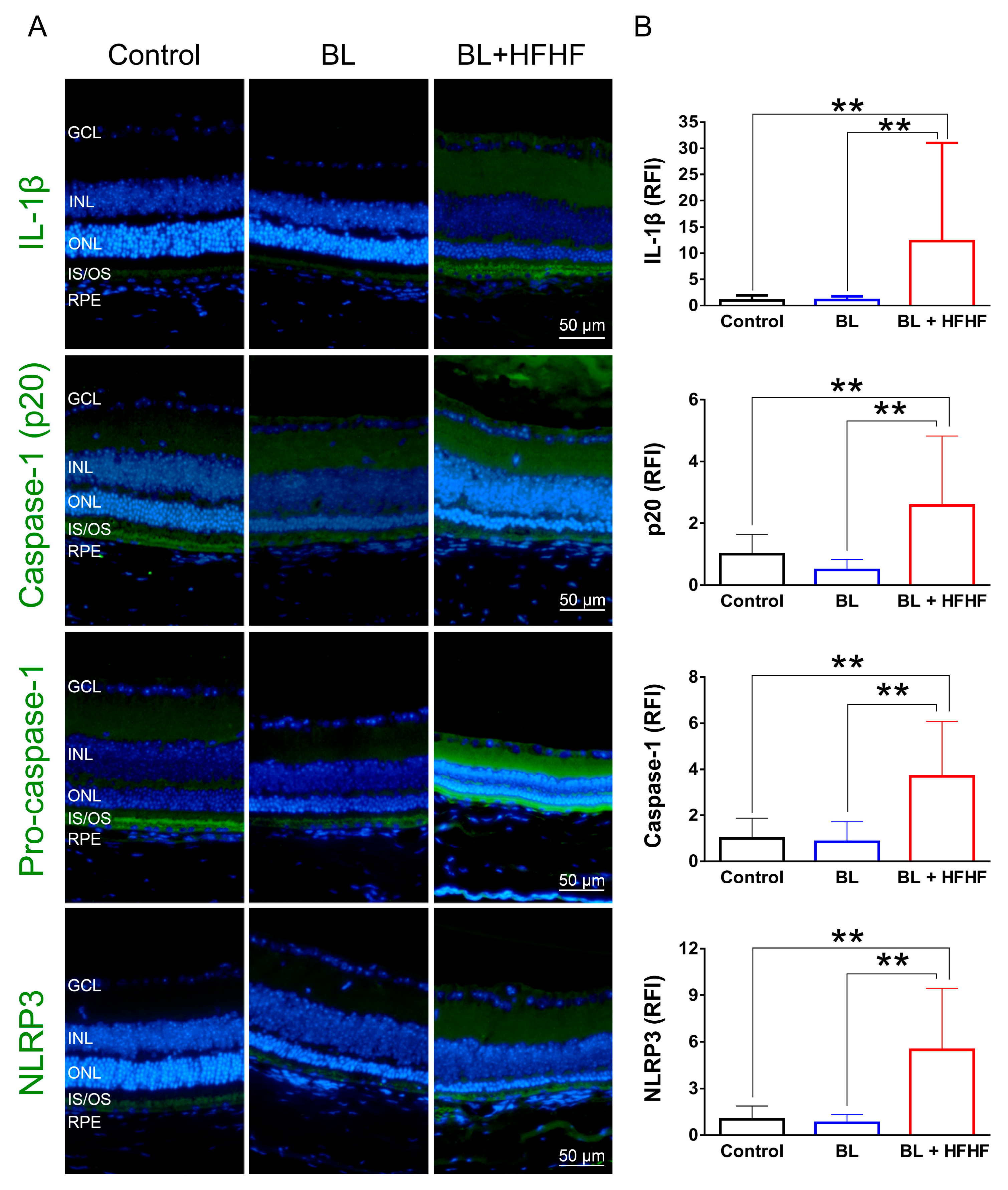
3.6. HFHF Diet Triggers NLRP3 Inflammasome Activation Under BL Exposure
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AGE | Advanced glycated end products |
| AMD | Age-related macular degeneration |
| AUC | Area under curve |
| BL | Blue light |
| BRB | Blood retinal barrier |
| CEL | Nε-(1-carboxyethyl)lysine |
| CML | Nε-(carboxymethyl)lysine |
| ERG | Electroretinogram |
| FFI | 2-(2-furoyl)-4(5)-(2-furanyl)-1H-imidazole |
| GFAP | Glial fibrillary acidic protein |
| H&E | Hematoxylin and eosin |
| HFHF | High-fructose and high-fat |
| IL-1β | Interleukin-1 beta |
| INL | Inner nuclear layer |
| IPGTT | The intraperitoneal glucose tolerance test |
| IS/OS | Inner/outer segment |
| LED | Light-emitting diodes |
| MDA | Malonaldehyde |
| MG-H1 | Nδ-(5-Methyl-4-imidazolon-2-yl)-L-ornithine |
| NFκB | Nuclear factor kappa-light-chain-enhancer of activated B cells |
| Nrf2 | Nuclear factor erythroid 2-related factor 2 |
| 8-OHdG | 8-hydroxy-2-deoxyguanosine |
| ONL | Outer nuclear layer |
| RAGE | Receptor for advanced glycated end product |
| RFI | Relative fluorescence intensity |
| ROS | Reactive oxygen species |
| RPE | Retinal pigment epithelium |
| TNF-α | Tumor necrosis factor-alpha |
| TUNEL | Terminal deoxynucleotidyl transferase dUTP nick end labeling |
| WAT | White adipose tissue |
Appendix A
| Antibody (Ab) | Company/Country | Catalog No. | Dilution |
|---|---|---|---|
| Anti-GFAP Ab | Merk/Germany | AB5804 | 1:1000 |
| Anti-rhodopsin Ab (Rho 4D2) | Abcam/UK | ab9887 | 1:1000 |
| Cleaved caspase-3 rabbit mAb (Asp175, 5A1E) | Cell signaling Technology/USA | 9664 | 1:1600 |
| IL-1β/IL-1F2 Ab | Novus Biologicals/USA | NB600-633 | 1:500 |
| TNF Alpha Ab | ProteinTech/USA | 60291-1-Ig | 1:250 |
| 8-OHdG Ab (15A3) | Novus Biologicals/USA | NB110-96878 | 1:750 |
| Anti-RAGE Ab | Abcam/UK | ab3611 | 1:200 |
| Anti-Nε-(carboxyethyl) lysine (CEL) Ab | COSMO BIO/Japan | AGE-M02 | 1:200 |
| Anti-AGEs monoclonal Ab | Trans Gentic/Japan | KH001 | 1:200 |
| Anti-Methylglyoxal (MG-H1) Monoclonal Ab | CELL BIOLABS/USA | STA-011 | 1:400 |
| Mouse Albumin Ab | Bethyl/USA | A90-134A | 1:800 |
| Phospho-NF-κB p65 (Ser536) (93H1) Rabbit mAb | Cell signaling Technology/USA | 3033 | 1:1600 |
| Recombinant Anti-NLRP3 Ab | Abcam/UK | ab263899 | 1:500 |
| Caspase-1 Ab | Affinity biosciences/ Ohio, USA | AF5418 | 1:200 |
| Cleaved-Caspase 1 (Asp296), p20 Ab | Affinity biosciences/USA | AF4005 | 1:200 |
| Alexa Fluor® 488 goat anti-rabbit igG H&L | Abcam/UK | ab150077 | 1:400 |
| Alexa Fluor® 488 goat anti-mouse igG H&L | Abcam/UK | ab150113 | 1:800 |
| Alexa Fluor® 568 | Abcam/UK | ab175473 | 1:400 |
Appendix B
References
- Kepios Pte. Ltd. Digital 2023 Global Digital Overview. 2023. [Google Scholar]
- Trott, M.; Driscoll, R.; Iraldo, E.; Pardhan, S. Changes and correlates of screen time in adults and children during the COVID-19 pandemic: A systematic review and meta-analysis. EClinicalMedicine 2022, 48. [Google Scholar] [CrossRef] [PubMed]
- Yeh, W.-J.; Chien, P.-T.; Wen, Y.-T.; Wu, C.-H. A comprehensive review of experimental models for investigating blue light-induced ocular damage: Insights into parameters, limitations, and new opportunities. Experimental Eye Research 2024, 110142. [Google Scholar] [CrossRef]
- Geiger, P.; Barben, M.; Grimm, C.; Samardzija, M. Blue light-induced retinal lesions, intraretinal vascular leakage and edema formation in the all-cone mouse retina. Cell death & disease 2015, 6, e1985–e1985. [Google Scholar]
- Shang, Y.-M.; Wang, G.-S.; Sliney, D.; Yang, C.-H.; Lee, L.-L. White light–emitting diodes (LEDs) at domestic lighting levels and retinal injury in a rat model. Environmental health perspectives 2014, 122, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Tao, J.-X.; Zhou, W.-C.; Zhu, X.-G. Mitochondria as potential targets and initiators of the blue light hazard to the retina. Oxidative medicine and cellular longevity 2019, 2019, 6435364. [Google Scholar] [CrossRef]
- Li, H.; Zhang, M.; Wang, D.; Dong, G.; Chen, Z.; Li, S.; Sun, X.; Zeng, M.; Liao, H.; Chen, H. Blue light from cell phones can cause chronic retinal light injury: the evidence from a clinical observational study and a SD rat model. BioMed Research International 2021, 2021, 3236892. [Google Scholar] [CrossRef]
- Ziółkowska, N.; Lewczuk, B.; Szyryńska, N.; Rawicka, A.; Vyniarska, A. Low-intensity blue light exposure reduces melanopsin expression in intrinsically photosensitive retinal ganglion cells and damages mitochondria in retinal ganglion cells in Wistar rats. Cells 2023, 12, 1014. [Google Scholar] [CrossRef]
- Rinninella, E.; Mele, M.C.; Merendino, N.; Cintoni, M.; Anselmi, G.; Caporossi, A.; Gasbarrini, A.; Minnella, A.M. The Role of Diet, Micronutrients and the Gut Microbiota in Age-Related Macular Degeneration: New Perspectives from the Gut⁻Retina Axis. Nutrients 2018, 10. [Google Scholar] [CrossRef]
- Clarkson-Townsend, D.A.; Douglass, A.J.; Singh, A.; Allen, R.S.; Uwaifo, I.N.; Pardue, M.T. Impacts of high fat diet on ocular outcomes in rodent models of visual disease. Exp Eye Res 2021, 204, 108440. [Google Scholar] [CrossRef]
- Joyal, J.S.; Gantner, M.L.; Smith, L.E.H. Retinal energy demands control vascular supply of the retina in development and disease: The role of neuronal lipid and glucose metabolism. Prog Retin Eye Res 2018, 64, 131–156. [Google Scholar] [CrossRef]
- Malesza, I.J.; Malesza, M.; Walkowiak, J.; Mussin, N.; Walkowiak, D.; Aringazina, R.; Bartkowiak-Wieczorek, J.; Mądry, E. High-Fat, Western-Style Diet, Systemic Inflammation, and Gut Microbiota: A Narrative Review. Cells 2021, 10. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, H.H.; Ramasamy, R.; Schmidt, A.M. Advanced glycation end products: building on the concept of the “common soil” in metabolic disease. Endocrinology 2020, 161, bqz006. [Google Scholar] [CrossRef]
- Aimaretti, E.; Chimienti, G.; Rubeo, C.; Di Lorenzo, R.; Trisolini, L.; Dal Bello, F.; Moradi, A.; Collino, M.; Lezza, A.M.S.; Aragno, M. Different effects of high-fat/high-sucrose and high-fructose diets on advanced glycation end-product accumulation and on mitochondrial involvement in heart and skeletal muscle in mice. Nutrients 2023, 15, 4874. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.-A.; Wu, C.-H.; Yen, G.-C. Perspective of advanced glycation end products on human health. Journal of agricultural and food chemistry 2018, 66, 2065–2070. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Z.; Tu, C.; Chen, X.; He, R. Advanced Glycation End Products in Disease Development and Potential Interventions. Antioxidants 2025, 14, 492. [Google Scholar] [CrossRef]
- Shen, C.Y.; Lu, C.H.; Wu, C.H.; Li, K.J.; Kuo, Y.M.; Hsieh, S.C.; Yu, C.L. The Development of Maillard Reaction, and Advanced Glycation End Product (AGE)-Receptor for AGE (RAGE) Signaling Inhibitors as Novel Therapeutic Strategies for Patients with AGE-Related Diseases. Molecules 2020, 25. [Google Scholar] [CrossRef] [PubMed]
- Varoniukaite, A.; Verkauskiene, R.; Simoniene, D.; Paskeviciene, D.; Balciuniene, V. Advanced glycation end products association with diabetic retinopathy severity. Acta Ophthalmologica 2025, 103. [Google Scholar] [CrossRef]
- Khoo, S.H.; Wu, P.R.; Yeh, K.T.; Hsu, S.L.; Wu, C.H. Biological and clinical significance of the AGE-RAGE axis in the aggressiveness and prognosis of prostate cancer. J Food Drug Anal 2023, 31, 664–682. [Google Scholar] [CrossRef]
- Ziółkowska, N.; Lewczuk, B.; Szyryńska, N.; Rawicka, A.; Vyniarska, A. Low-Intensity Blue Light Exposure Reduces Melanopsin Expression in Intrinsically Photosensitive Retinal Ganglion Cells and Damages Mitochondria in Retinal Ganglion Cells in Wistar Rats. Cells 2023, 12. [Google Scholar] [CrossRef]
- Gabrielle, P.H. Lipid metabolism and retinal diseases. Acta Ophthalmol 2022, 100 Suppl 269, 3–43. [Google Scholar] [CrossRef]
- Yan, Y.; Wu, Y.; Zhao, Y.; Yang, Y.; An, G.; Liu, Z.; Qi, D. A review on eye diseases induced by blue light: pathology, model, active ingredients and mechanisms. Frontiers in Pharmacology 2025, 16, 1513406. [Google Scholar] [CrossRef] [PubMed]
- Cougnard-Gregoire, A.; Merle, B.M.J.; Aslam, T.; Seddon, J.M.; Aknin, I.; Klaver, C.C.W.; Garhöfer, G.; Layana, A.G.; Minnella, A.M.; Silva, R.; et al. Blue Light Exposure: Ocular Hazards and Prevention-A Narrative Review. Ophthalmol Ther 2023, 12, 755–788. [Google Scholar] [CrossRef] [PubMed]
- Shang, Y.M.; Wang, G.S.; Sliney, D.H.; Yang, C.H.; Lee, L.L. Light-emitting-diode induced retinal damage and its wavelength dependency in vivo. Int J Ophthalmol 2017, 10, 191–202. [Google Scholar] [CrossRef]
- Hu, Z.; Zhang, Y.; Wang, J.; Mao, P.; Lv, X.; Yuan, S.; Huang, Z.; Ding, Y.; Xie, P.; Liu, Q. Knockout of Ccr2 alleviates photoreceptor cell death in rodent retina exposed to chronic blue light. Cell Death Dis 2016, 7, e2468. [Google Scholar] [CrossRef] [PubMed]
- Tosini, G.; Ferguson, I.; Tsubota, K. Effects of blue light on the circadian system and eye physiology. Molecular vision 2016, 22, 61. [Google Scholar] [CrossRef]
- Daugaard, S.; Markvart, J.; Bonde, J.P.; Christoffersen, J.; Garde, A.H.; Hansen, Å.M.; Schlünssen, V.; Vestergaard, J.M.; Vistisen, H.T.; Kolstad, H.A. Light exposure during days with night, outdoor, and indoor work. Annals of work exposures and health 2019, 63, 651–665. [Google Scholar] [CrossRef]
- Contín, M.A.; Arietti, M.M.; Benedetto, M.M.; Bussi, C.; Guido, M.E. Photoreceptor damage induced by low-intensity light: model of retinal degeneration in mammals. Molecular Vision 2013, 19, 1614. [Google Scholar]
- Dow, C.; Mancini, F.; Rajaobelina, K.; Boutron-Ruault, M.-C.; Balkau, B.; Bonnet, F.; Fagherazzi, G. Diet and risk of diabetic retinopathy: a systematic review. European journal of epidemiology 2018, 33, 141–156. [Google Scholar] [CrossRef]
- Orhan, C.; Er, B.; Deeh, P.B.D.; Bilgic, A.A.; Ojalvo, S.P.; Komorowski, J.R.; Sahin, K. Different Sources of Dietary Magnesium Supplementation Reduces Oxidative Stress by Regulation Nrf2 and NF-κB Signaling Pathways in High-Fat Diet Rats. Biol Trace Elem Res 2021, 199, 4162–4170. [Google Scholar] [CrossRef]
- Albouery, M.; Buteau, B.; Grégoire, S.; Martine, L.; Gambert, S.; Bron, A.M.; Acar, N.; Chassaing, B.; Bringer, M.A. Impact of a high-fat diet on the fatty acid composition of the retina. Exp Eye Res 2020, 196, 108059. [Google Scholar] [CrossRef] [PubMed]
- Keeling, E.; Lynn, S.A.; Koh, Y.M.; Scott, J.A.; Kendall, A.; Gatherer, M.; Page, A.; Cagampang, F.R.; Lotery, A.J.; Ratnayaka, J.A. A High Fat "Western-style" Diet Induces AMD-Like Features in Wildtype Mice. Mol Nutr Food Res 2022, 66, e2100823. [Google Scholar] [CrossRef] [PubMed]
- Clarkson-Townsend, D.A.; Douglass, A.J.; Singh, A.; Allen, R.S.; Uwaifo, I.N.; Pardue, M.T. Impacts of high fat diet on ocular outcomes in rodent models of visual disease. Experimental eye research 2021, 204, 108440. [Google Scholar] [CrossRef]
- Keeling, E.; Lynn, S.A.; Koh, Y.M.; Scott, J.A.; Kendall, A.; Gatherer, M.; Page, A.; Cagampang, F.R.; Lotery, A.J.; Ratnayaka, J.A. A High Fat “Western-style” Diet Induces AMD-like Features in Wildtype Mice. Molecular Nutrition & Food Research 2022, 66, 2100823. [Google Scholar]
- Chang, R.C.-A.; Shi, L.; Huang, C.C.-Y.; Kim, A.J.; Ko, M.L.; Zhou, B.; Ko, G.Y.-P. High-fat diet–induced retinal dysfunction. Investigative ophthalmology & visual science 2015, 56, 2367–2380. [Google Scholar]
- Hernandez-Castillo, C.; Shuck, S.C. Diet and Obesity-Induced Methylglyoxal Production and Links to Metabolic Disease. Chem Res Toxicol 2021, 34, 2424–2440. [Google Scholar] [CrossRef]
- Rowan, S.; Jiang, S.; Korem, T.; Szymanski, J.; Chang, M.L.; Szelog, J.; Cassalman, C.; Dasuri, K.; McGuire, C.; Nagai, R.; et al. Involvement of a gut-retina axis in protection against dietary glycemia-induced age-related macular degeneration. Proc Natl Acad Sci U S A 2017, 114, E4472–e4481. [Google Scholar] [CrossRef] [PubMed]
- Pan, C.; Banerjee, K.; Lehmann, G.L.; Almeida, D.; Hajjar, K.A.; Benedicto, I.; Jiang, Z.; Radu, R.A.; Thompson, D.H.; Rodriguez-Boulan, E.; et al. Lipofuscin causes atypical necroptosis through lysosomal membrane permeabilization. Proc Natl Acad Sci U S A 2021, 118. [Google Scholar] [CrossRef]
- O'Leary, F.; Campbell, M. The blood-retina barrier in health and disease. Febs j 2023, 290, 878–891. [Google Scholar] [CrossRef]
- Wei, L.; Mo, W.; Lan, S.; Yang, H.; Huang, Z.; Liang, X.; Li, L.; Xian, J.; Xie, X.; Qin, Y.; et al. GLP-1 RA Improves Diabetic Retinopathy by Protecting the Blood-Retinal Barrier through GLP-1R-ROCK-p-MLC Signaling Pathway. J Diabetes Res 2022, 2022, 1861940. [Google Scholar] [CrossRef]
- Chan, Y.J.; Hsiao, G.; Wan, W.N.; Yang, T.M.; Tsai, C.H.; Kang, J.J.; Lee, Y.C.; Fang, T.C.; Cheng, Y.W.; Li, C.H. Blue light exposure collapses the inner blood-retinal barrier by accelerating endothelial CLDN5 degradation through the disturbance of GNAZ and the activation of ADAM17. Fluids Barriers CNS 2023, 20, 31. [Google Scholar] [CrossRef] [PubMed]
- Rajagopal, R.; Bligard, G.W.; Zhang, S.; Yin, L.; Lukasiewicz, P.; Semenkovich, C.F. Functional Deficits Precede Structural Lesions in Mice With High-Fat Diet-Induced Diabetic Retinopathy. Diabetes 2016, 65, 1072–1084. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





