1. Introduction
Per- and polyfluoroalkyl substances (PFAS) are a class of synthetic chemicals increasingly detected in the environment and human tissues, prompting growing concern about their long-term effects on health, particularly when exposure occurs during vulnerable developmental windows [
1,
2,
3]. Beyond their persistence and bioaccumulation, PFAS have been shown to exert toxicity through a range of molecular mechanisms that transcend classical toxicokinetic models. Recent studies have highlighted that PFAS can disrupt both genomic and epigenomic integrity. This includes disruption in DNA methylation, histone post-translational modifications, chromatin remodeling, and DNA repair processes [
1,
4,
5,
6]. These effects are further amplified by PFAS-mediated modulation of nuclear receptor signaling [
7,
8]and by the dysregulation of non-coding RNAs, such as microRNAs and long non-coding RNAs [
9,
10,
11], which together contribute to aberrant transcriptional activity across multiple tissues, including the brain.
Importantly, these effects are not only immediate or reversible. An expanding body of research suggests that PFAS exposure may induce persistent, and in some cases transgenerational, reprogramming of gene expression via germline epigenetics and chromatin alterations [
12,
13,
14]. Such findings raise important concerns about the lasting and heritable consequences of early-life exposure. In this section, we synthesize current insights into the genotoxic and epigenetic mechanisms through which PFAS influence cellular function, developmental programming, and neurotoxicity. Particular emphasis is placed on mechanisms affecting neural gene regulation—including the suppression of neurotrophic factors like BDNF, impaired synaptogenesis, and chronic neuroinflammatory response—as well as systemic disruption that may converge on altered neurodevelopmental outcomes. Altogether, these findings underscore the role of chromatin-level dysregulation as a central mechanism of PFAS toxicity, with implications that extend across molecular, cellular, and generational scales.
2. PFAS, DNA Integrity, and Chromatin
Emerging research continues to shed light on how PFAS affect cellular and molecular systems in ways that extend beyond their persistence and bioaccumulation properties. One of the most concerning aspects of PFAS toxicity lies in their capacity to disrupt the structure and function of the genome and epigenome. These disruptions include changes in DNA methylation, the induction of DNA damage, alterations in histone modifications, and interference with nuclear receptor signaling, which collectively contribute to genomic instability and chromatin remodeling that can affect how genes are expressed across various biological systems.
PFAS and DNA Methylation
DNA methylation plays a fundamental role in gene expression regulation [
15], cellular differentiation [
16], and genomic stability [
17]. Increasing evidence suggests that PFAS exposure can interfere with these methylation processes, leading to broad and sometimes persistent changes in gene regulation.
A whole-genome methylome analysis conducted on MCF-10A human breast epithelial cells revealed that PFOS exposure altered DNA methylation, with over 12,000 differentially methylated CpG sites and more than 2,400 affected genes [
18]. Notably, tumor suppressor genes such as
CASC2 and
GACAT3 became hypermethylated, while oncogenes like
KMT2C were hypomethylated. In addition, PFOS modified the methylation status of genes involved in estrogen signaling pathways, such as
ESR1 and
GPER1, suggesting potential disruption of hormone-regulated gene expression [
18]. Similar effects have been observed with perfluorooctanoic acid (PFOA), particularly in developing neural cells. In embryonic mouse hypothalamic cells (mHypoE-N46), exposure to PFOA led to a dose-dependent decrease in cell viability and a significant increase in global DNA methylation levels [
6]. This hypermethylation was accompanied by altered expression of key genes involved in apoptosis (e.g.,
Bax,
Casp3,
Trp73), cell cycle regulation (e.g.,
Ccnb1,
Ccne1), and neurotrophic signaling pathways (e.g.,
Bdnf,
Ntrk2). Moreover, the expression of DNA methylation regulators, including DNA methyltransferases (
Dnmt1,
Dnmt3a,
Dnmt3b) and ten-eleven translocation enzymes (
Tet1,
Tet2,
Tet3), was significantly disrupted [
6]. The expression of the methyl-CpG binding protein 2 (
Mecp2), a critical reader of DNA methylation marks, was further reduced. In SH-SY5Y neuroblastoma cells, early exposure to low-dose PFOA before differentiation resulted in a persistent reduction in global DNA methylation, suggesting that PFAS can induce stable epigenetic reprogramming in neural cells [
19]
Evidence from human studies highlights PFAS-mediated alterations in placental and DNA methylation. Prenatal exposure to PFAS has been increasingly associated with epigenetic modifications that may influence early developmental processes, including those related to the nervous system. In a recent epigenome-wide association study, elevated placental concentrations of PFHxS, PFOA, and PFOS were linked to altered DNA methylation at specific CpG sites associated with genes involved in growth, cardiometabolic function, and neurodevelopment [
20]. Although the primary focus of the study was on cardiometabolic outcomes, the involvement of neurodevelopmentally relevant loci suggests that these epigenetic alterations may also have implications for brain development [
20]. Given the placenta’s essential role in regulating fetal nutrient, endocrine signaling, and developmental programming, such methylation modifications may indirectly affect neurodevelopmental. These findings support the hypothesis that the placenta may serve as a key mediator of environmental influences on the developing brain and highlight the importance of considering placental epigenetic marks as potential indicators of PFAS-related neurotoxicity [
20,
21,
22].
Additionally, PFAS exposure can induce epigenetic reprogramming in sperm by altering DNA methylation patterns, with potential consequences for offspring development. A recent study demonstrated that adult male mice exposed to an environmentally relevant PFAS mixture exhibited changes in sperm DNA methylation, notably at loci implicated in neurodevelopment, including
Ntrk2,
Bdnf, and Wnt pathway genes [
23]. These alterations were accompanied by persistent transcriptional dysregulation in the liver and adipose tissue of offspring, supporting the hypothesis that PFAS can reprogram developmental pathways via the paternal germline [
24]. Although the primary focus was on metabolic outcomes, the involvement of neurogenic loci points to broader neurodevelopmental implications. These insights also lay the groundwork for a more detailed exploration of PFAS-induced transgenerational effects, which will be addressed in a subsequent section.
PFAS-Induced DNA Damage and Genomic Instability
Beyond their impact on epigenetic regulation, PFAS exposure has also been shown to induce DNA damage, ultimately contributing to genomic instability and impairments in DNA repair mechanisms. This kind of damage is not trivial; it is a key event that can lead to mutagenesis, cancer, and apoptosis [
25]. One particularly illustrative example comes from the work of Qin et al. [
26], who investigated the effects of perfluorodecanoic acid (PFDA) on DNA integrity and repair mechanisms in ovarian epithelial cells. Their study showed that exposure to environmentally relevant concentrations of PFDA led to the accumulation of DNA double-strand breaks (DSBs) in both primary mouse ovarian epithelial cells and human IOSE-80 cells. Mechanistically, PFDA substances promoted the abnormal nuclear accumulation of cyclic GMP-AMP synthase (cGAS), a DNA sensor typically associated with immune signaling [
27]. When mislocalized to the nucleus, cGAS interferes with the homologous recombination (HR), a key repair pathway, thereby leaving cells more vulnerable to genomic instability.
In vivo findings from the same study supported these results, showing increased DSBs and nuclear accumulation of cGAS in the ovarian tissues of PFDA-exposed mice [
26]. Although cGAS-mediated interference constitutes one mechanism, oxidative stress emerges as a central driver of PFAS-induced genotoxicity at a broader scale. Several studies have highlighted the potential of PFAS compounds to induce various forms of DNA damage, either directly through oxidative stress (ROS) or indirectly through epigenetic modulation.
For instance, in the study by Teteltitla et al. [
4], oxidative stress-mediated DNA damage has been observed in reproductive cells, where exposure to 40 µM in cumulus cells triggered DNA fragmentation and impaired oocyte maturation, pointing to potential reproductive toxicity.
These effects extend to hepatic models, where PFAS mixtures have been shown to induce genotoxicity and epigenetic dysregulation in human liver cells (HepG2), reinforcing concerns regarding the cumulative toxicological effects of multiple PFAS exposures [
28]. Although short-chain PFAS exposure, once considered less harmful, has been found to significantly increase oxidative stress biomarkers across several human cell lines without causing direct DNA strand breaks, suggesting a more upstream mechanism for genomic instability [
29]. Similarly, Pierozan et al. [
30] reported that low concentrations of PFOS and PFOA mixtures synergistically induced not only oxidative stress and DNA/RNA damage but mitochondrial dysfunction in human breast epithelial cells, further supporting the idea that PFAS mixtures may present a cumulative genotoxic risk. In reproductive cells, PFAS exposure during mouse oocyte maturation led to an increase in γ-H2AX levels, a marker of DNA double-strand breaks, with damage severity correlating with carbon chain length and the presence of a sulfonate group [
31]. Although most of these studies center on hepatic, epithelial, and reproductive systems, emerging evidence indicates that the nervous system is not spared. In differentiated SH-SY5Y neuronal cells, PFAS mixtures led to increased production of ROS and mitochondrial dysfunction, both key early events in oxidative stress responses [
32]. Comparable findings in microglial cell lines confirmed that even short-chain PFAS compounds trigger oxidative stress [
33]. Additional work in SH-SY5Y cells showed disrupted redox and metabolic shifts [
34,
35], reinforcing the idea that PFAS can compromise neuronal oxidative homeostasis. However, it’s important to note that while these studies consistently show oxidative imbalance, direct measurement of DNA damage, such as strand breaks or chromatin instability, was not performed. One notable exception is the study by Running et al. [
5], which used transcriptomic and lipidomic profiling in PFAS-exposed neurons. The results revealed disruption in oxidative stress-related gene networks and lipid homeostasis, two processes that are closely linked to genomic stability. Still, direct evidence of DNA strand breaks or chromatin instability in neural systems remains lacking. Taken together, these findings underscore a significant knowledge gap. Although PFAS clearly induce DNA damage in neuronal cells, it’s still unclear. To address this, future studies should incorporate direct genotoxicity assays, such as the comet assay or γ-H2AX staining, in neural models. This will be essential to confirm whether oxidative stress induced by PFAS exposure translates into measurable DNA damage in neural cells, ultimately contributing to neurotoxicity.
PFAS and Chromatin Structure Modifications
Beyond their ability to induce DNA methylation changes and promote oxidative DNA damage, PFAS have also been implicated in the modulation of chromatin architecture through alterations in histone post-translational modifications. These modifications play a pivotal role in determining chromatin accessibility and transcriptional activity, thereby influencing gene expression at a fundamental level. In human breast epithelial cells, Pierozan et al. showed that exposure to PFOS and PFOA significantly disrupted histone landscapes. Their Western blot analyses revealed a reduction in H3K9 acetylation—a mark typically associated with active chromatin—alongside alterations in H3K9 demethylation and H3K4 trimethylation, both of which regulate chromatin accessibility and gene silencing [
36]. In neural systems, similar patterns of disruption have been observed. DA-like neurons derived from SH-SY5Y neuroblastoma cells and exposed to PFOA exhibited a persistent depletion of bivalent histone marks H3K4me3 and H3K27me3, detected via immunofluorescence and chromatin profiling [
19]. These marks are known to maintain a poised transcriptional state, and their depletion suggests a potential reprogramming of gene expression in neuronal contexts.
Critically, this line of evidence extends from
in vivo models to human populations. Tsai et al. reported that prenatal exposure to PFAS—including PFOA, PFNA, and PFUA—was associated with altered global histone methylation profiles in two-year-old children [
37]. Specifically, increased levels of the active mark H3K4me3 and decreased levels of the repressive mark H3K27me3 were observed in peripheral blood leukocytes, suggesting a shift toward transcriptionally active chromatin states early in life [
37].
Adding another layer of concern, recent research comparing the effects of PFOA and its replacement compound HFPO-TA revealed that both substances enhanced the expression of key steroidogenic genes (
Star and
Cyp11a1) in MLTC-1 cells through modulation. This occurred through changes in H3K4 and H3K9 methylation, with HFPO-TA exerting even stronger effects than PFOA, highlighting that this alternative compound may pose equal or greater risks to male reproductive health through similar epigenetic mechanisms [
38]. Together, these studies point to histone dysregulation as a conserved epigenetic mechanism by which PFAS may reprogram gene expression across diverse biological systems and developmental windows. Further studies are warranted to elucidate how these changes in histone marks intersect with broader chromatin dynamics, particularly in the developing brain.
PFAS and Nuclear Receptor-Mediated Chromatin Disruption
PFAS are increasingly recognized for their ability to modulate nuclear receptor (NR) signaling, a vital process that regulates metabolism, hormone balance, and xenobiotic regulation. In liver-derived models, compounds like PFOA have been shown to activate key nuclear receptors like PPARα, PPARγ, and estrogen receptors, leading to transcriptomic shifts consistent with nuclear receptor-mediated toxicity [
7]. Notably, the strength and specificity of this activation depend heavily on the chemical structure of PFAS molecules. Factors such as the length of the fluorocarbon chain and the nature of the head group significantly influence receptor binding and potency [
39,
40]. Computational screening has further expanded this understanding, predicting that thousands of PFAS and their mixtures may bind to orthosteric or allosteric sites across multiple nuclear receptor families—including PPARs, CAR, FXR, and ERα—suggesting a complex, structure-driven toxicological profile [
41]. Of particular concern is PPARγ, which appears highly sensitive to PFAS interference. Detailed structural analysis has revealed that PFAS can bind to the ligand-binding domain of PPARγ, thereby altering chromatin regulation and downstream gene transcription [
42]. Moreover, a secondary, noncanonical binding site on PPARγ was first identified through computational modeling, suggesting that PFAS may amplify transcriptional disruption via allosteric modulation [
43]. This mechanistic hypothesis was substantiated by structural studies showing that PFOA engages multiple ligand-binding pockets, including one near the activation function-2 (AF-2) surface critical for coactivator recruitment, thereby supporting its partial agonist activity [
8]. These findings highlight nuclear receptor interference as a conserved and central mode of action of PFAS, with implications that likely extend beyond hepatic systems to other receptor-rich tissues, including the brain.
Even more troubling is the expanding evidence that PFAS-induced activation of nuclear receptors such as PPARγ may trigger epigenetic remodeling. Beyond simply regulating transcription, PPARγ plays an active role in modifying the chromatin environment [
44] by recruiting chromatin-modifying enzymes such as CBP/p300 and HDACs, which govern key post-translational histone modifications [
45]. These enzymes dynamically reshape chromatin structure by adding or removing chemical tags, thereby influencing transcriptional accessibility [
46]. This positions PPARγ as a critical link between environmental signals and epigenetic reprogramming. Given its established role in neuronal differentiation and plasticity, PFAS-mediated disruption of PPARγ signaling may have profound consequences for neurodevelopment and brain function [
47]. More broadly, nuclear receptors appear to serve as molecular bridges between environmental exposure and chromatin-level changes, making them key regulators in the epigenetic response to PFAS. This is particularly relevant in the context of neurotoxicity, where further investigations are needed to validate these mechanisms within neuronal systems and understand their contribution to long-term brain dysfunction.
3. Role of Epigenetics and Genotoxicity in PFAS-Induced Neurotoxicity
Developmental exposure to PFAS has been linked to long-lasting neurotoxic effects, including impaired neuronal differentiation, disrupted synaptic plasticity, and cognitive deficits [
1,
2,
48]. Studies from both
in vitro neural models and
in vivo animal studies point to early-life PFAS exposure as a trigger for persistent epigenetic modifications [
49,
50], raising critical concerns about the potential for transgenerational transmission of neurotoxic outcomes [
49,
51]. Notably, these enduring effects cannot be fully attributed to PFAS bioaccumulation alone in the body, as blood levels decline over time while molecular and behavioral changes persist [
3,
52]. Furthermore, emerging data show that PFAS exposure can alter synaptic signaling, neurotrophic factor regulation, and inflammatory pathways in differentiating neurons, even after toxicant removal [
1,
2,
3,
48]. For instance, a study by Running et al. demonstrated that PFOA exposure significantly altered the expression of nearly 600 genes in SH-SY5Y neuronal-like cells, including those involved in synaptic growth and neural function. Of particular concern,
MANF (mesencephalic astrocyte-derived neurotrophic factor), crucial for neuronal survival, was consistently downregulated, while
TXNIP (thioredoxin-interacting protein), which is associated with neuronal apoptosis, was upregulated. These changes persisted beyond the exposure period, indicating long-term effects on neuronal gene expression [
5]. Given the growing body of evidence linking PFAS exposure to epigenetic dysregulation and genomic instability, understanding how these upstream molecular impairments contribute to neurodevelopmental disorders, cognitive disruption, and neurodegenerative diseases is pivotal for assessing long-term risk. This section explores how such early disturbances in DNA methylation, histone modifications, and genome integrity may reprogram neural gene expression and leave a lasting imprint on brain development. These upstream perturbations are increasingly implicated in the disruption of key neurodevelopmental endpoints—including BDNF signaling, synaptogenesis, neuroinflammation, mitochondrial function, calcium homeostasis, and neurotransmitter regulation— pathways critical to maintaining cognitive and neurological function.
BDNF as a Downstream Target of Epigenetic and Genotoxic Disruption
BDNF plays a central role in regulating neuronal differentiation, synaptic plasticity, and cognitive function [
53]. Its expression is finely controlled by epigenetic mechanisms, including DNA methylation, histone modifications, and microRNA (miRNA)-mediated regulation [
54,
55,
56,
57]. Disruptions in BDNF signaling have been reported following exposure to several environmental neurotoxicants such as the organophosphate diisopropylfluorophosphate (DFP) [
58], methylmercury, bisphenol S [
59], and combined manganese and lead exposure [
60]. These alterations have been associated with both neurodevelopmental and neurodegenerative disorders [
61,
62], positioning BDNF as a particularly sensitive and informative target for evaluating the neurotoxic potential of PFAS.
Recent
in vitro evidence supports the idea that PFAS, especially PFOS, may suppress BDNF expression through epigenetic mechanisms [
63,
64]. In human neuroblastoma SK-N-SH cells, PFOS exposure has been shown to significantly reduce BDNF mRNA and protein levels, coinciding with increased methylation at BDNF promoters I and IV [
63]. This hypermethylation was accompanied by shifts in DNA methyltransferase activity; PFOS decreases DNMT1, a maintenance methyltransferase, while increasing DNMT3B, responsible for de novo methylation, suggesting a repressive epigenetic shift in BDNF transcription [
63]. In parallel, PFOS also altered microRNA profiles, particularly upregulating miR-16, miR-22, and miR-30a-5p, all of which target BDNF transcripts, thereby reinforcing gene silencing through post-transcriptional repression [
63]. Functionally, these epigenetic disruptions impair BDNF–ERK–CREB signaling. In SH-SY5Y cells, PFOS exposure leads to a reduction in both phosphorylated ERK (p-ERK) and BDNF levels, while paradoxically increasing phosphorylated CREB (p-CREB) [
64]. These signaling imbalances are further accompanied by dose-dependent modulation of TrkB BDNF’s high-affinity receptor, whose expression is upregulated at lower PFOS concentrations but suppressed at higher doses [
64]. Among the implicated regulators, miR-22 appears particularly important, as its upregulation has been directly linked to decreased BDNF expression and impaired ERK-CREB signaling. Together, these data suggest that PFOS-mediated epigenetic regulation disrupts both the transcription and functional signaling of BDNF, potentially contributing to long-lasting neurodevelopmental deficits.
Beyond direct epigenetic regulation, NRF2 (Nuclear factor erythroid 2-related factor 2) dysfunction emerges as a key mediator of PFAS-induced BDNF suppression. As a transcription factor critical for neuronal homeostasis, synaptic plasticity, and neurotrophic signaling [
65,
66], NRF2 promotes BDNF transcription by repressing epigenetic silencing factors such as HDAC2, mSin3A, and MeCP2, thereby facilitating chromatin accessibility at BDNF promoter regions and thus, enhancing neuroprotection [
67]. This pathway appears particularly vulnerable to oxidative stress induced by PFAS. In both mouse astrocytes and zebrafish embryos, PFOS exposure has been shown to inhibit Nrf2 activation and increase ROS, implicating the Nrf2–MAPK axis in PFAS-mediated toxicity [
68,
69]. Given NRF2’s dual role in maintaining BDNF expression and regulating neuroinflammation tone, its suppression by PFAS may contribute to impaired neurodevelopment by simultaneously reducing neurotrophic support and exacerbating oxidative damage. Evidence from neurodegenerative models reinforces this link. In Parkinson’s disease, for instance, epigenetic silencing of
NRF2—e.g., via lncRNA MALAT1—has been shown to enhance inflammasome activation and progressive neuronal loss, further illustrating the sensitivity of NRF2-BDNF signaling to chromatin-level dysregulation [
70]. Similarly, in the Alzheimer’s disease model, DNA demethylation of the
NRF2 promoter restores
NRF2 expression and neuroprotection [
71]. Taken together, these findings illustrate how PFAS can disrupt BDNF signaling through both epigenetics and oxidative pathways, with
NRF2 suppression emerging as a key mediator. However, the mechanistic complexity of PFAS toxicity suggests that additional layers of regulation may be involved. In particular, the potential contribution of genotoxic mechanisms to BDNF dysregulation remains underexplored. Although no studies to date have directly linked PFAS-induced DNA damage to alterations in BDNF signaling, both pathways, epigenetic disruption and genomic instability have independently been implicated in PFAS-related neurotoxicity. Given BDNF’s sensitivity to genomic integrity and its regulation by chromatin structure, it is plausible that genotoxic stress may converge with epigenetic alterations to influence its expression. This convergence could further exacerbate neurodevelopmental vulnerability, particularly during early-life windows of heightened plasticity. Future studies are needed to explore this potential interaction.
PFAS-Induced Interference with Synaptic and Neurotransmitter Gene Networks
Emerging data suggest that synaptic development is particularly vulnerable to environmental disruption at the epigenetic level, including that caused by PFAS exposure. In zebrafish, PFNA was shown to impair gene networks critical to synaptogenesis, leading to deficits in neuronal connectivity and cognitive function [
72]. Transcriptomic profiling identified several key synaptic genes as targets of PFNA neurotoxicity. Notably, DLG4 (PSD-95), a postsynaptic scaffolding protein critical for excitatory synaptic structure, is significantly downregulated, suggesting impaired postsynaptic assembly. Likewise,
Grin2b, which encodes the NR2B b subunit of the NMDA receptor and regulates calcium-dependent synaptic plasticity, was suppressed, potentially compromising activity-driven synapse maturation. On the presynaptic side, PFNA reduces expression of
Syn1 (Synapsin I), a key regulator of vesicle mobilization and neurotransmitter release [
72].
While these transcriptional changes are well-documented, direct evidence linking PFAS exposure to epigenetic or genotoxic alteration at synaptic gene loci remains limited. Nonetheless, the regulation of synaptic genes is known to be highly responsive to histone acetylation and DNA methylation [
73,
74], both of which control activity-dependent gene expression essential for synaptic remodeling [
75,
76]. This raises a strong mechanistic possibility that PFAS-induced chromatin changes may contribute to impaired synaptic plasticity. Supporting this, persistent DNA damage in a Huntington’s disease model has been shown to suppress expression of core synaptic plasticity genes such as
Bdnf,
Arc, and
Egr1, linking genomic instability to impaired synaptic function [
77]. Given PFAS-associated oxidative and genotoxic stress [
26,
28,
78], these findings support a mechanistic hypothesis wherein PFAS may indirectly suppress synaptic gene expression via both epigenetic and DNA damage-associated pathways. Future studies should directly assess chromatin regulation at synaptic loci following PFAS exposure to define its neurodevelopmental consequences more precisely.
In parallel with its effects on synaptic architecture, PFAS may also disrupt neurotransmitter systems, further compromising neuronal communication through epigenetically mediated pathways. PFOS and PFOA, in particular, have been implicated in the disruption of neurotransmission, with a notable impact on the dopaminergic and glutamatergic systems. Experimental studies report that developmental exposure to these compounds reduces dopamine levels while elevating hippocampal glutamate, potentially triggering excitotoxicity and increasing vulnerability in dopaminergic neurons [
50,
79]. Although direct epigenetic analysis of PFAS effects on neurotransmission remains limited, converging evidence from other systems supports mechanistic plausibility. For instance, NMDA receptor hypofunction in schizophrenia has been linked to DNA methylation and histone modifications at glutamate receptor subunit genes [
80], and methamphetamine exposure reduces striatal glutamate receptor expression via histone deacetylation and miRNA dysregulation [
81]. Similarly, the differentiation of dopaminergic neurons is epigenetically regulated, with transcription factors such as Nurr1 and Pitx3 subject to methylation-sensitive control [
82].
Findings from other environmental toxicants further reinforce this model. Methylmercury has been shown to disrupt neurotransmitter-related gene expression through promoter methylation and chromatin alterations [
83], while PBDE-99 impairs cholinergic signaling by altering the transcription of neurotransmitter biosynthesis genes during early brain development [
84]. Likewise, atrazine interferes with dopaminergic signaling through miRNA regulation and AMPK-dependent epigenetic pathways [
85]. Taken together, these observations suggest that PFAS may impair neurotransmitter homeostasis not only through receptor or metabolic interference but also through epigenetic mechanisms. Dedicated studies are needed to map the chromatin landscape and transcriptional regulation of neurotransmission under PFAS exposure, particularly during critical windows of neurodevelopment.
Neuroinflammation and immunoepigenetic disruption by PFAS
Growing evidence positions per- and polyfluoroalkyl substances (PFAS), particularly perfluorooctane sulfonate (PFOS), as potent modulators of neuroinflammatory responses. These effects appear to arise from a complex interplay between immune activation, oxidative stress, and epigenetic regulation. At the molecular level, PFOS exposure has been shown to activate glial-mediated neuroinflammation, notably through upregulation of pro-inflammatory cytokines such as TNF-α, via the JAK2/STAT3 signaling axis [
86]. Supporting this, comparative proteomic analyses reveal that even newer-generation PFAS like GenX induce astrocyte inflammation and metabolic dysfunction, marked by altered expression of genes involved in mitochondrial respiration, cytokine production, and immune signaling —including IL6 upregulation and downregulation of redox-regulatory genes —suggesting a broader class-wide effect on glial reactivity [
87].
In vivo studies in larval zebrafish reveal that PFOS exposure sensitizes microglia to injury, elevating expression of the microglial marker
p2ry12, and increasing immune vigilance in the brain [
88]. Astrocyte-specific responses are similarly impacted, with PFOS and PFOA impairing NRF2-mediated antioxidant signaling and thereby exacerbating oxidative stress, a known trigger for inflammatory activation in the brain [
89].
Beyond the CNS, systemic inflammation adds another layer of concern. Subchronic PFOS exposure in adult mice elevates systemic levels of IL-6 and TNF-α, while additional studies show that PFAS pollutants engage the innate immune system through AIM2 inflammasome activation, pointing to inflammasome priming as a potential mechanistic bridge between PFAS exposure and neuroinflammation [
90]. In parallel, the NF-κB/TNF-α pathway, widely implicated in inflammation-related pathology, has been shown to be activated by PFOS in both hepatic and neuronal tissues, highlighting a conserved pro-inflammatory cascade across organ systems [
86,
91]. Notably, when PFOS exposure occurs prenatally and is combined with a high-fat diet, genomic reprogramming of neuromotor pathways has been observed in offspring, suggesting that early-life exposure may leave a lasting susceptibility to neuroinflammation and behavioral dysfunction [
92].
This neuroimmune activation is increasingly understood through the lens of epigenetic modulation. Human studies demonstrate that PFAS exposure is associated with differential DNA methylation at key immunoregulatory loci, including
RUNX3 (a transcription factor involved in T-cell differentiation and cytokine signaling),
NFKBIA (which encodes IκBα, a negative regulator of the NF-κB pathway), and
IL6R (the interleukin-6 receptor mediating inflammatory signaling), as well as with signatures of accelerated epigenetic in occupationally exposed individuals [
93].
In utero PFOS exposure in mice has been shown to induce epigenomic remodeling in fetal liver, with differential expression of genes such as
Ccl2 (encoding the chemokine MCP-1, involved in recruiting monocytes to inflammation sites),
Il1rn (interleukin-1 receptor antagonist, a regulator of IL-1 signaling and inflammation), and
Tnfrsf12a (also known as Fn14, a receptor for TWEAK, involved in TNF superfamily signaling and glial inflammation), all of which play critical role in cytokine signaling and glia inflammation [
94]. These findings reinforce the plausibility of developmental programming via PFAS-induced chromatin changes.
While direct causal links between PFAS-induced epigenetic alteration and neuroinflammation remain to be fully established, there is strong mechanistic support from the adjacent literature. Histone modifications such as H3K27ac have been implicated in microglial activation and transcriptional reprogramming [
95], while dynamic DNA methylation is known to regulate inflammatory gene expression in both astrocytes and microglia [
96,
97]. Epigenetic modulation of autophagy and cytokine signaling genes in glia has been shown to influence neuroinflammatory tone, suggesting that PFAS-induced chromatin remodeling may converge on similar regulatory pathways [
98].
These insights reveal chromatin remodeling as a potential mechanistic bridge between PFAS exposure and neuroimmune dysregulation, with implications that span both development and long-term neurotoxicity.
4. PFAS, ncRNA Dysregulation, and Neurotoxicity
ncRNAs, including miRNA and long non-coding RNAs lncRNAs, are emerging as key mediators of PFAS-induced neurotoxicity. These molecules are central to neural development and function, and mounting evidence indicates that PFAS exposure disrupts their expression and regulation [
99].
Epidemiological studies, such as those conducted in firefighters and adolescents, have identified PFAS-associated changes in plasma miRNA profiles, implicating key extracellular miRNAs in inflammatory and metabolic-related pathways [
9,
100,
101]. These findings highlight not only the systemic reach of PFAS but also the potential utility of circulating miRNA as a minimally invasive biomarker of exposure and effect. Parallel data from a pilot study in children and
in utero exposure models further support the role of PFAS in modulating miRNA expression during critical developmental windows, raising legitimate concerns about long-term effects on fetal growth and neurodevelopment [
102,
103].
Mechanistic studies have shown that PFOS disrupts miRNA profiles in human iPSC-derived neurons and PC12 cells, with consistent disruption of neurotrophic and apoptotic signaling pathways. In iPSC-derived neurons, PFOS exposure alters the extracellular miRNA profile consistent with early neurodegenerative changes. [
104]. Although specific miRNAs were not fully characterized in this system, complementary studies in PC12 cells have revealed that PFOS activates the pro-apoptotic transcription factor FoxO3a, leading to increased expression of Bcl-2 family proteins such as Bim and Bax. [
105]. Concomitantly,
miR-22 was significantly upregulated and directly targets BDNF transcripts for repression, even when CREB is phosphorylated, suggesting a miRNA-mediated block in BDNF signaling [
106]. Meanwhile,
miR-16, also involved in BDNF regulation and cell cycle control, is downregulated, potentially reflecting a failed compensatory mechanism that exacerbates cellular vulnerability [
106].
Animal studies provide further mechanistic insight into PFOS toxicity. Gestational PFOS exposure alters miRNA and lncRNA profiles in fetal rat brains and human trophoblasts, impairing synaptic development and angiogenesis [
107,
108,
109]. Notably,
MEG3,
XIST, and
H19, three epigenetically regulated lncRNAs, have been implicated in placental growth inhibition and neurodevelopmental dysregulation. These lncRNAs act either as scaffolds for chromatin modifiers or as miRNA sponges, indirectly modulating gene networks involved in cellular survival and differentiation. For instance, the
MEG3/miR-770/PTX3 axis and the
H19/miR-19a/19b module have been shown to mediate PFOS toxicity by targeting inflammatory and growth-related genes [
107,
110].
PFOS induces epigenetic silencing of
H19 via promoter hypermethylation, leading to reduced expression of its associated miRNAs
miR-19a and
miR-19b, which are crucial regulators of apoptosis and angiogenesis [
107]. The loss of
H19 not only reduces their direct gene-regulatory effects but also disrupts their role as competing endogenous RNA (ceRNAs), further amplifying dysregulation in target mRNA pathways. While predominantly studied in placental tissues, the
H19/miR-19a/b/PTEN axis is also active in the developing brain [
111,
112,
113,
114], suggesting a conserved mechanism underlying PFOS-induced neurotoxicity.
Similarly,
MEG3 is epigenetically repressed by PFOS via promoter hypermethylation as demonstrated in placental models [
110], thereby downregulating its intron product,
miR-770, which normally suppresses
PTX3, an inflammatory mediator. The resulting overexpression of
PTX3 contributes to both growth inhibition and inflammatory stress, effects mirrored in neural tissues, where both
MEG3 and
PTX3 are expressed in neural tissues and similar regulatory roles for
MEG3 in neuronal apoptosis and glial inflammation have been described [
110,
115,
116,
117]. This finding suggests that the
MEG3/miR-770/PTX3 axis may also be relevant to PFAS-induced neurotoxicity through inflammatory and epigenetic pathways.
System-level analysis adds to this picture. Integrated miRNA transcriptomics and proteomics have provided a broader perspective on ncRNA dysregulation due to PFAS exposure. In a mouse model of PFNA exposure, a coordinated repression of miRNAs alongside hepatotoxic proteins upregulation was observed, revealing ncRNA involvement in both direct organ toxicity and systemic metabolic effects [
118]. Notably, several of these altered genes and miRNAs —such as those regulating oxidative stress (
SOD1,
GPx) [
119], inflammation (
miR-155, miR-21) [
11,
120], and metabolic control (
PPARα) [
121]—are also expressed in neural tissues, reinforcing the notion of interconnected regulatory mechanism where PFAS-induced ncRNA dysregulation may exert systemic effects, with overlapping mechanisms in the liver and brain, particularly via pathways controlling redox balance, glial inflammation, and apoptosis.
Based on the evidence presented above, a coherent mechanistic model emerges: PFAS-induced neurotoxicity stems from epigenetic disruption of ncRNA networks essential for neurodevelopmental homeostasis. Hypermethylation of key lncRNAs (H19, MEG3) impairs miRNA-dependent regulation of inflammatory and apoptotic pathways, involving critical targets such as PTEN and PTX3. Concurrently, Disruption of the miR-22/miR-16 axis affects BDNF translation. This cascade impairs neurotrophic signaling, angiogenesis, and immune balance, with systemic implications, positioning ncRNA dysregulation as a central and actionable mechanism in PFAS neurotoxicity.
5. Transgenerational Effect of PFAS Exposure
Although direct evidence linking PFAS-induced transgenerational epigenetic changes to brain-specific outcomes remains limited, multiple experimental models have demonstrated that early-life PFAS exposure can reprogram the germline, resulting in heritable alterations to gene expression across generations. While these effects are most consistently observed in metabolic and reproductive tissues, several studies suggest that neurodevelopmental pathways may be secondarily affected. This section explores current insights into PFAS-induced epigenetic inheritance and its potential relevance to brain development across generations.
Animal studies have revealed that exposure to PFAS during critical developmental windows can induce transgenerational effects by altering DNA methylation in germ cells. For example, in a mouse model, maternal exposure to a PFAS mixture (PFOA, PFOS, PFHxS, PFNA) at 2.5 mg/L each in drinking water, relevant to environmental concentrations, resulted in persistent DNA methylation changes in F0 sperm. These epimutations were associated with long-term transcriptomic reprogramming in the liver and adipose tissue of F1 and F2 offspring [
122]. Key differentially methylated regions mapped genes involved in lipid metabolism (Pparα and Acsl1), immunity, and developmental patterning (Hox clusters) [
14]. Notably, these effects were transmitted across generations in the absence of continued PFAS exposure, emphasizing the role of inherited epigenetic modifications.
In human
in vitro models, PFOS exposure (1–10 µM) in spermatogonial stem cells led to downregulation of key spermatogenic genes, including
SYCP3,
PRM1, and
DAZL, and altered chromatin markers, including a loss of H3K4me3 and H3K9me2 (a heterochromatin-associated mark), and a reduction in global 5-methylcytosine levels [
123]. These chromatin changes suggest impaired germ cell integrity and potential disruption of epigenetic information transfer [
124]. Interestingly, supplementation with S-adenosylmethionine (SAM), a universal methyl donor, has been shown to partially restore DNA methylation balance and ameliorate PFOS-induced toxicity, highlighting possible avenues for intervention [
125].
Translational models like zebrafish and
C. elegans support these observations and offer mechanistic insights into heritable toxicity. In
C. elegans, PFAS disorganized nuage structures and RNA granules, such as P granules, critical for RNA silencing and small RNAs regulation during spermatogenesis, with downstream consequences for piRNAs and miRNAs localization and germline inheritance [
126]. Furthermore, low-dose exposure to PFBS and PFHxS (0.1–10 µM) altered the expression of
fat-7 and
ech-1, key regulators of fatty acid metabolism, along with changes in the localization and activity of the NHR-49 nuclear hormone receptor, suggesting persistent metabolic reprogramming [
12,
127]. Additional work in this model has demonstrated germline DNA damage, reproductive defects, and behavioral anomalies persisting into the F2 generation, including alteration in locomotion and chemotaxis, pointing to disrupted neuronal programming [
128,
129].
In zebrafish, developmental PFAS exposure led to gene expression changes in inflammation, neurodevelopment, and oxidative stress pathways in F1 and F2 offspring [
129], These heritable gene expression shifts were observed in pathways such as cytokine signaling and mitochondrial function, raising the possibility that early-life PFAS exposure can epigenetically program neuroimmune sensitivity across generations. Although the precise epigenetic carriers—such as DNA methylation marks or histone modifications—were not directly identified, the persistence of transcriptomic changes in the absence of continued exposure strongly implicates heritable chromatin alterations or disrupted germline epigenetic reprogramming as key drivers.
Complementing these findings, Cui et al. demonstrated that chronic low-level PFOS exposure in adult zebrafish (F0) led to hepatic steatosis and structural abnormalities in liver and intestinal tissues. In their offspring, genes such as
lepa,
kiss1, dgat1b, and
hb9 showed altered expression, with implications for neuroendocrine regulation [
130]. Similar findings were reported with F-53B, a PFOS analog, where parental exposure induced hepatotoxicity and—critically—altered the PPAR signaling pathway in unexposed offspring [
131]. Given the central role of PPARs in lipid metabolism and inflammatory gene regulation, these findings suggest that PFAS analogs may epigenetically rewire key metabolic-inflammatory circuits across generations.
PFAS-induced epigenetic effects also appear to extend to endocrine and reproductive systems. In fish, developmental exposure to PFBS has been shown to skew sex ratios, suggesting interference with early gonadal programming and sex hormone pathways [
132]. Combined parental exposure to PFOS and ZnO nanoparticles resulted in stress-related gene expression shifts in offspring, underscoring cumulative effects on endocrine and developmental signaling [
13]. Disruption of thyroid hormone homeostasis by PFAS — widely reported in recent reviews— is especially concerning, as thyroid hormones play a critical role in brain development and metabolism [134,135].
Although some of these studies did not directly assess specific epigenetic marks, the persistence of transcriptional dysregulation and phenotypic changes strongly supports the presence of heritable regulatory alterations. Zebrafish, in particular, have proven to be a powerful model for detecting compound-induced changes in DNA methylation during embryogenesis [
134]. Therefore, PFAS-induced transcriptomic changes in offspring—such as those affecting PPAR pathways—are likely driven by disrupted DNA methylation dynamics established during the developmental period and maintained across generations.
These findings position epigenetic disruption as a core mechanism through which PFAS may exert long-term, transgenerational effects. While definitive proof of brain-specific heritable toxicity is still evolving, converging data from transcriptomic, behavioral, and germline studies point toward plausible links between PFAS exposure and lasting changes in neurodevelopmental trajectories. Further studies incorporating a multigenerational profile of neural tissues are needed to clarify these mechanisms and assess potential risks.
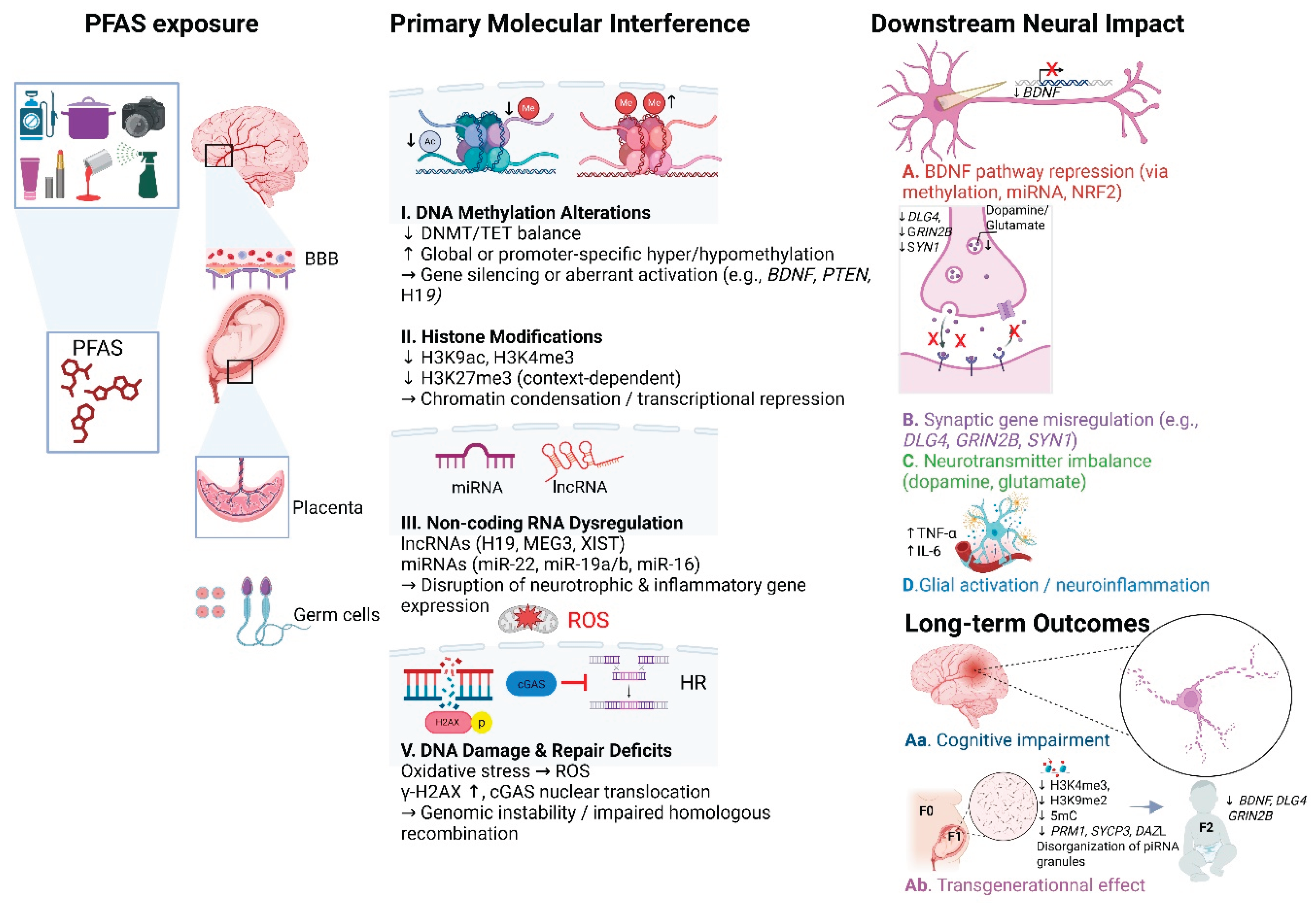
To synthesize the mechanisms described in all sections above,
Figure 1 illustrates the key molecular pathways through which PFAS disrupt neurodevelopment and epigenetic regulation across generations.
6. Conclusion
Current evidence paints a concerning picture of PFAS-induced neurotoxicity as a multifaceted process driven by interconnected epigenetic, genotoxic, and ncRNA-mediated mechanisms. Disruptions in DNA methylation and histone modifications—mediated through altered activity of key enzymes such as DNMTs, TETs, HDACs, and methylation readers—can destabilize chromatin architecture and misregulate gene expression across critical neurodevelopmental periods. In parallel, the dysregulation of non-coding RNAs contributes to the silencing of neurotrophic, inflammatory, and synaptic genes critical for brain development and function.
Compounding these effects, PFAS exposure induces oxidative DNA damage and impairs repair mechanisms, leading to cumulative genomic instability that may further destabilize neuronal homeostasis. Although most findings are derived from in vitro and animal models, growing evidence suggests that these molecular disturbances are not confined to directly exposed individuals. Instead, heritable changes in gene expression and epigenetic reprogramming are being increasingly observed in the offspring of exposed organisms, particularly through alterations in the germline.
Although brain-specific transgenerational data remain limited, persistent dysregulation of neurodevelopmental gene networks in exposed progeny suggests that PFAS-induced epigenetic reprogramming may have enduring consequences for brain health. These underscores are an urgent need for future research that integrates cell-type-specific epigenome mapping, detailed chromatin conformation studies, and multi-generational analysis of neural tissues.
Clarifying these mechanisms will not only deepen our understanding of PFAS-related neurotoxicity but will also help identify early biomarkers of exposure and guide the development of target interventions to protect vulnerable populations. As the field moves forward, the intersection of environmental exposure, epigenetic regulation, and brain development should remain a key focus for toxicological research and public health policy.
Author Contributions
N.K., R.S., and S.Y. conceptualized the work. N.k., wrote the first draft and prepared
Figure 1. All the authors revised the manuscript.
Funding
This work was supported by the University of Lorraine, France.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Starnes, H.M.; Rock, K.D.; Jackson, T.W.; Belcher, S.M. A Critical Review and Meta-Analysis of Impacts of Per- and Polyfluorinated Substances on the Brain and Behavior. Front. Toxicol. 2022, 4, 881584. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Qin, S.; Zeng, H.; Chou, W.; Oudin, A.; Kanninen, K.M.; Jalava, P.; Dong, G.; Zeng, X. Adverse Outcome Pathway for the Neurotoxicity of Per- and Polyfluoroalkyl Substances: A Systematic Review. Eco-Environment & Health 2024, 3, 476–493. [Google Scholar] [CrossRef]
- Bharal, B.; Ruchitha, C.; Kumar, P.; Pandey, R.; Rachamalla, M.; Niyogi, S.; Naidu, R.; Kaundal, R.K. Neurotoxicity of Per- and Polyfluoroalkyl Substances: Evidence and Future Directions. Science of The Total Environment 2024, 955, 176941. [Google Scholar] [CrossRef]
- Mario, T.; Yvonne, D.; Veronica, S.; Alejandro, D.; Juan, R.; Diana, F.; Edmundo, B.; Eduardo, C.; Mario, A.; Alma, L.; et al. Effects of Perfluorooctanoic Acid in Oxidative Stress Generation, DNA Damage in Cumulus Cells, and Its Impact on in Vitro Maturation of Porcine Oocytes. Environmental Toxicology 2022, 37, 1394–1403. [Google Scholar] [CrossRef]
- Running, L.; Cristobal, J.R.; Karageorgiou, C.; Camdzic, M.; Aguilar, J.M.N.; Gokcumen, O.; Aga, D.S.; Atilla-Gokcumen, G.E. Investigating the Mechanism of Neurotoxic Effects of PFAS in Differentiated Neuronal Cells through Transcriptomics and Lipidomics Analysis. ACS Chem. Neurosci. 2024, 15, 4568–4579. [Google Scholar] [CrossRef]
- Kim, H.; Hong, M.-W.; Bae, Y.; Lee, S.-J. Epigenetic Toxicity and Cytotoxicity of Perfluorooctanoic Acid and Its Effects on Gene Expression in Embryonic Mouse Hypothalamus Cells. Archives of Industrial Hygiene and Toxicology 2021, 72, 182–190. [Google Scholar] [CrossRef]
- Behr, A.-C.; Plinsch, C.; Braeuning, A.; Buhrke, T. Activation of Human Nuclear Receptors by Perfluoroalkylated Substances (PFAS). Toxicology in Vitro 2020, 62, 104700. [Google Scholar] [CrossRef] [PubMed]
- Pederick, J.L.; Frkic, R.L.; McDougal, D.P.; Bruning, J.B. A Structural Basis for the Activation of Peroxisome Proliferator-Activated Receptor Gamma (PPARγ) by Perfluorooctanoic Acid (PFOA). Chemosphere 2024, 354, 141723. [Google Scholar] [CrossRef]
- Stratakis, N.; Baumert, B.O.; Conti, D.; Wu, H.; Grandjean, P.; Nielsen, F.; Walker, D.I.; Valvi, D.; La Merrill, M.A.A.; Eckel, S.P.; et al. Associations between Liver PFAS Concentrations and Plasma Extracellular miRNAs in a Cohort of Adolescents Undergoing Bariatric Surgery. ISEE Conference Abstracts 2021, 2021, isee.2021.O-TO-140. [Google Scholar] [CrossRef]
- You, D.; Cohen, J.D.; Pustovalova, O.; Lewis, L.; Shen, L. Profiling Secreted miRNA Biomarkers of Chemical-Induced Neurodegeneration in Human iPSC-Derived Neurons. Toxicological Sciences 2022, 186, 221–241. [Google Scholar] [CrossRef]
- Zingale, V.D.; Gugliandolo, A.; Mazzon, E. MiR-155: An Important Regulator of Neuroinflammation. IJMS 2021, 23, 90. [Google Scholar] [CrossRef]
- Li, Z.; Yu, Z.; Yin, D. Multi- and Trans-Generational Disturbances of Perfluorobutane Sulfonate and Perfluorohexane Sulfonate on Lipid Metabolism in Caenorhabditis Elegans. Chemosphere 2021, 280, 130666. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Tang, J.; Xu, S.; Ge, J.; Dong, Y.; Li, H.; Jin, M. Parental Transfer of Perfluorooctane Sulfonate and ZnO Nanoparticles Chronic Co-Exposure and Inhibition of Growth in F1 Offspring. Regulatory Toxicology and Pharmacology 2018, 98, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Haimbaugh, A. Persistent Transcriptomic Effects Of Brief Developmental Exposure To Environmental Contaminants. Wayne State University Dissertations 2022. [Google Scholar]
- Moore, L.D.; Le, T.; Fan, G. DNA Methylation and Its Basic Function. Neuropsychopharmacol 2013, 38, 23–38. [Google Scholar] [CrossRef]
- Cedar, H.; Sabag, O.; Reizel, Y. The Role of DNA Methylation in Genome-Wide Gene Regulation during Development. Development 2022, 149, dev200118. [Google Scholar] [CrossRef]
- Besselink, N.; Keijer, J.; Vermeulen, C.; Boymans, S.; De Ridder, J.; Van Hoeck, A.; Cuppen, E.; Kuijk, E. The Genome-Wide Mutational Consequences of DNA Hypomethylation. Sci Rep 2023, 13, 6874. [Google Scholar] [CrossRef]
- Pierozan, P.; Höglund, A.; Theodoropoulou, E.; Karlsson, O. Perfluorooctanesulfonic Acid (PFOS) Induced Cancer Related DNA Methylation Alterations in Human Breast Cells: A Whole Genome Methylome Study. Science of The Total Environment 2024, 949, 174864. [Google Scholar] [CrossRef]
- Zhao, H.; Xie, J.; Wu, S.; Sánchez, O.F.; Zhang, X.; Freeman, J.L.; Yuan, C. Pre-Differentiation Exposure of PFOA Induced Persistent Changes in DNA Methylation and Mitochondrial Morphology in Human Dopaminergic-like Neurons. Environmental Pollution 2022, 308, 119684. [Google Scholar] [CrossRef]
- Everson, T.M.; Sehgal, N.; Barr, D.B.; Panuwet, P.; Yakimavets, V.; Perez, C.; Shankar, K.; Eick, S.M.; Pearson, K.J.; Andres, A. Placental PFAS Concentrations Are Associated with Perturbations of Placental DNA Methylation at Loci with Important Roles on Cardiometabolic Health 2024.
- Bronson, S.L.; Bale, T.L. The Placenta as a Mediator of Stress Effects on Neurodevelopmental Reprogramming. Neuropsychopharmacol 2016, 41, 207–218. [Google Scholar] [CrossRef]
- Shallie, P.D.; Naicker, T. The Placenta as a Window to the Brain: A Review on the Role of Placental Markers in Prenatal Programming of Neurodevelopment. Intl J of Devlp Neuroscience 2019, 73, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Maxwell, D.L.; Oluwayiose, O.A.; Houle, E.; Roth, K.; Nowak, K.; Sawant, S.; Paskavitz, A.L.; Liu, W.; Gurdziel, K.; Petriello, M.C.; et al. Mixtures of Per- and Polyfluoroalkyl Substances (PFAS) Alter Sperm Methylation and Long-Term Reprogramming of Offspring Liver and Fat Transcriptome. Environment International 2024, 186, 108577. [Google Scholar] [CrossRef]
- Maxwell, D.L.; Petriello, M.C.; Pilsner, J.R. PFAS Exposure and Male Reproductive Health: Implications for Sperm Epigenetics. Semin Reprod Med 2024, 42, 288–301. [Google Scholar] [CrossRef]
- Jackson, S.P.; Bartek, J. The DNA-Damage Response in Human Biology and Disease. Nature 2009, 461, 1071–1078. [Google Scholar] [CrossRef]
- Qin, Y.; Yuan, X.; Cui, Z.; Chen, W.; Xu, S.; Chen, K.; Wang, F.; Zheng, F.; Ni, H.; Shen, H.-M.; et al. Low Dose PFDA Induces DNA Damage and DNA Repair Inhibition by Promoting Nuclear cGAS Accumulation in Ovarian Epithelial Cells. Ecotoxicology and Environmental Safety 2023, 265, 115503. [Google Scholar] [CrossRef] [PubMed]
- Decout, A.; Katz, J.D.; Venkatraman, S.; Ablasser, A. The cGAS–STING Pathway as a Therapeutic Target in Inflammatory Diseases. Nat Rev Immunol 2021, 21, 548–569. [Google Scholar] [CrossRef]
- Ojo, A.F.; Peng, C.; Ng, J.C. Genotoxicity Assessment of Per- and Polyfluoroalkyl Substances Mixtures in Human Liver Cells (HepG2). Toxicology 2022, 482, 153359. [Google Scholar] [CrossRef]
- Solan, M.E.; Koperski, C.P.; Senthilkumar, S.; Lavado, R. Short-Chain per- and Polyfluoralkyl Substances (PFAS) Effects on Oxidative Stress Biomarkers in Human Liver, Kidney, Muscle, and Microglia Cell Lines. Environmental Research 2023, 223, 115424. [Google Scholar] [CrossRef] [PubMed]
- Pierozan, P.; Kosnik, M.; Karlsson, O. High-Content Analysis Shows Synergistic Effects of Low Perfluorooctanoic Acid (PFOS) and Perfluorooctane Sulfonic Acid (PFOA) Mixture Concentrations on Human Breast Epithelial Cell Carcinogenesis. Environment International 2023, 172, 107746. [Google Scholar] [CrossRef]
- Feng, J.; Soto-Moreno, E.J.; Prakash, A.; Balboula, A.Z.; Qiao, H. Adverse PFAS Effects on Mouse Oocyte in Vitro Maturation Are Associated with Carbon-chain Length and Inclusion of a Sulfonate Group. Cell Proliferation 2023, 56, e13353. [Google Scholar] [CrossRef]
- Ríos-Bonilla, K.M.; Aga, D.S.; Lee, J.; König, M.; Qin, W.; Cristobal, J.R.; Atilla-Gokcumen, G.E.; Escher, B.I. Neurotoxic Effects of Mixtures of Perfluoroalkyl Substances (PFAS) at Environmental and Human Blood Concentrations. Environ. Sci. Technol. 2024, acs.est.4c06017. [Google Scholar] [CrossRef] [PubMed]
- Solan, M.E.; Koperski, C.P.; Senthilkumar, S.; Lavado, R. Short-Chain per- and Polyfluoralkyl Substances (PFAS) Effects on Oxidative Stress Biomarkers in Human Liver, Kidney, Muscle, and Microglia Cell Lines. Environmental Research 2023, 223, 115424. [Google Scholar] [CrossRef]
- Obiako, P.C.; Ayisire, S.O.; Sayes, C.M. Impact of Perfluorooctanoic Acid (PFOA) and Perfluorobutanoic Acid (PFBA) on Oxidative Stress and Metabolic Biomarkers in Human Neuronal Cells (SH-SY5Y). Environment International 2024, 190, 108864. [Google Scholar] [CrossRef] [PubMed]
- Souders, C.L.; Sanchez, C.L.; Malphurs, W.; Aristizabal-Henao, J.J.; Bowden, J.A.; Martyniuk, C.J. Metabolic Profiling in Human SH-SY5Y Neuronal Cells Exposed to Perfluorooctanoic Acid (PFOA). NeuroToxicology 2021, 85, 160–172. [Google Scholar] [CrossRef]
- Pierozan, P.; Cattani, D.; Karlsson, O. Perfluorooctane Sulfonate (PFOS) and Perfluorooctanoic Acid (PFOA) Induce Epigenetic Alterations and Promote Human Breast Cell Carcinogenesis in Vitro. Arch Toxicol 2020, 94, 3893–3906. [Google Scholar] [CrossRef] [PubMed]
- Tsai, W.-J.; Hsieh, W.-S.; Chen, P.-C.; Liu, C.-Y. Prenatal Perfluoroalkyl Substance Exposure in Association with Global Histone Post-Translational Methylation in 2-Year-Old Children. Toxics 2024, 12, 876. [Google Scholar] [CrossRef]
- Li, F.; Yang, R.; Lu, L.; Hua, W.; Sun, Y.; Tian, M.; Lu, Y.; Huang, Q. Comparative Steroidogenic Effects of Hexafluoropropylene Oxide Trimer Acid (HFPO-TA) and Perfluorooctanoic Acid (PFOA): Regulation of Histone Modifications. Environmental Pollution 2024, 350, 124030. [Google Scholar] [CrossRef]
- Evans, N.; Conley, J.M.; Cardon, M.; Hartig, P.; Medlock-Kakaley, E.; Gray, L.E. In Vitro Activity of a Panel of Per- and Polyfluoroalkyl Substances (PFAS), Fatty Acids, and Pharmaceuticals in Peroxisome Proliferator-Activated Receptor (PPAR) Alpha, PPAR Gamma, and Estrogen Receptor Assays. Toxicology and Applied Pharmacology 2022, 449, 116136. [Google Scholar] [CrossRef]
- Kashobwe, L.; Sadrabadi, F.; Braeuning, A.; Leonards, P.E.G.; Buhrke, T.; Hamers, T. In Vitro Screening of Understudied PFAS with a Focus on Lipid Metabolism Disruption. Arch Toxicol 2024, 98, 3381–3395. [Google Scholar] [CrossRef]
- Roy, S.; Danasekaran, K.; Moran, J.; O’Brien, K.; Dakshanamurthy, S. Comprehensive Analysis and Large-Scale Screening of Binding Interactions Between PFAS and Their Mixtures with Nuclear Receptors 2024.
- Zhao, L.; Teng, M.; Zhao, X.; Li, Y.; Sun, J.; Zhao, W.; Ruan, Y.; Leung, K.M.Y.; Wu, F. Insight into the Binding Model of Per- and Polyfluoroalkyl Substances to Proteins and Membranes. Environment International 2023, 175, 107951. [Google Scholar] [CrossRef]
- Almeida, N.M.S.; Eken, Y.; Wilson, A.K. Binding of Per- and Polyfluoro-Alkyl Substances to Peroxisome Proliferator-Activated Receptor Gamma. ACS Omega 2021, 6, 15103–15114. [Google Scholar] [CrossRef]
- Feige, J.N.; Gelman, L.; Michalik, L.; Desvergne, B.; Wahli, W. From Molecular Action to Physiological Outputs: Peroxisome Proliferator-Activated Receptors Are Nuclear Receptors at the Crossroads of Key Cellular Functions. Progress in Lipid Research 2006, 45, 120–159. [Google Scholar] [CrossRef] [PubMed]
- Tontonoz, P.; Spiegelman, B.M. Fat and Beyond: The Diverse Biology of PPARγ. Annu. Rev. Biochem. 2008, 77, 289–312. [Google Scholar] [CrossRef]
- Steger, D.J.; Grant, G.R.; Schupp, M.; Tomaru, T.; Lefterova, M.I.; Schug, J.; Manduchi, E.; Stoeckert, C.J.; Lazar, M.A. Propagation of Adipogenic Signals through an Epigenomic Transition State. Genes Dev. 2010, 24, 1035–1044. [Google Scholar] [CrossRef]
- Mohanty, P.K.; Patel, R. Central Role of PPARγ in Alzheimer’s Disease: From Pathophysiology to Potential Therapies. AN 2025, 0, 6479. [Google Scholar] [CrossRef]
- Carstens, K.E.; Freudenrich, T.; Wallace, K.; Choo, S.; Carpenter, A.; Smeltz, M.; Clifton, M.S.; Henderson, W.M.; Richard, A.M.; Patlewicz, G.; et al. Evaluation of Per- and Polyfluoroalkyl Substances (PFAS) In Vitro Toxicity Testing for Developmental Neurotoxicity. Chem. Res. Toxicol. 2023, 36, 402–419. [Google Scholar] [CrossRef] [PubMed]
- Haimbaugh, A.; Wu, C.-C.; Akemann, C.; Meyer, D.N.; Connell, M.; Abdi, M.; Khalaf, A.; Johnson, D.; Baker, T.R. Multi- and Transgenerational Effects of Developmental Exposure to Environmental Levels of PFAS and PFAS Mixture in Zebrafish (Danio Rerio). Toxics 2022, 10, 334. [Google Scholar] [CrossRef]
- Kim, S.; Thapar, I.; Brooks, B.W. Epigenetic Changes by Per- and Polyfluoroalkyl Substances (PFAS). Environmental Pollution 2021, 279, 116929. [Google Scholar] [CrossRef]
- Abdulkadir, A.; Kandel, S.; Lewis, N.; Dauvergne, O.; Rosby, R.; Hossain, E. Epigenetic Consequences of In Utero PFAS Exposure: Implications for Development and Long-Term Health 2025.
- Olsen, G.W.; Mair, D.C.; Lange, C.C.; Harrington, L.M.; Church, T.R.; Goldberg, C.L.; Herron, R.M.; Hanna, H.; Nobiletti, J.B.; Rios, J.A.; et al. Per- and Polyfluoroalkyl Substances (PFAS) in American Red Cross Adult Blood Donors, 2000–2015. Environmental Research 2017, 157, 87–95. [Google Scholar] [CrossRef]
- Wang, C.S.; Kavalali, E.T.; Monteggia, L.M. BDNF Signaling in Context: From Synaptic Regulation to Psychiatric Disorders. Cell 2022, 185, 62–76. [Google Scholar] [CrossRef]
- Boulle, F.; Van Den Hove, D.L.A.; Jakob, S.B.; Rutten, B.P.; Hamon, M.; Van Os, J.; Lesch, K.-P.; Lanfumey, L.; Steinbusch, H.W.; Kenis, G. Epigenetic Regulation of the BDNF Gene: Implications for Psychiatric Disorders. Mol Psychiatry 2012, 17, 584–596. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.-W.; Chen, L. Epigenetic Regulation of BDNF Gene during Development and Diseases. Int J Mol Sci 2017, 18, 571. [Google Scholar] [CrossRef] [PubMed]
- Fuchikami, M.; Yamamoto, S.; Morinobu, S.; Takei, S.; Yamawaki, S. Epigenetic Regulation of BDNF Gene in Response to Stress. Psychiatry Investig 2010, 7, 251. [Google Scholar] [CrossRef]
- Ribeiro, A.C.R.; Jahr, F.M.; Hawkins, E.; Kronfol, M.M.; Younis, R.M.; McClay, J.L.; Deshpande, L.S. Epigenetic Histone Acetylation and Bdnf Dysregulation in the Hippocampus of Rats Exposed to Repeated, Low-Dose Diisopropylfluorophosphate. Life Sciences 2021, 281, 119765. [Google Scholar] [CrossRef]
- Onishchenko, N.; Karpova, N.; Sabri, F.; Castrén, E.; Ceccatelli, S. Long-lasting Depression-like Behavior and Epigenetic Changes of BDNF Gene Expression Induced by Perinatal Exposure to Methylmercury. Journal of Neurochemistry 2008, 106, 1378–1387. [Google Scholar] [CrossRef]
- Ke, T.; Tinkov, A.; Skalny, A.; Santamaria, A.; Rocha, J.; Bowman, A.; Chen, W.; Aschner, M. Epigenetics and Methylmercury-Induced Neurotoxicity, Evidence from Experimental Studies. Toxics 2023, 11, 72. [Google Scholar] [CrossRef]
- Wei, L.; He, H.; Yang, S.; Shi, Q.; Wang, X.; Huang, L.; Lu, J.; Shen, Y.; Zhi, K.; Xiang, J.; et al. Synergistic Suppression of BDNF via Epigenetic Mechanism Deteriorating Learning and Memory Impairment Caused by Mn and Pb Co-Exposure. Ecotoxicology and Environmental Safety 2024, 277, 116365. [Google Scholar] [CrossRef] [PubMed]
- Nagahara, A.H.; Tuszynski, M.H. Potential Therapeutic Uses of BDNF in Neurological and Psychiatric Disorders. Nat Rev Drug Discov 2011, 10, 209–219. [Google Scholar] [CrossRef]
- Onishchenko, N.; Karpova, N.; Sabri, F.; Castrén, E.; Ceccatelli, S. Long-lasting Depression-like Behavior and Epigenetic Changes of BDNF Gene Expression Induced by Perinatal Exposure to Methylmercury. Journal of Neurochemistry 2008, 106, 1378–1387. [Google Scholar] [CrossRef]
- Guo, X.-X.; He, Q.-Z.; Li, W.; Long, D.-X.; Pan, X.-Y.; Chen, C.; Zeng, H.-C. Brain-Derived Neurotrophic Factor Mediated Perfluorooctane Sulfonate Induced-Neurotoxicity via Epigenetics Regulation in SK-N-SH Cells. IJMS 2017, 18, 893. [Google Scholar] [CrossRef]
- Li, W.; He, Q.; Wu, C.; Pan, X.; Wang, J.; Tan, Y.; Shan, X.; Zeng, H. PFOS Disturbs BDNF-ERK-CREB Signalling in Association with Increased MicroRNA-22 in SH-SY5Y Cells. BioMed Research International 2015, 2015, 1–10. [Google Scholar] [CrossRef]
- Cuadrado, A. Brain-Protective Mechanisms of Transcription Factor NRF2: Toward a Common Strategy for Neurodegenerative Diseases. Annu Rev Pharmacol Toxicol 2022, 62, 255–277. [Google Scholar] [CrossRef] [PubMed]
- Sandberg, M.; Patil, J.; D’Angelo, B.; Weber, S.G.; Mallard, C. NRF2-Regulation in Brain Health and Disease: Implication of Cerebral Inflammation. Neuropharmacology 2014, 79, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Yao, W.; Lin, S.; Su, J.; Cao, Q.; Chen, Y.; Chen, J.; Zhang, Z.; Hashimoto, K.; Qi, Q.; Zhang, J. Activation of BDNF by Transcription Factor Nrf2 Contributes to Antidepressant-like Actions in Rodents. Transl Psychiatry 2021, 11, 140. [Google Scholar] [CrossRef] [PubMed]
- Ojo, A.F.; Peng, C.; Ng, J.C. Combined Effects of Mixed Per- and Polyfluoroalkyl Substances on the Nrf2-ARE Pathway in ARE Reporter-HepG2 Cells. Journal of Hazardous Materials 2022, 421, 126827. [Google Scholar] [CrossRef]
- Shi, X.; Zhou, B. The Role of Nrf2 and MAPK Pathways in PFOS-Induced Oxidative Stress in Zebrafish Embryos. Toxicological Sciences 2010, 115, 391–400. [Google Scholar] [CrossRef]
- Cai, L.-J.; Tu, L.; Huang, X.-M.; Huang, J.; Qiu, N.; Xie, G.-H.; Liao, J.-X.; Du, W.; Zhang, Y.-Y.; Tian, J.-Y. LncRNA MALAT1 Facilitates Inflammasome Activation via Epigenetic Suppression of Nrf2 in Parkinson’s Disease. Mol Brain 2020, 13, 130. [Google Scholar] [CrossRef]
- Cao, H.; Wang, L.; Chen, B.; Zheng, P.; He, Y.; Ding, Y.; Deng, Y.; Lu, X.; Guo, X.; Zhang, Y.; et al. DNA Demethylation Upregulated Nrf2 Expression in Alzheimer’s Disease Cellular Model. Front. Aging Neurosci. 2016, 7. [Google Scholar] [CrossRef]
- Liu, S.; Qiu, W.; Li, R.; Chen, B.; Wu, X.; Magnuson, J.T.; Xu, B.; Luo, S.; Xu, E.G.; Zheng, C. Perfluorononanoic Acid Induces Neurotoxicity via Synaptogenesis Signaling in Zebrafish. Environ. Sci. Technol. 2023, 57, 3783–3793. [Google Scholar] [CrossRef]
- Sultan, F.A.; Day, J.J. Epigenetic Mechanisms in Memory and Synaptic Function. Epigenomics 2011, 3, 157–181. [Google Scholar] [CrossRef]
- Xylaki, M.; Atzler, B.; Outeiro, T.F. Epigenetics of the Synapse in Neurodegeneration. Curr Neurol Neurosci Rep 2019, 19, 72. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Yang, Q.; Wu, L.; He, Y.; Zeng, N.; Wang, Z. Neurotoxic Effects of Per- and Polyfluoroalkyl Substances (PFAS) Mixture Exposure in Mice: Accumulations in Brain and Associated Changes of Behaviors, Metabolome, and Transcriptome. Journal of Hazardous Materials 2025, 489, 137699. [Google Scholar] [CrossRef]
- Patel, R.; Bradner, J.; Stout, K.; Caudle, W. Alteration to Dopaminergic Synapses Following Exposure to Perfluorooctane Sulfonate (PFOS), in Vitro and in Vivo. Medical Sciences 2016, 4, 13. [Google Scholar] [CrossRef]
- Spies, J.; Covarrubias-Pinto, A.; Carcamo, C.; Arancibia, Y.; Salazar, F.; Paredes-Martinez, C.; Otth, C.; Castro, M.; Zambrano, A. Modulation of Synaptic Plasticity Genes Associated to DNA Damage in a Model of Huntington’s Disease. Neurochem Res 2023, 48, 2093–2103. [Google Scholar] [CrossRef]
- Maxwell, D.L.; Oluwayiose, O.A.; Houle, E.; Roth, K.; Nowak, K.; Sawant, S.; Paskavitz, A.L.; Liu, W.; Gurdziel, K.; Petriello, M.C.; et al. Mixtures of Per- and Polyfluoroalkyl Substances (PFAS) Alter Sperm Methylation and Long-Term Reprogramming of Offspring Liver and Fat Transcriptome. Environment International 2024, 186, 108577. [Google Scholar] [CrossRef] [PubMed]
- Foguth, R.; Sepúlveda, M.S.; Cannon, J. Per- and Polyfluoroalkyl Substances (PFAS) Neurotoxicity in Sentinel and Non-Traditional Laboratory Model Systems: Potential Utility in Predicting Adverse Outcomes in Human Health. Toxics 2020, 8, 42. [Google Scholar] [CrossRef] [PubMed]
- Snyder, M.A.; Gao, W.-J. NMDA Receptor Hypofunction for Schizophrenia Revisited: Perspectives from Epigenetic Mechanisms. Schizophrenia Research 2020, 217, 60–70. [Google Scholar] [CrossRef]
- Jayanthi, S.; McCoy, M.T.; Chen, B.; Britt, J.P.; Kourrich, S.; Yau, H.-J.; Ladenheim, B.; Krasnova, I.N.; Bonci, A.; Cadet, J.L. Methamphetamine Downregulates Striatal Glutamate Receptors via Diverse Epigenetic Mechanisms. Biological Psychiatry 2014, 76, 47–56. [Google Scholar] [CrossRef]
- Liang, Z.; Liu, W.; Cao, M.; Cui, J.; Lan, J.; Ding, Y.; Zhang, T.; Yang, Z. Epigenetic Regulation-Mediated Disorders in Dopamine Transporter Endocytosis: A Novel Mechanism for the Pathogenesis of Parkinson’s Disease. Theranostics 2025, 15, 2250–2278. [Google Scholar] [CrossRef]
- Ke, T.; Tinkov, A.; Skalny, A.; Santamaria, A.; Rocha, J.; Bowman, A.; Chen, W.; Aschner, M. Epigenetics and Methylmercury-Induced Neurotoxicity, Evidence from Experimental Studies. Toxics 2023, 11, 72. [Google Scholar] [CrossRef]
- Hallgren, S.; Fredriksson, A.; Viberg, H. More Signs of Neurotoxicity of Surfactants and Flame Retardants – Neonatal PFOS and PBDE 99 Cause Transcriptional Alterations in Cholinergic Genes in the Mouse CNS. Environmental Toxicology and Pharmacology 2015, 40, 409–416. [Google Scholar] [CrossRef]
- Chen, X.; Hu, X.; Liu, H.; He, J.; Li, Y.; Zhang, X. Neurotoxic Effects of Atrazine on Dopaminergic System via miRNAs and Energy-Sensing Pathways. Mol Neurobiol 2025. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Nie, X.; Mao, J.; Zhang, Y.; Yin, K.; Jiang, S. Perfluorooctanesulfonate Induces Neuroinflammation through the Secretion of TNF-α Mediated by the JAK2/STAT3 Pathway. NeuroToxicology 2018, 66, 32–42. [Google Scholar] [CrossRef]
- Abu-Salah, A.; Cesur, M.F.; Anchan, A.; Ay, M.; Langley, M.R.; Shah, A.; Reina-Gonzalez, P.; Strazdins, R.; Çakır, T.; Sarkar, S. Comparative Proteomics Highlights That GenX Exposure Leads to Metabolic Defects and Inflammation in Astrocytes. Environ. Sci. Technol. 2024, 58, 20525–20539. [Google Scholar] [CrossRef] [PubMed]
- Paquette, S.E.; Martin, N.R.; Rodd, A.; Manz, K.E.; Allen, E.; Camarillo, M.; Weller, H.I.; Pennell, K.; Plavicki, J.S. Evaluation of Neural Regulation and Microglial Responses to Brain Injury in Larval Zebrafish Exposed to Perfluorooctane Sulfonate. Environ Health Perspect 2023, 131, 117008. [Google Scholar] [CrossRef]
- Alharthy, S.A.; Hardej, D. The Role of Transcription Factor Nrf2 in the Toxicity of Perfluorooctane Sulfonate (PFOS) and Perfluorooctanoic Acid (PFOA) in C57BL/6 Mouse Astrocytes. Environ Toxicol Pharmacol 2021, 86, 103652. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.-Q.; Liu, T.; Yang, S.; Sun, L.; Zhao, Z.-Y.; Li, L.-Y.; She, Y.-C.; Zheng, Y.-Y.; Ye, X.-Y.; Bao, Q.; et al. Perfluoroalkyl Substance Pollutants Activate the Innate Immune System through the AIM2 Inflammasome. Nat Commun 2021, 12, 2915. [Google Scholar] [CrossRef]
- Han, R.; Hu, M.; Zhong, Q.; Wan, C.; Liu, L.; Li, F.; Zhang, F.; Ding, W. Perfluorooctane Sulphonate Induces Oxidative Hepatic Damage via Mitochondria-Dependent and NF-κB/TNF-α-Mediated Pathway. Chemosphere 2018, 191, 1056–1064. [Google Scholar] [CrossRef]
- Hmila, I.; Hill, J.; Shalaby, K.E.; Ouararhni, K.; Abedsselem, H.; Modaresi, S.M.S.; Bihaqi, S.W.; Marques, E.; Sondhi, A.; Slitt, A.L.; et al. Perinatal Exposure to PFOS and Sustained High-Fat Diet Promote Neurodevelopmental Disorders via Genomic Reprogramming of Pathways Associated with Neuromotor Development. Ecotoxicology and Environmental Safety 2024, 272, 116070. [Google Scholar] [CrossRef]
- Nian, M.; Zhou, W.; Feng, Y.; Wang, Y.; Chen, Q.; Zhang, J. Emerging and Legacy PFAS and Cytokine Homeostasis in Women of Childbearing Age. Sci Rep 2022, 12, 6517. [Google Scholar] [CrossRef]
- Ho, T.C.; Wan, H.T.; Lee, W.K.; Lam, T.K.Y.; Lin, X.; Chan, T.F.; Lai, K.P.; Wong, C.K.C. Effects of In Utero PFOS Exposure on Epigenetics and Metabolism in Mouse Fetal Livers. Environ. Sci. Technol. 2023, 57, 14892–14903. [Google Scholar] [CrossRef]
- Huang, M.; Malovic, E.; Ealy, A.; Jin, H.; Anantharam, V.; Kanthasamy, A.; Kanthasamy, A.G. Microglial Immune Regulation by Epigenetic Reprogramming through Histone H3K27 Acetylation in Neuroinflammation. Front. Immunol. 2023, 14, 1052925. [Google Scholar] [CrossRef] [PubMed]
- Kaminska, B.; Mota, M.; Pizzi, M. Signal Transduction and Epigenetic Mechanisms in the Control of Microglia Activation during Neuroinflammation. Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease 2016, 1862, 339–351. [Google Scholar] [CrossRef] [PubMed]
- Neal, M.; Richardson, J.R. Epigenetic Regulation of Astrocyte Function in Neuroinflammation and Neurodegeneration. Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease 2018, 1864, 432–443. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, J.; Xing, Z.; Peng, C.; Li, D. Autophagy in Neuroinflammation: A Focus on Epigenetic Regulation. Aging Dis 2024, 15, 739–754. [Google Scholar] [CrossRef] [PubMed]
- Jauhari, A.; Singh, T.; Yadav, S. Neurodevelopmental Disorders and Neurotoxicity: MicroRNA in Focus. Journal of Chemical Neuroanatomy 2022, 120, 102072. [Google Scholar] [CrossRef]
- Furlong, M.A.; Liu, T.; Jung, A.; Beitel, S.; Hughes, J.; Krause, R.; Graber, J.M.; Calkins, M.M.; Calafat, A.M.; Botelho, J.C.; et al. Per- and Polyfluoroalkyl Substances (PFAS) and microRNA: An Epigenome-Wide Association Study in Firefighters. Environmental Research 2025, 121766. [Google Scholar] [CrossRef]
- Zhang, X.; Sands, M.; La Frano, M.; Spinella, M.J.; Masoud, F.; Fields, C.; Madak-Erdogan, Z.; Jensen, T.; Irudayaraj, J. MicroRNAs and PFAS: A Pilot Study in Blood Collected from Firefighters 2024.
- Larose, T.L.; Sætrom, P.; Martinussen, M.P.; Skogseth, H.; Sandanger, T.M.; Scélo, G.; McHale, C.M.; Jacobsen, G.W.; Smith, M.T. In Utero Exposure To Endocrine Disrupting Chemicals, Micro-Rna Profiles, And Fetal Growth: A Pilot Study Protocol. Journal of Public Health Research 2019, 8, jphr.2019.1550. [Google Scholar] [CrossRef]
- Li, Y.; Baumert, B.O.; Stratakis, N.; Goodrich, J.A.; Wu, H.; Liu, S.H.; Wang, H.; Beglarian, E.; Bartell, S.M.; Eckel, S.P.; et al. Exposure to Per- and Polyfluoroalkyl Substances and Alterations in Plasma microRNA Profiles in Children. Environmental Research 2024, 259, 119496. [Google Scholar] [CrossRef]
- You, D.; Cohen, J.D.; Pustovalova, O.; Lewis, L.; Shen, L. Profiling Secreted miRNA Biomarkers of Chemical-Induced Neurodegeneration in Human iPSC-Derived Neurons. Toxicological Sciences 2022, 186, 221–241. [Google Scholar] [CrossRef]
- Wu, P.; Ding, C.; Yan, M.; Qian, B.; Wang, W.; Sun, P.; Zhao, J. Perfluorooctane Sulfonate Induces Apoptosis via Activation of FoxO3a and Upregulation of Proapoptotic Bcl-2 Proteins in PC12 Cells. J. Toxicol. Sci. 2019, 44, 657–666. [Google Scholar] [CrossRef]
- Guo, X.-X.; He, Q.-Z.; Li, W.; Long, D.-X.; Pan, X.-Y.; Chen, C.; Zeng, H.-C. Brain-Derived Neurotrophic Factor Mediated Perfluorooctane Sulfonate Induced-Neurotoxicity via Epigenetics Regulation in SK-N-SH Cells. IJMS 2017, 18, 893. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Quan, X.; Chen, G.; Hong, J.; Wang, Q.; Xu, L.; Wang, B.; Yu, Z.; Yu, H.-M. PFOS-Induced Placental Cell Growth Inhibition Is Partially Mediated by lncRNA H19 through Interacting with miR-19a and miR-19b. Chemosphere 2020, 261, 127640. [Google Scholar] [CrossRef] [PubMed]
- Sonkar, R.; Kay, M.K.; Choudhury, M. PFOS Modulates Interactive Epigenetic Regulation in First-Trimester Human Trophoblast Cell Line HTR-8/SVneo. Chem. Res. Toxicol. 2019, 32, 2016–2027. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Liu, W.; Ma, J.; Yu, M.; Jin, Y.; Dai, J. Prenatal and Neonatal Exposure to Perfluorooctane Sulfonic Acid Results in Changes in miRNA Expression Profiles and Synapse Associated Proteins in Developing Rat Brains. Environ. Sci. Technol. 2012, 46, 6822–6829. [Google Scholar] [CrossRef]
- Li, J.; Quan, X.; Lei, S.; Chen, G.; Hong, J.; Huang, Z.; Wang, Q.; Song, W.; Yang, X. LncRNA MEG3 Alleviates PFOS Induced Placental Cell Growth Inhibition through Its Derived miR-770 Targeting PTX3. Environmental Pollution 2022, 293, 118542. [Google Scholar] [CrossRef]
- Gao, M.; Dong, Q.; Yang, Z.; Zou, D.; Han, Y.; Chen, Z.; Xu, R. Long Non-Coding RNA H19 Regulates Neurogenesis of Induced Neural Stem Cells in a Mouse Model of Closed Head Injury. Neural Regeneration Research 2024, 19, 872–880. [Google Scholar] [CrossRef]
- Groszer, M.; Erickson, R.; Scripture-Adams, D.D.; Dougherty, J.D.; Le Belle, J.; Zack, J.A.; Geschwind, D.H.; Liu, X.; Kornblum, H.I.; Wu, H. PTEN Negatively Regulates Neural Stem Cell Self-Renewal by Modulating G0 -G1 Cell Cycle Entry. Proc. Natl. Acad. Sci. U.S.A. 2006, 103, 111–116. [Google Scholar] [CrossRef]
- Han, C.-L.; Ge, M.; Liu, Y.-P.; Zhao, X.-M.; Wang, K.-L.; Chen, N.; Hu, W.; Zhang, J.-G.; Li, L.; Meng, F.-G. Long Non-Coding RNA H19 Contributes to Apoptosis of Hippocampal Neurons by Inhibiting Let-7b in a Rat Model of Temporal Lobe Epilepsy. Cell Death Dis 2018, 9, 617. [Google Scholar] [CrossRef]
- Horai, T.; Boku, S.; Okazaki, S.; Otsuka, I.; Ratta-apha, W.; Mouri, K.; Yamaki, N.; Hirata, T.; Hishimoto, A. miR-19b Is Elevated in Peripheral Blood of Schizophrenic Patients and Attenuates Proliferation of Hippocampal Neural Progenitor Cells. Journal of Psychiatric Research 2020, 131, 102–107. [Google Scholar] [CrossRef]
- Jeon, H.; Lee, S.; Lee, W.-H.; Suk, K. Analysis of Glial Secretome: The Long Pentraxin PTX3 Modulates Phagocytic Activity of Microglia. Journal of Neuroimmunology 2010, 229, 63–72. [Google Scholar] [CrossRef]
- Rodriguez-Grande, B.; Swana, M.; Nguyen, L.; Englezou, P.; Maysami, S.; Allan, S.M.; Rothwell, N.J.; Garlanda, C.; Denes, A.; Pinteaux, E. The Acute-Phase Protein PTX3 Is an Essential Mediator of Glial Scar Formation and Resolution of Brain Edema after Ischemic Injury. J Cereb Blood Flow Metab 2014, 34, 480–488. [Google Scholar] [CrossRef]
- Yi, J.; Chen, B.; Yao, X.; Lei, Y.; Ou, F.; Huang, F. Upregulation of the lncRNA MEG3 Improves Cognitive Impairment, Alleviates Neuronal Damage, and Inhibits Activation of Astrocytes in Hippocampus Tissues in Alzheimer’s Disease through Inactivating the PI3K/Akt Signaling Pathway. J of Cellular Biochemistry 2019, 120, 18053–18065. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yan, S.; Zhang, W.; Zhang, H.; Dai, J. Integrated Proteomic and miRNA Transcriptional Analysis Reveals the Hepatotoxicity Mechanism of PFNA Exposure in Mice. J. Proteome Res. 2015, 14, 330–341. [Google Scholar] [CrossRef]
- Sheldon, R.A.; Jiang, X.; Francisco, C.; Christen, S.; Vexler, Z.S.; Täuber, M.G.; Ferriero, D.M. Manipulation of Antioxidant Pathways in Neonatal Murine Brain. Pediatr Res 2004, 56, 656–662. [Google Scholar] [CrossRef]
- Ge, X.-T.; Lei, P.; Wang, H.-C.; Zhang, A.-L.; Han, Z.-L.; Chen, X.; Li, S.-H.; Jiang, R.-C.; Kang, C.-S.; Zhang, J.-N. miR-21 Improves the Neurological Outcome after Traumatic Brain Injury in Rats. Sci Rep 2014, 4, 6718. [Google Scholar] [CrossRef] [PubMed]
- Wójtowicz, S.; Strosznajder, A.K.; Jeżyna, M.; Strosznajder, J.B. The Novel Role of PPAR Alpha in the Brain: Promising Target in Therapy of Alzheimer’s Disease and Other Neurodegenerative Disorders. Neurochem Res 2020, 45, 972–988. [Google Scholar] [CrossRef] [PubMed]
- Maxwell, D.L.; Oluwayiose, O.A.; Houle, E.; Roth, K.; Nowak, K.; Sawant, S.; Paskavitz, A.L.; Liu, W.; Gurdziel, K.; Petriello, M.C.; et al. Mixtures of Per- and Polyfluoroalkyl Substances (PFAS) Alter Sperm Methylation and Long-Term Reprogramming of Offspring Liver and Fat Transcriptome. Environment International 2024, 186, 108577. [Google Scholar] [CrossRef]
- Steves, A.N.; Turry,Adam; Gill,Brittany; Clarkson-Townsend,Danielle; Bradner,Joshua M. ; Bachli,Ian; Caudle,W. Michael; Miller,Gary W.; Chan,Anthony W. S.; and Easley IV, C.A. Per- and Polyfluoroalkyl Substances Impact Human Spermatogenesis in a Stem-Cell-Derived Model. Systems Biology in Reproductive Medicine 2018, 64, 225–239. [Google Scholar] [CrossRef]
- Maxwell, D.L.; Petriello, M.C.; Pilsner, J.R. PFAS Exposure and Male Reproductive Health: Implications for Sperm Epigenetics. Semin Reprod Med 2024, 42, 288–301. [Google Scholar] [CrossRef]
- Tian, J.; Xu, H.; Zhang, Y.; Shi, X.; Wang, W.; Gao, H.; Bi, Y. SAM Targeting Methylation by the Methyl Donor, a Novel Therapeutic Strategy for Antagonize PFOS Transgenerational Fertilitty Toxicity. Ecotoxicology and Environmental Safety 2019, 184, 109579. [Google Scholar] [CrossRef] [PubMed]
- Bline, A.P. Investigation of Biomolecular Condensates as Novel Targets Mediating Germ Cell Toxicity from Per- and Polyfluoroalkyl Substance Exposure, UCLA, 2022.
- Li, Z.; Yu, Z.; Gao, P.; Yin, D. Multigenerational Effects of Perfluorooctanoic Acid on Lipid Metabolism of Caenorhabditis Elegans and Its Potential Mechanism. Sci Total Environ 2020, 703, 134762. [Google Scholar] [CrossRef]
- Cao, Z.; Dai, L.; Li, J.; Zhang, J.; Wang, X.; Xu, A.; Du, H. Reproductive and Germ-Cell Mutagenic Effects of Poly-and Perfluoroalkyl Substances (PFAS) to Caenorhabditis Elegans after Multigenerational Exposure. Science of The Total Environment 2024, 954, 176224. [Google Scholar] [CrossRef] [PubMed]
- Haimbaugh, A.; Wu, C.-C.; Akemann, C.; Meyer, D.N.; Connell, M.; Abdi, M.; Khalaf, A.; Johnson, D.; Baker, T.R. Multi- and Transgenerational Effects of Developmental Exposure to Environmental Levels of PFAS and PFAS Mixture in Zebrafish (Danio Rerio). Toxics 2022, 10, 334. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Lv, S.; Liu, J.; Nie, S.; Chen, J.; Dong, Q.; Huang, C.; Yang, D. Chronic Perfluorooctanesulfonic Acid Exposure Disrupts Lipid Metabolism in Zebrafish. Hum Exp Toxicol 2017, 36, 207–217. [Google Scholar] [CrossRef]
- Shi, G.; Cui, Q.; Wang, J.; Guo, H.; Pan, Y.; Sheng, N.; Guo, Y.; Dai, J. Chronic Exposure to 6:2 Chlorinated Polyfluorinated Ether Sulfonate Acid (F-53B) Induced Hepatotoxic Effects in Adult Zebrafish and Disrupted the PPAR Signaling Pathway in Their Offspring. Environmental Pollution 2019, 249, 550–559. [Google Scholar] [CrossRef]
- Chen, L.; Lam, J.C.W.; Hu, C.; Tsui, M.M.P.; Lam, P.K.S.; Zhou, B. Perfluorobutanesulfonate Exposure Skews Sex Ratio in Fish and Transgenerationally Impairs Reproduction. Environ. Sci. Technol. 2019, 53, 8389–8397. [Google Scholar] [CrossRef]
- Coperchini, F.; Teliti,Marsida; Greco,Alessia; Croce,Laura; and Rotondi, M. Per-Polyfluoroalkyl Substances (PFAS) as Thyroid Disruptors: Is There Evidence for Multi-Transgenerational Effects? Expert Review of Endocrinology & Metabolism 2024, 19, 307–315. [Google Scholar] [CrossRef]
- Bouwmeester, M.C.; Ruiter, S.; Lommelaars, T.; Sippel, J.; Hodemaekers, H.M.; Van Den Brandhof, E.-J.; Pennings, J.L.A.; Kamstra, J.H.; Jelinek, J.; Issa, J.-P.J.; et al. Zebrafish Embryos as a Screen for DNA Methylation Modifications after Compound Exposure. Toxicology and Applied Pharmacology 2016, 291, 84–96. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).