1. Introduction
The authentication of the geographical origin of food products has gained increasing attention in recent years due to growing concerns about food fraud, quality assurance, and consumer protection [
1,
2]. Edible nuts, such as almonds, walnuts, pistachios, and cashews, are high-value agricultural commodities that are frequently traded globally. Their market value and quality are often influenced by their origin, as environmental factors such as soil composition, climate, and agricultural practices can affect their chemical composition and sensory attributes [
3,
4]. Therefore, developing reliable methods for geographical classification is essential to ensure product authenticity and traceability.
Traditional analytical approaches for origin determination, including isotopic and chromatographic techniques, often require expensive instrumentation and time-consuming sample preparation [
5,
6,
7]. In contrast, rapid and simple chemical analysis methods—such as elemental profiling, spectroscopic measurements, and basic physicochemical tests—have emerged as promising alternatives due to their cost-effectiveness, accessibility, and ease of implementation [
8]. When combined with appropriate statistical or chemometric tools, these methods can provide sufficient discriminatory power to classify samples based on their geographic origin [
9,
10,
11].
In recent years, advanced analytical techniques such as ICP/MS, GC/MS, and FTIR have been widely applied for food authentication due to their high sensitivity and rapid analysis capabilities. However, despite their speed and accuracy, these methods still require sophisticated instrumentation and skilled personnel, limiting their routine use in on-site or resource-limited settings. As a result, there is growing interest in the development and application of simpler, cost-effective analytical methods that can be easily implemented for preliminary screening or large-scale classification [
12,
13,
14,
15].
This study aims to review and synthesize previous research related to the use of rapid and simple chemical analysis methods for the classification and geographical traceability of edible nuts. By consolidating existing findings, this work seeks to identify knowledge gaps and highlight nut types or regions that remain under-investigated. The outcome of this synthesis will support scientists in efficiently targeting future research and contribute to the development of comprehensive data systems on edible nuts. Moreover, it emphasizes the clear potential of rapid and simple chemical analysis techniques as powerful tools for the authentication of nut origin.
2. Classification of Edible Nuts
Edible nuts refer to seeds that can be consumed either raw or after processing, often characterized by their high nutritional value and frequent use as food. Botanically and culinarily, nuts are derived from various plant sources and can be classified into several major categories:
- –
Cereal grains: Examples include rice, wheat, corn, oats, and barley. These are the seeds of grasses belonging to the Poaceae family and are staple foods consumed globally. They serve as essential components of daily diets, providing carbohydrates, proteins, fiber, vitamins, and minerals, and are major energy sources in human nutrition.
- –
Legume seeds: Examples include soybeans, mung beans, red beans, lentils, and peas. These are the seeds of plants in the Fabaceae family. Legume seeds are typically used in side dishes and are less commonly consumed directly. They are rich in plant-based proteins, fiber, vitamins, and minerals, and are widely consumed worldwide as part of balanced diets.
- –
Oilseeds: Examples include walnuts, almonds, macadamia nuts, cashews, chia seeds, flaxseeds, and sunflower seeds. These seeds contain high oil content and can either be cold-pressed for edible oil or consumed directly as nutritious snacks. They are rich in healthy fats, essential fatty acids, vitamins, and minerals, offering significant health benefits.
- –
Fruit seeds: Examples include pumpkin seeds, melon seeds, and sesame seeds. These are the seeds found inside various fruits, often encased in a soft or hard shell. Some of these seeds are edible and nutrient-dense, while others may be inedible or even toxic. Fruit seeds are valued for their content of healthy fats, proteins, fiber, and essential micronutrients, playing an important role in human health and diet.
In this study, we focus exclusively on edible oilseeds, particularly those commonly consumed as snacks after simple processing such as roasting, and typically eaten whole. These nuts can be broadly classified into two main groups: true nuts (botanical nuts) and drupe nuts (culinary nuts). True nuts are dry, hard-shelled fruits that do not open at maturity to release the seed, whereas drupes are fruits with a fleshy outer layer surrounding a hard shell (pit or stone) that encases the seed (kernel). There are more than a dozen commercially significant nut types in this category, with popular examples including chestnuts, hazelnuts, acorns, almonds, walnuts, pecans, cashews, peanuts, pistachios, Brazil nuts, macadamia nuts, pine nuts, pili nuts, Marcona almonds, kola nuts, and saba nuts.
According to the Observatory of Economic Complexity (OEC) [
16], the total global export value of edible nuts reached approximately
$17.8 billion in 2023, corresponding to a trade volume of about 3.3 million tons. The United States was the leading exporter, accounting for
$8 billion, or 44.9% of global exports. European countries followed with
$2.72 billion (15.3%), then India (
$1.12 billion), China (
$1.06 billion), Canada (
$683.55 million), United Arab Emirates (
$553.89 million), Turkey (
$518.24 million), Vietnam (
$392.7 million), Mexico (
$353.8 million), Japan (
$335.39 million), and South Korea (
$293.99 million) [
17]. Major global importers include China (
$1.89 billion, 10.6%), Germany (
$1.81 billion, 10.1%), India (
$1.54 billion, 8.66%), Italy (
$1.3 billion, 7.31%), the United States (
$869 million, 4.88%), and Spain (
$822 million, 4.61%). Furthermore, the export value of edible nuts has grown steadily at an average annual rate of 1.5% from 2015 to 2024, highlighting the strong growth potential and economic importance of the nut industry.
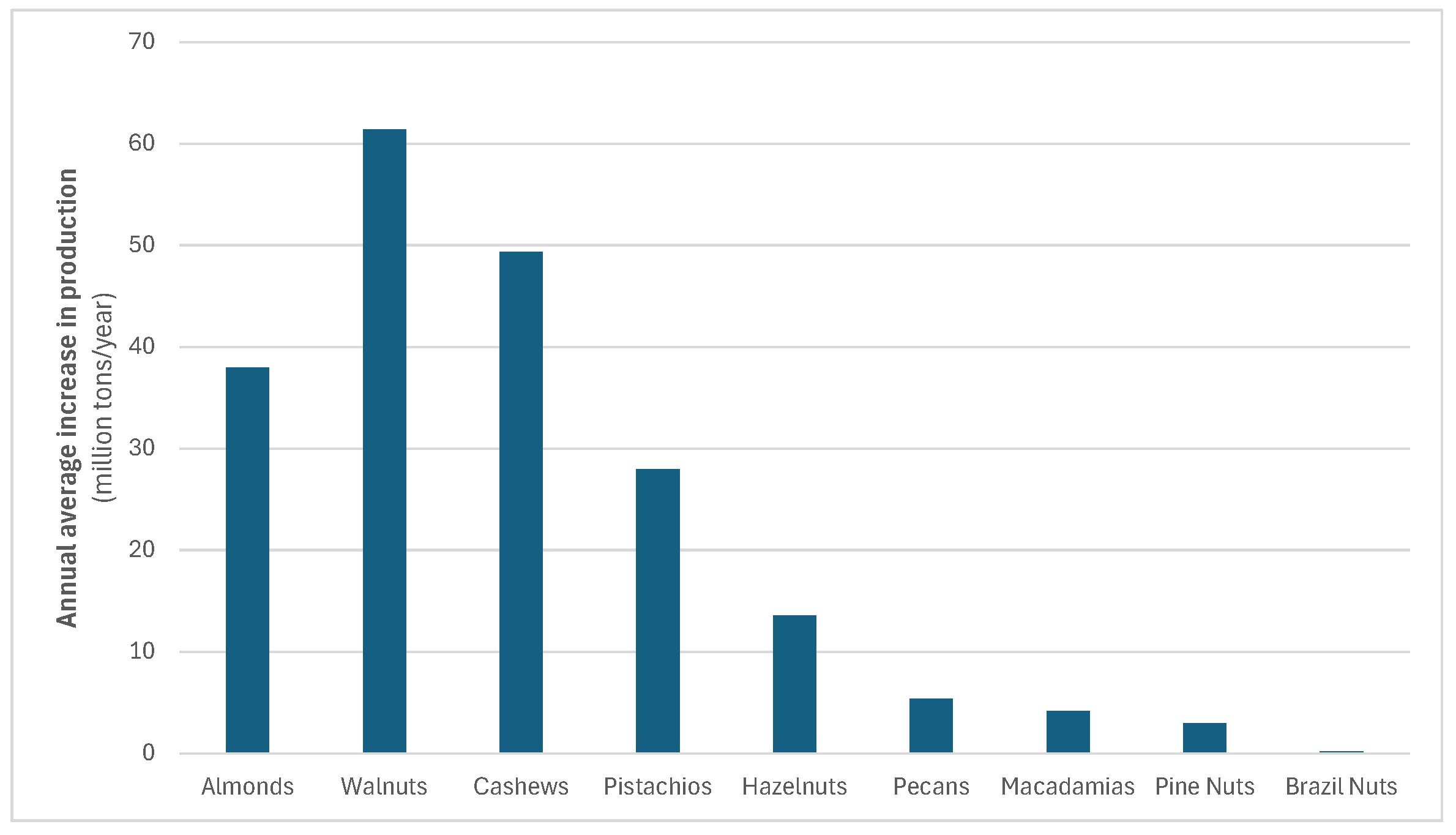
Among tree nuts, almonds had the highest global production, reaching over 1.4 million tons, followed by walnuts (1.156 million tons), cashews (1.095 million tons), pistachios (747.3 thousand tons), hazelnuts (585 thousand tons), pecans (164 thousand tons), and macadamias (78 thousand tons) [
18]. Almonds, walnuts, cashews, pistachios, and hazelnuts represent the top five most produced and consumed nut types worldwide. Their production volumes have shown consistent year-over-year growth, with average annual growth rates illustrated in
Figure 1. This trend reflects the increasing global demand for edible nuts.
3. Distribution of Previous Studies Related to Nuts
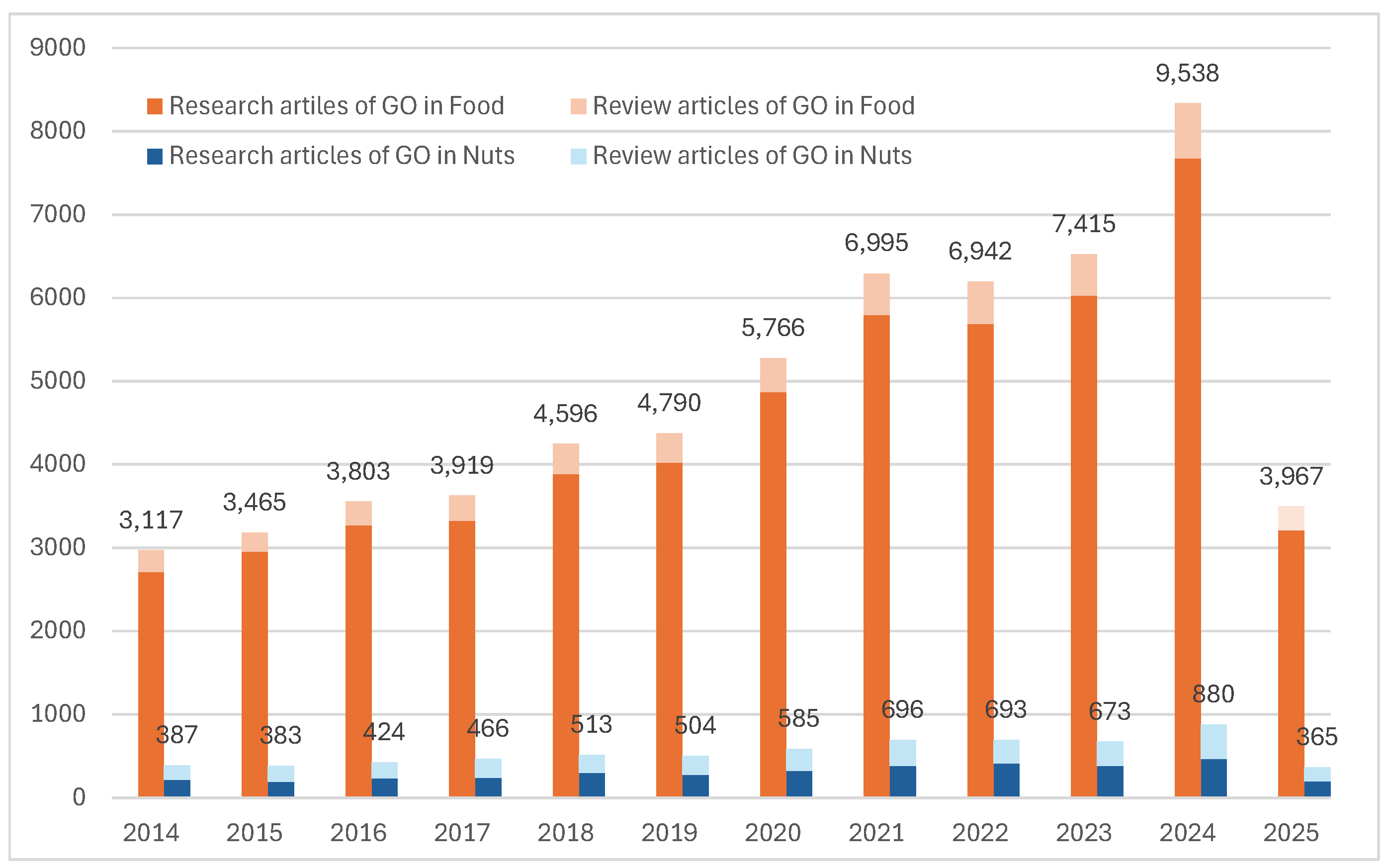
Approximately 100,000 documents (excluding book chapters) related to the keyword
“geographical origin” in food were found on the Elsevier platform alone. Among them, over 81,000 are research articles specifically addressing the geographical origin of food. The number of publications in 2024 has tripled compared to a decade ago (
Figure 2). Of these, studies on the geographical origin (GO) of edible nuts consistently account for around 10% of food-related GO research. This indicates that studies on food traceability in general—and on nuts in particular—are receiving increasing attention from the scientific community.
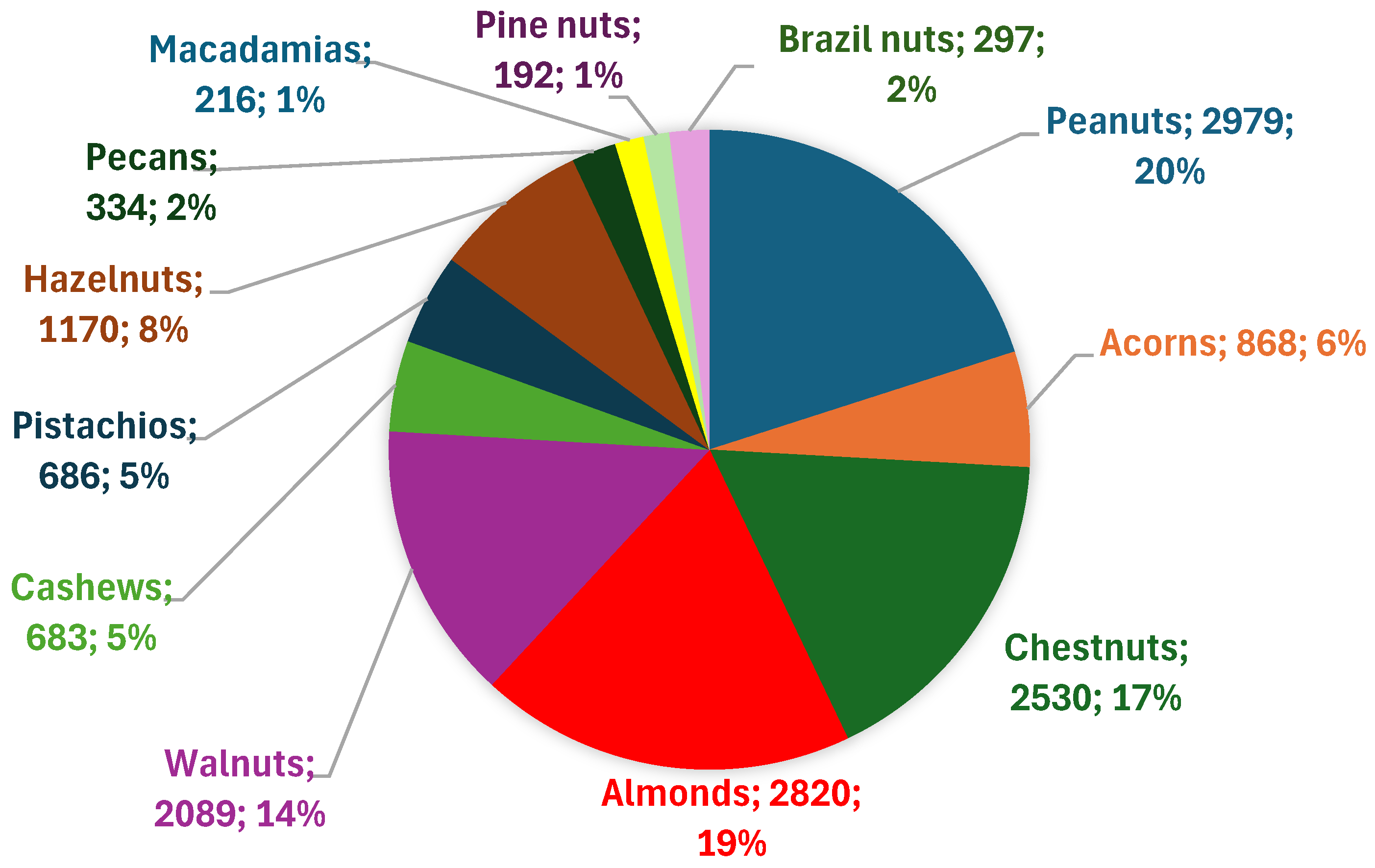
Among GO studies focusing on nuts, peanuts, almonds, walnuts, chestnuts, and hazelnuts are the most frequently studied, with corresponding publication counts of 2,979 (20%), 2,820 (19%), 2,089 (14%), 2,530 (17%), and 1,170 (8%), respectively, as of the end of March 2025. Together, these five nut types account for nearly 80% of all GO-related nut publications, while each of the remaining nut types accounts for less than 6% (
Figure 3). Review articles typically constitute about 20%–30% of the total number of publications for each nut type. Furthermore, the number of studies has increased steadily over the years. Since current research is concentrated on only a few types of nuts, expanding GO studies to other nut varieties—particularly those with unique regional characteristics—offers promising potential.
In recent years, chemical analysis methods combined with chemometric approaches have gained increasing interest for food discrimination studies [
5]. Commonly used rapid and simple chemical methods include ICP with MS detection [
7] and/or OES [
8], mass spectrometry (MS) [
9], and FTIR spectroscopy [
12]. Although these techniques differ in analytical procedure, sensitivity, and target analytes, they share the advantages of multi-compound detection, fast analysis time, and relatively simple procedures. As a result, these methods are highly suitable for classification and subsequent traceability of food products, where the collection of large sample sets is often necessary.
4. Rapid and Simple Chemical Analysis Methods
Recent advancements in analytical chemistry have led to the development of rapid and simple methods that are increasingly used for food authentication and geographical origin classification. These techniques offer a practical alternative to more complex or time-consuming approaches, enabling high-throughput screening with minimal sample preparation and reduced operational costs. In the context of edible nuts, such methods are particularly valuable due to the growing demand for traceability, quality assurance, and fraud prevention in global trade.
This section provides an overview of selected rapid and simple chemical analysis techniques—including ICP-MS, ICP-OES, FTIR, and GC-MS—that have shown promising results in the classification and origin identification of edible nuts. The principles, analytical capabilities, and applications of each method are discussed in detail below.
4.1. Application of Inductively Coupled Plasma Mass Spectrometry and Optical Emission Spectroscopy
Inductively Coupled Plasma Mass Spectrometry (ICP-MS) and Inductively Coupled Plasma Optical Emission Spectroscopy (ICP-OES) are two well-established elemental analysis techniques widely employed in food authenticity and traceability studies. These methods allow for the simultaneous quantification of multiple macro- and micro-elements, offering high sensitivity, precision, and throughput—features that make them ideal for classifying the geographical origin of edible nuts.
ICP-MS provides ultra-trace detection capabilities [
19], with limits of detection in the parts-per-trillion (ppt) to parts-per-billion (ppb) range, enabling the identification of minor variations in elemental profiles that may arise from differences in soil composition, climate, agricultural practices, and environmental exposure across regions. In contrast, ICP-OES, while generally less sensitive than ICP-MS, allows for the robust quantification of major elements with excellent repeatability and shorter analysis times, making it suitable for routine quality control.
Several studies have successfully applied ICP-MS and/or ICP-OES to classify nuts such as almonds, walnuts, and pistachios based on their elemental fingerprints. For example, variations in elements such as Ca, Mg, Fe, Zn, Cu, Sr, and Rb have been used as reliable discriminators of geographical origin. The elemental composition is often influenced by the mineral content of the soil in which the nut trees are grown, and thus serves as a natural marker for regional identification.
The integration of ICP data with multivariate chemometric techniques such as Principal Component Analysis (PCA), Linear Discriminant Analysis (LDA), or Partial Least Squares Discriminant Analysis (PLS-DA) has further enhanced the classification accuracy. These combinations allow for the development of predictive models capable of distinguishing nuts from different regions with high reliability.
Due to their versatility, relatively fast analysis times, and compatibility with a wide range of sample matrices, both ICP-MS and ICP-OES continue to be among the most widely used techniques for the rapid and cost-effective geographical classification of edible nuts. Their continued application is expected to contribute significantly to the development of traceability systems and food authentication platforms in the nut industry.
4.2. Application of Fourier Transform Infrared Spectroscopy (FTIR)
Fourier Transform Infrared Spectroscopy (FTIR) is a vibrational spectroscopic technique that measures the absorption of infrared radiation by molecular bonds within a sample, providing detailed information about its chemical composition and molecular structure. FTIR is widely recognized for its rapidity, minimal sample preparation, and non-destructive nature, making it a valuable tool for the analysis and classification of food products [
14,
15], including edible nuts.
In the context of edible nuts, FTIR can detect characteristic absorption bands corresponding to functional groups such as lipids, proteins, carbohydrates, and other organic compounds. The spectral fingerprint obtained from a nut sample reflects its biochemical profile, which is influenced by factors like species, geographic origin, cultivation practices, and environmental conditions.
Researchers have demonstrated the effectiveness of FTIR combined with chemometric methods—such as Principal Component Analysis (PCA), Partial Least Squares Discriminant Analysis (PLS-DA), and Support Vector Machines (SVM)—to classify nuts according to their geographical origin. These statistical tools help extract relevant spectral features and build predictive models that differentiate nut samples from various regions with high accuracy.
FTIR offers several advantages over other analytical techniques, including faster analysis times, lower operational costs, and the ability to analyze solid, liquid, or powdered samples directly without the need for extensive chemical reagents. However, the technique typically provides qualitative or semi-quantitative data and may require complementary methods to confirm elemental or compound-specific information.
Overall, FTIR represents a promising rapid and simple chemical analysis method for edible nut authentication and origin classification, especially when integrated within multi-analytical strategies aimed at ensuring food traceability and combating fraud.
4.3. Application of Gas Chromatography–Mass Spectrometry (GC-MS)
Gas Chromatography–Mass Spectrometry (GC-MS) is a powerful analytical technique that combines the separation capabilities of gas chromatography with the sensitive and selective detection offered by mass spectrometry [
20,
21,
22,
23,
24,
25]. GC-MS is widely used for the qualitative and quantitative analysis of volatile and semi-volatile organic compounds in complex food matrices [
26,
27,
28,
29,
30], making it particularly suitable for profiling the chemical composition of edible nuts.
In edible nut analysis, GC-MS can identify and quantify a range of organic compounds such as fatty acids, sterols, volatile oils, and flavor-related metabolites, which are often influenced by the nut’s variety, cultivation environment, and geographical origin. The unique chemical profiles derived from these compounds can serve as robust markers for origin authentication and classification.
The typical GC-MS procedure for nut samples involves sample preparation techniques such as solvent extraction, derivatization (if necessary), and injection into the chromatographic system. The separated compounds are then ionized and fragmented in the mass spectrometer, producing mass spectra that enable precise identification based on known spectral libraries.
By integrating GC-MS data with multivariate statistical analyses, including Principal Component Analysis (PCA) and Partial Least Squares Discriminant Analysis (PLS-DA), researchers can effectively discriminate nut samples from different regions or cultivars. This approach enhances the detection of subtle differences in chemical composition related to geographical origin.
Although GC-MS is highly sensitive and specific, it often requires longer analysis times and more extensive sample preparation compared to spectroscopic methods such as FTIR. Nevertheless, its ability to provide detailed molecular-level information makes it an indispensable tool in food authenticity studies, including the geographical classification of edible nuts.
5. Chemometric Tools for Data Interpretation
Chemometric tools are essential for extracting meaningful information from complex datasets generated by analytical techniques such as ICP-MS, ICP-OES, FTIR, and GC-MS. These statistical and mathematical methods are used to analyze, interpret, and visualize large volumes of chemical data, enabling the identification of patterns, classification of samples, and development of predictive models [
7].
In the context of edible nut authentication and geographical classification, chemometrics plays a crucial role in transforming raw analytical data into actionable insights. Each nut sample analyzed by chemical techniques yields a large set of variables—such as elemental concentrations or absorbance values across wavelengths—that cannot be easily interpreted without advanced data analysis.
Principal Component Analysis (PCA) is one of the most commonly used unsupervised methods. It reduces the dimensionality of the dataset by transforming correlated variables into a smaller number of uncorrelated principal components. PCA is effective in visualizing the natural grouping of nut samples based on their chemical characteristics and identifying potential outliers.
Partial Least Squares Discriminant Analysis (PLS-DA) and Linear Discriminant Analysis (LDA) are supervised classification techniques that build predictive models by finding directions in the data that best separate predefined classes, such as nut samples from different geographical regions. These methods are particularly useful when sample groups are known and the goal is to classify unknown samples.
Support Vector Machines (SVM) and Random Forest (RF) algorithms, which are examples of machine learning-based approaches, have also been increasingly applied to enhance classification accuracy. These methods can handle nonlinear relationships in the data and are robust against overfitting, especially when dealing with high-dimensional datasets.
Effective application of chemometric tools requires careful preprocessing of the data, including normalization, scaling, and sometimes feature selection to remove irrelevant or redundant variables. The choice of chemometric method depends on the nature of the dataset, the analytical technique used, and the classification objectives.
In summary, chemometric tools are indispensable for interpreting complex analytical data and building reliable models for the geographical classification of edible nuts. When combined with rapid and simple chemical analysis methods, these tools contribute significantly to the development of efficient and accurate food authentication systems.
6. Research Gaps and Future Perspectives
Despite significant progress in applying rapid and simple chemical analysis methods for the geographical classification of edible nuts, several research gaps remain that need to be addressed to further advance the field [
21,
22,
23,
24,
25,
26,
27,
28,
29,
30,
31,
32,
33,
34].
Current literature tends to focus on a few commercially important nuts such as almonds, walnuts, peanuts, and hazelnuts. Other edible nuts, including pili nuts, kola nuts, marcona almonds, and region-specific varieties, remain underexplored. Broadening the range of studied nut types would help develop a more comprehensive database and strengthen global traceability systems.
- 2.
Regional Bias and Uneven Sample Distribution:
Most studies concentrate on samples from a few geographic regions (e.g., the US, Europe, or East Asia), leading to a lack of representative global coverage. To ensure robust and generalizable classification models, future research should involve well-designed sampling strategies that include diverse geographical origins and environmental conditions.
- 3.
Lack of Standardized Protocols:
There is currently no universally accepted protocol for sample preparation, data acquisition, and chemometric analysis in the context of nut authentication. The absence of standardized methods makes it difficult to compare results across studies and hinders the development of interoperable databases. Establishing harmonized procedures would improve data reproducibility and model transferability.
- 4.
Integration of Multi-Omics and Hybrid Approaches:
Most existing studies rely on a single analytical technique (e.g., ICP-MS or FTIR) for classification. However, integrating multiple complementary techniques—such as combining elemental profiling with volatile compound analysis or spectral fingerprinting—may enhance classification accuracy and robustness. Future work should explore the potential of multi-modal approaches and machine learning algorithms to manage such integrated datasets.
- 5.
Need for Real-World Validation and Industrial Application:
While many studies demonstrate high classification accuracy under laboratory conditions, there is limited validation of these methods in real-world supply chains. Collaborations with industry stakeholders are essential to test the scalability and practicality of analytical tools under commercial settings, including post-harvest processing, packaging, and storage variations.
- 6.
Data Sharing and Open Access Repositories:
The creation of shared, open-access databases containing reference chemical profiles of nuts from different regions would support broader research and authentication efforts. Data sharing would also enable the development of AI-powered predictive models and facilitate international cooperation in food safety and fraud prevention.
In future research, efforts should focus on expanding nut coverage, improving methodological consistency, and integrating advanced chemometrics and artificial intelligence. These steps will not only close current knowledge gaps but also pave the way for reliable, rapid, and cost-effective systems for nut origin authentication—supporting food traceability, protecting regional products, and building consumer trust in global food markets [
35,
36,
37,
38,
39,
40].
7. Conclusions
The geographical classification of edible nuts plays a crucial role in ensuring food authenticity, quality control, and consumer trust. This review highlights the growing application of rapid and simple chemical analysis methods—particularly ICP-MS, ICP-OES, FTIR, and GC-MS—in combination with chemometric tools to differentiate nut origins based on their compositional fingerprints. These techniques offer significant advantages in terms of speed, sensitivity, and ease of implementation, making them highly suitable for routine screening and large-scale food traceability efforts. Despite the growing number of studies, research remains concentrated on a few major nut types, leaving clear opportunities for expanding origin-based classification to a broader range of regional and specialty nuts. Future work should focus on building comprehensive chemical databases, standardizing protocols, and integrating multi-analytical approaches to enhance the robustness and scalability of these methods in both scientific and industrial settings.
References
- Le, L. H. T., Tran-Lam, T.-T., Nguyen, H. Q., Quan, T. C., Nguyen, T. Q., Nguyen, D. T., & Dao, Y. H. (2021). A study on multi-mycotoxin contamination of commercial cashew nuts in Vietnam. Journal of Food Composition and Analysis, 102, 104066. [CrossRef]
- Nguyen-Quang, T., Bui-Quang, M., Pham-Van, T., Le-Van, N., Nguyen-Hoang, K., Nguyen-Minh, D., Phung-Thi, T., Le-Viet, A., Tran-Ha Minh, D., Nguyen-Tien, D., Hoang-Le, T.-A., & Truong-Ngoc, M. (2023). Classification of Vietnamese Cashew Nut (Anacardium occidentale L.) Products Using Statistical Algorithms Based on ICP/MS Data: A Study of Food Categorization. Journal of Analytical Methods in Chemistry, 2023, 1–13. [CrossRef]
- Vu-Duc, N., Nguyen-Quang, T., Le-Minh, T., Nguyen-Thi, X., Tran, T. M., Vu, H. A., Nguyen, L.-A., Doan-Duy, T., Van Hoi, B., Vu, C.-T., Le-Van, D., Phung-Thi, L.-A., Vu-Thi, H.-A., & Chu, D. B. (2019). Multiresidue Pesticides Analysis of Vegetables in Vietnam by Ultrahigh-Performance Liquid Chromatography in Combination with High-Resolution Mass Spectrometry (UPLC-Orbitrap MS). Journal of Analytical Methods in Chemistry, 2019, 1–12. [CrossRef]
- Le, V. N., Nguyen, Q. T., Nguyen, T. D., Nguyen, N. T., Janda, T., Szalai, G., & Le, T. G. (2020). The potential health risks and environmental pollution associated with the application of plant growth regulators in vegetable production in several suburban areas of Hanoi, Vietnam. Biologia Futura, 71(3), 323–331. [CrossRef]
- Nguyen-Quang, T., Bui-Quang, M., & Truong-Ngoc, M. (2021). Rapid Identification of Geographical Origin of Commercial Soybean Marketed in Vietnam by ICP-MS. Journal of Analytical Methods in Chemistry, 2021, 1–9. [CrossRef]
- Nguyen, T. Q., Tran-Lam, T.-T., Nguyen, H. Q., Dao, Y. H., & Le, G. T. (2021). Assessment of organic and inorganic arsenic species in Sengcu rice from terraced paddies and commercial rice from lowland paddies in Vietnam. Journal of Cereal Science, 102, 103346. [CrossRef]
- Nguyen, Q., Nguyen, T., Le, V., Nguyen, N., Truong, N., Hoang, M., Pham, T., & Bui, Q. (2023). Towards a Standardized Approach for the Geographical Traceability of Plant Foods Using Inductively Coupled Plasma Mass Spectrometry (ICP-MS) and Principal Component Analysis (PCA). Foods, 12(9), 1848. [CrossRef]
- Drivelos, S. A., & Georgiou, C. A. (2012). Multi-element and multi-isotope-ratio analysis to determine the geographical origin of foods in the European Union. TrAC Trends in Analytical Chemistry, 40, 38–51. [CrossRef]
- Nguyen Thi, K.-O., Do, H.-G., Duong, N.-T., Nguyen, T. D., & Nguyen, Q.-T. (2021). Geographical Discrimination of Curcuma longa L. in Vietnam Based on LC-HRMS Metabolomics. Natural Product Communications, 16(10). [CrossRef]
- Nguyen-Quang, T., Do-Hoang, G., & Truong-Ngoc, M. (2021). Multielement Analysis of Pakchoi (Brassica rapa L. ssp. chinensis) by ICP-MS and Their Classification according to Different Small Geographical Origins. Journal of Analytical Methods in Chemistry, 2021, 1–11. [CrossRef]
- Bui, M. Q., Quan, T. C., Nguyen, Q. T., Tran-Lam, T.-T., & Dao, Y. H. (2022). Geographical origin traceability of Sengcu rice using elemental markers and multivariate analysis. Food Additives & Contaminants: Part B, 15(3), 177–190. [CrossRef]
- Bui, Q. M., Nguyen, Q. T., Nguyen, T. T., Nguyen, H. M., Phung, T. T., Le, V. A., Truong, N. M., Mac, T. V., Nguyen, T. D., Hoang, L. T. A., Tran, H. M. D., Le, V. N., & Nguyen, M. D. (2024). Multivariate Statistical Analysis for the Classification of Sausages Based on Physicochemical Attributes, Using Attenuated Total Reflectance-Fourier Transform Infrared (ATR-FTIR) and Inductively Coupled Plasma-Mass Spectrometry (ICP-MS). Journal of Analytical Methods in Chemistry, 2024, 1–13. [CrossRef]
- Bui, D. T., Truong, N. M., Le, V. A., Nguyen, H. K., Bui, Q. M., Pham, V. T., & Nguyen, Q. T. (2023). Preserving the Authenticity of ST25 Rice (Oryza sativa) from the Mekong Delta: A Multivariate Geographical Characterization Approach. Stresses, 3(3), 653–664. [CrossRef]
- Minh, T. N., Van Thinh, P., Anh, H. L. T., Anh, L. V., Khanh, N. H., Van Nhan, L., Trung, N. Q., & Dat, N. T. (2024). Chemometric classification of Vietnamese green tea (Camellia sinensis) varieties and origins using elemental profiling and FTIR spectroscopy. International Journal of Food Science and Technology, 59(12), 9234–9244. [CrossRef]
- Truong Ngoc, M., Nguyen, Q. T., Pham, V. T., Hoang, L. T. A., Le, V. A., Le, V. N., Tran, H. M. D., & Nguyen, T. D. (2024). Assessing Vodka Authenticity and Origin in Vietnam’s Market: An Analytical Approach Using FTIR and ICP-MS with Multivariate Statistics. Journal of Analytical Methods in Chemistry, 2024(1). [CrossRef]
- https://oec.world/en/profile/hs/other-nuts.
- https://www.fas.usda.gov/data/commodities/tree-nuts.
- International Nuts and Dried Fruits, Nuts & Dried Fruits Statistical Yearbook 2022/23.
- Dang, T. T., Vo, T. A., Duong, M. T., Pham, T. M., Van Nguyen, Q., Nguyen, T. Q., Bui, M. Q., Syrbu, N. N., & Van Do, M. (2022). Heavy metals in cultured oysters (Saccostrea glomerata) and clams (Meretrix lyrata) from the northern coastal area of Vietnam. Marine Pollution Bulletin, 184, 114140. [CrossRef]
- Hai, Y. D., Tran-Lam, T.-T., Nguyen, T. Q., Vu, N. D., Ma, K. H., & Le, G. T. (2019). Acrylamide in daily food in the metropolitan area of Hanoi, Vietnam. Food Additives & Contaminants: Part B, 12(3), 159–166. [CrossRef]
- Duong, T. T., Nguyen, T. T. L., Dinh, T. H. V., Hoang, T. Q., Vu, T. N., Doan, T. O., Dang, T. M. A., Le, T. P. Q., Tran, D. T., Le, V. N., Nguyen, Q. T., Le, P. T., Nguyen, T. K., Pham, T. D., & Bui, H. M. (2021). Auxin production of the filamentous cyanobacterial Planktothricoides strain isolated from a polluted river in Vietnam. Chemosphere, 284, 131242. [CrossRef]
- Hanh, T. T. H., Anh, D. H., Huong, P. T. T., Thanh, N. V., Trung, N. Q., Cuong, T. V., Mai, N. T., Cuong, N. T., Cuong, N. X., Nam, N. H., & Minh, C. V. (2018). Crinane, augustamine, and β -carboline alkaloids from Crinum latifolium. Phytochemistry Letters, 24, 27–30. [CrossRef]
- Nu Nguyen, H. M., Khieu, H. T., Ta, N. A., Le, H. Q., Nguyen, T. Q., Do, T. Q., Hoang, A. Q., Kannan, K., & Tran, T. M. (2021). Distribution of cyclic volatile methylsiloxanes in drinking water, tap water, surface water, and wastewater in Hanoi, Vietnam. Environmental Pollution, 285, 117260. [CrossRef]
- Le, T. M., Pham, P. T., Nguyen, T. Q., Nguyen, T. Q., Bui, M. Q., Nguyen, H. Q., Vu, N. D., Kannan, K., & Tran, T. M. (2022). A survey of parabens in aquatic environments in Hanoi, Vietnam and its implications for human exposure and ecological risk. Environmental Science and Pollution Research, 29(31), 46767–46777. [CrossRef]
- Trinh, H. T., Marcussen, H., Hansen, H. C. B., Le, G. T., Duong, H. T., Ta, N. T., Nguyen, T. Q., Hansen, S., & Strobel, B. W. (2017). Screening of inorganic and organic contaminants in floodwater in paddy fields of Hue and Thanh Hoa in Vietnam. Environmental Science and Pollution Research, 24(8), 7348–7358. [CrossRef]
- D.T. Hanh, K. Kadomami, N. Matsuura, N.Q. Trung, Screening analysis of a thousand micro-pollutants in vietnamese rivers, In Proceedings of the 10th International Symposium on Southeast Asian Water Environment (2012), Hanoi, Vietnam, 8.-10. November, 2012.
- Truong, D. A., Trinh, H. T., Le, G. T., Phan, T. Q., Duong, H. T., Tran, T. T. L., Nguyen, T. Q., Hoang, M. T. T., & Nguyen, T. V. (2023). Occurrence and ecological risk assessment of organophosphate esters in surface water from rivers and lakes in urban Hanoi, Vietnam. Chemosphere, 331, 138805. [CrossRef]
- Nguyen, T. N., Trinh, H. T., Sam, L. H., Nguyen, T. Q., & Le, G. T. (2019). Halogen-free flame-retardant flexible polyurethane for textile coating: Preparation and characterisation. Fire and Materials, 44(2), 269–282. [CrossRef]
- Hoang, M. T. T., Le, G. T., Kiwao, K., Duong, H. T., Nguyen, T. Q., Phan, T. Q., Bui, M. Q., Truong, D. A., & Trinh, H. T. (2023). Occurrence and risk of human exposure to organophosphate flame retardants in indoor air and dust in Hanoi, Vietnam. Chemosphere, 328, 138597. [CrossRef]
- Nguyen, H. X., Nguyen, X. T., Mai, H. T. H., Nguyen, H. T., Vu, N. D., Pham, T. T. P., Nguyen, T. Q., Nguyen, D. T., Duong, N. T., Hoang, A. L. T., Nguyen, T. N., Le, N. V., Dao, H. V., Ngoc, M. T., & Bui, M. Q. (2024). A Comprehensive Evaluation of Dioxins and Furans Occurrence in River Sediments from a Secondary Steel Recycling Craft Village in Northern Vietnam. Molecules, 29(8), 1788. [CrossRef]
- Markus Amann, Zbigniew Klimont, T An Ha, Peter Rafaj, Gregor Kiesewetter, Adriana Gomez Sanabria, Binh Nguyen, TN Thi Thu, Kimminh Thuy, Wolfgang Schöpp, Jens Borken-Kleefeld, L Höglund-Isaksson, Fabian Wagner, Robert Sander, Chris Heyes, Janusz Cofala, Nguyen Quang Trung, Nguyen Tien Dat, Nguyen Ngoc Tung, Future Air Quality in Ha Noi and Northern Vietnam, http://pure.iiasa.ac.at/15803 (2019).
- Truong, A. H., Kim, M. T., Nguyen, T. T., Nguyen, N. T., & Nguyen, Q. T. (2018). Methane, Nitrous Oxide and Ammonia Emissions from Livestock Farming in the Red River Delta, Vietnam: An Inventory and Projection for 2000–2030. Sustainability, 10(10), 3826. [CrossRef]
- Nguyen, Q.-T., Le, T.-G., Bergonzo, P., & Tran, Q.-T. (2022). One-Step Fabrication of Nickel-Electrochemically Reduced Graphene Oxide Nanocomposites Modified Electrodes and Application to the Detection of Sunset Yellow in Drinks. Applied Sciences, 12(5), 2614. [CrossRef]
- Thang, P. Q., Muto, Y., Maeda, Y., Trung, N. Q., Itano, Y., & Takenaka, N. (2016). Increase in ozone due to the use of biodiesel fuel rather than diesel fuel. Environmental Pollution, 216, 400–407. [CrossRef]
- Quang, T. H., Phong, N. V., Anh, L. N., Hanh, T. T. H., Cuong, N. X., Ngan, N. T. T., Trung, N. Q., Nam, N. H., & Minh, C. V. (2020). Secondary metabolites from a peanut-associated fungus Aspergillus niger IMBC-NMTP01 with cytotoxic, anti-inflammatory, and antimicrobial activities. Natural Product Research, 36(5), 1215–1223. [CrossRef]
- Hanh, T. T. H., Hang, L. T. T., Huong Giang, V., Trung, N. Q., Thanh, N. V., Quang, T. H., & Cuong, N. X. (2021). Chemical constituents of Blumea balsamifera. Phytochemistry Letters, 43, 35–39. [CrossRef]
- Van, Pc. P., Ngo Van, H., Quang, M. B., Duong Thanh, N., Nguyen Van, D., Thanh, T. D., Tran Minh, N., Thi Thu, H. N., Quang, T. N., Thao Do, T., Thanh, L. P., Do Thi Thu, H., & Le Tuan, A. H. (2023). Stigmastane-type steroid saponins from the leaves of Vernonia amygdalina and their α -glucosidase and xanthine oxidase inhibitory activities. Natural Product Research, 38(4), 601–606. [CrossRef]
- Minh, T. N., Minh, B. Q., Duc, T. H. M., Thinh, P. V., Anh, L. V., Dat, N. T., Nhan, L. V., & Trung, N. Q. (2022). Potential Use of Moringa oleifera Twigs Extracts as an Anti-Hyperuricemic and Anti-Microbial Source. Processes, 10(3), 563. [CrossRef]
- Anh, B. T. K., Minh, N. N., Ha, N. T. H., Kim, D. D., Kien, N. T., Trung, N. Q., Cuong, T. T., & Danh, L. T. (2018). Field Survey and Comparative Study of Pteris Vittata and Pityrogramma Calomelanos Grown on Arsenic Contaminated Lands with Different Soil pH. Bulletin of Environmental Contamination and Toxicology, 100(5), 720–726. [CrossRef]
- Janda, T., Lejmel, M. A., Molnár, A. B., Majláth, I., Pál, M., Nguyen, Q. T., Nguyen, N. T., Le, V. N., & Szalai, G. (2020). Interaction between elevated temperature and different types of Na-salicylate treatment in Brachypodium dystachion. PLOS ONE, 15(1), e0227608. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).