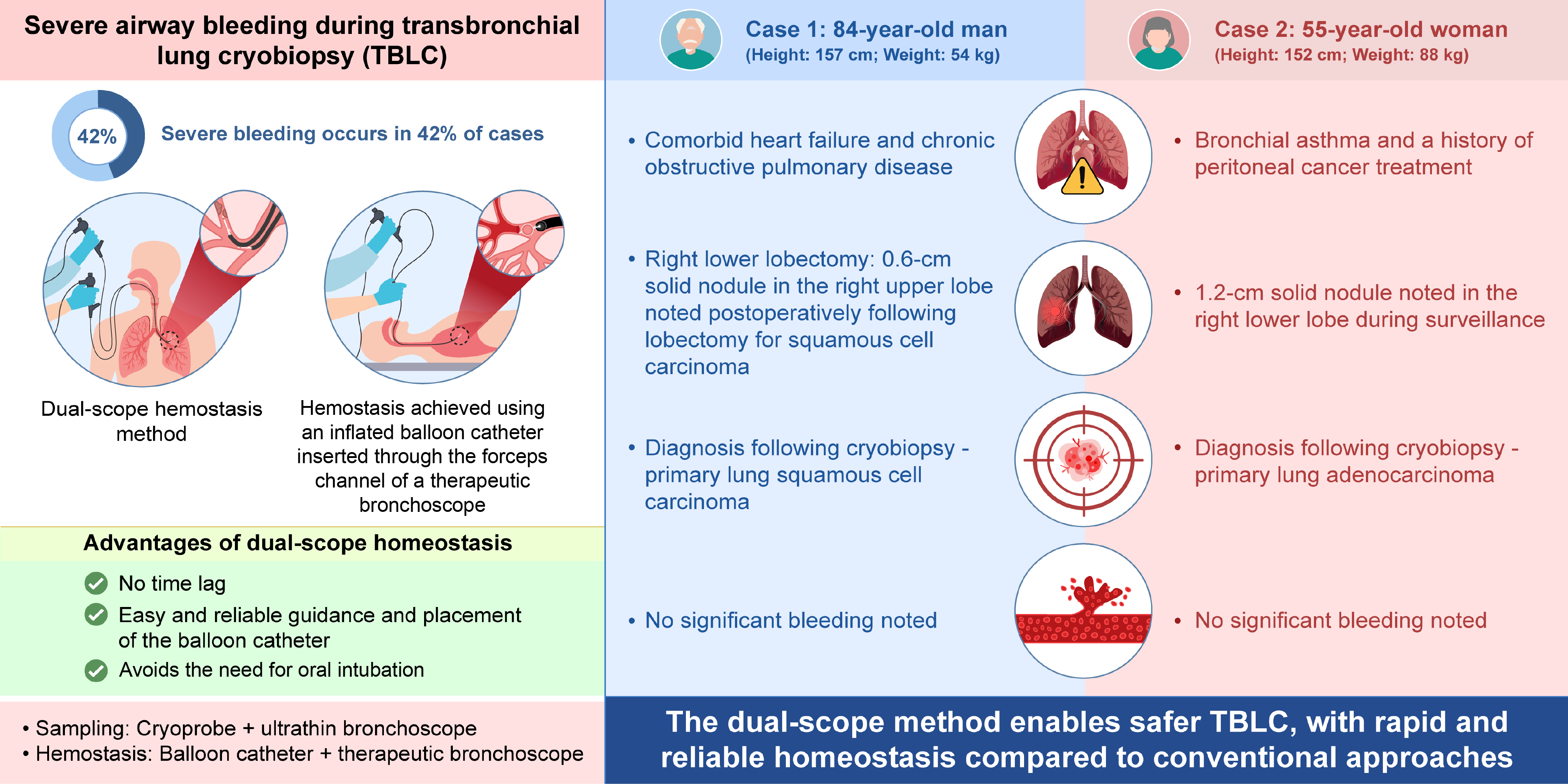
1. Introduction
Severe airway bleeding during transbronchial lung cryobiopsy (TBLC) is reported to occur in 42% of cases [
1]. As such bleeding can be fatal [
2], ensuring reliable hemostasis is critically important. Previously, we maintained hemostasis by combining the balloon occlusion technique [
3], wherein a 4-Fr Fogarty catheter is inserted through the suction hole above the intubation tube cuff following tracheal intubation using the two-scope technique [
4,
5]. In this technique, the pre-prepared second scope is inserted to achieve rapid bleeding control immediately after the first scope and cryoprobe are removed [
4,
5]. However, a temporary “blind time” cannot be avoided, during which the endobronchial lumen cannot be observed, and serious bleeding may occur [
6]. Therefore, the optimal hemostatic technique for TBLC, especially in peripheral pulmonary lesions, remains to be determined [
7].
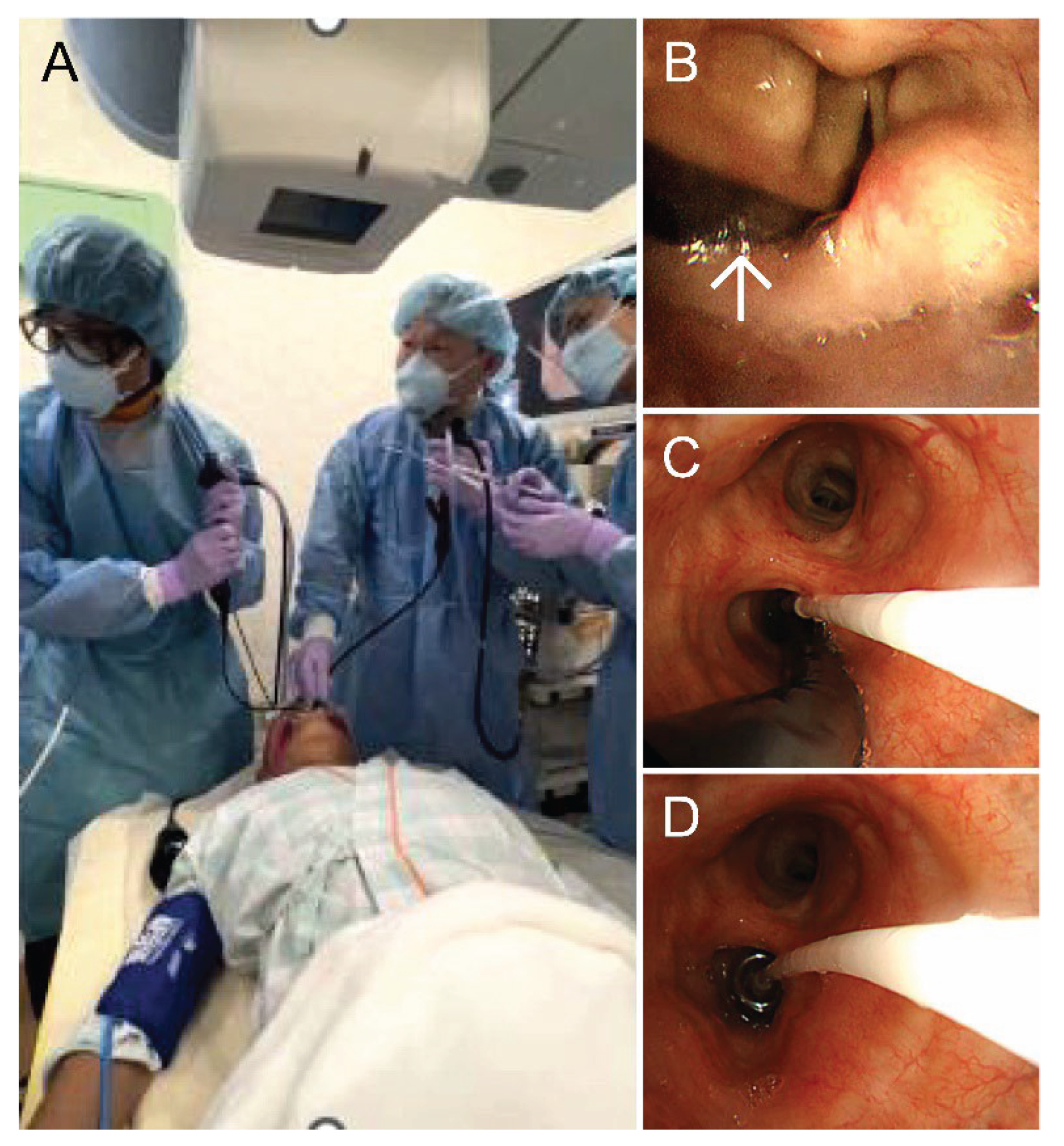
To address this issue, we developed the dual-scope hemostasis method, wherein therapeutic and ultrathin bronchoscopes are simultaneously inserted orally to reliably maintain hemostasis. An ultrathin bronchoscope (BF-MP290F, Olympus, Shinjuku, Japan) is used for sampling, and a therapeutic bronchoscope (BF-1TH1200, Olympus) is used for hemostasis. This approach utilizes single-use 1.1-mm cryoprobe (CRYO2, ERBE Electromedizin Gmbh, Tuebingen, Germany) and a balloon catheter (B5-2C, Olympus). The balloon catheter is inserted through the working channel of the therapeutic bronchoscope. Midazolam and fentanyl are used for sedation and analgesia. Additionally, 4% lidocaine is sprayed as a local anesthetic via a spray catheter. The dual-scope method steps are as follows: 1) The operator, hemostasis technician, and balloon technician are positioned on the head side of the patient (
Figure 1A). Local anesthesia is administered, and the tracheal and bronchial lumens are examined using the therapeutic bronchoscope. 2) The therapeutic bronchoscope is removed and replaced with an ultrathin bronchoscope, which is guided to the target lesion. 3) Simultaneously, the therapeutic bronchoscope is reinserted orally alongside the ultrathin bronchoscope (
Figure 1B). 4) The balloon catheter tip is advanced through the working channel of the therapeutic bronchoscope and positioned within the target bronchus (
Figure 1C). 5) A biopsy is performed using the cryoprobe, the balloon is inflated, and the ultrathin bronchoscope is removed under direct endoscopic observation (
Figure 1D). 6) The balloon is deflated, and hemostasis is confirmed. We report two cases in which cryobiopsy was safely performed using our method. This manuscript is a revised and elaborated version of the article titled “Dual Scope Method,” which was presented at the 28th Respiratory Intervention Seminar, held in Mie, Japan, on November 24th, 2024 [
8].
Written informed consent was obtained from the patients for the publication of this report.
2. Case Presentations
2.1. Case 1
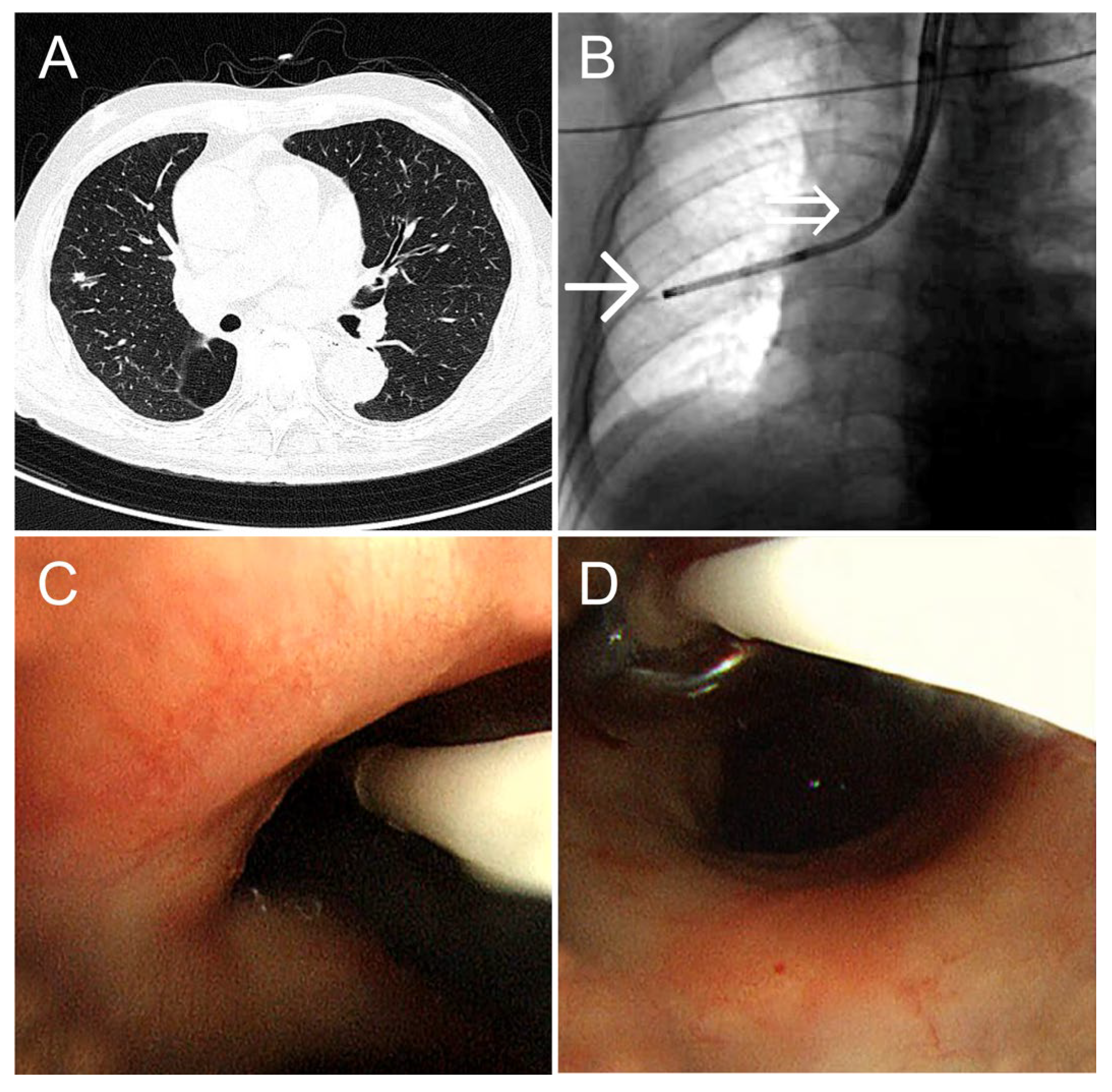
An 84-year-old man (height: 157 cm; weight: 54 kg) with comorbid heart failure and chronic obstructive pulmonary disease underwent a right lower lobectomy for right lower lobe squamous cell carcinoma. Postoperative follow-up computed tomography (CT) revealed a 0.6-cm solid nodule in the S3 periphery of the right upper lobe. A detailed examination was conducted. Fixing the balloon in the right upper lobe using the conventional method was deemed difficult; therefore, we selected the dual-scope method. An ultrathin bronchoscope was guided to the lesion, and cryobiopsy was performed using our method. Bleeding was completely controlled by 5 min of balloon occlusion at the right upper lobe bronchus. TBLC was performed in 31 min, maintaining oxygen saturation (SpO
2) at 99% (
Figure 2). The diagnosis was primary lung squamous cell carcinoma.
2.2. Case 2
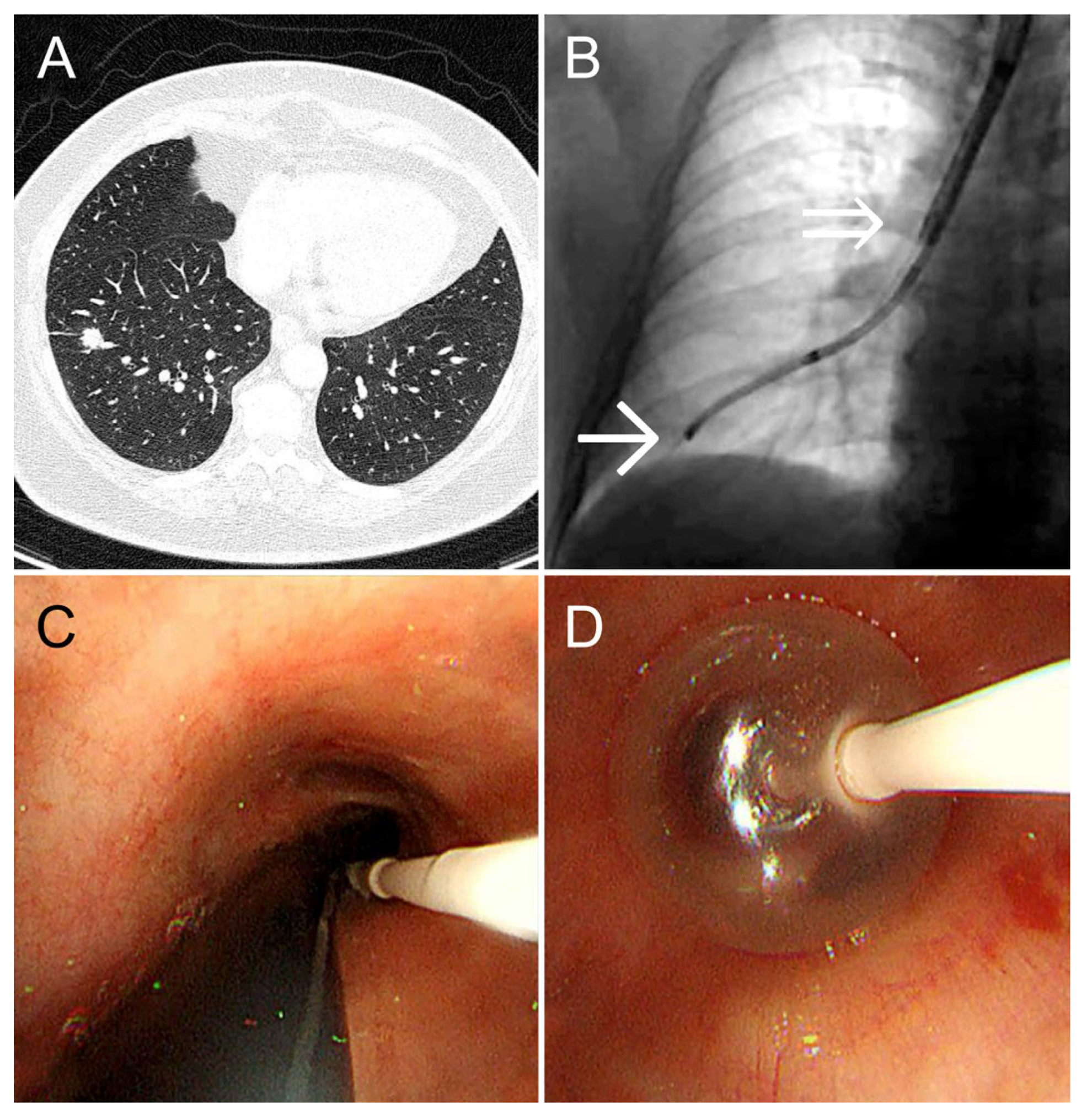
A 55-year-old woman (height: 152 cm; weight: 88 kg) with bronchial asthma and a history of treatment for peritoneal cancer underwent a follow-up CT scan for peritoneal cancer surveillance. The scan revealed a 1.2-cm solid nodule in the S8 periphery of the right lower lobe. She was referred to our department for diagnosis and treatment. Owing to her short stature and obesity, her tracheobronchial tree was relatively narrow, complicating tracheal intubation. Therefore, we selected the dual-scope method, which omits tracheal intubation. An ultrathin bronchoscope was guided to the lesion, and cryobiopsy was performed using our method, Bleeding was completely controlled by 3 min of balloon occlusion at the right basal bronchus. TBLC was performed in 31 min, maintaining SpO
2 at 98–99% (
Figure 3). The diagnosis was primary lung adenocarcinoma.
3. Discussion
Commonly used hemostatic methods for TBLC—the balloon occlusion [
3] and two-scope methods [
4,
5]—have significant drawbacks. Balloon occlusion involves challenges in guiding and securing the balloon, while the two-scope is hindered by a time lag during hemostasis. Additionally, both methods interrupt bronchoscopy and require invasive and complex tracheal intubation. Our dual-scope method offers three key advantages: 1) Real-time hemostasis: With the advantage of performing hemostasis reliably without time lag, the balloon condition and bleeding degree can be monitored in real time under endoscopic guidance, ensuring precise control. 2) Ease of balloon placement: The balloon catheter can be easily guided to the target bronchus and securely fixed by operating through the working channel of the therapeutic bronchoscope. For example, in Case 1, the lesion was located in the right upper lobe, making the conventional method challenging to employ [
8]. However, the dual-scope method allowed for smooth and reliable balloon fixation, effectively stopping the bleeding. In conventional methods, when selective placement in the right upper lobe bronchus is not feasible, a large balloon may be used to occlude the main bronchus, which can lead to hypoxemia. In contrast, our method maintains oxygenation by selectively occluding only the right upper lobe bronchus. 3) No tracheal intubation: Unlike conventional methods requiring prior creation of an artificial airway (e.g., tracheal intubation) to rapidly reinsert the bronchoscope after biopsy, the dual-scope method eliminates this step. The bronchoscope for hemostasis is pre-positioned, omitting the need for reinsertion and artificial airway creation, resulting in a simpler, minimally invasive procedure.
The dual-scope method has some limitations. First, omitting tracheal intubation may make it challenging to secure the airway and reinsert the bronchoscope in the event of massive bleeding. However, this method enables real-time monitoring under endoscopic guidance during hemostasis, significantly reducing this bleeding risk. Additionally, this was a small-scale study conducted at a single institution, and the effectiveness of this method has not yet been fully validated. Large-scale studies at other institutions are needed to confirm its utility.
4. Conclusions
The dual-scope method presented herein can provide reliable hemostasis and enable safe TBLC.
Author Contributions
Methodology, R.H, M.S; Supervision, S.O, K.A, M.K and I.N; Writing-Original Draft, R.H, Writing-review and editing, S.O, M.S, K.A, M.K and I.N
Funding
This research received no external funding.
Institutional Review Board Statement
Written informed consent was obtained from the patients for the publication of this report.
Informed Consent Statement
Written informed consent was obtained from the patients for the publication of this report.
Data Availability Statement
Data is contained within the article or supplementary material.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| TBLC |
Transbronchial lung cryobiopsy |
| CT |
Computed tomography |
| SpO2
|
Oxygen saturation |
References
- Lentz, R.J.; Argento, A.C.; Colby, T.V.; Rickman, O.B.; Maldonado, F. Transbronchial cryobiopsy for diffuse parenchymal lung disease: a state-of-the-art review of procedural techniques, current evidence, and future challenges. J. Thorac. Dis. 2017, 9, 2186–2203. [CrossRef]
- Sharp, C.; McCabe, M.; Adamali, H.; Medford, A. R. Use of transbronchial cryobiopsy in the diagnosis of interstitial lung disease—A systematic review and cost analysis. QJM. 2017, 110, 207–214. [CrossRef]
- Takashi, N.; Tamiko, T., Erina, T. Initial report of transbronchial lung cryobiopsy for diffuse parenchymal lung disease. J Japan Soc Respir Endosc, 40, 453-458. Initial report of transbronchial lung cryobiopsy for diffuse parenchymal lung disease. J Jpn. Soc. Respi. Endosc. 2018, 40, 453–458.
- Sriprasart, T.; Aragaki, A.; Baughman, R.; Wikenheiser-Brokamp, K.; Khanna, G.; Tanase, D.; Kirschner, M.; Benzaquen, S. A single US center experience of transbronchial lung cryobiopsy for diagnosing interstitial lung disease with a 2-scope technique. J. Bronchol. Interv. Pulmonol. 2017, 24, 131–135. [CrossRef]
- Nakai, T.; Watanabe, T.; Kaimi, Y.; Ogawa, K.; Matsumoto, Y.; Sawa, K.; Okamoto, A.; Sato, K.; Asai, K.; Matsumoto, Y.; Ohsawa, M.; Kawaguchi, T. Safety profile and risk factors for bleeding in transbronchial cryobiopsy using a two-scope technique for peripheral pulmonary lesions. BMC Pulm. Med. 2022, 10, 22:20. [CrossRef]
- Kho, S.S.; Chai, C.S.; Ismail, A.M. Modified two-scope technique for transbronchial lung cryobiopsy of peripheral pulmonary lesions. Respirol. Case Rep. 2024, 12, e01450. [CrossRef]
- Sryma, P.B.; Mittal, S.; Madan, N.K.; Tiwari, P.; Hadda, V.; Mohan, A.; Guleria, R.; Madan, K. Efficacy of radial endobronchial ultrasound (R-EBUS) guided transbronchial cryobiopsy for peripheral pulmonary lesions (PPL...s): A systematic review and meta-analysis. Pulmonology. 2023, 29, 50–64. [CrossRef]
- Hanawa, R.; Ozawa, T.; Fukunaga, M. Dual Scope Method ~クライオ生検でのより確実な止血を目指して~. Presented at the 28th Respiratory Intervention Seminar, Mie, Japan, November 24, 2024.
- Kho, S.S.; Chai, C.S.; Nyanti, L.E.; Ismail, A.M.B.; Tie, S.T. Combination of 1.1 mm flexible cryoprobe with conventional guide sheath and therapeutic bronchoscope in biopsy of apical upper lobe solitary pulmonary nodule. BMC Pulm. Med. 2020, 20, 158. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).