3. Results
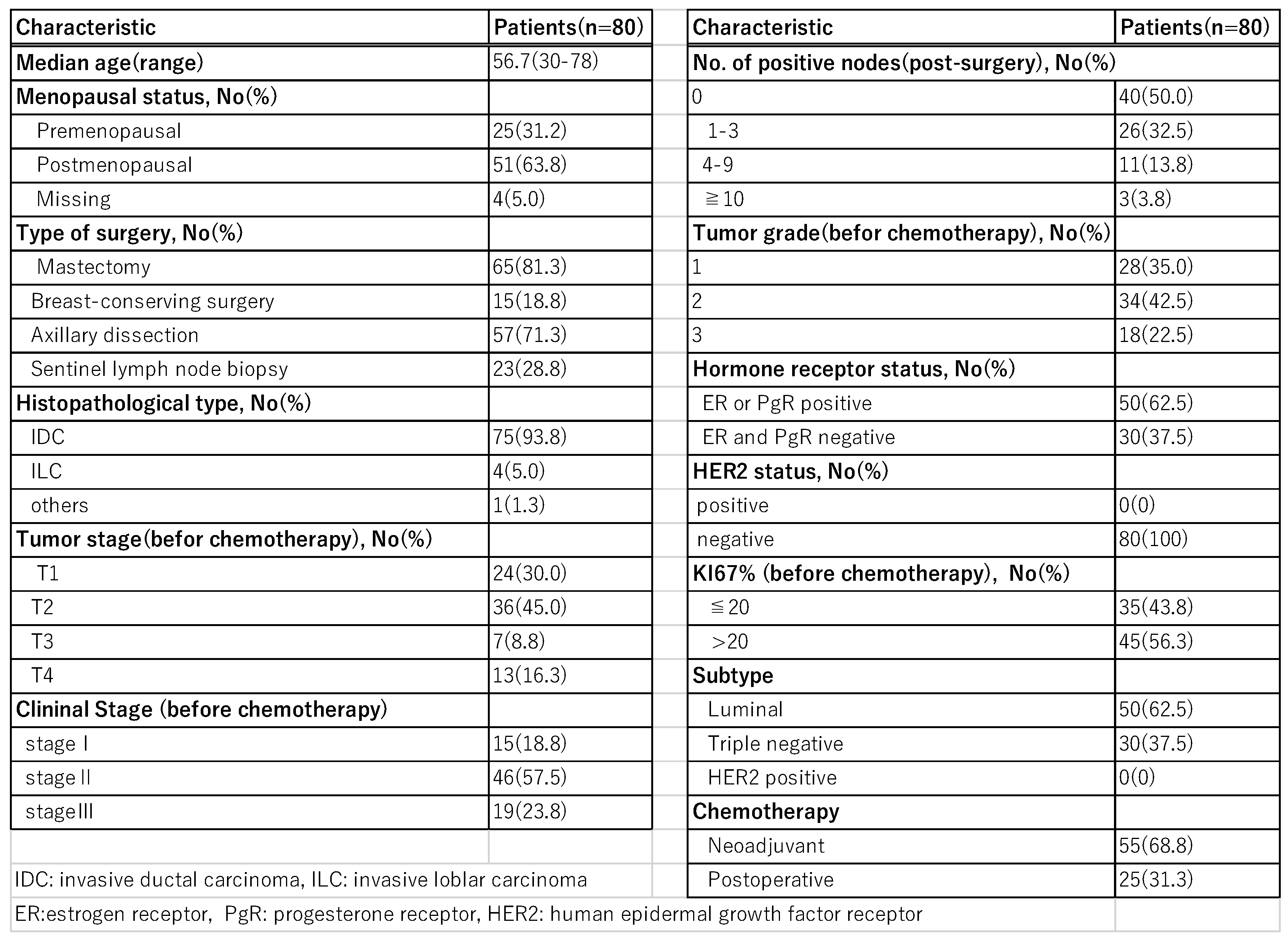
Patient’s background is shown in
Table 1. The age of the patients was 30~78 years (mean 56.7 years) and the observation period was 7~64 months (median 38 months). Subtypes were triple negative type (TNBC) in 30 cases and luminal type (Luminal) in 50 cases; HER2-positive cases were not included. Fifty-three patients (66.3%) were axillary lymph node-positive cases at the time of diagnosis, with clinical stage I in 15 cases, stage II in 46 cases, and stage III in 19 cases. 75 cases were invasive ductal carcinoma of the breast, 4 cases were invasive lobular carcinoma, and 1 case was invasive micropapillary carcinoma.
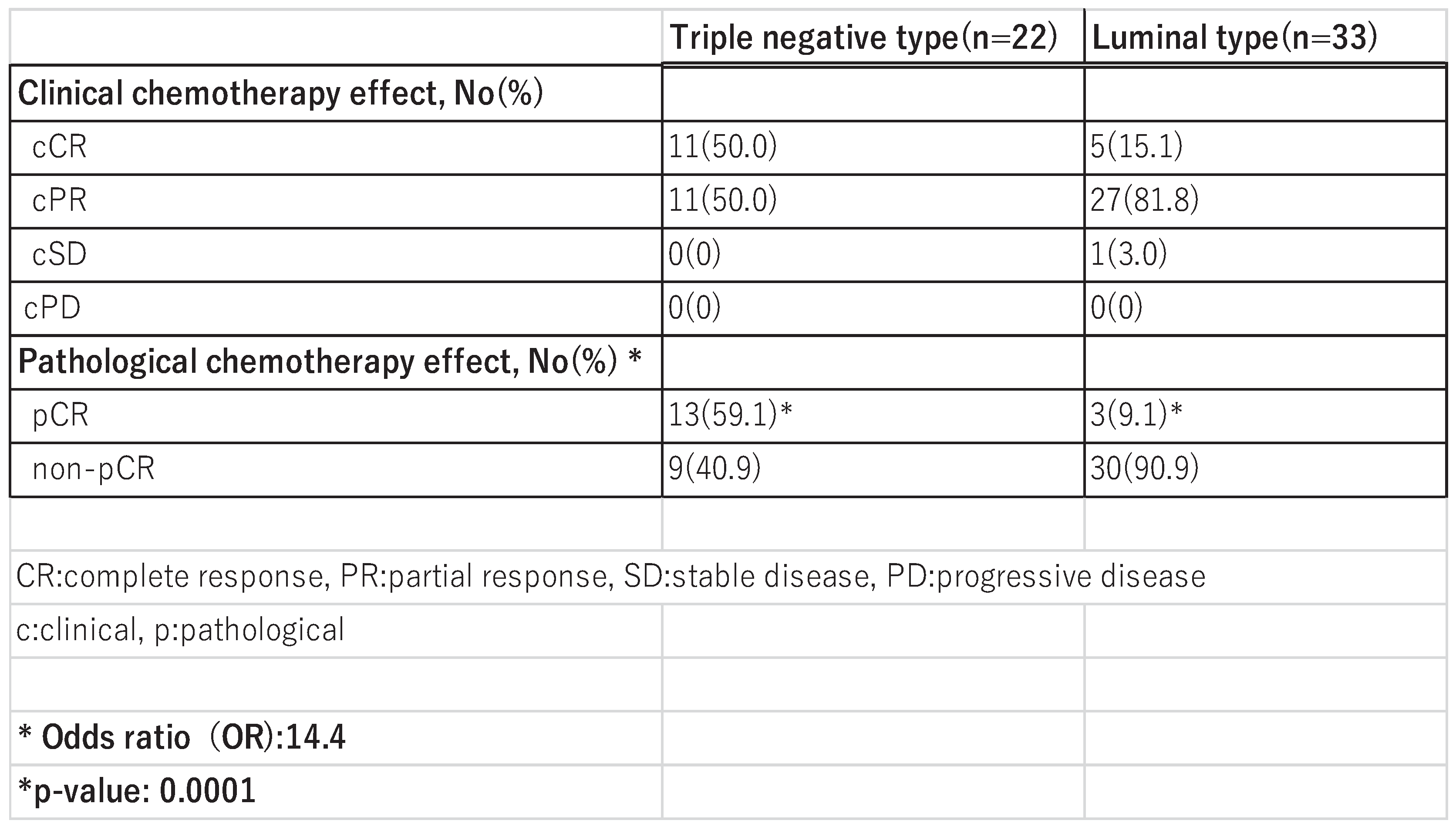
Of the 55 patients who received NAC, 22 were TNBC and 33 were Luminal (
Table 2). The overall pCR rate was 29.1% (16/55), 59.1% (13/22) in the TNBC group and 9.1% (3/33) in the Luminal group. The results of the Fisher’s test showed that the pCR rate in the TNBC group was statistically significantly higher than in the Luminal group (odds ratio: 14.4, p=0.0001).
The 8 cases of TNBC who did not undergo NAC were those who were considered to have non-invasive carcinoma preoperatively but were found to have invasion on postoperative pathology or those who preferred to go ahead with surgery because of the COVID-19 situation. Around 2020, there were cases where surgeries were postponed due to COVID-19 infections among medical staff, patients, and their families. As a result, patients sometimes requested to undergo surgery as early as possible. They were concerned that their surgeries might be canceled due to COVID-19.
The treatment completion rate was 82.5% (66/80), and 14 patients (17.5%) could not continue dd chemotherapy due to adverse events. The main adverse events are shown in
Table 3.
Hematologic adverse events, such as liver dysfunction (58.8%) and anemia (57.6%), were observed in more than half of the patients, but most were Grade 1 or 2. Thrombocytopenia was observed in 22.6% of patients, and leukopenia and neutropenia were observed in 18.8% of patients. Febrile neutropenia was observed in 7 patients (8.8%).
Non-hematologic complications included alopecia (100%), myalgia (70.1%), arthralgia (57.6%), and peripheral neuropathy (55.0%), which were observed in more than half of the patients. In addition, fatigue (43.8%), nausea (37.6%), and anorexia (27.6%) were also common.
Grade 3 or higher adverse events included leukopenia and neutropenia at 11.3%, febrile neutropenia at 8.8%, and thrombocytopenia at 1.3%. Non-hematologic adverse events included pneumonia at 5.0%, fatigue at 2.5%, anorexia, nausea, arthralgia, and myalgia were each 1.3%. The reasons for discontinuation were Grade 3/4 anemia and neutropenia, drug-induced pneumonia, pneumocystis pneumonia, liver dysfunction, and Grade 4 thrombocytopenia due to pegfilgrastim.
Two of the patients who discontinued treatment were diagnosed with pneumocystis pneumonia, which required to distinguish from COVID19-associated pneumonia. Due to severe pneumonia, oxygen administration and hospitalization for more than two weeks were required. After discharge, continued chemotherapy became difficult due to a decline in performance status, so chemotherapy was not continued and surgery was performed.
One patient diagnosed with thrombocytopenia due to pegfilgrastim was treated with pegfilgrastim body pod at the first time, and Grade 4 neutropenia and thrombocytopenia developed on day 8. Antibiotic therapy was administered for febrile neutropenia, and platelet counts decreased to 23,000/mm³, but no blood transfusion was given because the patient refused. The patient was kept under observation, and improvement to Grade 1 was observed by day 15. We also switched to subcutaneous injection of pegfilgrastim for this patient and reduced the dose of chemotherapy. Neutropenia improved, but thrombocytopenia similarly decreased to Grade 4. Considering thrombocytopenia associated with pegfilgrastim, we switched to a 3-week regimen without pegfilgrastim. This means that dd chemotherapy has been discontinued. After this change, thrombocytopenia remained at Grade 1. In our institution, several skinny women with low subcutaneous fat who received pegfilgrastim body pod developed Grade 4 febrile neutropenia. We switched to subcutaneous injection of pegfilgrastim, and Grade 4 neutropenia no longer occurred.
Other adverce events, such as liver dysfunction, myalgia/arthralgia, peripheral neuropathy, fatigue/malaise, and alopecia, with most cases showing symptomatic improvement after chemotherapy was completed.
There were three distant recurrence cases (3.8%), including one case of inflammatory breast carcinoma with TNBC whose pCR was obtained by NAC. Two months after surgery, multiple liver metastases were found, and the breast carcinoma was PD-L1 positive. Chemotherapy with an immune checkpoint inhibitor was ineffective, and the patient died four months after surgery. The other two patients had luminal type carcinoma with lymph node metastasis. Both patients had non-pCR after neoadjyuvant chemotherapy and died two years later due to liver and lung metastases, respectively.
4. Discussion
Dose dense chemotherapy is a combination of the same type and dose of chemotherapy with G-CSF and a shorter dosing interval. It has been shown to reduce recurrence and prolong survival, especially in high-risk breast cancer patients, but it is important to proceed with caution regarding adverse events [
1,
2,
3,
4,
5,
6,
7,
8,
9,
10,
11,
12].
Citron ML. et al. reported that the results of the CALGB9741 trial showed that 3-year DFS was 85% in the dd chemotherapy group and 81% in the standard therapy group (p = 0.010), and 3-year OS was 92% vs. 90% (p = 0.013), both superior in the dd chemotherapy group. In particular, the effect was more pronounced in premenopausal patients. Adverse events were within the expected range, and toxicity such as neutropenia was controllable with the use of G-CSF [
1]. Fornier MN et al. evaluated the efficacy and safety of sequential administration of paclitaxel and cyclophosphamide every two weeks after doxorubicin, compared with simultaneous administration of paclitaxel and cyclophosphamide. The study included breast cancer patients with lymph node metastasis, and the 5-year DFS was in the sequential administration group, although the difference was not statistically significant, a trend toward better outcomes was observed. Similarly, there was no significant difference between the two groups in terms of OS, but a slightly favorable trend was observed in the sequential administration group. Both the sequential administration group and the simultaneous administration group showed manageable toxicity, with the main adverse events being leukopenia, fatigue, and nausea. Neutropenia was alleviated by G-CSF. Sequential drug administration under a dose density schedule may be more effective than simultaneous administration [
2].
Venturini et al. compared every-2-week chemotherapy with standard every-3-week chemotherapy in women with early-stage breast cancer and lymph node metastasis. DFS and OS were significantly improved in the every-2-week chemotherapy group, with a particularly pronounced trend in premenopausal patients with lymph node metastasis and high risk of recurrence [
3]. Fornier M et al. reported in their review that dd chemotherapy is expected to be particularly effective in triple-negative breast cancer and premenopausal high-risk patients. At the same time, they emphasized the importance of side effect management and patient selection [
4]. Regarding long-term efficacy and safety, Blondeaux E et al. analyzed 15 years of follow-up data from the MIG-1 study and examined the effects on survival rates and recurrence rates. DFS was significantly improved in the dd chemotherapy group, and the recurrence-suppressing effect was sustained at 15 years. Regarding OS, there was no statistically significant difference between the two groups, but there was a tendency for the dose density group to be higher. In addition, although short-term toxicity was high, no increase in long-term adverse events was observed [
5].
Del Mastro et al. investigated the additional efficacy of fluorouracil (5-FU) as adjuvant chemotherapy and the efficacy of dose-dense chemotherapy in a 2 × 2 factorial, randomized phase 3 trial. Patients were divided into two groups: one receiving paclitaxel every two weeks in a dose-dense regimen and the other receiving paclitaxel every three weeks in a standard regimen following EC therapy with epirubicin and cyclophosphamide. Another group was divided into two subgroups: one receiving paclitaxel every two weeks and the other every three weeks following FEC therapy, which added 5-FU to EC therapy. This design constitutes a 2x2 factorial design. In the comparison between dose interval groups, the dose-dense administration group showed significantly improved disease-free survival (DFS) and overall survival (OS). The 5-year DFS was 88% in the dose-dense group vs. 85% in the standard group, and the 5-year OS was 96% in the dose-dense group vs. 94% in the standard group. In addition, the addition of 5-FU did not significantly affect disease-free survival or overall survival. Therefore, it was concluded that the addition of 5-FU does not improve treatment efficacy. In this study as well, dose density groups showed increased hematologic toxicity (neutropenia) and fatigue, but these were manageable with G-CSF and overall safety was reported to be within acceptable limits. Therefore, in adjuvant chemotherapy for high-risk recurrence, dose density strategies are recommended, but 5-FU can be omitted [
6]. Furthermore, Del Mastro L et al. have reported the results of this long-term follow-up study. After approximately 15 years of follow-up, the disease-free survival rate (DFS) was 64.4% in the dose-dense group and 60.0% in the standard interval group, showing a significant improvement (HR 0.82, P=0.0055)even in long-term follow-up. Overall survival (OS) was also significantly improved in the dose density group (78.5%) compared to the standard interval group (75.6%, hazard ratio [HR] 0.79, P=0.011). The addition of 5-FU did not result in significant improvement in DFS or OS, and it was concluded that the addition of 5-FU does not improve long-term prognosis. Regarding safety, no significant differences were observed in long-term cardiotoxicity or the incidence of secondary cancers. Subgroup analysis showed that dose density therapy was particularly effective in patients with lymph node-positive, premenopausal, and hormone receptor-negative disease [
7].
In addition, the efficacy of neoadjuvant chemotherapy has been evaluated in a clinical trial conducted by Baldini E et al., in which CEF (cyclophosphamide + epirubicin + 5-FU) or CMF was administered every 3 weeks or every 2 weeks for 6 doses. The response rate to neoadjuvant chemotherapy was 84% in the dose-dense group and 64% in the standard-dose group, demonstrating a significantly higher response rate in the dose-dense chemotherapy group (p < 0.01). The pathological complete response (pCR) rate also tended to be slightly higher in the dose-dense group. The percentage of patients who were able to undergo breast-conserving surgery was significantly higher in the dose dense group [
8]. Melichar B is also investigating the efficacy of dose-dense chemotherapy in preoperative chemotherapy. The clinical response rate was 91% in the dose-dense group and 75% in the standard group, with a significant difference (p = 0.03) in favor of the dose-dense group. The pCR rate was 25% in the dose-dense group and 11% in the standard-dose group, with a significantly higher rate in the dose-dense group (p < 0.05). The rate of breast-conserving surgery was also higher in the dose-dense group, and the possibility of breast conservation improved due to tumor shrinkage prior to surgery. Adverse events occurred, but were tolerable with concomitant supportive therapy. Notably, the pCR rate was higher in TNBC than in the Luminal group (43% vs. 6%). pCR was associated with significantly improved recurrence-free survival and OS, but notably, the difference was significant only in TNBC. It concludes that this treatment should be actively considered for high-risk groups such as young people and those with triple-negative breast cancer [
9].
Wang X et al. reported on the efficacy of dose-dense chemotherapy as neoadjuvant chemotherapy for triple-negative breast cancer. The treatment regimen consists of four doses of anthracycline every two weeks, followed by 12 weekly doses of paclitaxel as neoadjuvant chemotherapy. The clinical response rate (complete + partial response) was 85.7%. The pCR rate was 39.3% overall, with a higher pCR rate in patients with smaller tumor size and lymph node-negative status. They also reported that neoadjuvant chemotherapy for TNBC significantly increased the pCR rate in the dose dense chemotherapy group compared to the conventional chemotherapy group (45.3% vs. 34%) and also improved the pCR rate for axillary lymph node metastases (58% vs. 44%). The disease-free survival rate (DFS) at 30 months was significantly better in the pCR achieved group, and DFS prolongation was confirmed. Neoadjuvant chemotherapy with dose-dense chemotherapy has been a promising treatment option for triple-negative breast cancer. In particular, patients who achieved pCR showed significantly improved prognosis [
10].
Zhou W et al. conducted a meta-analysis limited to reliable randomized controlled trials (RCTs) to examine the effect of pure dose-dense chemotherapy (shortening the administration interval without changing or increasing the drug) on survival. In conclusion, overall survival was significantly improved in the dose-dense chemotherapy group with HR = 0.84 (95% CI: 0.75–0.95), and disease-free survival was also significantly improved in the dose-dense chemotherapy group with HR = 0.83 (95% CI: 0.75–0.91). In addition, the risk of distant recurrence was significantly lower (HR = 0.79 (95% CI: 0.69–0.91) in the dose-dense chemotherapy group. The incidence of neutropenia was high, but it was generally manageable with the use of G-CSF, and other adverse events were similar to those seen with standard treatment [
11]. They also reported limited efficacy in breast cancers of Luminal type with low Ki67.
In our study, too, the TNBC group was more likely to obtain a pCR, with a significant difference of 59% compared to the Luminal group, which had a pCR rate of 6%. This result supports the high efficacy of dose dense chemotherapy in TNBC. Although the number of cases was limited,the pCR rate for TNBC patients in this study was 59.1%, which is higher than the pCR rates shown in other clinical trials. On the other hand, the pCR rate for Luminal type was low at 9.1%, suggesting that the efficacy of dose dense chemotherapy in Luminal type is limited.
Additionally, previous reports have indicated that in TNBC, the presence of Insulin-like growth factor II mRNA-binding protein 3 (IMP3) expression is associated with poor response to chemotherapy [
12]. However, in recent regimens including dose-dense chemotherapy, there was no difference in the efficacy of preoperative chemotherapy based on the presence or absence of IMP3 expression, and dose-dense chemotherapy is considered effective even for more aggressive TNBC that was unresponsive to previous regimens [
13].
Adverse events were observed in many cases in the above clinical trials, similar to those seen with conventional chemotherapy. The 2022 Japanese Guidelines for the Management of Breast Cancer [
14] provide a literature review of adverse events associated with dose-dense chemotherapy. Anemia was analyzed in two randomized controlled trials (RCTs) involving 4,172 patients [
6,
7,
15]. In all grades, the incidence was 37.1% in the control group and 53.4% in the dose-dense group (risk difference (RD) 0.17, 95% CI 0.14–0.20). However, when limited to Grade 3 or higher anemia, no significant increase was observed, with 0.09% in the control group and 0.7% in the dose-dense group (RD 0.01, 95% CI -0.01 to 0.02). However, another study reported that all 116 triple-negative breast cancer patients who underwent dose-dense chemotherapy developed Grade 1 or higher anemia during treatment, with approximately 34.5% reaching Grade 3 and 37.9% requiring a blood transfusion. Based on these findings, it is considered necessary to carefully monitor blood sampling data [
16]. In our hospital, anemia during dose-dense chemotherapy improved naturally in all cases upon completion of chemotherapy. In the above Japanese guidelines for the treatment of breast cancer, the evaluation of febrile neutropenia was analyzed in one RCT involving 2,155 participants [
15]. The risk was reduced in the dose-dense chemotherapy group (1.4%) compared to the control group (3.6%) (RD -0.02, 95% CI -0.03 to -0.01). This result was considered to be due to the fact that pegfilgrastim was previously considered mandatory in dose-dense chemotherapy.
The incidence of adverse events was similar in our study, but febrile neutropenia was observed in 8.8% of cases (7/80). They required antibiotic treatment and hospitalization. At our institution, this febrile neutropenia occurred primarily after the first administration, and although the number of cases was small, it was observed in several cases of women who were skinny with little subcutaneous fat and received pegfilgrastim body pod.
In two cases of pneumocystis pneumonia, febrile neutropenia occurred when treatment was switched from anthracycline (4 times of dose-dense EC) to taxane (dose-dense paclitaxel). This pneumonia is also known as opportunistic infection, and it was thought that the administration of steroids every two weeks to prevent adverse events may have contributed to its development. Of these, one case was reported by Yagi [
17]. Pneumonia caused by Pneumocystis jiroveici pneumonia can appear to worsen despite effective treatment, and this coincided with the spread of COVID-19, making diagnosis and treatment difficult. During treatment for Pneumocystis jirovecii pneumonia, a phenomenon in which symptoms appear to worsen despite effective treatment is known as a “paradoxical response” or “immune reconstitution inflammatory syndrome (IRIS)” [
18].
Thrombocytopenia can also occur with dose-dense chemotherapy; however, in the Grade 4 thrombocytopenia case experienced in this study. Pegfilgrastim body pod was used at the first chemotherapy session, and the treatment was switched to a pegfilgrastim subcutaneous injection formulation for the second session, both of which resulted in Grade 4 thrombocytopenia. After discontinuing pegfilgrastim due to concerns about its effects, the thrombocytopenia remained at Grade 1, leading us to conclude that pegfilgrastim was the cause.
Pegfilgrastim stimulates hematopoietic stem cells in the bone marrow to promote the production of neutrophils. It is modified with PEG (polyethylene glycol), which slows its breakdown in the body and gives it a longer duration of action [
19,
20,
21,
22,
23,
24]. In a dose-dense regimen, it is important to manage white blood cell counts, and pegfilgrastim is an important adjuvant drug. However, patients using pegfilgrastim had a significantly higher risk of thrombocytopenia compared to non-users (adjusted odds ratio: 5.7, 95% confidence interval: 4.3-7.5) [
25]. Additionally, an analysis comparing adverse events between filgrastim and pegfilgrastim indicated that pegfilgrastim was associated with a higher incidence of thrombocytopenia.
Dose-dense chemotherapy has been reported to cause other adverse events, such as anemia, liver dysfunction, fatigue, muscle and joint pain, and peripheral neuropathy [
1,
2,
3,
4,
5,
6,
7,
11]. In this study, similar symptoms were observed, but many cases showed improvement after the completion of dose-dense chemotherapy. Regarding hair loss, our clinic does not use scalp cooling devices, and this has been approved by all patients.
Scalp cooling devices are medical devices designed to prevent chemotherapy-induced alopecia and are widely used in cancer treatment facilities around the world [
26,
27,28]. Alopecia in CTCAE v5.0 is defined as Grade 1 (mild) when hair loss is not noticeable or is sporadic, or when the patient does not require a wig or hat. Grade 2 (severe) is defined as hair loss that is noticeable (total or extensive) or when the use of a wig or hat may be necessary. VV Shah et al. analyzed these study results in randomized controlled trials and controlled clinical trials.
In controlled clinical trials, scalp cooling reduced the incidence of CIA by approximately 2.7-fold. In randomized controlled trials, scalp cooling reduced the incidence of chemotherapy-induced alopecia by approximately 3.9-fold. These results suggest that scalp cooling is an effective preventive measure against alopecia, a side effect of chemotherapy [
26].
Mangesh M. et al, showed the prevention of alopecia (grade 0 and grade I) was seen in 81%, while more than 50% hair loss (grade 2) was seen in 16.48% after completion of treatment [
27]. While there are reports that scalp cooling devices are effective in preventing hair loss, scalp cooling devices and cooling caps are expensive, may cause discomfort due to cooling, and require long cooling times, which can result in longer hospital stays.
Dustin HM et al. reported on the effects of scalp cooling, widely used as a preventive measure by the CIA, on quality of life (QOL). They systematically reviewed 13 studies (4 randomized controlled trials, 8 cohort studies, and 1 cross-sectional study, involving a total of 1,282 patients) that evaluated the effects of scalp cooling on the QOL of breast cancer patients. The most commonly used QOL assessment tools were the EORTC QLQ-C30 and QLQ-BR23 (46% each). Among the 13 studies, 4 (31%) reported a significant improvement in QOL due to scalp cooling. Eight (62%) reported that the improvement in QOL was not statistically significant or no improvement was observed. One study (7.7%) showed mixed results. Many studies have failed to clearly distinguish between the success of scalp cooling (prevention of hair loss) and its association with quality of life (QOL), evaluating all scalp cooling patients as a single group.
In reality, if scalp cooling treatment—which is expensive and may cause discomfort—fails to prevent hair loss, it could potentially lead to a decline in patients' QOL [28].
Although the number of cases examined was small, our dd chemotherapy showed a high pCR rate, especially in patients with TNBC. On the other hand, the limited efficacy of dd chemotherapy in luminal types suggests the importance of an individualized treatment strategy.
Regarding adverse events, the treatment completion rate was 82.5%, indicating that dd chemotherapy was generally well tolerated. However, neutropenia was tolerable in most patients with pegfilgrastim. However, although occurring in less than 10% of cases, it is important to note that febrile neutropenia may occur, and that serious complications such as pneumocystis pneumonia, which is considered to be caused by pegfilgrastim-induced thrombocytopenia or steroid administration, may rarely occur. By carefully managing adverse events, dose-dense chemotherapy for the treatment of early breast cancer with high risk of recurrence was completed in more than 80% of cases, and particularly in TNBC, it showed good pCR and is considered to have promising therapeutic effects.
But caution must be exercised with regard to bone marrow suppression such as anemia and thrombocytopenia, and it is important to use appropriate supportive care in combination with chemotherapy.