1. Introduction
Persons with primary or secondary immunodeficiency are at higher risk of severe and complicated primary infection with varicella zoster virus (VZV) as well as VZV reactivation [
1,
2,
3,
4,
5]. Among immunocompromised patients, especially those with transplants, malignancies or targeted therapies such as Janus kinase inhibitors have an up to 9-fold higher incidence of herpes zoster and postherpetic neuralgia relative to the total population [
4,
5,
6,
7,
8,
9,
10]. Particular attention must be paid to those immunocompromised patients who lack an adequate VZV-specific immunity, indicated by VZV seronegativity (i.e. antibodies below the assay’s cut-off/detection level), and are therefore at risk of severe VZV infection/disease. Seronegativity can originate from either lack of previous VZV infection or vaccination, primary vaccination failure or early antibody waning after infection or vaccination. Furthermore, a loss of antibodies and pre-formed long-lasting plasma cells as well as memory cells might follow immunoablation preceding hematopoietic stem cell transplantation (HSCT).
However, the use of the live-attenuated VZV vaccine to prevent varicella infection is contraindicated for severely immunocompromised persons [
7,
11,
12,
13,
14], thus leaving VZV-seronegative immunocompromised patients without an option to vaccinate against primary varicella infection and thus at increased risk.
With respect to endogenous varicella reactivation, i.e. herpes zoster, a recombinant subunit vaccine (rHZV-Shingrix
®) has been developed to prevent herpes zoster and postherpetic neuralgia. This vaccine replaced the former live-attenuated virus vaccine (Zostavax
®) used for herpes zoster prevention and is safe to use in immunocompromised patients [
6,
15]. The rHZV targeting the VZV glycoprotein E antigen (gE) contains the adjuvant AS01B to enhance immunogenicity and is licensed for adults at increased risk for VZV reactivation, due to aging (≥ 50 years) or immunosuppression (≥ 18 years) in a two-dose schedule [
15,
16]. This vaccine demonstrated an overall vaccine efficacy of 97.2% in preventing herpes zoster in individuals above the age of 50 years [
17]. In the elderly (≥ 70 years), vaccine efficacy was still 91.3% against zoster and 88.8% against post herpetic neuralgia [
18]. Even in immunocompromised individuals (≥ 18 years), such as patients after autologous HSCT or with hematologic malignancies, a robust immune response [
7,
19,
20,
21,
22] with high vaccine efficacy against zoster and post-herpetic neuralgia (68% and 87%, respectively) was shown [
23,
24].
The rHZV is not licensed to prevent VZV primary infection, but, as antibody determination is generally not required prior to vaccination, the vaccine might, in rare cases, also be applied to people without previous contact to VZV [
16]. Data on the immunogenicity after rHZV vaccination in initial VZV-seronegative immunocompromised patients are scarce and currently only available for solid-organ transplant patients [
5]. In these cases, the use of rHZV remains the only safe option for vaccination, albeit as off-label application. However, according to experts’ opinion, more evidence and data on the protection against varicella infection are required to support the recommendation to use rHZV for primary vaccination in immunocompromised patients [
16].
In the current study we aimed to retrospectively evaluate antibody responses in immunocompromised individuals who previously tested anti-VZV-IgG negative and received rHZV vaccination in our outpatient vaccination clinic for patients with medical risk conditions. Immunogenicity data were analyzed from a cohort of patients with underlying hemato-oncological malignancies or autoimmune diseases with their respective treatments following two or three doses of rHZV. Clinical outcome on the prevention of infection requires a separate evaluation, though.
2. Materials and Methods
Study Population and Study Design
For this retrospective explorative cohort study we first screened the database from our outpatient clinic at the Institute for Specific Prophylaxis and Tropical Medicine (ISPTM) for all patients aged 18 years and above, who received two or three doses of rHZV (Shingrix
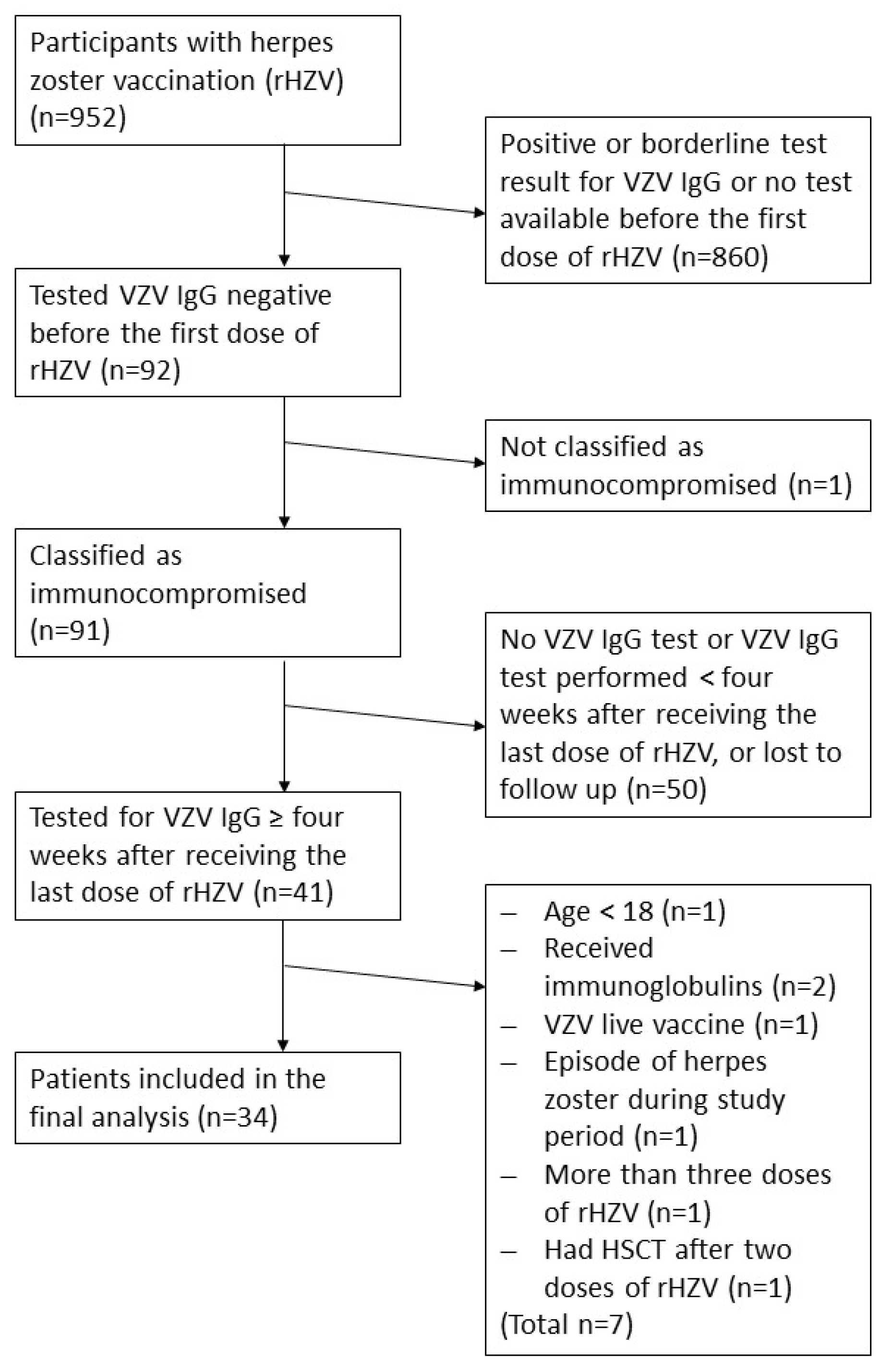
®; GlaxoSmithKline Biologicals (GSK)) intramuscularly (deltoid muscle) between March 01, 2018 and January 08, 2024 (n=952). Only immunocompromised individuals who tested seronegative for VZV-IgG antibodies (below the assay’s cut-off) before their first rHZV dose and with a VZV IgG test result at least four weeks after their last dose of rHZV were eligible, including one subject just before starting on immunosuppressive treatment. We excluded patients with intravenous human immunoglobulin substitution (IVIG) or varicella zoster immunoglobulin (VZIG) therapy in the patient’s history and one person who had a solid organ transplantation. For the analysis, 33 patients were available (
Figure 1). We assessed safety by retrospectively extracting documented adverse events from patient’s charts.
This study was approved by the ethics committee of the Medical University of Vienna (Ethics Nr.: 1255/2023) and was conducted in accordance with ICH and GCP guidelines and with the applicable local regulatory requirements.
Vaccination Schedule
Two doses of rHZV were applied i.m. in an interval of at least one month according to licensure. Only in HSCT patients, we initially offered three doses of rHZV at least one month apart following a publication on the immunogenicity and efficacy of three rHZV doses after autologous HSCT.[
25] Upon further published data in HSCT patients, a two-dose schedule has been implemented as routine schedule at our outpatient clinic. Patients with other diagnoses received the licensed two-dose regimen on a routine basis.
Analysis of Specific VZV IgG Levels
Anti-VZV-IgG antibody levels before the first, and at least four weeks after the last (second or third) dose of rHZV were quantified according to routine diagnostic testing at the Center of Virology, Medical University of Vienna, Austria. Antibodies were measured using a CE-IVD certified, commercially available ELISA with VZV-lysate pre-coated plates (Euroimmun, Germany) according to the manufacturer recommendations. As specified by the manufacturer, values <140 IU/l were assessed as negative, >200 IU/l as positive and the values in between as borderline. In eight cases VZV IgG levels were derived from an external laboratory, indicating values with respective interpretation according to the used assay system (n=5 negative according to the test cut-off, n=3 positive).
Endpoints
The primary endpoint was the quantitative result of VZV IgG ELISA more than four weeks after the last dose (second or third) rHZV in immunocompromised patients who tested negative before vaccination. The secondary endpoint was the percentage of seropositive participants defined by a positive IgG ELISA result after vaccination.
A key question of this study was, whether an antibody increase to a positive result, i.e. >200 IU/ml, can be established by vaccination with two or three doses of rHZV in these high-grade immunosuppressed patients in a routine clinical setting.
Statistical Analysis
The statistical analysis was performed using Stata 17.0 and figures were produced using GraphPad Prism 9.3.1. For calculation of the geometric mean concentration (GMC), a generalized linear model (GLM) was used with number of vaccinations as factor and time since last vaccination, age and sex as covariates. Wald 95% confidence intervals (CI) of the GMCs were computed adjusted for the covariates. In five cases, anti-VZV IgG concentrations below the assay’s cut-off level were reported from another lab. If no values were specified in these cases, an arbitrary value of 70 IU/l (half of the cut-off) was used. For analysis of fold increase, the log pre-vaccination concentrations were used as offset, otherwise the analysis was equivalent to that specified above.
All additional variables including the increase from negative to positive values were descriptively presented in total and as absolute and relative frequencies for nominal data and as median plus interquartile ranges (IQR) for metric data.
3. Results
Study Population and Demographic Data
Between March 01, 2018, until January 08, 2024, 952 individuals received rHZV vaccinations at our outpatient clinic with focus on immunocompromised patients. Of those, 91 initially tested seronegative for anti-VZV IgG (below the cut-off of the applied assay) before their first dose of rHZV, and classified as at risk patients. Of these, 41 patients were subsequently tested for VZV IgG at least four weeks after receiving their last (second or third) dose of rHZV. Further eight patients did not meet the inclusion criteria and were excluded. In total, 33 patients were included in the final analysis (
Figure 1).
The median age at the first dose of rHZV was 53.0 (43.0-61.0 IQR) years and 51.5% of the study group were female. The majority of patients (81.8%, n=27) had a chronic haematological disease and had received HSCT. Most HSCT recipients (64.7%, n=22) received three doses, starting three months after HSCT, on a routine basis following an early publication on HSCT patients (
Table 1). For all patients, the median interval between last dose of rHZV and blood draw for VZV IgG testing was 9.6 months (5.1-13.9 IQR). No severe adverse events following immunization were recorded in the patient charts.
Humoral Immune Response
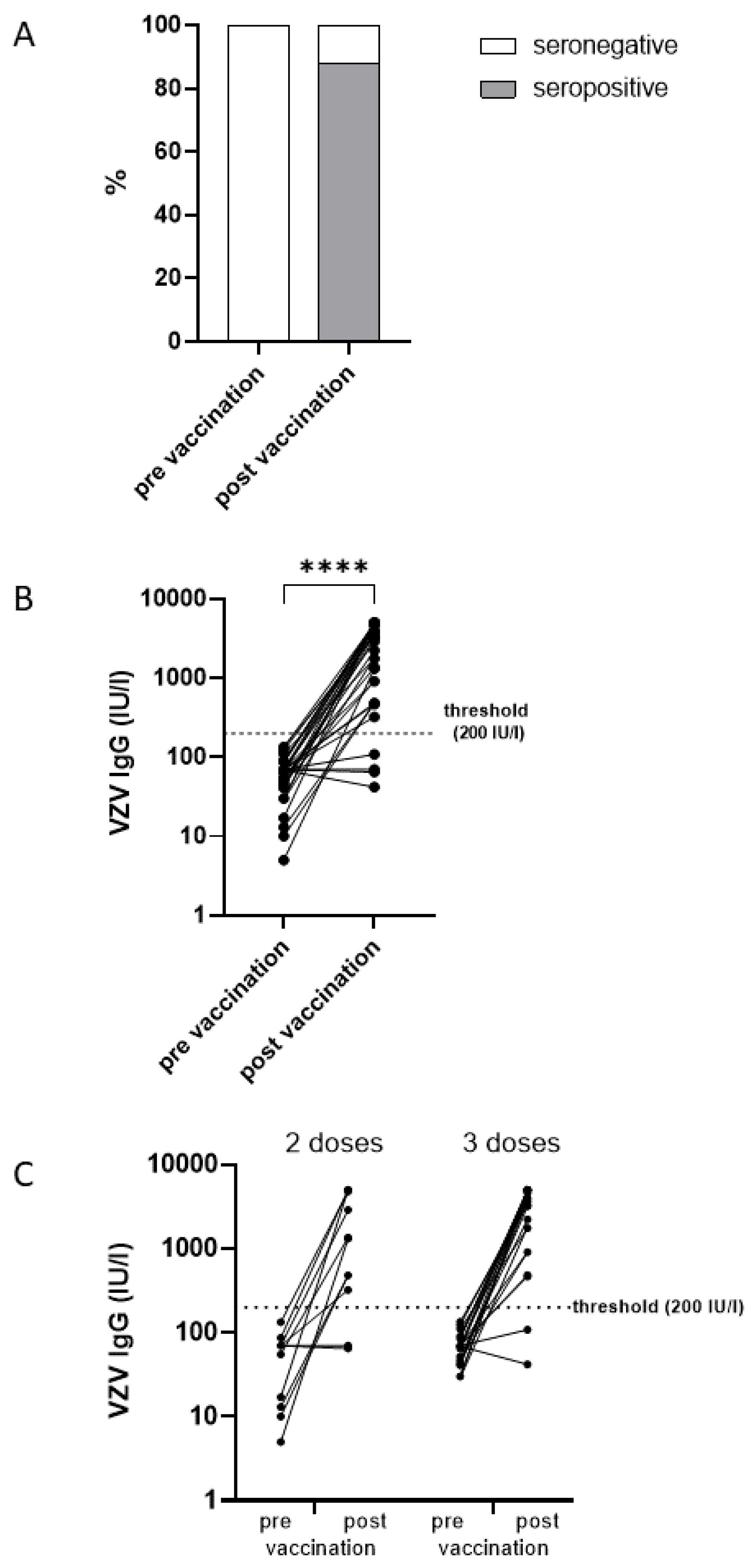
With respect to an antibody increase exceeding the assay’s cut-off level, 29 of the 33 individuals who previously tested negative (below 140 IU/l) achieved a positive VZV-specific IgG result (above 200 IU/l) at least four weeks after their last rHZV dose, resulting in a seropositivity rate of 88% (95% CI: 72-97%) (
Figure 2A). However, four patients (12%) remained seronegative after two (n=2) or three (n=2) doses of rHZV (
Table 2).
The GMC for all patients increased from 52 IU/l (95% CI: 40-68 IU/l;
Figure 2B) before vaccination to 1445 IU/l (95% CI: 969-2157 IU/l;
Figure 2B) after the last vaccination (corrected for distance from vaccination). Patients receiving two doses had significantly (p=0.032) lower GMC (751 IU/l 95% CI: 354-1550 IU/l vs 2001 IU/l 95% CI: 1201-3333 IU/l) than those with three doses (
Figure 2C). The antibody increase was highly significant (p<0.0001) with a GMC increase of 28-fold (95% CI: 17-43-fold) (
Figure 2B). Yet, the antibody increase did not differ significantly (p=0.533) between those receiving two doses (20-fold 95% CI: 9-46-fold) and those with three doses (32-fold 95% CI: 18-58-fold).
The interval between the IgG level assessment from the last vaccination had a significant impact on the IgG level (p=0.001). Per month increase of the interval, antibody levels decreased by 10.9% (95% CI: 5.4%-16.2%). Non-responders (n=4) had a median (IQR) of 20.9 (12.1-28.8) months between their last dose of rHZV and the time point of VZV IgG testing, while for responders (n=29) it was 9.1 months (4.1-11.7 months; p=0.010). However, also after correction for distance from last vaccination the non-responders had expected levels of VZV IgG below 200 IU/l at one month after vaccination.
Patients with HSCT tended to have higher increases of IgG concentrations than the other patients (29-fold, 95% CI: 17-50-fold vs 21-fold, 95% CI: 7-65-fold), though not statistically significant (p=0.069). Since it takes several months to years for the immune system to reestablish after HSCT, we analyzed the median (IQR) interval between HSCT (n=27) and the application of the first dose of rHZV; it was 10.0 (8.0-18.0) months for all patients, for non-responders (n=2) 7 (6 and 8 months) and for responders (n=25) 11.0 (8.0-18.5) months, respectively. Although non-responders received their first dose somewhat earlier with respect to HSCT than responders, this difference was not significant. Despite this difference, the interval between HSCT and first rHZV dose had no significant effect on increase of IgG concentrations (p=0.540) overall. Also allogeneic versus autologous HSCT showed no difference of the immune responses (p=0.321).
4. Discussion
Encountering VZV-seronegative immunocompromised patients in our outpatient clinic we aimed to retrospectively analyse their antibody responses to rHZV. Reasons for their VZV-seronegativity in our cohort remain unidentified but could have been due to the absence of previous VZV contact or vaccination (naïve persons), primary vaccination failure or the loss of antibodies after infection or vaccination due to early antibody waning in relation to the immunosuppressive therapy. Although we cannot investigate whether or which participants are truly naïve to VZV, they supposedly are at increased risk for infection and/or severe disease.
So far, rHZV is not licensed for primary vaccination against VZV in seronegative individuals. Due to the contraindication of live-attenuated varicella vaccination in immunocompromised patients, off-label use of rHZV remains the only option to induce antibody responses against VZV in this special patient cohort [
5,
16]. However, the protection capacity of the induced immune responses directed against the rHZV vaccine antigen glycoprotein E (gE) against primary varicella infection is yet unknown. So far, data on the antibody response following rHZV vaccination in VZV IgG negative persons under immunosuppression is limited to solid organ transplant recipients [
5]. Therefore, the aim of this study was to assess antibody responses and seropositivity rates following rHZV in patients with immunodeficiency or with immunosuppressive or immunomodulatory therapies who previously tested negative for VZV IgG.
In our retrospective cohort study, the majority of participants (88%) mounted a significant increase in VZV IgG levels and reached the threshold of seropositivity of the assay. Our results are supported by a study in 23 anti-VZV-gE-seronegative solid organ transplant (SOT) recipients who showed a significant increase in antibody immune response with a positive seroresponse in 55% four weeks after the administration of two doses of rHZV [
5]. To our knowledge, this former study is the only trial on immune responses to rHZV in gE-seronegative immunocompromised individuals so far. Although our study now provides more data on this topic, as a limitation, we assessed VZV-specific antibodies with an ELISA using lysate and not gE as the target antigen. However, an earlier report demonstrated a high correlation between a gE antigen-specific ELISA and an ELISA using VZV-lysate as target antigen to measure the VZV-specific humoral immune response [
26].
Due to the already published data, we excluded one person who received a solid organ transplantation from our analysis. Results from a phase 1/2 study described one individual post autologous HSCT who was initially seronegative against gE and seroconverted after the first dose of an rHZV adjuvanted with AS01E (containing half the amount of the adjuvant included in the now licensed vaccine containing AS01B) [
27]. In a subsequent phase 3 trial pre-vaccination seropositivity rates for gE are indicated in adult autologous HSCT recipients, but seroconversion rates for baseline negatives are not mentioned [
7]. Still, peak antibody levels one month after the last dose of rHZV were reported which subsequently declined.
We here demonstrate that the interval of IgG level assessment from the last vaccination had a significant impact on the IgG level with a decrease by 10.9% per month increase of the distance. Those patients we report as non-responders (n=4) had longer intervals (median 20.9 months) between their last dose of rHZV and the blood draw for VZV IgG testing than responders (n=29; 9.1 months), suggesting that fast antibody waning could have led to the seronegative results. However, further analysis following correction for interval-length from last vaccination rather points towards non-responsiveness (either intrinsic or extrinsic) and not early/fast antibody waning, as antibody concentrations were equal or even lower than pre-vaccination values in these patients.
Our study further showed that three vaccine doses led to higher antibody levels than two doses in highly immunocompromised patients. However, patient characteristics differed substantially as three doses were applied only to HSCT patients (according to Winston et. al.)[
25]. Practice changed at our outpatient department when more data on the immunogenicity and efficacy of a two-dose schedule for these patients became available [
7,
23]. All other patients received a two-dose schedule. Therefore, we cannot draw conclusions, which schedule may be superior with respect to quantity of antibody responses. Nevertheless, for the optimal management of non-responders to rHZV these patients will require monitoring for symptoms of VZV infection or reactivation to ensure prompt treatment. In addition, it will be crucial to clarify whether they might benefit from additional antiviral prophylaxis and/or an additional rHZV dose.
Importantly, we detected no safety concerns following the off-label use of two or three doses of rHZV in VZV seronegative individuals and therefore confirm previously reported data for SOT patients and patients after autologous HSCT [
5,
7,
23,
25].
Limitation of our study are the retrospective character of the study, the rather small sample size that does not allow for detailed subgroup analysis, and the use of a lysate rather than a gE-specific ELISA. A bias for the selection of included participants may arise from the fact that our clinic is specialized on immunocompromised patients that usually come on referral. Indeed, we used a test that was not optimised for the rHZV vaccine antigen gE, but the at least 5-fold (and up to 300-fold) increase in antibody levels in a lysate ELISA after rHZV indicates a strong humoral response against gE. Since assays to determine the cellular response to rHZV are not available on a routine basis, we were not able to present cellular analyses in our retrospective study. Thus we cannot exclude that our patients that did not reach antibody levels above the cut-off may nevertheless have a cellular response as described in the literature [
28]. Along these lines, data by L’Huillier et al from the SOT patients without a gE-specific seroresponse, demonstrated a significant increase of VZV-specific cellular responses following rHZV [
5].
Our study did not evaluate the clinical effectiveness of rHZV to prevent varicella infection or zoster reactivation in immunosuppressed individuals, but quantified the humoral immune responsiveness in patients with absent VZV-specific humoral immunity. Therefore we cannot predict if vaccination with rHZV in immunocompromised seronegative patients vaccinated with rHZV would benefit from a booster with the live-attenuated VZV vaccine upon re-established immunocompetence. In immunocompromised patients with previous VZV exposure, vaccine responders can be assumed to be protected against VZV reactivation based on data from patients with haematological malignancies and even after autologous HSCT [
7,
23,
25]. However, the long-term protection in the immunocompromised is still unclear and so far data are only available up to 21 months in highly immunocompromised patients [
23].
5. Conclusions
Taken together, our results suggest the use of rHZV in VZV seronegative immunocompromised patients as a safe option to induce immune responsiveness to VZV. The data further suggest antibody testing in high risk groups to identify non- or low-responders, who may require further vaccine doses or prophylactic antiviral treatment.
Further studies on the longevity of the humoral and cellular immune response in addition to the clinical efficacy are urgently needed to provide evidence-based recommendations for patients with contraindication to live-attenuated VZV vaccination. Ideally, data can be generated from patient groups with different underlying diagnoses and immunocompromising treatments.
Author Contributions
Conceptualization, AWa and U.W.; Methodology, MK, AWa; Software, AWe, MK, AWa; Validation, AWe, MK, AWa; Formal Analysis, AWe, MK, AWa; Investigation, AWe, IZ, MP, LW, MK, UW, AWa; Resources, UW; Data Curation, AWe, MK, AWa; Writing—Original Draft Preparation, AWe, MK, UW, AWa; Writing—Review & Editing, AWe, IZ, MP, LW, MK, UW, AWa; Visualization, AWe, MK, AWa; Supervision, MK, UW, AWa; Project Administration, AWe, IZ, MP; Funding Acquisition, n.a.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Institutional Review Board Statement
This study was approved by the ethics committee of the Medical University of Vienna (Ethics Nr.: 1255/2023) and was conducted in accordance with ICH and GCP guidelines and with the applicable local regulatory requirements.
Informed Consent Statement
Not applicable due to the retrospective study design.
Data Availability Statement
Data are made available conditional on ethics approval.
Conflicts of Interest
AWe, IZ, MP and LW declare no conflict of interest. MK has investigator initiated research grants from Pfizer and GSK (outside the current project). UW received funding from GSK, Pfizer and Themis to the Institute (outside the current project). AWa received lecture fees from GSK and has an investigator initiated research grant from GSK (outside the current project).
References
- Feldhoff, C. M., H. H. Balfour, Jr., R. L. Simmons, J. S. Najarian, and S. M. Mauer. "Varicella in Children with Renal Transplants." J Pediatr 98, no. 1 (1981): 25-31. [CrossRef]
- Fehr, T., W. Bossart, C. Wahl, and U. Binswanger. "Disseminated Varicella Infection in Adult Renal Allograft Recipients: Four Cases and a Review of the Literature." Transplantation 73, no. 4 (2002): 608-11. [CrossRef]
- Lynfield, R., J. T. Herrin, and R. H. Rubin. "Varicella in Pediatric Renal Transplant Recipients." Pediatrics 90, no. 2 Pt 1 (1992): 216-20. [CrossRef]
- Cullen, G., R. P. Baden, and A. S. Cheifetz. "Varicella Zoster Virus Infection in Inflammatory Bowel Disease." Inflamm Bowel Dis 18, no. 12 (2012): 2392-403. [CrossRef]
- L’Huillier, A. G., C. Hirzel, V. H. Ferreira, M. Ierullo, T. Ku, N. Selzner, J. Schiff, S. Juvet, C. Miao, D. S. Schmid, A. Humar, and D. Kumar. "Evaluation of Recombinant Herpes Zoster Vaccine for Primary Immunization of Varicella-Seronegative Transplant Recipients." Transplantation 105, no. 10 (2021): 2316-23. [CrossRef]
- López-Fauqued, M., M. Co-van der Mee, A. Bastidas, P. Beukelaers, A. F. Dagnew, J. J. Fernandez Garcia, A. Schuind, and F. Tavares-da-Silva. "Safety Profile of the Adjuvanted Recombinant Zoster Vaccine in Immunocompromised Populations: An Overview of Six Trials." Drug Saf 44, no. 7 (2021): 811-23. [CrossRef]
- Stadtmauer, E. A., K. M. Sullivan, M. El Idrissi, B. Salaun, A. Alonso Alonso, C. Andreadis, V. J. Anttila, A. J. Bloor, R. Broady, C. Cellini, A. Cuneo, A. F. Dagnew, E. Di Paolo, H. Eom, A. P. González-Rodríguez, A. Grigg, A. Guenther, T. C. Heineman, I. Jarque, J. Y. Kwak, A. Lucchesi, L. Oostvogels, M. Polo Zarzuela, A. E. Schuind, T. C. Shea, U. M. Sinisalo, F. Vural, L. Yáñez San Segundo, P. Zachée, and A. Bastidas. "Adjuvanted Recombinant Zoster Vaccine in Adult Autologous Stem Cell Transplant Recipients: Polyfunctional Immune Responses and Lessons for Clinical Practice." Hum Vaccin Immunother 17, no. 11 (2021): 4144-54. [CrossRef]
- Xu, Q., L. He, and Y. Yin. "Risk of Herpes Zoster Associated with Jak Inhibitors in Immune-Mediated Inflammatory Diseases: A Systematic Review and Network Meta-Analysis." Front Pharmacol 14 (2023): 1241954. [CrossRef]
- Schröder, C., D. Enders, T. Schink, and O. Riedel. "Incidence of Herpes Zoster Amongst Adults Varies by Severity of Immunosuppression." J Infect 75, no. 3 (2017): 207-15. [CrossRef]
- Chen, S. Y., J. A. Suaya, Q. Li, C. M. Galindo, D. Misurski, S. Burstin, and M. J. Levin. "Incidence of Herpes Zoster in Patients with Altered Immune Function." Infection 42, no. 2 (2014): 325-34. [CrossRef]
- Schrauder, A., C. Henke-Gendo, K. Seidemann, M. Sasse, G. Cario, A. Moericke, M. Schrappe, A. Heim, and A. Wessel. "Varicella Vaccination in a Child with Acute Lymphoblastic Leukaemia." Lancet 369, no. 9568 (2007): 1232. [CrossRef]
- Bhalla, P., G. N. Forrest, M. Gershon, Y. Zhou, J. Chen, P. LaRussa, S. Steinberg, and A. A. Gershon. "Disseminated, Persistent, and Fatal Infection Due to the Vaccine Strain of Varicella-Zoster Virus in an Adult Following Stem Cell Transplantation." Clin Infect Dis 60, no. 7 (2015): 1068-74. [CrossRef]
- Sartori, A. M. "A Review of the Varicella Vaccine in Immunocompromised Individuals." Int J Infect Dis 8, no. 5 (2004): 259-70. [CrossRef]
- Alexander, K. E., P. L. Tong, K. Macartney, R. Beresford, V. Sheppeard, and M. Gupta. "Live Zoster Vaccination in an Immunocompromised Patient Leading to Death Secondary to Disseminated Varicella Zoster Virus Infection." Vaccine 36, no. 27 (2018): 3890-93. [CrossRef]
- GlaxoSmithKline. "Summary of Product Characteristics (Sopc) Shingrix, Herpes Zoster Vaccine (Recombinant, Adjuvanted)." 2023.
- Federal Ministery Republic of Austria for Social Affairs, Health, Care and Consumer Protection. "Austrian Vaccination Recommendations 2022." 2022.
- Lal, H., A. L. Cunningham, O. Godeaux, R. Chlibek, J. Diez-Domingo, S. J. Hwang, M. J. Levin, J. E. McElhaney, A. Poder, J. Puig-Barberà, T. Vesikari, D. Watanabe, L. Weckx, T. Zahaf, and T. C. Heineman. "Efficacy of an Adjuvanted Herpes Zoster Subunit Vaccine in Older Adults." N Engl J Med 372, no. 22 (2015): 2087-96. [CrossRef]
- Cunningham, A. L., H. Lal, M. Kovac, R. Chlibek, S. J. Hwang, J. Díez-Domingo, O. Godeaux, M. J. Levin, J. E. McElhaney, J. Puig-Barberà, C. Vanden Abeele, T. Vesikari, D. Watanabe, T. Zahaf, A. Ahonen, E. Athan, J. F. Barba-Gomez, L. Campora, F. de Looze, H. J. Downey, W. Ghesquiere, I. Gorfinkel, T. Korhonen, E. Leung, S. A. McNeil, L. Oostvogels, L. Rombo, J. Smetana, L. Weckx, W. Yeo, and T. C. Heineman. "Efficacy of the Herpes Zoster Subunit Vaccine in Adults 70 Years of Age or Older." N Engl J Med 375, no. 11 (2016): 1019-32. [CrossRef]
- Racine, É, V. Gilca, R. Amini, M. Tunis, S. Ismail, and C. Sauvageau. "A Systematic Literature Review of the Recombinant Subunit Herpes Zoster Vaccine Use in Immunocompromised 18-49 year Old Patients." Vaccine 38, no. 40 (2020): 6205-14. [CrossRef]
- Vink, P., I. Delgado Mingorance, C. Maximiano Alonso, B. Rubio-Viqueira, K. H. Jung, J. F. Rodriguez Moreno, E. Grande, D. Marrupe Gonzalez, S. Lowndes, J. Puente, H. Kristeleit, D. Farrugia, S. A. McNeil, L. Campora, E. Di Paolo, M. El Idrissi, O. Godeaux, M. López-Fauqued, B. Salaun, T. C. Heineman, and L. Oostvogels. "Immunogenicity and Safety of the Adjuvanted Recombinant Zoster Vaccine in Patients with Solid Tumors, Vaccinated before or During Chemotherapy: A Randomized Trial." Cancer 125, no. 8 (2019): 1301-12. [CrossRef]
- Vink, P., J. M. Ramon Torrell, A. Sanchez Fructuoso, S. J. Kim, S. I. Kim, J. Zaltzman, F. Ortiz, J. M. Campistol Plana, A. M. Fernandez Rodriguez, H. Rebollo Rodrigo, M. Campins Marti, R. Perez, F. M. González Roncero, D. Kumar, Y. J. Chiang, K. Doucette, L. Pipeleers, M. L. Agüera Morales, M. L. Rodriguez-Ferrero, A. Secchi, S. A. McNeil, L. Campora, E. Di Paolo, M. El Idrissi, M. López-Fauqued, B. Salaun, T. C. Heineman, and L. Oostvogels. "Immunogenicity and Safety of the Adjuvanted Recombinant Zoster Vaccine in Chronically Immunosuppressed Adults Following Renal Transplant: A Phase 3, Randomized Clinical Trial." Clin Infect Dis 70, no. 2 (2020): 181-90. [CrossRef]
- Heineman, T. C., A. Cunningham, and M. Levin. "Understanding the Immunology of Shingrix, a Recombinant Glycoprotein E Adjuvanted Herpes Zoster Vaccine." Curr Opin Immunol 59 (2019): 42-48. [CrossRef]
- Bastidas, A., J. de la Serna, M. El Idrissi, L. Oostvogels, P. Quittet, J. López-Jiménez, F. Vural, D. Pohlreich, T. Zuckerman, N. C. Issa, G. Gaidano, J. J. Lee, S. Abhyankar, C. Solano, J. Perez de Oteyza, M. J. Satlin, S. Schwartz, M. Campins, A. Rocci, C. Vallejo Llamas, D. G. Lee, S. M. Tan, A. M. Johnston, A. Grigg, M. J. Boeckh, L. Campora, M. Lopez-Fauqued, T. C. Heineman, E. A. Stadtmauer, and K. M. Sullivan. "Effect of Recombinant Zoster Vaccine on Incidence of Herpes Zoster after Autologous Stem Cell Transplantation: A Randomized Clinical Trial." Jama 322, no. 2 (2019): 123-33. [CrossRef]
- Dagnew, A. F., O. Ilhan, W. S. Lee, D. Woszczyk, J. Y. Kwak, S. Bowcock, S. K. Sohn, G. Rodriguez Macías, T. J. Chiou, D. Quiel, M. Aoun, M. B. Navarro Matilla, J. de la Serna, S. Milliken, J. Murphy, S. A. McNeil, B. Salaun, E. Di Paolo, L. Campora, M. López-Fauqued, M. El Idrissi, A. Schuind, T. C. Heineman, P. Van den Steen, and L. Oostvogels. "Immunogenicity and Safety of the Adjuvanted Recombinant Zoster Vaccine in Adults with Haematological Malignancies: A Phase 3, Randomised, Clinical Trial and Post-Hoc Efficacy Analysis." Lancet Infect Dis 19, no. 9 (2019): 988-1000. [CrossRef]
- Winston, D. J., K. M. Mullane, O. A. Cornely, M. J. Boeckh, J. W. Brown, S. A. Pergam, I. Trociukas, P. Žák, M. D. Craig, G. A. Papanicolaou, J. D. Velez, J. Panse, K. Hurtado, D. A. Fernsler, J. E. Stek, L. Pang, S. C. Su, Y. Zhao, I. S. F. Chan, S. S. Kaplan, J. Parrino, I. Lee, Z. Popmihajlov, P. W. Annunziato, and A. Arvin. "Inactivated Varicella Zoster Vaccine in Autologous Haemopoietic Stem-Cell Transplant Recipients: An International, Multicentre, Randomised, Double-Blind, Placebo-Controlled Trial." Lancet 391, no. 10135 (2018): 2116-27. [CrossRef]
- Feyssaguet, M., V. Berthold, L. Helle, M. Povey, S. Ravault, S. Carryn, P. Gillard, and E. Di Paolo. "Comparison of a Glycoprotein E-Based Elisa with a Varicella-Zoster Whole-Virus Elisa for the Quantification of Varicella Vaccine Immune Responses in Young Children." Vaccine 38, no. 17 (2020): 3300-04. [CrossRef]
- Stadtmauer, E. A., K. M. Sullivan, F. M. Marty, S. S. Dadwal, G. A. Papanicolaou, T. C. Shea, S. B. Mossad, C. Andreadis, J. A. Young, F. K. Buadi, M. El Idrissi, T. C. Heineman, and E. M. Berkowitz. "A Phase 1/2 Study of an Adjuvanted Varicella-Zoster Virus Subunit Vaccine in Autologous Hematopoietic Cell Transplant Recipients." Blood 124, no. 19 (2014): 2921-9. [CrossRef]
- Katial, R. K., S. Ratto-Kim, K. V. Sitz, R. Moriarity, and R. J. M. Engler. "Varicella Immunity: Persistent Serologic Non-Response to Immunization." Annals of Allergy Asthma & Immunology 82, no. 5 (1999): 431-34. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).