1. Introduction
Electrochemical oxidation has emerged as a versatile and effective advanced oxidation process (AOP) for water and wastewater treatment [
1]. It is widely applied in disinfection and the degradation of persistent organic pollutants in various water matrices, including drinking water, swimming pools, and increasingly, municipal and industrial wastewater. This process relies on functional electrodes to generate highly reactive species such as hydroxyl radicals (•OH), hydrogen peroxide (H₂O₂), hypochlorous acid (HOCl), and hypochlorite (OCl⁻), which can directly or indirectly degrade a wide range of contaminants [
2,
3,
4].
However, when chloride ions (Cl⁻) are present—even at low concentrations—electrochemical oxidation may lead to the formation of undesirable disinfection by-products (DBPs), including chlorite (ClO₂⁻), chlorate (ClO₃⁻), and perchlorate (ClO₄⁻). These oxychlorine species are of particular concern due to their toxicity. Chlorate has been reported to cause oxidative damage to red blood cells and exhibit mutagenic properties in both bacterial and mammalian cells [
5,
6]. Perchlorate interferes with iodide uptake in the thyroid gland, potentially disrupting hormone synthesis and leading to adverse effects on the endocrine system [
7].
The formation pathway of chlorate and perchlorate during electrolysis involves a sequential oxidation process starting from chloride:
This multi-step transformation is strongly influenced by operational parameters such as current density, electrolyte composition, and most critically, the electrode material. For example, studies have shown that boron-doped diamond (BDD) and RuO₂ electrodes promote the formation of chlorate and perchlorate, whereas TiO₂ and IrO₂ anodes result in much lower levels [
2,
4,
5,
6,
7].
To address these challenges, a novel electrochemical platform known as ADEPT (Advanced Electrochemical Process Technology) has been developed. ADEPT utilizes specialized functional electrodes capable of generating reactive oxidants at high efficiency even under low chloride conditions. This makes it particularly suitable for decentralized water treatment systems where chloride levels are typically much lower than in seawater or industrial effluents. Despite the growing interest in ADEPT and similar systems, studies on the formation of DBPs under low-chloride scenarios remain limited, especially in the presence of organic solutes.
Organic matter can significantly influence DBP formation by acting as radical scavengers, intermediate stabilizers, or even precursors for secondary reactions. In this study, three representative organics—phenol (a model aromatic compound), acetate (a common low-molecular-weight organic acid), and dissolved organic carbon (DOC) (representing natural organic matter)—were selected to evaluate their effects on chlorine speciation and by-product formation during electrolysis.
In natural and engineered water systems, the presence of organic matter is nearly unavoidable [
8,
9,
10,
11,
12,
13,
14]. Organic solutes—including naturally occurring compounds such as humic substances, as well as anthropogenic pollutants like phenols and carboxylic acids [
15,
16]—can significantly influence the electrochemical oxidation process. These compounds may act as scavengers of reactive chlorine species (e.g., HOCl, Cl•), compete for oxidation with chloride ions, or form intermediate products that alter the pathway and rate of oxychlorine formation. In some cases, organic matter can suppress chlorate and perchlorate production, while in others it may enhance the formation of toxic by-products through secondary reactions.
Despite the recognized importance of organic matter in water treatment, few studies have systematically evaluated its role in the formation of chlorinated by-products under low-chloride electrolysis conditions [
17,
18,
19,
20,
21,
22,
23,
24,
25,
26,
27,
28,
29,
30,
31,
32,
33,
34,
35,
36,
37,
38,
39,
40,
41]. Understanding this interaction is essential for predicting DBP formation and optimizing operational conditions in real-world applications.
This study aims to investigate the formation of active chlorine species, chlorate, and perchlorate during electrolysis using ADEPT technology under low chloride concentrations. Special emphasis is placed on understanding how different types of organic solutes affect the production and transformation of these by-products. The findings are expected to support the development of safer and more efficient electrochemical water treatment systems by minimizing the formation of harmful oxychlorine species.
2. Materials and Methods
2.1. Preparing Chemicals and Samples
Sodium chloride (NaCl) is purchased from sigma-aldrich using as electrolytes and for stock standard of chloride (Cl-). Stock solutions (1000mg/l) of ClO2-, ClO3- and ClO4- were prepared for calibration using grade chemicals as sodium chlorite (NaClO2) and sodium chlorate (NaClO3) and sodium perchlorate (NaClO4) which purchased from Sigma- Aldrich. Postasium permanganate (KMnO4) was used as alternative standard for stock solution 1000mg/l of active chlorine and the agent solution DPD (N,N-Diethyl-P-Phenylenediamine,C10H10N2) were purchased from Sigma–Aldrich. The organic compounds as phenol, sodium acetate (CH3COONa.3H2O), glucose(C6H12O6.H2O) were purity chemicals using as effective factors on formation of active chlorine, chlorate and perchlorate which purchased from Sigma-Aldrich, J.T. Baker, Merck respectively. Deionized water with resistance < 18µƱ was using for preparing sample and chemicals.
Samples are prepared in 2litter for each experiment which using deionized water contain 50mg/l of Chloride and different concentration of effective factors. Concentration of effective factor as Phenol, CH
3COO
- are prepared in order are 5mg/l, 10mg/l, 50mg/l and 100mg/l. DOC solution is made by mixture from many compounds that solution properties nearly same natural condition of Copenhagen. They were prepared in Plant and Environment chemistry of Copenhagen University and the compounds species is show in
Table 1. DOC solution is filtered by 0.45µm cellulose filter beforeusing.The solution as electrolytes includes 50mg/l of Chloride and with change amount of DOC in range 0.1mg/l, 1.0mg/l, 5mg/l, 10mg/l, 25mg/l and 50mg/l. Solutions is neutrated at right pH before electrolysis. Flow rate is remained in constant 0.5l/min and current is change from 0.5 to 2.5 A.
2.2. Electrochemical Equipment and Procedure
Electrochemical system is shown in
Figure 1. The undivided reactor cell and electrodes are produced by Adept water Technology. The current is supplied by Voltage adjustable supplier (PS3020). Samples are pumped into electrolysis cell by Master Flex bump (American) at constant rate 500 ml/min. The experiments is performed continuously by pumping the sample from 2litter solution container in to cell and the current is set up at 0,5A, 1,0 A, 1,5 A, 2,0A and 2,5A during sample electrolysis. All of the experiments were performed using deionzied water. The inlet concentration of chlorour species is analyzed before electrolysis sample solution. At each current, the outlet electrolyzed solution is taken for measuring active chlorine and chlorate and perchlorate.
2.3. Analysis of the Sample Solutions
The concentration of active chlorine was measured using DPD (N,N-Diethyl-P-Phenylenediamine, C
10H
10N
2) method with UV/VIS Spectrometer Lambda 25 (Perkin Elmer) at wavelength 515nm. ClO
3-, ClO
4- and Cl
- were analyzed using Ion chromatography (Metrohm) with Column Metrosep A Supp 5, 100x4mm 61006.510 and Detector IC 819. The injection volume was 20µl and the eluent was 3.2mM Na
2CO
3 and 1,0mM NaHCO
3 pumped at a flow rate of 0.7ml min
-1[
8]. The H
2SO
4 2M was used as suppressor solution. Samples is filtered by membrance 0.45µm before measuring the ions by ion chromatography. The initial DOC concentration is determinated by 254 TOC meter of Shimazu and calculated as TOC concentration.
2.4. The Formation of Active Chlorite, Chlorate and Perchlorate and Effect of Initial Chloride Concentration
The formation ways of active chlorine, chlorite, chlorate and perchlorate results from electrochemical and chemicals reactions in electrolysis system that shown equations from (1) to (14)[
10]. The results of experiments on adept electrochemical technology shown concentration of active chlorine(HOCl, OCl
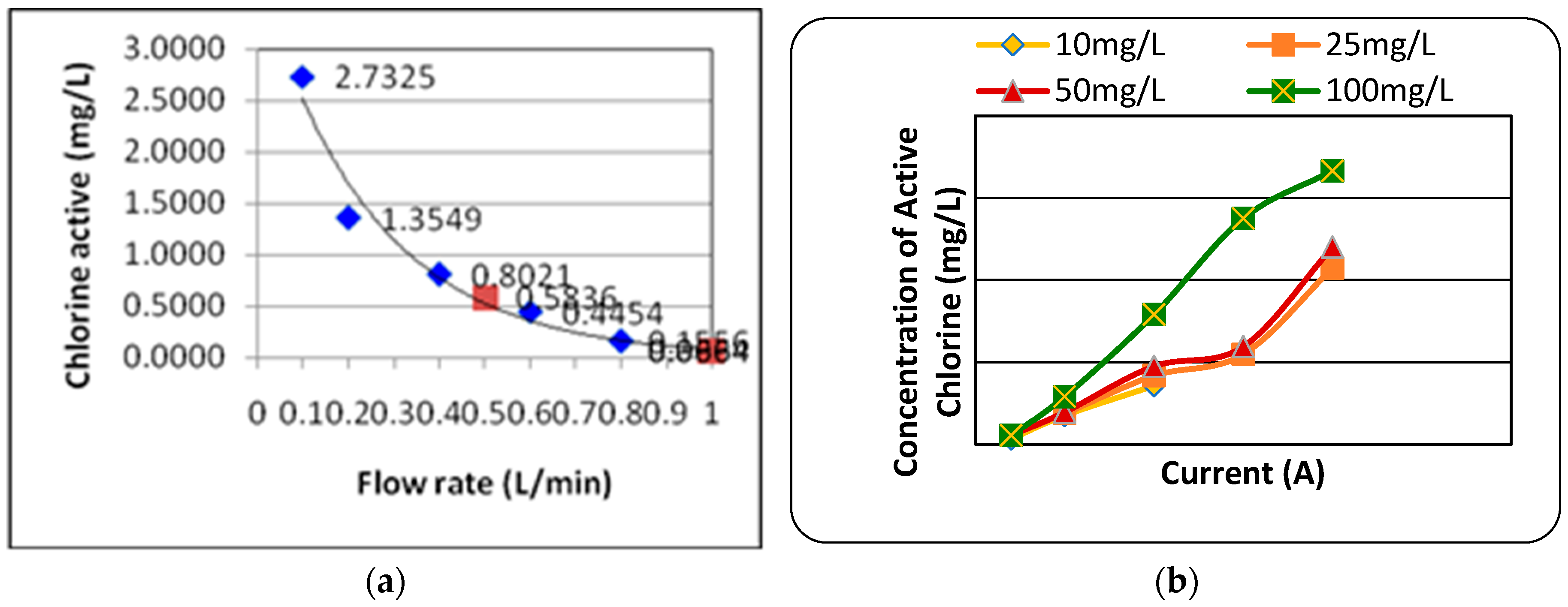
-) produced is depended on solution flow rate and electric current and initial concentration of chloride. In
Figure 2a, 2b, concentration of active chlorine was produced at rate flow of 0.1litter/min was highest 2.7325mg/l. Concentration of active chlorine decrease when flow rate increase until 1.0 litter/mins, lowest amount of active chlorine is 0.0664mg/l at 1.0 litter/mins rate. In
Figure 2b,the initial concentration of chloride and current effects the formation of active chlorine and by-products. Initial concentration of chloride increase, the formed active chlorine increase and the current increase, active chlorine also increase. In this experiments, chlorate is found in electrolyzed solution by ion chromatography but Chlorite and perchlorate is not found in electrolysis.
2.5. Effect of organic compounds as Phenol, Acetate and DOC solution
1. Effect of phenol
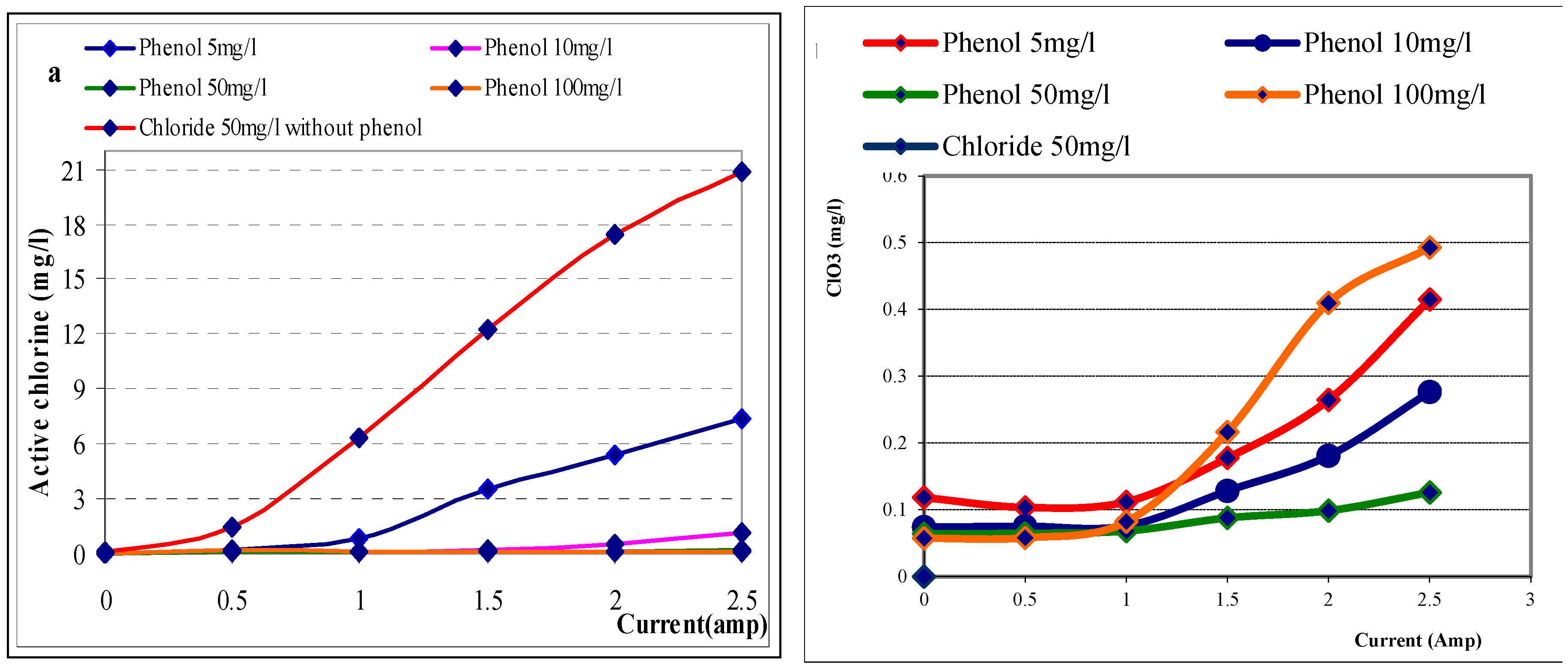
The electrochemical oxidation of phenol in presence of sodium chloride as electrolyte is investigated in many literatures recently. Phenol could been degradation by direction and indirection oxidation by electrochemical. In presence of chloride as electrolyte, the small fraction of phenol was oxidized by direct electrolysis, while complete degradation of phenol was achieved by indirect electrochemical oxidation. Many investigators have noted the formation of polymeric film is produced on anode surface in during direct oxidation (11,12,13,14) that cause electrochemical degradation rate of phenol is slow. The indirect electrochemical oxidation, phenol is oxidized by active chlorine, hypochlorous, hypochlorite ion and OH* when using chloride as electrolyte(14,15). In this working, the formation of active chlorine, chlorate, perchlorate in electrolysis are investigated in simultaneity presence of chloride (50mg/l) and phenol. In
Figure 3a, the formation of active chlorine is effected by concentration of phenol and electrolytic current. The formation of active chlorine decrease when phenol concentration increase at each current value. As using smallest phenol concentration of 5 mg/l, active chlorine at current of 0.5A, 1.0A, 1.5A, 2.0A, 2.5A was formed 0.098mg/l, 0,25mg/l, 0,48mg/l, 1,18 mg/l respectively. The active chlorine amount produced were increasedwhen using small phenol concentration that decrease from 10mg/l, 50mg/l and 100 mg/l, and active chlorine was decrease compare with samples without phenol. The results can be considered that the formatted active chlorine decrease when increase phenol amount from 5mg/l to 100 mg/l. The cause of active chlorine reduction is oxidation of phenol by special chlorous as shown in previous literatures. In
Figure 3b show that, the chlorate was found in most experiments when used current increase from 1.5 A to 2.5 A. Chlorate was increased when current increase but the effective of phenol on chlorate formation is not clearly.
2. Effect of Acetate
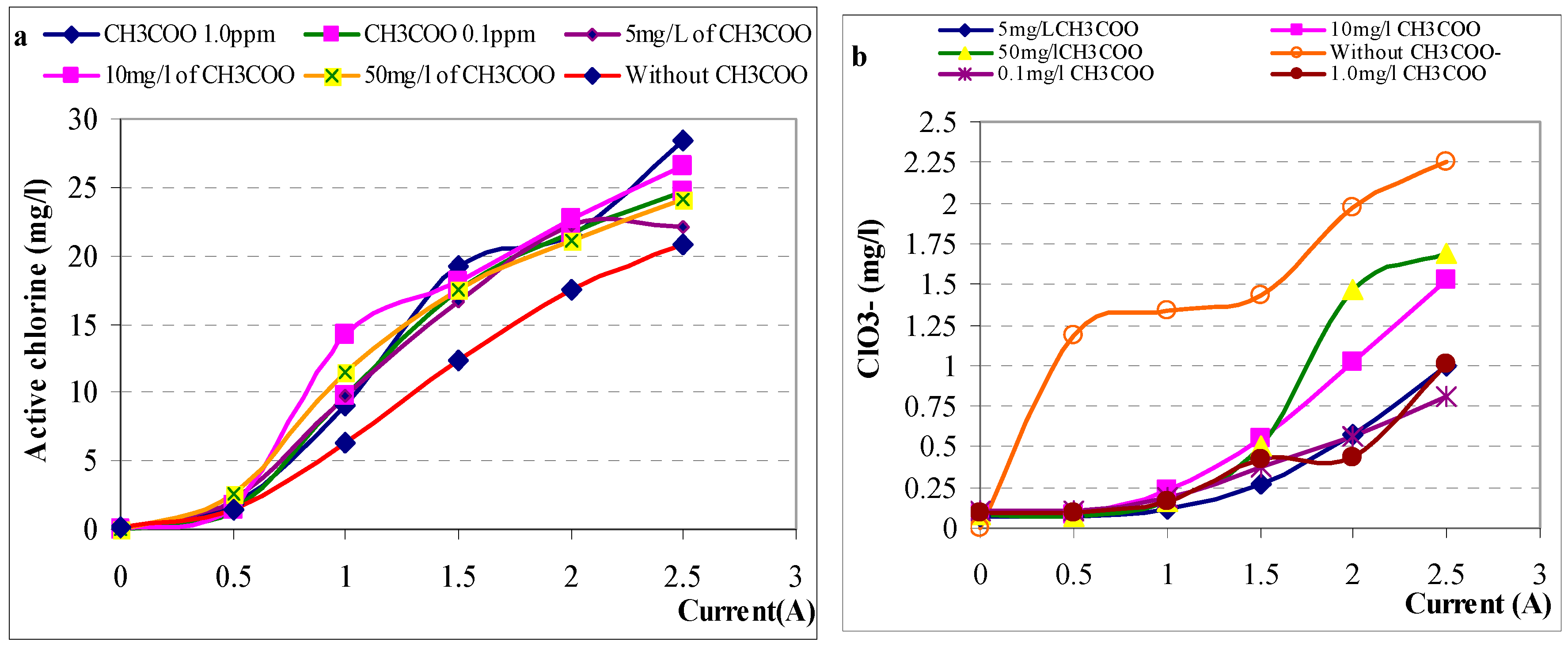
At both high and low concentration, acetate is not effect on formation of active chlorine in electrolysis samples. The concentration of active chlorine produced at different acetate concentration were nearly equivalent when current not change. The contrary of acetate effective, the active chlorine formation was depended on current as results shown in
Figure 4a. When current increase, concentration of active chlorine was produced increase in during electrolysis. The active chlorine concentration produced in sodium salt electrolysis contain acetate is higher than its amount in experiments without acetate. In
Figure 4b, chlorate was produced in presence of acetate as current increase from 1.0A to 2.5A and it was not detected at small current under 1A. The concentration of chlorate was lower compare to electrolysis sample without acetate.
3. Effect of DOC Solution
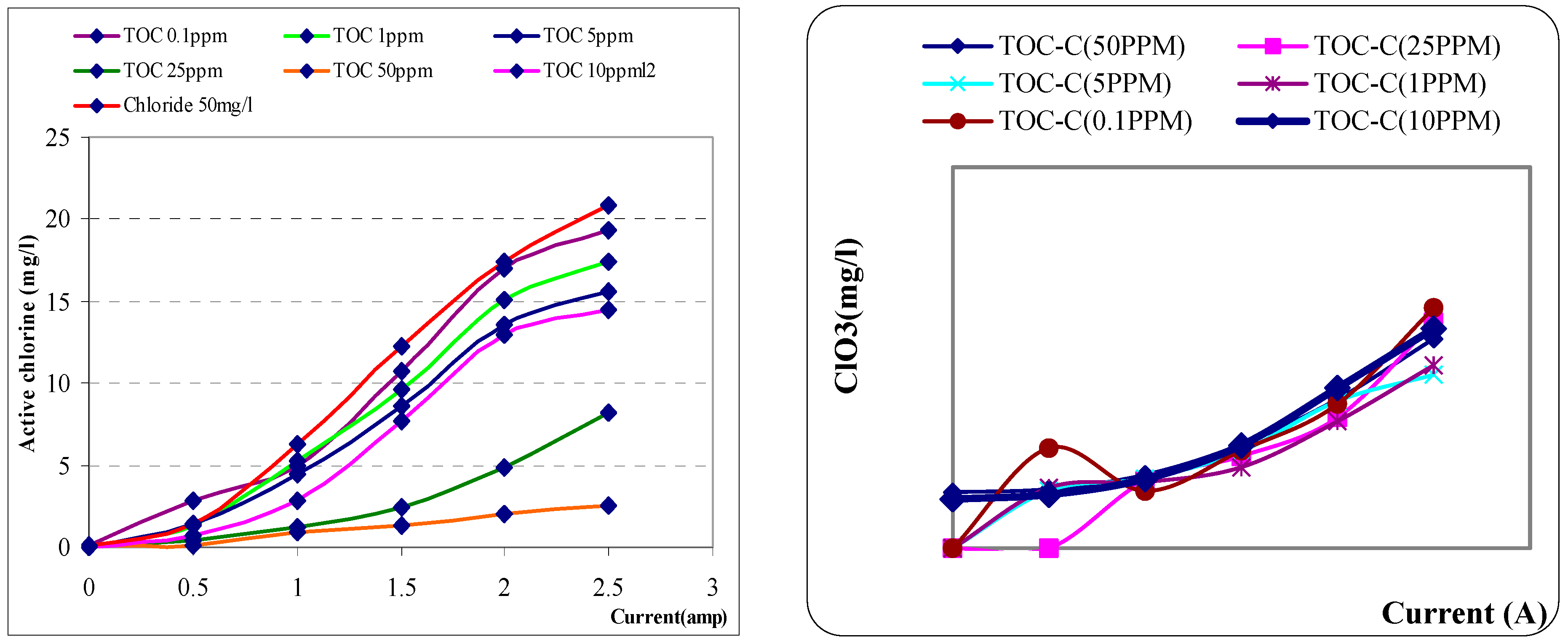
The results shown that initial concentration of TOC is strong effective in produce active chlorine in electrolysis sodium chloride salt. Formation of active chlorine is decrease when increase the concentration of DOC. Results are also shown that concentration of TOC is seemly constant in during electrolysis when current increase as below
Figure 5a. The formation of chlorate is also investigated in the experiments. The results shown that the chlorate formation is not effected by concentration of initial TOC amount. The produced chlorate concentration is seem similarly in each different current and initial TOC concentration. And highest level of chlorate is around 0.5mg/l at current 2.5 A at different TOC concentration as below
Figure 5b.
3. Conclusions
- With adept electrochemical technology equipment, the electrolysis process using small chloride concentration of 50mg/l at current 2,5 A can produce active chlorine and chlorate. The concentration of active chorine formed are depended on initial chloride concentration, flow rate and apply current.
- As electrolytes have presence of phenol, the results can be considered that the formatted active chlorine decrease when increase phenol amount from 5mg/l to 100 mg/l. The chlorate was found in most experiments when used current increase from 1.5 A to 2.5A. Chlorate was increased when current increase but the effective of phenol on chlorate formation is not clearly.
- At both high and low concentration, acetate is not effect on formation of active chlorine in electrolysis samples.The active chlorine concentration produced in sodium salt electrolysis contain acetate is higher than its amount in experiments without acetate. Chlorate was produced in presence of acetate as current increase from 1.0A to 2.5A and it was not detected at small current under 1A. The concentration of chlorate was lower compare to electrolysis sample without acetate.
- The results shown that initial concentration of TOC is strong effective in produce active chlorine in electrolysis sodium chloride salt. Formation of active chlorine is decrease when increase the concentration of DOC. The chlorate formation is not effected by concentration of initial TOC amount. The produced chlorate concentration is seem similarly in each different current and initial TOC concentration. And highest level of chlorate is around 0.5mg/l at current 2.5 A.
References
- Le, T. G. , Nguyen, N. T., Nguyen, Q. T., Laat, J. D., & Dao, H. Y. (2014). Effect of Chloride and Sulfate Ions on the Photoreduction Rate of Ferric Ion in UV Reactor Equipped with a Low Pressure Mercury Lamp. Journal of Advanced Oxidation Technologies, 17(2). [CrossRef]
- Czarnetzki, L. R. , & Janssen, L. J. J. (1992). Formation of hypochlorite, chlorate and oxygen during NaCl electrolysis from alkaline solutions at an RuO2/TiO2 anode. Journal of Applied Electrochemistry, 22(4), 315–324. [CrossRef]
- Bergmann, M. E. H. , & Koparal, A. S. ( 35(12), 1321–1329. [CrossRef]
- Bergmann, M. E. H. , & Rollin, J. (2007). Product and by-product formation in laboratory studies on disinfection electrolysis of water using boron-doped diamond anodes. Catalysis Today, 124(3–4), 198–203. [CrossRef]
- Bergmann, M. E. H. , Rollin, J. ( 54(7), 2102–2107. [CrossRef]
- Jung, Y. J. , Baek, K. W., Oh, B. S., & Kang, J.-W. (2010). An investigation of the formation of chlorate and perchlorate during electrolysis using Pt/Ti electrodes: The effects of pH and reactive oxygen species and the results of kinetic studies. Water Research, 44(18), 5345–5355. [CrossRef]
- Neodo, S. , Rosestolato, D., Ferro, S., & De Battisti, A. (2012). On the electrolysis of dilute chloride solutions: Influence of the electrode material on Faradaic efficiency for active chlorine, chlorate and perchlorate. Electrochimica Acta, 80, 282–291. [CrossRef]
- Qin, X. , He, Y. ( 266, 122387. [CrossRef]
- Duong, T. T. , Nguyen, T. M. ( 284, 131242. [CrossRef]
- Nu Nguyen, H. M. , Khieu, H. M. ( 285, 117260. [CrossRef]
- Le, T. M. , Pham, P. M. ( 29(31), 46767–46777. [CrossRef]
- Trinh, H. T. , Marcussen, H. W. ( 24(8), 7348–7358. [CrossRef]
- Truong, A. H. , Kim, M. T., Nguyen, T. T., Nguyen, N. T., & Nguyen, Q. T. (2018). Methane, Nitrous Oxide and Ammonia Emissions from Livestock Farming in the Red River Delta, Vietnam: An Inventory and Projection for 2000–2030. Sustainability, 10(10), 3826. [CrossRef]
- Hoang, M. T. T. , Le, G. T. ( 328, 138597. [CrossRef]
- D.T. Hanh, K. D.T. Hanh, K. Kadomami, N. Matsuura, N.Q. Trung, Screening analysis of a thousand micro-pollutants in vietnamese rivers, In Proceedings of the 10th International Symposium on Southeast Asian Water Environment (2012), Hanoi, Vietnam, 8.-10. November, 2012.
- Truong, D. A. , Trinh, H. V. ( 331, 138805. [CrossRef]
- Vu-Duc, N. , Nguyen-Quang, T., Le-Minh, T., Nguyen-Thi, X., Tran, T. M., Vu, H. A., Nguyen, L.-A., Doan-Duy, T., Van Hoi, B., Vu, C.-T., Le-Van, D., Phung-Thi, L.-A., Vu-Thi, H.-A., & Chu, D. B. (2019). Multiresidue Pesticides Analysis of Vegetables in Vietnam by Ultrahigh-Performance Liquid Chromatography in Combination with High-Resolution Mass Spectrometry (UPLC-Orbitrap MS). Journal of Analytical Methods in Chemistry, 2019, 1–12. [CrossRef]
- Hai, Y. D. , Tran-Lam, T.-T., Nguyen, T. Q., Vu, N. D., Ma, K. H., & Le, G. T. (2019). Acrylamide in daily food in the metropolitan area of Hanoi, Vietnam. Food Additives & Contaminants: Part B, 12(3), 159–166. [CrossRef]
- Hanh, T. T. H. , Anh, D. H., Huong, P. T. T., Thanh, N. V., Trung, N. Q., Cuong, T. V., Mai, N. T., Cuong, N. T., Cuong, N. X., Nam, N. H., & Minh, C. V. (2018). Crinane, augustamine, and β -carboline alkaloids from Crinum latifolium. Phytochemistry Letters, 24, 27–30. [CrossRef]
- Markus Amann, Zbigniew Klimont, T An Ha, Peter Rafaj, Gregor Kiesewetter, Adriana Gomez Sanabria, Binh Nguyen, TN Thi Thu, Kimminh Thuy, Wolfgang Schöpp, Jens Borken-Kleefeld, L Höglund-Isaksson, Fabian Wagner, Robert Sander, Chris Heyes, Janusz Cofala, Nguyen Quang Trung, Nguyen Tien Dat, Nguyen Ngoc Tung, Future Air Quality in Ha Noi and Northern Vietnam, http://pure.iiasa.ac.at/15803 (2019).
- Quang, T. H. , Phong, N. V., Anh, L. N., Hanh, T. T. H., Cuong, N. X., Ngan, N. T. T., Trung, N. Q., Nam, N. H., & Minh, C. V. (2020). Secondary metabolites from a peanut-associated fungus Aspergillus niger IMBC-NMTP01 with cytotoxic, anti-inflammatory, and antimicrobial activities. Natural Product Research, 36(5), 1215–1223. [CrossRef]
- Anh, B. T. K. , Minh, N. T. ( 100(5), 720–726. [CrossRef]
- Dang, T. T. , Vo, T. ( 184, 114140. [CrossRef]
- Hanh, T. T. H. , Hang, L. T. X. ( 43, 35–39. [CrossRef]
- Nguyen, T. N. , Trinh, H. T., Sam, L. H., Nguyen, T. Q., & Le, G. T. (2019). Halogen-free flame-retardant flexible polyurethane for textile coating: Preparation and characterisation. Fire and Materials, 44(2), 269–282. [CrossRef]
- Le, V. N. , Nguyen, Q. G. ( 71(3), 323–331. [CrossRef]
- Nguyen, Q.-T. , Le, T.-G., Bergonzo, P., & Tran, Q.-T. (2022). One-Step Fabrication of Nickel-Electrochemically Reduced Graphene Oxide Nanocomposites Modified Electrodes and Application to the Detection of Sunset Yellow in Drinks. Applied Sciences, 12(5), 2614. [CrossRef]
- Janda, T. , Lejmel, M. A., Molnár, A. B., Majláth, I., Pál, M., Nguyen, Q. T., Nguyen, N. T., Le, V. N., & Szalai, G. (2020). Interaction between elevated temperature and different types of Na-salicylate treatment in Brachypodium dystachion. PLOS ONE, 15(1), e0227608. [CrossRef]
- Nguyen, T. P. L. , Nguyen, V. T. ( 2020, 1–9. [CrossRef]
- Hoang, A. Q. , Trinh, H. T., Nguyen, H. M. N., Nguyen, T. Q., Nguyen, T. X., Duc, T. V., Nguyen, T. T., Do, T. Q., Minh, T. B., & Tran, T. M. (2022). Assessment of cyclic volatile methyl siloxanes (CVMSs) in indoor dust from different micro-environments in northern and central Vietnam. Environmental Geochemistry and Health, 45(5), 1711–1722. [CrossRef]
- Tran, T. V. , Vo, D.-V. N., Nguyen, D. T. C., Ching, Y. C., Nguyen, N. T., & Nguyen, Q. T. (2022). Effective mitigation of single-component and mixed textile dyes from aqueous media using recyclable graphene-based nanocomposite. Environmental Science and Pollution Research, 29(21), 32120–32141. [CrossRef]
- Le, L. H. T. , Tran-Lam, T.-T., Nguyen, H. Q., Quan, T. C., Nguyen, T. Q., Nguyen, D. T., & Dao, Y. H. (2021). A study on multi-mycotoxin contamination of commercial cashew nuts in Vietnam. Journal of Food Composition and Analysis, 102, 104066. [CrossRef]
- Bui, T. K. A. , Dang, D. C. ( 6(1), 47–51. [CrossRef]
- Nguyen-Quang, T. , Bui-Quang, M., & Truong-Ngoc, M. (2021). Rapid Identification of Geographical Origin of Commercial Soybean Marketed in Vietnam by ICP-MS. Journal of Analytical Methods in Chemistry, 2021, 1–9. [CrossRef]
- Van, Pc. P. , Ngo Van, H., Quang, M. B., Duong Thanh, N., Nguyen Van, D., Thanh, T. D., Tran Minh, N., Thi Thu, H. N., Quang, T. N., Thao Do, T., Thanh, L. P., Do Thi Thu, H., & Le Tuan, A. H. (2023). Stigmastane-type steroid saponins from the leaves of Vernonia amygdalina and their α -glucosidase and xanthine oxidase inhibitory activities. Natural Product Research, 38(4), 601–606. [CrossRef]
- Bui, M. Q. , Quan, T. C., Nguyen, Q. T., Tran-Lam, T.-T., & Dao, Y. H. (2022). Geographical origin traceability of Sengcu rice using elemental markers and multivariate analysis. Food Additives & Contaminants: Part B, 15(3), 177–190. [CrossRef]
- DARKÓ, É. , KHALIL, R. ( 57(4), 1035–1043. [CrossRef]
- Minh, T. N. , Minh, B. Q., Duc, T. H. M., Thinh, P. V., Anh, L. V., Dat, N. T., Nhan, L. V., & Trung, N. Q. (2022). Potential Use of Moringa oleifera Twigs Extracts as an Anti-Hyperuricemic and Anti-Microbial Source. Processes, 10(3), 563. [CrossRef]
- Thang, P. Q. , Muto, Y. ( 216, 400–407. [CrossRef]
- Nguyen, H. X. , Nguyen, X. Q. ( 29(8), 1788. [CrossRef]
- Hanh, T. T. H. , Anh, L. N. ( 45, 190–194. [CrossRef]
- Pérez, G. , Ibáñez, R. ( 197, 475–482. [CrossRef]
- Michalski, R. , Mathews B. (2007). Occurrence of Chlorite, Chlorate and Bromate in Disinfected Swimming Pool Water, Polish J. of Environ. Stud. Vol. 16, No. 2.
- Oturan, M. A. , & Brillas, E. ( 25(1), 1–18. [CrossRef]
- Polcaro, A. M. , Vacca, A., Mascia, M., & Ferrara, F. (2008). Product and by-product formation in electrolysis of dilute chloride solutions. Journal of Applied Electrochemistry, 38(7), 979–984. [CrossRef]
- de Souza, R. B. A. , & Ruotolo, L. A. M. (2013). Phenol Electrooxidation in Different Supporting Electrolytes Using Boron-Doped Diamond Anodes. International Journal of Electrochemical Science, 8(1), 643–657. [CrossRef]
- Li, X. , Cui, Y. ( 39(10), 1972–1981. [CrossRef] [PubMed]
- Yun, Jang-Hui, Shim, Yoon-Bo, Lee, Byeong-Seop, Choe, Se-Yong, & Won, Mi-Suk. (2012). Electrochemical Degradation of Phenol and 2-Chlorophenol Using Pt/Ti and Boron-Doped Diamond Electrodes. Bulletin of the Korean Chemical Society, 33(7), 2274–2278. [CrossRef]
- Rajkumar, D. , Guk Kim, J., & Palanivelu, K. (2005). Indirect Electrochemical Oxidation of Phenol in the Presence of Chloride for Wastewater Treatment. Chemical Engineering & Technology, 28(1), 98–105. [CrossRef]
- Zhang, F. , Li, M. ( 175, 349–355. [CrossRef]
- Scialdone, O. , Randazzo, S., Galia, A., & Silvestri, G. (2009). Electrochemical oxidation of organics in water: Role of operative parameters in the absence and in the presence of NaCl. Water Research, 43(8), 2260–2272. [CrossRef]
- Huang, Y.-H. , Shih, Y.-J., & Liu, C.-H. (2011). Oxalic acid mineralization by electrochemical oxidation processes. Journal of Hazardous Materials, 188(1–3), 188–192. [CrossRef]
- Greiner, P. , McLellan, C. ( 100(11), 68–74. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).