1. Introduction
The management of anesthesia during ERCP procedures in patients with Billroth II gastrectomy involves several challenges due to anatomical changes and the shared airway between the endoscopist and the anesthesiologist during the procedure.ERCP procedures can be performed under monitored anesthesia care with conscious sedation, deep sedation, or general anesthesia with endotracheal intubation [
1,
2]. However, ambulatory procedural sedation protocols are generally applied due to reasons such as patient admission costs and rapid patient recovery. In this rare but high-anesthesia-risk patient group, adequate sedation is necessary for the successful completion of the procedure. The depth of sedation in ERCP patients with Billroth II gastrectomy must be balanced between preventing pulmonary complications and ensuring procedural success.
Gastroparesis, hypomotility, remnant gastritis, and reflux esophagitis may occur after Billroth II surgery. In these patients, gastric pH increases due to reflux of duodenal contents into the stomach. Studies have shown that widespread gastritis is observed in the remaining stomach after Billroth II [
3]. In a study by Christodoulidis et al., bile reflux and alkaline gastritis were found in 54% of 74 patients who underwent Billroth II gastrectomy [
4]. Pulmonary aspiration is defined as the presence of bile secretions or particulate matter in the tracheobronchial tree. It most commonly results from passive regurgitation of gastric contents. In patients who have undergone Billroth II surgery and are sedated for ERCP, aspiration of gastric contents can lead to pneumonia due to decreased airway reflexes. In the literature, the frequency of aspiration during sedation has been reported to range between 0.01% and 1% [
5,
6]. Aspiration pneumonia is a significant cause of mortality.
During Billroth II surgery, Braun enteroenterostomy is an anastomosis performed between the afferent and efferent loops of the jejunum. Thanks to this procedure, gastritis, esophagitis, Barrett's esophagus, and cancer formation can be prevented. If a Braun anastomosis was performed during Billroth II surgery, the risk of reflux and pulmonary aspiration may be lower [
4].There are many studies in the literature on anesthesia methods in ERCP procedures, but studies on sedation methods used for ERCP in patients who have undergone Billroth II surgery are limited. Our primary aim is to compare patients who underwent monitored anesthesia care with conscious sedation (Group CS) to those who underwent deep sedation (Group DS) among these selected patients, during procedures performed by the same experienced (with more than ten years of experience and over a thousand cases per year) endoscopist and anesthesia specialist, in terms of airway complications including pulmonary aspiration. Our secondary objectives are to investigate procedural success, intensive care unit (ICU) need, hospital stay duration, and mortality during hospitalization.
2. Materials and Methods
2.1. Ethical Approval
Ethics committee approval number E1-23-4134 was obtained from the Ethics Committee of the Ankara City Hospital for this study.
2.2.Study Design and Setting
Between January 2020 and September 2023, patients who underwent ERCP procedures with conscious sedation and deep sedation in our hospital were retrospectively examined. Procedures performed by the same endoscopist and anesthesiologist within the specified period were included in the study. Procedures performed by different endoscopists, procedures in which the depth of sedation had to be changed during the same procedure, and procedures with incomplete records were excluded from the study. Some patients underwent the same procedure at different times with either conscious sedation or deep sedation; therefore, instead of the number of patients, the number of procedures was taken into account, and they were divided into Group CS and Group DS. The patients' demographic data, comorbid conditions, and American Society of Anesthesiologists (ASA) scores were recorded.
All patients underwent routine anesthesia monitoring with pulse oximetry, electrocardiogram, and non-invasive blood pressure measurements at 2-minute intervals. In Group CS, patients were administered midazolam (2 to 10 mg i.v.) and fentanyl (0.05 to 0.1 mg i.v.) titrated according to their age, accompanying diseases, and tolerance. In this group, the bispectral index (BIS) values of the patients were maintained in the range of 71–90.
In Group DS, after a bolus dose of propofol 0.5–1.0 mg/kg, the infusion dose was adjusted to 100–150 mcg/kg/min to maintain BIS values in the range of 61–70. All patients were provided with oxygen support via nasal cannula (4 L/min). Apnea monitoring was performed with a capnograph. SpO2 below 90% for a duration of ten seconds or a decrease in oxygen saturation of more than 5% from the baseline value during anesthesia was defined as desaturation. Patients who experienced apnea or desaturation were given a jaw thrust maneuver to secure the airway. During the procedure, patients who required intubation were recorded. A heart rate dropping below 50 beats/min was recorded as bradycardia, a heart rate exceeding 110 beats/min as tachycardia, a systolic blood pressure dropping below 90 mmHg or less than 85% of the baseline value as hypotension, and more than 20% above the baseline value as hypertension. Patients who were administered a vasopressor (5 mg ephedrine) due to hypotension during anesthesia were recorded. In the Billroth II surgery performed, those who had and had not undergone Braun anastomosis were recorded. Patient and endoscopist satisfaction were evaluated with "yes" and "no" answers to the questions. In the recovery phase, patients with an Aldrete score above 9 were considered to be fully recovered. Definite pulmonary aspiration symptoms were evaluated according to Bernardini et al.'s [
7] criteria, which include dyspnea, hypoxia, auscultation abnormalities, aspiration of gastric contents, bile fluid, or other non-respiratory secretions from the trachea, and the presence of new infiltrates on chest X-ray. We adapted the criteria used by Ezri et al. [
8] for possible pulmonary aspiration. These are: The occurrence of laryngospasm or bronchospasm, admission to the intensive care unit due to anesthesia, and the continuation of mechanical ventilation after surgery were considered in cases of clinical suspicion of pulmonary aspiration without the detection of bile secretions or solid particles.
2.3. Statistical Analysis
The data analysis was conducted using the IBM SPSS 27.0 (Armonk, NY: IBM Corp.) statistical software package. While evaluating the study data, descriptive statistical methods (frequency, percentage, mean, standard deviation, median, min-max) were used, and for the comparison of qualitative data, Pearson Chi-Square (χ2), Fisher's exact χ2, or Yates χ2 tests were used as appropriate. The normality of the data was evaluated using the Kolmogorov-Smirnov test, skewness-kurtosis, and graphical methods (histogram, Q-Q plot, Stem-and-Leaf, Boxplot). In the study, the Independent Samples t-test (t-test for independent groups) was used to evaluate quantitative data showing a normal distribution. The statistical significance level was accepted as p < 0.05.
Sample Size Calculation: (Calculated according to the need for airway maneuver)Power analysis was conducted using the G*Power 3.1.9.7 (Franz Faul, University of Kiel, Germany) statistical software package; n1 = 26 (P1 = 0.24), n2 = 37 (P2 = 0.62), OR = 5.12, α = 0.05, resulting in a power of 84%.
3. Results
A total of 63 ERCP procedures were performed on 28 patients who had undergone Billroth II gastrectomy. Of these, 37 procedures were performed under conscious sedation (Group CS), and 26 procedures under deep sedation (Group DS). Thirteen patients received only conscious sedation during repeated procedures, while 11 received only deep sedation. Four patients underwent some procedures with conscious sedation and others with deep sedation.The average age of the patients included in the study was 72.6 ± 10.4 years, with 7 women and 21 men. The average body mass index (BMI) was 20.3 ± 1.9 (
Table 1).Considering the procedures, 24 (38.1%) involved ASA II patients, and 39 (61.9%) involved ASA III patients. Among these procedures, comorbidities included 34.9% diabetes mellitus (DM), 71.4% hypertension (HT), 41.3% chronic heart disease, 23.8% chronic kidney disease, and 14.3% chronic pulmonary disease.
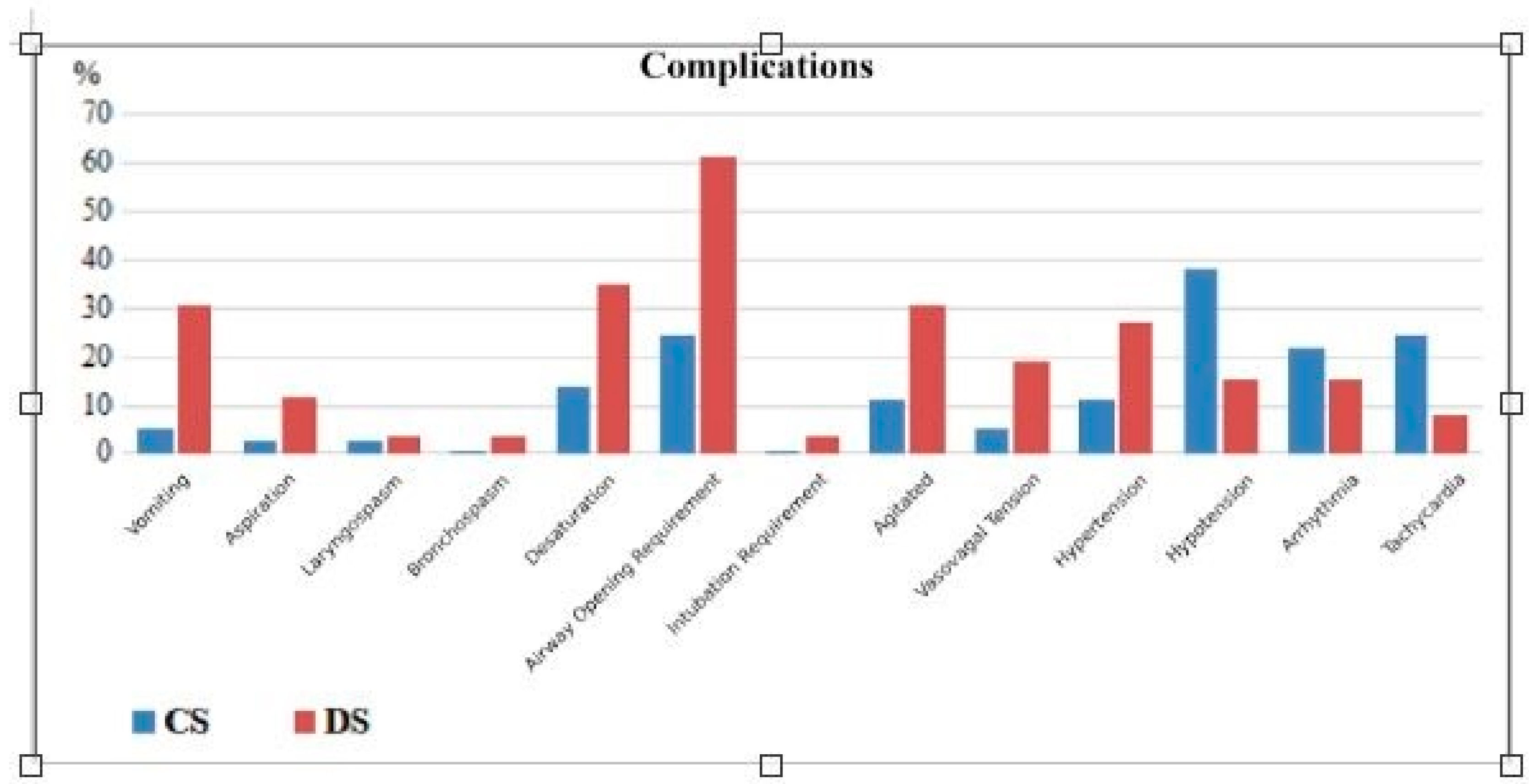
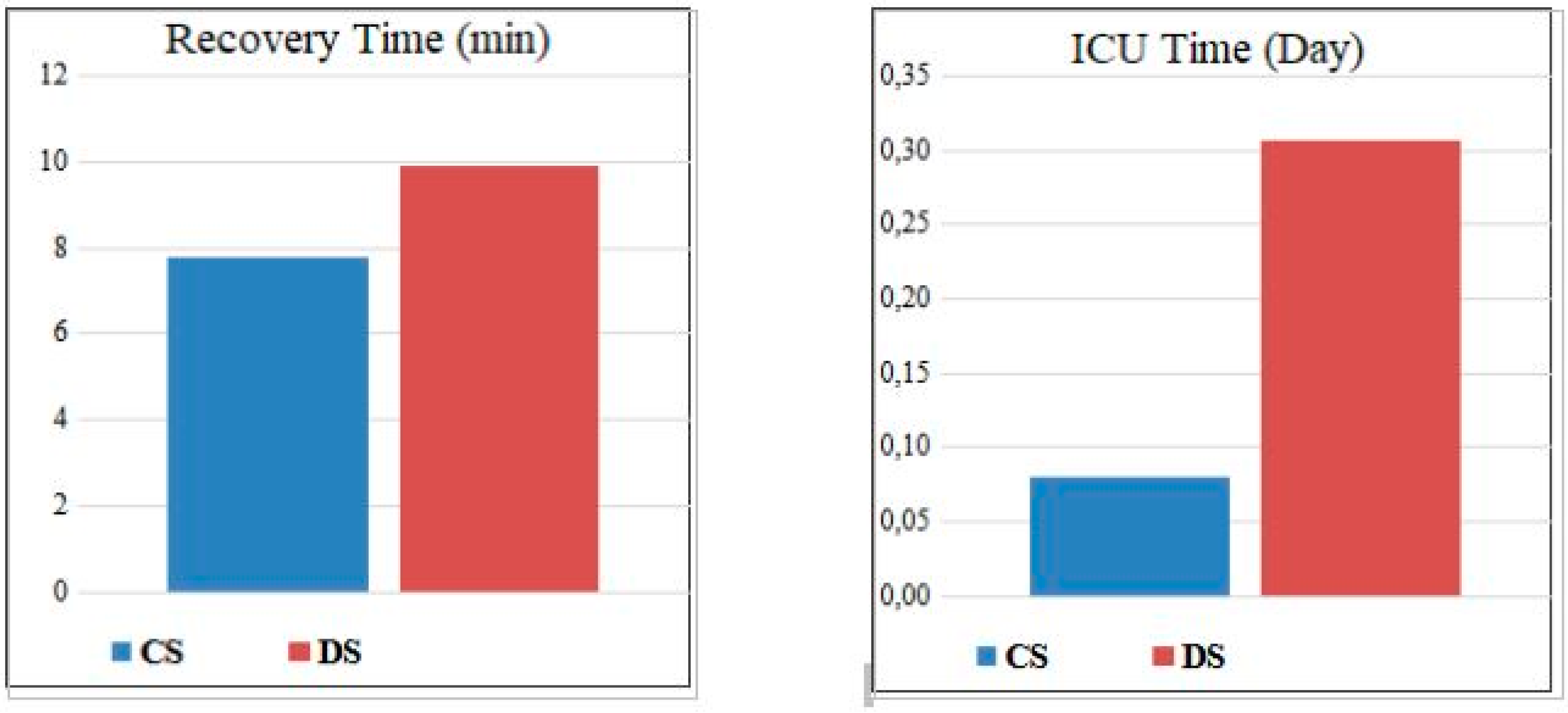
When comparing according to the type of anesthesia, it was found that in Group DS, the rate of chronic kidney disease was lower, the number of ASA II patients was higher, anesthesia duration was shorter, recovery time was longer, the vomiting rate was higher, and the need for airway maneuvers was greater. There was no statistically significant difference between the groups in terms of other variables (p > 0.05).
No statistically significant difference was found between Group CS and Group DS regarding pulmonary aspiration and other respiratory complications. Only in the deep sedation group, one patient developed a ruptured hydatid cyst followed by pulmonary aspiration, requiring post-procedural mechanical ventilation, which resulted in mortality. The requirements for non-invasive mechanical ventilation (Group CS 2.7%, Group DS 7.7%, p = 0.564) and oxygen via mask (Group CS 10.8%, Group DS 19.2%, p = 0.469) were similar between the groups (
Table 2).In the post-procedural intergroup comparison, similar rates of pulmonary infiltrate (Group CS 2.7%, Group DS 3.8%, p = 1.000), patient satisfaction, and endoscopist satisfaction were observed. The need for intensive care (Group CS 2.7%, Group DS 3.8%, p = 1.000) and the duration of intensive care stay were also similar between the groups (
Table 3).
Figure 1.
Comparison of complications between groups CS=Conscious sedation; DS= Deep sedation.
Figure 1.
Comparison of complications between groups CS=Conscious sedation; DS= Deep sedation.
Figure 2.
Comparison of recovery and intensive care hospitalization days of the groups CS=Conscious sedation; DS= Deep sedation.
Figure 2.
Comparison of recovery and intensive care hospitalization days of the groups CS=Conscious sedation; DS= Deep sedation.
4. Discussion
In patients who have undergone Billroth II gastrectomy and subsequently underwent ERCP performed by the same experienced endoscopist and anesthesiologist, we observed that conscious sedation and deep sedation were comparable in terms of pulmonary aspiration and other respiratory complications.
Contrary to our initial expectation of a lower aspiration rate in the conscious sedation group, the rates of aspiration were statistically similar between the two groups. Aspiration occurred in 3 patients in the deep sedation group (Group DS) and in 1 patient in the conscious sedation group (Group CS). The relatively small sample size likely contributed to the lack of statistical significance. In the conscious sedation group, midazolam use may have promoted reflux and aspiration by causing relaxation of the lower esophageal sphincter [
9]. Conversely, propofol—used for deep sedation—is known to have a lower emetogenic potential and acts as a potent 5-HT3 receptor blocker [
10]. Its antiemetic effects may be attributed to direct inhibition of the chemoreceptor trigger zone, vagal nuclei, and other central pathways involved in nausea and vomiting [
11]. These pharmacological properties could have contributed to the similar incidence of vomiting and pulmonary aspiration observed in the deep sedation group.
Some studies suggest that superficial sedation with propofol administered by the endoscopist results in fewer side effects compared to deep sedation performed by an anesthesiologist [
12]. However, an experienced anesthesiologist is better equipped to manage and intervene in sedation-related complications effectively. Unlike the study by Poincloux et al., which excluded patients with significant comorbidities, our cohort included elderly patients with multiple comorbid conditions, such as diabetes mellitus, hypertension, chronic heart disease, chronic kidney disease, and chronic pulmonary disease. These factors inherently increase the risk of aspiration, particularly in diabetic patients with autonomic neuropathy. Which was present in nine diabetic patients undergoing 22 ERCP procedures in our study.
A review by Gareval et al. supports deep sedation with propofol as a superior technique to conscious sedation for ERCP, citing better recovery profiles and lower failure rates, albeit emphasizing that such sedation should be administered or supervised by an anesthetist. [
13] In our study, anesthesia-related complications were low and comparable between groups. Although vomiting was more frequent in the deep sedation group, the need for airway interventions, aspiration, laryngospasm, bronchospasm, apnea, and intubation did not differ significantly. We attribute this safety anesthesia to our use of bispectral index (BIS) monitoring for titrating sedation depth. Park et al. demonstrated through a systematic meta-analysis that BIS monitoring during endoscopic procedures effectively prevents excessive propofol dosing while maintaining adequate sedation [
14]. Similarly, clinical studies confirm a strong correlation between BIS values and clinical sedation scales [
15]. Thus, BIS monitoring likely helped balance anesthetic safety and efficacy in our patients.
Airway maneuvers such as chin lifting were sufficient in most cases. Consistent with a systematic review and meta-analysis by Dhaliwal et al. on sedation preferences for ERCP, anesthesia durations were longer in the conscious sedation group, likely because procedure performance is more challenging in conscious patients [
16]. Conversely, recovery times were longer in the deep sedation group, but this did not prolonged respiratory support post-procedure.
A serious adverse event occurred in a patient undergoing deep sedation ERCP for cholangitis, who experienced a ruptured hydatid cyst and subsequent pulmonary aspiration, leading to mechanical ventilation and eventual mortality. Although mortality did not statistically differ between groups, this complication highlights the critical importance of vigilance by both anesthetists and endoscopists.
Technical success rates were similar between the groups. Key procedural steps—such as accessing the afferent loop, the papilla, and successful cannulation—were not impeded by sedation type, although altered anatomy after Billroth II surgery inherently complicates ERCP procedures [
17]. Only one procedure failure occurred, unrelated to sedation depth, underscoring the importance of the procedure being performed by an experienced endoscopist.
Braun enteroenterostomy performed during Billroth II surgery is known to reduce the risk of gastritis, esophagitis, reflux, and aspiration. In our cohort, Braun anastomosis was present in a small number of procedures (5 in Group CS, 4 in Group DS), and sedation type did not influence complication rates in these patients.
5. Limitations
The limitations of this study, in addition to being a retrospective study, were due to the relatively limited number of procedures because it involved a selected patient group. Despite being a center where a high number of ERCP procedures are performed, the relatively low number of ERCP procedures in patients who underwent Billroth II surgery did not sufficiently reflect in our statistical results. Further studies with a larger number of patients are needed in this regard.
6. Conclusions
In patients who have undergone Billroth II gastrectomy, both conscious sedation and deep sedation methods are safe and effective for ERCP procedures. There is no significant difference between the two methods in terms of complication rates, patient and endoscopist satisfaction, and clinical outcomes. The interrelatedness of respiratory complications and their contribution to poor post-procedural outcomes underscores the importance of careful monitoring, close follow-up, and early intervention in these patients.
Ethics approval and consent to participate
Local ethics committee approval was obtained.
Author Contributions
AL and MS—designed the research study; wrote the manuscript. AL and GE—performed the research. AL, MS, BO—analyzed the data. All authors read and approved the final manuscript.
Data Availability Statement
The data presented in this study are available from the corresponding author.
Acknowledgment
The authors thank all individuals who contributed to this paper.
Conflict of interest
No conflict of interest.
References
- Mbatshi, G. , Macken, E. J., De Schepper, H. U., Piessevaux, H., Deprez, P. H., Moreels, T. G. Comparison of side-viewing duodenoscope and single-balloon enteroscope to perform ERCP in patients with Billroth II gastrectomy. Acta gastroenterol. belg, 2017, 80, 493–497. [Google Scholar] [PubMed]
- Tringali, A. , Mutignani, M., Milano, A., Perri, V., Costamagna, G. No difference between supine and prone position for ERCP in conscious sedated patients: a prospective randomized study. Endoscopy; 493; 40, pp. 93–97. [Google Scholar]
- He, L. , Zhao, Y. Is Roux-en-Y or Billroth-II reconstruction the preferred choice for gastric cancer patients undergoing distal gastrectomy when Billroth I reconstruction is not applicable? A meta-analysis. Medicine 2019, 98, 493. [Google Scholar] [CrossRef] [PubMed]
- Christodoulidis, G. , Kouliou, M. N., Koumarelas, K. E., Argyriou, K., Karali, G. A., Tepetes, K. Billroth II anastomosis combined with brown anastomosis reduce reflux gastritis in gastric cancer patients. World Journal of Methodology 2024, 14, 89709. [Google Scholar] [CrossRef] [PubMed]
- Sidhu, R. , Turnbull, D., Newton, M., Thomas-Gibson, S., Sanders, D. S., Hebbar, S., et al. Deep sedation and anaesthesia in complex gastrointestinal endoscopy: a joint position statement endorsed by the British Society of Gastroenterology (BSG), Joint Advisory Group (JAG) and Royal College of Anaesthetists (RCoA). Frontline gastroenterology 2019, 141–147. [Google Scholar]
- Henriksson, A. M. , Thakrar, S. V. Anaesthesia and sedation for endoscopic retrograde cholangiopancreatography. BJA education 2022, 372. [Google Scholar]
- Bernardini A, Natalini G. Risk of pulmonary aspiration with laryngeal mask airway and tracheal tube: analysis on 65 712 procedures with positive pressure ventilation. Anaesthesia. 2009, 1289–1294.
- Ezri T, Szmuk P, Stein A, Konichezky S, Hagai T, Geva D. Peripartum generalanasthesia without tracheal intubation: incidence of aspiration pneumonia. Anaesthesia. 2000, 421–426.
- Campbell-Taylor, I. Benzodiazepines and pneumonia or aspiration pneumonitis. Thorax 2013, 68, 591. [Google Scholar] [CrossRef] [PubMed]
- Barann M, Göthert M, Fink K, Bönisch H. Inhibition by anaesthetics of 14C-guanidinium flux through the voltagegated sodium channel and the cation channel of the 5-HT3 receptor of N1E-115 neuroblastoma cells. NaunynSchmiedeberg’s Archives of Pharmacology 1993, 347, 125–132.
- Appadu BL, Strange PG, Lambert DG. Does propofol interact with D2 dopamine receptors? Anesthesia and Analgesia. 1994, 79, 1191–1192.
- Poincloux, L. , Laquière, A., Bazin, J. E., Monzy, F., Artigues, F., Bonny, C., et al. A randomized controlled trial of endoscopist vs. anaesthetist-administered sedation for colonoscopy. Digestive and Liver Disease 2011, 553–558. [Google Scholar] [CrossRef] [PubMed]
- Garewal, D. , Vele, L., Waikar, P. Anaesthetic considerations for endoscopic retrograde cholangio-pancreatography procedures. Current Opinion in Anesthesiology 2013, 475–480. [Google Scholar] [CrossRef] [PubMed]
- Park, S. W. , Lee, H., & Ahn, H. Bispectral index versus standard monitoring in sedation for endoscopic procedures: a systematic review and meta-analysis. Digestive diseases and sciences. 2016, 814–824. [Google Scholar]
- Yu, Y. H. , Han, D. S., Kim, H. S., Kim, E. K., Eun, C. S., Yoo, K. S., et al. Efficacy of bispectral index monitoring during balanced propofol sedation for colonoscopy: a prospective, randomized controlled trial. Digestive diseases and sciences 2013, 3576–3583. [Google Scholar] [CrossRef]
- Dhaliwal A, Dhindsa BS, Saghir SM, Ramai D, Chandan S, Mashiana H, et al. Choice of sedation in endoscopic retrograde cholangiopancreatography: is monitored anesthesia care as safe as general anesthesia? A systematic review and meta-analysis. Ann Gastroenterol 2021, 34, 879–887.
- Takano S, Fukasawa M, Shindo H, Takahashi E, Hirose S, Fukasawa Y, et al. Risk factors for perforation during endoscopic retrograde cholangiopancreatography in post-reconstruction intestinal tract. World J Clin Cases 2019, 10–18.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).