Submitted:
11 June 2025
Posted:
12 June 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Infection and Replication
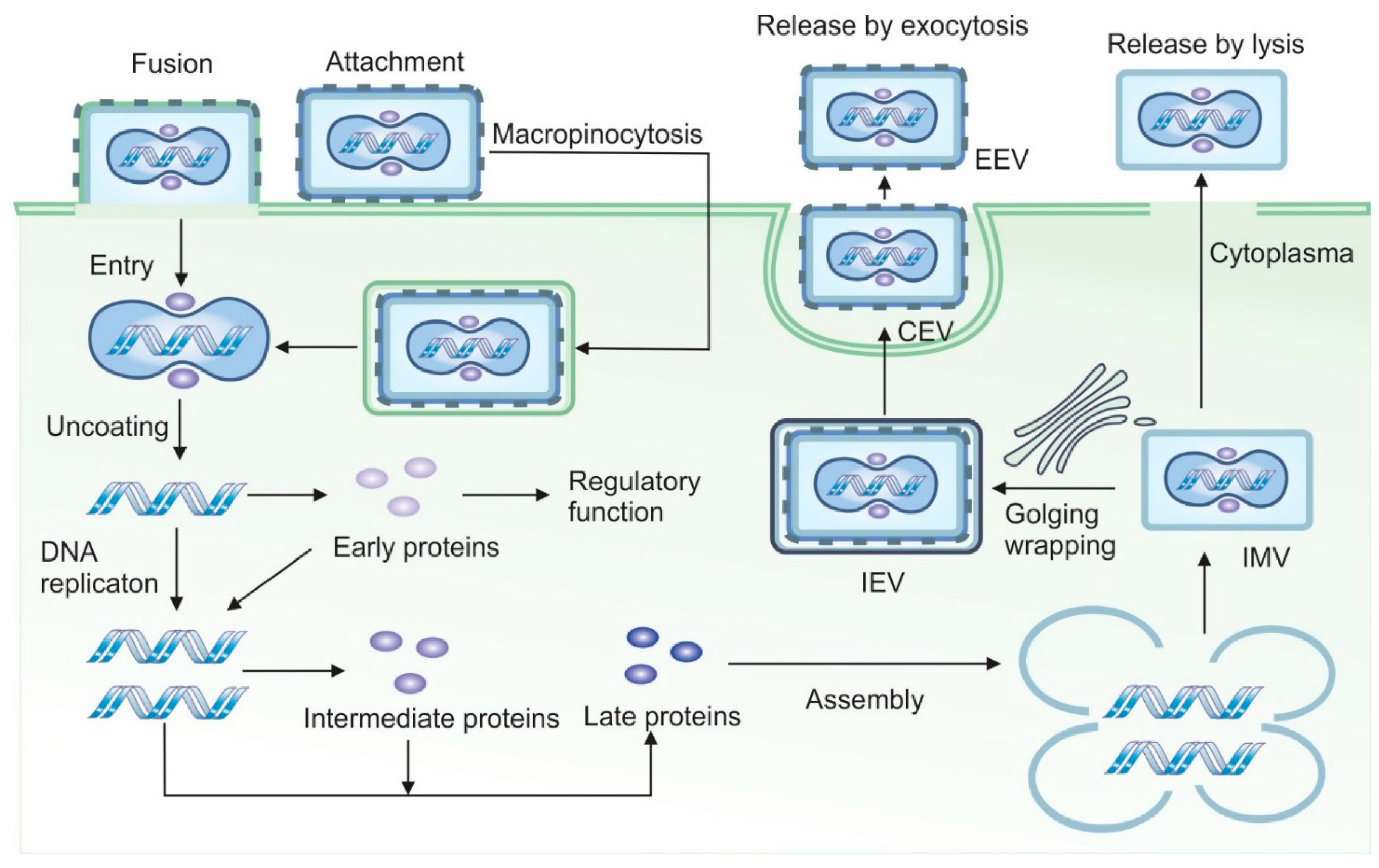
2.1. Viral Entry
2.2. Replication
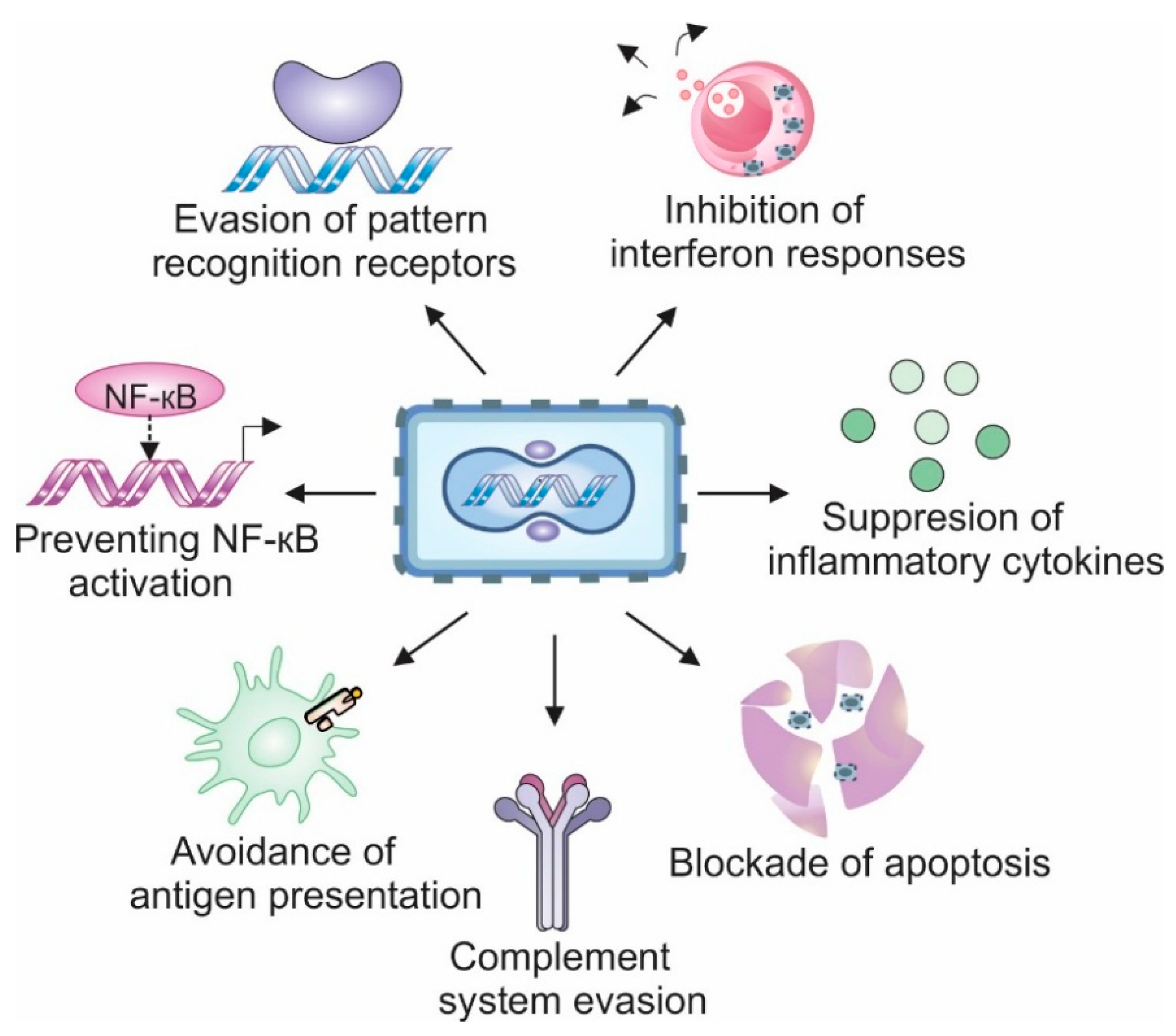
3. Strategies to Avoid Anti-Viral Mechanisms
3.1. DNA-PK and Viral DNA Detection
3.2. cGAS, STING and IRF3
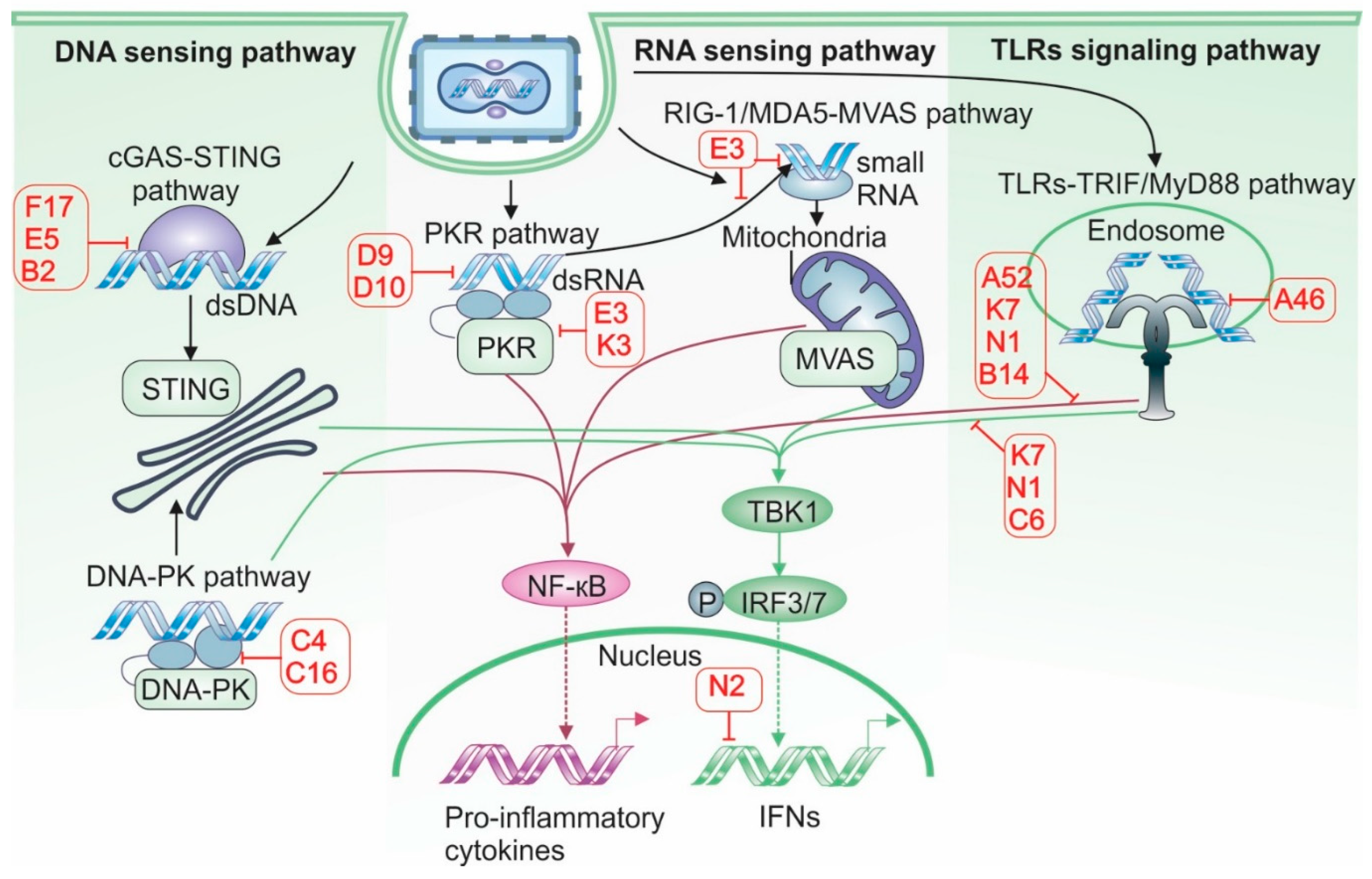
3.3. Double Stranded RNA Sensors
3.4. Toll-Like Receptor System
3.5. NF-kB, IFNs and Other Cytokines
3.6. Complement Evasion
3.7. Antigen Processing and Presentation
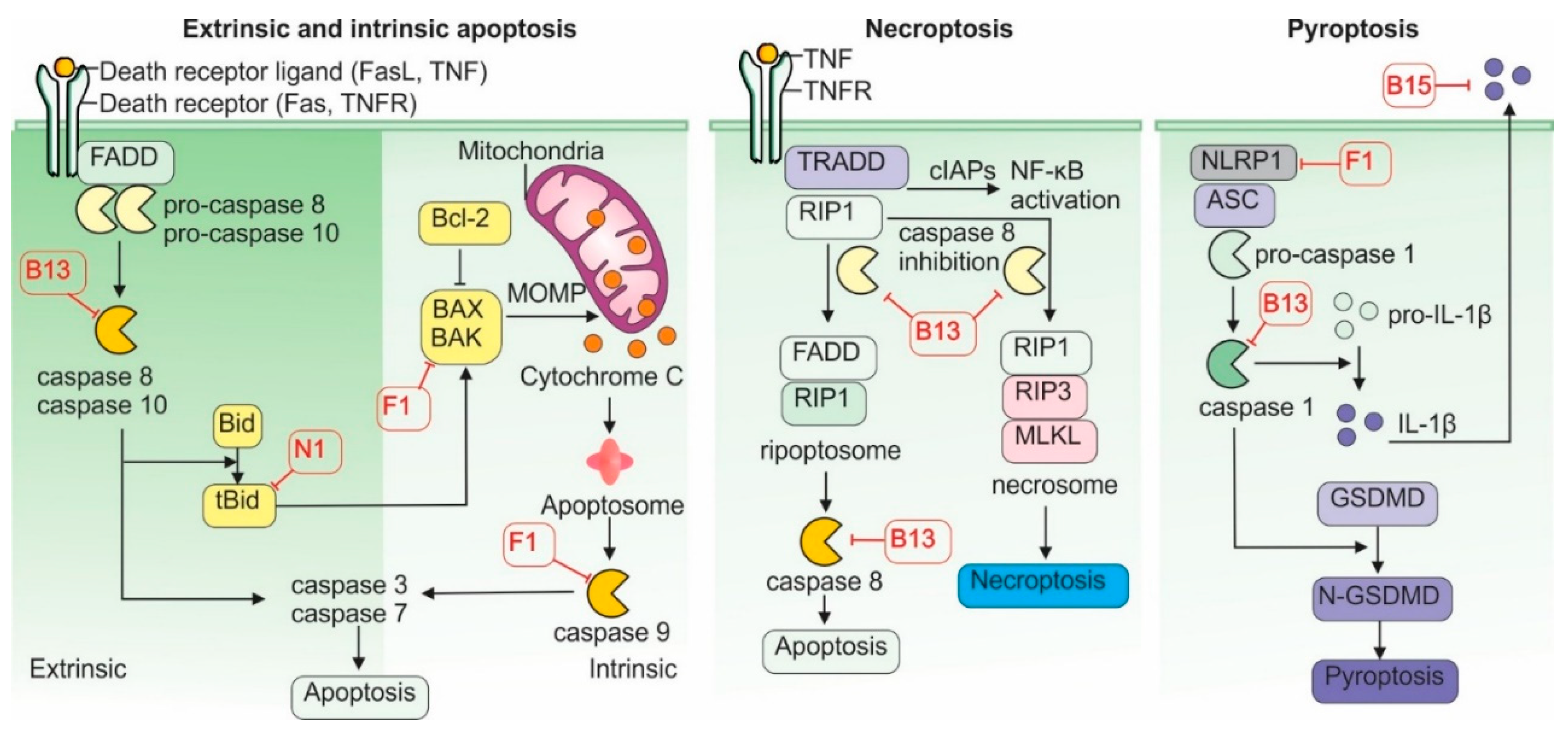
4. Strategies to Avoid Cell Death Mechanisms
5. Interactions with Immune Cells
5.1. Dendritic Cells
5.2. Macrophages
5.3. Neutrophils and NK Cells
5.4. B and T Lymphocytes
6. VACV as a Tool in Cancer Therapy
6.1. Advantages of VACV as an Anti-Cancer Agent
6.2. Chimeric VACV: CF33
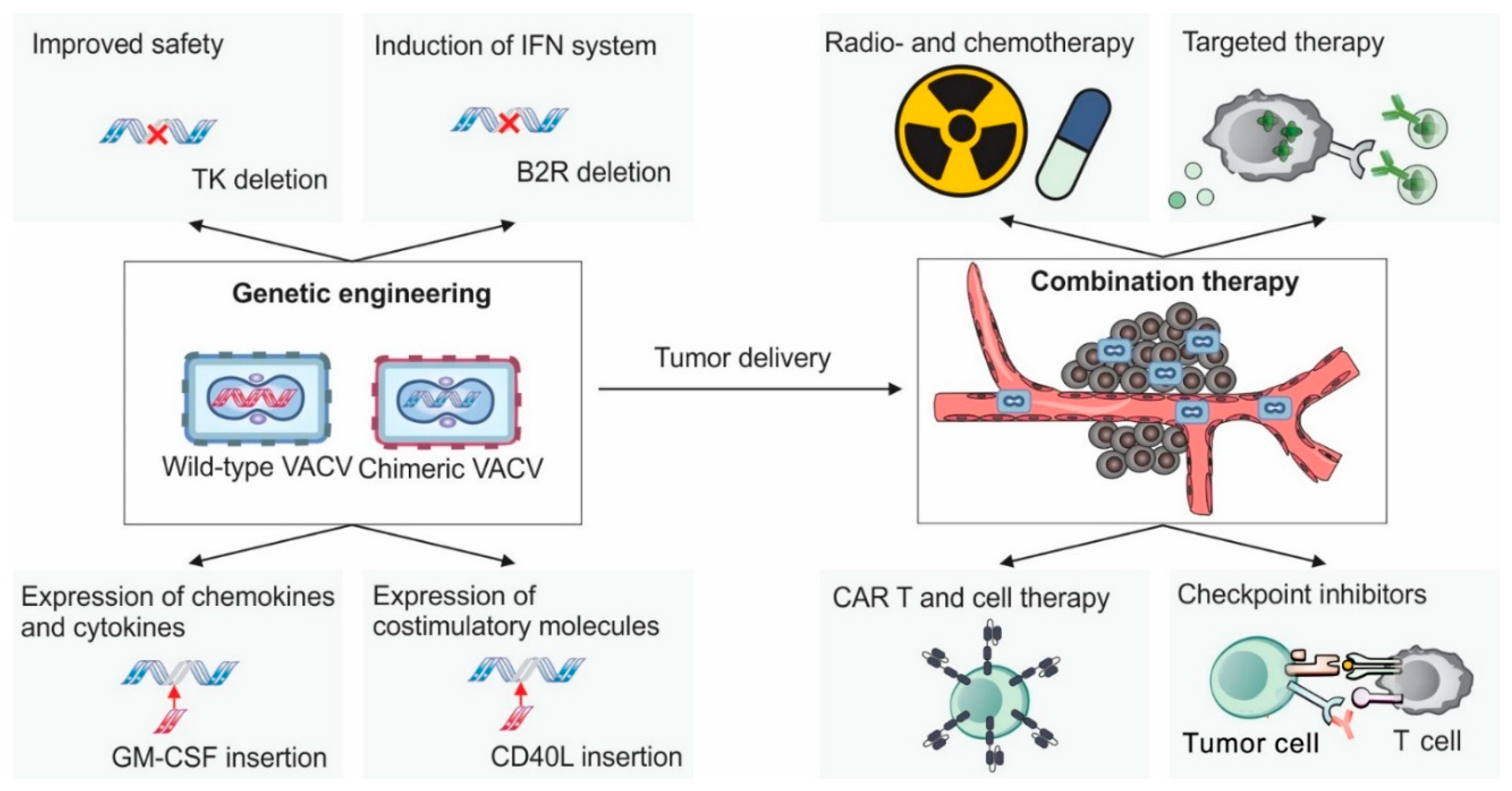
6.3. Genetic Engineering of VACV
6.4. Commonly Targeted Viral Genes for Modification in VACV-Based Oncolytic Viruses
6.5. Expression of Chemokines and Cytokines
| Deleted viral genes | Functions of deleted viral genes | Example of virus | Reference |
|---|---|---|---|
| TK (Thymidine kinase) | Provides material for viral DNA replication | vCB2 (vvLuc) | [136] |
| TK and VGF (Viral growth factor) | Supports viral replication, accelerates cell growth and metabolism | vvDD-GFP | [147] |
| SPI-1 (B22) and -2 (B13) | Inhibit apoptosis | vSP | [148] |
| TK, SPI-1 and -2 | Supports viral replication, inhibit apoptosis | vSPT | [149] |
| Soluble type I IFN receptor (B18R) | Inhibits mobilization of the immune cells | WR-delB18 | [150] |
| Soluble type I IFN receptor and TK | Inhibits mobilization of the immune cell, supports viral replication | ΔB18RΔTK | [150] |
| F14.5L, TK and HA | Promotes virulence in vivo, supports viral replication, inhibits complement-mediated lysis of infected cell | GLV-1h68 | [151] |
| TK, RR (ribonucleotide reductase) | Support viral replication by providing material for DNA synthesis | TG6002 | [152] |
| TK, N1L, and A41L | Supports viral replication, inhibits apoptosis, interferes with chemokine signaling | VVLΔTKΔN1LΔA41L | [153] |
| A49L | Inhibits NF-kB activation | vΔA49L | [154] |
| TK, F1L | Supports viral replication, Inhibits apoptosis | ΔTK/F1L | [155] |
| TK, B2R | Supports viral replication, Inhibits the cGAS/STING pathway | WR/TK−/ΔB2 | [156] |
6.6. Induction of the IFN System
6.7. Expression of Costimulatory Molecules
6.8. Other Strategies
7. Vaccinia Virus in Combination with Other Therapeutic Strategies
7.1. Combination with CAR-T Therapies
7.2. Combination with Checkpoint Inhibitors
7.3. Combination with Radio- and Chemotherapy
7.4. Combination with Small-Molecule Inhibitors
8. Clinical Trials and Potential Obstacles
9. Conclusions
Acknowledgments
Disclosure/Conflict of Interest
References
- Xu, L.; Sun, H.; Lemoine, N.R.; Xuan, Y.; Wang, P. Oncolytic Vaccinia Virus and Cancer Immunotherapy. Front. Immunol. 2024, 14. [CrossRef]
- Kaynarcalidan, O.; Moreno Mascaraque, S.; Drexler, I. Vaccinia Virus: From Crude Smallpox Vaccines to Elaborate Viral Vector Vaccine Design. Biomedicines 2021, 9, 1780. [CrossRef]
- Greseth, M.D.; Czarnecki, M.W.; Bluma, M.S.; Traktman, P. Isolation and Characterization of vΔI3 Confirm That Vaccinia Virus SSB Plays an Essential Role in Viral Replication. Journal of Virology 2018, 92, 10.1128/jvi.01719-17. [CrossRef]
- Wittek, R. VACCINIA VIRUS (POXVIRIDAE). In Encyclopedia of Virology (Second Edition); Granoff, A., Webster, R.G., Eds.; Elsevier: Oxford, 1999; pp. 1865–1872 ISBN 978-0-12-227030-7.
- Molteni, C.; Forni, D.; Cagliani, R.; Clerici, M.; Sironi, M. Genetic Ancestry and Population Structure of Vaccinia Virus. npj Vaccines 2022, 7, 1–9. [CrossRef]
- Moss, B. Poxvirus DNA Replication. Cold Spring Harb Perspect Biol 2013, 5, a010199. [CrossRef]
- Yang, Z.; Maruri-Avidal, L.; Sisler, J.; Stuart, C.A.; Moss, B. Cascade Regulation of Vaccinia Virus Gene Expression Is Modulated by Multistage Promoters. Virology 2013, 447, 10.1016/j.virol.2013.09.007. [CrossRef]
- Chou, W.; Ngo, T.; Gershon, P.D. An Overview of the Vaccinia Virus Infectome: A Survey of the Proteins of the Poxvirus-Infected Cell. J Virol 2012, 86, 1487–1499. [CrossRef]
- Laudermilch, E.; Chandran, K. MAVERICC: Marker-Free Vaccinia Virus Engineering of Recombinants through in Vitro CRISPR/Cas9 Cleavage. J Mol Biol 2021, 433, 166896. [CrossRef]
- Jacobs, B.L.; Langland, J.O.; Kibler, K.V.; Denzler, K.L.; White, S.D.; Holechek, S.A.; Wong, S.; Huynh, T.; Baskin, C.R. Vaccinia Virus Vaccines: Past, Present and Future. Antiviral Res 2009, 84, 1–13. [CrossRef]
- de Freitas, L.F.D.; Oliveira, R.P.; Miranda, M.C.G.; Rocha, R.P.; Barbosa-Stancioli, E.F.; Faria, A.M.C.; da Fonseca, F.G. The Virulence of Different Vaccinia Virus Strains Is Directly Proportional to Their Ability To Downmodulate Specific Cell-Mediated Immune Compartments In Vivo. J Virol 2019, 93, e02191-18. [CrossRef]
- Belongia, E.A.; Naleway, A.L. Smallpox Vaccine: The Good, the Bad, and the Ugly. Clin Med Res 2003, 1, 87–92.
- Meiser, A.; Boulanger, D.; Sutter, G.; Krijnse Locker, J. Comparison of Virus Production in Chicken Embryo Fibroblasts Infected with the WR, IHD-J and MVA Strains of Vaccinia Virus: IHD-J Is Most Efficient in Trans-Golgi Network Wrapping and Extracellular Enveloped Virus Release. J Gen Virol 2003, 84, 1383–1392. [CrossRef]
- Bengali, Z.; Townsley, A.C.; Moss, B. Vaccinia Virus Strain Differences in Cell Attachment and Entry. Virology 2009, 389, 132–140. [CrossRef]
- Carter, G.C.; Law, M.; Hollinshead, M.; Smith, G.L. Entry of the Vaccinia Virus Intracellular Mature Virion and Its Interactions with Glycosaminoglycans. Journal of General Virology 2005, 86, 1279–1290. [CrossRef]
- MacLeod, D.T.; Nakatsuji, T.; Wang, Z.; di Nardo, A.; Gallo, R.L. Vaccinia Virus Binds to the Scavenger Receptor MARCO on the Surface of Keratinocytes. J Invest Dermatol 2015, 135, 142–150. [CrossRef]
- Laliberte, J.P.; Weisberg, A.S.; Moss, B. The Membrane Fusion Step of Vaccinia Virus Entry Is Cooperatively Mediated by Multiple Viral Proteins and Host Cell Components. PLoS Pathog 2011, 7, e1002446. [CrossRef]
- Townsley, A.C.; Weisberg, A.S.; Wagenaar, T.R.; Moss, B. Vaccinia Virus Entry into Cells via a Low-pH-Dependent Endosomal Pathway. J Virol 2006, 80, 8899–8908. [CrossRef]
- Mercer, J.; Knébel, S.; Schmidt, F.I.; Crouse, J.; Burkard, C.; Helenius, A. Vaccinia Virus Strains Use Distinct Forms of Macropinocytosis for Host-Cell Entry. Proceedings of the National Academy of Sciences 2010, 107, 9346–9351. [CrossRef]
- Greseth, M.D.; Traktman, P. The Life Cycle of the Vaccinia Virus Genome. Annual Review of Virology 2022, 9, 239–259. [CrossRef]
- Schmidt, F.I.; Bleck, C.K.E.; Reh, L.; Novy, K.; Wollscheid, B.; Helenius, A.; Stahlberg, H.; Mercer, J. Vaccinia Virus Entry Is Followed by Core Activation and Proteasome-Mediated Release of the Immunomodulatory Effector VH1 from Lateral Bodies. Cell Reports 2013, 4, 464–476. [CrossRef]
- Tolonen, N.; Doglio, L.; Schleich, S.; Locker, J.K. Vaccinia Virus DNA Replication Occurs in Endoplasmic Reticulum-Enclosed Cytoplasmic Mini-Nuclei. MBoC 2001, 12, 2031–2046. [CrossRef]
- Moss, B. Cascade Regulation of Vaccinia Virus Gene Expression. In Regulation of Gene Expression in Animal Viruses; Carrasco, L., Sonenberg, N., Wimmer, E., Eds.; Springer US: Boston, MA, 1993; pp. 13–24 ISBN 978-1-4615-2928-6.
- Howell, L.M.; Gracie, N.P.; Newsome, T.P. Single-Cell Analysis of VACV Infection Reveals Pathogen-Driven Timing of Early and Late Phases and Host-Limited Dynamics of Virus Production. PLOS Pathogens 2024, 20, e1012423. [CrossRef]
- Liu, L.; Cooper, T.; Howley, P.M.; Hayball, J.D. From Crescent to Mature Virion: Vaccinia Virus Assembly and Maturation. Viruses 2014, 6, 3787–3808. [CrossRef]
- Boyle, K.A.; Stanitsa, E.S.; Greseth, M.D.; Lindgren, J.K.; Traktman, P. Evaluation of the Role of the Vaccinia Virus Uracil DNA Glycosylase and A20 Proteins as Intrinsic Components of the DNA Polymerase Holoenzyme *. Journal of Biological Chemistry 2011, 286, 24702–24713. [CrossRef]
- Rochester, S.C.; Traktman, P. Characterization of the Single-Stranded DNA Binding Protein Encoded by the Vaccinia Virus I3 Gene. J Virol 1998, 72, 2917–2926. [CrossRef]
- Garcia, A.D.; Moss, B. Repression of Vaccinia Virus Holliday Junction Resolvase Inhibits Processing of Viral DNA into Unit-Length Genomes. J Virol 2001, 75, 6460–6471. [CrossRef]
- Paran, N.; De Silva, F.S.; Senkevich, T.G.; Moss, B. Cellular DNA Ligase I Is Recruited to Cytoplasmic Vaccinia Virus Factories and Masks the Role of the Vaccinia Ligase in Viral DNA Replication. Cell Host Microbe 2009, 6, 563–569. [CrossRef]
- El Omari, K.; Solaroli, N.; Karlsson, A.; Balzarini, J.; Stammers, D.K. Structure of Vaccinia Virus Thymidine Kinase in Complex with dTTP: Insights for Drug Design. BMC Struct Biol 2006, 6, 22. [CrossRef]
- Topalis, D.; Collinet, B.; Gasse, C.; Dugué, L.; Balzarini, J.; Pochet, S.; Deville-Bonne, D. Substrate Specificity of Vaccinia Virus Thymidylate Kinase. The FEBS Journal 2005, 272, 6254–6265. [CrossRef]
- Gammon, D.B.; Gowrishankar, B.; Duraffour, S.; Andrei, G.; Upton, C.; Evans, D.H. Vaccinia Virus–Encoded Ribonucleotide Reductase Subunits Are Differentially Required for Replication and Pathogenesis. PLoS Pathog 2010, 6, e1000984. [CrossRef]
- Smith, G.L.; Vanderplasschen, A.; Law, M. The Formation and Function of Extracellular Enveloped Vaccinia Virus. Journal of General Virology 2002, 83, 2915–2931. [CrossRef]
- Smith, G.L.; Law, M. The Exit of Vaccinia Virus from Infected Cells. Virus Research 2004, 106, 189–197. [CrossRef]
- Zeh, H.J.; Downs-Canner, S.; McCart, J.A.; Guo, Z.S.; Rao, U.N.M.; Ramalingam, L.; Thorne, S.H.; Jones, H.L.; Kalinski, P.; Wieckowski, E.; et al. First-in-Man Study of Western Reserve Strain Oncolytic Vaccinia Virus: Safety, Systemic Spread, and Antitumor Activity. Molecular Therapy 2015, 23, 202–214. [CrossRef]
- Schwanke, H.; Stempel, M.; Brinkmann, M.M. Of Keeping and Tipping the Balance: Host Regulation and Viral Modulation of IRF3-Dependent IFNB1 Expression. Viruses 2020, 12, 733. [CrossRef]
- Cell Fate in Antiviral Response Arises in the Crosstalk of IRF, NF-κB and JAK/STAT Pathways | Nature Communications Available online: https://www.nature.com/articles/s41467-017-02640-8 (accessed on 18 April 2025).
- El-Jesr, M.; Teir, M.; Maluquer de Motes, C. Vaccinia Virus Activation and Antagonism of Cytosolic DNA Sensing. Front Immunol 2020, 11, 568412. [CrossRef]
- Peters, N.E.; Ferguson, B.J.; Mazzon, M.; Fahy, A.S.; Krysztofinska, E.; Arribas-Bosacoma, R.; Pearl, L.H.; Ren, H.; Smith, G.L. A Mechanism for the Inhibition of DNA-PK-Mediated DNA Sensing by a Virus. PLoS Pathog 2013, 9, e1003649. [CrossRef]
- Scutts, S.R.; Ember, S.W.; Ren, H.; Ye, C.; Lovejoy, C.A.; Mazzon, M.; Veyer, D.L.; Sumner, R.P.; Smith, G.L. DNA-PK Is Targeted by Multiple Vaccinia Virus Proteins to Inhibit DNA Sensing. Cell Rep 2018, 25, 1953-1965.e4. [CrossRef]
- Chiu, Y.-H.; MacMillan, J.B.; Chen, Z.J. RNA Polymerase III Detects Cytosolic DNA and Induces Type I Interferons through the RIG-I Pathway. Cell 2009, 138, 576–591. [CrossRef]
- Valentine, R.; Smith, G.L. Inhibition of the RNA Polymerase III-Mediated dsDNA-Sensing Pathway of Innate Immunity by Vaccinia Virus Protein E3. Journal of General Virology 2010, 91, 2221–2229. [CrossRef]
- Unterholzner, L.; Keating, S.E.; Baran, M.; Horan, K.A.; Jensen, S.B.; Sharma, S.; Sirois, C.M.; Jin, T.; Latz, E.; Xiao, T.S.; et al. IFI16 Is an Innate Immune Sensor for Intracellular DNA. Nat Immunol 2010, 11, 997–1004. [CrossRef]
- Almine, J.F.; O’Hare, C.A.J.; Dunphy, G.; Haga, I.R.; Naik, R.J.; Atrih, A.; Connolly, D.J.; Taylor, J.; Kelsall, I.R.; Bowie, A.G.; et al. IFI16 and cGAS Cooperate in the Activation of STING during DNA Sensing in Human Keratinocytes. Nat Commun 2017, 8, 14392. [CrossRef]
- Ablasser, A.; Schmid-Burgk, J.L.; Hemmerling, I.; Horvath, G.L.; Schmidt, T.; Latz, E.; Hornung, V. Cell Intrinsic Immunity Spreads to Bystander Cells via the Intercellular Transfer of cGAMP. Nature 2013, 503, 530–534. [CrossRef]
- Luteijn, R.D.; Zaver, S.A.; Gowen, B.G.; Wyman, S.K.; Garelis, N.E.; Onia, L.; McWhirter, S.M.; Katibah, G.E.; Corn, J.E.; Woodward, J.J.; et al. SLC19A1 Transports Immunoreactive Cyclic Dinucleotides. Nature 2019, 573, 434–438. [CrossRef]
- Seo, G.J.; Yang, A.; Tan, B.; Kim, S.; Liang, Q.; Choi, Y.; Yuan, W.; Feng, P.; Park, H.-S.; Jung, J.U. Akt Kinase-Mediated Checkpoint of cGAS DNA Sensing Pathway. Cell Rep 2015, 13, 440–449. [CrossRef]
- Meade, N.; Furey, C.; Li, H.; Verma, R.; Chai, Q.; Rollins, M.G.; DiGiuseppe, S.; Naghavi, M.H.; Walsh, D. Poxviruses Evade Cytosolic Sensing through Disruption of an mTORC1-mTORC2 Regulatory Circuit. Cell 2018, 174, 1143-1157.e17. [CrossRef]
- Yang, N.; Wang, Y.; Dai, P.; Li, T.; Zierhut, C.; Tan, A.; Zhang, T.; Xiang, J.Z.; Ordureau, A.; Funabiki, H.; et al. Vaccinia E5 Is a Major Inhibitor of the DNA Sensor cGAS. Nat Commun 2023, 14, 2898. [CrossRef]
- Eaglesham, J.B.; Pan, Y.; Kupper, T.S.; Kranzusch, P.J. Viral and Metazoan Poxins Are cGAMP-Specific Nucleases That Restrict cGAS-STING Signaling. Nature 2019, 566, 259–263. [CrossRef]
- Unterholzner, L.; Sumner, R.P.; Baran, M.; Ren, H.; Mansur, D.S.; Bourke, N.M.; Randow, F.; Smith, G.L.; Bowie, A.G. Vaccinia Virus Protein C6 Is a Virulence Factor That Binds TBK-1 Adaptor Proteins and Inhibits Activation of IRF3 and IRF7. PLoS Pathog 2011, 7, e1002247. [CrossRef]
- Schröder, M.; Baran, M.; Bowie, A.G. Viral Targeting of DEAD Box Protein 3 Reveals Its Role in TBK1/IKKɛ-Mediated IRF Activation. EMBO J 2008, 27, 2147–2157. [CrossRef]
- Ferguson, B.J.; Benfield, C.T.O.; Ren, H.; Lee, V.H.; Frazer, G.L.; Strnadova, P.; Sumner, R.P.; Smith, G.L. Vaccinia Virus Protein N2 Is a Nuclear IRF3 Inhibitor That Promotes Virulence. J Gen Virol 2013, 94, 2070–2081. [CrossRef]
- Willis, K.L.; Langland, J.O.; Shisler, J.L. Viral Double-Stranded RNAs from Vaccinia Virus Early or Intermediate Gene Transcripts Possess PKR Activating Function, Resulting in NF-κB Activation, When the K1 Protein Is Absent or Mutated *. Journal of Biological Chemistry 2011, 286, 7765–7778. [CrossRef]
- García, M.A.; Gil, J.; Ventoso, I.; Guerra, S.; Domingo, E.; Rivas, C.; Esteban, M. Impact of Protein Kinase PKR in Cell Biology: From Antiviral to Antiproliferative Action. Microbiol Mol Biol Rev 2006, 70, 1032–1060. [CrossRef]
- Szczerba, M.; Subramanian, S.; Trainor, K.; McCaughan, M.; Kibler, K.V.; Jacobs, B.L. Small Hero with Great Powers: Vaccinia Virus E3 Protein and Evasion of the Type I IFN Response. Biomedicines 2022, 10, 235. [CrossRef]
- Cao, J.; Varga, J.; Deschambault, Y. Poxvirus Encoded eIF2α Homolog, K3 Family Proteins, Is a Key Determinant of Poxvirus Host Species Specificity. Virology 2020, 541, 101–112. [CrossRef]
- Yu, H.; Bruneau, R.C.; Brennan, G.; Rothenburg, S. Battle Royale: Innate Recognition of Poxviruses and Viral Immune Evasion. Biomedicines 2021, 9, 765. [CrossRef]
- Rivas, C.; Gil, J.; Mělková, Z.; Esteban, M.; Díaz-Guerra, M. Vaccinia Virus E3L Protein Is an Inhibitor of the Interferon (IFN)-Induced 2-5A Synthetase Enzyme. Virology 1998, 243, 406–414. [CrossRef]
- Chan, Y.K.; Gack, M.U. RIG-I-like Receptor Regulation in Virus Infection and Immunity. Curr Opin Virol 2015, 12, 7–14. [CrossRef]
- Deng, L.; Dai, P.; Parikh, T.; Cao, H.; Bhoj, V.; Sun, Q.; Chen, Z.; Merghoub, T.; Houghton, A.; Shuman, S. Vaccinia Virus Subverts a Mitochondrial Antiviral Signaling Protein-Dependent Innate Immune Response in Keratinocytes through Its Double-Stranded RNA Binding Protein, E3. Journal of Virology 2008, 82, 10735–10746. [CrossRef]
- Valentine, R.; Smith, G.L. Inhibition of the RNA Polymerase III-Mediated dsDNA-Sensing Pathway of Innate Immunity by Vaccinia Virus Protein E3. Journal of General Virology 2010, 91, 2221–2229. [CrossRef]
- Botos, I.; Segal, D.M.; Davies, D.R. The Structural Biology of Toll-like Receptors. Structure 2011, 19, 447–459. [CrossRef]
- El-Zayat, S.R.; Sibaii, H.; Mannaa, F.A. Toll-like Receptors Activation, Signaling, and Targeting: An Overview. Bulletin of the National Research Centre 2019, 43, 187. [CrossRef]
- Harte, M.T.; Haga, I.R.; Maloney, G.; Gray, P.; Reading, P.C.; Bartlett, N.W.; Smith, G.L.; Bowie, A.; O’Neill, L.A.J. The Poxvirus Protein A52R Targets Toll-like Receptor Signaling Complexes to Suppress Host Defense. J Exp Med 2003, 197, 343–351. [CrossRef]
- Stack, J.; Haga, I.R.; Schröder, M.; Bartlett, N.W.; Maloney, G.; Reading, P.C.; Fitzgerald, K.A.; Smith, G.L.; Bowie, A.G. Vaccinia Virus Protein A46R Targets Multiple Toll-like–Interleukin-1 Receptor Adaptors and Contributes to Virulence. Journal of Experimental Medicine 2005, 201, 1007–1018. [CrossRef]
- Dempsey, A.; Keating, S.E.; Carty, M.; Bowie, A.G. Poxviral Protein E3–Altered Cytokine Production Reveals That DExD/H-Box Helicase 9 Controls Toll-like Receptor–Stimulated Immune Responses. J Biol Chem 2018, 293, 14989–15001. [CrossRef]
- Dai, P.; Cao, H.; Merghoub, T.; Avogadri, F.; Wang, W.; Parikh, T.; Fang, C.-M.; Pitha, P.M.; Fitzgerald, K.A.; Rahman, M.M.; et al. Myxoma Virus Induces Type I Interferon Production in Murine Plasmacytoid Dendritic Cells via a TLR9/MyD88-, IRF5/IRF7-, and IFNAR-Dependent Pathway. Journal of Virology 2011, 85, 10814–10825. [CrossRef]
- DiPerna, G.; Stack, J.; Bowie, A.G.; Boyd, A.; Kotwal, G.; Zhang, Z.; Arvikar, S.; Latz, E.; Fitzgerald, K.A.; Marshall, W.L. Poxvirus Protein N1L Targets the I-κB Kinase Complex, Inhibits Signaling to NF-κB by the Tumor Necrosis Factor Superfamily of Receptors, and Inhibits NF-κB and IRF3 Signaling by Toll-like Receptors *. Journal of Biological Chemistry 2004, 279, 36570–36578. [CrossRef]
- Albarnaz, J.D.; Ren, H.; Torres, A.A.; Shmeleva, E.V.; Melo, C.A.; Bannister, A.J.; Brember, M.P.; Chung, B.Y.-W.; Smith, G.L. Molecular Mimicry of NF-κB by Vaccinia Virus Protein Enables Selective Inhibition of Antiviral Responses. Nat Microbiol 2022, 7, 154–168. [CrossRef]
- Mansur, D.S.; Motes, C.M. de; Unterholzner, L.; Sumner, R.P.; Ferguson, B.J.; Ren, H.; Strnadova, P.; Bowie, A.G.; Smith, G.L. Poxvirus Targeting of E3 Ligase β-TrCP by Molecular Mimicry: A Mechanism to Inhibit NF-κB Activation and Promote Immune Evasion and Virulence. PLOS Pathogens 2013, 9, e1003183. [CrossRef]
- Smith, G.L.; Benfield, C.T.O.; Maluquer de Motes, C.; Mazzon, M.; Ember, S.W.J.; Ferguson, B.J.; Sumner, R.P. Vaccinia Virus Immune Evasion: Mechanisms, Virulence and Immunogenicity. Journal of General Virology 2013, 94, 2367–2392. [CrossRef]
- Symons, J.A.; Alcamí, A.; Smith, G.L. Vaccinia Virus Encodes a Soluble Type I Interferon Receptor of Novel Structure and Broad Species Specificity. Cell 1995, 81, 551–560. [CrossRef]
- Mann, B.A.; Huang, J.H.; Li, P.; Chang, H.-C.; Slee, R.B.; O’Sullivan, A.; Mathur, A.; Yeh, N.; Klemsz, M.J.; Brutkiewicz, R.R.; et al. Vaccinia Virus Blocks Stat1-Dependent and Stat1-Independent Gene Expression Induced by Type I and Type II Interferons. J Interferon Cytokine Res 2008, 28, 367–379. [CrossRef]
- Alvarez-de Miranda, F.J.; Alonso-Sánchez, I.; Alcamí, A.; Hernaez, B. TNF Decoy Receptors Encoded by Poxviruses. Pathogens 2021, 10, 1065. [CrossRef]
- Symons, J.A.; Tscharke, D.C.; Price, N.; Smith, G.L. A Study of the Vaccinia Virus Interferon-Gamma Receptor and Its Contribution to Virus Virulence. J Gen Virol 2002, 83, 1953–1964. [CrossRef]
- Reading, P.C.; Smith, G.L. Vaccinia Virus Interleukin-18-Binding Protein Promotes Virulence by Reducing Gamma Interferon Production and Natural Killer and T-Cell Activity. J Virol 2003, 77, 9960–9968. [CrossRef]
- Alcami, A.; Smith, G.L. A Soluble Receptor for Interleukin-1β Encoded by Vaccinia Virus: A Novel Mechanism of Virus Modulation of the Host Response to Infection. Cell 1992, 71, 153–167. [CrossRef]
- Charles A Janeway, J.; Travers, P.; Walport, M.; Shlomchik, M.J. The Complement System and Innate Immunity. In Immunobiology: The Immune System in Health and Disease. 5th edition; Garland Science, 2001.
- Bernet, J.; Mullick, J.; Panse, Y.; Parab, P.B.; Sahu, A. Kinetic Analysis of the Interactions between Vaccinia Virus Complement Control Protein and Human Complement Proteins C3b and C4b. Journal of Virology 2004, 78, 9446–9457. [CrossRef]
- Li, P.; Wang, N.; Zhou, D.; Yee, C.S.K.; Chang, C.-H.; Brutkiewicz, R.R.; Blum, J.S. Disruption of MHC Class II-Restricted Antigen Presentation by Vaccinia Virus1. The Journal of Immunology 2005, 175, 6481–6488. [CrossRef]
- Rehm, K.E.; Connor, R.F.; Jones, G.J.B.; Yimbu, K.; Roper, R.L. Vaccinia Virus A35R Inhibits MHC Class II Antigen Presentation. Virology 2010, 397, 176. [CrossRef]
- Orzalli, M.H.; Kagan, J.C. Apoptosis and Necroptosis as Host Defense Strategies to Prevent Viral Infection. Trends Cell Biol 2017, 27, 800–809. [CrossRef]
- Gregory, C.D.; Devitt, A. The Macrophage and the Apoptotic Cell: An Innate Immune Interaction Viewed Simplistically? Immunology 2004, 113, 1–14. [CrossRef]
- Veyer, D.L.; Carrara, G.; Maluquer de Motes, C.; Smith, G.L. Vaccinia Virus Evasion of Regulated Cell Death. Immunology Letters 2017, 186, 68–80. [CrossRef]
- Maluquer de Motes, C.; Cooray, S.; Ren, H.; Almeida, G.M.F.; McGourty, K.; Bahar, M.W.; Stuart, D.I.; Grimes, J.M.; Graham, S.C.; Smith, G.L. Inhibition of Apoptosis and NF-κB Activation by Vaccinia Protein N1 Occur via Distinct Binding Surfaces and Make Different Contributions to Virulence. PLoS Pathog 2011, 7, e1002430. [CrossRef]
- Liu, Y.; Pan, R.; Ouyang, Y.; Gu, W.; Xiao, T.; Yang, H.; Tang, L.; Wang, H.; Xiang, B.; Chen, P. Pyroptosis in Health and Disease: Mechanisms, Regulation and Clinical Perspective. Sig Transduct Target Ther 2024, 9, 1–28. [CrossRef]
- Gerlic, M.; Faustin, B.; Postigo, A.; Yu, E.C.-W.; Proell, M.; Gombosuren, N.; Krajewska, M.; Flynn, R.; Croft, M.; Way, M.; et al. Vaccinia Virus F1L Protein Promotes Virulence by Inhibiting Inflammasome Activation. Proceedings of the National Academy of Sciences 2013, 110, 7808–7813. [CrossRef]
- Gerlic, M.; Faustin, B.; Postigo, A.; Yu, E.C.-W.; Proell, M.; Gombosuren, N.; Krajewska, M.; Flynn, R.; Croft, M.; Way, M.; et al. Vaccinia Virus F1L Protein Promotes Virulence by Inhibiting Inflammasome Activation. Proceedings of the National Academy of Sciences 2013, 110, 7808–7813. [CrossRef]
- Liu, Y.; Liu, T.; Lei, T.; Zhang, D.; Du, S.; Girani, L.; Qi, D.; Lin, C.; Tong, R.; Wang, Y. RIP1/RIP3-Regulated Necroptosis as a Target for Multifaceted Disease Therapy (Review). Int J Mol Med 2019, 44, 771–786. [CrossRef]
- Koehler, H.S.; Jacobs, B.L. Subversion of Programed Cell Death by Poxviruses. Curr Top Microbiol Immunol 2023, 442, 105–131. [CrossRef]
- Chahroudi, A.; Chavan, R.; Koyzr, N.; Waller, E.K.; Silvestri, G.; Feinberg, M.B. Vaccinia Virus Tropism for Primary Hematolymphoid Cells Is Determined by Restricted Expression of a Unique Virus Receptor. J Virol 2005, 79, 10397–10407. [CrossRef]
- Engelmayer, J.; Larsson, M.; Subklewe, M.; Chahroudi, A.; Cox, W.I.; Steinman, R.M.; Bhardwaj, N. Vaccinia Virus Inhibits the Maturation of Human Dendritic Cells: A Novel Mechanism of Immune Evasion1. The Journal of Immunology 1999, 163, 6762–6768. [CrossRef]
- Object, object Vaccinia Virus Infection of Mature Dendritic Cells Results in Activation of Virus-Specific Naïve CD8+ T Cells: A Potential Mechanism for Direct Presentation.
- Yao, Y.; Li, P.; Singh, P.; Thiele, A.T.; Wilkes, D.S.; Renukaradhya, G.J.; Brutkiewicz, R.R.; Travers, J.B.; Luker, G.D.; Hong, S.-C.; et al. Vaccinia Virus Infection Induces Dendritic Cell Maturation but Inhibits Antigen Presentation by MHC Class II. Cell Immunol 2007, 246, 92–102. [CrossRef]
- Byrd, D.; Shepherd, N.; Lan, J.; Hu, N.; Amet, T.; Yang, K.; Desai, M.; Yu, Q. Primary Human Macrophages Serve as Vehicles for Vaccinia Virus Replication and Dissemination. J Virol 2014, 88, 6819–6831. [CrossRef]
- Zhou, D.; Xu, W.; Ding, X.; Guo, H.; Wang, J.; Zhao, G.; Zhang, C.; Zhang, Z.; Wang, Z.; Wang, P.; et al. Transient Inhibition of Neutrophil Functions Enhances the Antitumor Effect of Intravenously Delivered Oncolytic Vaccinia Virus. Cancer Sci 2024, 115, 1129–1140. [CrossRef]
- Duffy, D.; Perrin, H.; Abadie, V.; Benhabiles, N.; Boissonnas, A.; Liard, C.; Descours, B.; Reboulleau, D.; Bonduelle, O.; Verrier, B.; et al. Neutrophils Transport Antigen from the Dermis to the Bone Marrow, Initiating a Source of Memory CD8+ T Cells. Immunity 2012, 37, 917–929. [CrossRef]
- Abboud, G.; Tahiliani, V.; Desai, P.; Varkoly, K.; Driver, J.; Hutchinson, T.E.; Salek-Ardakani, S. Natural Killer Cells and Innate Interferon Gamma Participate in the Host Defense against Respiratory Vaccinia Virus Infection. Journal of Virology 2015, 90, 129–141. [CrossRef]
- Dokun, A.O.; Kim, S.; Smith, H.R.; Kang, H.S.; Chu, D.T.; Yokoyama, W.M. Specific and Nonspecific NK Cell Activation during Virus Infection. Nat Immunol 2001, 2, 951–956. [CrossRef]
- Chisholm, S.E.; Reyburn, H.T. Recognition of Vaccinia Virus-Infected Cells by Human Natural Killer Cells Depends on Natural Cytotoxicity Receptors. J Virol 2006, 80, 2225–2233. [CrossRef]
- Gillard, G.O.; Bivas-Benita, M.; Hovav, A.-H.; Grandpre, L.E.; Panas, M.W.; Seaman, M.S.; Haynes, B.F.; Letvin, N.L. Thy1+ Nk Cells from Vaccinia Virus-Primed Mice Confer Protection against Vaccinia Virus Challenge in the Absence of Adaptive Lymphocytes. PLOS Pathogens 2011, 7, e1002141. [CrossRef]
- Shepherd, N.; Lan, J.; Li, W.; Rane, S.; Yu, Q. Primary Human B Cells at Different Differentiation and Maturation Stages Exhibit Distinct Susceptibilities to Vaccinia Virus Binding and Infection. J Virol 2019, 93, e00973-19. [CrossRef]
- Goulding, J.; Bouge, R.; Tahiliani, V.; Croft, M.; Salek-Ardakani, S. CD8 T Cells Are Essential for Recovery from a Respiratory Vaccinia Virus Infection. J Immunol 2012, 189, 2432–2440. [CrossRef]
- Bernasconi, N.L.; Traggiai, E.; Lanzavecchia, A. Maintenance of Serological Memory by Polyclonal Activation of Human Memory B Cells. Science 2002, 298, 2199–2202. [CrossRef]
- Liu, L.; Zhong, Q.; Tian, T.; Dubin, K.; Athale, S.K.; Kupper, T.S. Epidermal Injury and Infection during Poxvirus Immunization Is Crucial for the Generation of Highly Protective T Cell-Mediated Immunity. Nat Med 2010, 16, 224–227. [CrossRef]
- Sánchez-Puig, J.M.; Sánchez, L.; Roy, G.; Blasco, R. Susceptibility of Different Leukocyte Cell Types to Vaccinia Virus Infection. Virol J 2004, 1, 10. [CrossRef]
- Hu, Z.; Molloy, M.J.; Usherwood, E.J. CD4+ T-Cell Dependence of Primary CD8+ T-Cell Response against Vaccinia Virus Depends upon Route of Infection and Viral Dose. Cell Mol Immunol 2016, 13, 82–93. [CrossRef]
- Dai, R.; Huang, X.; Yang, Y. γδT Cells Are Required for CD8+ T Cell Response to Vaccinia Viral Infection. Front. Immunol. 2021, 12. [CrossRef]
- Demkowicz, W.E.; Littaua, R.A.; Wang, J.; Ennis, F.A. Human Cytotoxic T-Cell Memory: Long-Lived Responses to Vaccinia Virus. J Virol 1996, 70, 2627–2631.
- Mercer, J.; Knébel, S.; Schmidt, F.I.; Crouse, J.; Burkard, C.; Helenius, A. Vaccinia Virus Strains Use Distinct Forms of Macropinocytosis for Host-Cell Entry. Proceedings of the National Academy of Sciences 2010, 107, 9346–9351. [CrossRef]
- Yaghchi, C.A.; Zhang, Z.; Alusi, G.; Lemoine, N.R.; Wang, Y. Vaccinia Virus, a Promising New Therapeutic Agent for Pancreatic Cancer. Immunotherapy 2015, 7, 1249–1258. [CrossRef]
- Lei, W.; Ye, Q.; Hao, Y.; Chen, J.; Huang, Y.; Yang, L.; Wang, S.; Qian, W. CD19-Targeted BiTE Expression by an Oncolytic Vaccinia Virus Significantly Augments Therapeutic Efficacy against B-Cell Lymphoma. Blood Cancer J. 2022, 12, 1–10. [CrossRef]
- Smith, G.L.; Moss, B. Infectious Poxvirus Vectors Have Capacity for at Least 25 000 Base Pairs of Foreign DNA. Gene 1983, 25, 21–28. [CrossRef]
- Gallardo, F.; Schmitt, D.; Brandely, R.; Brua, C.; Silvestre, N.; Findeli, A.; Foloppe, J.; Top, S.; Kappler-Gratias, S.; Quentin-Froignant, C.; et al. Fluorescent Tagged Vaccinia Virus Genome Allows Rapid and Efficient Measurement of Oncolytic Potential and Discovery of Oncolytic Modulators. Biomedicines 2020, 8, 543. [CrossRef]
- Hiley, C.T.; Yuan, M.; Lemoine, N.R.; Wang, Y. Lister Strain Vaccinia Virus, a Potential Therapeutic Vector Targeting Hypoxic Tumours. Gene Ther 2010, 17, 281–287. [CrossRef]
- Ma, J.; Ramachandran, M.; Jin, C.; Quijano-Rubio, C.; Martikainen, M.; Yu, D.; Essand, M. Characterization of Virus-Mediated Immunogenic Cancer Cell Death and the Consequences for Oncolytic Virus-Based Immunotherapy of Cancer. Cell Death Dis 2020, 11, 1–15. [CrossRef]
- Fucikova, J.; Kepp, O.; Kasikova, L.; Petroni, G.; Yamazaki, T.; Liu, P.; Zhao, L.; Spisek, R.; Kroemer, G.; Galluzzi, L. Detection of Immunogenic Cell Death and Its Relevance for Cancer Therapy. Cell Death Dis 2020, 11, 1–13. [CrossRef]
- O’Leary, M.P.; Choi, A.H.; Kim, S.-I.; Chaurasiya, S.; Lu, J.; Park, A.K.; Woo, Y.; Warner, S.G.; Fong, Y.; Chen, N.G. Novel Oncolytic Chimeric Orthopoxvirus Causes Regression of Pancreatic Cancer Xenografts and Exhibits Abscopal Effect at a Single Low Dose. J Transl Med 2018, 16, 110. [CrossRef]
- Choi, A.H.; O’Leary, M.P.; Lu, J.; Kim, S.-I.; Fong, Y.; Chen, N.G. Endogenous Akt Activity Promotes Virus Entry and Predicts Efficacy of Novel Chimeric Orthopoxvirus in Triple-Negative Breast Cancer. Mol Ther Oncolytics 2018, 9, 22–29. [CrossRef]
- Chaurasiya, S.; Yang, A.; Zhang, Z.; Lu, J.; Valencia, H.; Kim, S.-I.; Woo, Y.; Warner, S.G.; Olafsen, T.; Zhao, Y.; et al. A Comprehensive Preclinical Study Supporting Clinical Trial of Oncolytic Chimeric Poxvirus CF33-hNIS-Anti-PD-L1 to Treat Breast Cancer. Mol Ther Methods Clin Dev 2021, 24, 102–116. [CrossRef]
- Warner, S.G.; Kim, S.-I.; Chaurasiya, S.; O’Leary, M.P.; Lu, J.; Sivanandam, V.; Woo, Y.; Chen, N.G.; Fong, Y. A Novel Chimeric Poxvirus Encoding hNIS Is Tumor-Tropic, Imageable, and Synergistic with Radioiodine to Sustain Colon Cancer Regression. Mol Ther Oncolytics 2019, 13, 82–92. [CrossRef]
- Yang, A.; Zhang, Z.; Chaurasiya, S.; Park, A.K.; Jung, A.; Lu, J.; Kim, S.-I.; Priceman, S.; Fong, Y.; Woo, Y. Development of the Oncolytic Virus, CF33, and Its Derivatives for Peritoneal-Directed Treatment of Gastric Cancer Peritoneal Metastases. J Immunother Cancer 2023, 11, e006280. [CrossRef]
- Chaurasiya, S.; Yang, A.; Zhang, Z.; Lu, J.; Valencia, H.; Kim, S.-I.; Woo, Y.; Warner, S.G.; Olafsen, T.; Zhao, Y.; et al. A Comprehensive Preclinical Study Supporting Clinical Trial of Oncolytic Chimeric Poxvirus CF33-hNIS-Anti-PD-L1 to Treat Breast Cancer. Mol Ther Methods Clin Dev 2021, 24, 102–116. [CrossRef]
- Chen, C.; Park, A.K.; Monroy, I.; Ren, Y.; Kim, S.-I.; Chaurasiya, S.; Priceman, S.J.; Fong, Y. Using Oncolytic Virus to Retask CD19-Chimeric Antigen Receptor T Cells for Treatment of Pancreatic Cancer: Toward a Universal Chimeric Antigen Receptor T-Cell Strategy for Solid Tumor. J Am Coll Surg 2024, 238, 436–447. [CrossRef]
- Earl, P.L.; Moss, B.; Wyatt, L.S. Generation of Recombinant Vaccinia Viruses. Curr Protoc Protein Sci 2017, 89, 5.13.1-5.13.18. [CrossRef]
- Laudermilch, E.; Chandran, K. MAVERICC: Marker-Free Vaccinia Virus Engineering of Recombinants through in Vitro CRISPR/Cas9 Cleavage. J Mol Biol 2021, 433, 166896. [CrossRef]
- Domi, A.; Moss, B. Cloning the Vaccinia Virus Genome as a Bacterial Artificial Chromosome in Escherichia Coli and Recovery of Infectious Virus in Mammalian Cells. Proc Natl Acad Sci U S A 2002, 99, 12415–12420. [CrossRef]
- Yuan, M.; Zhang, W.; Wang, J.; Al Yaghchi, C.; Ahmed, J.; Chard, L.; Lemoine, N.R.; Wang, Y. Efficiently Editing the Vaccinia Virus Genome by Using the CRISPR-Cas9 System. J Virol 2015, 89, 5176–5179. [CrossRef]
- Xu, L.; Sun, H.; Lemoine, N.R.; Xuan, Y.; Wang, P. Oncolytic Vaccinia Virus and Cancer Immunotherapy. Front. Immunol. 2024, 14. [CrossRef]
- Pfeffer, C.M.; Singh, A.T.K. Apoptosis: A Target for Anticancer Therapy. Int J Mol Sci 2018, 19, 448. [CrossRef]
- de Queiroz, N.M.G.P.; Xia, T.; Konno, H.; Barber, G.N. Ovarian Cancer Cells Commonly Exhibit Defective STING Signaling Which Affects Sensitivity to Viral Oncolysis. Molecular Cancer Research 2019, 17, 974–986. [CrossRef]
- Aye, Y.; Li, M.; Long, M.J.C.; Weiss, R.S. Ribonucleotide Reductase and Cancer: Biological Mechanisms and Targeted Therapies. Oncogene 2015, 34, 2011–2021. [CrossRef]
- Bitter, E.E.; Townsend, M.H.; Erickson, R.; Allen, C.; O’Neill, K.L. Thymidine Kinase 1 through the Ages: A Comprehensive Review. Cell & Bioscience 2020, 10, 138. [CrossRef]
- Potts, K.G.; Irwin, C.R.; Favis, N.A.; Pink, D.B.; Vincent, K.M.; Lewis, J.D.; Moore, R.B.; Hitt, M.M.; Evans, D.H. Deletion of F4L (Ribonucleotide Reductase) in Vaccinia Virus Produces a Selective Oncolytic Virus and Promotes Anti-Tumor Immunity with Superior Safety in Bladder Cancer Models. EMBO Mol Med 2017, 9, 638–654. [CrossRef]
- Puhlmann, M.; Brown, C.K.; Gnant, M.; Huang, J.; Libutti, S.K.; Alexander, H.R.; Bartlett, D.L. Vaccinia as a Vector for Tumor-Directed Gene Therapy: Biodistribution of a Thymidine Kinase-Deleted Mutant. Cancer Gene Ther 2000, 7, 66–73. [CrossRef]
- Breitbach, C.J.; Arulanandam, R.; De Silva, N.; Thorne, S.H.; Patt, R.; Daneshmand, M.; Moon, A.; Ilkow, C.; Burke, J.; Hwang, T.-H.; et al. Oncolytic Vaccinia Virus Disrupts Tumor-Associated Vasculature in Humans. Cancer Research 2013, 73, 1265–1275. [CrossRef]
- Lai, A.C.-K.; Pogo, B.G.-T. Attenuated Deletion Mutants of Vaccinia Virus Lacking the Vaccinia Growth Factor Are Defective in Replication in Vivo. Microbial Pathogenesis 1989, 6, 219–226. [CrossRef]
- DeHaven, B.C.; Gupta, K.; Isaacs, S.N. The Vaccinia Virus A56 Protein: A Multifunctional Transmembrane Glycoprotein That Anchors Two Secreted Viral Proteins. Journal of General Virology 2011, 92, 1971–1980. [CrossRef]
- Izmailyan, R.; Chang, W. Vaccinia Virus WR53.5/F14.5 Protein Is a New Component of Intracellular Mature Virus and Is Important for Calcium-Independent Cell Adhesion and Vaccinia Virus Virulence in Mice. J Virol 2008, 82, 10079–10087. [CrossRef]
- Zhang, Q.; Liang, C.; Yu, Y.A.; Chen, N.; Dandekar, T.; Szalay, A.A. The Highly Attenuated Oncolytic Recombinant Vaccinia Virus GLV-1h68: Comparative Genomic Features and the Contribution of F14.5L Inactivation. Mol Genet Genomics 2009, 282, 417–435. [CrossRef]
- Papatriantafyllou, M. GM-CSF in Focus. Nat Rev Immunol 2011, 11, 370–371. [CrossRef]
- Deng, L.; Fan, J.; Guo, M.; Huang, B. Oncolytic and Immunologic Cancer Therapy with GM-CSF-Armed Vaccinia Virus of Tian Tan Strain Guang9. Cancer Letters 2016, 372, 251–257. [CrossRef]
- Xuan, Y.; Yan, W.; Wang, R.; Wang, X.; Guo, Y.; Dun, H.; Huan, Z.; Xu, L.; Han, R.; Sun, X.; et al. GM-CSF and IL-21-Armed Oncolytic Vaccinia Virus Significantly Enhances Anti-Tumor Activity and Synergizes with Anti-PD1 Immunotherapy in Pancreatic Cancer. Front Immunol 2025, 15, 1506632. [CrossRef]
- Li, J.; O’Malley, M.; Urban, J.; Sampath, P.; Guo, Z.S.; Kalinski, P.; Thorne, S.H.; Bartlett, D.L. Chemokine Expression From Oncolytic Vaccinia Virus Enhances Vaccine Therapies of Cancer. Molecular Therapy 2011, 19, 650–657. [CrossRef]
- Liu, Z.; Ravindranathan ,Roshni; Li ,Jun; Kalinski ,Pawel; Guo ,Z. Sheng; and Bartlett, D.L. CXCL11-Armed Oncolytic Poxvirus Elicits Potent Antitumor Immunity and Shows Enhanced Therapeutic Efficacy. OncoImmunology 2016, 5, e1091554. [CrossRef]
- McCart, J.A.; Ward, J.M.; Lee, J.; Hu, Y.; Alexander, H.R.; Libutti, S.K.; Moss, B.; Bartlett, D.L. Systemic Cancer Therapy with a Tumor-Selective Vaccinia Virus Mutant Lacking Thymidine Kinase and Vaccinia Growth Factor Genes. Cancer Res 2001, 61, 8751–8757.
- Guo, Z.S.; Naik, A.; O’Malley, M.E.; Popovic, P.; Demarco, R.; Hu, Y.; Yin, X.; Yang, S.; Zeh, H.J.; Moss, B.; et al. The Enhanced Tumor Selectivity of an Oncolytic Vaccinia Lacking the Host Range and Antiapoptosis Genes SPI-1 and SPI-2. Cancer Res 2005, 65, 9991–9998. [CrossRef]
- Yang, S.; Guo, Z.S.; O’Malley, M.E.; Yin, X.; Zeh, H.J.; Bartlett, D.L. A New Recombinant Vaccinia with Targeted Deletion of Three Viral Genes: Its Safety and Efficacy as an Oncolytic Virus. Gene Ther 2007, 14, 638–647. [CrossRef]
- Kirn, D.H.; Wang, Y.; Le Boeuf, F.; Bell, J.; Thorne, S.H. Targeting of Interferon-Beta to Produce a Specific, Multi-Mechanistic Oncolytic Vaccinia Virus. PLoS Med 2007, 4, e353. [CrossRef]
- Eradication of Solid Human Breast Tumors in Nude Mice with an Intravenously Injected Light-Emitting Oncolytic Vaccinia Virus | Cancer Research | American Association for Cancer Research Available online: https://aacrjournals.org/cancerres/article/67/20/10038/533730/Eradication-of-Solid-Human-Breast-Tumors-in-Nude (accessed on 28 May 2025).
- Foloppe, J.; Kempf, J.; Futin, N.; Kintz, J.; Cordier, P.; Pichon, C.; Findeli, A.; Vorburger, F.; Quemeneur, E.; Erbs, P. The Enhanced Tumor Specificity of TG6002, an Armed Oncolytic Vaccinia Virus Deleted in Two Genes Involved in Nucleotide Metabolism. Mol Ther Oncolytics 2019, 14, 1–14. [CrossRef]
- Jia, Y.; Wang, Y.; Zhao, G.; Yang, Y.; Yan, W.; Wang, R.; Han, B.; Wang, L.; Zhang, Z.; Chen, L.; et al. Novel Oncolytic Vaccinia Virus Armed with Interleukin-27 Is a Potential Therapeutic Agent for the Treatment of Murine Pancreatic Cancer. J Immunother Cancer 2025, 13, e010341. [CrossRef]
- Neidel, S.; Torres, A.A.; Ren, H.; Smith, G.L. Leaky Scanning Translation Generates a Second A49 Protein That Contributes to Vaccinia Virus Virulence. J Gen Virol 2020, 101, 533–541. [CrossRef]
- Pelin, A.; Foloppe, J.; Petryk, J.; Singaravelu, R.; Hussein, M.; Gossart, F.; Jennings, V.A.; Stubbert, L.J.; Foster, M.; Storbeck, C.; et al. Deletion of Apoptosis Inhibitor F1L in Vaccinia Virus Increases Safety and Oncolysis for Cancer Therapy. Mol Ther Oncolytics 2019, 14, 246–252. [CrossRef]
- Riederer, S.; del Canizo, A.; Navas, J.; Peter, M.G.; Link, E.K.; Sutter, G.; Rojas, J.J. Improving Poxvirus-Mediated Antitumor Immune Responses by Deleting Viral cGAMP-Specific Nuclease. Cancer Gene Ther 2023, 30, 1029–1039. [CrossRef]
- Ge, Y.; Wang, H.; Ren, J.; Liu, W.; Chen, L.; Chen, H.; Ye, J.; Dai, E.; Ma, C.; Ju, S.; et al. Oncolytic Vaccinia Virus Delivering Tethered IL-12 Enhances Antitumor Effects with Improved Safety. J Immunother Cancer 2020, 8, e000710. [CrossRef]
- Shakiba, Y.; Vorobyev, P.O.; Yusubalieva, G.M.; Kochetkov, D.V.; Zajtseva, K.V.; Valikhov, M.P.; Kalsin, V.A.; Zabozlaev, F.G.; Semkina, A.S.; Troitskiy, A.V.; et al. Oncolytic Therapy with Recombinant Vaccinia Viruses Targeting the Interleukin-15 Pathway Elicits a Synergistic Response. Molecular Therapy - Oncolytics 2023, 29, 158–168. [CrossRef]
- Chen, L.; Chen, H.; Ye, J.; Ge, Y.; Wang, H.; Dai, E.; Ren, J.; Liu, W.; Ma, C.; Ju, S.; et al. Intratumoral Expression of Interleukin 23 Variants Using Oncolytic Vaccinia Virus Elicit Potent Antitumor Effects on Multiple Tumor Models via Tumor Microenvironment Modulation. Theranostics 2021, 11, 6668–6681. [CrossRef]
- Chard, L.S.; Maniati, E.; Wang, P.; Zhang, Z.; Gao, D.; Wang, J.; Cao, F.; Ahmed, J.; El Khouri, M.; Hughes, J.; et al. A Vaccinia Virus Armed with Interleukin-10 Is a Promising Therapeutic Agent for Treatment of Murine Pancreatic Cancer. Clinical Cancer Research 2015, 21, 405–416. [CrossRef]
- Mittal, S.K.; Cho, K.-J.; Ishido, S.; Roche, P.A. Interleukin 10 (IL-10)-Mediated Immunosuppression. J Biol Chem 2015, 290, 27158–27167. [CrossRef]
- Holicek, P.; Guilbaud, E.; Klapp, V.; Truxova, I.; Spisek, R.; Galluzzi, L.; Fucikova, J. Type I Interferon and Cancer. Immunol Rev 2024, 321, 115–127. [CrossRef]
- Riederer, S.; del Canizo, A.; Navas, J.; Peter, M.G.; Link, E.K.; Sutter, G.; Rojas, J.J. Improving Poxvirus-Mediated Antitumor Immune Responses by Deleting Viral cGAMP-Specific Nuclease. Cancer Gene Ther 2023, 30, 1029–1039. [CrossRef]
- Hirvinen, M.; Capasso, C.; Guse, K.; Garofalo, M.; Vitale, A.; Ahonen, M.; Kuryk, L.; Vähä-Koskela, M.; Hemminki, A.; Fortino, V.; et al. Expression of DAI by an Oncolytic Vaccinia Virus Boosts the Immunogenicity of the Virus and Enhances Antitumor Immunity. Molecular Therapy - Oncolytics 2016, 3. [CrossRef]
- Wang, X.; Zhou, N.; Liu, T.; Jia, X.; Ye, T.; Chen, K.; Li, G. Oncolytic Vaccinia Virus Expressing White-Spotted Charr Lectin Regulates Antiviral Response in Tumor Cells and Inhibits Tumor Growth In Vitro and In Vivo. Marine Drugs 2021, 19, 292. [CrossRef]
- Hinterberger, M.; Giessel, R.; Fiore, G.; Graebnitz, F.; Bathke, B.; Wennier, S.; Chaplin, P.; Melero, I.; Suter, M.; Lauterbach, H.; et al. Intratumoral Virotherapy with 4-1BBL Armed Modified Vaccinia Ankara Eradicates Solid Tumors and Promotes Protective Immune Memory. J Immunother Cancer 2021, 9, e001586. [CrossRef]
- Parviainen, S.; Ahonen, M.; Diaconu, I.; Hirvinen, M.; Karttunen, Å.; Vähä-Koskela, M.; Hemminki, A.; Cerullo, V. CD40 Ligand and tdTomato-Armed Vaccinia Virus for Induction of Antitumor Immune Response and Tumor Imaging. Gene Ther 2014, 21, 195–204. [CrossRef]
- Yang, N.; Wang, Y.; Liu, S.; Tariq, S.B.; Luna, J.M.; Mazo, G.; Tan, A.; Zhang, T.; Wang, J.; Yan, W.; et al. OX40L-Expressing Recombinant Modified Vaccinia Virus Ankara Induces Potent Antitumor Immunity via Reprogramming Tregs. Journal of Experimental Medicine 2023, 220, e20221166. [CrossRef]
- Yu, F.; Wang, X.; Guo, Z.S.; Bartlett, D.L.; Gottschalk, S.M.; Song, X.-T. T-Cell Engager-Armed Oncolytic Vaccinia Virus Significantly Enhances Antitumor Therapy. Molecular Therapy 2014, 22, 102–111. [CrossRef]
- DePeaux, K.; Rivadeneira, D.B.; Lontos, K.; Dean, V.G.; Gunn, W.G.; Watson, M.J.; Yao, T.; Wilfahrt, D.; Hinck, C.; Wieteska, L.; et al. An Oncolytic Virus–Delivered TGFβ Inhibitor Overcomes the Immunosuppressive Tumor Microenvironment. Journal of Experimental Medicine 2023, 220, e20230053. [CrossRef]
- Frentzen, A.; Yu, Y.A.; Chen, N.; Zhang, Q.; Weibel, S.; Raab, V.; Szalay, A.A. Anti-VEGF Single-Chain Antibody GLAF-1 Encoded by Oncolytic Vaccinia Virus Significantly Enhances Antitumor Therapy. Proceedings of the National Academy of Sciences 2009, 106, 12915–12920. [CrossRef]
- Brown, C.E.; Mackall, C.L. CAR T Cell Therapy: Inroads to Response and Resistance. Nat Rev Immunol 2019, 19, 73–74. [CrossRef]
- Aalipour, A.; Boeuf, F.L.; Tang, M.; Murty, S.; Simonetta, F.; Lozano, A.X.; Shaffer, T.M.; Bell, J.C.; Gambhir, S.S. Viral Delivery of CAR Targets to Solid Tumors Enables Effective Cell Therapy. Molecular Therapy - Oncolytics 2020, 17, 232–240. [CrossRef]
- Park, A.K.; Fong, Y.; Kim, S.-I.; Yang, J.; Murad, J.P.; Lu, J.; Jeang, B.; Chang, W.-C.; Chen, N.G.; Thomas, S.H.; et al. Effective Combination Immunotherapy Using Oncolytic Viruses to Deliver CAR Targets to Solid Tumors. Sci Transl Med 2020, 12, eaaz1863. [CrossRef]
- Moon, E.K.; Wang ,Liang-Chuan S.; Bekdache ,Kheng; Lynn ,Rachel C.; Lo ,Albert; Thorne ,Stephen H.; and Albelda, S.M. Intra-Tumoral Delivery of CXCL11 via a Vaccinia Virus, but Not by Modified T Cells, Enhances the Efficacy of Adoptive T Cell Therapy and Vaccines. OncoImmunology 2018, 7, e1395997. [CrossRef]
- Sivanandam, V.; LaRocca, C.J.; Chen, N.G.; Fong, Y.; Warner, S.G. Oncolytic Viruses and Immune Checkpoint Inhibition: The Best of Both Worlds. Mol Ther Oncolytics 2019, 13, 93–106. [CrossRef]
- Chaurasiya, S.; Chen, N.G.; Fong, Y. Oncolytic Viruses and Immunity. Curr Opin Immunol 2018, 51, 83–90. [CrossRef]
- Sun, Y.; Zhang, Z.; Zhang, C.; Zhang, N.; Wang, P.; Chu, Y.; Dunmall, L.S.C.; Lemoine, N.R.; Wang, Y. An Effective Therapeutic Regime for Treatment of Glioma Using Oncolytic Vaccinia Virus Expressing IL-21 in Combination with Immune Checkpoint Inhibition. Molecular Therapy - Oncolytics 2022, 26, 105–119. [CrossRef]
- Lou, J.; Dong, J.; Xu, R.; Zeng, H.; Fang, L.; Wu, Y.; Liu, Y.; Wang, S. Remodeling of the Tumor Microenvironment Using an Engineered Oncolytic Vaccinia Virus Improves PD-L1 Inhibition Outcomes. Bioscience Reports 2021, 41, BSR20204186. [CrossRef]
- Liu, Z.; Ravindranathan, R.; Kalinski, P.; Guo, Z.S.; Bartlett, D.L. Rational Combination of Oncolytic Vaccinia Virus and PD-L1 Blockade Works Synergistically to Enhance Therapeutic Efficacy. Nat Commun 2017, 8, 14754. [CrossRef]
- Wang, G.; Kang, X.; Chen, K.S.; Jehng, T.; Jones, L.; Chen, J.; Huang, X.F.; Chen, S.-Y. An Engineered Oncolytic Virus Expressing PD-L1 Inhibitors Activates Tumor Neoantigen-Specific T Cell Responses. Nat Commun 2020, 11, 1395. [CrossRef]
- Semmrich, M.; Marchand, J.-B.; Fend, L.; Rehn, M.; Remy, C.; Holmkvist, P.; Silvestre, N.; Svensson, C.; Kleinpeter, P.; Deforges, J.; et al. Vectorized Treg-Depleting αCTLA-4 Elicits Antigen Cross-Presentation and CD8+ T Cell Immunity to Reject ‘Cold’ Tumors. J Immunother Cancer 2022, 10, e003488. [CrossRef]
- Zuo, S.; Wei, M.; Xu, T.; Kong, L.; He, B.; Wang, S.; Wang, S.; Wu, J.; Dong, J.; Wei, J. An Engineered Oncolytic Vaccinia Virus Encoding a Single-Chain Variable Fragment against TIGIT Induces Effective Antitumor Immunity and Synergizes with PD-1 or LAG-3 Blockade. J Immunother Cancer 2021, 9, e002843. [CrossRef]
- Advani, S.J.; Buckel, L.; Chen, N.G.; Scanderbeg, D.J.; Geissinger, U.; Zhang, Q.; Yu, Y.A.; Aguilar, R.J.; Mundt, A.J.; Szalay, A.A. Preferential Replication of Systemically Delivered Oncolytic Vaccinia Virus in Focally Irradiated Glioma Xenografts. Clinical Cancer Research 2012, 18, 2579–2590. [CrossRef]
- Storozynsky, Q.T.; Agopsowicz, K.C.; Noyce, R.S.; Bukhari, A.B.; Han, X.; Snyder, N.; Umer, B.A.; Gamper, A.M.; Godbout, R.; Evans, D.H.; et al. Radiation Combined with Oncolytic Vaccinia Virus Provides Pronounced Antitumor Efficacy and Induces Immune Protection in an Aggressive Glioblastoma Model. Cancer Letters 2023, 562, 216169. [CrossRef]
- Dai, M.H.; Liu, S.L.; Chen, N.G.; Zhang, T.P.; You, L.; Q. Zhang, F.; Chou, T.C.; Szalay, A.A.; Fong, Y.; Zhao, Y.P. Oncolytic Vaccinia Virus in Combination with Radiation Shows Synergistic Antitumor Efficacy in Pancreatic Cancer. Cancer Letters 2014, 344, 282–290. [CrossRef]
- Chen, W.-Y.; Chen, Y.-L.; Lin, H.-W.; Chang, C.-F.; Huang, B.-S.; Sun, W.-Z.; Cheng, W.-F. Stereotactic Body Radiation Combined with Oncolytic Vaccinia Virus Induces Potent Anti-Tumor Effect by Triggering Tumor Cell Necroptosis and DAMPs. Cancer Letters 2021, 523, 149–161. [CrossRef]
- Gholami, S.; Haddad, D.; Chen, C.-H.; Chen, N.G.; Zhang, Q.; Zanzonico, P.B.; Szalay, A.A.; Fong, Y. Novel Therapy for Anaplastic Thyroid Carcinoma Cells Using an Oncolytic Vaccinia Virus Carrying the Human Sodium Iodide Symporter. Surgery 2011, 150, 1040–1047. [CrossRef]
- Gholami, S.; Chen, C.-H.; Lou, E.; De Brot, M.; Fujisawa, S.; Chen, N.G.; Szalay, A.A.; Fong, Y. Vaccinia Virus GLV-1h153 Is Effective in Treating and Preventing Metastatic Triple-Negative Breast Cancer. Annals of Surgery 2012, 256, 437. [CrossRef]
- Haddad, D.; Chen, N.G.; Zhang, Q.; Chen, C.-H.; Yu, Y.A.; Gonzalez, L.; Carpenter, S.G.; Carson, J.; Au, J.; Mittra, A.; et al. Insertion of the Human Sodium Iodide Symporter to Facilitate Deep Tissue Imaging Does Not Alter Oncolytic or Replication Capability of a Novel Vaccinia Virus. J Transl Med 2011, 9, 36. [CrossRef]
- Mansfield, D.C.; Kyula, J.N.; Rosenfelder, N.; Chao-Chu, J.; Kramer-Marek, G.; Khan, A.A.; Roulstone, V.; McLaughlin, M.; Melcher, A.A.; Vile, R.G.; et al. Oncolytic Vaccinia Virus as a Vector for Therapeutic Sodium Iodide Symporter Gene Therapy in Prostate Cancer. Gene Ther 2016, 23, 357–368. [CrossRef]
- Ottolino-Perry, K.; Mealiea, D.; Sellers, C.; Acuna, S.A.; Angarita, F.A.; Okamoto, L.; Scollard, D.; Ginj, M.; Reilly, R.; McCart, J.A. Vaccinia Virus and Peptide-Receptor Radiotherapy Synergize to Improve Treatment of Peritoneal Carcinomatosis. Molecular Therapy - Oncolytics 2023, 29, 44–58. [CrossRef]
- Huang, B.; Sikorski, R.; Kirn, D.H.; Thorne, S.H. Synergistic Anti-Tumor Effects between Oncolytic Vaccinia Virus and Paclitaxel Are Mediated by the IFN Response and HMGB1. Gene Ther 2011, 18, 164–172. [CrossRef]
- Yu, Y.A.; Galanis, C.; Woo, Y.; Chen, N.; Zhang, Q.; Fong, Y.; Szalay, A.A. Regression of Human Pancreatic Tumor Xenografts in Mice after a Single Systemic Injection of Recombinant Vaccinia Virus GLV-1h68. Molecular Cancer Therapeutics 2009, 8, 141–151. [CrossRef]
- Lun, X.Q.; Jang, J.-H.; Tang, N.; Deng, H.; Head, R.; Bell, J.C.; Stojdl, D.F.; Nutt, C.L.; Senger, D.L.; Forsyth, P.A.; et al. Efficacy of Systemically Administered Oncolytic Vaccinia Virotherapy for Malignant Gliomas Is Enhanced by Combination Therapy with Rapamycin or Cyclophosphamide. Clinical Cancer Research 2009, 15, 2777–2788. [CrossRef]
- Hofmann, E.; Weibel, S.; Szalay, A.A. Combination Treatment with Oncolytic Vaccinia Virus and Cyclophosphamide Results in Synergistic Antitumor Effects in Human Lung Adenocarcinoma Bearing Mice. Journal of Translational Medicine 2014, 12, 197. [CrossRef]
- Berchtold, S.; Beil, J.; Raff, C.; Smirnow, I.; Schell, M.; D’Alvise, J.; Gross, S.; Lauer, U.M. Assessing and Overcoming Resistance Phenomena against a Genetically Modified Vaccinia Virus in Selected Cancer Cell Lines. International Journal of Molecular Sciences 2020, 21, 7618. [CrossRef]
- Seubert, C.M.; Stritzker, J.; Hess, M.; Donat, U.; Sturm, J.B.; Chen, N.; Hof, J.M. von; Krewer, B.; Tietze, L.F.; Gentschev, I.; et al. Enhanced Tumor Therapy Using Vaccinia Virus Strain GLV-1h68 in Combination with a β-Galactosidase-Activatable Prodrug Seco-Analog of Duocarmycin SA. Cancer Gene Ther 2011, 18, 42–52. [CrossRef]
- Lee, S.; Yang, W.; Kim, D.K.; Kim, H.; Shin, M.; Choi, K.U.; Suh, D.S.; Kim, Y.H.; Hwang, T.-H.; Kim, J.H. Inhibition of MEK-ERK Pathway Enhances Oncolytic Vaccinia Virus Replication in Doxorubicin-Resistant Ovarian Cancer. Molecular Therapy - Oncolytics 2022, 25, 211–224. [CrossRef]
- Ferguson, M.S.; Dunmall, L.S.C.; Gangeswaran, R.; Marelli, G.; Tysome, J.R.; Burns, E.; Whitehead, M.A.; Aksoy, E.; Alusi, G.; Hiley, C.; et al. Transient Inhibition of PI3Kδ Enhances the Therapeutic Effect of Intravenous Delivery of Oncolytic Vaccinia Virus. Molecular Therapy 2020, 28, 1263–1275. [CrossRef]
- MacTavish, H.; Diallo, J.-S.; Huang, B.; Stanford, M.; Boeuf, F.L.; Silva, N.D.; Cox, J.; Simmons, J.G.; Guimond, T.; Falls, T.; et al. Enhancement of Vaccinia Virus Based Oncolysis with Histone Deacetylase Inhibitors. PLOS ONE 2010, 5, e14462. [CrossRef]
- Kim, M.; Nitschké, M.; Sennino, B.; Murer, P.; Schriver, B.J.; Bell, A.; Subramanian, A.; McDonald, C.E.; Wang, J.; Cha, H.; et al. Amplification of Oncolytic Vaccinia Virus Widespread Tumor Cell Killing by Sunitinib through Multiple Mechanisms. Cancer Research 2018, 78, 922–937. [CrossRef]
- Heo, J.; Breitbach, C.J.; Moon, A.; Kim, C.W.; Patt, R.; Kim, M.K.; Lee, Y.K.; Oh, S.Y.; Woo, H.Y.; Parato, K.; et al. Sequential Therapy With JX-594, A Targeted Oncolytic Poxvirus, Followed by Sorafenib in Hepatocellular Carcinoma: Preclinical and Clinical Demonstration of Combination Efficacy. Molecular Therapy 2011, 19, 1170–1179. [CrossRef]
- Chen, N.G.; and Szalay, A.A. Oncolytic Vaccinia Virus: A Theranostic Agent for Cancer. Future Virology 2010, 5, 763–784. [CrossRef]
- Heo, J.; Reid, T.; Ruo, L.; Breitbach, C.J.; Rose, S.; Bloomston, M.; Cho, M.; Lim, H.Y.; Chung, H.C.; Kim, C.W.; et al. Randomized Dose-Finding Clinical Trial of Oncolytic Immunotherapeutic Vaccinia JX-594 in Liver Cancer. Nat Med 2013, 19, 329–336. [CrossRef]
- Moehler, M.; Heo, J.; Lee, H.C.; Tak, W.Y.; Chao, Y.; Paik, S.W.; Yim, H.J.; Byun, K.S.; Baron, A.; Ungerechts, G.; et al. Vaccinia-Based Oncolytic Immunotherapy Pexastimogene Devacirepvec in Patients with Advanced Hepatocellular Carcinoma after Sorafenib Failure: A Randomized Multicenter Phase IIb Trial (TRAVERSE). Oncoimmunology 2019, 8, 1615817. [CrossRef]
- Abou-Alfa, G.K.; Galle, P.R.; Chao, Y.; Erinjeri, J.; Heo, J.; Borad, M.J.; Luca, A.; Burke, J.; Pelusio, A.; Agathon, D.; et al. PHOCUS: A Phase 3, Randomized, Open-Label Study of Sequential Treatment with Pexa-Vec (JX-594) and Sorafenib in Patients with Advanced Hepatocellular Carcinoma. Liver Cancer 2023, 13, 256–272. [CrossRef]
- Toulmonde, M.; Cousin, S.; Kind, M.; Guegan, J.-P.; Bessede, A.; Le Loarer, F.; Perret, R.; Cantarel, C.; Bellera, C.; Italiano, A. Randomized Phase 2 Trial of Intravenous Oncolytic Virus JX-594 Combined with Low-Dose Cyclophosphamide in Patients with Advanced Soft-Tissue Sarcoma. J Hematol Oncol 2022, 15, 149. [CrossRef]
- Heo, J.; Breitbach, C.; Cho, M.; Hwang, T.-H.; Kim, C.W.; Jeon, U.B.; Woo, H.Y.; Yoon, K.T.; Lee, J.W.; Burke, J.; et al. Phase II Trial of Pexa-Vec (Pexastimogene Devacirepvec; JX-594), an Oncolytic and Immunotherapeutic Vaccinia Virus, Followed by Sorafenib in Patients with Advanced Hepatocellular Carcinoma (HCC). JCO 2013, 31, 4122–4122. [CrossRef]
- Kim, S.-G.; Ha, H.K.; Lim, S.; De Silva, N.S.; Pelusio, A.; Mun, J.H.; Patt, R.H.; Breitbach, C.J.; Burke, J.M. Phase II Trial of Pexa-Vec (Pexastimogene Devacirepvec; JX-594), an Oncolytic and Immunotherapeutic Vaccinia Virus, in Patients with Metastatic, Refractory Renal Cell Carcinoma (RCC). JCO 2018, 36, 671–671. [CrossRef]
- Immunization Strategy With Intra-Tumoral Injections of Pexa-Vec With Ipilimumab in Metastatic / Advanced Solid Tumors. (ISI-JX) Available Online: Https://Clinicaltrials.Gov/Study/NCT02977156?term=NCT02977156&rank=1 (Accessed on 8/5/2025).
- Monge, C.; Xie, C.; Myojin, Y.; Coffman, K.; Hrones, D.M.; Wang, S.; Hernandez, J.M.; Wood, B.J.; Levy, E.B.; Juburi, I.; et al. Phase I/II Study of PexaVec in Combination with Immune Checkpoint Inhibition in Refractory Metastatic Colorectal Cancer. J Immunother Cancer 2023, 11, e005640. [CrossRef]
- Lauer, U.M.; Schell, M.; Beil, J.; Berchtold, S.; Koppenhöfer, U.; Glatzle, J.; Königsrainer, A.; Möhle, R.; Nann, D.; Fend, F.; et al. Phase I Study of Oncolytic Vaccinia Virus GL-ONC1 in Patients with Peritoneal Carcinomatosis. Clinical Cancer Research 2018, 24, 4388–4398. [CrossRef]
- Pedersen, J.V.; Karapanagiotou, E.M.; Biondo, A.; Tunariu, N.; Puglisi, M.; Denholm, K.A.; Sassi, S.; Mansfield, D.; Yap, T.A.; De Bono, J.S.; et al. A Phase I Clinical Trial of a Genetically Modified and Imageable Oncolytic Vaccinia Virus GL-ONC1 with Clinical Green Fluorescent Protein (GFP) Imaging. JCO 2011, 29, 2577–2577. [CrossRef]
- Mell, L.K.; Brumund, K.T.; Daniels, G.A.; Advani, S.J.; Zakeri, K.; Wright, M.E.; Onyeama, S.-J.; Weisman, R.A.; Sanghvi, P.R.; Martin, P.J.; et al. Phase I Trial of Intravenous Oncolytic Vaccinia Virus (GL-ONC1) with Cisplatin and Radiotherapy in Patients with Locoregionally Advanced Head and Neck Carcinoma. Clinical Cancer Research 2017, 23, 5696–5702. [CrossRef]
- Krug, L.M.; Zauderer, M.G.; Adusumili, P.S.; McGee, E.; Sepkowitz, K.; Klang, M.; Yu, Y.A.; Scigalla, P.; Rusch, V.W. Phase I Study of Intra-Pleural Administration of GL-ONC1, an Oncolytic Vaccinia Virus, in Patients with Malignant Pleural Effusion. JCO 2015, 33, 7559–7559. [CrossRef]
- Chintala, N.K.; Choe, J.K.; McGee, E.; Bellis, R.; Saini, J.K.; Banerjee, S.; Moreira, A.L.; Zauderer, M.G.; Adusumilli, P.S.; Rusch, V.W. Correlative Analysis from a Phase I Clinical Trial of Intrapleural Administration of Oncolytic Vaccinia Virus (Olvi-Vec) in Patients with Malignant Pleural Mesothelioma. Front Immunol 2023, 14, 1112960. [CrossRef]
- Holloway, R.W.; Mendivil, A.A.; Kendrick, J.E.; Abaid, L.N.; Brown, J.V.; LeBlanc, J.; McKenzie, N.D.; Mori, K.M.; Ahmad, S. Clinical Activity of Olvimulogene Nanivacirepvec–Primed Immunochemotherapy in Heavily Pretreated Patients With Platinum-Resistant or Platinum-Refractory Ovarian Cancer. JAMA Oncol 2023, 9, 903–908. [CrossRef]
- Genelux Clinical Trial Summary. Available Online: Https://Genelux.Com/Clinical-Trials-Summary/ (Accessed on May 8, 2025).
- GL-ONC1 Administered Intravenously Prior to Surgery to Patients With Solid Organ Cancers. Available Online: Https://Clinicaltrials.Gov/Study/NCT02714374?intr=NCT02714374&rank=1 (Accessed on May 8, 2025).
- Expanded Access to Provide GL-ONC1 for the Treatment of Advanced Cancers With No Standard of Care. Available Online: Https://Clinicaltrials.Gov/Study/NCT03420430?term=NCT03420430&rank=1 (Accessed on May 8, 2025).
- Safety and Efficacy of the ONCOlytic VIRus Armed for Local Chemotherapy, TG6002/5-FC, in Recurrent Glioblastoma Patients (ONCOVIRAC). Available Online: Https://Clinicaltrials.Gov/Study/NCT03294486?intr=NCT03294486&rank=1 (Accessed on May 8, 2025).
- Bendjama, K.; Cassier, P.; Moreno, V.; Doger, B.; Calvo, E.; de Miguel, M.; Jungels, C.; Erbs, P.; Carpentier, D.; Sadoun, A. Abstract LB179: Oncolytic Virus TG6002 Locates to Tumors after Intravenous Infusion and Induces Tumor-Specific Expression of a Functional pro-Drug Activating Enzyme in Patients with Advanced Gastrointestinal Carcinomas. Cancer Research 2021, 81, LB179. [CrossRef]
- West, E.J.; Sadoun, A.; Bendjama, K.; Erbs, P.; Smolenschi, C.; Cassier, P.A.; de Baere, T.; Sainte-Croix, S.; Brandely, M.; Melcher, A.A.; et al. A Phase I Clinical Trial of Intrahepatic Artery Delivery of TG6002 in Combination with Oral 5-Fluorocytosine in Patients with Liver-Dominant Metastatic Colorectal Cancer. Clin Cancer Res 2025, 31, 1243–1256. [CrossRef]
- Study of TBio-6517 Given Alone or in Combination With Pembrolizumab in Solid Tumors (RAPTOR). Available Online: Https://Clinicaltrials.Gov/Study/NCT04301011?term=NCT04301011&rank=1 (Accessed on May 8, 2025).
- Chang, C.-L.; Ma, B.; Pang, X.; Wu, T.-C.; Hung, C.-F. Treatment With Cyclooxygenase-2 Inhibitors Enables Repeated Administration of Vaccinia Virus for Control of Ovarian Cancer. Molecular Therapy 2009, 17, 1365–1372. [CrossRef]
- Evgin, L.; Acuna, S.A.; Souza, C.T. de; Marguerie, M.; Lemay, C.G.; Ilkow, C.S.; Findlay, C.S.; Falls, T.; Parato, K.A.; Hanwell, D.; et al. Complement Inhibition Prevents Oncolytic Vaccinia Virus Neutralization in Immune Humans and Cynomolgus Macaques. Molecular Therapy 2015, 23, 1066–1076. [CrossRef]
- Lee, N.; Jeon, Y.-H.; Yoo, J.; Shin, S.; Lee, S.; Park, M.-J.; Jung, B.-J.; Hong, Y.-K.; Lee, D.-S.; Oh, K. Generation of Novel Oncolytic Vaccinia Virus with Improved Intravenous Efficacy through Protection against Complement-Mediated Lysis and Evasion of Neutralization by Vaccinia Virus-Specific Antibodies. J Immunother Cancer 2023, 11, e006024. [CrossRef]
| Trial# Name, strain and sponsor |
Gene Inactivation | Transgene | Phase (route) | Number of patients (cancer type) | Design | Toxicity | Response | Ref. |
|---|---|---|---|---|---|---|---|---|
|
NCT00554372 JX-594 (Pexa-Vec/TG6006) Wyeth SillaJen, Inc. |
TK | GM-CSF lacZ |
Phase II (intratumoral) | 30 (Advanced Hepatocellular Carcinoma, HCC) |
Two groups treated with high dose 1x109 PFU and low dose 1x108 PFU, respectively | Fever, chills, fatigue, nausea. Also: hypertension in 10% of patients and thrombocytopenia in 7% |
62.5% of high dose group showed tumor necrosis on MRI vs 35.7% of low-dose group. Median overall survival (OS): 14.1 months for high dose group and 6.7 months for low dose group. Historical controls: 6-8 months | [205] |
|
NCT01387555 JX-594 (Pexa-Vec/TG6006) Wyeth SillaJen, Inc. |
TK | GM-CSF lacZ |
Phase IIb (Intravenous infusion + later Intratumoral injections) | 129 (Advanced HCC, refractory or ineligible to sorafenib) |
Two groups: Virus + Best Supportive Care (BSC) vs BSC alone. Pexa-Vec was given as a single intravenous (IV) infusion followed by up to 5 IT injections. at 1x109 PFU each |
Fever, chills, fatigue, nausea. Also: hypertension in 8% of patients and thrombocytopenia in 6% |
No statistically significant difference between two groups. Median OS: ~4.2 months (Virus+BSC group) vs. 4.4 months (BSC only) Study terminated early as it did not meet its primary survival endpoint and lacked clear benefit |
[206] |
|
NCT02562755 JX-594 (Pexa-Vec/TG6006) Wyeth SillaJen, Inc. |
TK | GM-CSF lacZ |
Phase III (intratumoral) |
459 patients (Advanced HCC) |
Three intratumoral doses at 1x109 PFU plus sorafenib orally (400mg BID) vs sorafenib alone | Pyrexia, diarrhea, decreased appetite, nausea, fatigue | No survival benefit, trial was stopped for futility after interim analysis. Median OS: ~ 12.7 months (virus+sorafenib) vs 14.0 months (sorafenib) |
[207] |
|
NCT02630368 JX-594 (Pexa-Vec/TG6006) Wyeth SillaJen, Inc. |
TK | GM-CSF lacZ |
Phase II (intravenous) |
20 patients (Soft tissue sarcoma) | Dose of 1x109 PFU every 2 weeks for the first 3 injections and then every 3 weeks combined with low-dose cyclophosphamide, compared to the patients treated with low-dose phosphamide only |
Fever and fatigue, single case of grade 3 lymphopenia and grade 3 fever. | Both progression free survival (PFS) and OS were lower in the JX-594+cyclophosphamide group vs the cyclophosphamide-only group. | [208] |
|
NCT01171651 JX-594 (Pexa-Vec/TG6006) Wyeth SillaJen, Inc. |
TK | GM-CSF lacZ |
Phase II (Intravenous and Intratumoral) |
25 patients with unresectable primary HCC |
One intravenous dose followed by two intratumoral doses each once a week followed by sorafenib at Day 25 | Fever, chills, headache and nausea | 47% of patients displayed responses after treatment with virus only, after including sorafenib, responses were observed in 75% of patients | [209] |
|
Unspecified JX-594 (Pexa-Vec/TG6006) Wyeth SillaJen, Inc. |
TK | GM-CSF lacZ |
Phase II (Intravenous) |
17 patients with with Metastatic, Refractory Renal Cell Carcinoma (RCC). |
5 weekly intravenous infusions, followed by extra doses if response is observed | Transient flu-like illness, vomiting, chills, nausea | 76% of patients displayed disease control including one complete response | [210] |
|
NCT02977156 JX-594 (Pexa-Vec/TG6006) Wyeth Transgene |
TK | GM-CSF lacZ |
Phase I (Intravenous and Intratumoral) |
22 patients with Metastatic / Advanced Solid Tumors |
Single intravenous injection at 1x109 PFU followed by up to 4 intratumoral injections every week followed by treatment with ipilimumab |
No data available | The trial is marked as complete but no information about efficacy has been revealed. Since the asset is no longer in the company’s pipeline, it’s likely that efficacy was not satisfactory | [211] |
|
NCT03206073 JX-594 (Pexa-Vec/TG6006) Wyeth SillaJen, Inc. |
TK | GM-CSF lacZ |
Phase I/II (Intravenous and Intratumoral) |
34 patients with refractory metastatic colorectal cancer |
Four doses every 2 weeks at 3x108 or 1x109 PFU followed by treatment with durvalumab. 18 patients received additional single dose of tremelimumab on day 1 | Fever and decreased lymphocyte count | Disease control rate was 12.5% in the group without tremelimumab and 16.7% in the group with tremelimumab. OS was 5.2 months with tremelimumab and 7.5 months without tremelimumab. | [212] |
|
NCT01443260 GL-ONC1 (GLV-1h68) Lister Genelux, Inc. |
TK, hemagglutinin, F14.5L | Ruc-GFP, β-glucuronidase, and β-galactosidase | Phase I (intraperitoneal) | 9 patients (7 with peritoneal carcinomatosis and 2 with peritoneal mesothelioma) |
Three doses ranging from 107 to 109 PFU per cycle via intraperitoneal infusion | Decrease in lymphocyte count, increase in C-reactive protein (CRP), pyrexia, and abdominal pain | Stable disease observed in ~30% of evaluable patients (no complete/partial responses). Median OS ~5 months vs 3-6 months in historical controls |
[213] |
|
NCT00794131 GL-ONC1 (GLV-1h68) Lister Genelux, Inc. |
TK, hemagglutinin, F14.5L | Ruc-GFP, β-glucuronidase, and β-galactosidase | Phase I (Intravenous) |
24 patients with advanced solid tumors (including colorectal, head/neck, and lung tumors) | IIV infusion (dose-escalation study) 3 infusions at doses ranging from 105 to 109 PFU |
Pyrexia, musculoskeletal pain, fatigue, nausea. | Stable disease observed in 33% patients, but no partial or complete response | [214] |
|
NCT01584284 GL-ONC1 (GLV-1h68) Lister Genelux, Inc. |
TK, hemagglutinin, F14.5L | Ruc-GFP, β-glucuronidase, and β-galactosidase | Phase I (Intravenous) |
19 patients with Advanced Head and Neck Carcinoma |
3 cohorts of patients treated with single dose at 3x108, 1x109 and 3x109 PFU. 1 cohort treated with 2 doses at 3x109 PFU and 1 cohort treated with 4 doses at 3x109 PFU every 5-7 days. Virus treatment combined with cis-platin and radiotherapy |
Nausea, fatigue, mucositis, dysphagia | Favorable results in terms of PFS and OS were observed in the virus treated group compared with historical data. | [215] |
|
NCT01766739 GL-ONC1 (GLV-1h68) Lister Genelux, Inc. |
TK, hemagglutinin, F14.5L | Ruc-GFP, β-glucuronidase, and β-galactosidase | Phase I (Intraperitoneal) | 18 patients with malignant pleural effusions from mesothelioma, breast or non-small cell lung cancer | Single doses of 1x107, 1x108, 1x109, or 3x109 PFU |
Fever, chills and flu-like symptoms | Virus was detected in tumor tissues, tumor cell density decreased, while density of immune cells increased. Following completion of the trial, patients received subsequent other therapies so it’s hard to precisely determine the effect of virus alone | [216,217] |
|
NCT02759588 GL-ONC1 (GLV-1h68) Lister Genelux, Inc. |
TK, hemagglutinin, F14.5L | Ruc-GFP, β-glucuronidase, and β-galactosidase | Phase II (Intraperitoneal) |
27 patients with ovarian cancer | 3 × 109 PFU administered on 2 consecutive days followed by platinum doublet chemotherapy with or without bevacizumab | Pyrexia, abdominal pain and nausea | 54% evaluable by RECIST 1.1 had an objective response, with a median PFS of 11.0 months. | [218] |
|
NCT02714374 GL-ONC1 (GLV-1h68) Lister Genelux, Inc. |
TK, hemagglutinin, F14.5L | Ruc-GFP, β-glucuronidase, and β-galactosidase | Phase I (Intravenous) |
5 patients with solid organ cancers | 1 × 109 PFU administered intravenously prior to surgery | Limited data, according to the company’s website, it was well tolerated | Study terminated due to insufficient funding. According to company’s website, virus replication and increased infiltration by immune cells were detected in treated patients | [219,220] |
|
NCT03420430 GL-ONC1 (GLV-1h68) Lister Genelux, Inc. |
TK, hemagglutinin, F14.5L | Ruc-GFP, β-glucuronidase, and β-galactosidase | Expanded access (Intravenous) |
4 patients with solid tumors and 6 patients with blood tumors with no standard of care treatment available | IV administration, dose not officially revealed. | Limited data, according to the company’s website, it was well tolerated | No official results posted. | [219,221] |
|
NCT03294486 TG6002 Copenhagen Transgene |
TK, RR | cytosine deaminase and uracil phosphoribosyl transferase | Phase I/IIa (Intravenous) |
78 patients with recurrent glioblastoma | 3 weekly IV infusions at the dose of 1x105 PFU combined with fluorocytosine (5-FC) | Limited data. According to the company, toxicity was low and included usual mild symptoms (fever, fatigue, etc.) | Limited data. According to the official company website, the results were favorable. However, the asset was dropped from the pipeline, suggesting that efficacy was not high enough | [222] |
|
NCT03724071 TG6002 Copenhagen Transgene |
TK, RR | cytosine deaminase and uracil phosphoribosyl transferase | Phase I (Intravenous) |
15 patients with gastric carcinoma | 3 infusions once a week at different doses 3x108, 1x109 and 3x109 PFU combined with 5-Fluorocytosine | None of the patients displayed signs of vaccinia induced disease. | Virus and transgene expression were detected in tumors. No other data available probably due to low efficacy. TG6002 is no longer in the company’s pipeline | [223] |
|
Eudra-CT 2018-004103-39 TG6002 Copenhagen Transgene |
TK, RR | cytosine deaminase and uracil phosphoribosyl transferase | Phase I (Intrahepatic Artery Delivery) |
15 patients with Liver-Dominant Metastatic Colorectal Cancer |
Two infusions 43 days apart with the dose range from 1x106 to 1x109 PFU combined with 5-fluorocytosine | Gastrointestinal disorders, pyrexia, fatigue. | Virus and transgene expression were detected in tumors No patients had an objective response based on a 10-week disease control rate | [224] |
|
Unspecified vvDD Western Reserve NCI |
TK, VGF | NA | Phase I (Intratumoral) | 17 patients with advanced solid tumors | Single injections of the virus with the dose range from 3x107 to 3x109 PFU | Fever, nausea, fatigue | Virus replication detected in tumors, but true clinical benefit was not achieved in any of the patients | [35] |
|
NCT04301011 Tbio-6517 Copenhagen Turnstone Biologics |
25 kb deletion from the virus genome including 32 genes | anti-CTLA-4 antibody, FLT3 ligand (fms7 -like tyrosine kinase 3 ligand; FLT3L), and membrane-bound IL-12 (p35 subunit) | Phase I/IIa (Intratumorally or Intravenously) |
27 Patients with advanced solid tumors | Administered Intratumorally or Intravenously Alone and in Combination with Pembrolizumab, No info on dose revealed |
Lack of information | Study was terminated and the asset was withdrawn from pipeline. Official reason not revealed | [225] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





