1. Introduction
sFLC Biology
In 1847 Henry Bence Jones reported finding a novel protein in the urine of a patient with mollities ossium, a fatal condition characterized by softening and deformities of bone. (Jones, 1848) Early in the 20th century the proteins identified by Bence Jones were determined to be produced by neoplastic plasma cells. (Kyle, 2008) Serum free light chain (sFLC) measurements replaced urinary Bence Jones protein analysis for screening and monitoring multiple myeloma. (Jenner, 2014)
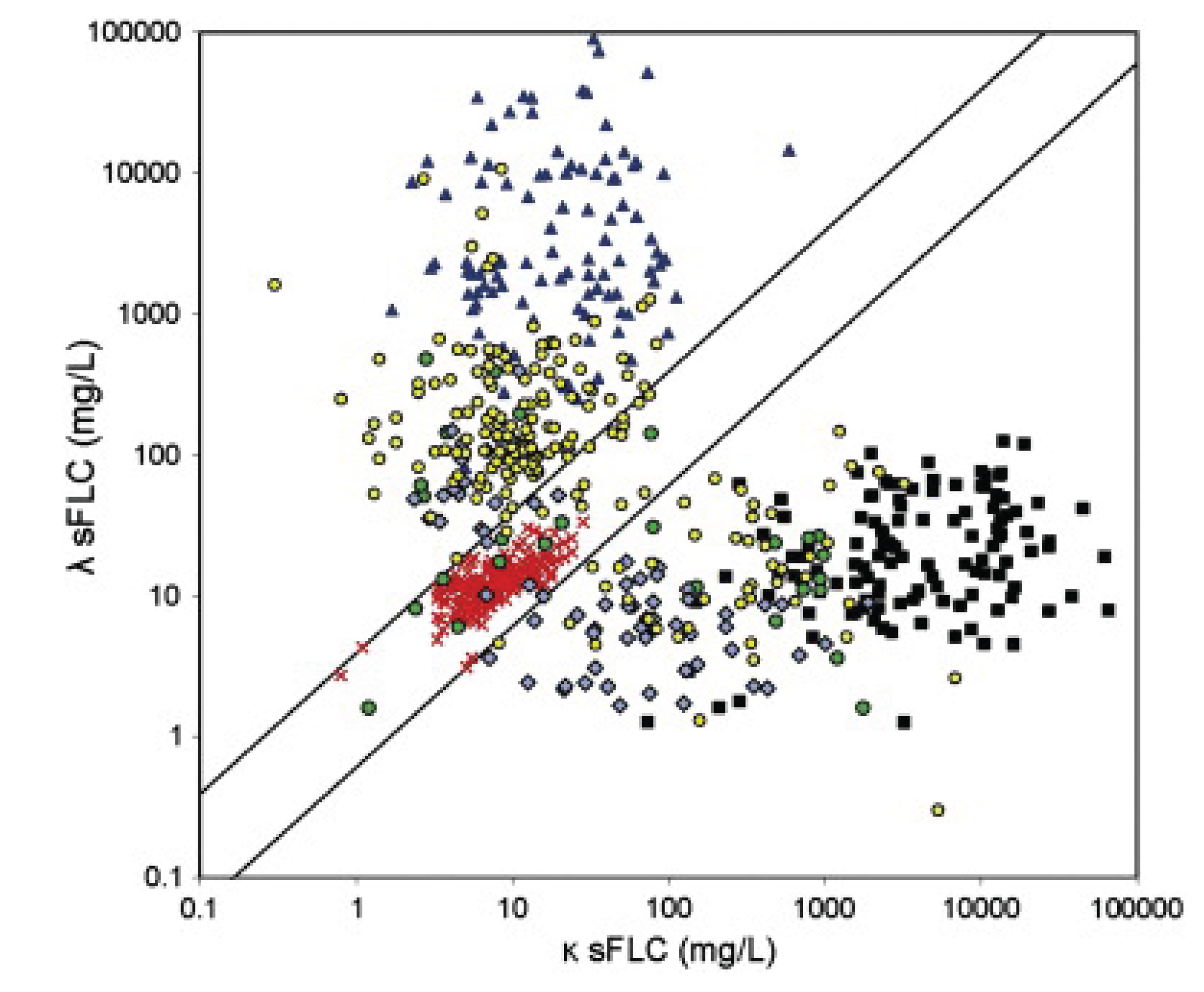
Figure 1.
The diagonal lines define the normal ratio boundary 0.26-1.65 with outliers clustering according to k, l and sFLCR. (Jenner,
2014). Jenner
Figure 1 (Open Access under CC BY-NC-ND License).
https://creativecommons.org/licenses/by-nc-nd/3.0/. 1. Normal Sera: Balanced k/λ ratio (0.26–1.65), low sFLC levels (0.1–10 mg/L).
Red crosses. 2. k Light Chain Multiple Myeloma: Extremely high k sFLC (100–100,000 mg/L), low λ, k/λ >> 1.65.
Black squares. 3. λ Light Chain Multiple Myeloma: Extremely high λ sFLC (100–100,000 mg/L), low k, k/λ << 0.26.
Blue triangles. 4. Non Secretory Multiple Myeloma: Variable sFLC (0.1–1,000 mg/L), often abnormal ratios, heterogeneous pattern.
Green circles. 5. Intact Immunoglobulin Multiple Myeloma: Elevated sFLC (10–10,000 mg/L), abnormal ratios, less extreme than LCMM.
Blue diamonds. 6. AL Amyloidosis: Moderate sFLC elevations (10–10,000 mg/L), often λ-dominant, abnormal ratios.
Yellow circles.
Figure 1.
The diagonal lines define the normal ratio boundary 0.26-1.65 with outliers clustering according to k, l and sFLCR. (Jenner,
2014). Jenner
Figure 1 (Open Access under CC BY-NC-ND License).
https://creativecommons.org/licenses/by-nc-nd/3.0/. 1. Normal Sera: Balanced k/λ ratio (0.26–1.65), low sFLC levels (0.1–10 mg/L).
Red crosses. 2. k Light Chain Multiple Myeloma: Extremely high k sFLC (100–100,000 mg/L), low λ, k/λ >> 1.65.
Black squares. 3. λ Light Chain Multiple Myeloma: Extremely high λ sFLC (100–100,000 mg/L), low k, k/λ << 0.26.
Blue triangles. 4. Non Secretory Multiple Myeloma: Variable sFLC (0.1–1,000 mg/L), often abnormal ratios, heterogeneous pattern.
Green circles. 5. Intact Immunoglobulin Multiple Myeloma: Elevated sFLC (10–10,000 mg/L), abnormal ratios, less extreme than LCMM.
Blue diamonds. 6. AL Amyloidosis: Moderate sFLC elevations (10–10,000 mg/L), often λ-dominant, abnormal ratios.
Yellow circles.
Produced by B lymphocytes, immunoglobulins consist of two heavy and two light polypeptide chains. The heavy chains come in IgG, IgM, IgA, IgD, and IgE varieties. The light chains have two forms, kappa (k) and lambda (l). Light chains are produced in greater quantities than heavy chains with kappa produced in quantities double that of lambda chains. The range of normal values for serum kappa chains is 3.3-19.4 mg/L and 5.7-26.6 mg/L for lambda, with a normal ratio of 0.26-1.65. (Katzman, 2002)
Clinical Relevance: Neoplasia Versus Inflammation
Serum free light chain determinations have had an expanding role in medical diagnostics and disease management for both neoplastic and, more recently, inflammatory disorders. Jenner compiled data from multiple sources to identify a “broad spectrum” of neoplastic monoclonal plasma cell variants including k Light Chain Multiple Myeloma (kLCMM), λ Light Chain Multiple Myeloma (λ LCMM), Non Secretory Multiple Myeloma (NSMM), Intact Immunoglobulin Multiple Myeloma (IIMM), and AL Amyloidosis (ALM). Numerical data for k, l, and sFLCR were presented as a dot-plot. ( Jenner, 2014)
Cases with sFLCR in the range < 0.26 or >1.65 are associated with hematopoietic neoplasms and para or preneoplastic conditions.
1 The sFLC abnormalities have been studied in the following plasma cell neoplastic or paraneoplastic conditions (
Table 1) which is a small sample of the 39 B-cell and 23 T-cell neoplasms recognized by the World Health Organization. (Salama, 2023)
Gudowska-Sawczuk and Mroczko performed a comprehensive search of the literature up to 2023 in which they identified FLCs as biomarkers of inflammatory diseases including SARS-CoV-2 infection as well as monoclonal gammopathies,
Table 2. (Gudowska-Sawczuk, 2023)
Use of sFLCk, sFLCl and sFLCR measurement continues to expand as a diagnostic and management tool that gives insight about function of terminally differentiated B-cells known as plasma cells. These measurements provide some insight into the functioning of cells that are active in responding to inflammatory conditions and a growing array of neoplastic and pre or para neoplastic conditions.
Recent studies highlight the diagnostic utility of sFLC levels in various conditions, including
CNS disorders, type 2 diabetes, cardiac disorders, renal disorders, protein deposition in multiple organs, and following COVID-19 vaccination.
Hegen, et al., reported on cerebrospinal fluid kappa free light chains as useful biomarker in multiple sclerosis from diagnosis to prediction of disease activity. (Hegen, 2022)
Demortiere, et al. found FLC levels in patients with inaugural optic neuritis were useful to sort out multiple sclerosis (MS), myelin oligodendrocyte glycoprotein antibody-associated disease (MOGAD) and neuromyelitis spectrum disorder (NMOSD). (Demortiere, 2025)
Bracco et al. found the presence of FLC in cerebrospinal fluid of multiple sclerosis patients implicated a recent immunological stimulation leading to increased synthesis of FLC within the central nervous system. (Bracco, 1987)
Matsumori et al. found that sFLCR was a “…more specific and sensitive for the diagnosis of T2D than HbA1c, and thus represents a potentially promising biomarker of inflammation.” (Matsumori, 2020)
Basile, et al. found sFLCR > 0.63 was associated with left ventricular ejection fraction improvement in a small series of patients with NSTEMI, STEMI and stable angina at one year follow up. (Basile, 2019)
Nakao, H. reported an increase in IgA nephropathy following COVID-19 mRNA vaccination. (Nakao, 2023)
Park and Kwon pointed out the role of monoclonal FLC in producing kidney damage in Monoclonal Gammopathy of Renal Significance (MGRS) without multiple myeloma or other forms of neoplasia. (Park, 2023).
Martins, et al. reported on 23 cases of non-myeloma light chain cast nephropathy (non MM-LCCN) pointing out that malignancy develops later in 43% of cases. (Martins, 2024).
Lan, et al. presented a case of light chain proximal tubulopathy (LCPT) and light chain cast nephropathy (LCCN) in a 49 year-old patient with acute kidney injury associated with of lambda light chain multiple myeloma (LCMM). (Lan, 2024).
Cassano, et al. reported on light chain deposition disease (LCDD) in which non-amyloid monoclonal light chains are deposited in different organs particularly kidney where monoclonal immunoglobulins are deposited in vascular basement membranes, glomerular basement membranes and tubular basement membranes in patients with plasma cell dyscrasias but also monoclonal gammopathy of unknown significance (MGUS). (Cassano, 2025).
Gudowska-Sawczuk, et al. found COVID-19 vaccinated subjects had higher sFLC levels than COVID-19 patients and unvaccinated controls. (Gudowska-Sawczuk, 2022).
Gudowska-Sawczuk, et al. conclude,
… abnormal levels of k and λ FLCs, as well as the ratio of k:λ, are usually the result of disturbances in the synthesis of immunoglobulins as an effect of overactive inflammatory reactions. Therefore, it seems that k and λ FLCs may be significant diagnostic and prognostic biomarkers of selected diseases. (Gudowska-Sawczuk,2022)
The desired outcome from study of sFLC is identification of a biomarker for diseases that affect the immune system with more specificity and sensitivity than the common indicators of inflammation such as the erythrocyte sedimentation rate (ESR) and C reactive protein (CRP) with the additional value in diagnosis and management of neoplasms of terminally differentiated B-cells.
This article will examine sFLC patterns (sFLCk, sFLCl, and sFLCR) following vaccination with Pfizer/BioNTech’s BNT162b2 and Moderna’s mRNA1273 using cases drawn from the VAERS database.
2. Materials and Methods
The Vaccine Adverse Event Reporting System (VAERS), maintained by the Centers for Disease Control and Prevention (CDC) and the Food and Drug Administration (FDA), catalogs reports of adverse events following COVID-19 vaccination. VAERS cases are voluntarily submitted by individuals, including medical professionals, who suspect a possible causal relationship between the reported conditions and administered COVID-19 vaccines.
Search terms included all FLC-related identifiers and free-text field searches for FLC reporting in Adverse Event Descriptions and Lab Data sections of VAERS reports. Specific conditions searched included multiple organ system related diagnoses such as renal disorders, CNS disorders, hematological disorders, and cardiac disorders. A master spreadsheet was constructed and analyzed using Grok3 (xAI) an AI tool designed for data analysis, proofing and preparation of tables.
In total, 75 cases of sFLC abnormalities were identified. This study examines 25 unique VAERS cases reporting free light chain (FLC) abnormalities potentially associated with adverse events following COVID-19 mRNA vaccination, with data updated as of June 21, 2025. The following framework was used to analyze the quantitative VAERS data. Normal versus abnormal sFLCR was used as an ordering principle to analyze data with subset analysis according to sFLCk and/or sFLCl levels.
Table 3.
sFLCR: Inflammation vs. Neoplasm.
Table 3.
sFLCR: Inflammation vs. Neoplasm.
| Type |
sFLCR |
sFLC |
| Inflammation |
0.26–1.65 |
k, λ, k & λ |
| Neoplasia |
<0.26 or >1.65 |
k, λ, k & λ |
3. Results
The average time to onset was 50 days and less than or equal to 7 days in 45% (10/22) of these cases. Average values for sFLCk/sFLCλ/sFLCR are 96.1/95.3/9.86, well above normal for sFLCk, sFLCl and FLCR. (
Table 4)
In two cases the average sFLCR was elevated at 2.07, sFLCk was normal (7.05 mg/L), and sFLCl was low at 3.49 mg/L suggesting immunosuppression as there was no clonal proliferation.
It has been reported that a ratio over 100 indicates elevated risk of progression to multiple myeloma. (Aklaghi, 2025) The cohort featured in this paper had one case with a sFLCR > 100 that was a myeloma case. Cases LC# 60 (lymphoma) and LC# 63 (myeloma) had normal ratios.
Cases in which the ratio ≤1.65 but in which sFLCk and/or sFLCl exceed the normal range should be considered for serial monitoring. Malignant transformation is reported to occur in up to 30% over 20 years for non IgM Monoclonal Gammopathy of Unknown Significance (MGUS) patients with two risk factors, elevated ratio and high serum monoclonal protein greater than or equal to 1.5 g per deciliter. (Kyle, et al. 2018) Bird et al. concluded,
Clinicians responsible for monitoring patients should be aware that the risk of progression to myeloma or other LPD remains lifelong and that risk never disappears even if the M-protein remains stable. Bird, 2009
Case LC #11 (VAERS ID# 2619645) received five doses of COVID-19 vaccines, three Moderna and two Pfizer, and was subsequently identified as having Monoclonal Gammopathy of Unknown Significance on the basis of elevation of sFLCk (2.75 mg/dl) with normal sFLCR (1.31). Serial sFLC determinations should be considered in such a case.
Case# LC 63 (VAERS ID# 2509414 was a 60 year old male who presented 1 week after a second dose of BNT162b2 with weight gain, proteinuria, and hypoalbumenia. Urinary Bence Jones proteins were positive, both sFLCk (300 mg/L) and sFLCl (384 mg/L) were elevated but the ratio was normal at 0.78. Bone marrow biopsy demonstrated 10% plasma cell aggregates with amyloid deposits consistent with myeloma.
Case LC# 24 (VAERS ID# 2516749) was a 41 year old woman with HLAB27+ arthritis in remission had onset of nephrotic syndrome 7-10 days after dose four of BNT162b2 with lower extremity edema, hematuria, proteinuria, hypoalbuminemia, and elevated sFLCk (37.74 mg/L) and sFLCl (37.95 mg/L) with sFLCR normal (0.99). Renal biopsy revealed focal segmental glomerulosclerosis (FSGS).
LC# 42 (VAERS ID# 2267797) was a 47 year old woman received three doses of mRNA1273 6/4/2021, 7/2/2021 and the date of the third dose was not given. In November of 2021 she had onset of anemia, an IgG monoclonal band, and an elevated sFLCl (173 mg/L) with sFLCR of 0.09. Bone marrow biopsy showed 7-9% monoclonal plasma cell infiltration.
Case LC #16 (VAERS ID# 2130959) was a 46 year old woman with past history of hypothyroidism and labile hypertension who had onset of severe bilateral knee pain and flu-like symptoms 31 days after her second dose of mRNA1273. A rheumatology consultation was obtained who obtained sFLCs showing sFLCk was 72.87 mg/dL with a ratio of 165.61.
After consultation with a hematologist a bone marrow biopsy confirmed Kappa Light Chain Multiple Myeloma. She was rated as permanently disabled due to the chronic and progressive nature of multiple myeloma.
4. Discussion
COVID-19, COVID-19 Vaccines and sFLC Patterns
Using WBC differential fluorescence (WDF), Malecka-Gieldowska et al. observed a 3-fold increase in kappa light chain synthesis in SARS-CoV-2-infected ICU patients compared to non-infected ICU cases, distinguishing COVID-19 ICU from non-ICU patients. (Malecka-Gieldowska, 2021) Cell population data (CPD) revealed a variation leucocyte size, granularity, and amount of genetic material in the three groups.
COVID ICU patients had the greatest size, granularity, and nucleic acid content in neutrophils and lymphocytes when compared with other groups. In contrast, patients without SARS-CoV-2 infection hospitalized in the ICU had the lowest values of the mentioned parameters.
These findings suggest that SARS-CoV-2 infection stimulates sFLC production, particularly in severe cases.
Gudowska-Sawczuk et al. extended this analysis, reporting significantly higher sFLC concentrations in COVID-19 patients and vaccinated controls compared to unvaccinated controls (p<0.001). (Gudowska-Sawczuk, 2022) The current study, analyzing 75 VAERS cases, identifies even higher sFLC levels post-COVID-19 vaccination in cases of adverse reactions (mean kFLC: 94.6 mg/L, λFLC: 95.31 mg/L, k:λ ratio: 9.46,
Table 5) compared to COVID-19 ICU (kFLC: 47.03 mg/L, λFLC: 34.71 mg/L) and non-ICU patients (kFLC: 24.62 mg/L, λFLC: 25.83 mg/L). These associations suggest a gradient of immune challenge, from natural infection to reported adverse events following COVID-19 vaccination.
The gradient of sFLC elevation—highest in VAERS reported vaccine adverse event cases, followed by COVID-19 ICU, non-ICU, vaccinated controls, and non-COVID controls suggests increasing immune challenge from SARS-CoV-2 infection to vaccination. This work identifies sFLC abnormalities as a potentially useful biomarker for COVID-19 vaccine related adverse events.
The current study identified 25 cases with complete sFLC data out of 75 total cases with reported free light chain disorders using the search criteria based upon out of range values. This small sample was remarkably diverse with the following six patterns identified.
Table 6.
Six Patterns Identified.
Table 6.
Six Patterns Identified.
| Pattern |
N |
Kappa |
Lambda |
Ratio |
| 1. Normal r, Kappa Elevated |
3 |
32.70 |
23.04 |
1.41 |
| 2. Normal r, Kappa & Lambda Elevated |
7 |
105.38 |
111.61 |
1.00 |
| 3. Elevated r, Kappa Elevated |
5 |
103.48 |
17.06 |
9.45 |
| 4. Elevated r, Kappa & Lambda Elevated |
5 |
158.64 |
74.69 |
34.74 |
| 5. Low r, Lambda Elevated |
3 |
64.48 |
231.67 |
0.09 |
| 6. Elevated r, Lambda Decreased8
|
2 |
7.05 |
3.49 |
2.07 |
| Avg. |
|
94.64 |
95.319
|
9.46 |
Compared to traditional inflammatory markers like ESR and CRP, sFLC offers greater sensitivity for detecting immune dysregulation, supporting its potential for monitoring neoplasia or inflammation.
As demonstrated in this review cases with abnormal ratios (red) are used clinically in the context of hematological neoplasms both for diagnosis and monitoring. Less clear is the clinical use in cases with normal sFLCR but with out of range values for sFLCk, or sFLCk + sFLCl. These cases must be considered carefully as abnormal ratios are not a perfect indicator of potential neoplasia as the case reports indicate.
Furthermore, as shown by Gudowska-Sawczuk and Mroczko and others sFLCs may be useful for diagnosis and management of inflammatory conditions extending the value of this biomarker to a wider assortment of medical conditions. (Gudowska-Sawczuk, 2022) One limitation is that the COVID-19 illness and COVID-19 vaccination research reported by Malecka-Gieldowska et al. and Gudowska-Sawczuk et al. was applied to groups and not individuals. Longitudinal data is needed.
Limitations
The VAERS database was designed as an early warning system for adverse events following vaccination. VAERS data can be useful for hypothesis generation but not for establishing causation. Attempts to use adverse event and lab data from VAERS for clinical analysis in this report was abandoned due to incomplete data in complex cases. Neoplasia in hematopoietic cells often requires extensive efforts to reach a definitive diagnosis and VAERS is not sophisticated to address complex clinical aspects of these medical conditions. Attention was focused on patterns of sFLC abnormalities as found in 25 cases with quantitative data.
Case selection based on abnormal sFLC values introduces bias toward complex medical cases. Small sample size, inconsistent data collection and absent longitudinal data reduce analytic potential while multiple co-morbidities and limited follow-up complicate analysis of underlying medical conditions. Some case reports are highly detailed, some have been published as case reports, yet others have very limited information.
5. Conclusions
1. The current data set serves to illustrate use of sFLC in clinical decision making and not to connect sFLC abnormalities causally to COVID19 vaccines. To overcome constraints in VAERS, more sophisticated tools are needed.
2. Malecka-Gieldowska, et al. showed a connection between cellular changes in lymphocytes during COVID-19 illness and after COVID-19 vaccination. (Malecka-Gieldowska, 2021) Additional research into the mechanism of these interactions should be pursued.
3. sFLC sensitivity and complexity as a biomarker supports its potential for diagnosing and monitoring inflammatory, preneoplastic and neoplastic conditions. as demonstrated by application to COVID-19 illness and COVID-19 vaccines by Malecka-Gieldowska, et al. and Gudowska-Sawczuk, et al.
4. Cases with out of bounds values for one or both sFLCs should receive careful medical consideration. sFLCR should not be used as the sole clinical indication of concern. Important exceptions to sFLCR guided clinical practice occur. Attention should be given to sFLCk and sFLCl in clinical decision making and not solely focused on cases with abnormal k/l ratios.
5. The complexity of sFLC tests with multiple patterns of abnormality has the potential for application to a broader sample of medical illnesses for diagnosis, to follow activity of a disease, and to identify progression to neoplasm as is the case with Monoclonal Gammopathy of Unknown Significance (MGUS).
Funding
There was no external funding.
Institutional Review Board Statement
This study used publicly available data provided by the Centers for Disease Control and Prevention and the U.S. Food and Drug Administration.
Data Availability Statement
Data are available on request.
Conflicts of Interest
None.
References
- Aklaghi, K., Maclachlan, K., Korde, N., Mailankody, S., Lesokhin, A., Hassoun, H., Lu, S., Patel, D., Shah, U., Tan, C., Hultcrantz, M., Iyengar, N., Shah, G. L., Scordo, M., Lahoud, O. B., Chung, D. J., & Landau, H. J. (2025). Evaluating serum free light chain ratio as a biomarker in multiple myeloma. Haematologica, 110(1), 326–338. [CrossRef]
- Basile, U., La Rosa, G., Napodano, C., Pocino, K., Cappannoli, L., Gulli, F., Cianfrocca, C., Di Stasio, E., & Biasucci, L. M. (2019). Free light chains: A novel biomarker of cardiovascular disease. A pilot study. European Review for Medical and Pharmacological Sciences, 23(6), 2563–2569. [CrossRef]
- Bird, J., Behrens, J., Westin, J., Turesson, I., Drayson, M., Beetham, R., D’Sa, S., Soutar, R., Waage, A., Gulbrandsen, N., Gregersen, H., Low, E. and (2009), UK Myeloma Forum (UKMF) and Nordic Myeloma Study Group (NMSG): guidelines for the investigation of newly detected M-proteins and the management of monoclonal gammopathy of undetermined significance (MGUS). British Journal of Haematology, 147: 22-42. [CrossRef]
- Bracco, F., Gallo, P., Menna, R., Battistin, L., & Tavolato, B. (1987). Free light chains in the CSF in multiple sclerosis. Journal of Neurology, 234(5), 303–307. [CrossRef]
- Cassano, R., Ferraro, S., Stella, A., Buda, G., Orciuolo, E., & Petrini, M. (2025). Light chain deposition disease: Pathogenesis, clinical characteristics, and treatment strategies. Annals of Hematology, 104(7), 2083–2093. [CrossRef]
- Davids, M. S., Murali, M. R., & Kuter, D. J. (2010). Serum free light chain analysis. American Journal of Hematology, 85(10), 787–790. [CrossRef]
- Demortiere, S., Marignier, R., Bertheaume, N., Vukusic, S., D’Hardivilliers, F., & Lebrun-Frenay, C. (2025). Diagnostic utility of kappa free light chain index in adults with inaugural optic neuritis. Neurology: Neuroimmunology & Neuroinflammation, 12(1), Article e200386. [CrossRef]
- Dispenzieri, A. (2019). POEMS syndrome: 2019 update on diagnosis, risk-stratification, and management. American Journal of Hematology, 94(7), 812–827. [CrossRef]
- Fend, F., Dogan, A., & Cook, J. R. (2023). Plasma cell neoplasms and related entities—evolution in diagnosis and classification. Virchows Archiv, 482(1), 163–177. [CrossRef]
- Gertz, M. A. (2024). Immunoglobulin light chain amyloidosis: 2024 update on diagnosis, prognosis, and treatment. American Journal of Hematology, 99(2), 309–324. [CrossRef]
- Gudowska-Sawczuk, M., & Mroczko, B. (2023). Free light chains k and λ as new biomarkers of selected diseases. International Journal of Molecular Sciences, 24(11), Article 9531. [CrossRef]
- Gudowska-Sawczuk, M., Moniuszko-Malinowska, A., Pączek, S., Guziejko, K., Chorąży, M., & Mroczko, B. (2022). Evaluation of free light chains (FLCs) synthesis in response to exposure to SARS-CoV-2. International Journal of Molecular Sciences, 23(19), Article 11589. [CrossRef]
- Hegen, H., Arrambide, G., Gnanapavan, S., Kaplan, B., Khalil, M., Saadeh, R., Teunissen, C., Tumani, H., Villar, L. M., Willrich, M. A. V., & Zettl, U. K. (2022). Cerebrospinal fluid kappa free light chains for the diagnosis of multiple sclerosis: A consensus statement. Multiple Sclerosis Journal, 29(2), 182–195. [CrossRef]
- Jenner, E. (2014). Serum free light chains in clinical laboratory diagnostics. Clinica Chimica Acta, 427, 15–20. [CrossRef]
- Jones, H. B. (1848). On a new substance occurring in the urine of a patient with mollities ossium. Philosophical Transactions of the Royal Society, 138, 55–62. [CrossRef]
- Kaplan, B., Livneh, A., & Sela, B. A. (2011). Immunoglobulin free light chain dimers in human diseases. The Scientific World Journal, 11, 726–735. [CrossRef]
- Katzmann, J., Clark, R., Abraham, R., Bryant, Lymp, S., Bradwell, A., Kyle, R. Serum Reference Intervals and Diagnostic Ranges for Free κ and Free λ Immunoglobulin Light Chains: Relative Sensitivity for Detection of Monoclonal Light Chains, Clinical Chemistry, Volume 48, Issue 9, 1 September 2002, Pages 1437–1444. [CrossRef]
- Katzman, J. A., Kyle, R. A., Benson, J., Larson, D. R., Snyder, M. R., Lust, J. A., Rajkumar, S. V., & Dispenzieri, A. (2009). Screening panels for detection of monoclonal gammopathies. Clinical Chemistry, 55(8), 1517–1522. [CrossRef]
- Kyle, R. A., & Rajkumar, S. V. (2008). Multiple myeloma. Blood, 111(6), 2962–2972. [CrossRef]
- Kyle, R. A., Larson, D. R., Therneau, T. M., Dispenzieri, A., Kumar, S., Cerhan, J. R., & Rajkumar, S. V. (2018). Long-term follow-up of monoclonal gammopathy of undetermined significance. New England Journal of Medicine, 378(3), 241–249. [CrossRef]
- Lan, M., Guo, Y., Wang, C., Wang, X., Li, J., & Wang, Y. (2024). Lambda light chain–restricted non-crystalline proximal tubulopathy with cast nephropathy in multiple myeloma: A case report and literature review. BMC Nephrology, 25(1), Article 325. [CrossRef]
- Malecka-Gieldowska, M., Folta, M., Wisniewska, A., & Czyzewska, E. (2021). Cell population data and serum polyclonal immunoglobulin free light chains in the assessment of COVID-19 severity. Viruses, 13(7), Article 1381. [CrossRef]
- Martins, C., Gibier, J., Leroy, X., Bridoux, F., Touchard, G., Joly, D., Royal, V., Goujon, J. M., & Sirac, C. (2024). Non-myeloma light chain cast nephropathy: A multicenter retrospective study on clinicopathological characteristics. Haematologica, 109(8), 2557–2566. [CrossRef]
- Matsumori, A., Shimada, T., Shimada, M., & Drayson, M. T. (2020). Immunoglobulin free light chains: An inflammatory biomarker of diabetes. Inflammation Research, 69(7), 715–718. [CrossRef]
- Nakao, H., Koseki, T., Kato, K., Yamada, S., Tsuboi, N., Takahashi, K., & Mizuno, T. (2023). COVID-19 mRNA vaccination is associated with IgA nephropathy: An analysis of the Japanese adverse drug event report database. Journal of Pharmacy & Pharmaceutical Sciences, 26, Article 11453. [CrossRef]
- Park, K., & Kwon, S. (2024). Monoclonal gammopathy of renal significance from the perspective of nephrologists. Blood Research, 59(1), Article 28. [CrossRef]
- Salama, M., & Hoffman, R. (2023). Progress in the classification of hematopoietic and lymphoid neoplasms: Clinical implications. In R. Hoffman (Ed.), Hematology: Basic principles and practice (8th ed., pp. 800–812). Elsevier. [CrossRef]
- xAI. (n.d.). Grok 3 (Version 3) [Artificial intelligence language model]. xAI. https://x.ai.
- Zhu, L., Hu, Q., Zhang, L., & Li, A. (2024). The role of minimal residual disease and serum free light chain ratio in the management of multiple myeloma. Discover Oncology, 15(1), Article 229. [CrossRef]
| 1 |
Neoplastic and pre or para neoplastic conditions are combined for this analysis. |
| 2 |
Outlier removed LC #29 l = 5975. |
| 3 |
Reference values: sFLCk 3.3-19.4 mg/L, sFLCl 5.7-26.6 mg/L, sFLCR= 0.26-1.65 |
| 4 |
Elevated sFLC values with abnormal ratios are in red. |
| 5 |
Outlier removed LC # 29 l = 5975 |
| 6 |
Malecka-Gieldowska, 2021 |
| 7 |
Gudowska-Sawczuk, 2022 |
| 8 |
Possible suppression of l producing plasma cells |
| 9 |
Outlier removed LC # 29 l = 5975 |
Table 1.
Neoplastic and paraneoplastic conditions potentially having out of reference values for sFLCR.
Table 1.
Neoplastic and paraneoplastic conditions potentially having out of reference values for sFLCR.
|
Classification of Plasma Cell Neoplasms and Paraneoplastic Conditions (Katzman, 2009; Davids, 2010; Kaplan, 2011; Kyle, 2018; Dispenzieri, 2019; Zhu, 2024; Gertz, 2024; Aklaghi, 2025) |
| 1. Non-IgM MGUS |
| 2. Smoldering myeloma |
| 3. Multiple myeloma |
| 4. Solitary bone plasmacytoma |
| 5. Solitary extraosseous plasmacytoma |
| 6. Immunoglobulin light chain amyloidosis |
| 7. Localized AL amyloidosis |
| 8. Waldenström macroglobulinemia |
| 9. Light chain deposition disease (LCDD) |
| 10. POEMS syndrome (polyneuropathy, organomegaly, endocrinopathy, M-protein, skin changes) |
Table 2.
Conditions associated with Abnormal sFLC.
Table 2.
Conditions associated with Abnormal sFLC.
| Monoclonal Gammopathies |
Diabetes |
| Multiple sclerosis |
Cardiovascular disease |
| SARS-CoV-2 infection |
Rheumatoid arthritis |
| HCV |
Sjogren’s syndrome |
| HBV |
SLE |
| HIV |
Lung cancer |
| Lyme Disease |
Breast cancer |
| Tick-born encephalitis |
Bowel Disease |
Table 4.
sFLC data for Quantitative Cases n = 25.
Table 4.
sFLC data for Quantitative Cases n = 25.
| |
sFLCk |
sFLCl |
sFLCR |
| Avg |
96.13
|
95.32
|
9.863
|
| Min |
4.27 |
0.44 |
0.01 |
| Max |
338 |
5975 |
165.61 |
Table 5.
Comparison of sFLC for VAERS adverse event reports following vaccination, COVID-19 ICU, COVID-19 non-ICU, Vaccinated Controls and Non Vaccinated Controls.
4
Table 5.
Comparison of sFLC for VAERS adverse event reports following vaccination, COVID-19 ICU, COVID-19 non-ICU, Vaccinated Controls and Non Vaccinated Controls.
4
| Group |
kFLC (mg/L) |
λFLC (mg/L) |
k/λ Ratio |
Reported AEs after COVID-19 Vaccine
n = 25 |
94.6 (7.05–300) |
95.31 (3.49–384)5
|
9.46 (0.09–165.61) |
COVID-19 ICU6
n = 45 |
47.03 (43.52–64.76) |
34.71 (30.66–47.23) |
1.34 (1.20–1.52) |
COVID-19 non-ICU2
n = 43 |
24.62 (21.22–36.45) |
25.83 (19.26–28.38) |
1.27 (1.06–1.35) |
Vaccinated Controls7
n = 20 |
17.83 ± 3.03 (12.10–23.70) |
13.22 ± 3.87 (9.24–22.00) |
1.40 ± 0.24 (0.88–1.77) |
Mild COVID-193
n = 80, (67 vaccinated) |
16.76 ± 5.51 (5.25-42.50) |
16.38 ± 6.17 (6.32-36.50) |
1.10 ± 0.28 (0.44-1.94) |
Non-Vaccinated Controls2
n = 20 |
10.25 ± 2.13 (6.28–15.04) |
10.26 ± 2.76 (6.84–18.89) |
1.03 ± 0.22 (0.51–1.41) |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).