Submitted:
10 June 2025
Posted:
11 June 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Methods
2.1. The Human Torso and the Human Heart Modeling
2.2. Activation Isochrones Modeling
3. Results
4. Conclusions
References
- D.S. Farina, O. Skipa, C. Kaltwasser, O. Dossel and W.R. Bauer “Personalized Model of Cardiac Electrophysiology of a Patient” IJBEM (2005);7(1): 303-306.
- M. Seger “Modeling the Electrical Function of the Human Heart”, Ph.D. Thesis, Institute of Biomedical Engineering, University for Health Sciences, Medical Informatics and Technology, Austria (2006).
- M. Lorange, and R. M. Gulrajani “A computer Heart Model Incorporating Anisotropic Propagation” Journal of Electrocardiology, (1993);26(4):245-261.
- C. Hintermuller “Development of a Multi-Lead ECG Array for Noninvasive Imaging of the Cardiac Electrophysiology”, Ph.D. Thesis, Institute of Biomedical Engineering, University for Health Sciences, Medical Informatics and Technology,Austria, (2006).
- L. Cheng “Non-Invasive Electrical Imaging of the Heart”, Ph.D. Thesis, The University of Auckland,New Zealand (2001).
- B. He, and D. Wu “Imaging and Visualization of 3-D Cardiac Electric Activity” IEEE Tran. Inf Tech. Biomed. 2001; 5(3): 181-186. [CrossRef]
- Z. Liu, C. Liu, and B. He “Noninvasive Reconstruction of Three-Dimensional Ventricular Activation Sequence From the Inverse Solution of Distributed Equivalent Current Density” IEEE Trans. Med. Imag. (2006); 25(10): 1307-1318. [CrossRef]
- B. He, C. Liu ,and Y. Zhang “Three-Dimensional Cardiac Electrical Imaging From Intracavity Recordings” IEEE Trans. Biomed. Eng. (2007); 54(8): 1454-1460. [CrossRef]
- Elaff “Effect of the Conduction Network Structure of the Heart on Modeling of the Body Surface Potential Map and the ECG”. Preprints 2025, 2025060714. [CrossRef]
- R.L. Winslow, D.F. Scollan, J.L. Greenstein, C.K. Yung, W. Baumgartner, G. Bhanot, D.L. Gresh and B.E. Rogowitz “Mapping, modeling,and visual exploration of structure-function relationships in the heart” IBM Sys J.,(2001); 40(2):342-359. [CrossRef]
- ELAFF “Modeling of 3D Inhomogeneous Human Body from Medical Images”, World Journal of Advanced Engineering Technology and Sciences. 2025, 15(02): 2010-2017. [CrossRef]
- ELAFF “Modeling of the Human Heart in 3D Using DTI Images”, World Journal of Advanced Engineering Technology and Sciences, 2025, 15(02), 2450-2459. [CrossRef]
- IAI El-Aff “Extraction of human heart conduction network from diffusion tensor MRI” The 7th IASTED International Conference on Biomedical Engineering, 217-22.
- ELAFF “Modeling the Human Heart Conduction Network in 3D using DTI Images”, World Journal of Advanced Engineering Technology and Sciences, 2025, 15(02), 2565–2575. [CrossRef]
- ELAFF “Modeling of realistic heart electrical excitation based on DTI scans and modified reaction diffusion equation” Turkish Journal of Electrical Engineering and Computer Sciences: 2018, 26(3): Article 2. [CrossRef]
- ELAFF “Modeling of The Excitation Propagation of The Human Heart”, World Journal of Biology Pharmacy and Health Sciences, 2025, 22(02): 512–519. [CrossRef]
- ELAFF “Effect of the material properties on modeling of the excitation propagation of the human heart”, World Journal of Biology Pharmacy and Health Sciences, 2025, 22(3): 088–094. [CrossRef]
- D. Durrer, R.TH. D. Durrer, R.TH. Van Dam, G.E. Freud, M.J. Janse, F.L. Meijler and R.C. Arzbaecher “Total Excitation of the Isolated Human Heart” Circulation, (1970); 41(6):899-912.
- ELAFF “Modeling of the Body Surface Potential Map for Anisotropic Human Heart Activation”, Research Square, 2025.
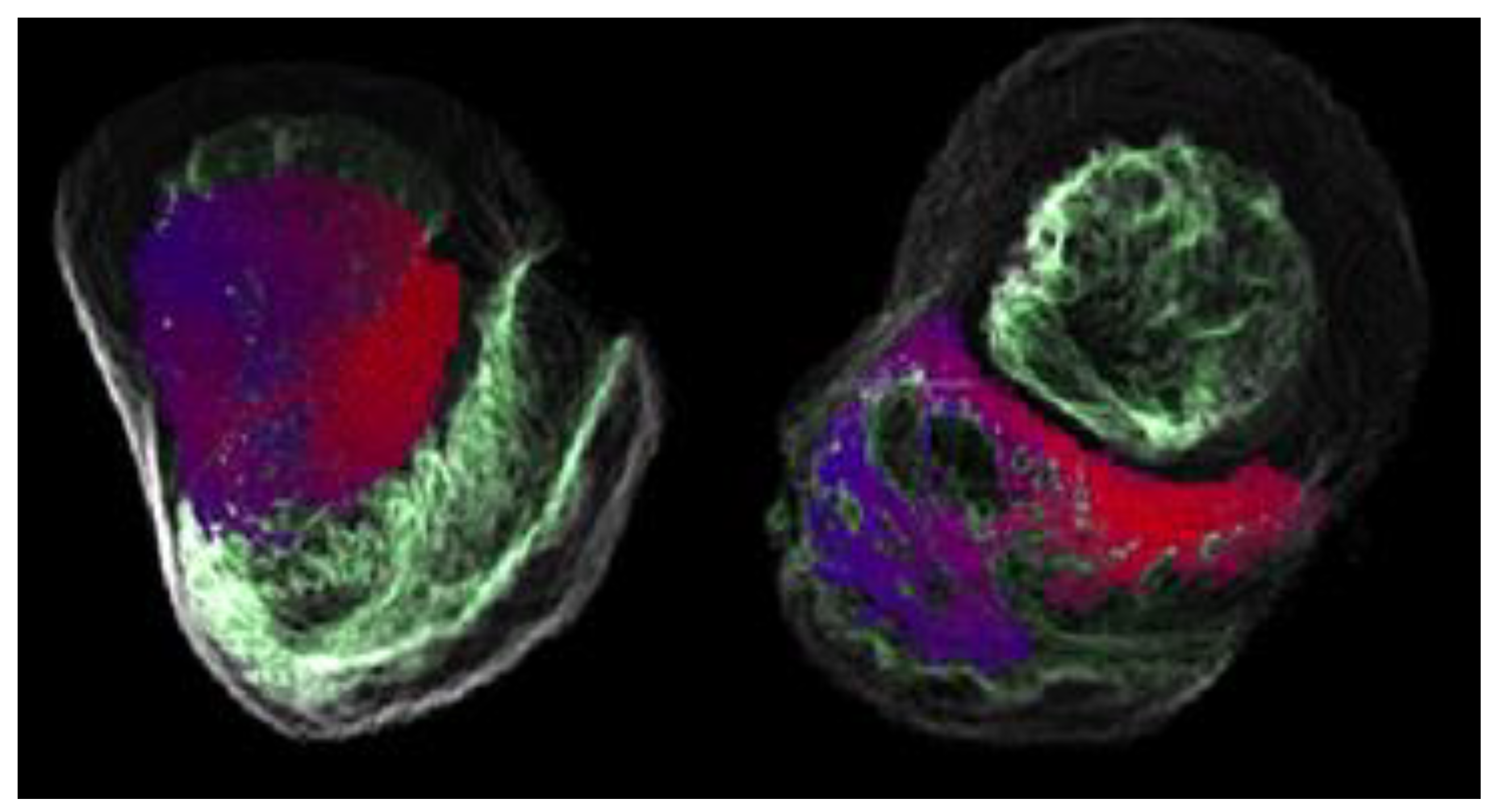
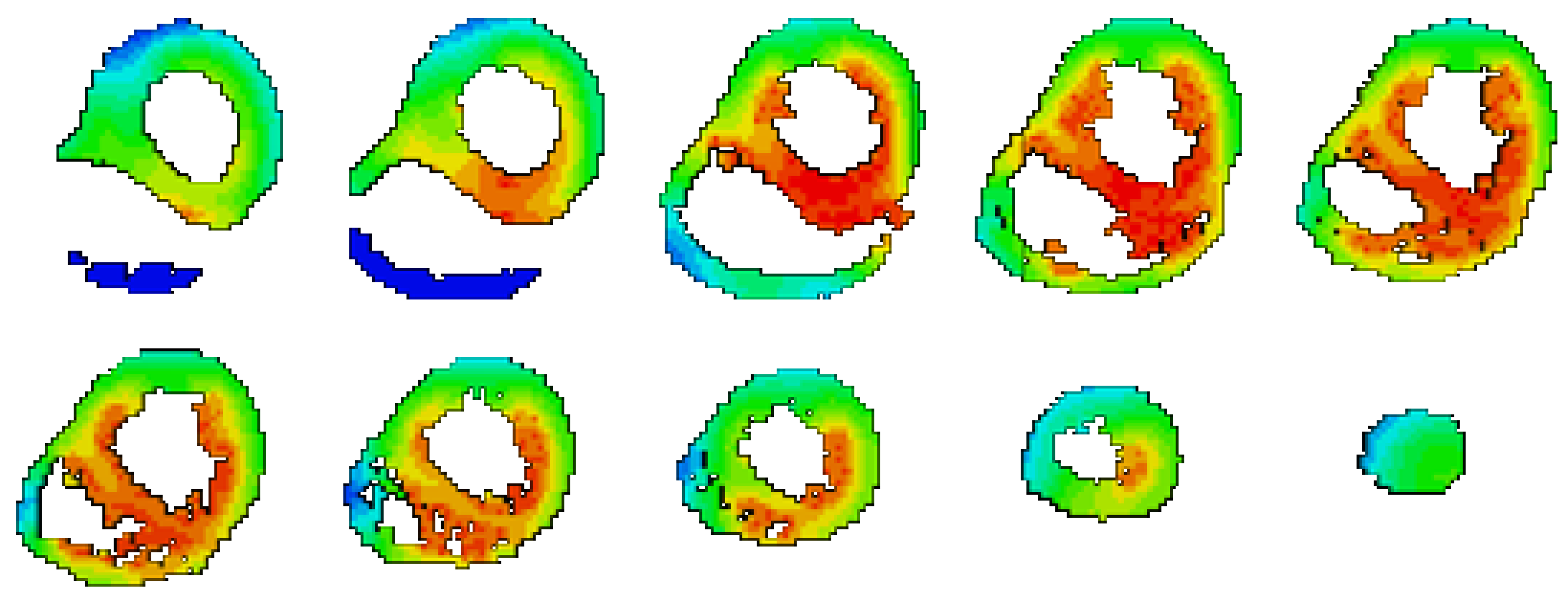
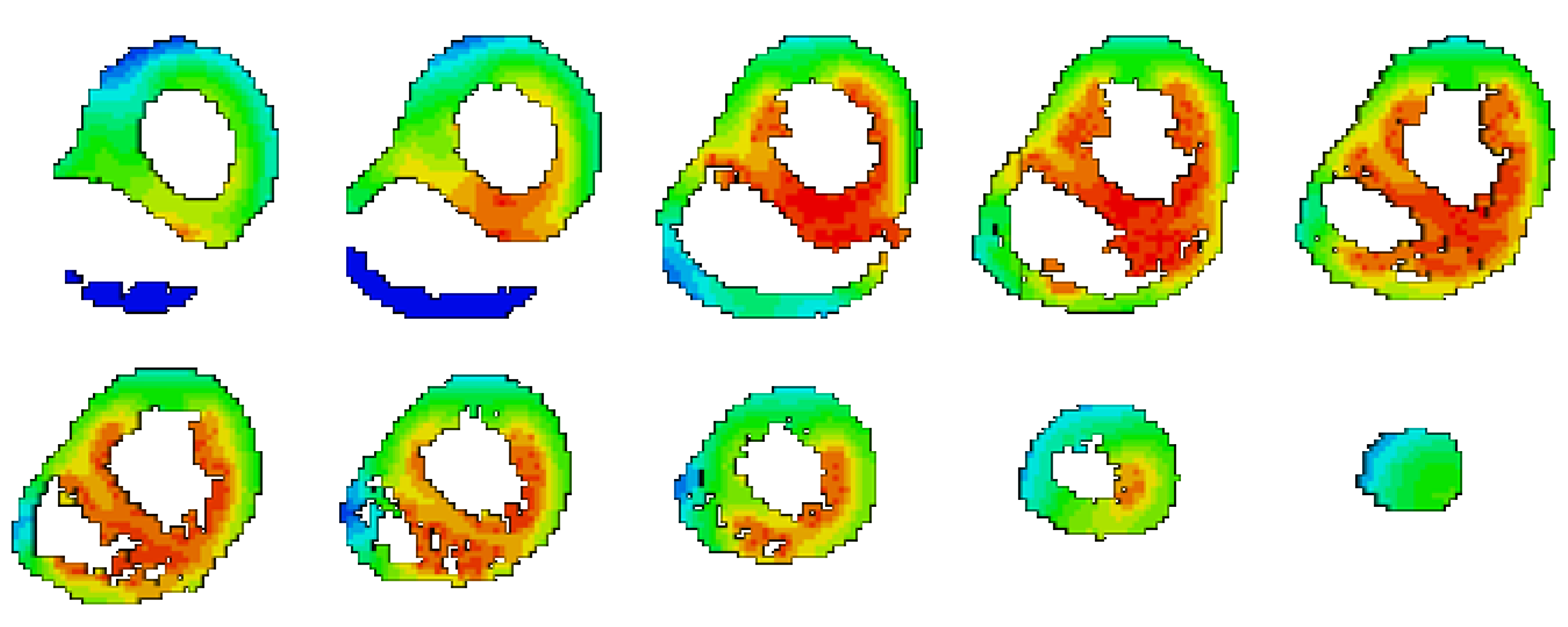

| ID | CC | RE |
| 1 | 0.999 | 0.217 |
| 2 | 0.997 | 0.185 |
| 3 | 0.999 | 0.121 |
| 4 | 0.995 | 0.141 |
| 5 | 0.991 | 0.144 |
| 6 | 0.972 | 0.242 |
| 7 | 0.970 | 0.283 |
| 8 | 0.987 | 0.209 |
| 9 | 0.995 | 0.145 |
| 10 | 0.996 | 0.103 |
| 11 | 0.997 | 0.081 |
| 12 | 0.997 | 0.080 |
| 13 | 0.996 | 0.084 |
| 14 | 0.995 | 0.102 |
| 15 | 0.994 | 0.118 |
| 16 | 0.994 | 0.140 |
| 17 | 0.992 | 0.159 |
| 18 | 0.986 | 0.223 |
| 19 | 0.960 | 0.321 |
| 20 | 0.891 | 0.481 |
| 21 | 0.904 | 0.434 |
| 22 | 0.965 | 0.281 |
| 23 | 0.975 | 0.291 |
| Mean | 0.980 | 0.199 |
| SD | 0.027 | 0.106 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





