Introduction
Modern agriculture faces the critical challenge of maintaining high productivity to meet the food demands of a rapidly growing global population. Nitrogen fertilizers play an essential role in this process; however, their excessive use has led to serious environmental problems [
7]. When nitrogen is applied beyond the absorption capacity of crops, it is converted into potent greenhouse gases such as nitrous oxide (N₂O) and released into the atmosphere [
6]. Nitrous oxide possesses a global warming potential approximately 298 times greater than that of carbon dioxide (CO₂) and thus significantly contributes to climate change [
11]. Additionally, nitrate (NO₃⁻) leaching contaminates water bodies, threatening aquatic ecosystems and compromising groundwater quality, which can be detrimental to drinking water supplies [
3].
To address these issues, it is globally imperative to maintain agricultural productivity while reducing nitrogen fertilizer inputs. Improving nitrogen use efficiency (NUE) has emerged as a key strategy to achieve this goal [
10]. Enhancing the NUE of staple crops such as rice is particularly critical for ensuring global food security and protecting the environment [
13]. Previous studies have explored various breeding and biotechnological approaches to improve rice NUE, among which leveraging symbiotic interactions between rice and nitrogen-fixing microorganisms has gained attention as an environmentally sustainable and effective solution [
14].
Root exudates secreted by rice plants play a pivotal role in promoting such symbiotic interactions [
2]. These exudates stimulate the growth and biofilm formation of nitrogen-fixing microorganisms, thereby facilitating biological nitrogen fixation and enhancing nitrogen availability to the plant [
5,
8]. However, significant differences exist among rice cultivars in both the quantity and composition of root exudates, which in turn affect the efficiency of these microbial interactions [
1,
4]. Therefore, identifying exudate-derived compounds that favor nitrogen-fixing microorganisms is critical for improving NUE in rice.
In this study, we aim to identify compounds within rice root exudates that promote biofilm formation by nitrogen-fixing microorganisms, thereby enhancing symbiotic interactions and improving rice NUE. To achieve this, we conducted a comprehensive screening of a natural compound library comprising 1,597 substances to identify candidates that stimulate biofilm formation and nitrogen fixation activity. Furthermore, we plan to investigate the biosynthetic pathways of the selected compounds and employ gene editing technologies to enhance their secretion in rice roots, thereby maximizing beneficial plant-microbe interactions.
The compounds identified in this study have the potential to contribute to rice breeding efforts focused on improving NUE. Ultimately, this approach is expected to reduce nitrogen fertilizer usage and support the development of sustainable agricultural systems. Compared to conventional fertilizer reduction strategies, our method offers a more sustainable and long-term solution. Moreover, the findings of this study could be extended to other major cereal crops, contributing to a paradigm shift in global agricultural practices.
Materials and Methods
Selection of Nitrogen-Fixing Microorganisms
To identify microbial strains capable of enhancing nitrogen fixation efficiency through symbiosis with rice, nine nitrogen-fixing microorganisms were obtained from the Korean Agricultural Culture Collection (KACC,
https://genebank.rda.go.kr) (
Table 1). Among them,
Gluconacetobacter diazotrophicus (KACC 12358) was selected as a reference strain, based on the findings of [
15], which reported its superior nitrogen-fixing ability through biofilm formation. The microorganisms were selected from genera known for nitrogen fixation, choosing species available in the KACC repository.
Analysis of Microbial Growth Characteristics
To evaluate the growth characteristics of each nitrogen-fixing microorganism, the optimal growth medium was first selected among NA, R2A, and RAE media. To determine colony-forming units (CFU), samples were serially diluted, plated onto solid media, and incubated under appropriate conditions. After incubation, the number of colonies was counted, and CFUs were calculated considering the dilution factors. Specifically, precultures were adjusted to an OD600 of 0.3 using a spectrophotometer, followed by serial dilutions up to 10⁻⁹. Dilutions from 10⁻⁵ to 10⁻⁹ were plated onto solid media, with each dilution divided into five sections per plate and 20 µL aliquots dispensed into each section. Each condition was prepared in triplicate. After plating, the plates were air-dried under sterile conditions before incubation at optimal temperatures, and colony formation was monitored.
Growth curves of each microorganism were also determined by measuring OD600 at the early, mid, and late stages of cultivation. Specifically, precultures were adjusted to an OD600 of 0.01, and 200 µL of each culture was inoculated into a 96-well plate, with three replicates per strain. Cultures were incubated for up to 96 hours, and growth was monitored over time to assess proliferation rates and growth patterns under the tested conditions [
9].
Acquisition of Natural Compound Library
To identify compounds that enhance biofilm formation by nitrogen-fixing microorganisms from rice root exudates, a total of 1,597 natural compounds were obtained from the Korea Chemical Bank (
https://chembank.org) (
Table S1). These compounds were prepared at a concentration of 5 mM and were used for screening to evaluate their effects on biofilm formation.
Final Selection of Compounds
Among the 50 compounds selected from the primary screening, compounds were finally selected based on their differential effects on biofilm formation between the selected strains and the control strain, Gluconacetobacter diazotrophicus (KACC 12358), and their biosynthesis in rice. These compounds are expected to enhance nitrogen use efficiency (NUE) in rice and will be utilized in future studies to strengthen the symbiotic interaction between rice and nitrogen-fixing microorganisms.
Results
Optimization of Cultivation Conditions and Microbial Identification Results
In this study, the optimal cultivation conditions for 9 nitrogen-fixing microorganisms were selected by comparing cultivation on NA, R2A, and RAE media. The growth characteristics of the strains on each medium were evaluated, and the results showed that most strains exhibited the most vigorous growth on R2A medium, particularly
Azoarcus indigens (KACC 11682) and Herbaspirillum strains, which displayed the most prominent growth effects. Based on these results, R2A medium was selected as the most suitable medium for the cultivation of the 9 nitrogen-fixing microorganisms. After selecting the optimal medium, each strain was cultured on R2A medium, and CFU (OD600=0.3) was measured to quantitatively assess the growth. The CFU calculation showed that the growth of all strains was higher on R2A medium compared to other media, suggesting that this medium provides the most suitable environment for microbial proliferation and colony formation (
Figure S2).
Microbial identification was performed using 16S rRNA sequencing, and bands of the expected size were confirmed for all strains (
Figure S1). This indicates that successful 16S rRNA-based identification of each strain was achieved, supporting the purity and accuracy of the microbial identification. These results demonstrate the successful establishment of optimal cultivation conditions for the 9 nitrogen-fixing microorganisms used in the study and the successful identification of the microorganisms.
Growth Curve Analysis
The growth curves of the 9 nitrogen-fixing microorganisms were analyzed, and the results showed that
Azoarcus indigens (KACC 11682) exhibited stable growth at 37 °C, while the other 8 strains grew stably at 30 °C (
Figure S3). In particular,
Herbaspirillum frisingense (KACC 15012) showed the fastest growth rate, reaching its peak OD600 value after 24 hours. In contrast,
Azoarcus indigens (KACC 11682) showed slower growth. This was in contrast to the visual observation, where a large amount of suspended material, such as biofilm, was observed. The
Gluconacetobacter diazotrophicus (KACC 12358) strain, which had previously demonstrated nitrogen fixation and biofilm formation ability in the study by [
15], showed a growth pattern similar to that of
Herbaspirillum frisingense (KACC 15012), but with relatively slower growth.
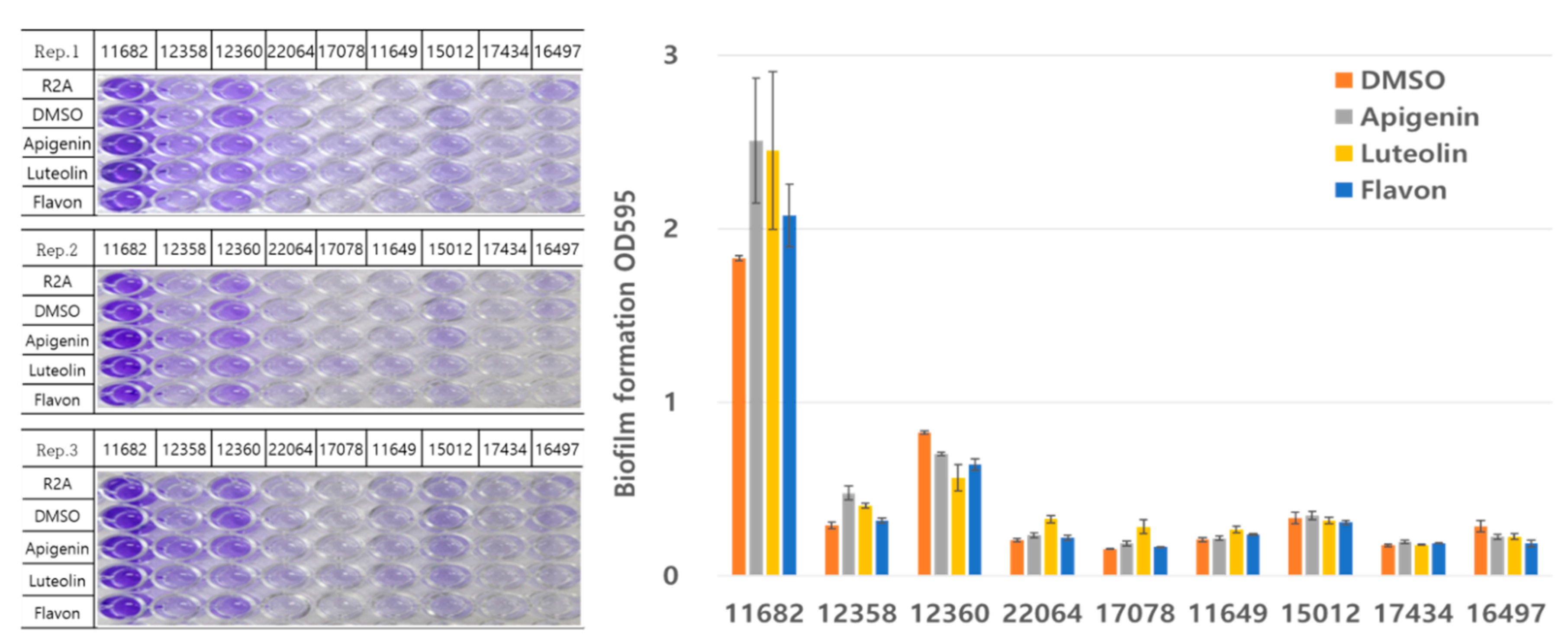
Natural Compound Screening Results
As shown in
Figure 2, screening of 1,597 natural compounds revealed the top 50 compounds that most significantly enhanced biofilm formation in
Azoarcus indigens (KACC 11682). The selected compounds demonstrated a biofilm formation ability more than 500% higher than the control strain,
Gluconacetobacter diazotrophicus (KACC 12358) (
Table S2). The compound with the highest activity, oxytetracycline hydrochloride, showed a tendency to be 1,793% higher in comparison. This compound, known as an antibiotic synthesized by actinomycetes, was identified in this study as a biofilm-promoting substance.
Among the compounds synthesized in rice, alkaloids and flavonoids were most frequently identified, with cardamomin being a representative compound. This compound showed a 245% increase in relative comparison. Cardamomin was selected from the flavonoid group due to its position at the top of the biosynthetic pathway, which strategically aligns with ongoing research on chalcones in flavonoid biosynthesis. Furthermore, it was ranked fifth in biofilm formation ability when tested alone in Azoarcus indigens (KACC 11682), indicating a relatively high level of activity.
Ultimately, the compounds identified in this study are considered to have high potential for improving nitrogen use efficiency (NUE) in rice. Additionally, these compounds demonstrated a strong association with specific rice gene pathways, and they are expected to play a key role in enhancing the symbiotic interaction between rice and nitrogen-fixing microorganisms in future research.
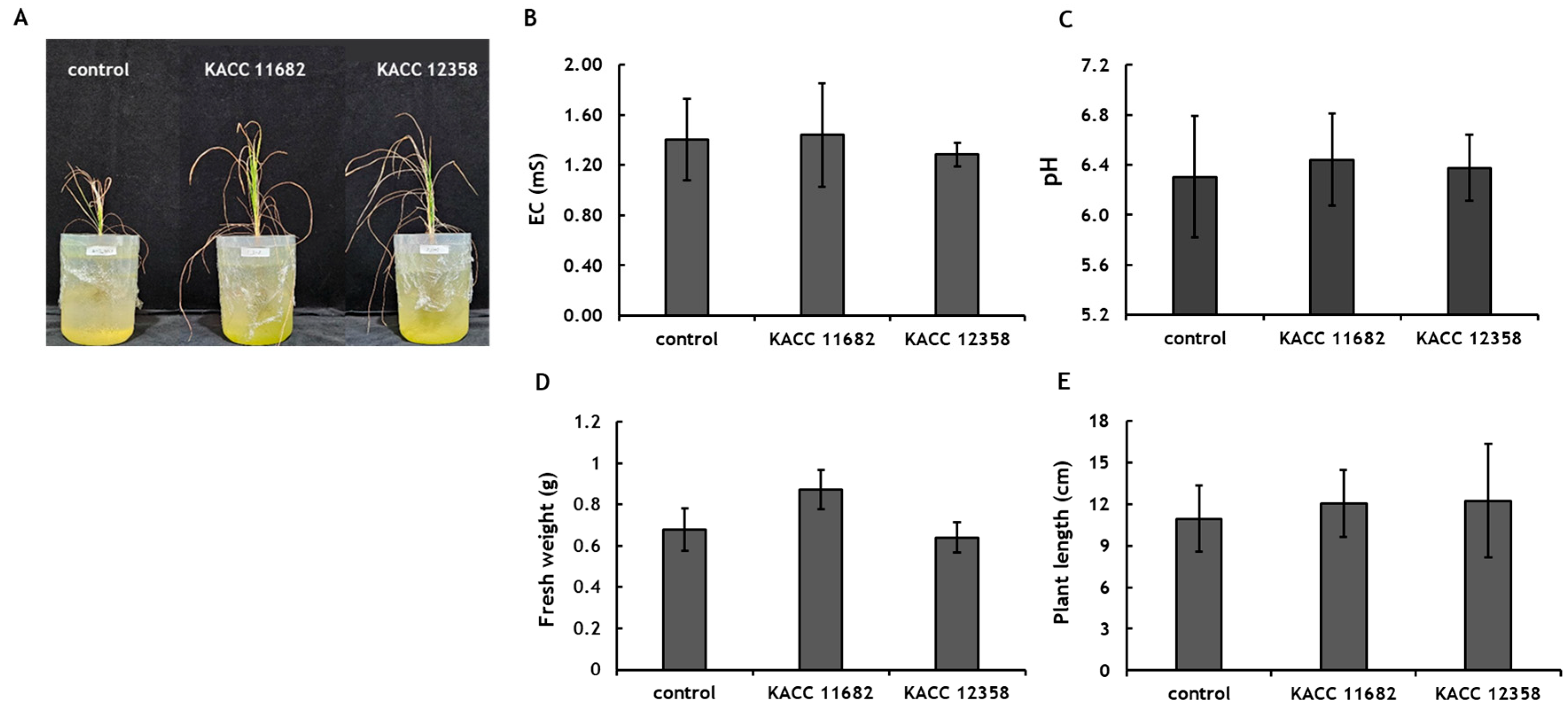
Effect of Nitrogen-Fixing Microorganisms on Rice Growth
In the growth promotion test using the Samgwang rice variety, rice inoculated with
Azoarcus indigens (KACC 11682) showed approximately a 128% increase in biomass compared to the untreated control group (
Figure 3). Furthermore, rice inoculated with
Azoarcus indigens (KACC 11682) exhibited a trend of about 136% greater biomass than rice inoculated with
Gluconacetobacter diazotrophicus (KACC 12358). However, a contrasting trend was observed in plant height, with rice inoculated with
Gluconacetobacter diazotrophicus (KACC 12358) showing about a 112% increase in plant height compared to the control, though the difference was not statistically significant.
Discussion
In this study, we identified a novel nitrogen-fixing microorganism, Azoarcus indigens (KACC 11682), aimed at enhancing rice nitrogen use efficiency (NUE) and evaluated its impact on rice growth. The strain Azoarcus indigens (KACC 11682) demonstrated excellent biofilm formation ability, suggesting its potential to effectively perform nitrogen fixation through symbiotic interactions with rice. Compared to Gluconacetobacter diazotrophicus (KACC 12358), which was used in previous studies, Azoarcus indigens (KACC 11682) exhibited significantly superior biofilm formation. This superior biofilm formation may play a critical role in maximizing the nitrogen use efficiency of rice through strong interactions with rice roots.
From the natural compound screening, 50 compounds that promote biofilm formation were identified in both Azoarcus indigens (KACC 11682) and Gluconacetobacter diazotrophicus (KACC 12358), with one compound selected based on KEGG database analysis for its association with specific rice gene pathways. This suggests that natural compounds may play an important role in enhancing the interaction between rice and nitrogen-fixing microorganisms.
Moreover, the significant increase in biomass observed in rice inoculated with nitrogen-fixing microorganisms strongly indicates that these microorganisms contribute to rice growth and nitrogen use efficiency improvement. These results suggest the potential for leveraging symbiotic interactions with nitrogen-fixing microorganisms to improve rice NUE.
Future studies will focus on evaluating the long-term effects of these compounds and microorganisms, assessing their applicability in real agricultural settings, and validating their efficacy across various rice cultivars.
Conclusions
This study identified a novel strain, Azoarcus indigens (KACC 11682), that can enhance symbiotic interactions between rice and nitrogen-fixing microorganisms, promoting rice growth and improving nitrogen use efficiency (NUE). Furthermore, the study demonstrated the potential of using natural compounds to strengthen these interactions, offering a promising strategy to improve rice NUE and reduce negative environmental impacts.
Future research will focus on evaluating the applicability of these findings in real agricultural settings and conducting further validation across various rice cultivars, with the aim of developing concrete strategies for sustainable agricultural practices.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org, Figure S1: Optimal cultivation conditions and CFU determination for nitrogen-fixing microorganisms, Figure S2: Design and validation of specific primers for species identification within the 16S rRNA region, Figure S3: Growth curve determination for each nitrogen-fixing microorganism, Table S1: Information on 1,597 natural compounds obtained from the Korean Compound Bank, Table S2: Biofilm formation levels of 1,597 natural compounds.
Author Contributions
JHO was responsible for the conceptualization of the study; JHO developed the methodology for the research; JHO, EK, and MC contributed to the software development; EK, and MC conducted formal analysis; EK, and MC were involved in the investigation process; JHO, EK, and MC provided necessary resources for the study; JHO and EK curated the data used in the research; JHO and EK took the lead in preparing the original draft of the manuscript; JHO participated in the writing, review, and editing of the paper; JHO provided supervision throughout the study; JHO handled project administration. JHO secured funding for the study; All authors have reviewed and agreed upon the final version of the manuscript.
Funding
This work was supported by agrant from the National Institute of Agricultural Sciences Program (ProjectNo.PJ017406012025), Rural Development Administration, Republic of Korea.
Acknowledgments
Not applicable.
Conflicts of Interest
The authors declare no competing interests. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Aulakh, M.S; Wassmann, R. Impact of agricultural management on emissions of greenhouse gases: Nitrogen cycles in rice fields. Plant and Soil 2001, 228, 15–28. [Google Scholar]
- Bais, H.P.; Weir, T.L.; Perry, L.G.; Gilroy, S.; Vivanco, J.M. The role of root exudates in rhizosphere interactions with plants and other organisms. Annual Review of Plant Biology 2006, 57, 233–266. [Google Scholar] [CrossRef]
- Camargo, J.A.; Alonso, Á. Ecological and toxicological effects of inorganic nitrogen pollution in aquatic ecosystems: A global assessment. Environment International 2006, 32, (6): 831–849. [Google Scholar] [CrossRef]
- Cassman, K.G.; Dobermann, A.; Walters, D.T.; Yang, H. Meeting cereal demand while protecting natural resources and improving environmental quality. Annual Review of Environment and Resources 2003, 28, (1): 315–358. [Google Scholar] [CrossRef]
- Dakora, F.D.; Phillips, D.A. Root exudates as mediators of mineral acquisition in low-nutrient environments. Plant and Soil 2002, 245, 35–47. [Google Scholar] [CrossRef]
- Davidson, E.A. The contribution of manure and fertilizer nitrogen to atmospheric nitrous oxide since 1860. Nature Geoscience 2009, 2, (9): 659–662. [Google Scholar] [CrossRef]
- Galloway, J.N.; Townsend, A.R.; Erisman, J.W.; Bekunda, M.; Cai, Z.; Freney, J.R.; Martinelli, L.A.; Seitzinger, S.P.; Sutton, M.A. Transformation of the nitrogen cycle: Recent trends, questions, and potential solutions. Science 2008, 320, (5878): 889–892. [Google Scholar] [CrossRef]
- Garnett, T.; Appleby, M.C.; Balmford, A.; Bateman, I.J.; Benton, T.G.; Bloomer, P.; Godfray, H.C.J. Sustainable intensification in agriculture: Premises and policies. Science 2013, 341, (6141): 33–34. [Google Scholar] [CrossRef]
- Madigan, M.T.; Bender, K.S.; Buckley, D.H.; Sattley, W.M.; Stahl, D.A. Brock Biology of Microorganisms (15th ed.). 2018, Pearson. https://lib.ugent.be/catalog/rug01:002399713.
- Raun, W.R.; Johnson, G.V. Improving nitrogen use efficiency for cereal production. Agronomy Journal 1999, 91, (3): 357–363. [Google Scholar] [CrossRef]
- Ravishankara, A.R.; Daniel, J.S.; Portmann, R.W. Nitrous oxide (N2O): the dominant ozone-depleting substance emitted in the 21st century. Science 2009, 326, (5949): 123–125. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G. ; Kumar, S, MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Molecular Biology and Evolution 2021, 38, (7): 3022–3027. [Google Scholar] [CrossRef]
- Tilman, D.; Balzer, C.; Hill, J.; Befort, B.L. Global food demand and the sustainable intensification of agriculture. Proceedings of the National Academy of Sciences 2011, 108, (50): 20260–20264. [Google Scholar] [CrossRef]
- Yang, C.; Yang, L.; Yang, Y.; Ouyang, Z. Rice root growth and nutrient uptake as influenced by organic manure in continuously and alternately flooded paddy soils. Plant and Soil 2017, 417, 567–575. [Google Scholar] [CrossRef]
- Yan, Y.; Wang, Y.; Tao, Y; He, J.; Chen, R.; Ma, Y.; Yang, L.; Xie, Q; Chen, X. Genetic modification of flavone biosynthesis in rice enhances biofilm formation of soil diazotrophic bacteria and biological nitrogen fixation. Nature Communications 2022, 13: 4275. [CrossRef]
- Yoshida, S.; Forno, D.A.; Cock, J.H. Routine procedure for growing rice plants in culture solution. Laboratory Manual for Physiological Studies of Rice, International Rice Research Institute 1976 Los Baños, 61–66. Available online: https://ci.nii.ac.jp/naid/20000862586/.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).