Submitted:
04 June 2025
Posted:
04 June 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Cell Lines
2.2. Plasmid Construction and Establishment of Stable Transfectants
2.3. Hybridoma Production
2.4. Flow Cytometry
2.5. Determination of Dissociation Constant Values Using Flow Cytometry
2.6. Western Blotting
2.7. Immunohistochemistry (IHC) Using Cell Blocks
3. Results
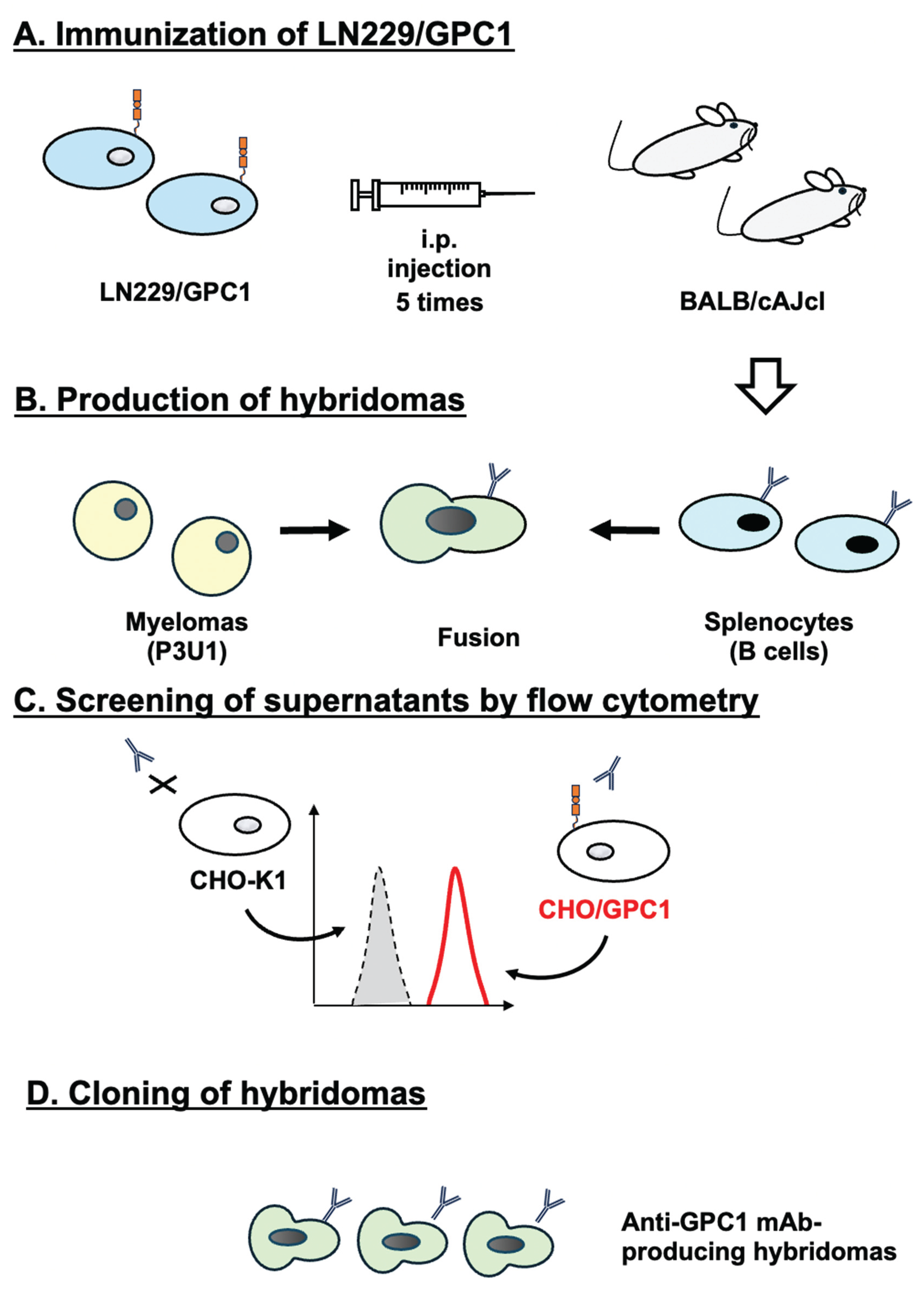
3.1. Development of Anti-GPC1 mAbs Using the CBIS Method
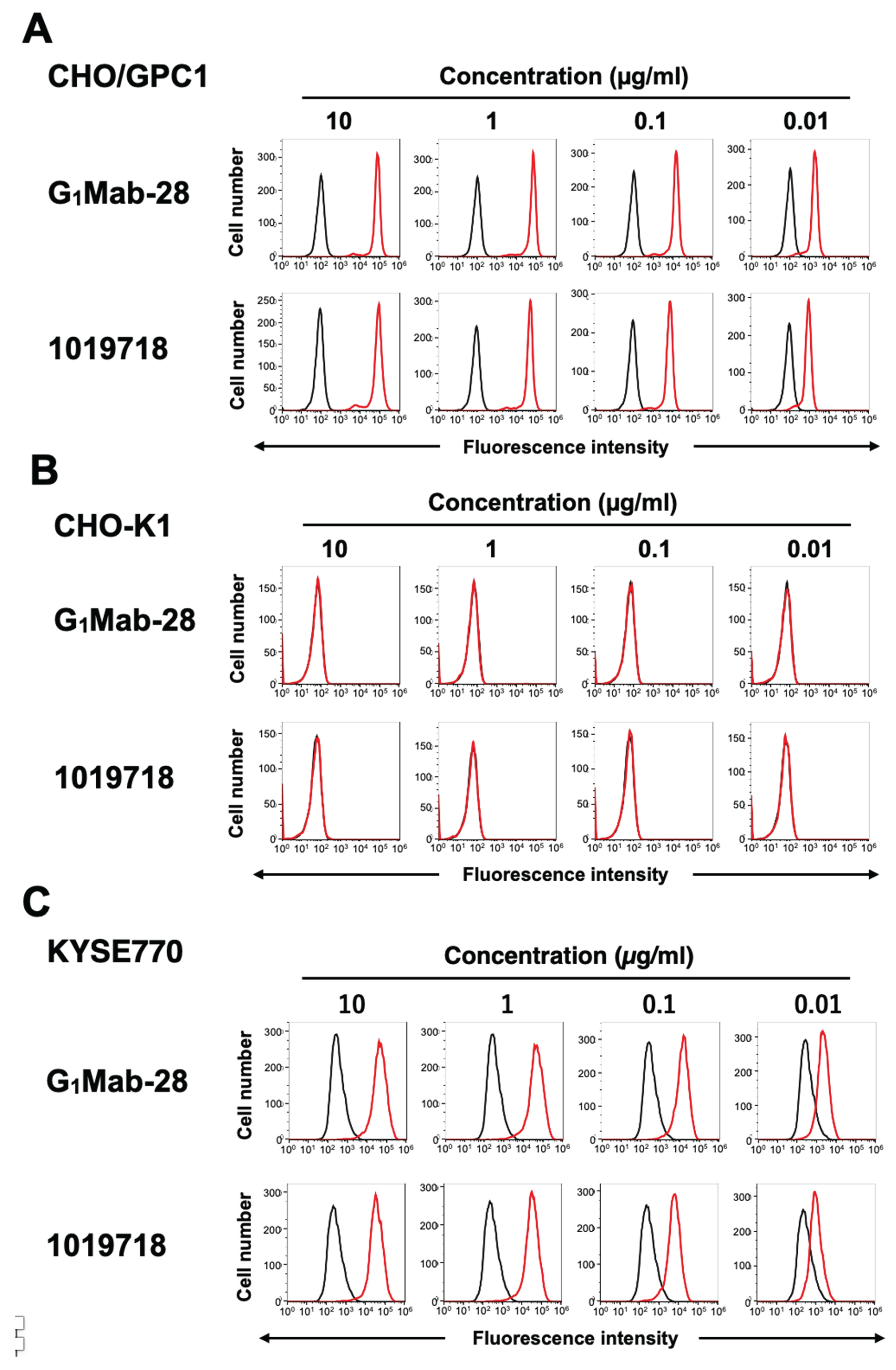
3.2. Flow Cytometry Using Anti-GPC1 mAbs
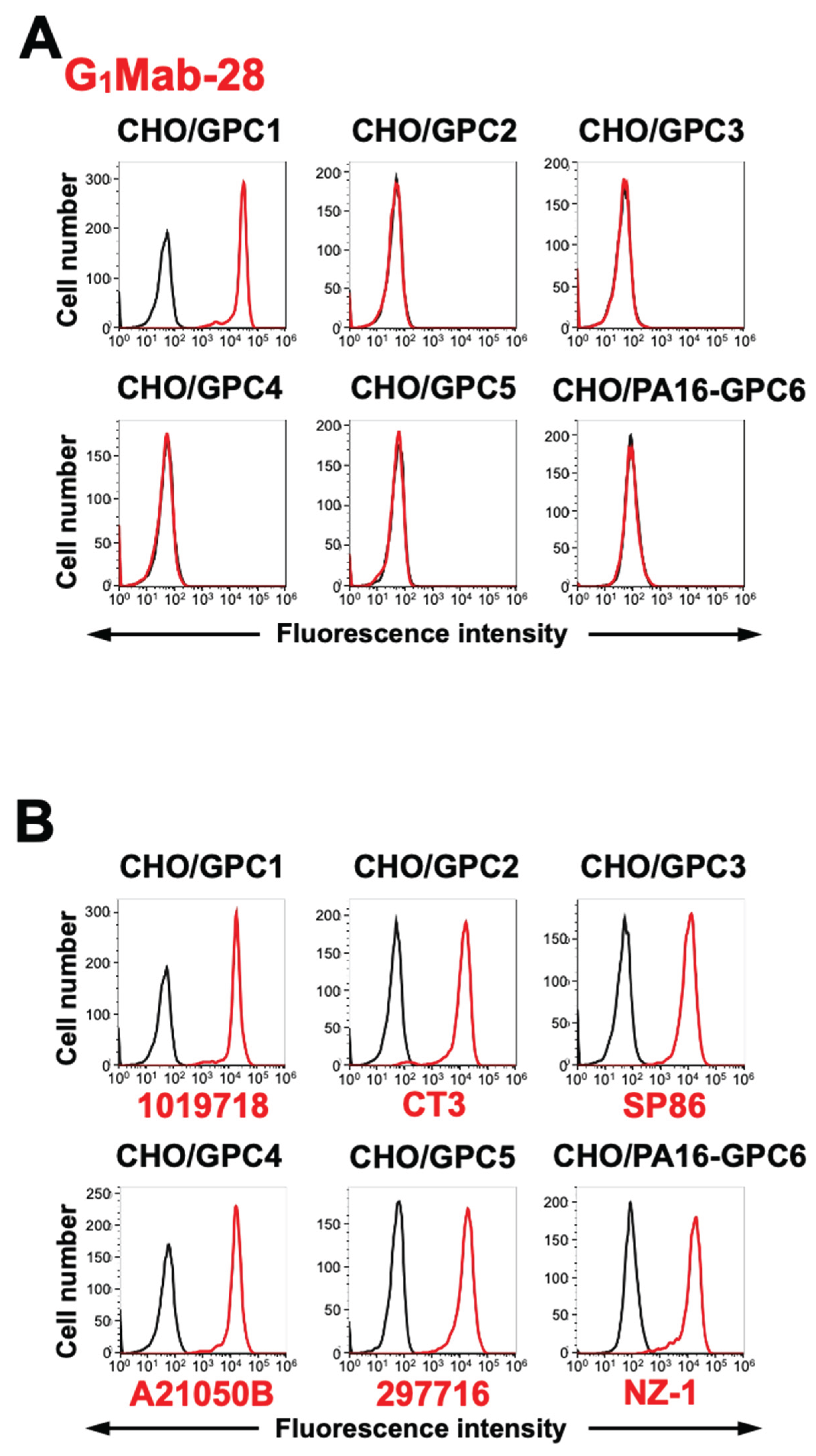
3.3. Specificity of G1Mab-28 Against GPC Family Members
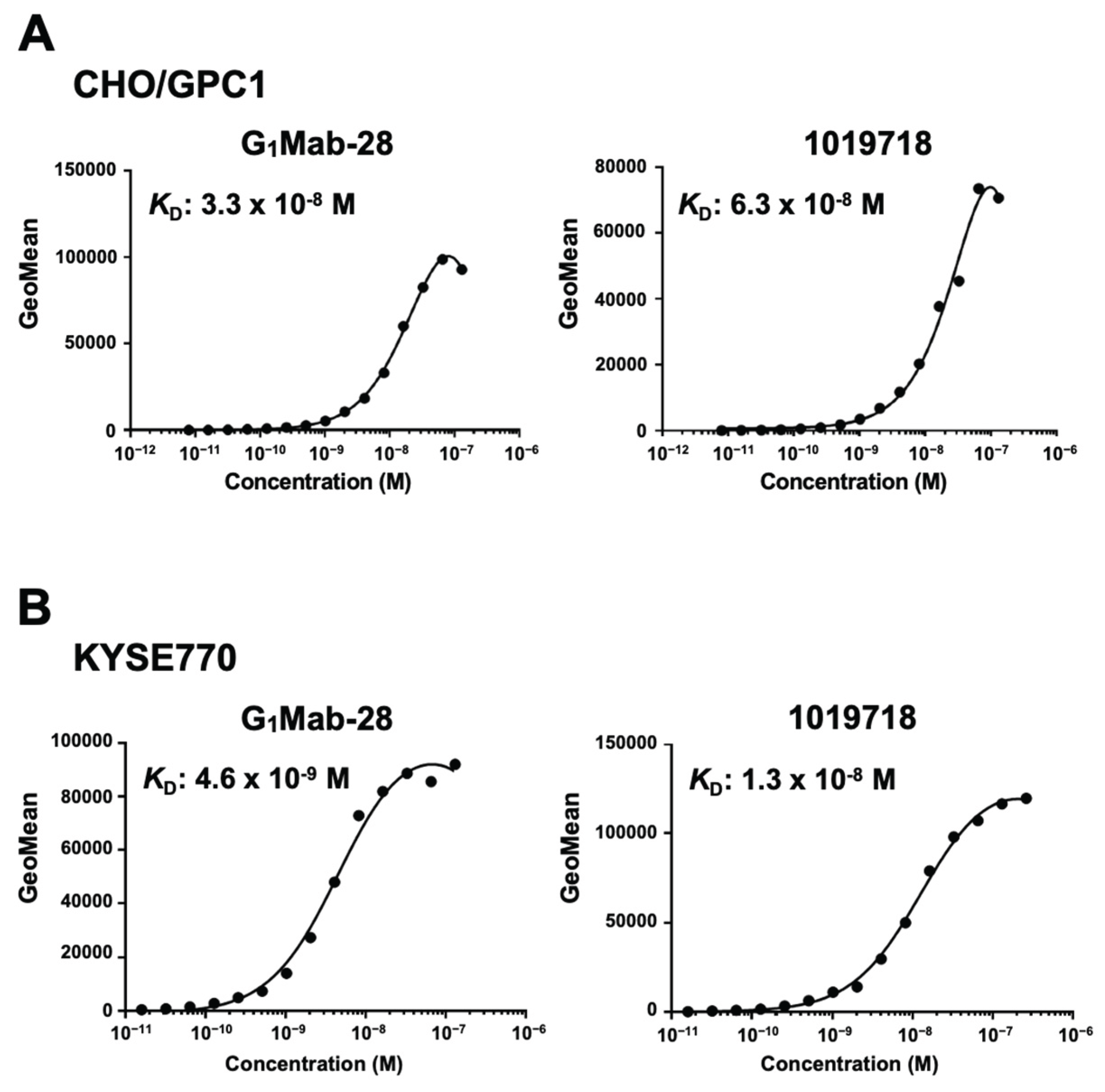
3.4. Determination of KD Values of G1Mab-28 by Flow Cytometry
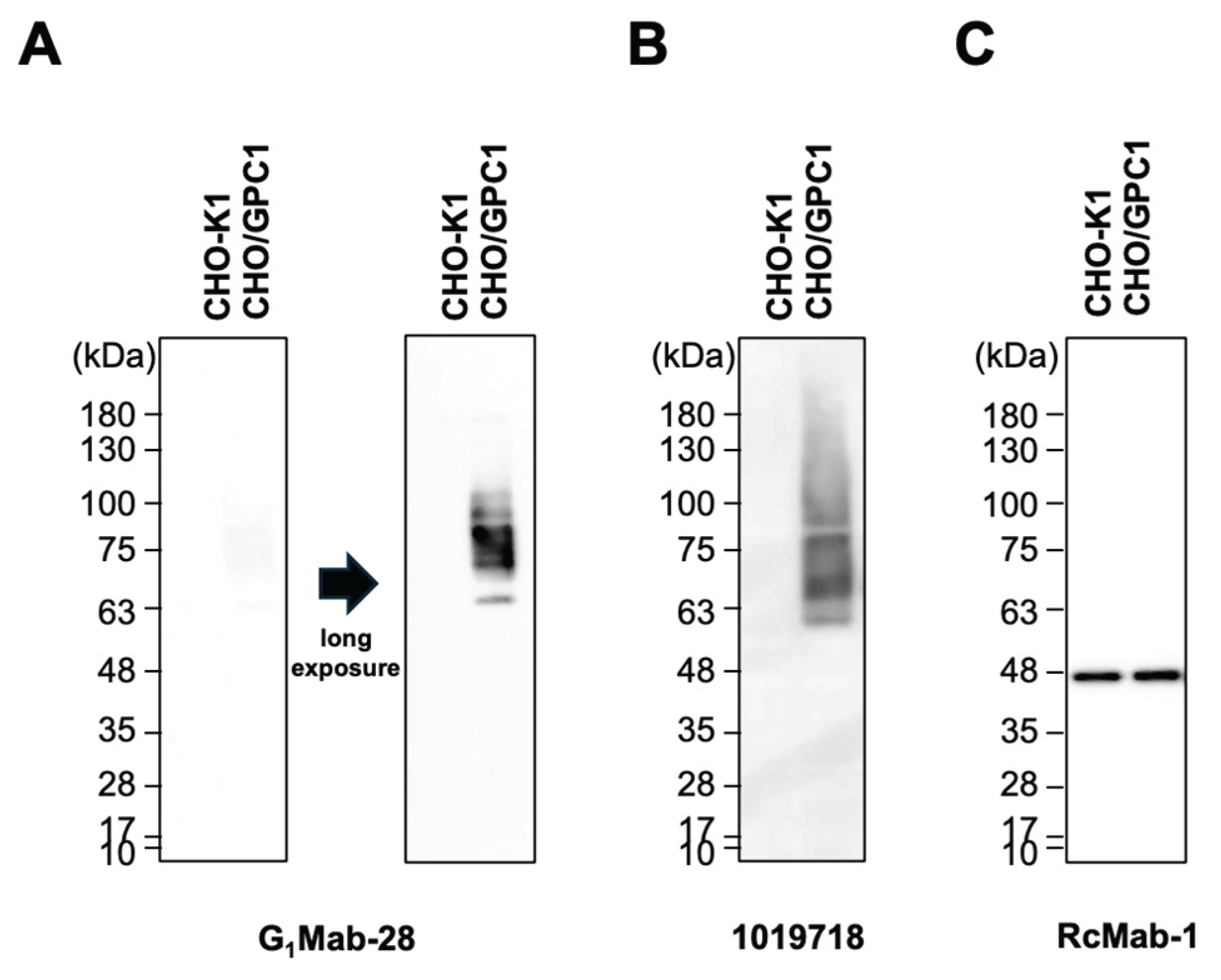
3.5. Western Blotting Using G1Mab-28
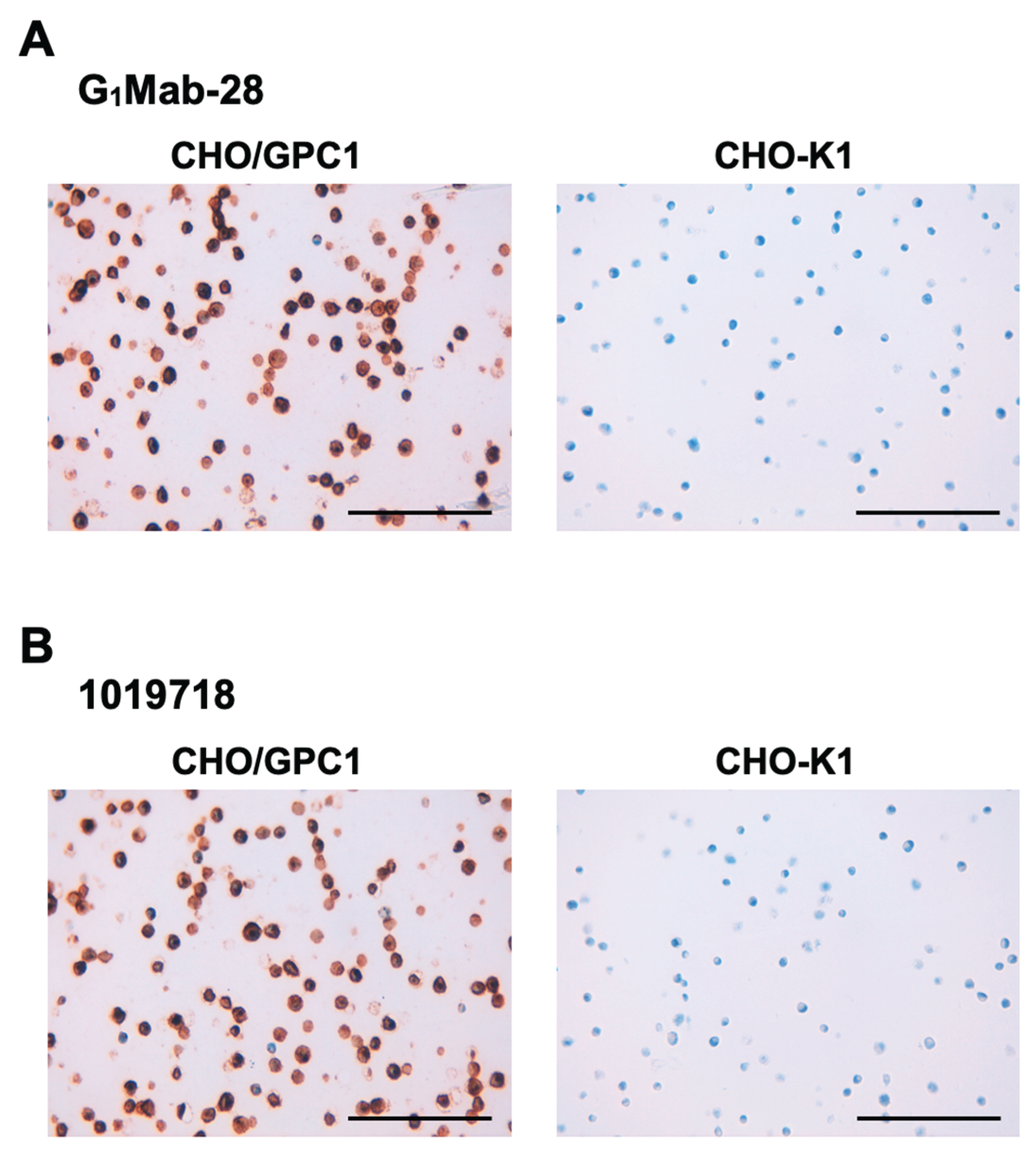
3.6. IHC Using G1Mab-28 in FFPE Cell Sections
4. Discussion
Supplementary Materials
Author Contributions
Funding Information
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Filmus, J.; Capurro, M.; Rast, J. Glypicans. Genome Biol 2008;9(5): 224. [CrossRef]
- Filmus, J. The function of glypicans in the mammalian embryo. Am J Physiol Cell Physiol 2022;322(4): C694-c698.
- Pan, J.; Ho, M. Role of glypican-1 in regulating multiple cellular signaling pathways. Am J Physiol Cell Physiol 2021;321(5): C846-c858. [CrossRef]
- Li, N.; Spetz, M.R.; Ho, M. The Role of Glypicans in Cancer Progression and Therapy. J Histochem Cytochem 2020;68(12): 841-862. [CrossRef]
- Kawahara, R.; Granato, D.C.; Yokoo, S.; et al. Mass spectrometry-based proteomics revealed Glypican-1 as a novel ADAM17 substrate. J Proteomics 2017;151: 53-65. [CrossRef]
- Traister, A.; Shi, W.; Filmus, J. Mammalian Notum induces the release of glypicans and other GPI-anchored proteins from the cell surface. Biochem J 2008;410(3): 503-511. [CrossRef]
- Ghosh, S.; Huda, P.; Fletcher, N.; et al. Clinical development of an anti-GPC-1 antibody for the treatment of cancer. Expert Opin Biol Ther 2022;22(5): 603-613. [CrossRef]
- Lund, M.E.; Campbell, D.H.; Walsh, B.J. The Role of Glypican-1 in the Tumour Microenvironment. Adv Exp Med Biol 2020;1245: 163-176.
- Wang, S.; Qiu, Y.; Bai, B. The Expression, Regulation, and Biomarker Potential of Glypican-1 in Cancer. Front Oncol 2019;9: 614.
- Hara, H.; Takahashi, T.; Serada, S.; et al. Overexpression of glypican-1 implicates poor prognosis and their chemoresistance in oesophageal squamous cell carcinoma. Br J Cancer 2016;115(1): 66-75. [CrossRef]
- Kai, Y.; Amatya, V.J.; Kushitani, K.; et al. Glypican-1 is a novel immunohistochemical marker to differentiate poorly differentiated squamous cell carcinoma from solid predominant adenocarcinoma of the lung. Transl Lung Cancer Res 2021;10(2): 766-775. [CrossRef]
- Saito, T.; Sugiyama, K.; Hama, S.; et al. High Expression of Glypican-1 Predicts Dissemination and Poor Prognosis in Glioblastomas. World Neurosurg 2017;105: 282-288. [CrossRef]
- Duan, L.; Hu, X.Q.; Feng, D.Y.; Lei, S.Y.; Hu, G.H. GPC-1 may serve as a predictor of perineural invasion and a prognosticator of survival in pancreatic cancer. Asian J Surg 2013;36(1): 7-12. [CrossRef]
- Russell, P.J.; Ow, K.T.; Tam, P.N.; et al. Immunohistochemical characterisation of the monoclonal antibody BLCA-38 for the detection of prostate cancer. Cancer Immunol Immunother 2004;53(11): 995-1004. [CrossRef]
- Matsuda, K.; Maruyama, H.; Guo, F.; et al. Glypican-1 is overexpressed in human breast cancer and modulates the mitogenic effects of multiple heparin-binding growth factors in breast cancer cells. Cancer Res 2001;61(14): 5562-5569.
- Suzuki, H.; Tanaka, T.; Li, G.; et al. Development of a Sensitive Anti-Mouse CCR5 Monoclonal Antibody for Flow Cytometry. Monoclon Antib Immunodiagn Immunother 2024;43(4): 96-100. [CrossRef]
- Tanaka, T.; Nanamiya, R.; Takei, J.; et al. Development of Anti-Mouse CC Chemokine Receptor 8 Monoclonal Antibodies for Flow Cytometry. Monoclon Antib Immunodiagn Immunother 2021;40(2): 65-70. [CrossRef]
- Nanamiya, R.; Takei, J.; Asano, T.; et al. Development of Anti-Human CC Chemokine Receptor 9 Monoclonal Antibodies for Flow Cytometry. Monoclon Antib Immunodiagn Immunother 2021;40(3): 101-106. [CrossRef]
- Nanamiya, R.; Suzuki, H.; Kaneko, M.K.; Kato, Y. Development of an Anti-EphB4 Monoclonal Antibody for Multiple Applications Against Breast Cancers. Monoclon Antib Immunodiagn Immunother 2023;42(5): 166-177. [CrossRef]
- Itai, S.; Yamada, S.; Kaneko, M.K.; et al. Establishment of EMab-134, a Sensitive and Specific Anti-Epidermal Growth Factor Receptor Monoclonal Antibody for Detecting Squamous Cell Carcinoma Cells of the Oral Cavity. Monoclon Antib Immunodiagn Immunother 2017;36(6): 272-281. [CrossRef]
- Goto, N.; Suzuki, H.; Tanaka, T.; et al. Development of a Novel Anti-CD44 Monoclonal Antibody for Multiple Applications against Esophageal Squamous Cell Carcinomas. Int J Mol Sci 2022;23(10). [CrossRef]
- Fujii, Y.; Kaneko, M.; Neyazaki, M.; et al. PA tag: a versatile protein tagging system using a super high affinity antibody against a dodecapeptide derived from human podoplanin. Protein Expr Purif 2014;95: 240-247. [CrossRef]
- Ikota, H.; Nobusawa, S.; Arai, H.; et al. Evaluation of IDH1 status in diffusely infiltrating gliomas by immunohistochemistry using anti-mutant and wild type IDH1 antibodies. Brain Tumor Pathol 2015;32(4): 237-244. [CrossRef]
- Xiao, D.; Dong, Z.; Zhen, L.; et al. Combined Exosomal GPC1, CD82, and Serum CA19-9 as Multiplex Targets: A Specific, Sensitive, and Reproducible Detection Panel for the Diagnosis of Pancreatic Cancer. Mol Cancer Res 2020;18(2): 300-310. [CrossRef]
- Tripathi, A.D.; Katiyar, S.; Mishra, A. Glypican1: A potential cancer biomarker for nanotargeted therapy. Drug Discov Today 2023;28(8): 103660. [CrossRef]
- Pan, J.; Li, N.; Renn, A.; et al. GPC1-Targeted Immunotoxins Inhibit Pancreatic Tumor Growth in Mice via Depletion of Short-lived GPC1 and Downregulation of Wnt Signaling. Mol Cancer Ther 2022;21(6): 960-973. [CrossRef]
- Walker, K.Z.; Russell, P.J.; Kingsley, E.A.; Philips, J.; Raghavan, D. Detection of malignant cells in voided urine from patients with bladder cancer, a novel monoclonal assay. J Urol 1989;142(6): 1578-1583. [CrossRef]
- Gurney, H.; Sabanathan, D.; Gillatt, D.; et al. MILGa-01: A first-in-human study assessing the safety and tolerability of chMIL-38 in metastatic prostate, bladder, and pancreatic cancers. Journal of Clinical Oncology 2017;35(6_suppl): e565-e565. [CrossRef]
- Sabanathan, D.; Campbell, D.H.; Velonas, V.M.; et al. Safety and tolerability of Miltuximab(®) - a first in human study in patients with advanced solid cancers. Asia Ocean J Nucl Med Biol 2021;9(2): 86-100. [CrossRef]
- Ghosh, S.; Fletcher, N.L.; Huda, P.; et al. Pharmacokinetics and Biodistribution of (89)Zr-Miltuximab and Its Antibody Fragments as Glypican-1 Targeting Immuno-PET Agents in Glioblastoma. Mol Pharm 2023;20(3): 1549-1563. [CrossRef]
- Yeh, M.C.; Tse, B.W.C.; Fletcher, N.L.; et al. Targeted beta therapy of prostate cancer with (177)Lu-labelled Miltuximab® antibody against glypican-1 (GPC-1). EJNMMI Res 2020;10(1): 46. [CrossRef]
- Polikarpov, D.M.; Campbell, D.H.; Lund, M.E.; et al. The feasibility of Miltuximab®-IRDye700DX-mediated photoimmunotherapy of solid tumors. Photodiagnosis Photodyn Ther 2020;32: 102064.
- Lund, M.E.; Howard, C.B.; Thurecht, K.J.; et al. A bispecific T cell engager targeting Glypican-1 redirects T cell cytolytic activity to kill prostate cancer cells. BMC Cancer 2020;20(1): 1214. [CrossRef]
- Uchida, S.; Serada, S.; Suzuki, Y.; et al. Glypican-1-targeted antibody-drug conjugate inhibits the growth of glypican-1-positive glioblastoma. Neoplasia 2024;50: 100982. [CrossRef]
- Yokota, K.; Serada, S.; Tsujii, S.; et al. Anti-Glypican-1 Antibody-drug Conjugate as Potential Therapy Against Tumor Cells and Tumor Vasculature for Glypican-1-Positive Cholangiocarcinoma. Mol Cancer Ther 2021;20(9): 1713-1722. [CrossRef]
- Nishigaki, T.; Takahashi, T.; Serada, S.; et al. Anti-glypican-1 antibody-drug conjugate is a potential therapy against pancreatic cancer. Br J Cancer 2020;122(9): 1333-1341.
- Matsuzaki, S.; Serada, S.; Hiramatsu, K.; et al. Anti-glypican-1 antibody-drug conjugate exhibits potent preclinical antitumor activity against glypican-1 positive uterine cervical cancer. Int J Cancer 2018;142(5): 1056-1066.
- Li, N.; Quan, A.; Li, D.; et al. The IgG4 hinge with CD28 transmembrane domain improves V(H)H-based CAR T cells targeting a membrane-distal epitope of GPC1 in pancreatic cancer. Nat Commun 2023;14(1): 1986.
- 39. Kato, D.; Yaguchi, T.; Iwata, T.; et al. GPC1 specific CAR-T cells eradicate established solid tumor without adverse effects and synergize with anti-PD-1 Ab. Elife 2020;9. [CrossRef]
- Paul, S.; Konig, M.F.; Pardoll, D.M.; et al. Cancer therapy with antibodies. Nat Rev Cancer 2024;24(6): 399-426.
- Kaneko, M.K.; Suzuki, H.; Kato, Y. Establishment of a Novel Cancer-Specific Anti-HER2 Monoclonal Antibody H(2)Mab-250/H(2)CasMab-2 for Breast Cancers. Monoclon Antib Immunodiagn Immunother 2024;43(2): 35-43.
- Arimori, T.; Mihara, E.; Suzuki, H.; et al. Locally misfolded HER2 expressed on cancer cells is a promising target for development of cancer-specific antibodies. Structure 2024;32(5): 536-549.e535. [CrossRef]
- Tanaka, T.; Suzuki, H.; Ohishi, T.; Kaneko, M.K.; Kato, Y. A Cancer-Specific Anti-Podoplanin Monoclonal Antibody, PMab-117-mG(2a) Exerts Antitumor Activities in Human Tumor Xenograft Models. Cells 2024;13(22).
- Suzuki, H.; Ohishi, T.; Kaneko, M.K.; Kato, Y. A Humanized and Defucosylated Antibody against Podoplanin (humLpMab-23-f) Exerts Antitumor Activities in Human Lung Cancer and Glioblastoma Xenograft Models. Cancers (Basel) 2023;15(20). [CrossRef]
- Kato, Y.; Kaneko, M.K. A cancer-specific monoclonal antibody recognizes the aberrantly glycosylated podoplanin. Sci Rep 2014;4: 5924. [CrossRef]
- Suzuki, H.; Ohishi, T.; Tanaka, T.; Kaneko, M.K.; Kato, Y. A Cancer-Specific Monoclonal Antibody against Podocalyxin Exerted Antitumor Activities in Pancreatic Cancer Xenografts. Int J Mol Sci 2023;25(1). [CrossRef]
- Kaneko, M.K.; Suzuki, H.; Ohishi, T.; et al. Antitumor Activities of a Humanized Cancer-Specific Anti-HER2 Monoclonal Antibody, humH(2)Mab-250 in Human Breast Cancer Xenografts. Int J Mol Sci 2025;26(3). [CrossRef]
- Suzuki, H.; Ohishi, T.; Tanaka, T.; Kaneko, M.K.; Kato, Y. Anti-HER2 Cancer-Specific mAb, H(2)Mab-250-hG(1), Possesses Higher Complement-Dependent Cytotoxicity than Trastuzumab. Int J Mol Sci 2024;25(15). [CrossRef]
- Kaneko, M.K.; Suzuki, H.; Ohishi, T.; et al. A Cancer-Specific Monoclonal Antibody against HER2 Exerts Antitumor Activities in Human Breast Cancer Xenograft Models. Int J Mol Sci 2024;25(3). [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




