Submitted:
04 June 2025
Posted:
04 June 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
2.1. Baseline Characteristics of the Patients
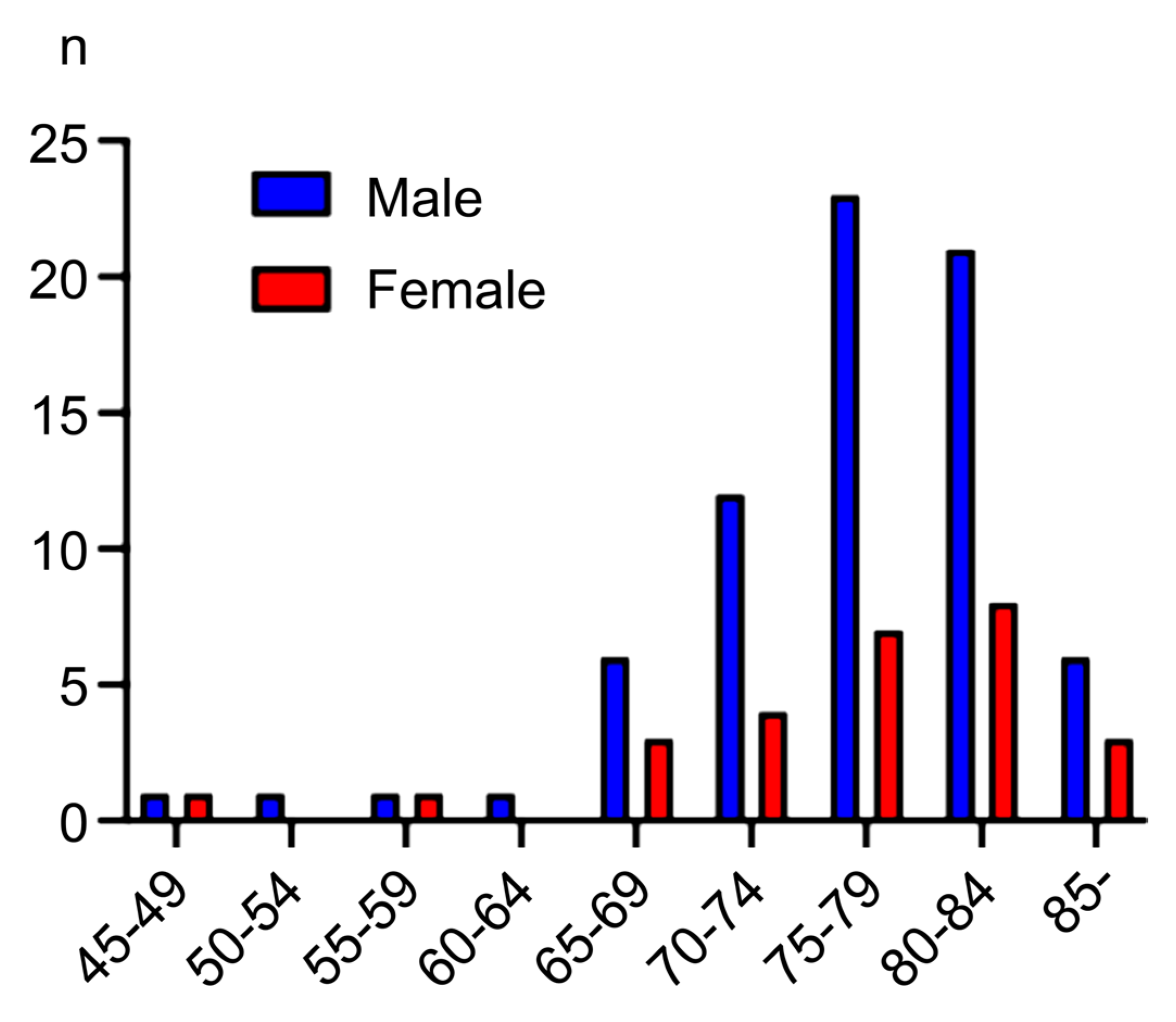
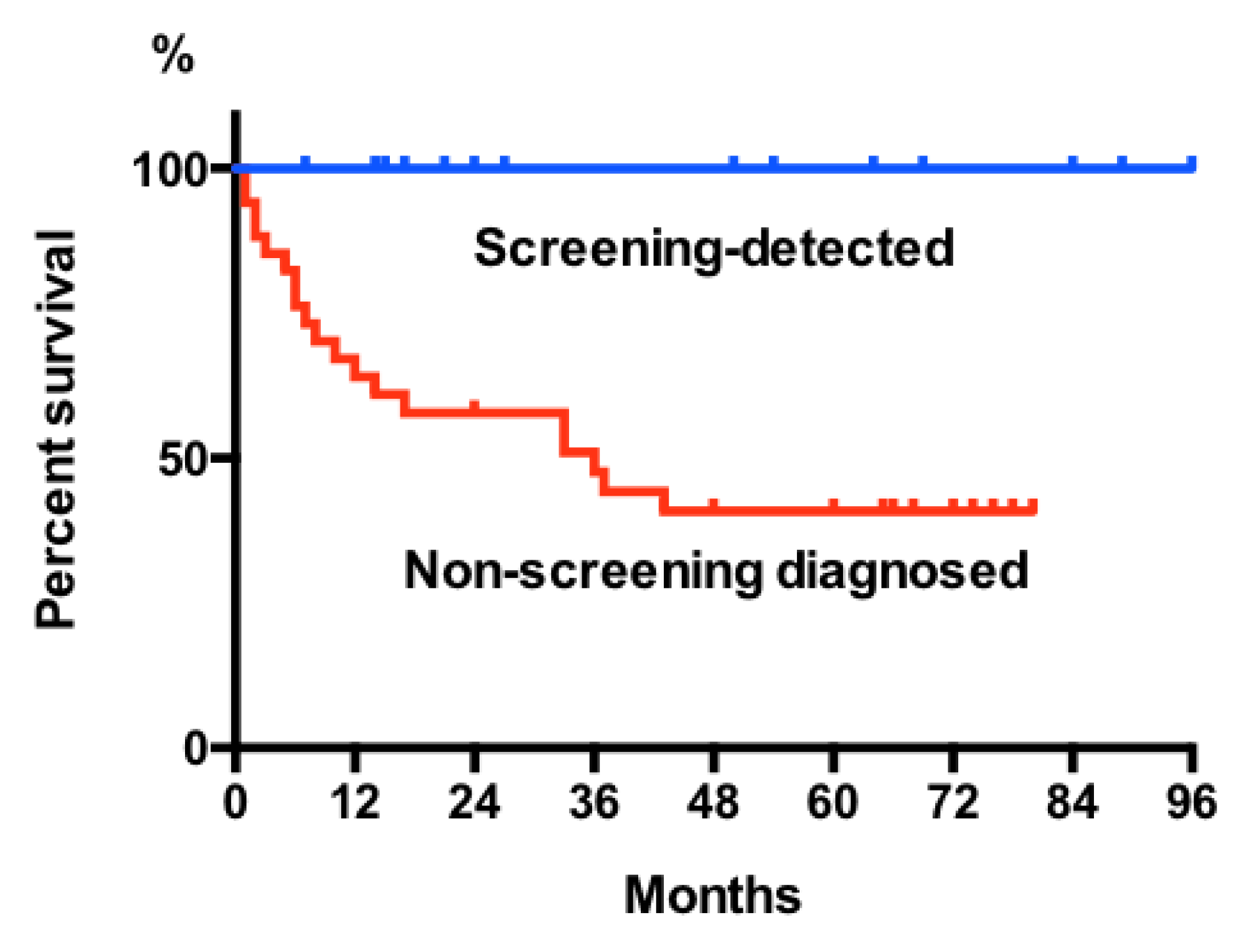
2.2. Stages, Treatment, and Outcome of Population-Based Screening Detected GCs
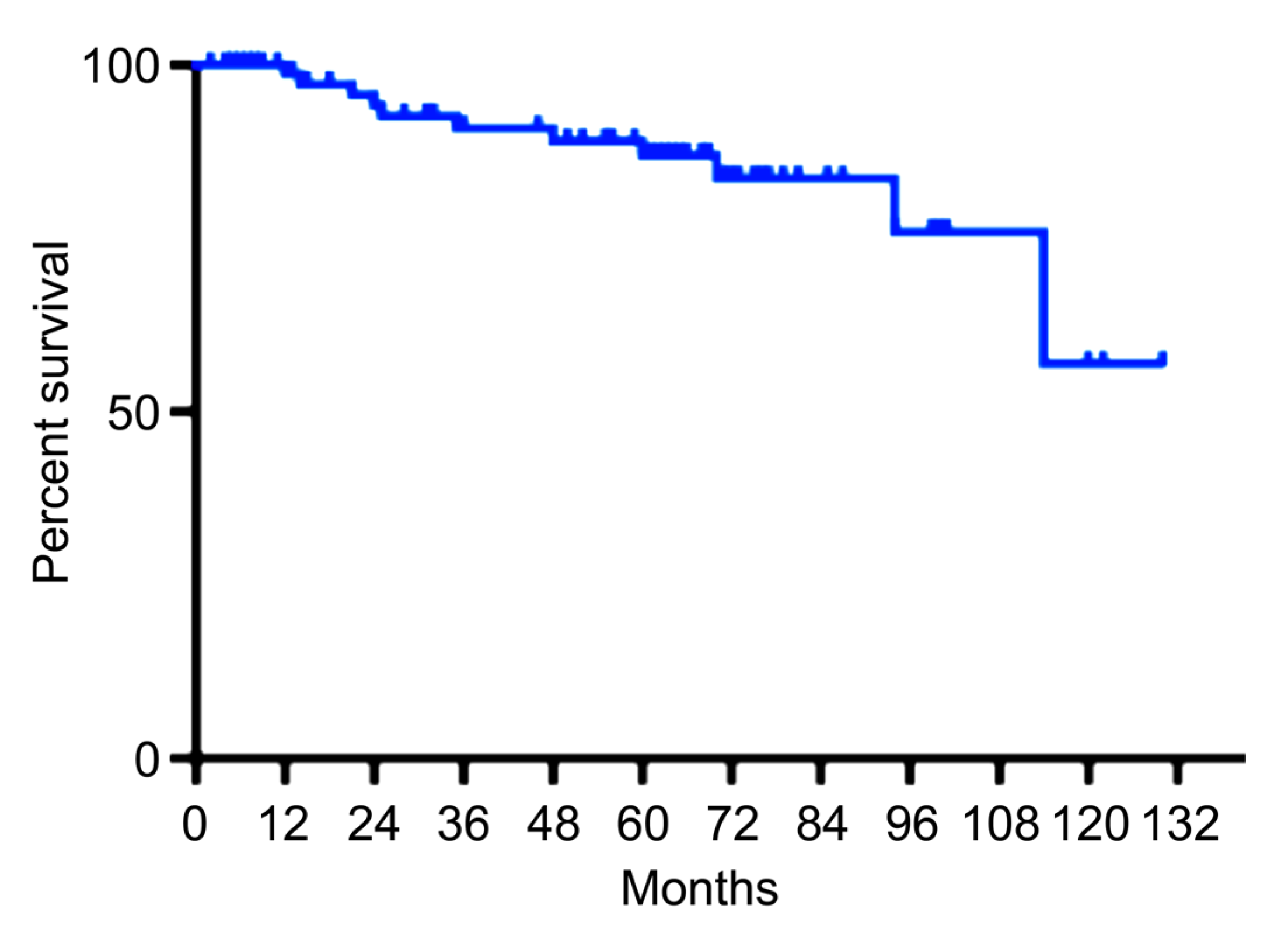

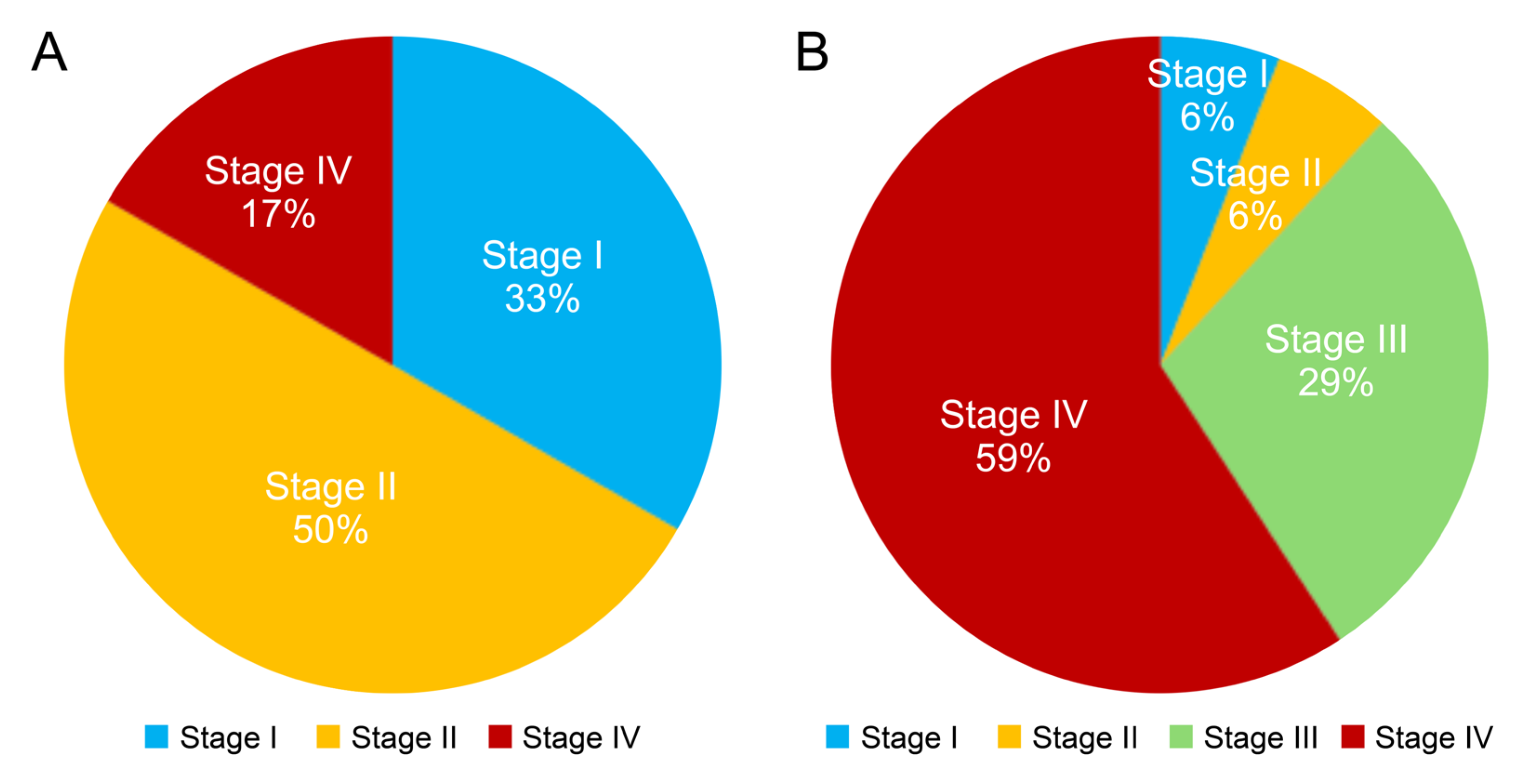
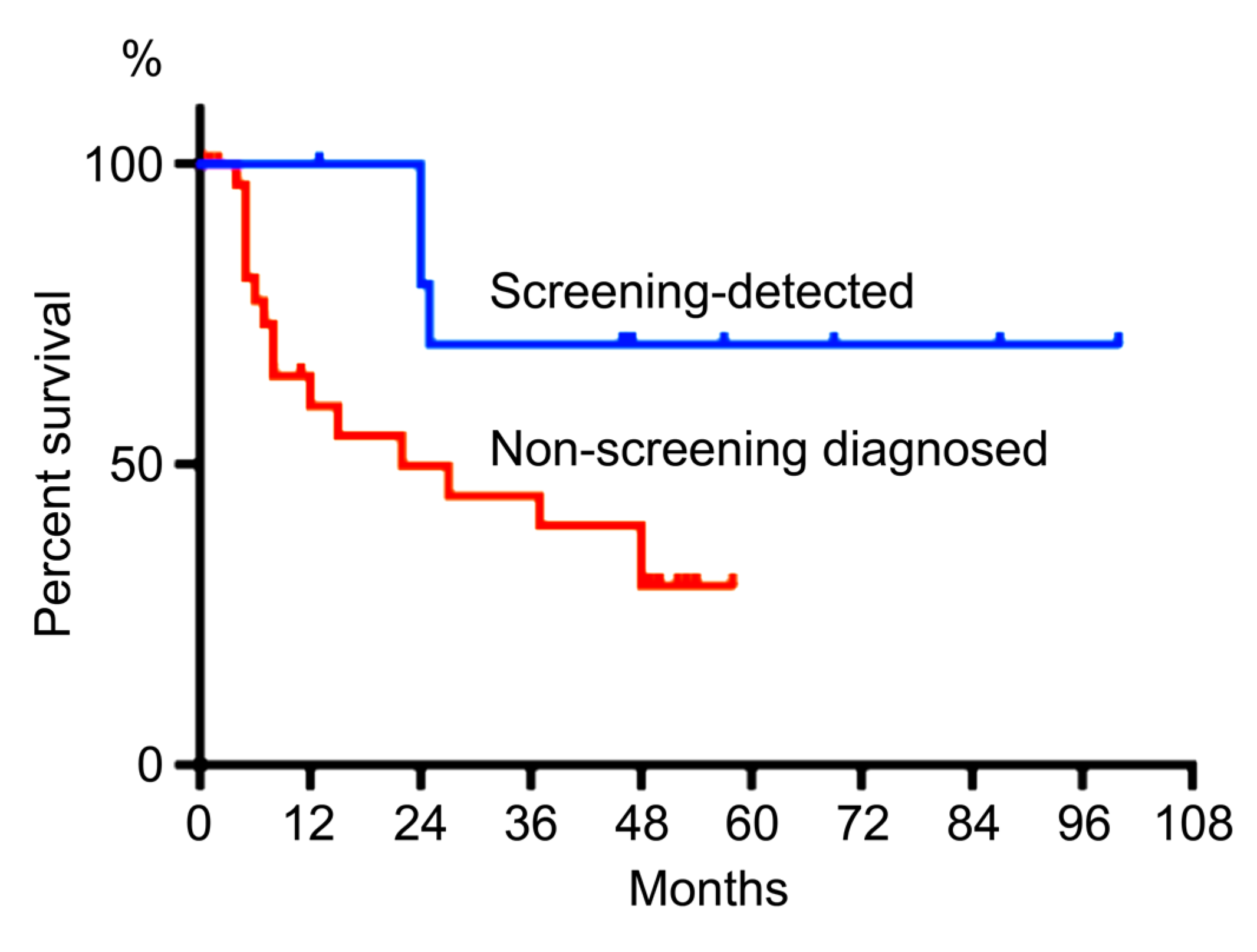
3. Discussion
4. Patients and Methods
4.1. Population-Based GC Screening of Kawasaki City
4.2. Patients
4.3. Helicobacter pylori (Hp) Infection Status
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgements
Conflicts of Interest
Abbreviations
| GC | gastric cancer |
| EC | esophageal cancer |
| Hp | Helicobacter pylori |
| SCC | squamous cell carcinomas |
| ESD | endoscopic submucosal dissection |
| eCura | endoscopic curability |
| DG | distal gastrectomy |
| TG | total gastrectomy |
| CRT | chemoradiotherapy |
| UGI | upper gastrointestinal |
Appendix A
Appendix B
| Screening detected advanced GC | Non-screening diagnosed advanced GC | ||
| (n=11) | (n=34) | ||
| Age mean(range) | 71.0 (47-86) | 71.2 (42-86) | |
| Sex (male/female) | 8 / 3 | 24 / 10 | |
| Stage | I | 3 | 2 |
| II | 6 | 2 | |
| III | 10 | ||
| IV | 2 | 20 | |
| Treatment | surgery | 11 | 12 |
| chemotherapy | 16 | ||
| BSC | 6 |
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021, 71, 209–249. [CrossRef]
- National Cancer Center. Cancer statistics in Japan 2023. Available online: https://ganjoho.jp/public/qa_links/report/statistics/2023_en.html (accessed on 26 May 2025).
- Sun, D.; Mülder, D.T.; Li, Y.; Nieboer, D.; Park, J.Y.; Suh, M.; Hamashima, C.; Han, W.; O’Mahony, J.F.; Lansdorp-Vogelaar, I. The effect of nationwide organized cancer screening programs on gastric cancer mortality: A synthetic control study. Gastroenterology 2024, 166, 503–514. [CrossRef]
- Fukao, A.; Tsubono, Y.; Tsuji, I.; Hisamichi, S.; Sugahara, N.; Takano, A. The evaluation of screening for gastric cancer in Miyagi Prefecture, Japan: a population-based case-control study. Int J Cancer 1995, 60, 45–48. [CrossRef]
- Oshima, A. A critical review of cancer screening programs in Japan. Int J Technol Assess Health Care 1994, 10, 346–358. [CrossRef]
- Hamashima, C. Cancer screening guidelines and policy making: 15 years of experience in cancer screening guideline development in Japan. Jpn J Clin Oncol 2018, 48, 278–286. [CrossRef]
- Kawasaki City Health and Welfare Bureau. Annual Reports of Results of Kawasaki City Gastric and Colon Cancer Screening. (in Japanese).
- Japanese Society for Gastrointestinal Cancer Screening. Nationwide survey of gastric cancer screening status. Available online: https://www.jsgcs.or.jp/files/uploads/2020zenkoku_naisikyo.pdf (in Japanese, last accessed Oct. 19, 2024).
- Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer 2011, 14, 101.
- 10. Japanese Gastric Cancer Association. Japanese Gastric Cancer Treatment Guidelines 2021: 6th edition. Gastric Cancer 2023, 26, 1.
- Ministry of Heal, Labor and Welfare. Japanese life expectancy at major ages groups. Available online: https://www.mhlw.go.jp/toukei/saikin/hw/life/life23/dl/life23-02.pdf (in Japanese, last accessed May 26. 2025).
- Huang, R.J.; Epplein, M. An Approach to the primary and secondary prevention of gastric cancer in the United States. Clin Gast Hepatol 2021, 20, 2218–2228.e2.
- Chang, E.T.; Clarke, C.A.; Colditz, G.A.; Kurian, A.W.; Hubbell, E. Avoiding lead-time bias by estimating stage-specific proportions of cancer and non-cancer deaths. Cancer Causes Control 2024, 35, 849–864. [CrossRef]
- Hamashima, C.; Ogoshi, K.; Okamoto, M.; Shabana, M.; Kishimoto, T.; Fukao, A. A community-based, case-control study evaluating mortality reduction from gastric cancer by endoscopic screening in Japan. PLOS ONE 2013, 8, e79088. [CrossRef]
- Hamashima, C.; Shabana, M.; Okada, K.; Okamoto, M.; Osaki, Y. Mortality reduction from gastric cancer by endoscopic and radiographic screening. Cancer Sci 2015, 106, 1744–1749. [CrossRef]
- Kim, Y.; Jun, J.K.; Choi, K.S.; Lee, H.Y.; Park, E.C. Overview of the National Cancer screening programme and the cancer screening status in Korea. Asian Pac J Cancer Prev 2011, 12, 725–730.
- Jun, J.K.; Choi, K.S.; Lee, H.Y.; Suh, M.; Park, B.; Song, S.H.; Jung, K.W.; Lee, C.W.; Choi, I.J.; Park, E.C.; et al. Effectiveness of the Korean National Cancer Screening Program in reducing gastric cancer mortality. Gastroenterology 2017, 152, 1319–1328.e7. [CrossRef]
- Zhang, X.; Li, M.; Chen, S.; Hu, J.; Guo, Q.; Liu, R.; Zheng, H.; Jin, Z.; Yuan, Y.; Xi, Y.; et al. Endoscopic screening in Asian countries Is associated with reduced gastric cancer mortality: a meta-analysis and systematic review. Gastroenterology 2018, 155, 347–354.e9. [CrossRef]
- Uemura, N.; Okamoto, S.; Yamamoto, S.; Matsumura, N.; Yamaguchi, S.; Yamakido, M.; Taniyama, K.; Sasaki, N.; Schlemper, R.J. Helicobacter pylori infection and the development of gastric cancer. N Engl J Med 2001, 345, 784–789. [CrossRef]
- Take, S.; Mizuno, M.; Ishiki, K.; Kusumoto, C.; Imada, T.; Hamada, F.; Yoshida, T.; Yokota, K.; Mitsuhashi, T.; Okada, H. Risk of gastric cancer in the second decade of follow-up after Helicobacter pylori eradication. J Gastroenterol 2020, 55, 281–288. [CrossRef]
- Graham, D.Y. History of Helicobacter pylori, duodenal ulcer, gastric ulcer and gastric cancer. World J Gastroenterol 2014, 20, 5191–5204. [CrossRef]
- Nagy, P.; Johansson, S.; Molloy-Bland, M. Systematic review of time trends in the prevalence of Helicobacter pylori infection in China and the USA. Gut Pathog 2016, 8, 8. [CrossRef]
- Hirayama, Y.; Kawai, T.; Otaki, J.; Kawakami, K.; Harada, Y. Prevalence of Helicobacter pylori infection with healthy subjects in Japan. J Gastroenterol Hepatol 2014, 29(Suppl 4), 16–19. [CrossRef]
- Kamada, T.; Haruma, K.; Ito, M.; Inoue, K.; Manabe, N.; Matsumoto, H.; Kusunoki, H.; Hata, J.; Yoshihara, M.; Sumii, K.; et al. Time trends in Helicobacter pylori infection and atrophic gastritis over 40 years in Japan. Helicobacter 2015, 20, 192–198. [CrossRef]
- Yanaoka, K.; Oka, M.; Mukoubayashi, C.; Yoshimura, N.; Enomoto, S.; Iguchi, M.; Magari, H.; Utsunomiya, H.; Tamai, H.; Arii, K.; et al. Cancer high-risk subjects identified by serum pepsinogen tests: outcomes after 10-year follow-up in asymptomatic middle-aged males. Cancer Epidemiol Biomarkers Prev 2008, 17, 838–845. [CrossRef]
- Terasawa, T.; Nishida, H.; Kato, K.; Miyashiro, I.; Yoshikawa, T.; Takaku, R.; Hamashima, C. Prediction of gastric cancer development by serum pepsinogen test and Helicobacter pylori seropositivity in Eastern Asians: a systematic review and meta-analysis. PLOS ONE 2014, 9, e109783. [CrossRef]
- Hamashima, C.; Systematic Review Group and Guideline Development Group for Gastric Cancer Screening Guidelines. Update version of the Japanese Guidelines for Gastric Cancer Screening. Jpn J Clin Oncol 2018, 48, 673–683. [CrossRef]
- Domper Arnal, M.J.; Ferrández Arenas, Á.; Lanas Arbeloa, Á. Esophageal cancer: risk factors, screening and endoscopic treatment in Western and Eastern countries. World J Gastroenterol 2015, 21, 7933–7943. [CrossRef]
- Kim, J.H.; Han, K.D.; Lee, J.K.; Kim, H.S.; Cha, J.M.; Park, S.; Kim, J.S.; Kim, W.H.; Big Data Research Group (BDRG) of the Korean Society of Gastroenterology (KSG). Association between the National Cancer Screening Programme (NSCP) for gastric cancer and oesophageal cancer mortality. Br J Cancer 2020, 123, 480–486. [CrossRef]
- Goto, E.; Ishikawa, H.; Okuhara, T.; Kiuchi, T. Relationship of health literacy with utilization of health-care services in a general Japanese population. Prev Med Rep 2019, 14, 100811. [CrossRef]
- Japan Esophageal Society. Esophageal cancer practice guidelines 2022. Esophagus 2023, 20, 343-389. [CrossRef]
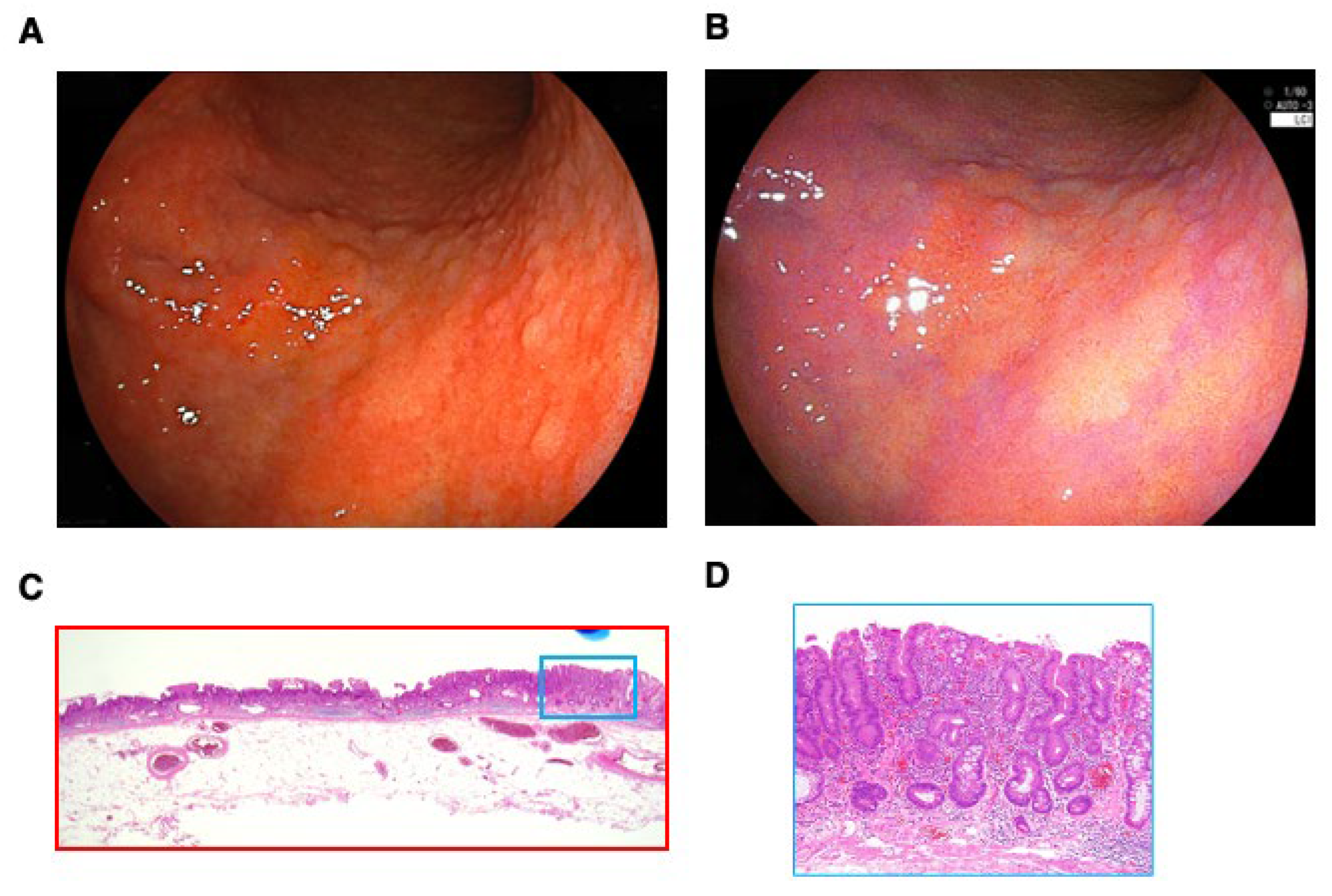
| Stage grouping | n | Treatment | n | |
| Gastric cancer | Stage IA | 69 | ESD | 63 |
| DG | 6 | |||
| StageIB | 25 | DG/TG | 13 | |
| ESD | 6 | |||
| ESD→DG | 6 | |||
| StageⅡA | 6 | DG/TG | 6 | |
| StageⅣB | 2 | DG/TG | 2 | |
| Esophageal cancer | Stage 0 (T1a) | 11 | ESD | 9 |
| thoracic esophagectomy | 1 | |||
| CRT | 1 | |||
| Stage I (T1b) | 4 | ESD | 1 | |
| CRT | 3 | |||
| Stage II | 2 | thoracic esophagectomy | 2 | |
| Stage III | 1 | CRT | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





