1. Introduction
The application of REs in fields such as electronic information, new energy, and environmental protection is constantly expanding, which has promoted the continuous progress of the theory and technology of rare earth separation, achieving the effects of cost reduction, quality improvement, and efficiency increase [
1]. Therefore, various separation methods have been proposed to achieve high efficiency of rare earths purification separation from impurity ions, including solvent extraction, ion exchange, and precipitation methods [
2,
3,
4,
5]. Among them, the precipitation separation of REs plays a very important role in the production of high-purity REs, because it is the main technology for separating REs from impurity ions in solution, such as Fe
3+, Al
3+, Mg
2+, Ca
2+, and the coexisting anions [
6,
7]. At the early stage of rare earth industrializing process, oxalic acid precipitation was once the most widely used method because of its good separation selectivity, large precipitation crystal particles, and simple solid-liquid separation. However, oxalic acid is toxic with high price, and the treatment of acidic wastewater is difficult [
8]. Replacing oxalic acid precipitation with ammnium bicarbonate (ABC) precipitation can reduce production cost, facilitate the subsequent wastewater treatment and material recycling, realize green and clean production [
9]. Therefore, extensive research has been conducted on the mechanism and process of rare earth precipitation separation using ABC, and a series of precipitation separation methods have been proposed and applied for different rare earth solutions and target product requirements [
10]. Among them, the research work of Nanchang University is the most systematic, and the results of promotion and application are also very effective [
11].
In recent years, the supply of rare earth mine products has been restricted, and the product quality is more poor. Meanwhile, to reduce ammonia nitrogen pollution, raw materials of Ca, Mg, and Na are used to replace ammonium, resulting in relatively high impurity ions in the mine products and separation products in separation enterprises. So, the current precipitation methods are difficult to meet the product quality requirements [
12,
13]. In fact, these impurities can be separated by extraction methods, but adding an additional separation step will significantly increase the separation cost, especially for high-abundance lanthanum-cerium products, which cannot be afforded [
14]. Therefore, how to improve the separation effect of REs from impurity ions based on the simple precipitation separation process and equipment by optimizing the operating conditions and process flow and adding a small amount of auxiliary reagents is the most realistic and effective research idea [
15].
The precipitation separation of REs from Ca and Mg by ABC can be achieved via controlling the precipitation conditions [
1]. This study conducts a comparative study on the effect of Al and Fe impurities on the separation of lanthanum-cerium from Ca and Mg using ABC as precipitant. It is found that the co-precipitation property of Ca and Mg is related to the coexisting high-valent ions Fe
3+ and Al
3+. To obtain qualified lanthanum-cerium carbonate products, it is necessary to completely remove the high-valent (Fe and Al) impurity ions in advance. Therefore, this paper studies the direction and mutual influence laws of low-valent (Ca and Mg) and high-valent (Fe and Al) impurity ions during the precipitation of RECl
3 with ABC. Based on these laws, the precipitation process and conditions for separating them from Al, Fe, Ca, and Mg and preparing qualified lanthanum-cerium carbonate products are proposed for achieving the expected goal and providing theoretical support for the precipitation separation of rare earth from impurities.
2. Materials and Methods
2.1. Materials and Reagents
The actual solution of RECl3 used was taken from Jiangxi Golden Century Rare Earth New Materials Co., Ltd, and its main composition is Mg (1.30 g/L), Al (2.31 g/L), Ca (2. 02 g/L), Fe (0.23 g/L), other ions (1.96 g/L), and RE (72.62 g/L), where the rare earth content accounted for 90.28%. At the same time, simulated RECl3 with or without Fe and Al was prepared. The reagents, such as ABC and TAC, were analytical grade reagents purchased from Xilong Science Co. The concentration of impurities and REs were determined by an inductively coupled plasma mass spectrometer (ICP-MS, Thermo Fisher) and EDTA titration respectively.
2.2. Experimental Process
Simulated solution of RECl3 were taken into a beaker, TAC (or other comparative reagents) was added and stirred for mixing. Then ABC was slowly added under stirring and monitoring the pH by a PHS-3C pH meter (Anhui Lei Magnet Instruments Co, Ltd.). At different controlling pH values, separating the liquid and solid by filtration, the filtrates were used to determine the contents of REs, Al, Fe, Ca and Mg ions. After filtration, the filtrates were used to prepare high purity of RE carbonates by a precipitation process using ABC as precipitant. The final RE2O3 were obtained by calcinating the RE carbonates in a muffle furnace at 900-1000 ℃ (Nanjing Boyuntong Instrument Co., Ltd.).
3. Results and Discussion
3.1. Method and Conditions for Separating Lanthanum and Cerium from Ca and Mg by Ammonium Bicarbonate Precipitation
Simulated solution of RECl
3 with concentrations of Mg (1.30 g/L), Ca (2.0 g/L) and REs (72.62 g/L) were used to investigate the effect of pH and aging time on the precipitation percentage and the RE
2O
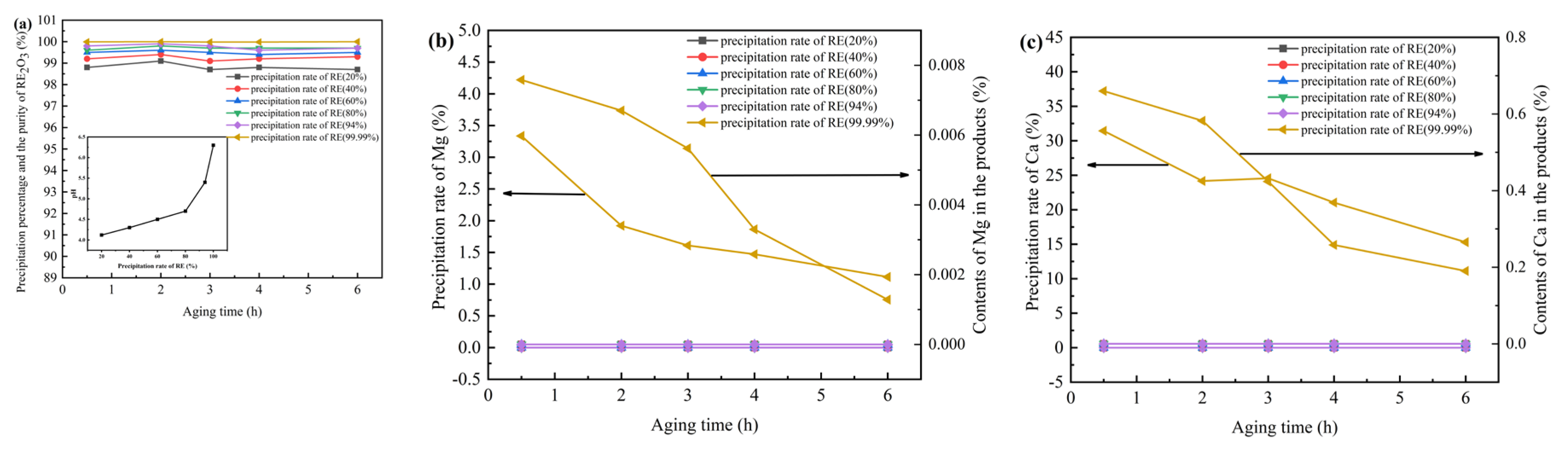
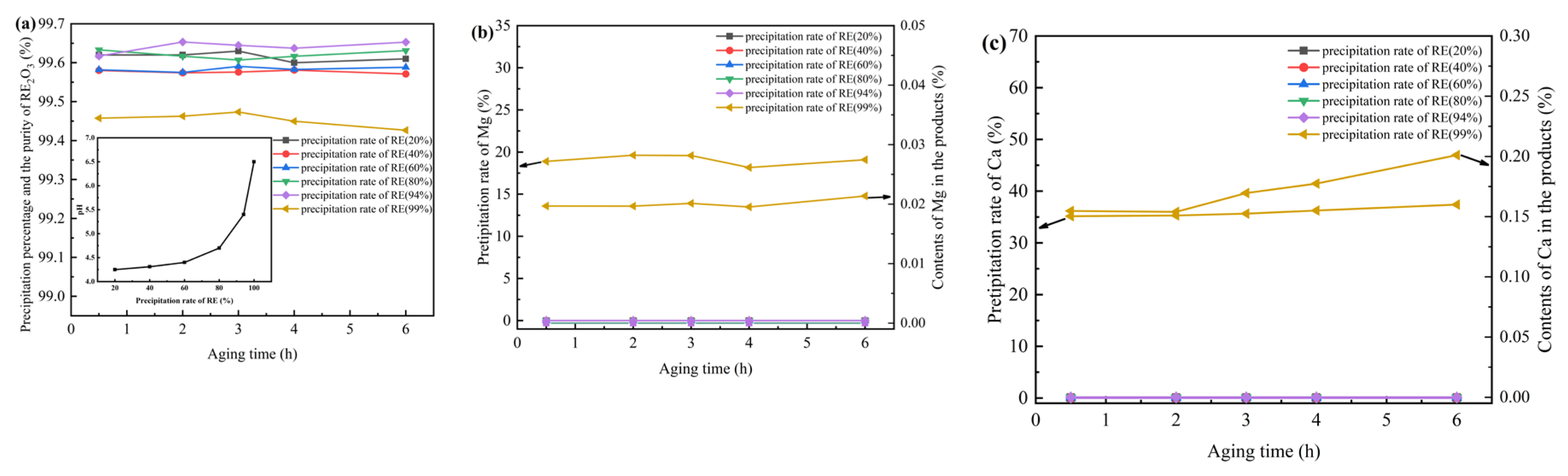
3 purity of obtained products by fractional precipitation with ABC as well as the contents of Ca, and Mg. The precipitation percentages of REs, Ca, and Mg under different addition conditions of ammonium bicarbonate solution, pH and aging time were determined and compared in
Figure 1. It was found that as the dosage of ammonium bicarbonate increased, the pH value of the solution increased. At the beginning, the increase rate was slow. When the pH is higher than 5, the increase rate increased sharply.
Figure 1 (b, c) respectively show the precipitation percentages of Ca and Mg in the solution and the content of Ca and Mg in the products under different aging times when the dosage of ammonium bicarbonate is controlled for different precipitation percentages of REs. It was found that when the rare earth precipitation percentage was higher than 94%, Ca can be precipitated and entered the products with a small amount of Mg. Meanwhile, it decreased as the aging time increasing. Therefore, as long as the rare earth precipitation percentage is controlled to be less than 94% or the pH is less than 6, neither Ca nor Mg will enter the precipitation, and the precipitation separation from REs can be achieved, with the product purity approaching 100%. When the rare earth precipitation percentage is higher than 94%, Ca and Mg will enter the rare earth precipitation and affect the purity of the products. However, by extending the aging time, the co-precipitation of Ca and Mg can be reduced and the purity of the products can be improved.
3.2. The Effect of Al and Fe on the Precipitation Separation of Lanthanum and Cerium from Ca and Mg by abc
The pH of the initial feed solution was 3, and the concentrations of Ca, Mg, Al, Fe and REs were 1.82 g/L, 1.17 g/L, 2.04 g/L, 0.16 g/L and 65.79 g/L respectively. Gradually add 2% ammonium bicarbonate solution to this solution, measure the content of each ion in the supernatant at different pH values, and calculate the precipitation percentage of each ion.
As the results we reported previously that with the increase of pH from 3 to 4.4, the precipitation percentages of Al and Fe increased significantly. And when pH exceeds 3.8, Ca and Mg in the solution begin to precipitate in advance, and the precipitation percentage increased with the increase of the precipitation percentages of Fe and Al. Furthermore, Fe has been completely precipitated at pH=4.12, but the precipitation percentage of Al is only about 60%. Further increased the pH to 4.4, the removal effect of Al was not improvement obviously. At pH=4.6, the precipitation percentage of Al was 69%, and that of REs was 6.67%.
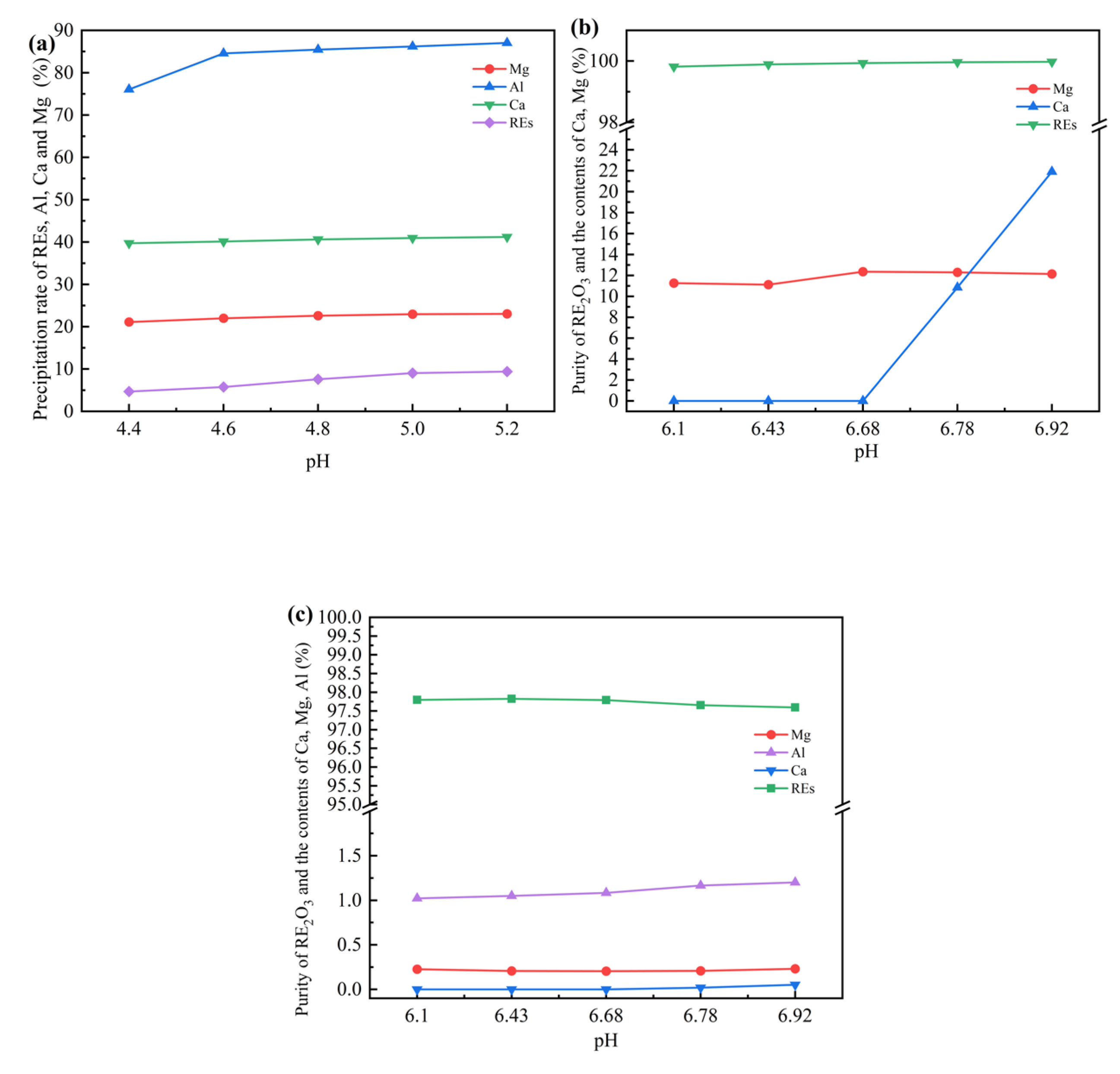
Figure 2(a) shows the variation of the precipitation percentages of REs, Al and Ca Mg with pH when further precipitated with ammonium bicarbonate after Fe was completely precipitated (pH=4.12) and filtered out. At pH=4.6, the Al precipitation percentage was 85% and the rare earth loss percentage was 5.71%. Therefore, by filtering out the Fe slag and then sedimentation, the removal percentage of Al can be increased, and the loss of REs is small. However, the removal percentage of Al is still not high, less than 90%. Compared with the rare earth solutions containing only Ca and Mg impurities, as the pH increases, Ca and Mg will enter the precipitate in advance via co-precipitation due to the presence of a small amount of Al, resulting in an increase in the impurity content in the final product.
Therefore, it is considered to filter the Al slag at pH=4.6, and then precipitate the filtrate with ammonium bicarbonate until pH=6.1. The ion concentrations in the supernatant were Mg=0.28 g/L, Ca=0.65 g/L and RE=0.067 g/L respectively. The pH was further increased to 6.43, 6.68, 6.78 and 6.92 respectively. After aging at room temperature for 2 hours and filtering by suction, the contents of each ion in the supernatant were determined and the precipitation percentages was calculated. The precipitate was washed several times, dried and calcined at 950 ℃ to obtain rare earth oxides. After dissolution, the contents of rare earth and non-rare earth impurities were determined. The results shown in
Figure 2(b) demonstrated that when the pH value is above 6, REs can almost be precipitated completely with a small amount of Al in the solution. Ca began to precipitate at pH=6.68, while the changes of other impurities were not obvious. Through the product analysis results in
Figure 2(c), it was found that with the increase of pH, the content of impurity Ca in the precipitate gradually increased along with the content of Al, and the purity of rare earth decreased. The purity of the obtained product was the highest when pH=6.43, reaching 97.83%. The contents of Al and Mg were 1.05% and 0.21% respectively, and the contents of Ca and Fe were lower than the detection limits. Because the solubility product constant K
sp of Ca carbonate is much smaller than that of Mg carbonate, Ca is more likely to precipitate than Mg, and impurities such as Mg mainly enter the product as co-precipitates. The results indicated that the presence of Fe will affect the precipitation of Al, and Al will affect the precipitation of Ca and Mg.
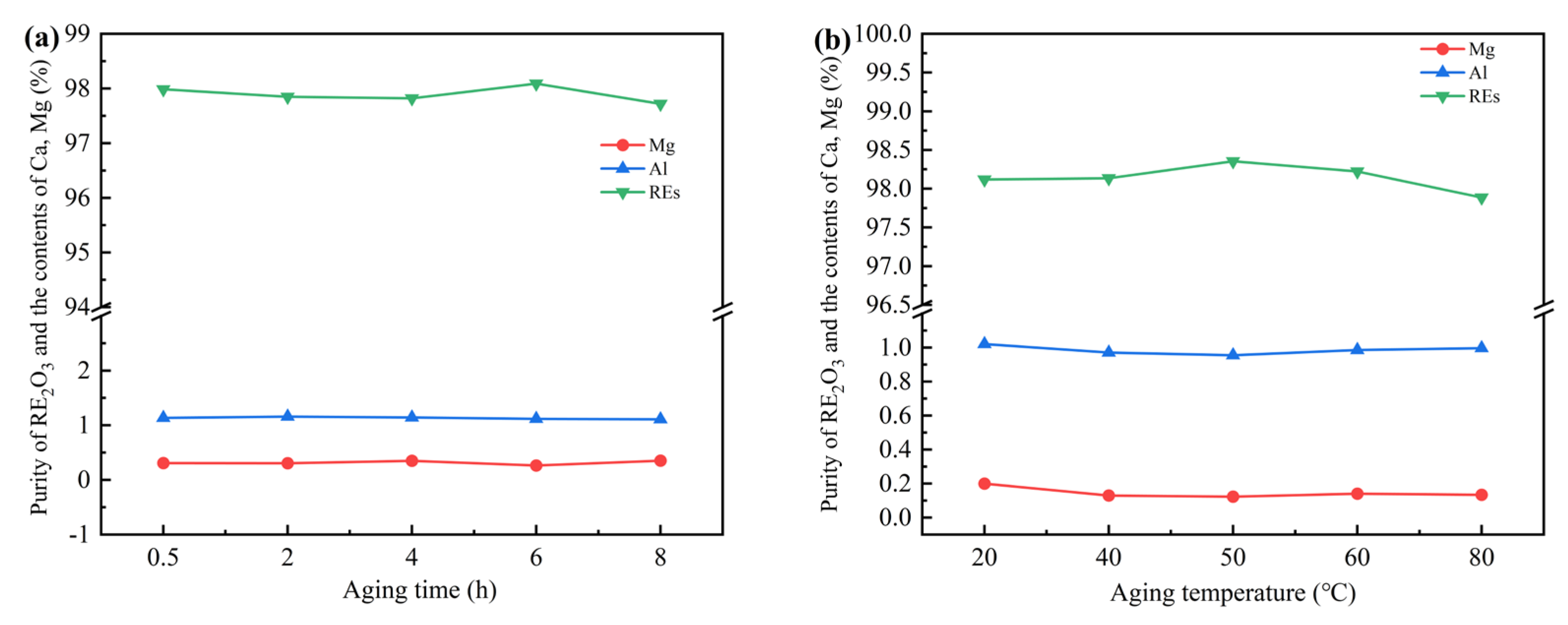
To increase the purity of RE
2O
3, the effect of aging time and aging temperature on it at pH=6.43 after filtered Fe and Al was studied. The results in
Figure 3. show that an appropriate aging time was conducive to the stability and growth of the precipitated particles, dissolving the impurity ions carried due to excessive precipitation rate, and reducing the residue of impurities in the precipitates. However, an excessively long aging time may cause the impurity ions adsorbed on the surface of the precipitated particles to re-enter the solution, thereby reducing the purity of RE
2O
3. Meanwhile, appropriately increasing the aging temperature can reduce impurity ions such as Mg entering the precipitates. But excessively high temperatures may cause the impurity ions adsorbed on the surface of the precipitated particles to re-enter the solution, thereby reducing the purity of RE
2O
3. So, the purity of RE
2O
3 was the highest at 6 hours aging time and 50 ℃ aging temperature with proportion of RE 98.51%, Al 0.9%, Mg 0.13%, without Ca and Fe.
3.3. The Effect of Precipitation Percentage of REs on the Purity of RE2O3 and the Contents of Ca, and Mg by Fractional Precipitation of RECl3 with ABC
RE
2O
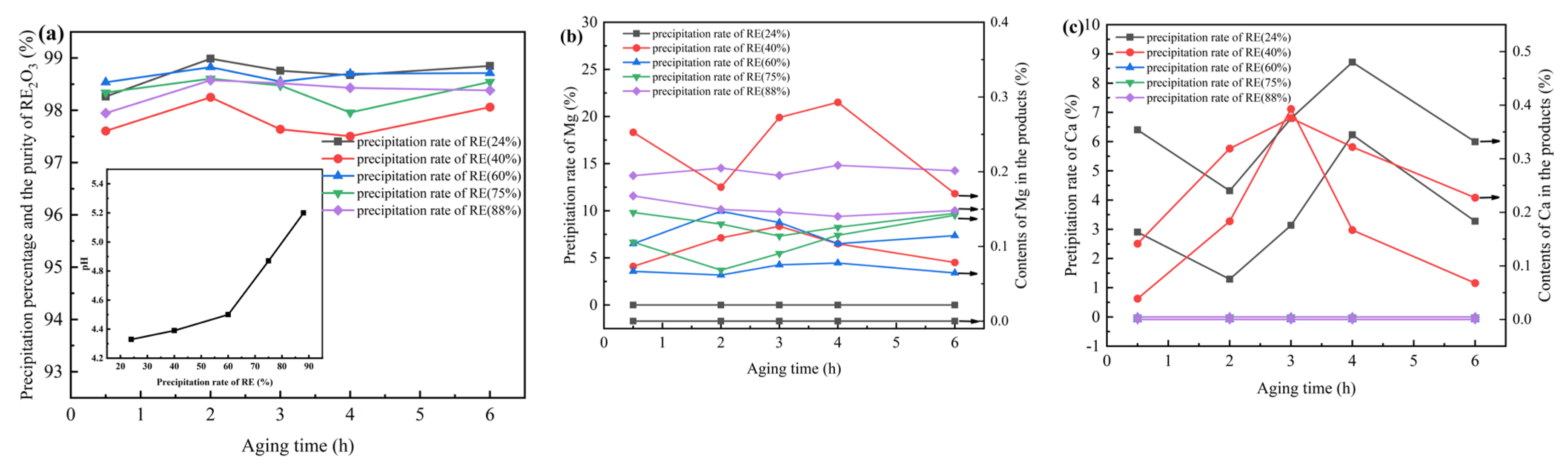
3 products obtained by above one-step precipitation method still contained a small amount of impurities such as Al and Mg. To study the influence of co-existing ions (Al, Fe, Ca and Mg), fractional precipitation was adopted after filtered out the precipitate of Fe and Al. The results shown in
Figure 4. indicated that the precipitation percentage of Mg increased with that of REs, about 10% when the precipitation percentage of REs between 40% and 75%, and 15% for rare earth precipitation percentage at around 88%. Compared with the fractional precipitation of RECl
3 containing only Ca and Mg, Mg precipitated prematurely due to a small amount of impurities of Al.
Interestingly, the co-precipitation of Ca was occurred when the precipitation percentage of REs is lower that 40% or higher that 90%. No Ca isolated When the precipitation percentage between 60% and 88%. However, when ammonium bicarbonate was excess, Ca entered the precipitate in the form of Ca carbonate prematurely due to the presence of a small amount of Al. Therefore, to separate Mg and Ca from REs, controlling the precipitation percentage of REs is important. In addition, the aging time also show a significant impact. When the REs precipitation percentage was set at 60%, the purity of RE2O3 was the highest with RE purity of 98.83%, Al 0.73%, Mg 0.06%, without Ca and Fe.
3.4. The Effect of Precipitation Percentage of REs on the Purity of RE2O3 and the Contents of Ca, and Mg by Fractional Precipitation of RECl3 with ABC and TAC
The fractional precipitation method could improve the separation effect of REs from Fe and Ca, but the separation of REs from Al and Mg was still not thorough. Therefore, when precipitating Fe and Al, an appropriate amount of TAC was added, then the filtrate was used to precipitate REs with ammonium bicarbonate. The results in
Figure 5 indicated that adding a small amount of TAC significantly improved the precipitation percentage of Al. When the precipitation percentage of REs was controlled below 99%, Ca and Mg can not be precipitated via co-precipitation. When the REs precipitation percentage was controlled at 94%, the purity of RE
2O
3 was the highest, reaching 99.66%, only containing 0.09% of Al, without Ca, Mg and Fe remaining. We reported a method in the previous paper by raising removal percentage of Al by the coordination of Fe, Al with a small amount of TAC. Its dosage ratio and pH control range were very important. TAC could not only be used as a purifying agent to coordinate with Al and Fe ions for lowering the precipitation pH of Al and Fe, but also lower the crystallization rate of REs precipitation and reduce the co-precipitation of Ca, Mg with REs. So the separation selectivity of REs from Al, Fe, Ca, Mg was improved and high-purity of RE
2O
3 products with low impurities content can be obtained.
3.5. Coupling of Continuous Precipitation Crystallization and Coordination Assisted Precipitation Separation of REs from Impurities by ABC and TAC
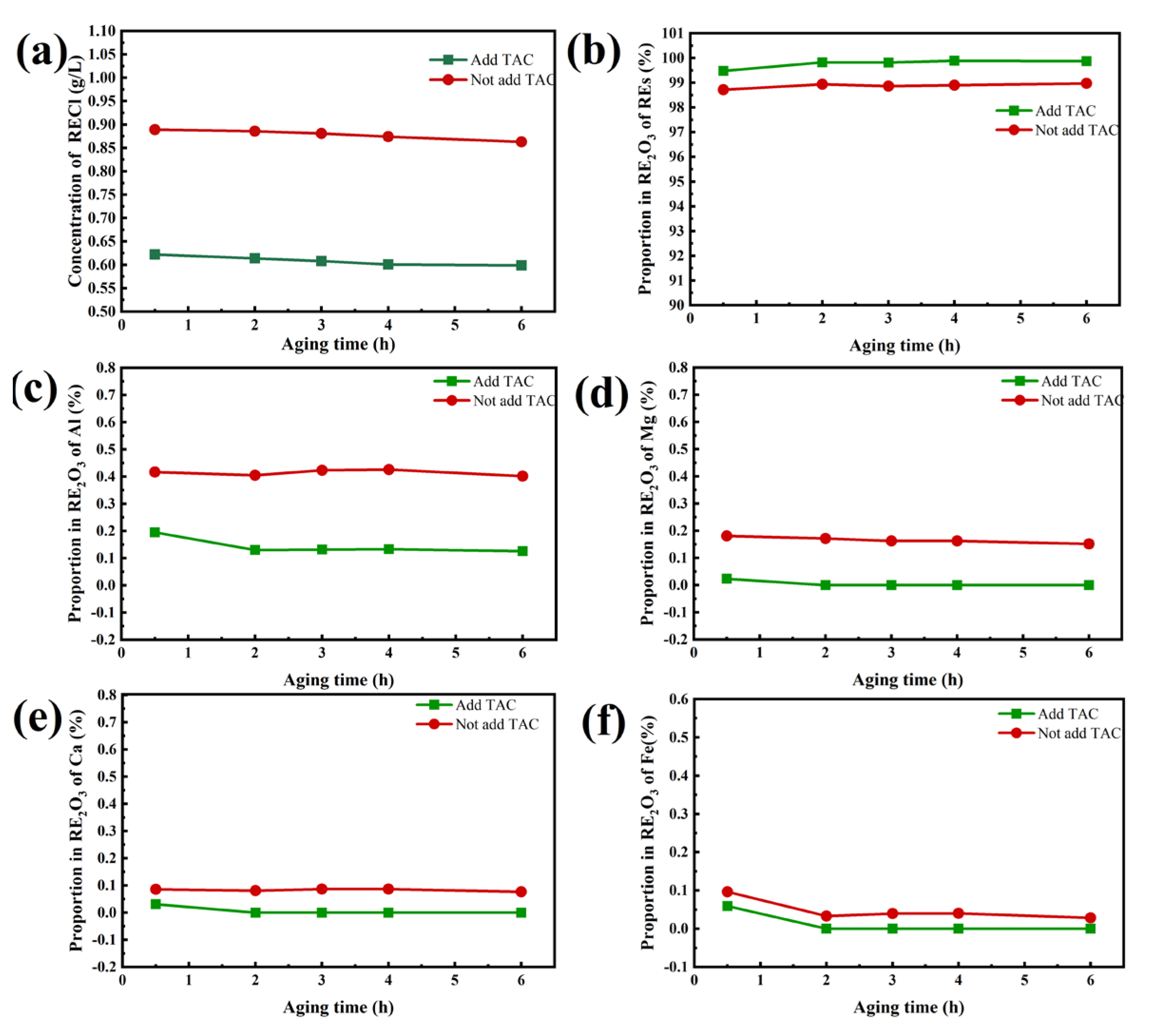
The above results prove that the control of TAC dosage and precipitation pH in the previous stage is very important in the precipitation separation of REs from Ca, Mg, Fe and Al by ammonium bicarbonate with the coordination of TAC. Meanwhile, the control of precipitating agent dosage, as well as the pH range is also important, which can be achieved by using continuous precipitation crystallization. In a continuous precipitation crystallization technology, the role of seed crystals is fully utilized to accelerate the precipitation and crystallization rate of rare earth carbonate. The method of injecting and discharging simultaneously can effectively reduce the entrainment of impurity ions in the product, and it is also convenient to control the feeding ratio and the pH value of the solution. For this purpose, we compared the continuous crystallization effects in the two cases of impurity removal with and without the addition of TAC. It can be seen from
Figure 6(a) that REs concentration in the supernatant decreased with the increase of aging time, and the rare earth concentration was the lowest at a 6 hours of aging time. During this process, REs concentration in the supernatant for continuous precipitation was 0.599 g/L with TAC added and 0.863 g/L without TAC added, and REs concentrations after impurity removal were 19.66 g/L and 19.43 g/L, with REs loss rate 5% and 7% respectively. REs loss percentage is lower in the continuous precipitation process with the addition of TAC. During the continuous precipitation process, the volume ratio of the precipitating agent (10%NH
4HCO
3) to the feed liquid is approximately 55:100.
Figure 6(b-f) showed the purity of RE
2O
3 obtained after calcination at 950 ℃ for 1 hour under different aging times with or without TAC during the impurity removal process when the rare earth precipitation percentage was controlled at 94%. The results showed that the purity of RE
2O
3 reached 98.93% with 0.41% of Al, 0.03% of Fe, 0.08% of Ca, 0.16% of Mg when the aging time was 6 hours without adding TAC. However, the purity of RE
2O
3 was found to be 99.87%, only with 0.03% of Al, without Fe, Ca and Mg, when the aging time was 6 hours adding TAC. It can be seen that adding TAC during the impurity removal process can effectively reduce the contents of Ca, Mg, Al, Fe in RE
2O
3, improve the purity of products, and the effect of continuous precipitation crystallization is even better.
4. Conclusions
Using ammonium bicarbonate to precipitate lanthanum and cerium in solution containing only Ca and Mg impurities, as long as the rare earth precipitation percentage is controlled to be less than 94% (pH less than 6), neither Ca nor Mg can enter the precipitation, Ca and Mg can be separated well from REs. The product purity is close to 100%.
For lanthanum and cerium solution containing Al, Fe, Ca and Mg, the presence of Fe and Al will cause Ca and Mg to precipitate prematurely, affecting the purity of the product. It is necessary to remove Fe and Al impurities first. After Fe is precipitated and filtered out by adjusting pH to 4.12, 90% of Al with 6% of REs can be precipitated by adjusting pH to 4.6. If 100% of REs were precipitated at pH=6.43 by one step precipitation method, the product purity is good, but there still exist a small amount of Al and Mg in the product, affecting the product purity. When the rare earth precipitation percentage is between 60% and 88%, no Ca can enter the precipitation, only a small amount of Mg is carried into the precipitation. At this time, the rare earth purity is 98.83%, the Mg content is 0.06%, and the Al content is 0.73%.
Introducing TAC for removing Fe and Al, REs can be separated from Ca and Mg by fractional precipitation method. When the rare earth precipitation percentage is 94%, the purity of RE2O3 is up to 99.66% with only 0.09% of Al. After removing Fe and Al from the RECl3 solution, an continuous precipitation crystallization method was employed to precipitate REs. The purity of RE2O3 was 99.87%, only with 0.03% of Al, without Ca, Mg and Fe.
Author Contributions
Data curation, LiHui Liu and Jinfei Shi; Formal analysis, Zhenghui Zhu, Fen Nie and Jinfei Shi; Investigation, Yanzhu Liu, Zhenghui Zhu, Fen Nie, LiHui Liu and Yongxiu Li; Methodology, Yanzhu Liu, Fen Nie, LiHui Liu and Yongxiu Li; Supervision, Yongxiu Li; Validation, Jinfei Shi; Writing – original draft, Yanzhu Liu and Zhenghui Zhu; Writing – review & editing, Yongxiu Li. All authors have read and agreed to the published version of the manuscript.
Funding
This work is supported financially by National Key Research Development Program of China (grant number 2022YFC2905201).
Acknowledgments
The authors sincerely thank DP Li and J Li for their support of this article.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| RECl3
|
rare earth chloride solution |
| RE2O3
|
rare earth oxide |
| ABC |
ammonium bicarbonate |
| TAC |
triammonium citrate |
| REs |
rare earths |
References
- Li, Y.X.; Zhu, W.C.; Zhang, L.Z.; Liu, Y.Z. Rare Earth Metallurgy and EnvFemental Protection, 1st ed.; Chemical Industrial Press: Beijing, China, 2024; Volume 1, ISBN 978-7-122-46001-1. [Google Scholar]
- He, Q.; Qiu, J.; Chen, J.F.; Zan, M.M.; Xiao, Y.F. Progress in green and efficient enrichment of rare earth from leaching liquor of ion adsorption type rare earth ores. Journal of Rare Earths 2022, 40, 353–364. [Google Scholar] [CrossRef]
- He, Y.F.; Gong, J.A.; Sun, J.E. Study on the Extraction Separation Methods of Rare Earth Elements. Huaxue Tongbao 2023, 86, 386–396. [Google Scholar]
- Hu, Y.W.; Wang, L.M.; Cao, Z.; Zhang, W.B. Research Progress on Rare Earth Ore Metallurgy and Separation Technology in China. Conservation and Utilization of Mineral Resources 2020, 40, 151–161. [Google Scholar]
- Preston, J.S.; Du Preez, A.C. The separation of europium from a middle rare earth concentrate by combined chemical reduction, precipitation and solvent-extraction methods. Journal of Chemical Technology and Biotechnology 1996, 65, 93–101. [Google Scholar] [CrossRef]
- Al-Hadeethi, Y.; Ahmed, M.; Al-Heniti, S.H.; Sayyed, M.I.; Rammah, Y.S. Rare earth Co-Doped tellurite glass ceramics: Potential use in optical and radiation shielding applications. Ceramics International 2020, 46, 19198–19208. [Google Scholar] [CrossRef]
- Li, L.; Li, G.l.; Tian, F.; Wang, Z.Q.; Yan, S.H.; Li, X.G. Preparation of high purity Gd for novel functional materials. Materials Protection. 2013, 46, 28–29. [Google Scholar]
- Ahmad, N.; Yang, X.B.; Honaker, R. Parametric study and speciation analysis of rare earth precipitation using oxalic acid in a chloride solution system. Minerals Engineering 2022, 176, 107352. [Google Scholar]
- Li, Y.X.; He, X.B.; Gu, Z.Y.; Hu, P.G. Precipitation Reaction of RECl3 with NH4HCO3 and Co-Precipitation Behavior of Accompanying Impurity lons. Chinese Rare Earth 1999, 21–24. [Google Scholar]
- Luo, W.Q. Discussion on New Processes for Rare Earth Separation and Purification in High-End Application Fields. Shanxi Metallurgy. 2023, 46, 87–89. [Google Scholar]
- Li, Y.X.; Li, M.; He, X.B.; Hu, P.G.; Gu, Z.Y. precipitation and crystallization process of rare earth carbonate. Transaction of Nonferrous Soc. China 1999, 169–174. [Google Scholar]
- Rahman, M.M.; Awual, M.R.; Asiri, A.M. Preparation and evaluation of composite hybrid nanomaterials for rare-earth elements separation and recovery. Separation and Purification Technology 2020, 253, 117515. [Google Scholar] [CrossRef]
- Li, X.J.; Yu, J.X.; Wang, R.; OuYang, Z.; Liu, Y.; Hu, Q.Z.; Chen, S.Y.; Zhou, F.; Chi, R. Distribution of Residual Leaching Agent and Main Co-existing lons in Weathered Crust Elution Deposited Rare Earth Tailings. Mining And Metallurgical Engineering. 2023, 43, 103–109. [Google Scholar]
- Li, T.; Cui, K.H.; Sui, N.; Huang, K. Interface salt effect based on competition adsorption ofco-existing cations in process of rare earth solvent extraction. The Chinese Journal Nonferrous Metals. 2023, 33, 4201–4213. [Google Scholar]
- Cai, L.X. Study on the influence of Citric Acid Dosage and pH Value on the Leaching Rate of Rare Earth and Aluminum during Ammonium Sulfate Leaching of lon-Type Rare Earth. World Nonferrous Metals. 2024, 193–195. [Google Scholar]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).