Submitted:
28 May 2025
Posted:
29 May 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Nailfold Capillaroscopy
2.1. History and Modern Implementations
2.2. Long COVID as an Example
2.3. Sepsis and Septic Shock
2.5. Future Directions
3. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Pretorius, E., Oberholzer, H. M., van der Spuy, W. J. and Meiring, J. H. (2010) The changed ultrastructure of fibrin networks during use of oral contraception and hormone replacement. J Thromb Thrombolysis. 30, 502-506. [CrossRef]
- Pretorius, E., Swanepoel, A. C., Oberholzer, H. M., van der Spuy, W. J., Duim, W. and Wessels, P. F. (2011) A descriptive investigation of the ultrastructure of fibrin networks in thrombo-embolic ischemic stroke. J Thromb Thrombolysis. 31, 507-513. [CrossRef]
- Pretorius, E., Vermeulen, N., Bester, J., Lipinski, B. and Kell, D. B. (2013) A novel method for assessing the role of iron and its functional chelation in fibrin fibril formation: the use of scanning electron microscopy. Toxicol Mech Methods. 23, 352-359. [CrossRef]
- Pretorius, E., Mbotwe, S., Bester, J., Robinson, C. J. and Kell, D. B. (2016) Acute induction of anomalous and amyloidogenic blood clotting by molecular amplification of highly substoichiometric levels of bacterial lipopolysaccharide. J R Soc Interface. 123, 20160539. [CrossRef]
- Kell, D. B. and Pretorius, E. (2017) Proteins behaving badly. Substoichiometric molecular control and amplification of the initiation and nature of amyloid fibril formation: lessons from and for blood clotting. Progr Biophys Mol Biol. 123, 16-41. [CrossRef]
- Pretorius, E., Page, M. J., Hendricks, L., Nkosi, N. B., Benson, S. R. and Kell, D. B. (2018) Both lipopolysaccharide and lipoteichoic acids potently induce anomalous fibrin amyloid formation: assessment with novel Amytracker™ stains. J R Soc Interface. 15, 20170941. [CrossRef]
- Biancalana, M., Makabe, K., Koide, A. and Koide, S. (2009) Molecular mechanism of thioflavin-T binding to the surface of beta-rich peptide self-assemblies. J Mol Biol. 385, 1052-1063. [CrossRef]
- Biancalana, M. and Koide, S. (2010) Molecular mechanism of Thioflavin-T binding to amyloid fibrils. Biochim Biophys Acta. 1804, 1405-1412. [CrossRef]
- Amdursky, N., Erez, Y. and Huppert, D. (2012) Molecular rotors: what lies behind the high sensitivity of the thioflavin-T fluorescent marker. Acc Chem Res. 45, 1548-1557. [CrossRef]
- Gade Malmos, K., Blancas-Mejia, L. M., Weber, B., Buchner, J., Ramirez-Alvarado, M., Naiki, H. and Otzen, D. (2017) ThT 101: a primer on the use of thioflavin T to investigate amyloid formation. Amyloid. 24, 1-16. [CrossRef]
- Kell, D. B. and Pretorius, E. (2018) No effects without causes. The Iron Dysregulation and Dormant Microbes hypothesis for chronic, inflammatory diseases. Biol Rev. 93, 1518-1557. [CrossRef]
- de Waal, G. M., Engelbrecht, L., Davis, T., de Villiers, W. J. S., Kell, D. B. and Pretorius, E. (2018) Correlative Light-Electron Microscopy detects lipopolysaccharide and its association with fibrin fibres in Parkinson's Disease, Alzheimer's Disease and Type 2 Diabetes Mellitus. Sci Rep. 8, 16798. [CrossRef]
- Pretorius, E., Bester, J. and Kell, D. B. (2016) A bacterial component to Alzheimer-type dementia seen via a systems biology approach that links iron dysregulation and inflammagen shedding to disease J Alzheimers Dis. 53, 1237-1256. [CrossRef]
- Pretorius, E., Bester, J., Page, M. J. and Kell, D. B. (2018) The potential of LPS-binding protein to reverse amyloid formation in plasma fibrin of individuals with Alzheimer-type dementia. Frontiers Aging Neurosci. 10, 257. [CrossRef]
- Adams, B., Nunes, J. M., Page, M. J., Roberts, T., Carr, J., Nell, T. A., Kell, D. B. and Pretorius, E. (2019) Parkinson’s disease: a systemic inflammatory disease accompanied by bacterial inflammagens. Front Ag Neurosci. 11, 210. [CrossRef]
- Pretorius, E., Page, M. J., Mbotwe, S. and Kell, D. B. (2018) Lipopolysaccharide-binding protein (LBP) can reverse the amyloid state of fibrin seen or induced in Parkinson’s disease. PlosOne. 13, e0192121. [CrossRef]
- Pretorius, E., Page, M. J., Engelbrecht, L., Ellis, G. C. and Kell, D. B. (2017) Substantial fibrin amyloidogenesis in type 2 diabetes assessed using amyloid-selective fluorescent stains. Cardiovasc Diabetol. 16, 141. [CrossRef]
- Pretorius, E., Venter, C., Laubscher, G. J., Lourens, P. J., Steenkamp, J. and Kell, D. B. (2020) Prevalence of readily detected amyloid blood clots in ‘unclotted’ Type 2 Diabetes Mellitus and COVID-19 plasma: A preliminary report. Cardiovasc Diabetol. 19, 193. [CrossRef]
- Pretorius, E., Akeredolu, O.-O., Soma, P. and Kell, D. B. (2017) Major involvement of bacterial components in rheumatoid arthritis and its accompanying oxidative stress, systemic inflammation and hypercoagulability. Exp Biol Med. 242, 355-373. [CrossRef]
- Kell, D. B. and Pretorius, E. (2015) The simultaneous occurrence of both hypercoagulability and hypofibrinolysis in blood and serum during systemic inflammation, and the roles of iron and fibrin(ogen). Integr Biol. 7, 24-52. [CrossRef]
- Kell, D. B., Laubscher, G. J. and Pretorius, E. (2022) A central role for amyloid fibrin microclots in long COVID/PASC: origins and therapeutic implications. Biochem J. 479, 537-559. [CrossRef]
- Kell, D. B. and Pretorius, E. (2023) Are fibrinaloid microclots a cause of autoimmunity in Long Covid and other post-infection diseases? Biochem J. 480, 1217-1240. [CrossRef]
- Nunes, J. M., Kruger, A., Proal, A., Kell, D. B. and Pretorius, E. (2022) The occurrence of hyperactivated platelets and fibrinaloid microclots in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS). Pharmaceuticals (Basel). 15, 931. [CrossRef]
- Turner, S., Khan, M. A., Putrino, D., Woodcock, A., Kell, D. B. and Pretorius, E. (2023) Long COVID: pathophysiological factors and abnormal coagulation. Trends Endocrinol Metab. 34, 321-344. [CrossRef]
- Kell, D. B., Lip, G. Y. H. and Pretorius, E. (2024) Fibrinaloid Microclots and Atrial Fibrillation. Biomedicines. 12, 891. [CrossRef]
- Klingstedt, T., Shirani, H., Åslund, K. O. A., Cairns, N. J., Sigurdson, C. J., Goedert, M. and Nilsson, K. P. R. (2013) The structural basis for optimal performance of oligothiophene-based fluorescent amyloid ligands: conformational flexibility is essential for spectral assignment of a diversity of protein aggregates. Chemistry. 19, 10179-10192. [CrossRef]
- Nilsson, K. P., Lindgren, M. and Hammarström, P. (2012) A pentameric luminescent-conjugated oligothiophene for optical imaging of in vitro-formed amyloid fibrils and protein aggregates in tissue sections. Methods Mol Biol. 849, 425-434. [CrossRef]
- Stepanchuk, A., Tahir, W., Nilsson, K. P. R., Schatzl, H. M. and Stys, P. K. (2021) Early detection of prion protein aggregation with a fluorescent pentameric oligothiophene probe using spectral confocal microscopy. J Neurochem. 156, 1033-1048. [CrossRef]
- Laubscher, G. J., Lourens, P. J., Venter, C., Kell, D. B. and Pretorius, E. (2021) TEG®, Microclot and Platelet Mapping for Guiding Early Management of Severe COVID-19 Coagulopathy. J Clin Med. 10, 5381. [CrossRef]
- Pretorius, E., Vlok, M., Venter, C., Bezuidenhout, J. A., Laubscher, G. J., Steenkamp, J. and Kell, D. B. (2021) Persistent clotting protein pathology in Long COVID/ Post-Acute Sequelae of COVID-19 (PASC) is accompanied by increased levels of antiplasmin. Cardiovasc Diabetol. 20, 172. [CrossRef]
- retorius, E., Venter, C., Laubscher, G. J., Kotze, M. J., Oladejo, S., Watson, L. R., Rajaratnam, K., Watson, B. W. and Kell, D. B. (2022) Prevalence of symptoms, comorbidities, fibrin amyloid microclots and platelet pathology in individuals with Long COVID/ Post-Acute Sequelae of COVID-19 (PASC) Cardiovasc Diabetol. 21, 148. [CrossRef]
- Turner, S., Laubscher, G. J., Khan, M. A., Kell, D. B. and Pretorius, E. (2023) Accelerating discovery: A novel flow cytometric method for detecting fibrin(ogen) amyloid microclots using long COVID as a model Heliyon. 9, e19605. [CrossRef]
- alton, C. F., de Oliveira, M. I. R., Stafford, P., Peake, N., Kane, B., Higham, A., Singh, D., Jackson, N., Davies, H., Price, D., Duncan, R., Tattersall, N., Barnes, A. and Smith, D. P. (2024) Increased fibrinaloid microclot counts in platelet-poor plasma are associated with Long COVID. medRxiv, 2024.2004.2004.24305318. [CrossRef]
- Pretorius, E., Nunes, M., Pretorius, J. and Kell, D. B. (2024) Flow Clotometry: Measuring Amyloid Microclots in ME/CFS, Long COVID, and Healthy Samples with Imaging Flow Cytometry. Research Square. https://www.researchsquare.com/article/rs-4507472/v4507471. [CrossRef]
- Pretorius, E., Thierry, A., Sanchez, C., Ha, T., Pastor, B., Mirandola, A., Pisareva, E., Prevostel, C., Laubscher, G., Usher, T., Venter, C., Turner, S., Waters, M. and Kell, D. B. (2024) Circulating microclots are structurally associated with Neutrophil Extracellular Traps and their amounts are strongly elevated in long COVID patients. Res Square. https://www.researchsquare.com/article/rs-4666650/v4666651. [CrossRef]
- Turner, S., Naidoo, C. A., Usher, T. J., Kruger, A., Venter, C., Laubscher, G. J., Khan, M. A., Kell, D. B. and Pretorius, E. (2024) Increased Levels of Inflammatory and Endothelial Biomarkers in Blood of Long COVID Patients Point to Thrombotic Endothelialitis. Semin Thromb Hemost. 50, 288-294. [CrossRef]
- Nunes, J. M., Kell, D. B. and Pretorius, E. (2023) Cardiovascular and haematological pathology in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS): a role for Viruses. Blood Rev. 60, 101075. [CrossRef]
- Schofield, J., Abrams, S. T., Jenkins, R., Lane, S., Wang, G. and Toh, C. H. (2024) Microclots, as defined by amyloid-fibrinogen aggregates, predict risks of disseminated intravascular coagulation and mortality. Blood Adv. 8, 2499-2508. [CrossRef]
- Marfella, R., Prattichizzo, F., Sardu, C., Fulgenzi, G., Graciotti, L., Spadoni, T., D'Onofrio, N., Scisciola, L., La Grotta, R., Frigé, C., Pellegrini, V., Municinò, M., Siniscalchi, M., Spinetti, F., Vigliotti, G., Vecchione, C., Carrizzo, A., Accarino, G., Squillante, A., Spaziano, G., Mirra, D., Esposito, R., Altieri, S., Falco, G., Fenti, A., Galoppo, S., Canzano, S., Sasso, F. C., Matacchione, G., Olivieri, F., Ferraraccio, F., Panarese, I., Paolisso, P., Barbato, E., Lubritto, C., Balestrieri, M. L., Mauro, C., Caballero, A. E., Rajagopalan, S., Ceriello, A., D'Agostino, B., Iovino, P. and Paolisso, G. (2024) Microplastics and nanoplastics in atheromas and cardiovascular events. N Engl J Med. 390, 900-910. [CrossRef]
- Wang, S., Lu, W., Cao, Q., Tu, C., Zhong, C., Qiu, L., Li, S., Zhang, H., Lan, M., Qiu, L., Li, X., Liu, Y., Zhou, Y. and Liu, J. (2023) Microplastics in the Lung Tissues Associated with Blood Test Index. Toxics. 11, 759. [CrossRef]
- Zhao, B., Rehati, P., Yang, Z., Cai, Z., Guo, C. and Li, Y. (2024) The potential toxicity of microplastics on human health. Sci Total Environ. 912, 168946. [CrossRef]
- Kell, D. B. and Pretorius, E. (2022) The potential role of ischaemia-reperfusion injury in chronic, relapsing diseases such as rheumatoid arthritis, long COVID and ME/CFS: evidence, mechanisms, and therapeutic implications. Biochem J. 479, 1653-1708. [CrossRef]
- Kell, D. B., Khan, M. A., Kane, B., Lip, G. Y. H. and Pretorius, E. (2024) Possible role of fibrinaloid microclots in Postural Orthostatic Tachycardia Syndrome (POTS): focus on Long COVID. J Personalised Medicine. 14, 170. [CrossRef]
- Kell, D. B. and Pretorius, E. (2024) Potential roles of fibrinaloid microclots in fibromyalgia syndrome. OSF preprint. https://osf.io/9e2y5/.
- Kruger, A., Vlok, M., Turner, S., Venter, C., Laubscher, G. J., Kell, D. B. and Pretorius, E. (2022) Proteomics of fibrin amyloid microclots in Long COVID/ Post-Acute Sequelae of COVID-19 (PASC) shows many entrapped pro-inflammatory molecules that may also contribute to a failed fibrinolytic system. Cardiovasc Diabetol. 21, 190. [CrossRef]
- Kell, D. B. and Pretorius, E. (2024) Proteomic evidence for amyloidogenic cross-seeding in fibrinaloid microclots. Int J Mol Sci. 25, 10809. [CrossRef]
- Ząbczyk, M., Stachowicz, A., Natorska, J., Olszanecki, R., Wiśniewski, J. R. and Undas, A. (2019) Plasma fibrin clot proteomics in healthy subjects: Relation to clot permeability and lysis time. J Proteomics. 208, 103487. [CrossRef]
- Kell, D. B. and Pretorius, E. (2025) The proteome content of blood clots observed under different conditions: successful role in predicting clot amyloid(ogenicity). Molecules. 30, 668. [CrossRef]
- Pretorius, E., Windberger, U. B., Oberholzer, H. M. and Auer, R. E. (2010) Comparative ultrastructure of fibrin networks of a dog after thrombotic ischaemic stroke. Onderstepoort J Vet Res. 77, E1-4. [CrossRef]
- Pretorius, E., Steyn, H., Engelbrecht, M., Swanepoel, A. C. and Oberholzer, H. M. (2011) Differences in fibrin fiber diameters in healthy individuals and thromboembolic ischemic stroke patients. Blood Coagul Fibrinolysis. 22, 696-700. [CrossRef]
- Pretorius, E. (2011) The use of a desktop scanning electron microscope as a diagnostic tool in studying fibrin networks of thrombo-embolic ischemic stroke. Ultrastruct Pathol. 35, 245-250. [CrossRef]
- Grixti, J. M., Chandran, A., Pretorius, J. H., Walker, M., Sekhar, A., Pretorius, E. and Kell, D. B. (2024) The clots removed from ischaemic stroke patients by mechanical thrombectomy are amyloid in nature. medRxiv, 10.1101/2024.1111.1101.24316555v24316551. [CrossRef]
- Grixti, J. M., Chandran, A., Pretorius, J. H., Walker, M., Sekhar, A., Pretorius, E. and Kell, D. B. (2025) Amyloid presence in acute ischemic stroke thrombi: observational evidence for fibrinolytic resistance. Stroke, online. [CrossRef]
- Bagot, C. N. and Arya, R. (2008) Virchow and his triad: a question of attribution. Br J Haematol. 143, 180-190. [CrossRef]
- Gonzalez-Gonzalez, F. J., Ziccardi, M. R. and McCauley, M. D. (2021) Virchow's Triad and the Role of Thrombosis in COVID-Related Stroke. Front Physiol. 12, 769254. [CrossRef]
- Mehta, J. L., Calcaterra, G. and Bassareo, P. P. (2020) COVID-19, thromboembolic risk, and Virchow's triad: Lesson from the past. Clin Cardiol. 43, 1362-1367. [CrossRef]
- Choi, T. Y., Jun, J. H., Park, B., Lee, J. A., You, S. S., Jung, J. Y. and Lee, M. S. (2016) Concept of blood stasis in Chinese medical textbooks: A systematic review. Eur J Integr Med. 8, 158-164. [CrossRef]
- Huang, H., Pan, J., Han, Y., Zeng, L., Liang, G., Yang, W. and Liu, J. (2021) Chinese Herbal Medicines for Promoting Blood Circulation and Removing Blood Stasis for Preventing Deep Venous Thrombosis after Total Hip Arthroplasty: A Systematic Review and Meta-Analysis. Comb Chem High Throughput Screen. 24, 893-907. [CrossRef]
- Li, H. Q., Wei, J. J., Xia, W., Li, J. H., Liu, A. J., Yin, S. B., Wang, C., Song, L., Wang, Y., Zheng, G. Q. and Fan, J. P. (2015) Promoting blood circulation for removing blood stasis therapy for acute intracerebral hemorrhage: a systematic review and meta-analysis. Acta Pharmacol Sin. 36, 659-675. [CrossRef]
- Park, M. S., Kim, J., Kim, K. H., Yoo, H. R., Chae, I., Lee, J., Joo, I. H. and Kim, D. H. (2023) Modern concepts and biomarkers of blood stasis in cardio- and cerebrovascular diseases from the perspectives of Eastern and Western medicine: a scoping review protocol. JBI Evid Synth. 21, 214-222. [CrossRef]
- Kell, D. B., Pretorius, E. and Zhao, H. (2025) A direct relationship between ‘blood stasis’ and fibrinaloid microclots in chronic, inflammatory and vascular diseases, and some traditional natural products approaches to treatment. Pharmaceuticals. 18, 712. [CrossRef]
- Jomova, K., Raptova, R., Alomar, S. Y., Alwasel, S. H., Nepovimova, E., Kuca, K. and Valko, M. (2023) Reactive oxygen species, toxicity, oxidative stress, and antioxidants: chronic diseases and aging. Arch Toxicol. 97, 2499-2574. [CrossRef]
- Biswas, S. K. (2016) Does the Interdependence between Oxidative Stress and Inflammation Explain the Antioxidant Paradox? Oxid Med Cell Longev. 2016, 5698931. [CrossRef]
- Fischer, R. and Maier, O. (2015) Interrelation of oxidative stress and inflammation in neurodegenerative disease: role of TNF. Oxid Med Cell Longev. 2015, 610813. [CrossRef]
- Gambini, J. and Stromsnes, K. (2022) Oxidative Stress and Inflammation: From Mechanisms to Therapeutic Approaches. Biomedicines. 10, 753. [CrossRef]
- Hussain, T., Tan, B., Yin, Y., Blachier, F., Tossou, M. C. B. and Rahu, N. (2016) Oxidative Stress and Inflammation: What Polyphenols Can Do for Us? Oxid Med Cell Longev. 2016, 7432797. [CrossRef]
- Kell, D. B. (2009) Iron behaving badly: inappropriate iron chelation as a major contributor to the aetiology of vascular and other progressive inflammatory and degenerative diseases. BMC Med Genom. 2, 2. [CrossRef]
- Liguori, I., Russo, G., Curcio, F., Bulli, G., Aran, L., Della-Morte, D., Gargiulo, G., Testa, G., Cacciatore, F., Bonaduce, D. and Abete, P. (2018) Oxidative stress, aging, and diseases. Clin Interv Aging. 13, 757-772. [CrossRef]
- McGarry, T., Biniecka, M., Veale, D. J. and Fearon, U. (2018) Hypoxia, oxidative stress and inflammation. Free Radic Biol Med. 125, 15-24. [CrossRef]
- Reuter, S., Gupta, S. C., Chaturvedi, M. M. and Aggarwal, B. B. (2010) Oxidative stress, inflammation, and cancer: how are they linked? Free Radic Biol Med. 49, 1603-1616. [CrossRef]
- Siti, H. N., Kamisah, Y. and Kamsiah, J. (2015) The role of oxidative stress, antioxidants and vascular inflammation in cardiovascular disease (a review). Vascul Pharmacol. 71, 40-56. [CrossRef]
- Zhazykbayeva, S., Pabel, S., Mugge, A., Sossalla, S. and Hamdani, N. (2020) The molecular mechanisms associated with the physiological responses to inflammation and oxidative stress in cardiovascular diseases. Biophys Rev. 12, 947-968. [CrossRef]
- Grixti, J. M., Theron, C. W., Salcedo-Sora, J. E., Pretorius, E. and Kell, D. B. (2024) Automated microscopic measurement of fibrinaloid microclots and their degradation by nattokinase, the main natto protease. J Exp Clin Appl Chin Med. 5, 30-55. [CrossRef]
- Cracowski, J. L. and Roustit, M. (2020) Human Skin Microcirculation. Compr Physiol. 10, 1105-1154. [CrossRef]
- Jung, F., Pindur, G., Ohlmann, P., Spitzer, G., Sternitzky, R., Franke, R. P., Leithauser, B., Wolf, S. and Park, J. W. (2013) Microcirculation in hypertensive patients. Biorheology. 50, 241-255. [CrossRef]
- Jung, C. and Kelm, M. (2015) Evaluation of the microcirculation in critically ill patients. Clin Hemorheol Microcirc. 61, 213-224. [CrossRef]
- Morf, S., Amann-Vesti, B., Forster, A., Franzeck, U. K., Koppensteiner, R., Uebelhart, D. and Sprott, H. (2005) Microcirculation abnormalities in patients with fibromyalgia - measured by capillary microscopy and laser fluxmetry. Arthritis Res Ther. 7, R209-216. [CrossRef]
- Lutze, S., Westphal, T., Jünger, M. and Arnold, A. (2024) Microcirculation disorders of the skin. J Dtsch Dermatol Ges. 22, 236-264. [CrossRef]
- Radic, M., Thomas, J., McMillan, S. and Frech, T. (2021) Does sublingual microscopy correlate with nailfold videocapillaroscopy in systemic sclerosis? Clin Rheumatol. 40, 2263-2266. [CrossRef]
- Jakhar, D., Grover, C., Singal, A. and Das, G. K. (2020) Nailfold Capillaroscopy and Retinal Findings in Patients with Systemic Sclerosis: Is There An Association? Indian Dermatol Online J. 11, 382-386. [CrossRef]
- Briers, D., Duncan, D. D., Hirst, E., Kirkpatrick, S. J., Larsson, M., Steenbergen, W., Stromberg, T. and Thompson, O. B. (2013) Laser speckle contrast imaging: theoretical and practical limitations. J Biomed Opt. 18, 066018. [CrossRef]
- Couturier, A., Bouvet, R., Cracowski, J. L. and Roustit, M. (2022) Reproducibility of high-resolution laser speckle contrast imaging to assess cutaneous microcirculation for wound healing monitoring in mice. Microvasc Res. 141, 104319. [CrossRef]
- Hellmann, M., Kalinowski, L. and Cracowski, J. L. (2022) Laser speckle contrast imaging to assess microcirculation. Cardiol J. 29, 1028-1030. [CrossRef]
- Lazaridis, A., Triantafyllou, A., Mastrogiannis, K., Malliora, A., Doumas, M. and Gkaliagkousi, E. (2023) Assessing skin microcirculation in patients at cardiovascular risk by using laser speckle contrast imaging. A narrative review. Clin Physiol Funct Imaging. 43, 211-222. [CrossRef]
- Linkous, C., Pagan, A. D., Shope, C., Andrews, L., Snyder, A., Ye, T. and Valdebran, M. (2023) Applications of Laser Speckle Contrast Imaging Technology in Dermatology. JID Innov. 3, 100187. [CrossRef]
- Senarathna, J., Rege, A., Li, N. and Thakor, N. V. (2013) Laser Speckle Contrast Imaging: theory, instrumentation and applications. IEEE Rev Biomed Eng. 6, 99-110. [CrossRef]
- Cutolo, M. and Smith, V. (2013) State of the art on nailfold capillaroscopy: a reliable diagnostic tool and putative biomarker in rheumatology? Rheumatology (Oxford). 52, 1933-1940. [CrossRef]
- El Miedany, Y., Ismail, S., Wadie, M. and Hassan, M. (2022) Nailfold capillaroscopy: tips and challenges. Clin Rheumatol. 41, 3629-3640. [CrossRef]
- Grover, C., Jakhar, D., Mishra, A. and Singal, A. (2022) Nail-fold capillaroscopy for the dermatologists. Indian J Dermatol Venereol Leprol. 88, 300-312. [CrossRef]
- Karbalaie, A., Emrani, Z., Fatemi, A., Etehadtavakol, M. and Erlandsson, B. E. (2019) Practical issues in assessing nailfold capillaroscopic images: a summary. Clin Rheumatol. 38, 2343-2354. [CrossRef]
- Rodriguez-Reyna, T. S., Bertolazzi, C., Vargas-Guerrero, A., Gutiérrez, M., Hernández-Molina, G., Audisio, M., Roverano, S., González de Urizar, M., Diaz Coto, J. F., Herrera Velasco, B. E., Cornejo Ortega, M. P., Sapag Durán, A. M., Villegas Guzmán, J. E., Medina Quintero, L. F., Sabelli, M., Sapag Durán, S., Cutolo, M. and PANLAR Capillaroscopy Group. (2019) Can nailfold videocapillaroscopy images be interpreted reliably by different observers? Results of an inter-reader and intra-reader exercise among rheumatologists with different experience in this field. Clin Rheumatol. 38, 205-210. [CrossRef]
- Gracia Tello, B. D. C., SáezComet, L., Lledó, G., Freire Dapena, M., Mesa, M. A., Martín-Cascón, M., Guillén Del Castillo, A., Martínez Robles, E., Simeón-Aznar, C. P., Todolí Parra, J. A., Varela, D. C., Maldonado Vélez, G., Marin Ballvé, A., Aramburu Llorente, J., Pérez Abad, L. and Ramos Ibáñez, E. (2024) Capi-score: a quantitative algorithm for identifying disease patterns in nailfold videocapillaroscopy. Rheumatology (Oxford). 63, 3315-3321. [CrossRef]
- Emrani, Z., Karbalaie, A., Fatemi, A., Etehadtavakol, M. and Erlandsson, B. E. (2017) Capillary density: An important parameter in nailfold capillaroscopy. Microvasc Res. 109, 7-18. [CrossRef]
- Karbalaie, A., Abtahi, F., Fatemi, A., Etehadtavakol, M., Emrani, Z. and Erlandsson, B. E. (2017) Elliptical broken line method for calculating capillary density in nailfold capillaroscopy: Proposal and evaluation. Microvasc Res. 113, 1-8. [CrossRef]
- Kintrup, S., Listkiewicz, L., Arnemann, P. H. and Wagner, N. M. (2024) Nailfold videocapillaroscopy - a novel method for the assessment of hemodynamic incoherence on the ICU. Crit Care. 28, 400. [CrossRef]
- El Miedany, Y., Ismail, S., Wadie Fawzy, M., Muller-Ladner, U., Giacomelli, R., Liakouli, V., Hermann, W., Fathy, N., El Gaafary, M., Fouad, N. A., Saber, S. and Abu-Zaid, M. H. (2023) Towards a consensus on the clinical applications and interpretations of the nailfold capillaroscopy standards in clinical practice: An initiative by the Egyptian Society of Microcirculation. Arch Rheumatol. 38, 451-460. [CrossRef]
- El Miedany, Y., Ismail, S., Wadie, M., Muller-Ladneru, U., Giacomelli, R., Liakouli, V., Hermann, W., Fathy, N., El Gaafary, M., Fouad, N. A., Saber, S. and Abu-Zaid, M. H. (2024) Development of a core domain set for nailfold capillaroscopy reporting. Reumatol Clin (Engl Ed). 20, 345-352. [CrossRef]
- Etehad Tavakol, M., Fatemi, A., Karbalaie, A., Emrani, Z. and Erlandsson, B. E. (2015) Nailfold Capillaroscopy in Rheumatic Diseases: Which Parameters Should Be Evaluated? Biomed Res Int. 2015, 974530. [CrossRef]
- Bertolazzi, C., Cutolo, M., Smith, V. and Gutierrez, M. (2017) State of the art on nailfold capillaroscopy in dermatomyositis and polymyositis. Semin Arthritis Rheum. 47, 432-444. [CrossRef]
- Cutolo, M., Melsens, K., Wijnant, S., Ingegnoli, F., Thevissen, K., De Keyser, F., Decuman, S., Muller-Ladner, U., Piette, Y., Riccieri, V., Ughi, N., Vandecasteele, E., Vanhaecke, A. and Smith, V. (2018) Nailfold capillaroscopy in systemic lupus erythematosus: A systematic review and critical appraisal. Autoimmun Rev. 17, 344-352. [CrossRef]
- Smith, V., Herrick, A. L., Ingegnoli, F., Damjanov, N., De Angelis, R., Denton, C. P., Distler, O., Espejo, K., Foeldvari, I., Frech, T., Garro, B., Gutierrez, M., Gyger, G., Hachulla, E., Hesselstrand, R., Iagnocco, A., Kayser, C., Melsens, K., Muller-Ladner, U., Paolino, S., Pizzorni, C., Radic, M., Riccieri, V., Snow, M., Stevens, W., Sulli, A., van Laar, J. M., Vonk, M. C., Vanhaecke, A., Cutolo, M., Eular Study Group on Microcirculation in Rheumatic Diseases and The Scleroderma Clinical Trials Consortium Group on, C. (2020) Standardisation of nailfold capillaroscopy for the assessment of patients with Raynaud's phenomenon and systemic sclerosis. Autoimmun Rev. 19, 102458. [CrossRef]
- Smith, V., Ickinger, C., Hysa, E., Snow, M., Frech, T., Sulli, A. and Cutolo, M. (2023) Nailfold capillaroscopy. Best Pract Res Clin Rheumatol. 37, 101849. [CrossRef]
- Patil, A. and Sood, I. (2020) Nailfold Capillaroscopy in Rheumatic Diseases. Intech Open, 72602. [CrossRef]
- Ocampo-Garza, S. S., Villarreal-Alarcon, M. A., Villarreal-Trevino, A. V. and Ocampo-Candiani, J. (2019) Capillaroscopy: A Valuable Diagnostic Tool. Actas Dermosifiliogr (Engl Ed). 110, 347-352. [CrossRef]
- Dundar, H. A., Adrovic, A., Demir, S., Demir, F., Cakmak, F., Ayaz, N. A., Sözeri, B., Bilginer, Y., Kasapçopur, O. and Unsal, E. (2024) Description of the characteristics of the nailfold capillary structure in healthy children: a multi-centric study. Rheumatology (Oxford). 63, SI152-SI159. [CrossRef]
- Altorok, N., Wang, Y. and Kahaleh, B. (2014) Endothelial dysfunction in systemic sclerosis. Curr Opin Rheumatol. 26, 615-620. [CrossRef]
- Matucci-Cerinic, M., Kahaleh, B. and Wigley, F. M. (2013) Review: evidence that systemic sclerosis is a vascular disease. Arthritis Rheum. 65, 1953-1962. [CrossRef]
- Moschetti, L., Piantoni, S., Vizzardi, E., Sciatti, E., Riccardi, M., Franceschini, F. and Cavazzana, I. (2022) Endothelial Dysfunction in Systemic Lupus Erythematosus and Systemic Sclerosis: A Common Trigger for Different Microvascular Diseases. Front Med (Lausanne). 9, 849086. [CrossRef]
- Mostmans, Y., Cutolo, M., Giddelo, C., Decuman, S., Melsens, K., Declercq, H., Vandecasteele, E., De Keyser, F., Distler, O., Gutermuth, J. and Smith, V. (2017) The role of endothelial cells in the vasculopathy of systemic sclerosis: A systematic review. Autoimmun Rev. 16, 774-786. [CrossRef]
- Ota, Y. and Kuwana, M. (2020) Endothelial cells and endothelial progenitor cells in the pathogenesis of systemic sclerosis. Eur J Rheumatol. 7, S139-S146. [CrossRef]
- Patnaik, E., Lyons, M., Tran, K. and Pattanaik, D. (2023) Endothelial Dysfunction in Systemic Sclerosis. Int J Mol Sci. 24, 14385. [CrossRef]
- Silva, I., Teixeira, A., Oliveira, J., Almeida, I., Almeida, R., Aguas, A. and Vasconcelos, C. (2015) Endothelial Dysfunction and Nailfold Videocapillaroscopy Pattern as Predictors of Digital Ulcers in Systemic Sclerosis: a Cohort Study and Review of the Literature. Clin Rev Allergy Immunol. 49, 240-252. [CrossRef]
- Matucci-Cerinic, M., Hughes, M., Taliani, G. and Kahaleh, B. (2021) Similarities between COVID-19 and systemic sclerosis early vasculopathy: A "viral" challenge for future research in scleroderma. Autoimmun Rev. 20, 102899. [CrossRef]
- Kuchler, T., Gunthner, R., Ribeiro, A., Hausinger, R., Streese, L., Wohnl, A., Kesseler, V., Negele, J., Assali, T., Carbajo-Lozoya, J., Lech, M., Schneider, H., Adorjan, K., Stubbe, H. C., Hanssen, H., Kotilar, K., Haller, B., Heemann, U. and Schmaderer, C. (2023) Persistent endothelial dysfunction in post-COVID-19 syndrome and its associations with symptom severity and chronic inflammation. Angiogenesis. 26, 547-563. [CrossRef]
- Santoro, L., Zaccone, V., Falsetti, L., Ruggieri, V., Danese, M., Miro, C., Di Giorgio, A., Nesci, A., D'Alessandro, A., Moroncini, G. and Santoliquido, A. (2023) Role of Endothelium in Cardiovascular Sequelae of Long COVID. Biomedicines. 11, 2239. [CrossRef]
- Xu, S. W., Ilyas, I. and Weng, J. P. (2023) Endothelial dysfunction in COVID-19: an overview of evidence, biomarkers, mechanisms and potential therapies. Acta Pharmacol Sin. 44, 695-709. [CrossRef]
- Aljadah, M., Khan, N., Beyer, A. M., Chen, Y., Blanker, A. and Widlansky, M. E. (2024) Clinical Implications of COVID-19-Related Endothelial Dysfunction. JACC Adv. 3, 101070. [CrossRef]
- Perico, L., Benigni, A. and Remuzzi, G. (2024) SARS-CoV-2 and the spike protein in endotheliopathy. Trends Microbiol. 32, 53-67. [CrossRef]
- Wu, X., Xiang, M., Jing, H., Wang, C., Novakovic, V. A. and Shi, J. (2024) Damage to endothelial barriers and its contribution to long COVID. Angiogenesis. 27, 5-22. [CrossRef]
- Kruger, A., Joffe, D., Lloyd-Jones, G., Khan, M. A., Šalamon, Š., Laubscher, G. J., Putrino, D., Kell, D. B. and Pretorius, E. (2025) Vascular pathogenesis in acute and long covid: current insights and therapeutic outlook Semin Throm Hemost. 51, 256-271. [CrossRef]
- Çakmak, F., Demirbuga, A., Demirkol, D., Gümüs, S., Torun, S. H., Kayaalp, G. K., Ömeroglu, R. E., Somer, A., Uysalol, M., Yıldız, R. and Ayaz, N. A. (2021) Nailfold capillaroscopy: A sensitive method for evaluating microvascular involvement in children with SARS-CoV-2 infection. Microvasc Res. 138, 104196. [CrossRef]
- Jud, P., Gressenberger, P., Muster, V., Avian, A., Meinitzer, A., Strohmaier, H., Sourij, H., Raggam, R. B., Stradner, M. H., Demel, U., Kessler, H. H., Eller, K. and Brodmann, M. (2021) Evaluation of Endothelial Dysfunction and Inflammatory Vasculopathy After SARS-CoV-2 Infection-A Cross-Sectional Study. Front Cardiovasc Med. 8, 750887. [CrossRef]
- Natalello, G., De Luca, G., Gigante, L., Campochiaro, C., De Lorenzis, E., Verardi, L., Paglionico, A., Petricca, L., Martone, A. M., Calvisi, S., Ripa, M., Cavalli, G., Della-Torre, E., Tresoldi, M., Landi, F., Bosello, S. L., Gremese, E. and Dagna, L. (2021) Nailfold capillaroscopy findings in patients with coronavirus disease 2019: Broadening the spectrum of COVID-19 microvascular involvement. Microvasc Res. 133, 104071. [CrossRef]
- Wollina, U., Kanitakis, J. and Baran, R. (2021) Nails and COVID-19 - A comprehensive review of clinical findings and treatment. Dermatol Ther. 34, e15100. [CrossRef]
- Armağan, B., Özdemir, B., Aypak, A., Akıncı, E., Karakaş, Ö., Güven, S. C., Küçükşahin, O., Omma, A. and Erden, A. (2022) Evaluation of Coronavirus Disease-2019 Patients with Nailfold Capillaroscopy. Namik Kemal Med J 10, 80-86. [CrossRef]
- Mostmans, Y., Smith, V., Cutolo, M., Melsens, K., Battist, S., Benslimane, A., Corazza, F., Richert, B., Michel, O. and Kolivras, A. (2022) Nailfold videocapillaroscopy and serum vascular endothelial growth factor in probable COVID-19-induced chilblains: a cross-sectional study to assess microvascular impairment. Br J Dermatol. 187, 1017-1019. [CrossRef]
- Rosei, C. A., Gaggero, A., Fama, F., Malerba, P., Chiarini, G., Nardin, M., Brami, V., Rossini, C., Coschignano, M. A., Porteri, E., Salvetti, M., Muiesan, M. L., Rizzoni, D. and De Ciuceis, C. (2022) Skin capillary alterations in patients with acute SarsCoV2 infection. J Hypertens. 40, 2385-2393. [CrossRef]
- Sulli, A., Gotelli, E., Bica, P. F., Schiavetti, I., Pizzorni, C., Aloe, T., Grosso, M., Barisione, E., Paolino, S., Smith, V. and Cutolo, M. (2022) Detailed videocapillaroscopic microvascular changes detectable in adult COVID-19 survivors. Microvasc Res. 142, 104361. [CrossRef]
- Cutolo, M., Sulli, A., Smith, V. and Gotelli, E. (2023) Emerging nailfold capillaroscopic patterns in COVID-19: from acute patients to survivors. Reumatismo. 74, 139-143. [CrossRef]
- Mondini, L., Confalonieri, P., Pozzan, R., Ruggero, L., Trotta, L., Lerda, S., Hughes, M., Bellan, M., Confalonieri, M., Ruaro, B., Salton, F. and Tavano, S. (2023) Microvascular Alteration in COVID-19 Documented by Nailfold Capillaroscopy. Diagnostics (Basel). 13. [CrossRef]
- Kaplan, H., Cengiz, G., Şaş, S. and Kara, H. (2024) Comparison of nailfold capillaroscopy findings in COVID-19 survivors with and without rheumatic disease: a case-control study. Cucurova Med J. 49, 71-80. [CrossRef]
- Kastarli Bakay, O. S., Cetin, N., Bakay, U., Cinar, G. and Goksin, S. (2025) A Window into the Vascular Endothelium in Covid-19: Nails. Dermatol Pract Concept. 15. [CrossRef]
- Wilkinson, S., Wilkinson, J., Grace, A., Lyon, D., Mellor, M., Yunus, T., Manning, J., Dinsdale, G., Berks, M., Knight, S., Bakerly, N., Gebril, A., Dark, P., Herrick, A., Taylor, C., Dickinson, M. and Murray, A. (2025) Imaging the microvasculature using nailfold capillaroscopy in patients with coronavirus disease-2019; A cross-sectional study. Microvasc Res. 159, 104796. [CrossRef]
- Bunch, C. M., Moore, E. E., Moore, H. B., Neal, M. D., Thomas, A. V., Zackariya, N., Zhao, J., Zackariya, S., Brenner, T. J., Berquist, M., Buckner, H., Wiarda, G., Fulkerson, D., Huff, W., Kwaan, H. C., Lankowicz, G., Laubscher, G. J., Lourens, P. J., Pretorius, E., Kotze, M. J., Moolla, M. S., Sithole, S., Maponga, T. G., Kell, D. B., Fox, M., Gillespie, L., Khan, R. Z., Mamczak, C. N., March, R., Macias, R., Bull, B. S. and Walsh, M. M. (2022) Immuno-thrombotic Complications of COVID-19: Implications for Timing of Surgery and Anticoagulation. Front Surg. 9, 889999. [CrossRef]
- Grobbelaar, L. M., Venter, C., Vlok, M., Ngoepe, M., Laubscher, G. J., Lourens, P. J., Steenkamp, J., Kell, D. B. and Pretorius, E. (2021) SARS-CoV-2 spike protein S1 induces fibrin(ogen) resistant to fibrinolysis: implications for microclot formation in COVID-19. Biosci Rep. 41, BSR20210611. [CrossRef]
- Grobbelaar, L. M., Kruger, A., Venter, C., Burger, E. M., Laubscher, G. J., Maponga, T. G., Kotze, M. J., Kwaan, H. C., Miller, J. B., Fulkerson, D., Huff, W., Chang, E., Wiarda, G., Bunch, C. M., Walsh, M. M., Raza, S., Zamlut, M., Moore, H. B., Moore, E. E., Neal, M. D., Kell, D. B. and Pretorius, E. (2022) Relative hypercoagulopathy of the SARS-CoV-2 Beta and Delta variants when compared to the less severe Omicron variants is related to TEG parameters, the extent of fibrin amyloid microclots, and the severity of clinical illness. Semin Thromb Haemost. 48, 858-868. [CrossRef]
- Grobler, C., Maphumulo, S. C., Grobbelaar, L. M., Bredenkamp`, J., Laubscher, J., Lourens, P. J., Steenkamp, J., Kell, D. B. and Pretorius, E. (2020) COVID-19: The Rollercoaster of Fibrin(ogen), D-dimer, von Willebrand Factor, P-selectin and Their Interactions with Endothelial Cells, Platelets and Erythrocytes. Int J Mol Sci. 21, 5168. [CrossRef]
- Cousins, C. C., Alosco, M. L., Cousins, H. C., Chua, A., Steinberg, E. G., Chapman, K. R., Bing-Canar, H., Tripodis, Y., Knepper, P. A., Stern, R. A. and Pasquale, L. R. (2018) Nailfold Capillary Morphology in Alzheimer's Disease Dementia. J Alzheimers Dis. 66, 601-611. [CrossRef]
- Ciaffi, J., Ajasllari, N., Mancarella, L., Brusi, V., Meliconi, R. and Ursini, F. (2020) Nailfold capillaroscopy in common non-rheumatic conditions: A systematic review and applications for clinical practice. Microvasc Res. 131, 104036. [CrossRef]
- Grobler, C., van Tongeren, M., Gettemans, J., Kell, D. and Pretorius, E. (2023) Alzheimer-type dementia: a systems view provides a unifying explanation of its development. J Alz Dis. 91, 43-70. [CrossRef]
- Pretorius, L., Kell, D. B. and Pretorius, E. (2018) Iron Dysregulation and Dormant Microbes as Causative Agents for Impaired Blood Rheology and Pathological Clotting in Alzheimer's Type Dementia. Front Neurosci. 12, 851. [CrossRef]
- Deshayes, S., Auboire, L., Jaussaud, R., Lidove, O., Parienti, J. J., Triclin, N., Imbert, B., Bienvenu, B. and Aouba, A. (2015) Prevalence of Raynaud phenomenon and nailfold capillaroscopic abnormalities in Fabry disease: a cross-sectional study. Medicine (Baltimore). 94, e780. [CrossRef]
- Faro, D. C., Di Pino, F. L. and Monte, I. P. (2024) Inflammation, Oxidative Stress, and Endothelial Dysfunction in the Pathogenesis of Vascular Damage: Unraveling Novel Cardiovascular Risk Factors in Fabry Disease. Int J Mol Sci. 25, 8273. [CrossRef]
- Faro, D. C., Di Pino, F. L., Rodolico, M. S., Costanzo, L., Losi, V., Di Pino, L. and Monte, I. P. (2024) Relationship between Capillaroscopic Architectural Patterns and Different Variant Subgroups in Fabry Disease: Analysis of Cases from a Multidisciplinary Center. Genes (Basel). 15, 1101. [CrossRef]
- Wasik, J. S., Simon, R. W., Meier, T., Steinmann, B. and Amann-Vesti, B. R. (2009) Nailfold capillaroscopy: Specific features in Fabry disease. Clin Hemorheol Microcirc. 42, 99-106. [CrossRef]
- De Martinis, M., Sirufo, M. M. and Ginaldi, L. (2018) Raynaud's phenomenon and nailfold capillaroscopic findings in anorexia nervosa. Curr Med Res Opin. 34, 547-550. [CrossRef]
- Sirufo, M. M., Ginaldi, L. and De Martinis, M. (2021) Peripheral Vascular Abnormalities in Anorexia Nervosa: A Psycho-Neuro-Immune-Metabolic Connection. Int J Mol Sci. 22, 5043. [CrossRef]
- Matsuda, S., Kotani, T., Wakura, R., Suzuka, T., Kuwabara, H., Kiboshi, T., Wada, Y., Shiba, H., Hata, K., Shoda, T., Hirose, Y. and Takeuchi, T. (2023) Examination of nailfold videocapillaroscopy findings in ANCA-associated vasculitis. Rheumatology (Oxford). 62, 747-757. [CrossRef]
- Triggianese, P., D'Antonio, A., Nesi, C., Kroegler, B., Di Marino, M., Conigliaro, P., Modica, S., Greco, E., Nucci, C., Bergamini, A., Chimenti, M. S. and Cesareo, M. (2023) Subclinical microvascular changes in ANCA-vasculitides: the role of optical coherence tomography angiography and nailfold capillaroscopy in the detection of disease-related damage. Orphanet J Rare Dis. 18, 184. [CrossRef]
- Screm, G., Mondini, L., Confalonieri, P., Salton, F., Trotta, L., Barbieri, M., Mari, M., Reccardini, N., Della Porta, R., Kodric, M., Bandini, G., Hughes, M., Bellan, M., Lerda, S., Confalonieri, M. and Ruaro, B. (2024) Nailfold Capillaroscopy Analysis Can Add a New Perspective to Biomarker Research in Antineutrophil Cytoplasmic Antibody-Associated Vasculitis. Diagnostics (Basel). 14, 254. [CrossRef]
- Sullivan, M. M., Abril, A., Aslam, N., Ball, C. T. and Berianu, F. (2024) Nailfold videocapillaroscopy in antineutrophil cytoplasmic antibody-associated vasculitis. Arthritis Res Ther. 26, 4. [CrossRef]
- Arslan Uku, S., Demir, B., Cicek, D. and Inan Yuksel, E. (2021) Assessment of nail findings in children with atopic dermatitis. Clin Exp Dermatol. 46, 1511-1517. [CrossRef]
- Aytekin, S., Yuksel, E. P., Aydin, F., Senturk, N., Ozden, M. G., Canturk, T. and Turanli, A. Y. (2014) Nailfold capillaroscopy in Behçet disease, performed using videodermoscopy. Clin Exp Dermatol. 39, 443-447. [CrossRef]
- Mercadé-Torras, J. M., Guillén-Del-Castillo, A., Buján, S. and Solans-Laque, R. (2024) Nailfold videocapillaroscopy abnormalities and vascular manifestations in Behçet’s syndrome. Clin Exp Rheumatol. 42, 2065-2070. [CrossRef]
- Monoe, K., Takahashi, A., Abe, K., Kanno, Y., Watanabe, H. and Ohira, H. (2014) Evaluation of nail fold capillaroscopy findings in patients with primary biliary cirrhosis. Hepatol Res. 44, E129-136. [CrossRef]
- Kim, M. (2024) Nail fold capillaroscopy as a potential tool to evaluate breast tumor. J Anal Sci Technol. 15, 35. [CrossRef]
- Screm, G., Mondini, L., Salton, F., Confalonieri, P., Trotta, L., Barbieri, M., Romallo, A., Galantino, A., Hughes, M., Lerda, S., Confalonieri, M. and Ruaro, B. (2024) Vascular Endothelial Damage in COPD: Where Are We Now, Where Will We Go? Diagnostics (Basel). 14. [CrossRef]
- Corrado, A., Carpagnano, G. E., Gaudio, A., Foschino-Barbaro, M. P. and Cantatore, F. P. (2010) Nailfold capillaroscopic findings in systemic sclerosis related lung fibrosis and in idiopathic lung fibrosis. Joint Bone Spine. 77, 570-574. [CrossRef]
- Yuksel, E. P., Yuksel, S., Soylu, K. and Aydin, F. (2019) Microvascular abnormalities in asymptomatic chronic smokers: A videocapillaroscopic study. Microvasc Res. 124, 51-53. [CrossRef]
- Mostmans, Y., Maurer, M., Richert, B., Smith, V., Melsens, K., De Maertelaer, V., Saidi, I., Corazza, F. and Michel, O. (2024) Chronic spontaneous urticaria: Evidence of systemic microcirculatory changes. Clin Transl Allergy. 14, e12335. [CrossRef]
- Bernardino, V., Rodrigues, A., Lladó, A. and Panarra, A. (2020) Nailfold capillaroscopy and autoimmune connective tissue diseases in patients from a Portuguese nailfold capillaroscopy clinic. Rheumatol Int. 40, 295-301. [CrossRef]
- Munteanu, A., Kundnani, N. R. and Caraba, A. (2024) Nailfold capillaroscopy abnormalities and pulmonary hypertension in mixed connective tissue disease and systemic sclerosis patients. Eur Rev Med Pharmacol Sci. 28, 1314-1326. [CrossRef]
- Tang, Z., Yang, F., Wu, H., Zhao, Y., Shen, J., Hong, H., Yin, F., Ma, X., Geng, L., Xu, X., Wei, Y. and Zhang, H. (2025) Alterations in nailfold videocapillaroscopy among patients with connective tissue diseases combined with pulmonary arterial hypertension: A cross-sectional study. Sci Rep. 15, 8647. [CrossRef]
- Maslianitsyna, A., Ermolinskiy, P., Lugovtsov, A., Pigurenko, A., Sasonko, M., Gurfinkel, Y. and Priezzhev, A. (2021) Multimodal Diagnostics of Microrheologic Alterations in Blood of Coronary Heart Disease and Diabetic Patients. Diagnostics (Basel). 11, 76. [CrossRef]
- Manfredi, A., Sebastiani, M., Cassone, G., Pipitone, N., Giuggioli, D., Colaci, M., Salvarani, C. and Ferri, C. (2015) Nailfold capillaroscopic changes in dermatomyositis and polymyositis. Clin Rheumatol. 34, 279-284. [CrossRef]
- J. D. and Sontheimer, R. D. (2016) Proximal nailfold microhemorrhage events are manifested as distal cuticular (eponychial) hemosiderin-containing deposits (CEHD) (syn. Maricq sign) and can aid in the diagnosis of dermatomyositis and systemic sclerosis. Dermatol Online J. 22. [CrossRef]
- Cutolo, M. and Smith, V. (2021) Detection of microvascular changes in systemic sclerosis and other rheumatic diseases. Nat Rev Rheumatol. 17, 665-677. [CrossRef]
- Monfort, J. B., Chasset, F., Barbaud, A., Frances, C. and Senet, P. (2021) Nailfold capillaroscopy findings in cutaneous lupus erythematosus patients with or without digital lesions and comparison with dermatomyositis patients: A prospective study. Lupus. 30, 1207-1213. [CrossRef]
- Pachman, L. M., Morgan, G., Klein-Gitelman, M. S., Ahsan, N. and Khojah, A. (2023) Nailfold capillary density in 140 untreated children with juvenile dermatomyositis: an indicator of disease activity. Pediatr Rheumatol Online J. 21, 118. [CrossRef]
- Flatley, E. M., Collins, D., Lukowiak, T. M. and Miller, J. H. (2024) Nailfold microscopy in adult-onset dermatomyositis in association with myositis antibodies. Arch Dermatol Res. 317, 34. [CrossRef]
- Trevisan, G., Bonin, S., Tucci, S. and Bilancini, S. (2024) Dermatomyositis: nailfold capillaroscopy patterns and a general survey. Acta Dermatovenerol Alp Pannonica Adriat. 33, 69-79. [CrossRef]
- Xu, H. and Qian, J. (2025) The role of nailfold video-capillaroscopy in the assessment of dermatomyositis. Rheumatology (Oxford). 64, 2987-2994. [CrossRef]
- Yılmaz Tuğan, B., Sönmez, H. E., Güngör, M., Yüksel, N. and Karabaş, L. (2022) Preclinical ocular microvascular changes in juvenile dermatomyositis: A pilot optical coherence tomography angiography study. Microvasc Res. 143, 104382. [CrossRef]
- Piette, Y., Reynaert, V., Vanhaecke, A., Bonroy, C., Gutermuth, J., Sulli, A., Cutolo, M. and Smith, V. (2022) Standardised interpretation of capillaroscopy in autoimmune idiopathic inflammatory myopathies: A structured review on behalf of the EULAR study group on microcirculation in Rheumatic Diseases. Autoimmun Rev. 21, 103087. [CrossRef]
- Abdelmaksoud, A. A., Daifallah, S. M., Salah, N. Y. and Saber, A. S. (2022) Nail fold microangiopathy in adolescents with type 1 diabetes: Relation to diabetic vascular complications. Microcirculation. 29, e12771. [CrossRef]
- Kaminska-Winciorek, G., Deja, G., Polańska, J. and Jarosz-Chobot, P. (2012) Diabetic microangiopathy in capillaroscopic examination of juveniles with diabetes type 1. Postepy Hig Med Dosw (Online). 66, 51-59.
- Shah, R., Petch, J., Nelson, W., Roth, K., Noseworthy, M. D., Ghassemi, M. and Gerstein, H. C. (2023) Nailfold capillaroscopy and deep learning in diabetes. J Diabetes. 15, 145-151. [CrossRef]
- Abd El-Khalik, D. M., Hafez, E. A., Hassan, H. E., Mahmoud, A. E., Ashour, D. M. and Morshedy, N. A. (2022) Nail Folds Capillaries Abnormalities Associated With Type 2 Diabetes Mellitus Progression and Correlation With Diabetic Retinopathy. Clin Med Insights Endocrinol Diabetes. 15, 11795514221122828. [CrossRef]
- Ahmad, S., Pai, V. V., Sharath, A., Ghodge, R. and Shukla, P. (2023) Qualitative analysis of nailfold capillaries in diabetes and diabetic retinopathy using dermatoscope in patients with coloured skin. Indian J Dermatol Venereol Leprol, 1-8. [CrossRef]
- Maldonado, G., Guerrero, R., Paredes, C. and Rios, C. (2017) Nailfold capillaroscopy in diabetes mellitus. Microvasc Res. 112, 41-46. [CrossRef]
- Pretorius, E., Oberholzer, H. M., van der Spuy, W. J., Swanepoel, A. C. and Soma, P. (2011) Qualitative scanning electron microscopy analysis of fibrin networks and platelet abnormalities in diabetes. Blood Coagul Fibrinol. 22, 463-467. [CrossRef]
- Pretorius, E., Bester, J., Vermeulen, N., Alummoottil, S., Soma, P., Buys, A. V. and Kell, D. B. (2015) Poorly controlled type 2 diabetes is accompanied by significant morphological and ultrastructural changes in both erythrocytes and in thrombin-generated fibrin: implications for diagnostics. Cardiovasc Diabetol. 134, 30. [CrossRef]
- Pazos-Moura, C. C., Moura, E. G., Bouskela, E., Torres Filho, I. P. and Breitenbach, M. M. (1990) Nailfold capillaroscopy in non-insulin dependent diabetes mellitus: blood flow velocity during rest and post-occlusive reactive hyperaemia. Clin Physiol. 10, 451-461. [CrossRef]
- Maldonado, G., Chacko, A., Lichtenberg, R., Ionescu, M. and Rios, C. (2022) Nailfold capillaroscopy in diabetes mellitus: a case of neo-angiogenesis after achieving normoglycemia. Oxf Med Case Reports. 2022, omac088. [CrossRef]
- Lisco, G. and Triggiani, V. (2023) Computerized nailfold video-capillaroscopy in type 2 diabetes: A cross-sectional study on 102 outpatients. J Diabetes. 15, 890-899. [CrossRef]
- Elumalai, S., Krishnamoorthi, N., Periyasamy, N., Farazullah, M., Raj, K. and Mahadevan, S. (2024) Analysis of microvascular pattern in diabetes mellitus condition using the nailfold capillaroscopy images. Proc Inst Mech Eng H. 238, 340-347. [CrossRef]
- Bakirci, S., Celik, E., Acikgoz, S. B., Erturk, Z., Tocoglu, A. G., Imga, N. N., Kaya, M. and Tamer, A. (2019) The evaluation of nailfold videocapillaroscopy findings in patients with type 2 diabetes with and without diabetic retinopathy. North Clin Istanb. 6, 146-150. [CrossRef]
- Uyar, S., Balkarlı, A., Erol, M. K., Yeşil, B., Tokuç, A., Durmaz, D., Görar, S. and Çekin, A. H. (2016) Assessment of the Relationship between Diabetic Retinopathy and Nailfold Capillaries in Type 2 Diabetics with a Noninvasive Method: Nailfold Videocapillaroscopy. J Diabetes Res. 2016, 7592402. [CrossRef]
- Chao, C. Y. L., Zheng, Y. P. and Cheing, G. L. Y. (2012) The association between skin blood flow and edema on epidermal thickness in the diabetic foot. Diabetes Technol Ther. 14, 602-609. [CrossRef]
- Yilmaz, U., Ayan, A., Uyar, S., Inci, A., Ozer, H., Yilmaz, F. T., Demirtas, G., Kok, M. and Tokuc, A. (2022) Capillaroscopic appearance of nailfold vasculature of diabetic nephropathy patients. Arch Endocrinol Metab. 66, 295-302. [CrossRef]
- Hsu, P. C., Liao, P. Y., Huang, S. W., Chang, H. H., Chiang, J. Y. and Lo, L. C. (2025) Nailfold capillary abnormalities as indicators of diabetic nephropathy progression: a cross-sectional study in type 2 diabetes. Ann Med. 57, 2458766. [CrossRef]
- Haak, E. S., Usadel, K. H., Kohleisen, M., Yilmaz, A., Kusterer, K. and Haak, T. (1999) The effect of alpha-lipoic acid on the neurovascular reflex arc in patients with diabetic neuropathy assessed by capillary microscopy. Microvasc Res. 58, 28-34. [CrossRef]
- Gou, H. and Liu, J. (2025) Non-ocular biomarkers for early diagnosis of diabetic retinopathy by non-invasive methods. Front Endocrinol (Lausanne). 16, 1496851. [CrossRef]
- Mahajan, M., Kaur, T., Singh, K. and Mahajan, B. B. (2024) Evaluation of nail fold capillaroscopy changes in patients with diabetic retinopathy and healthy controls, and its correlation with disease duration, HbA1c levels and severity of diabetic retinopathy: An observational study. Indian J Dermatol Venereol Leprol. 90, 782-788. [CrossRef]
- Okabe, T., Kunikata, H., Yasuda, M., Kodama, S., Maeda, Y., Nakano, J., Takeno, D., Fuse, N. and Nakazawa, T. (2024) Relationship between nailfold capillaroscopy parameters and the severity of diabetic retinopathy. Graefes Arch Clin Exp Ophthalmol. 262, 759-768. [CrossRef]
- Shikama, M., Sonoda, N., Morimoto, A., Suga, S., Tajima, T., Kozawa, J., Maeda, N., Otsuki, M., Matsuoka, T. A., Shimomura, I. and Ohno, Y. (2021) Association of crossing capillaries in the finger nailfold with diabetic retinopathy in type 2 diabetes mellitus. J Diabetes Investig. 12, 1007-1014. [CrossRef]
- Hughes, M. and Herrick, A. L. (2017) Digital ulcers in systemic sclerosis. Rheumatology (Oxford). 56, 14-25. [CrossRef]
- Hughes, M., Allanore, Y., Chung, L., Pauling, J. D., Denton, C. P. and Matucci-Cerinic, M. (2020) Raynaud phenomenon and digital ulcers in systemic sclerosis. Nat Rev Rheumatol. 16, 208-221. [CrossRef]
- Herrick, A. L. (2021) Raynaud's phenomenon and digital ulcers: advances in evaluation and management. Curr Opin Rheumatol. 33, 453-462. [CrossRef]
- Apti Sengun, O., Ergun, T., Guctekin, T. and Alibaz Oner, F. (2023) Endothelial dysfunction, thrombophilia, and nailfold capillaroscopic features in livedoid vasculopathy. Microvasc Res. 150, 104591. [CrossRef]
- Angeloudi, E., Bekiari, E., Pagkopoulou, E., Anyfanti, P., Doumas, M., Garyfallos, A. and Dimitroulas, T. (2022) Study of Peripheral Microcirculation Assessed by Nailfold Video-Capillaroscopy and Association with Markers of Endothelial Dysfunction and Inflammation in Rheumatoid Arthritis. Mediterr J Rheumatol. 33, 375-379. [CrossRef]
- Frödin, T., Bengtsson, A. and Skogh, M. (1988) Nail fold capillaroscopy findings in patients with primary fibromyalgia. Clin Rheumatol. 7, 384-388. [CrossRef]
- Bennett, R. M., Clark, S. R., Campbell, S. M., Ingram, S. B., Burckhardt, C. S., Nelson, D. L. and Porter, J. M. (1991) Symptoms of Raynaud's syndrome in patients with fibromyalgia. A study utilizing the Nielsen test, digital photoplethysmography, and measurements of platelet alpha 2-adrenergic receptors. Arthritis Rheum. 34, 264-269. [CrossRef]
- Scolnik, M., Vasta, B., Hart, D. J., Shipley, J. A., McHugh, N. J. and Pauling, J. D. (2016) Symptoms of Raynaud's phenomenon (RP) in fibromyalgia syndrome are similar to those reported in primary RP despite differences in objective assessment of digital microvascular function and morphology. Rheumatol Int. 36, 1371-1377. [CrossRef]
- Esen, E. and Çetin, A. (2017) Microvascular functions in patients with fibromyalgia syndrome: effects of physical exercise. Turk J Phys Med Rehabil. 63, 215-223. [CrossRef]
- Choi, D. H. and Kim, H. S. (2015) Quantitative analysis of nailfold capillary morphology in patients with fibromyalgia. Korean J Intern Med. 30, 531-537. [CrossRef]
- Appelman, B., Charlton, B. T., Goulding, R. P., Kerkhoff, T. J., Breedveld, E. A., Noort, W., Offringa, C., Bloemers, F. W., van Weeghel, M., Schomakers, B. V., Coelho, P., Posthuma, J. J., Aronica, E., Joost Wiersinga, W., van Vugt, M. and Wüst, R. C. I. (2024) Muscle abnormalities worsen after post-exertional malaise in long COVID. Nat Commun. 15, 17. [CrossRef]
- Coşkun Benlidayı, I., Kayacan Erdoğan, E. and Sarıyıldız, A. (2021) The evaluation of nailfold capillaroscopy pattern in patients with fibromyalgia. Arch Rheumatol. 36, 341-348. [CrossRef]
- Salah, N. Y. (2020) Vascular endothelial growth factor (VEGF), tissue inhibitors of metalloproteinase-1 (TIMP-1) and nail fold capillaroscopy changes in children and adolescents with Gaucher disease; relation to residual disease severity. Cytokine. 133, 155120. [CrossRef]
- Dima, A., Berza, I., Popescu, D. N. and Parvu, M. I. (2021) Nailfold capillaroscopy in systemic diseases: short overview for internal medicine. Rom J Intern Med. 59, 201-217. [CrossRef]
- Herrick, A. L., Berks, M. and Taylor, C. J. (2021) Quantitative nailfold capillaroscopy-update and possible next steps. Rheumatology (Oxford). 60, 2054-2065. [CrossRef]
- Komai, M., Takeno, D., Fujii, C., Nakano, J., Ohsaki, Y. and Shirakawa, H. (2024) Nailfold Capillaroscopy: A Comprehensive Review on Its Usefulness in Both Clinical Diagnosis and Improving Unhealthy Dietary Lifestyles. Nutrients. 16, 1914. [CrossRef]
- Mansueto, N., Rotondo, C., Corrado, A. and Cantatore, F. P. (2021) Nailfold capillaroscopy : a comprehensive review on common findings and clinical usefulness in non-rheumatic disease. J Med Invest. 68, 6-14. [CrossRef]
- Abularrage, C. J., Sidawy, A. N., Aidinian, G., Singh, N., Weiswasser, J. M. and Arora, S. (2005) Evaluation of the microcirculation in vascular disease. J Vasc Surg. 42, 574-581. [CrossRef]
- Cousins, C. C., Chou, J. C., Greenstein, S. H., Brauner, S. C., Shen, L. Q., Turalba, A. V., Houlihan, P., Ritch, R., Wiggs, J. L., Knepper, P. A. and Pasquale, L. R. (2019) Resting nailfold capillary blood flow in primary open-angle glaucoma. Br J Ophthalmol. 103, 203-207. [CrossRef]
- Yüksel, S., Yüksel, E. P. and Meriç, M. (2021) Abnormal nailfold videocapillaroscopic findings in heart failure patients with preserved ejection fraction. Clin Hemorheol Microcirc. 77, 115-121. [CrossRef]
- Pancar, G. S. and Kaynar, T. (2020) Nailfold capillaroscopic changes in patients with chronic viral hepatitis. Microvasc Res. 129, 103970. [CrossRef]
- Mishra, A., Grover, C., Singal, A., Narang, S. and Das, G. K. (2021) Nailfold capillary changes in newly diagnosed hypertensive patients: An observational analytical study. Microvasc Res. 136, 104173. [CrossRef]
- Kubo, S., Todoroki, Y., Nakayamada, S., Nakano, K., Satoh, M., Nawata, A., Satoh, Y., Miyagawa, I., Saito, K., Smith, V., Cutolo, M. and Tanaka, Y. (2019) Significance of nailfold videocapillaroscopy in patients with idiopathic inflammatory myopathies. Rheumatology (Oxford). 58, 120-130. [CrossRef]
- Sambataro, D., Sambataro, G., Libra, A., Vignigni, G., Pino, F., Fagone, E., Fruciano, M., Gili, E., Pignataro, F., Del Papa, N. and Vancheri, C. (2020) Nailfold Videocapillaroscopy is a Useful Tool to Recognize Definite Forms of Systemic Sclerosis and Idiopathic Inflammatory Myositis in Interstitial Lung Disease Patients. Diagnostics (Basel). 10, 253. [CrossRef]
- Mugii, N., Hamaguchi, Y., Horii, M., Fushida, N., Ikeda, T., Oishi, K., Yahata, T., Someya, F. and Matsushita, T. (2023) Longitudinal changes in nailfold videocapillaroscopy findings differ by myositis-specific autoantibody in idiopathic inflammatory myopathy. Rheumatology (Oxford). 62, 1326-1334. [CrossRef]
- Bogojevic, M., Markovic Vlaisavljevic, M., Medjedovic, R., Strujic, E., Pravilovic Lutovac, D. and Pavlov-Dolijanovic, S. (2024) Nailfold Capillaroscopy Changes in Patients with Idiopathic Inflammatory Myopathies. J Clin Med. 13, 5550. [CrossRef]
- Sieiro Santos, C., Tandaipan, J. L., Castillo, D., Codes, H., Martínez-Martínez, L., Magallares, B., Moya, P., Mariscal, A., Park, H. S., Díaz-Torné, C., Fernandez-Sanchez, S. P., Bernardez, J., Corominas, H., Diez Alvarez, E. and Castellvi, I. (2024) Nailfold videocapillaroscopy findings correlate with lung outcomes in idiopathic inflammatory myopathies-related interstitial lung disease. Rheumatology (Oxford), keae669. [CrossRef]
- Gedik, B., Erol, M. K., Bulut, M., Dogan, B., Bozdogan, Y. C., Ekinci, R. and Ayan, A. (2024) Proximal nailfold videocapillaroscopy findings of patients with idiopathic macular telangiectasia type 2. Indian J Ophthalmol. 72, S148-S152. [CrossRef]
- Aggarwal, B., Gandhi, V., Singal, A., Aggarwal, A. and Saha, S. (2024) Nail fold capillaroscopy in leprosy: Unveiling the microvascular changes. Microvasc Res. 155, 104712. [CrossRef]
- Gotelli, E., Campitiello, R., Pizzorni, C., Sammori, S., Aitella, E., Ginaldi, L., De Martinis, M., Carubbi, F., Di Ruscio, E., Cuomo, G., Martinelli, E., Marrone, S., De Angelis, R., Giuggioli, D., Guiducci, S., Ingegnoli, F., Riccieri, V., Sebastiani, M., Sulli, A., Smith, V., Cutolo, M., Study Group on, C. and Microcirculation in Rheumatic Diseases of the Italian Society of Rheumatology (CAPSIR). (2025) Multicentre retrospective detection of nailfold videocapillaroscopy abnormalities in long covid patients. RMD Open. 11. [CrossRef]
- Kell, D. B., Khan, M. A. and Pretorius, E. (2024) Fibrinaloid microclots in Long COVID: assessing the actual evidence properly. Res Pract Thromb Haemost. 8, 102566. [CrossRef]
- Zhao, T., Lin, F. A. and Chen, H. P. (2020) Pattern of Nailfold Capillaroscopy in Patients With Systemic Lupus Erythematosus. Arch Rheumatol. 35, 568-574. [CrossRef]
- Bergkamp, S. C., Schonenberg-Meinema, D., Nassar-Sheikh Rashid, A., Melsens, K., Vanhaecke, A., Boumans, M. J. H., Hissink Muller, P. C. E., Cutolo, M., Kuijpers, T. W., van den Berg, J. M. and Smith, V. (2021) Reliable detection of subtypes of nailfold capillary haemorrhages in childhood-onset systemic lupus erythematosus. Clin Exp Rheumatol. 39, 1126-1131. [CrossRef]
- Schonenberg-Meinema, D., Bergkamp, S. C., Nassar-Sheikh Rashid, A., Gruppen, M. P., Middelkamp-Hup, M. A., Armbrust, W., Dolman, K., Hak, A. E., Hissink Muller, P. C. E., van Onna, M., Swart, J. F., Kuijpers, T. W., Kamphuis, S. S. M., Smith, V. and van den Berg, J. M. (2022) Nailfold capillary scleroderma pattern may be associated with disease damage in childhood-onset systemic lupus erythematosus: important lessons from longitudinal follow-up. Lupus Sci Med. 9, e000572. [CrossRef]
- Makarem, Y. S., Selim, Z. I., Ismail, S., Imam Mekkawy, A., Galal, H. and El Nouby, F. H. (2025) Nailfold capillaroscopy changes in systemic lupus erythematosus patients: Correlation with disease activity and anti-uridin1-ribonucleoprotein antibodies. Reumatol Clin (Engl Ed). 21, 501840. [CrossRef]
- Gasser, P. and Meienberg, O. (1991) Finger microcirculation in classical migraine. A video-microscopic study of nailfold capillaries. Eur Neurol. 31, 168-171. [CrossRef]
- Hegyalijai, T., Meienberg, O., Dubler, B. and Gasser, P. (1997) Cold-induced acral vasospasm in migraine as assessed by nailfold video-microscopy: prevalence and response to migraine prophylaxis. Angiology. 48, 345-349. [CrossRef]
- de Villiers, S., Bester, J., Kell, D. B. and Pretorius, E. (2019) Erythrocyte health and the possible role of amyloidogenic blood clotting in the evolving haemodynamics of female migraine-with-aura pathophysiology: Results from a pilot study. Frontiers Neurol. 10, 1262. [CrossRef]
- Bourquard, A., Pablo-Trinidad, A., Butterworth, I., Sánchez-Ferro, Á., Cerrato, C., Humala, K., Fabra Urdiola, M., Del Rio, C., Valles, B., Tucker-Schwartz, J. M., Lee, E. S., Vakoc, B. J., Padera, T. P., Ledesma-Carbayo, M. J., Chen, Y. B., Hochberg, E. P., Gray, M. L. and Castro-González, C. (2018) Non-invasive detection of severe neutropenia in chemotherapy patients by optical imaging of nailfold microcirculation. Sci Rep. 8, 5301. [CrossRef]
- McKay, G. N., Mohan, N., Butterworth, I., Bourquard, A., Sánchez-Ferro, Á., Castro-González, C. and Durr, N. J. (2020) Visualization of blood cell contrast in nailfold capillaries with high-speed reverse lens mobile phone microscopy. Biomed Opt Express. 11, 2268-2276. [CrossRef]
- Arslan, N. G. and Pancar, G. S. (2021) Nailfold capillaroscopic changes of sleep apnea patients. Microvasc Res. 137, 104177. [CrossRef]
- van Vuuren, M. J., Nell, T. A., Carr, J. A., Kell, D. B. and Pretorius, E. (2021) Iron dysregulation and inflammagens related to oral and gut health are central to the development of Parkinson’s disease. Biomolecules. 11, 30. [CrossRef]
- Ersozlu, E. D., Bakirci, S., Sunu, C., Erturk, Z., Acikgoz, S. B. and Tamer, A. (2022) Use of nailfold video capillaroscopy in polycythemia vera. Arch Rheumatol. 37, 404-410. [CrossRef]
- Pinal-Fernandez, I., Fonollosa-Pla, V. and Selva-O'Callaghan, A. (2016) Improvement of the nailfold capillaroscopy after immunosuppressive treatment in polymyositis. QJM. 109, 205-206. [CrossRef]
- Shenavandeh, S. and Rashidi, F. (2022) Nailfold capillaroscopy changes with disease activity in patients with inflammatory myositis including overlap myositis, pure dermatomyositis, and pure polymyositis. Reumatologia. 60, 42-52. [CrossRef]
- Pacini, G., Schenone, C., Pogna, A., Ferraiolo, A., Ferrero, S., Gustavino, C., Carmisciano, L., Pizzorni, C., Paolino, S., Gotelli, E., Sulli, A., Smith, V. and Cutolo, M. (2022) Full longitudinal nailfold videocapillaroscopy analysis of microvascular changes during normal pregnancy. Microvasc Res. 141, 104343. [CrossRef]
- Rusavy, Z., Pitrova, B., Korecko, V. and Kalis, V. (2015) Changes in capillary diameters in pregnancy-induced hypertension. Hypertens Pregnancy. 34, 307-313. [CrossRef]
- Thevissen, K., Demir, M., Cornette, J. and Gyselaers, W. (2022) Nailfold Video Capillaroscopy in Pregnant Women With and Without Cardiovascular Risk Factors. Front Med (Lausanne). 9, 904373. [CrossRef]
- Thevissen, K. and Gyselaers, W. (2017) Capillaroscopy in pregnancy. Expert Rev Med Devices. 14, 961-967. [CrossRef]
- Graceffa, D., Amorosi, B., Maiani, E., Bonifati, C., Chimenti, M. S., Perricone, R. and Di Carlo, A. (2013) Capillaroscopy in psoriatic and rheumatoid arthritis: a useful tool for differential diagnosis. Arthritis. 2013, 957480. [CrossRef]
- Fink, C., Kilian, S., Bertlich, I., Hoxha, E., Bardehle, F., Enk, A. and Haenssle, H. A. (2018) Evaluation of capillary pathologies by nailfold capillaroscopy in patients with psoriasis vulgaris: study protocol for a prospective, controlled exploratory study. BMJ Open. 8, e021595. [CrossRef]
- Bardehle, F., Sies, K., Enk, A., Rosenberger, A., Fink, C. and Haenssle, H. (2021) Nailfold videocapillaroscopy identifies microvascular pathologies in psoriasis vulgaris: Results of a prospective controlled study. J Dtsch Dermatol Ges. 19, 1736-1744. [CrossRef]
- Lazar, L. T., Guldberg-Moller, J., Lazar, B. T. and Mogensen, M. (2023) Nailfold capillaroscopy as diagnostic test in patients with psoriasis and psoriatic arthritis: A systematic review. Microvasc Res. 147, 104476. [CrossRef]
- Cafaro, G., Bursi, R., Valentini, V., Hansel, K., Perricone, C., Venerito, V., Bistoni, O., Sebastiano, M., Topini, F., Stingeni, L., Gerli, R. and Bartoloni, E. (2024) Combined semiquantitative nail-enthesis complex ultrasonography and capillaroscopy in psoriasis and psoriatic arthritis. Front Immunol. 15, 1505322. [CrossRef]
- Santhosh, P., Riyaz, N., Bagde, P., Binitha, M. P. and Sasidharanpillai, S. (2021) A Cross-Sectional Study of Nailfold Capillary Changes in Psoriasis. Indian Dermatol Online J. 12, 873-878. [CrossRef]
- Sivasankari, M., Arora, S., Vasdev, V. and Mary, E. M. (2021) Nailfold capillaroscopy in psoriasis. Med J Armed Forces India. 77, 75-81. [CrossRef]
- Smits, A. J., Isebia, K., Combee-Duffy, C., van der Wal, S., Nossent, E. J., Boonstra, A., Vonk-Noordegraaf, A., Bogaard, H. J. and Serné, E. H. (2024) Low nailfold capillary density in patients with pulmonary arterial hypertension and chronic thromboembolic pulmonary hypertension: biomarker of clinical outcome? Sci Rep. 14, 19467. [CrossRef]
- Sugimoto, T., Dohi, Y., Yoshida, Y., Mokuda, S. and Hirata, S. (2023) Ameliorated nailfold capillary morphology of patients with pulmonary arterial hypertension in systemic sclerosis, treated with riociguat. Rheumatol Adv Pract. 7, rkad011. [CrossRef]
- Brunner-Ziegler, S., Dassler, E., Muller, M., Pratscher, M., Forstner, N. F. M., Koppensteiner, R., Schlager, O. and Jilma, B. (2024) Capillaroscopic differences between primary Raynaud phenomenon and healthy controls indicate potential microangiopathic involvement in benign vasospasms. Vasc Med. 29, 200-207. [CrossRef]
- Kapten, K., Orczyk, K. and Smolewska, E. (2021) The effect of vitamin D3 and thyroid hormones on the capillaroscopy-confirmed microangiopathy in pediatric patients with a suspicion of systemic connective tissue disease-a single-center experience with Raynaud phenomenon. Rheumatol Int. 41, 1485-1493. [CrossRef]
- Koenig, M., Joyal, F., Fritzler, M. J., Roussin, A., Abrahamowicz, M., Boire, G., Goulet, J. R., Rich, E., Grodzicky, T., Raymond, Y. and Senecal, J. L. (2008) Autoantibodies and microvascular damage are independent predictive factors for the progression of Raynaud's phenomenon to systemic sclerosis: a twenty-year prospective study of 586 patients, with validation of proposed criteria for early systemic sclerosis. Arthritis Rheum. 58, 3902-3912. [CrossRef]
- Smith, V., Vanhaecke, A., Herrick, A. L., Distler, O., Guerra, M. G., Denton, C. P., Deschepper, E., Foeldvari, I., Gutierrez, M., Hachulla, E., Ingegnoli, F., Kubo, S., Muller-Ladner, U., Riccieri, V., Sulli, A., van Laar, J. M., Vonk, M. C., Walker, U. A., Cutolo, M. and EULAR Study Group on Microcirculation in Rheumatic Diseases. (2019) Fast track algorithm: How to differentiate a "scleroderma pattern" from a "non-scleroderma pattern". Autoimmun Rev. 18, 102394. [CrossRef]
- Nawaz, I., Nawaz, Y., Nawaz, E., Manan, M. R. and Mahmood, A. (2022) Raynaud's Phenomenon: Reviewing the Pathophysiology and Management Strategies. Cureus. 14, e21681. [CrossRef]
- Roberts-Thomson, P. J., Patterson, K. A. and Walker, J. G. (2023) Clinical utility of nailfold capillaroscopy. Intern Med J. 53, 671-679. [CrossRef]
- Amaral, M. C., Paula, F. S., Caetano, J., Ames, P. R. and Alves, J. D. (2024) Re-evaluation of nailfold capillaroscopy in discriminating primary from secondary Raynaud's phenomenon and in predicting systemic sclerosis: a randomised observational prospective cohort study. Expert Rev Clin Immunol. 20, 665-672. [CrossRef]
- Wu, P. C., Huang, M. N., Kuo, Y. M., Hsieh, S. C. and Yu, C. L. (2013) Clinical applicability of quantitative nailfold capillaroscopy in differential diagnosis of connective tissue diseases with Raynaud's phenomenon. J Formos Med Assoc. 112, 482-488. [CrossRef]
- Herrick, A. L. and Murray, A. (2018) The role of capillaroscopy and thermography in the assessment and management of Raynaud's phenomenon. Autoimmun Rev. 17, 465-472. [CrossRef]
- Herrick, A. L. and Wigley, F. M. (2020) Raynaud's phenomenon. Best Pract Res Clin Rheumatol. 34, 101474. [CrossRef]
- Herrick, A. L., Dinsdale, G. and Murray, A. (2020) New perspectives in the imaging of Raynaud's phenomenon. Eur J Rheumatol. 7, S212-S221. [CrossRef]
- van Roon, A. M., Smit, A. J., van Roon, A. M., Bootsma, H. and Mulder, D. J. (2016) Digital ischaemia during cooling is independently related to nailfold capillaroscopic pattern in patients with Raynaud's phenomenon. Rheumatology (Oxford). 55, 1083-1090. [CrossRef]
- do Rosário e Souza, E. J. and Kayser, C. (2015) Nailfold capillaroscopy: relevance to the practice of rheumatology. Rev Bras Reumatol. 55, 264-271. [CrossRef]
- Chojnowski, M. M., Felis-Giemza, A. and Olesińska, M. (2016) Capillaroscopy – a role in modern rheumatology. Reumatologia. 54, 67-72. [CrossRef]
- Anyfanti, P., Angeloudi, E., Dara, A., Arvanitaki, A., Bekiari, E., Kitas, G. D. and Dimitroulas, T. (2022) Nailfold Videocapillaroscopy for the Evaluation of Peripheral Microangiopathy in Rheumatoid Arthritis. Life (Basel). 12, 1167. [CrossRef]
- Eden, M., Wilkinson, S., Murray, A., Bharathi, P. G., Vail, A., Taylor, C. J., Payne, K. and Herrick, A. L. (2022) Nailfold capillaroscopy: a survey of current UK practice and 'next steps' to increase uptake among rheumatologists. Rheumatology (Oxford). 62, 335-340. [CrossRef]
- Ingegnoli, F., Cornalba, M., De Angelis, R., Guiducci, S., Giuggioli, D., Pizzorni, C., Riccieri, V., Sebastiani, M., Sulli, A. and Cutolo, M. (2022) Nailfold capillaroscopy in the rheumatological current clinical practice in Italy: results of a national survey. Reumatismo. 74, 97-102. [CrossRef]
- Angeloudi, E., Anyfanti, P., Dara, A., Pagkopoulou, E., Bekiari, E., Sgouropoulou, V., Garyfallos, A., Doumas, M., Kitas, G. D. and Dimitroulas, T. (2023) Peripheral nailfold capillary microscopic abnormalities in rheumatoid arthritis are associated with arterial stiffness: Results from a cross-sectional study. Microvasc Res. 150, 104576. [CrossRef]
- Anghel, D., Sirbu, C. A., Petrache, O. G., Opris-Belinski, D., Negru, M. M., Bojinca, V. C., Plesa, C. F. and Ionita Radu, F. (2023) Nailfold Videocapillaroscopy in Patients with Rheumatoid Arthritis and Psoriatic Arthropathy on ANTI-TNF-ALPHA Therapy. Diagnostics (Basel). 13, 2079. [CrossRef]
- Schonenberg-Meinema, D. Schonenberg-Meinema, D., Cutolo, M. and Smith, V. (2024) Capillaroscopy in the daily clinic of the pediatric rheumatologist. Best Pract Res Clin Rheumatol. 38, 101978. [CrossRef]
- Anghel, D., Prioteasă, O. G., Nicolau, I. N., Bucurică, S., Belinski, D. O., Popescu, G. G., Ghinescu, M. C., Bobircă, A., Groşeanu, M. L. and Bojincă, V. C. (2025) The Role of Nailfold Videocapillaroscopy in the Diagnosis and Monitoring of Interstitial Lung Disease Associated with Rheumatic Autoimmune Diseases. Diagnostics (Basel). 15, 362. [CrossRef]
- Bezuidenhout, J., Venter, C., Roberts, T., Tarr, G., Kell, D. and Pretorius, E. (2020) The Atypical Fibrin Fibre Network in Rheumatoid Arthritis and its Relation to Autoimmunity, Inflammation and Thrombosis. bioRxiv, 2020.2005.2028.121301v121301. [CrossRef]
- Pretorius, E., Oberholzer, H. M., van der Spuy, W. J., Swanepoel, A. C. and Soma, P. (2012) Scanning electron microscopy of fibrin networks in rheumatoid arthritis: a qualitative analysis. Rheumatol Int. 32, 1611-1615. [CrossRef]
- Acemoğlu, Ş. Ş. Z., Türk, I., Aşık, M. A., Bircan, A. Ö., Deniz, P. P., Arslan, D., Hanta, I. and Ünal, I. (2023) Microvascular damage evaluation based on nailfold videocapillarosopy in sarcoidosis. Clin Rheumatol. 42, 1951-1957. [CrossRef]
- Cattelan, F., Hysa, E., Gotelli, E., Pizzorni, C., Bica, P. F., Grosso, M., Barisione, E., Paolino, S., Carmisciano, L., Sulli, A., Smith, V. and Cutolo, M. (2022) Microvascular capillaroscopic abnormalities and occurrence of antinuclear autoantibodies in patients with sarcoidosis. Rheumatol Int. 42, 2199-2210. [CrossRef]
- Chianese, M., Screm, G., Confalonieri, P., Salton, F., Trotta, L., Da Re, B., Romallo, A., Galantino, A., D'Oria, M., Hughes, M., Bandini, G., Confalonieri, M., Baratella, E., Mondini, L. and Ruaro, B. (2024) Nailfold Video-Capillaroscopy in Sarcoidosis: New Perspectives and Challenges. Tomography. 10, 1547-1563. [CrossRef]
- Paolino, S., Goegan, F., Cimmino, M. A., Casabella, A., Pizzorni, C., Patane, M., Schenone, C., Tomatis, V., Sulli, A., Gotelli, E., Smith, V. and Cutolo, M. (2020) Advanced microvascular damage associated with occurence of sarcopenia in systemic sclerosis patients: results from a retrospective cohort study. Clin Exp Rheumatol. 38 Suppl 125, 65-72.
- Zhang, L., Mao, D. and Zhang, Q. (2021) Correlation between sarcopenia and nailfold microcirculation, serum 25-hydroxycholecalciferol (vitamin D3) and IL-17 levels in female patients with rheumatoid arthritis. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 165, 264-269. [CrossRef]
- Miranda, M., Balarini, M., Caixeta, D. and Bouskela, E. (2016) Microcirculatory dysfunction in sepsis: pathophysiology, clinical monitoring, and potential therapies. Am J Physiol Heart Circ Physiol. 311, H24-35. [CrossRef]
- Kell, D. B. and Pretorius, E. (2018) To what extent are the terminal stages of sepsis, septic shock, SIRS, and multiple organ dysfunction syndrome actually driven by a toxic prion/amyloid form of fibrin? Semin Thromb Hemost. 44, 224-238. [CrossRef]
- De Backer, D., Creteur, J., Preiser, J. C., Dubois, M. J. and Vincent, J. L. (2002) Microvascular blood flow is altered in patients with sepsis. Am J Respir Crit Care Med. 166, 98-104. [CrossRef]
- Sapozhnikov, M., Rehman, M., Johnson, C., Daich, J., Salciccioli, L., Gillette, P. and Lazar, J. M. (2019) Characterization of microvascular disease in patients with sickle cell disease using nailfold capillaroscopy. Microvasc Res. 125, 103877. [CrossRef]
- Melsens, K., Leone, M. C., Paolino, S., Elewaut, D., Gerli, R., Vanhaecke, A., Peene, I., Cutolo, M. and Smith, V. (2020) Nailfold capillaroscopy in Sjögren's syndrome: a systematic literature review and standardised interpretation. Clin Exp Rheumatol. 38 Suppl 126, 150-157.
- Lercara, A., Malattia, C., Hysa, E., Gattorno, M., Cere, A., Lavarello, C., Vojinovic, T., Gotelli, E., Paolino, S., Sulli, A., Pizzorni, C., Smith, V. and Cutolo, M. (2024) Microvascular status in juvenile Sjögren's disease: the first nailfold videocapillaroscopy investigation. Clin Rheumatol. 43, 733-741. [CrossRef]
- Soulaidopoulos, S., Triantafyllidou, E., Garyfallos, A., Kitas, G. D. and Dimitroulas, T. (2017) The role of nailfold capillaroscopy in the assessment of internal organ involvement in systemic sclerosis: A critical review. Autoimmun Rev. 16, 787-795. [CrossRef]
- Paxton, D. and Pauling, J. D. (2018) Does nailfold capillaroscopy help predict future outcomes in systemic sclerosis? A systematic literature review. Semin Arthritis Rheum. 48, 482-494. [CrossRef]
- Nikolova Lambova, S. and Müller-Ladner, U. (2019) Nailfold capillaroscopy in systemic sclerosis – state of the art: The evolving knowledge about capillaroscopic abnormalities in systemic sclerosis. J Scleroderm Rel Dis. 4, 200-211. [CrossRef]
- Smith, V., Vanhaecke, A., Vandecasteele, E., Guerra, M., Paolino, S., Melsens, K. and Cutolo, M. (2020) Nailfold Videocapillaroscopy in Systemic Sclerosis-related Pulmonary Arterial Hypertension: A Systematic Literature Review. J Rheumatol. 47, 888-895. [CrossRef]
- Paolino, S., Gotelli, E., Goegan, F., Casabella, A., Ferrari, G., Patane, M., Albertelli, M., Gatto, F., Pizzorni, C., Cattelan, F., Sulli, A., Smith, V. and Cutolo, M. (2021) Body composition and bone status in relation to microvascular damage in systemic sclerosis patients. J Endocrinol Invest. 44, 255-264. [CrossRef]
- van Leeuwen, N. M., Ciaffi, J., Schoones, J. W., Huizinga, T. W. J. and de Vries-Bouwstra, J. K. (2021) Contribution of Sex and Autoantibodies to Microangiopathy Assessed by Nailfold Videocapillaroscopy in Systemic Sclerosis: A Systematic Review of the Literature. Arthritis Care Res (Hoboken). 73, 722-731. [CrossRef]
- Minopoulou, I., Theodorakopoulou, M., Boutou, A., Arvanitaki, A., Pitsiou, G., Doumas, M., Sarafidis, P. and Dimitroulas, T. (2021) Nailfold Capillaroscopy in Systemic Sclerosis Patients with and without Pulmonary Arterial Hypertension: A Systematic Review and Meta-Analysis. J Clin Med. 10, 1528. [CrossRef]
- Mandujano, A. and Golubov, M. (2022) Animal Models of Systemic Sclerosis: Using Nailfold Capillaroscopy as a Potential Tool to Evaluate Microcirculation and Microangiopathy: A Narrative Review. Life (Basel). 12, 703. [CrossRef]
- Hysa, E., Campitiello, R., Sammori, S., Gotelli, E., Cere, A., Pesce, G., Pizzorni, C., Paolino, S., Sulli, A., Smith, V. and Cutolo, M. (2023) Specific Autoantibodies and Microvascular Damage Progression Assessed by Nailfold Videocapillaroscopy in Systemic Sclerosis: Are There Peculiar Associations? An Update. Antibodies (Basel). 12, 3. [CrossRef]
- Ma, Z., Mulder, D. J., Gniadecki, R., Cohen Tervaert, J. W. and Osman, M. (2023) Methods of Assessing Nailfold Capillaroscopy Compared to Video Capillaroscopy in Patients with Systemic Sclerosis-A Critical Review of the Literature. Diagnostics (Basel). 13, 2204. [CrossRef]
- De Angelis, R., Riccieri, V., Cipolletta, E., Del Papa, N., Ingegnoli, F., Bosello, S., Spinella, A., Pellegrino, G., de Pinto, M., Papa, S., Armentaro, G. and Giuggioli, D. (2024) Significant nailfold capillary loss and late capillaroscopic pattern are associated with pulmonary arterial hypertension in systemic sclerosis. Rheumatology (Oxford). 63, 1616-1623. [CrossRef]
- Zanatta, E., Famoso, G., Boscain, F., Montisci, R., Pigatto, E., Polito, P., Schiavon, F., Iliceto, S., Cozzi, F., Doria, A. and Tona, F. (2019) Nailfold avascular score and coronary microvascular dysfunction in systemic sclerosis: A newsworthy association. Autoimmun Rev. 18, 177-183. [CrossRef]
- Lambova, S. N. and Muller-Ladner, U. (2019) Nailfold capillaroscopy in systemic sclerosis - state of the art: The evolving knowledge about capillaroscopic abnormalities in systemic sclerosis. J Scleroderma Relat Disord. 4, 200-211. [CrossRef]
- Hammoda, R. M., El-Gharbawy, N. H., Khalifa, A. A., Moharram, A. A. and Elziaty, R. A. (2024) Neutrophil-to-lymphocyte ratio: association with microcirculatory changes detected by nailfold capillaroscopy in scleroderma patients and its relation to disease severity. Eqyptian Rheumatol Rehab. 52, 4. [CrossRef]
- Vanhaecke, A., Cutolo, M., Distler, O., Riccieri, V., Allanore, Y., Denton, C. P., Hachulla, E., Ingegnoli, F., Deschepper, E., Avouac, J., Jordan, S., Launay, D., Melsens, K., Pizzorni, C., Sulli, A., Vasile, M., Herrick, A. L., Smith, V., in, E. S. G. o. M. and Rheumatic Diseases. (2022) Nailfold capillaroscopy in SSc: innocent bystander or promising biomarker for novel severe organ involvement/progression? Rheumatology (Oxford). 61, 4384-4396. [CrossRef]
- Smith, V., Vanhaecke, A., Guerra, M. G., Melsens, K., Vandecasteele, E., Paolino, S. and Cutolo, M. (2020) May capillaroscopy be a candidate tool in future algorithms for SSC-ILD: Are we looking for the holy grail? A systematic review. Autoimmun Rev. 19, 102619. [CrossRef]
- Andracco, R., Irace, R., Zaccara, E., Vettori, S., Maglione, W., Riccardi, A., Pignataro, F., Ferrara, R., Sambataro, D., Sambataro, G., Vitali, C., Valentini, G. and Del Papa, N. (2017) The cumulative number of micro-haemorrhages and micro-thromboses in nailfold videocapillaroscopy is a good indicator of disease activity in systemic sclerosis: a validation study of the NEMO score. Arthritis Res Ther. 19, 133. [CrossRef]
- Pignataro, F., Maglione, W., Minniti, A., Sambataro, D., Sambataro, G., Campanaro, F., Valentini, G., Vitali, C. and Del Papa, N. (2019) NEMO score in nailfold videocapillaroscopy is a good tool to assess both steady state levels and overtime changes of disease activity in patients with systemic sclerosis: a comparison with the proposed composite indices for this disease status entity. Arthritis Res Ther. 21, 258. [CrossRef]
- Del Papa, N., Pignataro, F., Maglione, W., Minniti, A., Sambataro, D., Sambataro, G., Valentini, G., Caporali, R. and Vitali, C. (2020) High NEMO score values in nailfold videocapillaroscopy are associated with the subsequent development of ischaemic digital ulcers in patients with systemic sclerosis. Arthritis Res Ther. 22, 237. [CrossRef]
- Crescenzi, D., Balducci, D., Mazzetti, M., Menghini, D., Gelardi, C., Pedini, V., Mezzanotte, C., Tarantino, G., Benedetti, A., Danieli, M. G., Marzioni, M. and Maroni, L. (2025) Use of nailfold capillaroscopy for the early diagnosis of systemic sclerosis in patients with primary biliary cholangitis. Ann Gastroenterol. 38, 187-194. [CrossRef]
- Riccieri, V., Vasile, M., Iannace, N., Stefanantoni, K., Sciarra, I., Vizza, C. D., Badagliacca, R., Poscia, R., Papa, S., Mezzapesa, M., Nocioni, M. and Valesini, G. (2013) Systemic sclerosis patients with and without pulmonary arterial hypertension: a nailfold capillaroscopy study. Rheumatology (Oxford). 52, 1525-1528. [CrossRef]
- Lim, M. W. S., Setjiadi, D., Dobbin, S. J. H., Lang, N. N., Delles, C. and Connelly, P. J. (2023) Nailfold video-capillaroscopy in the study of cardiovascular disease: a systematic review. Blood Press Monit. 28, 24-32. [CrossRef]
- Callard, F. and Perego, E. (2021) How and why patients made Long Covid. Soc Sci Med. 268, 113426. [CrossRef]
- Al-Aly, Z. and Topol, E. (2024) Solving the puzzle of Long Covid. Science. 383, 830-832. [CrossRef]
- Al-Aly, Z., Davis, H., McCorkell, L., Soares, L., Wulf-Hanson, S., Iwasaki, A. and Topol, E. J. (2024) Long COVID science, research and policy. Nat Med. 30, 2148-2164. [CrossRef]
- Aiyegbusi, O. L., Hughes, S. E., Turner, G., Rivera, S. C., McMullan, C., Chandan, J. S., Haroon, S., Price, G., Davies, E. H., Nirantharakumar, K., Sapey, E., Calvert, M. J. and T. L. C. Study Group. (2021) Symptoms, complications and management of long COVID: a review. J R Soc Med. 114, 428-442. [CrossRef]
- Al-Aly, Z., Xie, Y. and Bowe, B. (2021) High-dimensional characterization of post-acute sequelae of COVID-19. Nature. 594, 259-264. [CrossRef]
- Hayes, L. D., Ingram, J. and Sculthorpe, N. F. (2021) More Than 100 Persistent Symptoms of SARS-CoV-2 (Long COVID): A Scoping Review. Front Med (Lausanne). 8, 750378. [CrossRef]
- Davis, H. E., McCorkell, L., Vogel, J. M. and Topol, E. J. (2023) Long COVID: major findings, mechanisms and recommendations. Nat Rev Microbiol. 21, 133-146. [CrossRef]
- Nalbandian, A., Desai, A. D. and Wan, E. Y. (2023) Post-COVID-19 Condition. Annu Rev Med. 74, 55-64. [CrossRef]
- Greenhalgh, T., Sivan, M., Perlowski, A. and Nikolich, J. Ž. (2024) Long COVID: a clinical update. Lancet. 404, 707-724. [CrossRef]
- Komaroff, A. L. and Lipkin, W. I. (2023) ME/CFS and Long COVID share similar symptoms and biological abnormalities: road map to the literature. Front Med. 10, 1187163. [CrossRef]
- Poole-Wright, K., Guennouni, I., Sterry, O., Evans, R. A., Gaughran, F. and Chalder, T. (2023) Fatigue outcomes following COVID-19: a systematic review and meta-analysis. BMJ Open. 13, e063969. [CrossRef]
- Chang, J. C. (2019) Sepsis and septic shock: endothelial molecular pathogenesis associated with vascular microthrombotic disease. Thromb J. 17, 10. [CrossRef]
- Guven, G., Hilty, M. P. and Ince, C. (2020) Microcirculation: Physiology, Pathophysiology, and Clinical Application. Blood Purif. 49, 143-150. [CrossRef]
- Wei, J. X., Jiang, H. L. and Chen, X. H. (2023) Endothelial cell metabolism in sepsis. World J Emerg Med. 14, 10-16. [CrossRef]
- Cusack, R., O'Neill, S. and Martin-Loeches, I. (2022) Effects of Fluids on the Sublingual Microcirculation in Sepsis. J Clin Med. 11, 7277. [CrossRef]
- Ince, C. (2005) The microcirculation is the motor of sepsis. Crit Care. 9 Suppl 4, S13-19. [CrossRef]
- Lipińska-Gediga, M. (2016) Sepsis and septic shock-is a microcirculation a main player? Anaesthesiol Intensive Ther. 48, 261-265. [CrossRef]
- De Backer, D., Ricottilli, F. and Ospina-Tascón, G. A. (2021) Septic shock: a microcirculation disease. Curr Opin Anaesthesiol. 34, 85-91. [CrossRef]
- De Backer, D. (2023) Novelties in the evaluation of microcirculation in septic shock. J Intensive Med. 3, 124-130. [CrossRef]
- Guo, Q., Liu, D., Wang, X. and Chinese Critical Ultrasound Study Group. (2024) Early peripheral perfusion monitoring in septic shock. Eur J Med Res. 29, 477. [CrossRef]
- Wang, H., Ding, H., Wang, Z. Y. and Zhang, K. (2024) Research progress on microcirculatory disorders in septic shock: A narrative review. Medicine (Baltimore). 103, e37273. [CrossRef]
- Chua, M. T. and Kuan, W. S. (2016) Venous-to-arterial carbon dioxide differences and the microcirculation in sepsis. Ann Transl Med. 4, 62. [CrossRef]
- Colbert, J. F. and Schmidt, E. P. (2016) Endothelial and Microcirculatory Function and Dysfunction in Sepsis. Clin Chest Med. 37, 263-275. [CrossRef]
- Charlton, M., Sims, M., Coats, T. and Thompson, J. P. (2017) The microcirculation and its measurement in sepsis. J Intensive Care Soc. 18, 221-227. [CrossRef]
- Duranteau, J., De Backer, D., Donadello, K., Shapiro, N. I., Hutchings, S. D., Rovas, A., Legrand, M., Harrois, A. and Ince, C. (2023) The future of intensive care: the study of the microcirculation will help to guide our therapies. Crit Care. 27, 190. [CrossRef]
- Hawiger, J., Veach, R. A. and Zienkiewicz, J. (2015) New paradigms in sepsis: from prevention to protection of failing microcirculation. J Thromb Haemost. 13, 1743-1756. [CrossRef]
- Opal, S. M. and van der Poll, T. (2015) Endothelial barrier dysfunction in septic shock. J Intern Med. 277, 277-293. [CrossRef]
- Potter, E. K., Hodgson, L., Creagh-Brown, B. and Forni, L. G. (2019) Manipulating the Microcirculation in Sepsis - the Impact of Vasoactive Medications on Microcirculatory Blood Flow: A Systematic Review. Shock. 52, 5-12. [CrossRef]
- Colantuoni, A., Martini, R., Caprari, P., Ballestri, M., Capecchi, P. L., Gnasso, A., Lo Presti, R., Marcoccia, A., Rossi, M. and Caimi, G. (2020) COVID-19 Sepsis and Microcirculation Dysfunction. Front Physiol. 11, 747. [CrossRef]
- Tang, A. L., Shen, M. J. and Zhang, G. Q. (2022) Intestinal microcirculation dysfunction in sepsis: pathophysiology, clinical monitoring, and therapeutic interventions. World J Emerg Med. 13, 343-348. [CrossRef]
- Yajnik, V. and Maarouf, R. (2022) Sepsis and the microcirculation: the impact on outcomes. Curr Opin Anaesthesiol. 35, 230-235. [CrossRef]
- Vallet, B. (2003) Bench-to-bedside review: endothelial cell dysfunction in severe sepsis: a role in organ dysfunction? Crit Care. 7, 130-138. [CrossRef]
- Xing, K., Murthy, S., Liles, W. C. and Singh, J. M. (2012) Clinical utility of biomarkers of endothelial activation in sepsis--a systematic review. Crit Care. 16, R7. [CrossRef]
- Spronk, P. E., Zandstra, D. F. and Ince, C. (2004) Bench-to-bedside review: sepsis is a disease of the microcirculation. Crit Care. 8, 462-468. [CrossRef]
- Joffre, J. and Hellman, J. (2021) Oxidative Stress and Endothelial Dysfunction in Sepsis and Acute Inflammation. Antioxid Redox Signal. 35, 1291-1307. [CrossRef]
- Joffre, J., Hellman, J., Ince, C. and Ait-Oufella, H. (2020) Endothelial Responses in Sepsis. Am J Respir Crit Care Med. 202, 361-370. [CrossRef]
- Dolmatova, E. V., Wang, K., Mandavilli, R. and Griendling, K. K. (2021) The effects of sepsis on endothelium and clinical implications. Cardiovasc Res. 117, 60-73. [CrossRef]
- Damiani, E., Carsetti, A., Casarotta, E., Domizi, R., Scorcella, C., Donati, A. and Adrario, E. (2023) Microcirculation-guided resuscitation in sepsis: the next frontier? Front Med (Lausanne). 10, 1212321. [CrossRef]
- Dilken, O., Ergin, B. and Ince, C. (2020) Assessment of sublingual microcirculation in critically ill patients: consensus and debate. Ann Transl Med. 8, 793. [CrossRef]
- Tang, A., Shi, Y., Dong, Q., Wang, S., Ge, Y., Wang, C., Gong, Z., Zhang, W. and Chen, W. (2024) Prognostic Value of Sublingual Microcirculation in Sepsis: A Systematic Review and Meta-analysis. J Intensive Care Med. 39, 1221-1230. [CrossRef]
- Ahmed, S., Zimba, O. and Gasparyan, A. Y. (2020) Thrombosis in Coronavirus disease 2019 (COVID-19) through the prism of Virchow's triad. Clin Rheumatol. 39, 2529-2543. [CrossRef]
- Wolberg, A. S., Aleman, M. M., Leiderman, K. and Machlus, K. R. (2012) Procoagulant activity in hemostasis and thrombosis: Virchow's triad revisited. Anesth Analg. 114, 275-285. [CrossRef]
- Luo, X., Xie, J., Huang, L., Gan, W. and Chen, M. (2023) Efficacy and safety of activating blood circulation and removing blood stasis of Traditional Chinese Medicine for managing renal fibrosis in patients with chronic kidney disease: a systematic review and Meta-analysis. J Tradit Chin Med. 43, 429-440. [CrossRef]
- Chen, K. J. (2012) Blood stasis syndrome and its treatment with activating blood circulation to remove blood stasis therapy. Chin J Integr Med. 18, 891-896. [CrossRef]
- Liao, J., Wang, J., Liu, Y., Li, J., Duan, L., Chen, G. and Hu, J. (2016) Modern researches on Blood Stasis syndrome 1989-2015: A bibliometric analysis. Medicine (Baltimore). 95, e5533. [CrossRef]
- Yan, J., Dong, Y., Niu, L., Cai, J., Jiang, L., Wang, C. and Xing, J. (2021) Clinical effect of Chinese herbal medicine for removing blood stasis combined with acupuncture on sequelae of cerebral infarction. Am J Transl Res. 13, 10843-10849.
- Rosenthal, L., Hernandez, P. and Vaamonde, D. (2022) Traditional Chinese medicine, Ayurveda, and fertility Fertility, Pregnancy, and Wellness, 209-247. [CrossRef]
- Birch, S., Alraek, T., Lee, M. S., Lee, J. A. and Kim, T.-H. (2021) Understanding blood stasis in traditional East Asian medicine: a comparison of Asian and Western sources. Eur J Integr Med. 44, 101341. [CrossRef]
- Zhai, X., Wang, X., Wang, L., Xiu, L., Wang, W. and Pang, X. (2020) Treating Different Diseases With the Same Method-A Traditional Chinese Medicine Concept Analyzed for Its Biological Basis. Front Pharmacol. 11, 946. [CrossRef]
- Yan, D.-X. (2015) Aging and blood stasis: a new TCM approach to geriatrics. Blue Poppy Press, Boulder, CO.
- Ishida, H., Takamatsu, M., Tsuji, K. and Kosuge, T. (1987) Studies on active substances in herbs used for oketsu ("stagnant blood") in Chinese medicine. V. On the anticoagulative principle in moutan cortex. Chem Pharm Bull (Tokyo). 35, 846-848. [CrossRef]
- Matsumoto, C., Kojima, T., Ogawa, K., Kamegai, S., Oyama, T., Shibagaki, Y., Kawasaki, T., Fujinaga, H., Takahashi, K., Hikiami, H., Goto, H., Kiga, C., Koizumi, K., Sakurai, H., Muramoto, H., Shimada, Y., Yamamoto, M., Terasawa, K., Takeda, S. and Saiki, I. (2008) A Proteomic Approach for the Diagnosis of 'Oketsu' (blood stasis), a Pathophysiologic Concept of Japanese Traditional (Kampo) Medicine. Evid Based Complement Alternat Med. 5, 463-474. [CrossRef]
- Morita, A., Murakami, A., Watanabe, Y., Tamura, Y., Suganami, A., Shiko, Y., Kawasaki, Y., Nakaguchi, T., Ochi, S., Okudaira, K., Hirasaki, Y. and Namik, T. (2020) The association in Kampo medicine between Oketsu (blood stasis) and sublingual vein width of the tongue on a tongue image analyzing system. Tradit Kampo Med. 7, 108-112. [CrossRef]
- Morita, A., Murakami, A., Noguchi, K., Watanabe, Y., Nakaguchi, T., Ochi, S., Okudaira, K., Hirasaki, Y. and Namiki, T. (2021) Combination Image Analysis of Tongue Color and Sublingual Vein Improves the Diagnostic Accuracy of Oketsu (Blood Stasis) in Kampo Medicine. Front Med (Lausanne). 8, 790542. [CrossRef]
- Park, B., You, S., Jung, J., Lee, J. A., Yun, K. J. and Lee, M. S. (2015) Korean studies on blood stasis: an overview. Evid Based Complement Alternat Med. 2015, 316872. [CrossRef]
- Yi, M., Li, Q., Zhao, Y., Nie, S., Wu, N. and Wang, D. (2019) Metabolomics study on the therapeutic effect of traditional Chinese medicine Xue-Fu-Zhu-Yu decoction in coronary heart disease based on LC-Q-TOF/MS and GC-MS analysis. Drug Metab Pharmacokinet. 34, 340-349. [CrossRef]
- Zhao, Y., Nie, S., Yi, M., Wu, N., Wang, W., Zhang, Z., Yao, Y. and Wang, D. (2019) UPLC-QTOF/MS-based metabolomics analysis of plasma reveals an effect of Xue-Fu-Zhu-Yu capsules on blood-stasis syndrome in CHD rats. J Ethnopharmacol. 241, 111908. [CrossRef]
- He, H., Chen, G., Gao, J., Liu, Y., Zhang, C., Liu, C., Li, H., He, Q., Li, J. and Wang, J. (2018) Xue-Fu-Zhu-Yu capsule in the treatment of qi stagnation and blood stasis syndrome: a study protocol for a randomised controlled pilot and feasibility trial. Trials. 19, 515. [CrossRef]
- Chen, S., Wu, X., Li, T., Cheng, W., Han, X., Li, Y., Wang, B., Teng, Y., Zhao, M. and Wang, Y. (2022) The Improvement of Cardiac and Endothelial Functions of Xue-Fu-Zhu-Yu Decoction for Patients with Acute Coronary Syndrome: A Meta-Analysis of Randomized Controlled Trials. Evid Based Complement Alternat Med. 2022, 2671343. [CrossRef]
- Xue, D. J., Zhen, Z., Wang, K. X., Zhao, J. L., Gao, Y., Chen, Y. P., Shen, Y. B., Peng, Z. Z., Guan, D. G. and Huang, T. (2022) Uncovering the potential mechanism of Xue Fu Zhu Yu Decoction in the treatment of intracerebral hemorrhage. BMC Complement Med Ther. 22, 103. [CrossRef]
- Kuo, C. E., Hsu, S. F., Chen, C. C., Wu, S. Y., Hung, Y. C., Hsu, C. Y., Tsai, I. J. and Hu, W. L. (2023) Prescription characteristics of Xue-Fu-Zhu-Yu-Tang in pain management: a population-based study using the National Health Insurance Research Database in Taiwan. Front Pharmacol. 14, 1233156. [CrossRef]
- Hikiami, H., Goto, H., Sekiya, N., Hattori, N., Sakakibara, I., Shimada, Y. and Terasawa, K. (2003) Comparative efficacy of Keishi-bukuryo-gan and pentoxifylline on RBC deformability in patients with "oketsu" syndrome. Phytomedicine. 10, 459-466. [CrossRef]
- Tomita, T., Hirayama, A., Matsui, H. and Aoyagi, K. (2017) Effect of Keishibukuryogan, a Japanese Traditional Kampo Prescription, on Improvement of Microcirculation and Oketsu and Induction of Endothelial Nitric Oxide: A Live Imaging Study. Evid Based Complement Alternat Med. 2017, 3620130. [CrossRef]
- Brzezińska, O. E., Rychlicki-Kicior, K. A. and Makowska, J. S. (2024) Automatic assessment of nailfold capillaroscopy software: a pilot study. Reumatologia. 62, 346-350. [CrossRef]
- Karbalaie, A., Etehadtavakol, M., Abtahi, F., Fatemi, A., Emrani, Z. and Erlandsson, B. E. (2018) Image enhancement effect on inter and intra-observer reliability of nailfold capillary assessment. Microvasc Res. 120, 100-110. [CrossRef]
- Dinsdale, G., Moore, T., O'Leary, N., Tresadern, P., Berks, M., Roberts, C., Manning, J., Allen, J., Anderson, M., Cutolo, M., Hesselstrand, R., Howell, K., Pizzorni, C., Smith, V., Sulli, A., Wildt, M., Taylor, C., Murray, A. and Herrick, A. L. (2017) Intra-and inter-observer reliability of nailfold videocapillaroscopy - A possible outcome measure for systemic sclerosis-related microangiopathy. Microvasc Res. 112, 1-6. [CrossRef]
- Gracia Tello, B., Ramos Ibáñez, E., Fanlo Mateo, P., Sáez Cómet, L., Martínez Robles, E., Ríos Blanco, J. J., Mari Alfonso, B., Espinosa Garriga, G., Todolí Parra, J., Ortego Centeno, N., Callejas Rubio, J. L., Freire Dapena, M., Marín Ballvé, A., Selva-O'Callaghan, A., Guillén Del Castillo, A., Simeón Aznar, C. P. and Fonollosa Pla, V. (2022) The challenge of comprehensive nailfold videocapillaroscopy practice: a further contribution. Clin Exp Rheumatol. 40, 1926-1932. [CrossRef]
- Niizawa, T., Yokemura, K., Kusaka, T., Sugashi, T., Miura, I., Kawagoe, K. and Masamoto, K. (2022) Automated capillary flow segmentation and mapping for nailfold video capillaroscopy. Microcirculation. 29, e12753. [CrossRef]
- Helmy, M., Truong, T. T., Jul, E. and Ferreira, P. (2023) Deep learning and computer vision techniques for microcirculation analysis: A review. Patterns (N Y). 4, 100641. [CrossRef]
- Kornaev, A. V., Dremin, V. V., Kornaeva, E. P. and Volkov, M. V. (2022) Application of deep convolutional and long short-term memory neural networks to red blood cells motion detection and velocity approximation. Proc SPIE. 12194, 1605-7422. [CrossRef]
- Bharathi, P. G., Berks, M., Dinsdale, G., Murray, A., Manning, J., Wilkinson, S., Cutolo, M., Smith, V., Herrick, A. L. and Taylor, C. J. (2023) A deep learning system for quantitative assessment of microvascular abnormalities in nailfold capillary images. Rheumatology (Oxford). 62, 2325-2329. [CrossRef]
- Gracia Tello, B. C., Ramos Ibañez, E., Saez Comet, L., Guillen Del Castillo, A., Simeón Aznar, C. P., Selva-O'Callaghan, A., Espinosa, G., Lledó, G., Freire Dapena, M., Martinez Robles, E., Rios, J. J., Todolí Parra, J. A., Marí Alfonso, B., Ortego Centeno, N., Marín Ballvé, A., Callejas Rubio, J. L., Fonollosa Plá, V. and Fanlo, P. (2023) External clinical validation of automated software to identify structural abnormalities and microhaemorrhages in nailfold videocapillaroscopy images. Clin Exp Rheumatol. 41, 1605-1611. [CrossRef]
- Emam, O. S., Ebadi Jalal, M., Garcia-Zapirain, B. and Elmaghraby, A. S. (2024) Artificial Intelligence Algorithms in Nailfold Capillaroscopy Image Analysis: A Systematic Review. medRxiv, 2024.2007.2028.24311154. [CrossRef]
- Kassani, P. H., Ehwerhemuepha, L., Martin-King, C., Kassab, R., Gibbs, E., Morgan, G. and Pachman, L. M. (2024) Artificial intelligence for nailfold capillaroscopy analyses - a proof of concept application in juvenile dermatomyositis. Pediatr Res. 95, 981-987. [CrossRef]
- Tuncer, S. A., Yildirim, M., Tuncer, T. and Mulayim, M. K. (2024) YOLOv8-Based System for Nail Capillary Detection on a Single-Board Computer. Diagnostics (Basel). 14, 1843. [CrossRef]
- Ebadi Jalal, M., Emam, O. S., Castillo-Olea, C., Garcia-Zapirain, B. and Elmaghraby, A. (2025) Abnormality detection in nailfold capillary images using deep learning with EfficientNet and cascade transfer learning. Sci Rep. 15, 2068. [CrossRef]
- Ozturk, L., Laclau, C., Boulon, C., Mangin, M., Braz-Ma, E., Constans, J., Dari, L. and Le Hello, C. (2025) Analysis of nailfold capillaroscopy images with artificial intelligence: Data from literature and performance of machine learning and deep learning from images acquired in the SCLEROCAP study. Microvasc Res. 157, 104753. [CrossRef]
- Takimoto, B., Bito, K., Hari, S., Taguchi, H. and Haneishi, H. (2023) Observation and density estimation of a large number of skin capillaries using wide-field portable video capillaroscopy and semantic segmentation. J Biomed Opt. 28, 106003. [CrossRef]
- Li, X., Cen, M., Xu, J., Zhang, H. and Xu, X. S. (2022) Improving feature extraction from histopathological images through a fine-tuning ImageNet model. J Pathol Inform. 13, 100115. [CrossRef]
- Chawla, S., Nakov, P., Ali, A., Hall, W., Khalil, I., Ma, X., Taha Sencar, H., Weber, I., Wooldridge, M. and Yu, T. (2023) Ten years after ImageNet: a 360 degrees perspective on artificial intelligence. R Soc Open Sci. 10, 221414. [CrossRef]
- Deng, J., Dong, W., Socher, R., Li, L.-J., Li, K. and Li, F.-F. (2009) ImageNet: a large-scale hierarchical image database. IEEE Conf. on Computer Vision and Pattern Recognition, 248–255. [CrossRef]
- im, S., Chen, J., Cheng, T., Gindulyte, A., He, J., He, S., Li, Q., Shoemaker, B. A., Thiessen, P. A., Yu, B., Zaslavsky, L., Zhang, J. and Bolton, E. E. (2025) PubChem 2025 update. Nucleic Acids Res. 53, D1516-D1525. [CrossRef]
- Fleming, J., Magana, P., Nair, S., Tsenkov, M., Bertoni, D., Pidruchna, I., Lima Afonso, M. Q., Midlik, A., Paramval, U., Žídek, A., Laydon, A., Kovalevskiy, O., Pan, J., Cheng, J., Avsec, Ž., Bycroft, C., Wong, L. H., Last, M., Mirdita, M., Steinegger, M., Kohli, P., Varadi, M. and Velankar, S. (2025) AlphaFold Protein Structure Database and 3D-Beacons: New Data and Capabilities. J Mol Biol, 168967. [CrossRef]
- Varadi, M., Bertoni, D., Magana, P., Paramval, U., Pidruchna, I., Radhakrishnan, M., Tsenkov, M., Nair, S., Mirdita, M., Yeo, J., Kovalevskiy, O., Tunyasuvunakool, K., Laydon, A., Žídek, A., Tomlinson, H., Hariharan, D., Abrahamson, J., Green, T., Jumper, J., Birney, E., Steinegger, M., Hassabis, D. and Velankar, S. (2024) AlphaFold Protein Structure Database in 2024: providing structure coverage for over 214 million protein sequences. Nucleic Acids Res. 52, D368-D375. [CrossRef]
- Berman, H. M. and Burley, S. K. (2025) Protein Data Bank (PDB): Fifty-three years young and having a transformative impact on science and society. Q Rev Biophys. 58, e9. [CrossRef]
- Holzinger, A., Biemann, C., Pattichis, C. S. and Kell, D. B. (2017) What do we need to build explainable AI systems for the medical domain? arXiv, 1712.09923v09921. [CrossRef]
- Arron, H. E., Marsh, B. D., Kell, D. B., Khan, M. A., Jaeger, B. R. and Pretorius, E. (2024) Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: the biology of a neglected disease. Front Immunol. 15, 1386607. [CrossRef]
- Legler, F., Meyer-Arndt, L., Modl, L., Kedor, C., Freitag, H., Stein, E., Hoppmann, U., Rust, R., Wittke, K., Siebert, N., Behrens, J., Thiel, A., Konietschke, F., Paul, F., Scheibenbogen, C. and Bellmann-Strobl, J. (2023) Long-term symptom severity and clinical biomarkers in post-COVID-19/chronic fatigue syndrome: results from a prospective observational cohort. EClinicalMedicine. 63, 102146. [CrossRef]
- van Campen, C. L. M. C., Rowe, P. C. and Visser, F. C. (2021) Orthostatic Symptoms and Reductions in Cerebral Blood Flow in Long-Haul COVID-19 Patients: Similarities with Myalgic Encephalomyelitis/Chronic Fatigue Syndrome. Medicina (Kaunas). 58, 28. [CrossRef]
- Haffke, M., Freitag, H., Rudolf, G., Seifert, M., Doehner, W., Scherbakov, N., Hanitsch, L., Wittke, K., Bauer, S., Konietschke, F., Paul, F., Bellmann-Strobl, J., Kedor, C., Scheibenbogen, C. and Sotzny, F. (2022) Endothelial dysfunction and altered endothelial biomarkers in patients with post-COVID-19 syndrome and chronic fatigue syndrome (ME/CFS). J Transl Med. 20, 138. [CrossRef]
- Newton, D. J., Kennedy, G., Chan, K. K., Lang, C. C., Belch, J. J. and Khan, F. (2012) Large and small artery endothelial dysfunction in chronic fatigue syndrome. Int J Cardiol. 154, 335-336. [CrossRef]
- Nunes, J. M., Kell, D. B. and Pretorius, E. (2024) Herpesvirus Infection of Endothelial Cells as a Systemic Pathological Axis in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome. Viruses. 16, 572. [CrossRef]
- Nunes, J. M., Vlok, M., Proal, A., Kell, D. B. and Pretorius, E. (2024) Data-independent LC-MS/MS analysis of ME/CFS plasma reveals a dysregulated coagulation system, endothelial dysfunction, downregulation of complement machinery. Cardiovasc. Diabetol. 23, 254. [CrossRef]
- McLaughlin, M., Sanal-Hayes, N. E. M., Hayes, L. D., Berry, E. C. and Sculthorpe, N. F. (2023) People With Long COVID and Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS) Exhibit Similarly Impaired Vascular Function. Am J Med. 138, 530-566. [CrossRef]
- Wirth, K. J. and Löhn, M. (2024) Microvascular Capillary and Precapillary Cardiovascular Disturbances Strongly Interact to Severely Affect Tissue Perfusion and Mitochondrial Function in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome Evolving from the Post COVID-19 Syndrome. Medicina (Kaunas). 60, 194. [CrossRef]
- Vaes, A. W., De Boever, P., Franssen, F. M. E., Uszko-Lencer, N., Vanfleteren, L. and Spruit, M. A. (2023) Endothelial function in patients with COPD: an updated systematic review of studies using flow-mediated dilatation. Expert Rev Respir Med. 17, 53-69. [CrossRef]
- Garcia, D. J., Chagnot, A., Wardlaw, J. M. and Montagne, A. (2023) A Scoping Review on Biomarkers of Endothelial Dysfunction in Small Vessel Disease: Molecular Insights from Human Studies. Int J Mol Sci. 24, 13114. [CrossRef]
- Garcia, V. P., Fandl, H. K., Wegerson, K. N., Berry, A. R., Ruzzene, S. T., Greiner, J. J., Stockelman, K. A., Dow, C. A., Park, A. J., Stauffer, B. L. and DeSouza, C. A. (2025) Elevated circulating endothelial cell-derived microvesicles: a biomarker of endothelial vasomotor dysfunction in adults with obesity. J Appl Physiol (1985). 138, 1143-1149. [CrossRef]
- Mohebbi, A., Haybar, H., Nakhaei Moghaddam, F., Rasti, Z., Vahid, M. A. and Saki, N. (2023) Biomarkers of endothelial dysfunction are associated with poor outcome in COVID-19 patients: A systematic review and meta-analysis. Rev Med Virol. 33, e2442. [CrossRef]
- Zhang, J. (2022) Biomarkers of endothelial activation and dysfunction in cardiovascular diseases. Rev Cardiovasc Med. 23, 73. [CrossRef]
- Attia, A. B. E., Moothanchery, M., Li, X., Yew, Y. W., Thng, S. T. G., Dinish, U. S. and Olivo, M. (2021) Microvascular imaging and monitoring of hemodynamic changes in the skin during arterial-venous occlusion using multispectral raster-scanning optoacoustic mesoscopy. Photoacoustics. 22, 100268. [CrossRef]
- Geisler, E. L., Brannen, A., Pressler, M., Perez, J., Kane, A. A. and Hallac, R. R. (2022) 3D imaging of vascular anomalies using raster-scanning optoacoustic mesoscopy. Lasers Surg Med. 54, 1269-1277. [CrossRef]
- Nitkunanantharajah, S., Haedicke, K., Moore, T. B., Manning, J. B., Dinsdale, G., Berks, M., Taylor, C., Dickinson, M. R., Jüstel, D., Ntziachristos, V., Herrick, A. L. and Murray, A. K. (2020) Three-dimensional optoacoustic imaging of nailfold capillaries in systemic sclerosis and its potential for disease differentiation using deep learning. Sci Rep. 10, 16444. [CrossRef]
- Böke, J. S., Popp, J. and Krafft, C. (2022) Optical photothermal infrared spectroscopy with simultaneously acquired Raman spectroscopy for two-dimensional microplastic identification. Sci Rep. 12, 18785. [CrossRef]
- Gvazava, N., Konings, S. C., Cepeda-Prado, E., Skoryk, V., Umeano, C. H., Dong, J., Silva, I. A. N., Rylander Ottosson, D., Leigh, N. D., Darcy Elizabeth Wagner and Klementieva, O. (2023) Label-Free High-Resolution Photothermal Optical Infrared Spectroscopy for Spatiotemporal Chemical Analysis in Fresh, Hydrated Living Tissues and Embryos. J Am Chem Soc. 145, 24796-24808. [CrossRef]
- Lima, C., Ahmed, S., Xu, Y., Muhamadali, H., Parry, C., McGalliard, R. J., Carrol, E. D. and Goodacre, R. (2022) Simultaneous Raman and infrared spectroscopy: a novel combination for studying bacterial infections at the single cell level. Chem Sci. 13, 8171-8179. [CrossRef]
- Lima, C., Muhamadali, H. and Goodacre, R. (2023) Monitoring Phenotype Heterogeneity at the Single-Cell Level within Bacillus Populations Producing Poly-3-hydroxybutyrate by Label-Free Super-resolution Infrared Imaging. Anal Chem. 95, 17733-17740. [CrossRef]
- Richardson, P. I. C., Horsburgh, M. J. and Goodacre, R. (2024) Benchmarking classification abilities of novel optical photothermal IR spectroscopy at the single-cell level with bulk FTIR measurements. Anal Methods. 16, 5419-5425. [CrossRef]
- Davison, A. K., Dinsdale, G., New, P., Manning, J., Patrick, H., Taxiarchi, V. P., Dixon, W. G., Vail, A., Murray, A. K., Dickinson, M., Taylor, C. and Herrick, A. L. (2022) Feasibility study of mobile phone photography as a possible outcome measure of systemic sclerosis-related digital lesions. Rheumatol Adv Pract. 6, rkac105. [CrossRef]
- Madenidou, A. V., Dinsdale, G., Samaranayaka, M., Muir, L., Dixon, W. G. and Herrick, A. L. (2023) Smartphone images of digital ulcers provide a clear picture of disease progression for the first rheumatology visit. Rheumatology (Oxford). 62, e153-e154. [CrossRef]
- Davison, A. K., Krishan, A., New, R. P., Murray, A., Dinsdale, G., Manning, J., Hall, F., Pauling, J. D., Vail, A., Kearney, K., Patrick, H., Hughes, M., Dixon, W., Dickinson, M., Taylor, C. and Herrick, A. L. (2024) Development of a measuring app for systemic sclerosis-related digital ulceration (SALVE: Scleroderma App for Lesion VErification). Rheumatology (Oxford). 63, 3297-3305. [CrossRef]
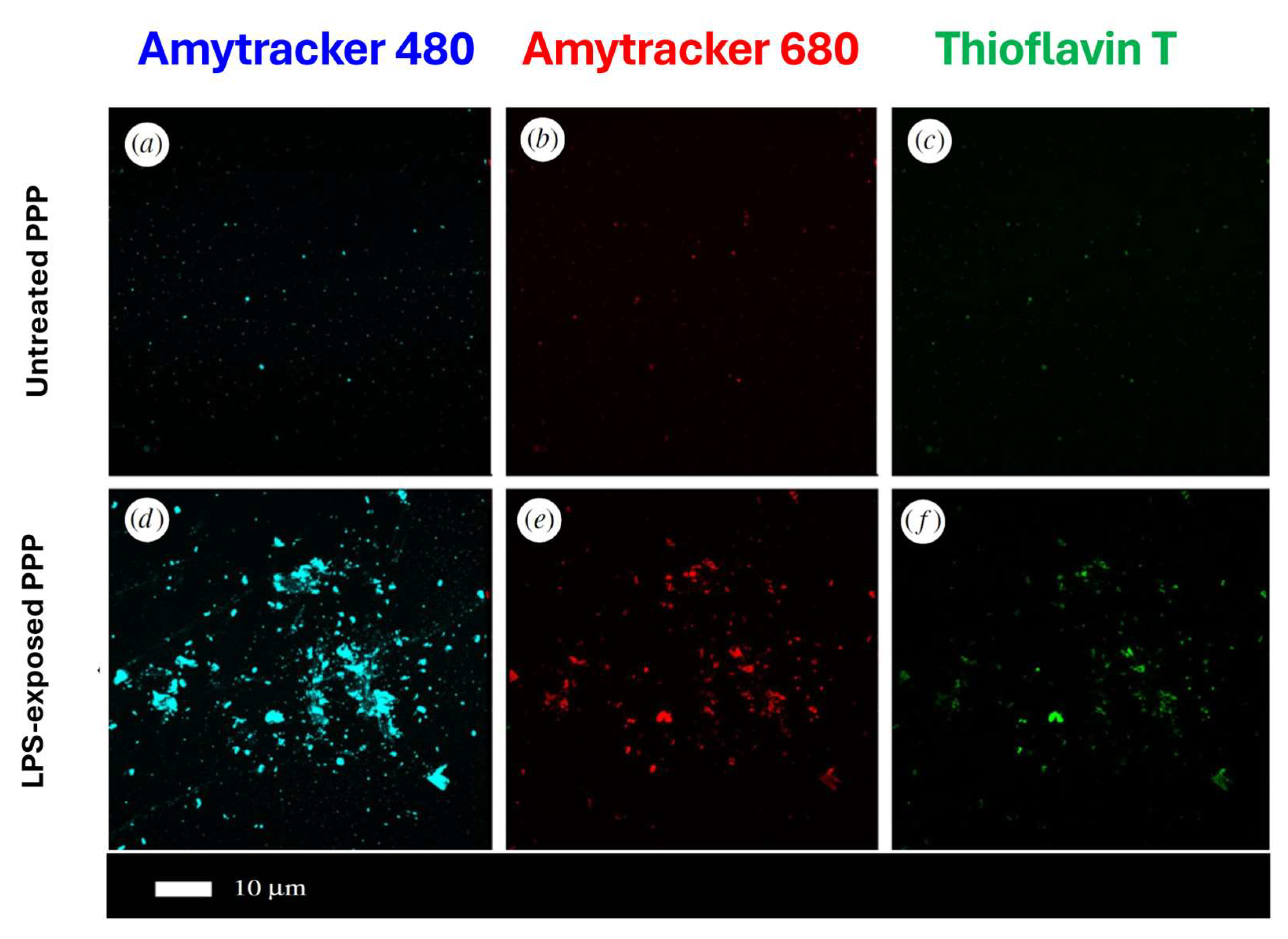
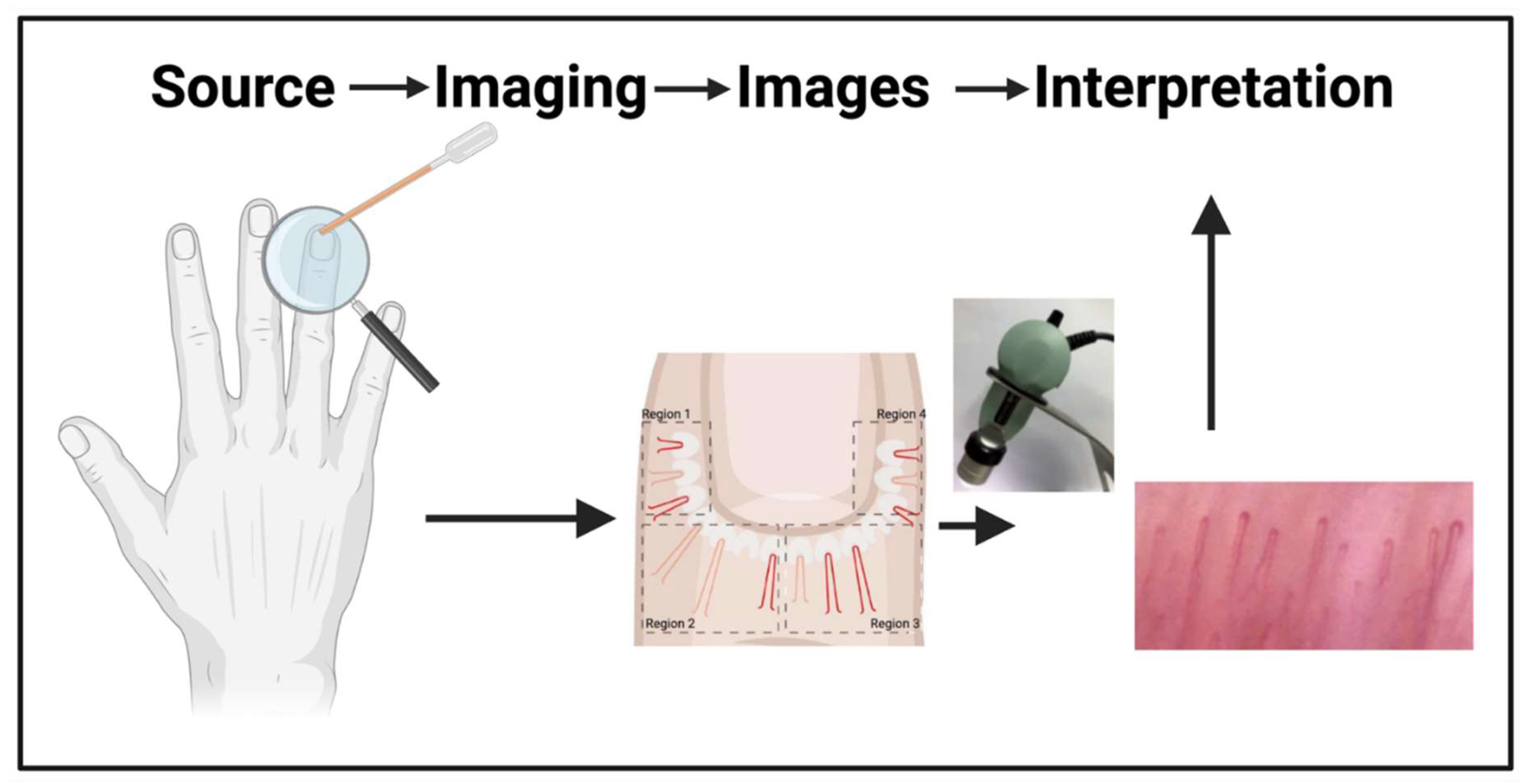
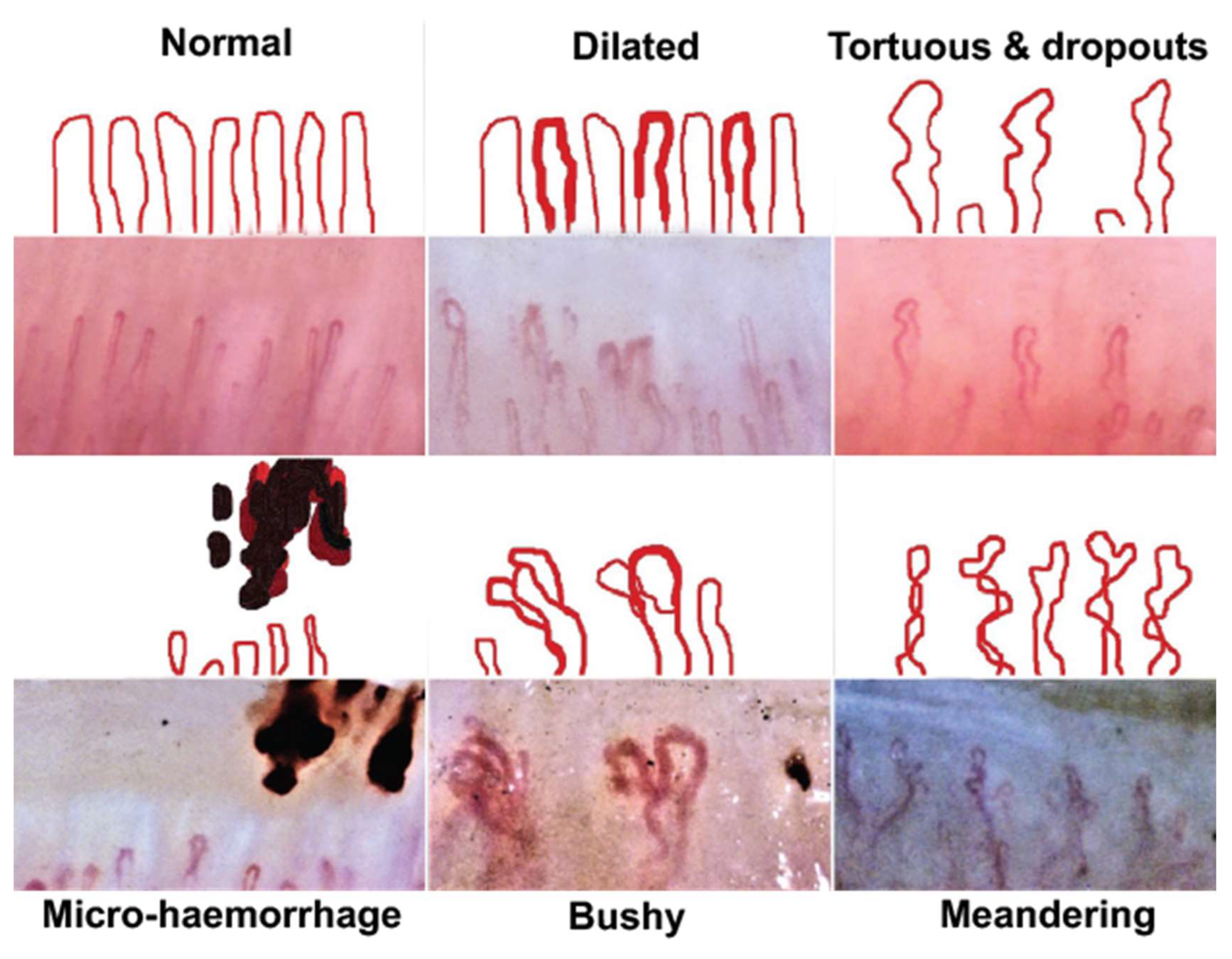
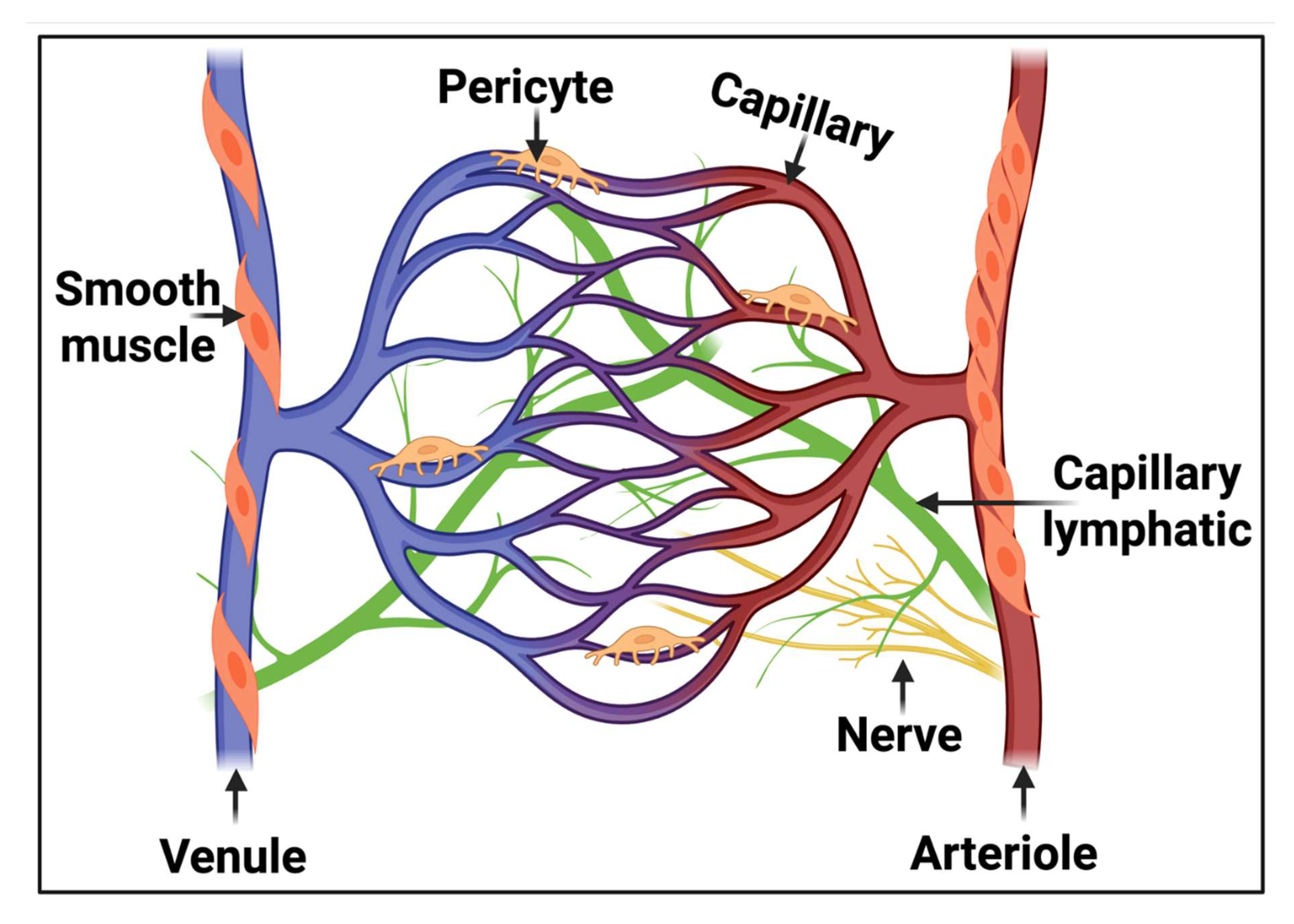
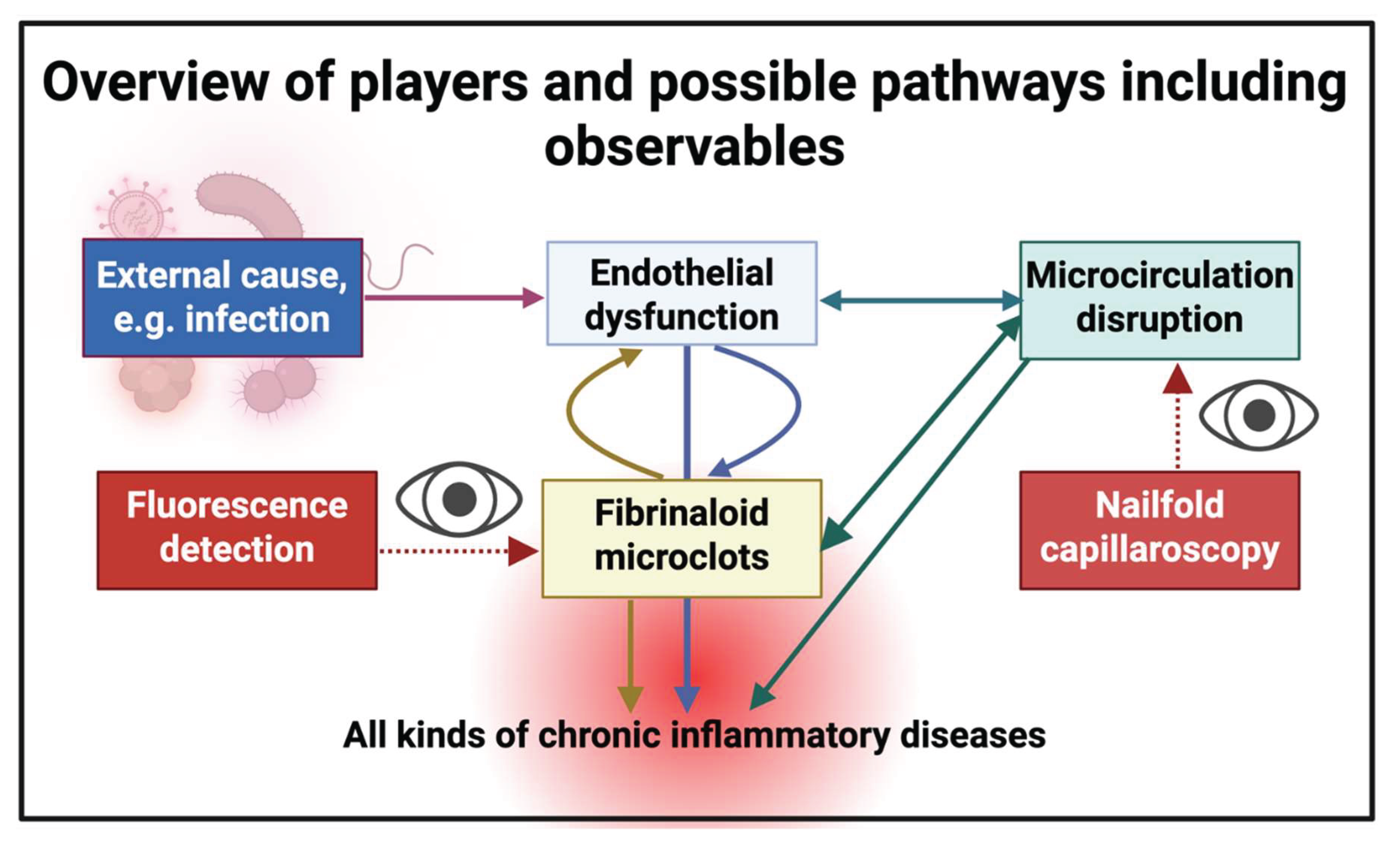
| Disease or Syndrome | Comments | Selected Nailfold Capillaroscopy References | Selected Fibrinaloid Microclot References (Where Tested) |
|---|---|---|---|
| Acute COVID-19 | Capillary tortuosity, meandering, haemosiderin deposition and microhaemorrhage increased, capillary density and length and blood flow decreased | [121,122,123,124,125,126,127,128,129,130,131,132,133] | [18,29,134,135,136,137] |
| Alzheimer’s dementia | Greater nailfold capillary tortuosity in individuals with Alzheimer’s dementia | [138,139] | [12,13,14,140,141] |
| Anderson-Fabry disease | Also known as Fabry disease. Promising but underexplored. Subclasses detected depending on the a-galactosidase A variant. | [142,143,144,145] | |
| Anorexia nervosa | Related to connective tissue disorders | [146,147] | |
| Antineutrophil Cytoplasmic Antibody-Associated (ANCA) Vasculitis | Neoangiogenesis, capillary loss, microhaemorrhages, and bushy and enlarged capillaries |
[148,149,150,151] | |
| Atopic dermatitis | Many more lesion, e.g. pitting, capillary density, tortuosity | [152] | |
| Behçet’s disease | Increases in capillary dilatation, tortuous and branched capillaries, and microhaemorrhages | [153,154] | |
| Biliary cirrhosis | Multiple abnormalities | [155] | |
| Cancer | Not yet widely deployed but some suggestive data imply it could have diagnostic or prognostic value | [156] | |
| Chronic obstructive pulmonary disorder (COPD) | Review | [157] (but cf. [158]) | |
| Chronic smokers | Abnormalities more common in chronic smokers | [159] | |
| Chronic urticaria | Lower capillary density, more capillary malformations, and more irregular capillary dilations | [160] | |
| Connective tissue disorders | Various | [161,162,163] | |
| Coronary heart disease | Assessment of capillary blood flow and relation to erythrocyte aggregation | [164] | |
| Dermatomyositis | Diminished capillary density and abnormal capillary morphology (including enlargement) in patients.Can be related to antibody levels. Haemosiderin deposits can occur. | [99,165,166,167,168,169,170,171,172,173,174] | |
| Diabetes mellitus, type 1 | General | [175,176] | |
| Use of deep learning | [177] | ||
|
Diabetes mellitus, type 2 |
Capillary dilatation, avascular zones and tortuous capillaries | [178,179,180] | [12,17,18,181,182] |
| Peak capillary blood flow velocity (CBFV) post-occlusion much lower | [183] | ||
| Use of deep learning | [177] | ||
| Changes closely related to quality of glucose control | [184,185] | ||
| Capillaries larger but less dense | [186] | ||
| Various differences | [187,188] | ||
| Diabetic complications | Listed separately below | ||
| Diabetic foot | Significant difference in pulp of big toe | [189] | |
| Diabetic nephropathy | Significant correlation between lowered glomerular filtration rate and e.g. reduced fundus transparency and visibility of the sub-venous plexus | [190,191] | |
| Diabetic neuropathy | Improved by a-lipoic acid | [192] | |
| Diabetic retinopathy | Decreased capillary length, width number and turbidity, crossing capillaries, and other abnormalities | [178,179,193,194,195,196] | |
| Digital ulcers | A common accompaniment to systemic sclerosis and Raynaud’s disease | [197,198,199] | |
| Endothelial dysfunction | Reviews | [112,123] | [120] |
| In livedoid vasculopathy | [200] | ||
| Association with rheumatoid arthritis | [201] | ||
| Fibromyalgia | Raynaud’s disease and impaired micrvascular function common in patients with fibromyalgia, where nailfold abnormalities are common | [202,203,204,205] | |
| Fewer capillaries, apical limb width and capillary width decreased | [77,206] | See [44], and for amyloid deposition in skeletal muscle [207] | |
| Mean capillary loop diameter, micro-aneurysm number, avascular areas, and neoangiogenic capillaries significantly higher |
[208] | ||
| Gaucher disease | Various subtypes. Microangiopathy the most obvious NFC finding | [209] | |
| General reviews | [76,89,95,210,211,212] | ||
| Non-rheumatic diseases | [213] | ] | |
| Vascular disease | [214] | ||
| Glaucoma | Huge decrease in capillary blood flow | [215] | |
| Heart failure | Greater abnormalities with preserved ejection fraction | [216] | |
| Hepatitis, viral | Changes in avascular area, capillary dilatation, capillary tortuosity and capillary enlargement observed | [217] | |
| Hypertension (general) | Many differences, including lowered capillary density and other morphological changes | [218] | |
| Idiopathic inflammatory myopathy | Significant differences, including between subtypes | [219,220,221,222,223] | |
| Idiopathic macular telangiectasia type 2 | Increased capillary tortuosity, ‘bizarre’ capillaries and microhaemorrhage in the patient group compared to the controls | [224] | |
| Leprosy | Capillary dropouts most frequent, followed by tortuous, receding, and dilated capillaries | [225] | |
| Long COVID | Compares Long COVID patients without and with systemic sclerosis. Long COVID patients show more microvascular alterations than recovered COVID patients | [226] | [21,24,30,31,32,33,34,35,43,45,120,227] |
| Lupus (systemic lupus erythematosus) | [100,168,228,229,230,231] | ||
| Migraine | Seemingly little studied recently by nailfold capillaroscopy, but major differences observed vs controls, especially when cold-induced | [232,233] | [234] |
| Neutropenia | Indirect assessment from videocapillaroscopy | [235,236] | |
| Obstructive sleep apnoea | All capillaroscopy findings were significantly higher in the patient group | [237] | |
| Parkinson’s disease | Seemingly not yet studied by nailfold capillaroscopy in any detail | [12,16,238] | |
| Polycythemia vera |
Increase in diameter of vessels | [239] | |
| Polymyositis | Scleroderma pattern predominated. Improved after treatment. | [99,165,174,240,241] | |
| Pre-eclampsia | Change in capillary length and density with pregnancy-induced hypertension, whether pre-eclamptic or not. Some relationship to maternal blood pressure [242]. | [243] | |
| Decreased capillary density in pre-eclamptics | [244] | ||
| Review of capillaroscopy in pregnancy | [245] | ||
| Psoriasis | [246,247,248,249,250] [251,252] | ||
| Pulmonary arterial hypertension |
(Can accompany systemic sclerosis.) Lowered capillary density | [218,253,254] | |
| Raynaud’s disease or Raynaud’s phenomenon | Common accompaniment of systemic sclerosis. NFC especially used in discriminating primary Raynaud’s from secondary Raynaud’s due to systematic sclerosis | [199,255,256,257,258,259,260,261,262,263,264,265,266] | |
| Standardisation | [101] | ||
| Rheumatology, especially rheumatoid arthritis | Reviews | [87,246,267,268,269,270,271,272,273,274,275] | [19,276,277] |
| Sarcoidosis | Early but promising | [278,279,280] | |
| Sarcopenia | Common in rheumatoid arthritis and systemic sclerosis | [281] [282] | |
| Sepsis and septic shock | See section 2.3 | [283] | [38] and see [284] |
| Decreased capillary density sublingually | [285] | ||
| Sickle cell disease | Lower capillary density and more dilated capillaries | [286] | |
| Sjögren’s syndrome | [287,288] | ||
| Systemic sclerosis | It is inceasingly seen as a vasculopathy [109]. Reviews on the role of NFC in the adjacent column. Arguably the commonest area of study. | [102,167,289,290,291,292,293,294,295,296,297,298,299,300,301,302,303,304] | |
| Use of the NEMO score (the number of microhaemorrhages plus micro-thromboses) |
[305,306,307] | ||
| Patients with primary biliary cholangitis | [308] | ||
| Differences when pulmonary arterial hypertension is also present | [309,310] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





