Submitted:
29 May 2025
Posted:
29 May 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
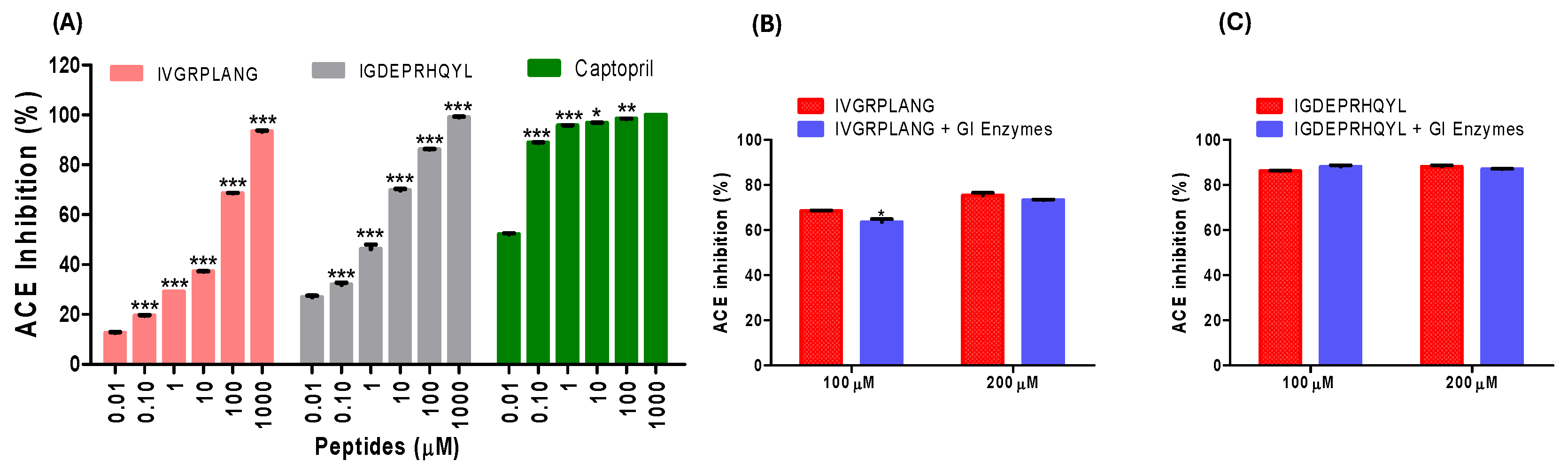
2.1. ACE Inhibitory Activity and Digestive Stability of IVGRPLANG and IGDEPRHQYL Peptides
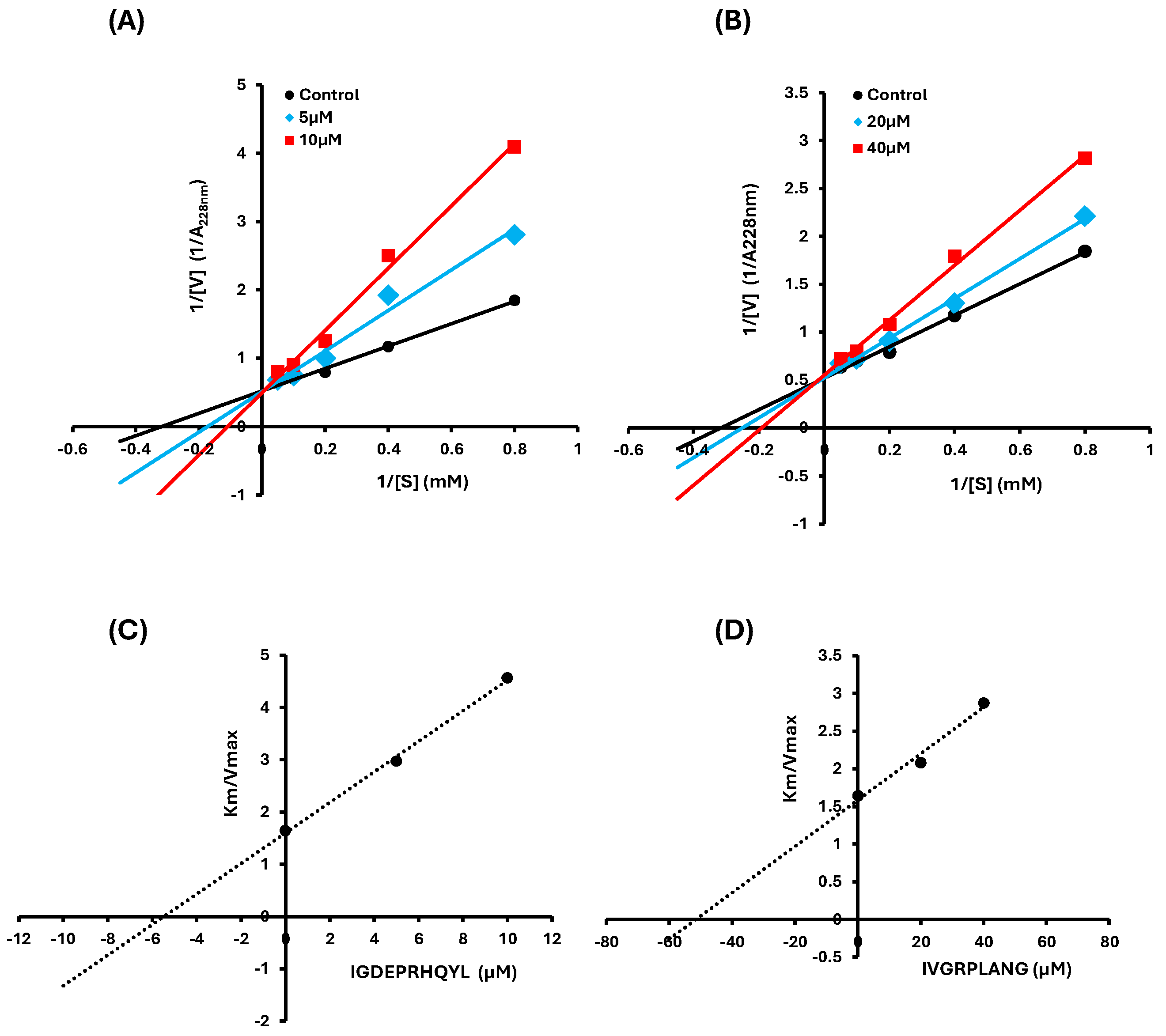
2.2. Inhibition Pattern of IVGRPLANG and IGDEPRHQYL Peptides
2.3. In Silico Screening of IVGRPLANG and IGDEPRHQYL Peptide with Angiotensin Converting Enzyme
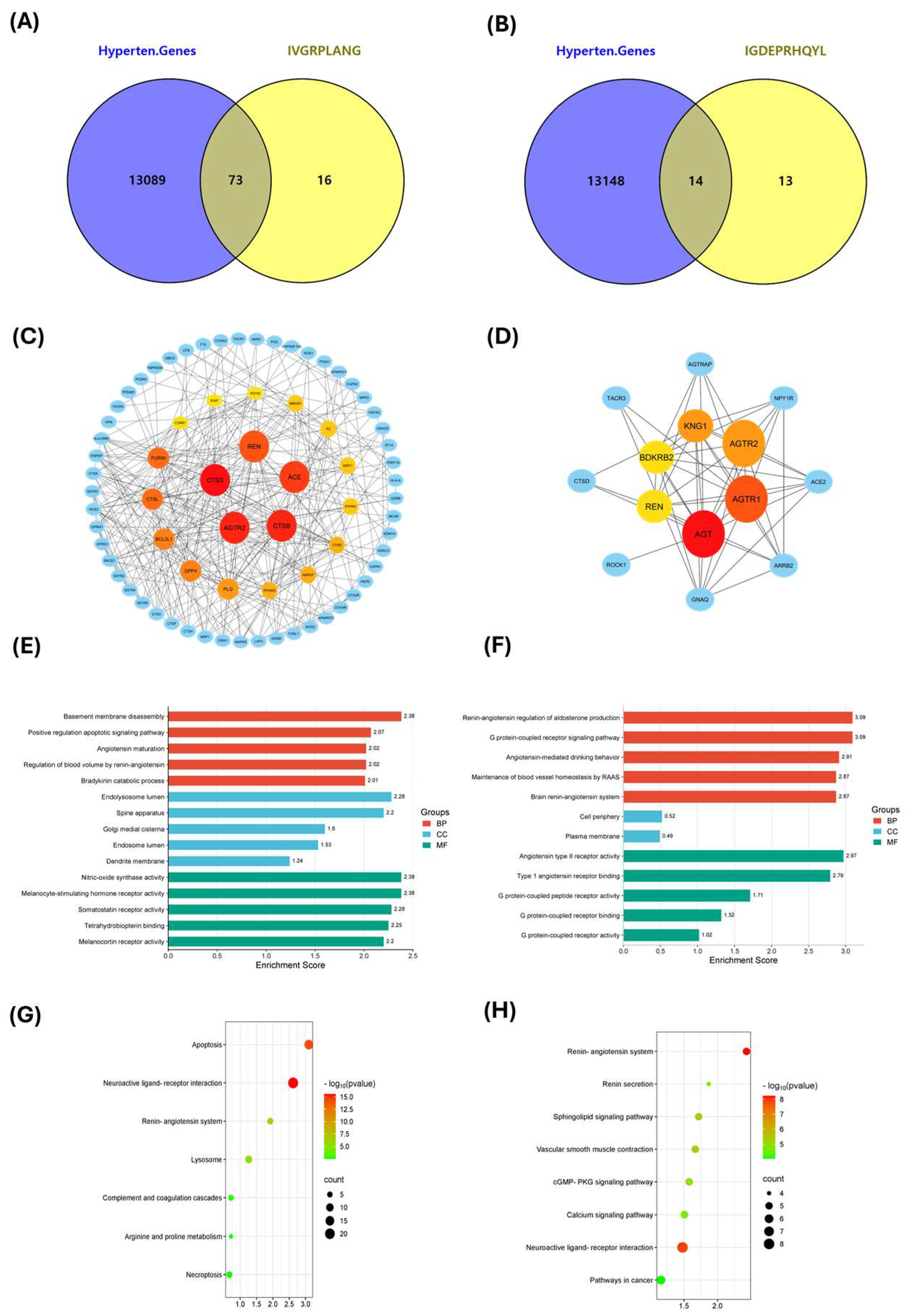
2.4. Exploring Potential Hypertension-Related Targets for IVGRPLANG and IGDEPRHQYL Peptides and Performing GO and KEGG Analyses
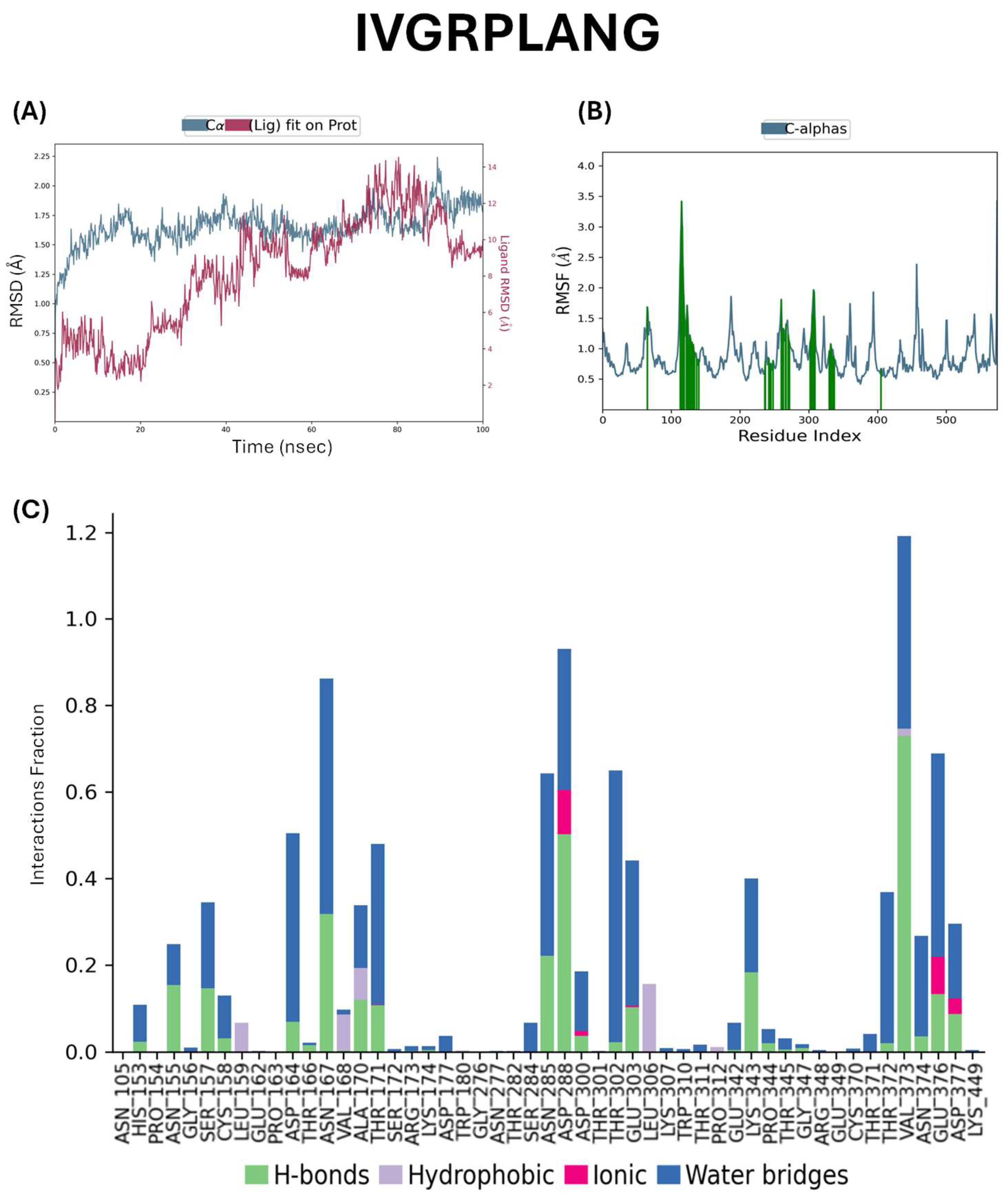
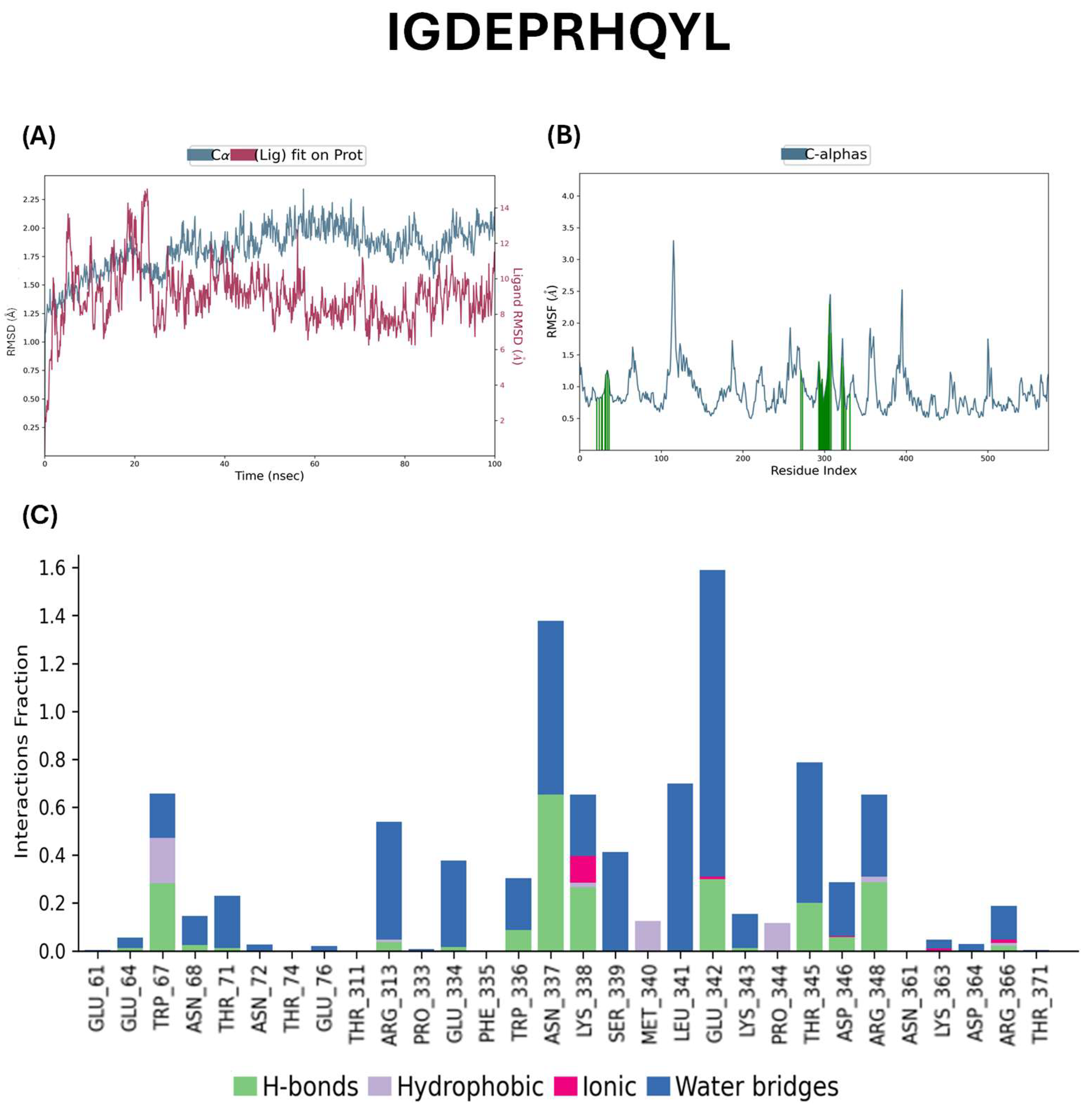
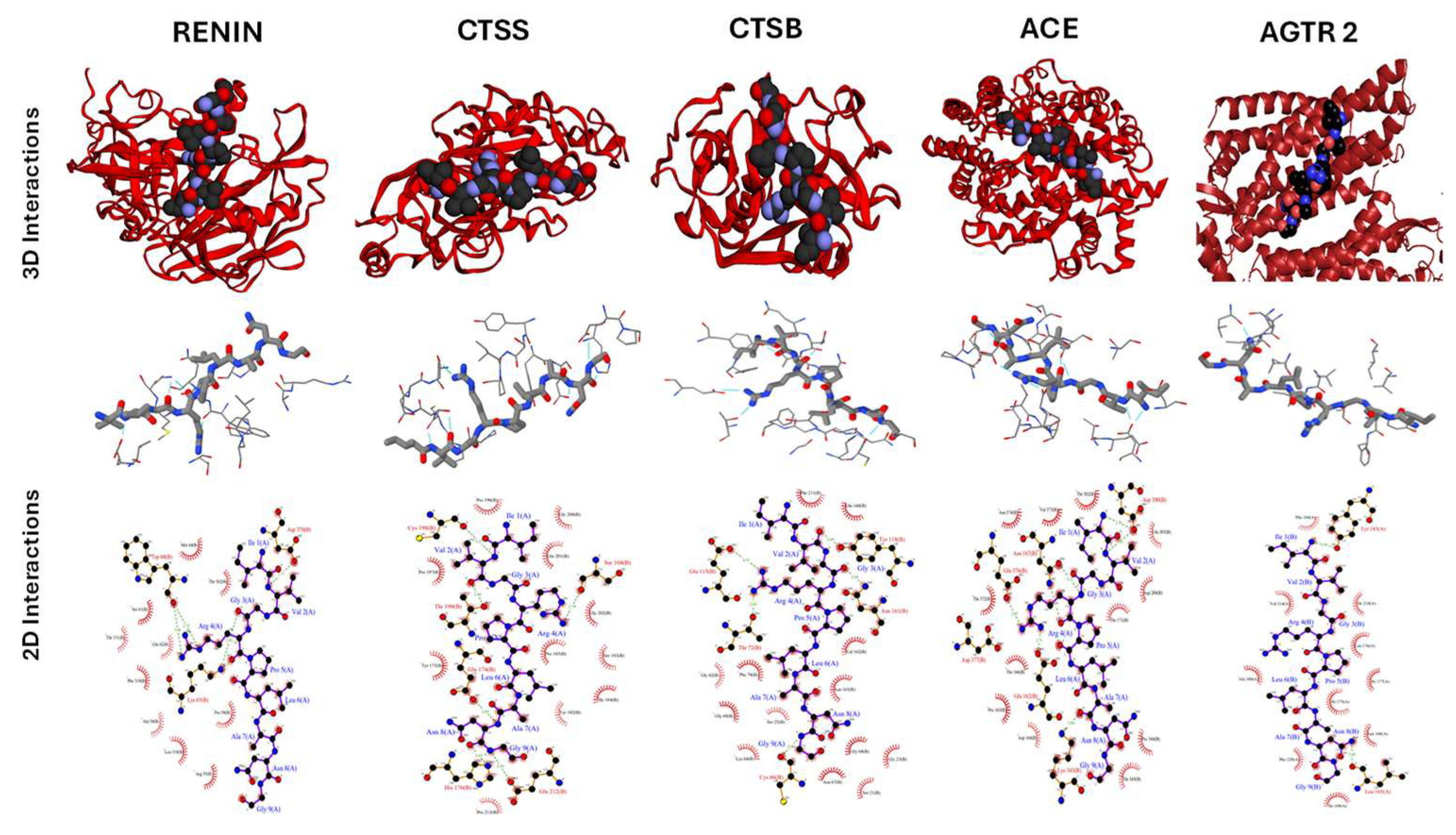
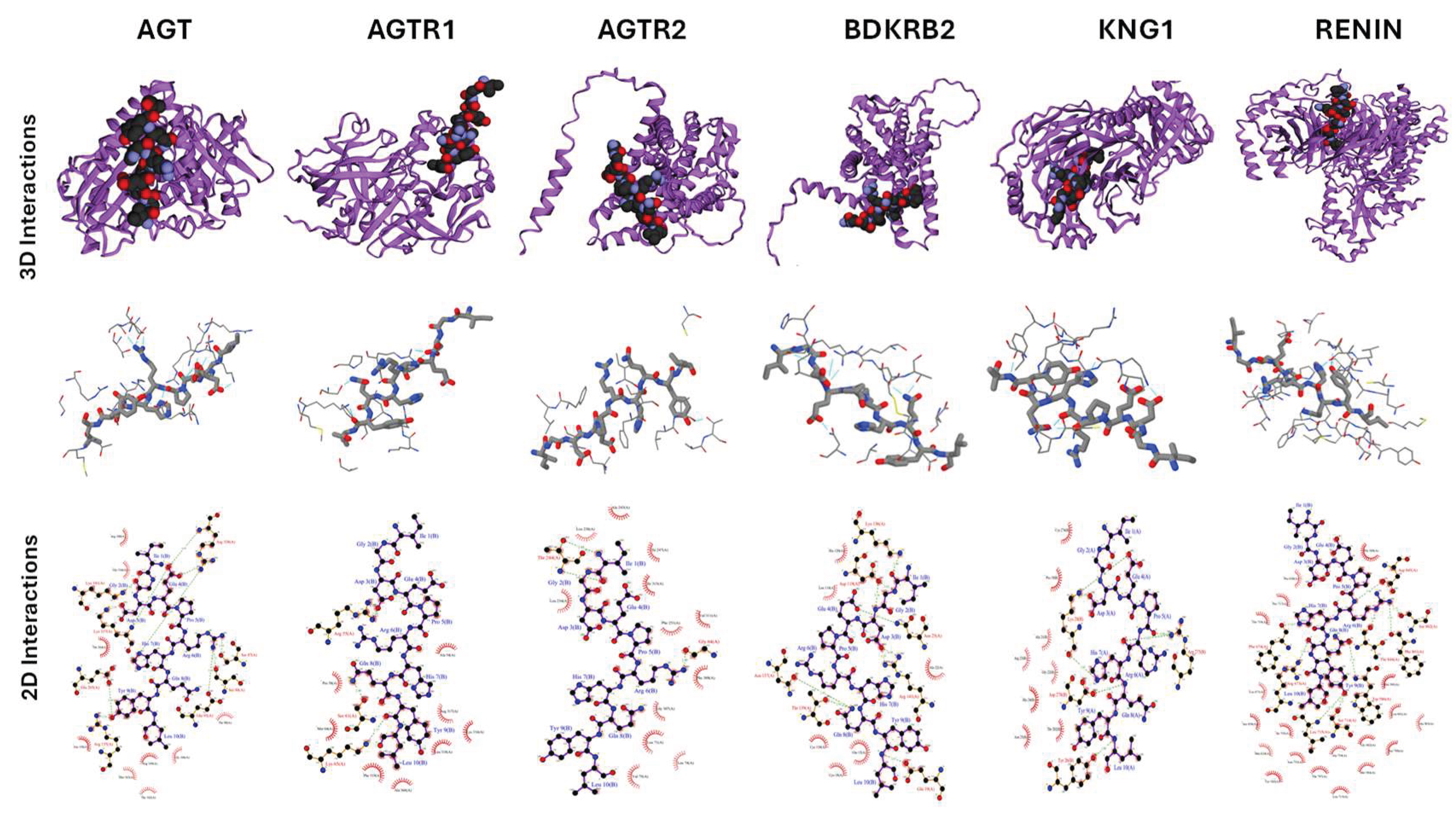
2.5. Docking Studies of Potential Hypertensive Target Proteins with IGDEPRHQYL and IVGRPLANG Peptide
3. Discussion
4. Materials and Methods
4.1. Peptide Synthesis
4.2. ACE Inhibitory Effect
4.3. Digestive Stability Assay
4.4. Lineweaver Bark Plot & Secondary Plot
4.5. Molecular Dynamic Simulations
4.6. Peptide Mining Antihypertensive Targets
4.7. GO & KEGG Analysis
4.8. Molecular Docking
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- M. Kontis, V.; Mathers, C.D.; Rehm, J.; Stevens, G.A.; Shield, K.D.; Bonita, R.; Riley, L.M.; Poznyak, V.; Beaglehole, R.; Ezzati, Contribution of Six Risk Factors to Achieving the 25 × 25 Non-Communicable Disease Mortality Reduction Target: A Modelling Study, (2014).
- World Health Organization. NCD Global Monitoring Framework, WHO Geneva, Switz. (2013).
- M. Danaei, G.; Ding, E.L.; Mozaffarian, D.; Taylor, B.; Rehm, J.; Murray, C.J.; Ezzati, The preventable causes of death in the United States: Comparative risk assessment of dietary, lifestyle, and metabolic risk factors., PLoS Med. (2009).
- C.J. Lopez, A.D.; Mathers, C.D.; Ezzati, M.; Jamison, D.T.; Murray, Global and regional burden of disease and risk factors, 2001: Systematic analysis of population health data., Lancet. (2006).
- World Health Organization. Global Health Risks-Mortality and Burden of Disease Attributable to Selected Major Risks, World Heal. Organ. Geneva, Switz. (2015).
- V. Yadav, S.; Boddula, R.; Genitta, G.; Bhatia, V.; Bansal, B.; Kongara, S.; Ramesh, Prevalence & risk factors of pre-hypertension & hypertension in an affluent north Indian population., Indian J. Med. Res. (2008).
- M.R. Kitt J, Fox R, Tucker KL, New approaches in hypertension management: a review of current and developing technologies and their potential impact on hypertension care., Curr Hypertens Rep. (2019).
- N.K. Patel, S.A.; Winkel, M.; Ali, M.K.; Narayan, K.V.; Mehta, Cardiovascular mortality associated with 5 leading risk factors: National and state preventable fractions estimated from survey data., Ann. Intern. Med. (2015).
- A.H. Te Riet, L.; van Esch, J.H.; Roks, A.J.; van den Meiracker, A.H.; Danser, Hypertension: Renin-angiotensin-aldosterone system alterations., Circ. Res. (2015).
- Soffer, R.L. Angiotensin-Converting Enzyme and the Regulation of Vasoactive Peptides. Annu. Rev. Biochem. 1976, 45, 73–94. [Google Scholar] [CrossRef] [PubMed]
- C.Y. Ju, D.T.; Kuo, W.W.; Ho, T.J.; Chang, R.L.; Lin, W.T.; Day, C.H.; Viswanadha, V.V.P.; Liao, P.H.; Huang, Bioactive peptide VHVV upregulates the long-term memory-related biomarkers in adult spontaneously hypertensive rats., Int. J. Mol. Sci. (2019).
- B. Zhao, Y.Q.; Zhang, L.; Tao, J.; Chi, C.F.; Wang, Eight antihypertensive peptides from the protein hydrolysate of Antarctic krill (Euphausia superba): Isolation, identification, and activity evaluation on human umbilical vein endothelial cells (HUVECs)., Food Res. Int. (2019).
- Veterans Administration Cooperative Study Group on Antihypertensive Agents. Low-dose captopril for the treatment of mild to moderate hypertension., Arch Intern Med. (1984).
- Zusman, R.M. Renin- and non-renin–mediated antihypertensive actions of converting enzyme inhibitors. Kidney Int. 1984, 25, 969–983. [Google Scholar] [CrossRef] [PubMed]
- L.R. Jimsheena, V.K.; Gowda, Angiotensin I-converting enzyme (ACE) inhibitory peptides derived from Arachin by simulated gastric digestion, Food Chem. (2011).
- M. Zhao, Y.; Li, B.; Dong, S.; Liu, Z.; Zhao, X.; Wang, J.; Zeng, A novel ACE inhibitory peptide isolated from Acaudina molpadioidea hydrolysate., Peptides. (2009).
- Y.K. Song, L.; Shen, H.X.; Xiao, Y.; Luo, Preparation of angiotensin I converting enzyme inhibitory peptides from pearl mussel meat., Food Sci. Technol. (2007).
- M.Y. Gu, R.Z.; Li, C.Y.; Liu, W.Y.; Yi, W.X.; Cai, Angiotensin I-converting enzyme inhibitory activity of low-molecular-weight peptides from atlantic Salmon (Salmo salar L.) skin., Food Res. Int. (2011).
- B. Lin, L.; Lv, S.; Li, Angiotensin-I-converting enzyme (ACE)-inhibitory and antihypertensive properties of squid skin gelatin hydrolysates., Food Chem. (2012).
- B.C. He, H.L.; Chen, X.L.; Sun, C.Y.; Zhang, Y.Z.; Zhou, Analysis of novel angiotensin-I-converting enzyme inhibitory peptides from protease-hydrolyzed marine shrimp Acetes chinensis, J. Pept. Sci. (2006).
- C. Liu, X., Zhang, M., Jia, A., Zhang, Y., Zhu, H., Zhang, C., ... & Liu, Purification and characterization of angiotensin I converting enzyme inhibitory peptides from jellyfish Rhopilema esculentum, Food Res. Int. (2013).
- Nisa, S.A.; Vinu, D.; Krupakar, P.; Govindaraju, K.; Sharma, D.; Vivek, R. Jellyfish venom proteins and their pharmacological potentials: A review. Int. J. Biol. Macromol. 2021, 176, 424–436. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Bae, S.; Hwang, D.H.; Prakash, R.L.M.; Kim, J.-H.; Hong, I.-H.; Kim, W.H.; Rho, I.R.; Kim, E.; Kang, C. Nemopilema nomurai jellyfish venom attenuates phenotypic modulation of PDGF BB-induced vascular smooth muscle cells and κ-carrageenan-induced rat tail thrombosis. Toxicon 2023, 233, 107266. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, I.; Lee, H.; Pyo, M.J.; Heo, Y.; Chae, J.; Yum, S.S.; Kang, C.; Kim, E. Proteomic Investigation to Identify Anticancer Targets of Nemopilema nomurai Jellyfish Venom in Human Hepatocarcinoma HepG2 Cells. Toxins 2018, 10, 194. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Bae, S.K.; Kim, M.; Pyo, M.J.; Kim, M.; Yang, S.; Won, C.-K.; Yoon, W.D.; Han, C.H.; Kang, C.; et al. Anticancer Effect of Nemopilema nomurai Jellyfish Venom on HepG2 Cells and a Tumor Xenograft Animal Model. Evidence-Based Complement. Altern. Med. 2017, 2017, 2752716. [Google Scholar] [CrossRef] [PubMed]
- Pamela Berilyn T, In vitro angiotensin I converting enzyme inhibition by a peptide isolated from Chiropsalmus quadrigatus Haeckel (box jellyfish) venom hydrolysate., Toxicon. (2016).
- Prakash, R.L.M.; Ravi, D.A.; Hwang, D.H.; Kang, C.; Kim, E. Identification of New Angiotensin-Converting Enzyme Inhibitory Peptides Isolated from the Hydrolysate of the Venom of Nemopilema nomurai Jellyfish. Toxins 2024, 16, 410. [Google Scholar] [CrossRef] [PubMed]
- Kit-WST, D.A. Technical Manual;, Dojindo Mol. Technol. Kumamoto, Japan. (2013).
- Z.Y. Dong, Y., Yan, W., Zhang, Y. Q., & Dai, A novel angiotensin-converting enzyme (ACE) inhibitory peptide from tilapia skin: Preparation, identification and its potential antihypertensive mechanism., Food Chem. (2024).
- F.A.P.. C. Bordon, K.D.C.F.; Cologna, C.T.; Fornari-Baldo, E.C.; Pinheiro-Júnior, E.L.; Cerni, F.A.; Amorim, F.G.; Anjolette, I.A. F.A.; Wiezel, G.A.; Cardoso, From animal poisons and venoms to medicines: Achievements, challenges and perspectives in drug discovery, Front. Pharmacol. (2020).
- M. Fujita, H., Yokoyama, K., & Yoshikawa, Classification and antihypertensive activity of angiotensin I-converting enzyme inhibitory peptides derived from food proteins., J. Food Sci. (2000).
- Priyanto, A.D.; Doerksen, R.J.; Chang, C.-I.; Sung, W.-C.; Widjanarko, S.B.; Kusnadi, J.; Lin, Y.-C.; Wang, T.-C.; Hsu, J.-L. Screening, discovery, and characterization of angiotensin-I converting enzyme inhibitory peptides derived from proteolytic hydrolysate of bitter melon seed proteins. J. Proteom. 2015, 128, 424–435. [Google Scholar] [CrossRef] [PubMed]
- X.D. J. Wu, Characterization of inhibition and stability of soy-protein-derived angiotensin I-converting enzyme inhibitory peptides, Food Res. Int. (2002).
- S.J. Bincy Bhaskar, B. B., Laxmi Ananthanarayan, L. A., & Sahayog Jamdar, Purification, identification, and characterization of novel angiotensin I-converting enzyme (ACE) inhibitory peptides from alcalase digested horse gram flour., (2019).
- Forghani, B.; Zarei, M.; Ebrahimpour, A.; Philip, R.; Bakar, J.; Hamid, A.A.; Saari, N. Purification and characterization of angiotensin converting enzyme-inhibitory peptides derived from Stichopus horrens : Stability study against the ACE and inhibition kinetics. J. Funct. Foods 2016, 20, 276–290. [Google Scholar] [CrossRef]
- Setayesh-Mehr, Z.; Asoodeh, A. The inhibitory activity of HL-7 and HL-10 peptide from scorpion venom ( Hemiscorpius lepturus ) on angiotensin converting enzyme: Kinetic and docking study. Bioorganic Chem. 2017, 75, 30–37. [Google Scholar] [CrossRef] [PubMed]
- B.S. Tsai, J. S., Chen, J. L., & Pan, ACE-inhibitory peptides identified from the muscle protein hydrolysate of hard clam (Meretrix lusoria)., Process Biochem. (2008).
- W. Chen, J., Wang, Y., Zhong, Q., Wu, Y., & Xia, Purification and characterization of a novel angiotensin-I converting enzyme (ACE) inhibitory peptide derived from enzymatic hydrolysate of grass carp protein., Peptides. (2012).
- U.S. Azam F., Alabdullah N.H., Ehmedat H.M., Abulifa A.R., Taban I., NSAIDs as potential treatment option for preventing amyloid β toxicity in Alzheimer’s disease: an investigation by docking, molecular dynamics, and DFT studies., J. Biomol. Struct. Dyn. (2018).
- T.T. Azam F., Abodabos H.S., Taban I.M., Rfieda A.R., Mahmood D., Anwar M.J., Khan S., Sizochenko N., Poli G., Rutin as promising drug for the treatment of Parkinson’s disease: an assessment of MAO-B inhibitory potential by docking, molecular dynamics and DFT studies., Mol. Simul. (2019).
- G.J.L. Hospital A., Goñi J.R., Orozco M., Molecular dynamics simulations: advances and applications., Adv. Appl. Bioinform. Chem. (2015).
- D. Zhu, J., Li, H., Xu, Y., & Wang, Construction of fucoxanthin vector based on binding of whey protein isolate and its subsequent complex coacervation with lysozyme, J. Agric. Food Chem. (2019).
- L.J. Roche, D. B., & McGuffin, In silico identification and characterization of protein-ligand binding sites., Comput. Des. Ligand Bind. Proteins. (2016).
- T. Patel, S., Rauf, A., Khan, H., & Abu-Izneid, Renin-angiotensin-aldosterone (RAAS): The ubiquitous system for homeostasis and pathologies., Biomed. Pharmacother. (2017).
- A.R.A. Sharma, J. N., Uma, K., Noor, A. R., & Rahman, Blood pressure regulation by the kallikrein-kinin system., Gen. Pharmacol. Vasc. Syst. (1996).
- B.M. Maheshwari, S., & Patel, Unravelling the role of cathepsins in cardiovascular diseases., Mol. Biol. Rep. (2024).
- B. Pan, D., Cao, J., Guo, H., & Zhao, Studies on purification and the molecular mechanism of a novel ACE inhibitory peptide from whey protein hydrolysate, Food Chem. (2012).
| Peptide |
Concentration (µM) |
Vmax | Km |
Ki (µM) |
Mode of Inhibition |
| IVGRPLANG | 0 | 1.919 ± 0.00019 |
3.145 ± 0.0196 |
51.389 | - |
| 20 | 1.918 ± 0.00021 |
3.982 ± 0.00383 |
Competitive | ||
| 40 | 1.811 ± 0.00076 |
5.175 ± 0.02686 |
Competitive | ||
| IGDEPRHQYL | 0 | 1.919 ± 0.00019 |
3.147 ± 0.0196 |
5.457 | - |
| 5 | 1.953 ± 0.00613 |
5.801 ± 0.10923 |
Competitive | ||
| 10 | 2.015 ± 0.05609 |
9.198 ± 0.39909 |
Competitive |
|
Targets for IVGRPLANG |
Cluster Number |
Cluster Sizea |
HADDOCK Scoreb | Overall RMSDc | Z-Scored |
| Renin | 4 | 15 | -65.4 ± 2.5 | 2.3 ± 0.0 | -1.2 |
| Cathepsin S | 1 | 42 | -54.5 ± 4.1 | 0.3 ± 0.2 | -1.9 |
| Cathepsin B | 8 | 7 | -27.8 ± 6.2 | 2.8 ± 0.0 | -2.2 |
| Angiotensin Converting Enzyme | 1 | 20 | -17.2 ± 5.1 | 1.5 ± 0.0 | -2.1 |
| Angiotensin II Receptor type 2 | 3 | 18 | -29.5 ± 5.3 | 0.4 ± 0.3 | -2.5 |
|
Targets for IGDEPRHQYL |
|||||
| Angiotensin | 3 | 18 | -72.2 ± 1.8 | 2.2 ± 0.1 | -1.3 |
| Angiotensin II Receptor type 1 | 3 | 12 | -60.3 ± 3.2 | 3.0 ± 0.0 | -1.7 |
| Angiotensin II Receptor type 2 | 3 | 12 | -36.3 ± 4.0 | 0.8 ± 0.2 | -2.9 |
| Renin | 2 | 42 | -67.3 ± 3.5 | 2.2 ± 0.2 | -1 |
| Bradykinin receptor 2 |
1 | 36 | -87.4 ± 2.8 | 2.7 ± 0.1 | -1.9 |
| Kininogen I | 2 | 44 | -115.3 ± 8.3 | 0.3 ± 0.2 | -1.7 |
| Protein Name | Gene Symbol | PDB ID |
| Angiotensin | AGT | 2WXW |
| Angiotensin Receptor 1 | AGTR1 | 4YAY |
| Angiotensin Receptor 2 | AGTR2 | 5UNF |
| Kininogen 1 | KNG1 | 7F6H |
| Angiotensin-Converting Enzyme 2 | ACE2 | 6M1D |
| Angiotensin-Converting Enzyme | ACE | 6M1D |
| Cathepsin S | CTSS | 4P6G |
| Cathepsin B | CTSB | 1GMY |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





