Submitted:
27 May 2025
Posted:
28 May 2025
You are already at the latest version
Abstract
Keywords:
Introduction
A Review of FATE Pathophysiology: Current Evidence and Knowledge Gap
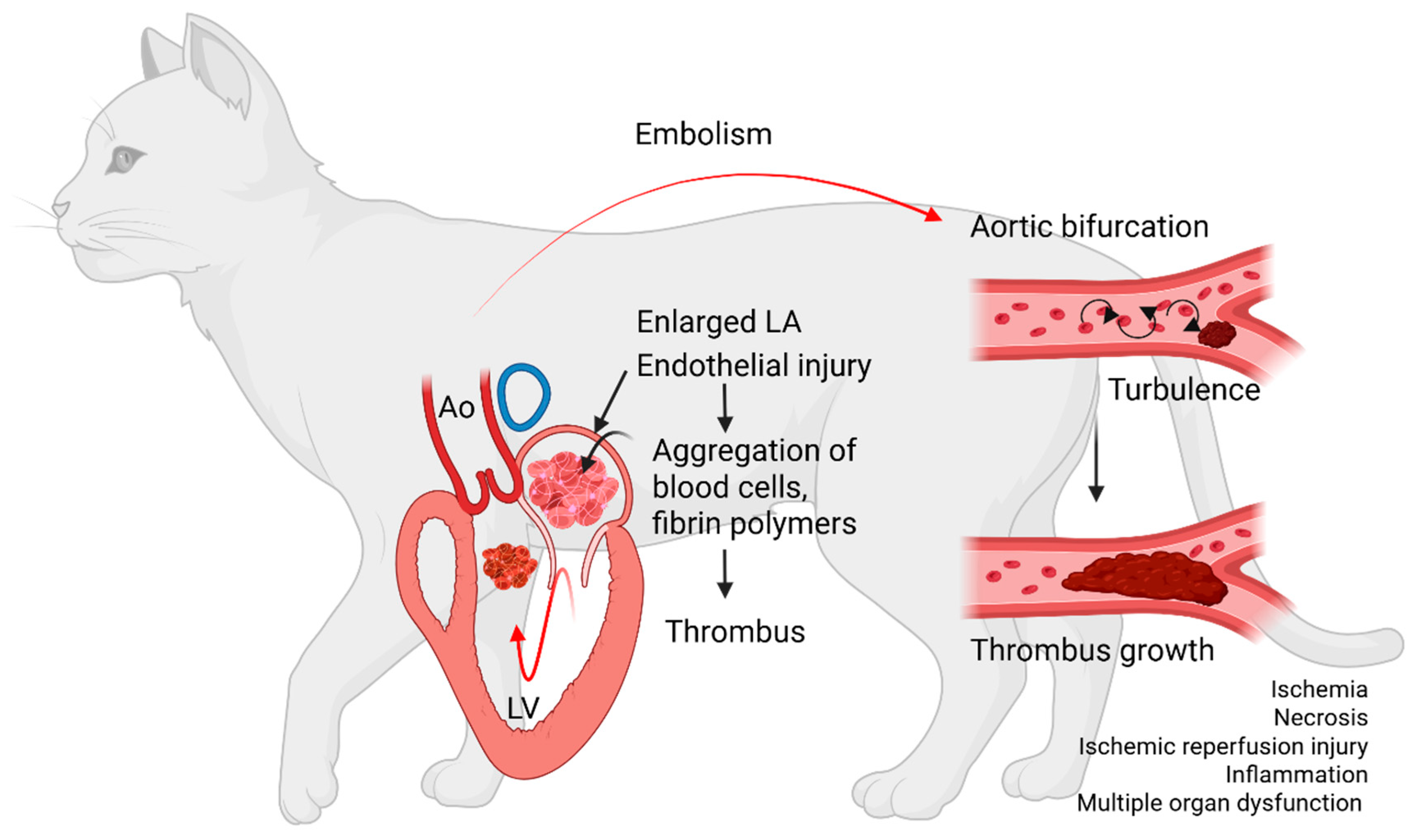
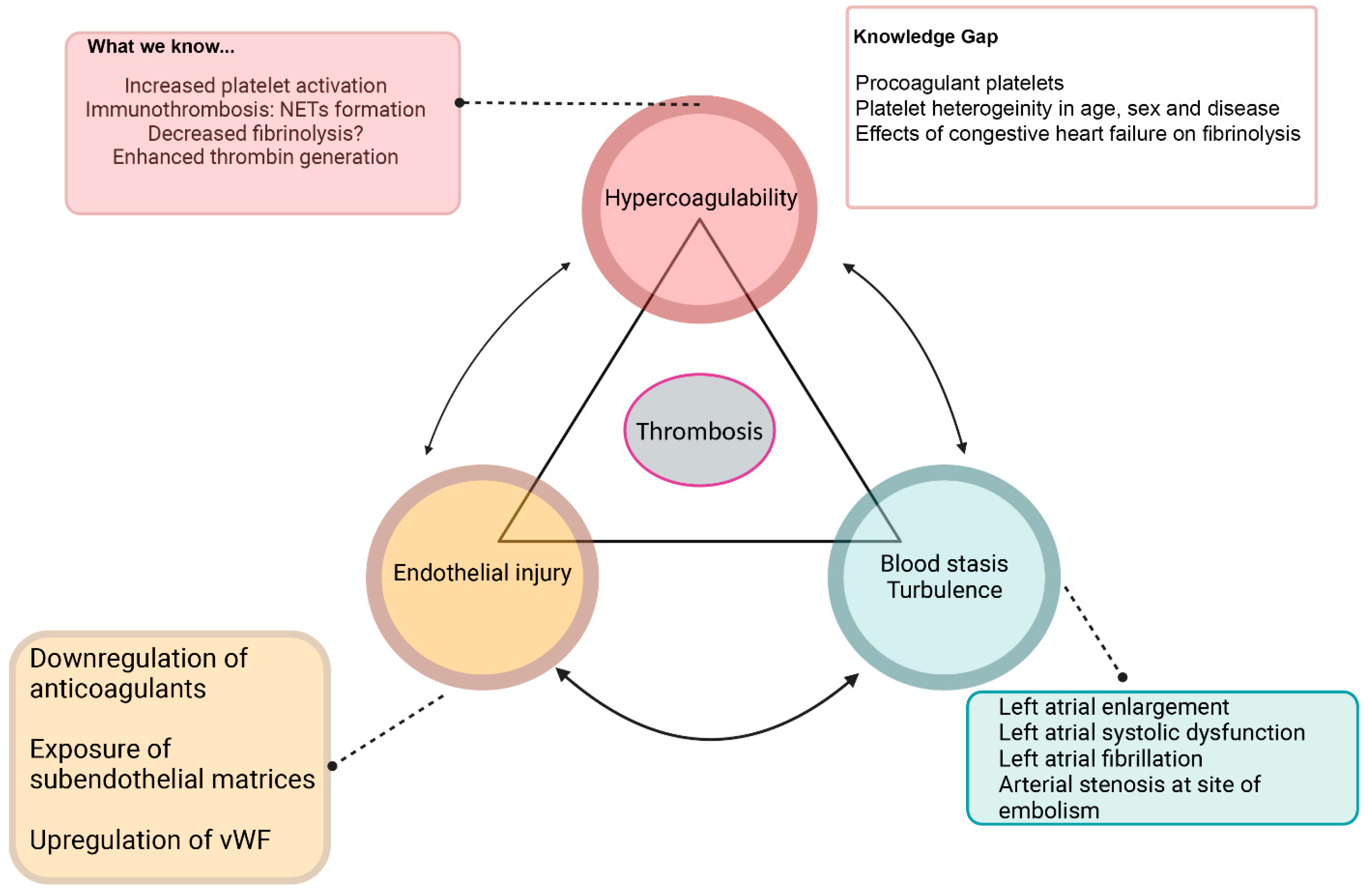
- 1. Hypercoagulability
- Platelet activation
- Systemic hypercoagulability
- Alternations in fibrinolysis
- Immunothrombosis
- 2. Blood stasis
- 3. Endothelial injury
Knowledge Gap and Future Research Directions in FATE Pathogenesis:
- 1. Procoagulant platelets
- 2. Immunothrombosis
- 3. Congestive heart failure and fibrinolysis
- 4. Blood flow stasis and endothelial injury
Current Risk Factors of FATE
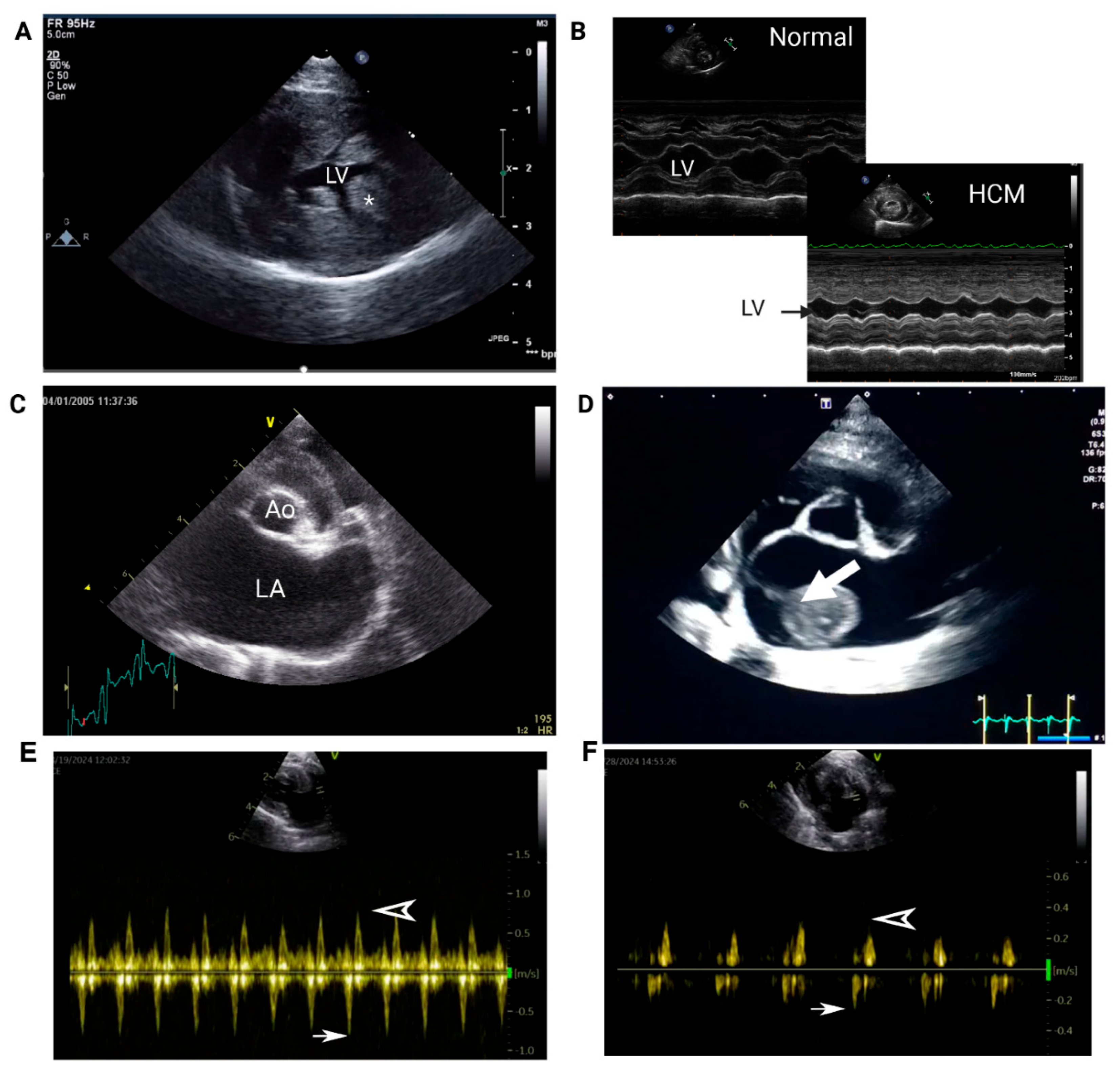
- 1. Echocardiography
- 2. Cardiac Biomarkers
Knowledge Gaps and Future Directions
- 1. Platelet heterogeneity as a risk factor of FATE
- 2. Hematological variables as risk factors of FATE
- 3. NETs as biomarkers of FATE
- 4. Other Biomarkers of FATE
Current Recommendations of Primary and Secondary FATE Prevention
Knowledge Gap and Future Directions in Optimizing Prevention Strategies
- 1. Genetic testing
- 2. Platelet function testing in clinical practice
- 3. Monitoring of anticoagulants
- 4. Viscoelastic testing to monitor antithrombotic therapies
Novel Antithrombotic Therapies
- 1. Rapamycin
- 2. Non-anticoagulated Heparins
- 3. Thrombolytic therapy
- 4. Histone Deacetylase Inhibitors and scavenging
Conclusion
Funding
Declaration statement
Ethical approval
Informed consent
References
- Smith SA, Tobias AH. Feline arterial thromboembolism: an update. Vet Clin Small Anim Pract. 2004 Sep 1;34(5):1245–71. [CrossRef]
- Rush JE, Freeman LM, Fenollosa NK, Brown DJ. Population and survival characteristics of cats with hypertrophic cardiomyopathy: 260 cases (1990-1999). J Am Vet Med Assoc. 2002 Jan 15;220(2):202–7. [CrossRef]
- Schoeman JP. Feline Distal Aortic Thromboembolism: A Review of 44 Cases (1990–1998). J Feline Med Surg. 1999 Dec;1(4):221–31. [CrossRef]
- Smith SA, Tobias AH, Jacob KA, Fine DM, Grumbles PL. Arterial Thromboembolism in Cats: Acute Crisis in 127 Cases (1992–2001) and Long-Term Management with Low-Dose Aspirin in 24 Cases. J Vet Intern Med. 2003;17(1):73–83.
- Lo ST, Walker AL, Georges CJ, Li RH, Stern JA. Dual therapy with clopidogrel and rivaroxaban in cats with thromboembolic disease. J Feline Med Surg. 2022 Apr 1;24(4):277–83. [CrossRef]
- Hogan DF, Fox PR, Jacob K, Keene B, Laste NJ, Rosenthal S, et al. Secondary prevention of cardiogenic arterial thromboembolism in the cat: the double-blind, randomized, positive-controlled feline arterial thromboembolism; clopidogrel vs. aspirin trial (FAT CAT). J Vet Cardiol. 2015 Dec;17:S306–17. [CrossRef]
- Fox PR, Keene BW, Lamb K, Schober KA, Chetboul V, Luis Fuentes V, et al. International collaborative study to assess cardiovascular risk and evaluate long-term health in cats with preclinical hypertrophic cardiomyopathy and apparently healthy cats: The REVEAL Study. J Vet Intern Med. 2018;32(3):930–43. [CrossRef]
- Shaverdian M, Li RHL. Preventing Cardiogenic Thromboembolism in Cats: Literature Gaps, Rational Recommendations, and Future Therapies. Vet Clin North Am Small Anim Pract. 2023 Nov 1;53(6):1309–23.
- Scharf RE. Platelet Signaling in Primary Haemostasis and Arterial Thrombus Formation: Part 1. Hämostaseologie. 2018 Oct 23;38:203–10. [CrossRef]
- Gale AJ. Continuing Education Course #2: Current Understanding of Hemostasis. Toxicol Pathol. 2011 Jan 1;39(1):273–80. [CrossRef]
- Tablin F, Schumacher T, Pombo M, Marion CT, Huang K, Norris JW, et al. Platelet Activation in Cats with Hypertrophic Cardiomyopathy. J Vet Intern Med. 2014 Mar;28(2):411–8. [CrossRef]
- Li RHL, Stern JA, Ho V, Tablin F, Harris SP. Platelet Activation and Clopidogrel Effects on ADP -Induced Platelet Activation in Cats with or without the A31P Mutation in MYBPC 3 . J Vet Intern Med. 2016 Sep;30(5):1619–29.
- Helenski CA, Ross JN. Platelet Aggregation in Feline Cardiomyopathy. J Vet Intern Med. 1987 Jan;1(1):24–8. [CrossRef]
- Tan AWK, Li RHL, Ueda Y, Stern JA, Hussain M, Haginoya S, et al. Platelet Priming and Activation in Naturally Occurring Thermal Burn Injuries and Wildfire Smoke Exposure Is Associated With Intracardiac Thrombosis and Spontaneous Echocardiographic Contrast in Feline Survivors. Front Vet Sci [Internet]. 2022 Jul 14 [cited 2025 Jan 27];9. Available from: https://www.frontiersin.org/journals/veterinary-science/articles/10.3389/fvets.2022.892377/full. [CrossRef]
- Bédard C, Lanevschi-Pietersma A, Dunn M. Evaluation of coagulation markers in the plasma of healthy cats and cats with asymptomatic hypertrophic cardiomyopathy. Vet Clin Pathol. 2007;36(2):167–72. [CrossRef]
- Wells M, Sheffield W, Blajchman M. The Clearance of Thrombin-antithrombin and Related Serpin-enzyme Complexes from the Circulation: Role of Various Hepatocyte Receptors. Thromb Haemost. 1999;81(03):325–37. [CrossRef]
- Smith SA. The cell-based model of coagulation. J Vet Emerg Crit Care. 2009;19(1):3–10.
- Johnson AJ, Rozanski EA, De Laforcade AM, Davila C, Rush JE, Guillaumin J. Viscoelastic coagulation monitoring parameters in cats with acute arterial thromboembolism. J Vet Intern Med. 2024 Jul;38(4):2045–51. [CrossRef]
- Moses IA, Hallowell TC, Johnson JA. Feline aortic thromboembolism with and without congestive heart failure did not exhibit hypercoagulability using a novel viscoelastic coagulation monitor. Am J Vet Res. 2024 Aug 1;85(8):ajvr.24.03.0065. [CrossRef]
- Rosati T, Jandrey KE, Stern JA, Nguyen N, Li RHL. Evaluation of clopidogrel response in healthy cats using a novel viscoelastic test and thromboelastography. Front Vet Sci. 2024 Jun 18;11:1371781. [CrossRef]
- Hennink I, Peters L, van Geest G, Adamik KN. Evaluation of a Viscoelastic Coagulation Monitoring System (VCM Vet®) and Its Correlation with Thromboelastometry (ROTEM®) in Diseased and Healthy Dogs. Animals. 2023 Jan;13(3):405.
- Buriko Y, Chalifoux NV, Clarkin-Breslin R, Silverstein DC. Comparison of a viscoelastic point-of-care coagulation monitor with thromboelastography in sick dogs with hemostatic abnormalities. Vet Clin Pathol. 2023 Jun;52(2):217–27. [CrossRef]
- Kane KK. Fibrinolysis--a review. Ann Clin Lab Sci. 1984 Nov 1;14(6):443–9.
- Busato F, Drigo M, Zoia A. Reduced risk of arterial thromboembolism in cats with pleural effusion due to congestive heart failure. J Feline Med Surg. 2022 Aug;24(8):e142–52. [CrossRef]
- Li RHL, Tablin F. A Comparative Review of Neutrophil Extracellular Traps in Sepsis. Front Vet Sci [Internet]. 2018 Nov 28 [cited 2025 Jan 28];5. Available from: https://www.frontiersin.org/journals/veterinary-science/articles/10.3389/fvets.2018.00291/full. [CrossRef]
- Ryan TAJ, O’Neill LAJ. Innate immune signaling and immunothrombosis: New insights and therapeutic opportunities. Eur J Immunol. 2022 Jul;52(7):1024–34. [CrossRef]
- Clark SR, Ma AC, Tavener SA, McDonald B, Goodarzi Z, Kelly MM, et al. Platelet TLR4 activates neutrophil extracellular traps to ensnare bacteria in septic blood. Nat Med. 2007 Apr;13(4):463–9.
- Li P, Li M, Lindberg MR, Kennett MJ, Xiong N, Wang Y. PAD4 is essential for antibacterial innate immunity mediated by neutrophil extracellular traps. J Exp Med. 2010 Aug 23;207(9):1853–62. [CrossRef]
- McDonald B, Davis RP, Kim SJ, Tse M, Esmon CT, Kolaczkowska E, et al. Platelets and neutrophil extracellular traps collaborate to promote intravascular coagulation during sepsis in mice. Blood. 2017 Mar 9;129(10):1357–67. [CrossRef]
- Pilsczek FH, Salina D, Poon KKH, Fahey C, Yipp BG, Sibley CD, et al. A Novel Mechanism of Rapid Nuclear Neutrophil Extracellular Trap Formation in Response to Staphylococcus aureus. J Immunol. 2010 Dec 15;185(12):7413–25. [CrossRef]
- Nicolai L, Leunig A, Brambs S, Kaiser R, Weinberger T, Weigand M, et al. Immunothrombotic Dysregulation in COVID-19 Pneumonia Is Associated With Respiratory Failure and Coagulopathy. Circulation. 2020 Sep 22;142(12):1176–89. [CrossRef]
- Stark K, Massberg S. Interplay between inflammation and thrombosis in cardiovascular pathology. Nat Rev Cardiol. 2021 Sep;18(9):666–82. [CrossRef]
- Mesa MA, Vasquez G. NETosis. Autoimmune Dis. 2013;2013:1–7.
- Schulz C, Massberg S. Demystifying the prothrombotic role of NETs. Blood. 2017 Feb 23;129(8):925–6. [CrossRef]
- Zhang Y, Wang C, Yu M, Zhao X, Du J, Li Y, et al. Neutrophil extracellular traps induced by activated platelets contribute to procoagulant activity in patients with colorectal cancer. Thromb Res. 2019 Aug;180:87–97. [CrossRef]
- Zhou P, Li T, Jin J, Liu Y, Li B, Sun Q, et al. Interactions between neutrophil extracellular traps and activated platelets enhance procoagulant activity in acute stroke patients with ICA occlusion. eBioMedicine [Internet]. 2020 Mar 1 [cited 2025 Jan 28];53. Available from: https://www.thelancet.com/journals/ebiom/article/PIIS2352-3964(20)30046-3/fulltext. [CrossRef]
- Li RHL, Fabella A, Nguyen N, Kaplan JL, Ontiveros E, Stern JA. Circulating neutrophil extracellular traps in cats with hypertrophic cardiomyopathy and cardiogenic arterial thromboembolism. J Vet Intern Med. 2023 Mar;37(2):490–502. [CrossRef]
- Fuchs TA, Bhandari AA, Wagner DD. Histones induce rapid and profound thrombocytopenia in mice. Blood. 2011 Sep 29;118(13):3708–14. [CrossRef]
- Semeraro F, Ammollo CT, Morrissey JH, Dale GL, Friese P, Esmon NL, et al. Extracellular histones promote thrombin generation through platelet-dependent mechanisms: involvement of platelet TLR2 and TLR4. Blood. 2011 Aug 18;118(7):1952–61. [CrossRef]
- Schober KE, Maerz I. Assessment of Left Atrial Appendage Flow Velocity and its Relation to Spontaneous Echocardiographic Contrast in 89 Cats with Myocardial Disease. J Vet Intern Med. 2006 Jan;20(1):120–30.
- Neubauer K, Zieger B. Endothelial cells and coagulation. Cell Tissue Res. 2022 Mar;387(3):391–8.
- De Wouwer MV, Collen D, Conway EM. Thrombomodulin-Protein C-EPCR System: Integrated to Regulate Coagulation and Inflammation. Arterioscler Thromb Vasc Biol. 2004 Aug;24(8):1374–83.
- Ciaramella P, Piantedosi D, Lindquist E, Loria AD, Cortese L, Skeels M, et al. Plasma Thrombomodulin (TM) Concentration in Cats with Cardiomyopathies. Vet Res Commun. 2006 Aug;30(S1):289–91. [CrossRef]
- Oliver JJ, Webb DJ, Newby DE. Stimulated Tissue Plasminogen Activator Release as a Marker of Endothelial Function in Humans. Arterioscler Thromb Vasc Biol. 2005 Dec;25(12):2470–9. [CrossRef]
- Cambronero F, Vilchez JA, García-Honrubia A, Ruiz-Espejo F, Moreno V, Hernández-Romero D, et al. Plasma levels of Von Willebrand factor are increased in patients with hypertrophic cardiomyopathy. Thromb Res. 2010 Jul;126(1):e46–50. [CrossRef]
- Stokol T, Brooks M, Rush JE, Rishniw M, Erb H, Rozanski E, et al. Hypercoagulability in Cats with Cardiomyopathy. J Vet Intern Med. 2008 May;22(3):546–52. [CrossRef]
- Cheng WC, Wilkie L, Kurosawa TA, Dobromylskyj M, Priestnall SL, Luis Fuentes V, et al. Immunohistological Evaluation of Von Willebrand Factor in the Left Atrial Endocardium and Atrial Thrombi from Cats with Cardiomyopathy. Animals. 2021 Apr 26;11(5):1240. [CrossRef]
- Agbani EO, Poole AW. Procoagulant platelets: generation, function, and therapeutic targeting in thrombosis. Blood. 2017 Nov 16;130(20):2171–9.
- Nechipurenko DY, Receveur N, Yakimenko AO, Shepelyuk TO, Yakusheva AA, Kerimov RR, et al. Clot Contraction Drives the Translocation of Procoagulant Platelets to Thrombus Surface. Arterioscler Thromb Vasc Biol. 2019 Jan;39(1):37–47. [CrossRef]
- Prodan CI, Stoner JA, Dale GL. Lower Coated-Platelet Levels Are Associated With Increased Mortality After Spontaneous Intracerebral Hemorrhage. Stroke. 2015 Jul;46(7):1819–25. [CrossRef]
- Prodan CI, Vincent AS, Dale GL. Coated-platelet levels are elevated in patients with transient ischemic attack. Transl Res J Lab Clin Med. 2011 Jul;158(1):71–5. [CrossRef]
- Pasalic L, Wing-Lun E, Lau JK, Campbell H, Pennings GJ, Lau E, et al. Novel assay demonstrates that coronary artery disease patients have heightened procoagulant platelet response. J Thromb Haemost JTH. 2018 Jun;16(6):1198–210. [CrossRef]
- Shaverdian M, Nguyen N, Li RHL. A novel technique to characterize procoagulant platelet formation and evaluate platelet procoagulant tendency in cats by flow cytometry. Front Vet Sci [Internet]. 2024 Dec 16 [cited 2025 Mar 30];11. Available from: https://www.frontiersin.org/journals/veterinary-science/articles/10.3389/fvets.2024.1480756/full. [CrossRef]
- Li RH, Nguyen N, Stern JA, Duler LM. Neutrophil extracellular traps in feline cardiogenic arterial thrombi: a pilot study. J Feline Med Surg. 2022 Jun 1;24(6):580–6. [CrossRef]
- Sreeramkumar V, Adrover JM, Ballesteros I, Cuartero MI, Rossaint J, Bilbao I, et al. Neutrophils scan for activated platelets to initiate inflammation. Science. 2014 Dec 5;346(6214):1234–8. [CrossRef]
- Etulain J, Martinod K, Wong SL, Cifuni SM, Schattner M, Wagner DD. P-selectin promotes neutrophil extracellular trap formation in mice. Blood. 2015 Jul 9;126(2):242–6. [CrossRef]
- Carestia A, Kaufman T, Rivadeneyra L, Landoni VI, Pozner RG, Negrotto S, et al. Mediators and molecular pathways involved in the regulation of neutrophil extracellular trap formation mediated by activated platelets. J Leukoc Biol. 2016 Jan 1;99(1):153–62. [CrossRef]
- Welles EG, Boudreaux MK, Crager CS, Tyler JW. Platelet function and antithrombin, plasminogen, and fibrinolytic activities in cats with heart disease. 1994 May 1 [cited 2025 Mar 30]; Available from: https://avmajournals.avma.org/view/journals/ajvr/55/5/ajvr.1994.55.05.619.xml.
- Siostrzonek P, Koppensteiner R, Gössinger H, Zangeneh M, Heinz G, Kreiner G, et al. Hemodynamic and hemorheologic determinants of left atrial spontaneous echo contrast and thrombus formation in patients with idiopathic dilated cardiomyopathy. Am Heart J. 1993 Feb;125(2 Pt 1):430–4. [CrossRef]
- Kirby R, Linklater A. Monitoring and Intervention for the Critically Ill Small Animal: The Rule of 20. John Wiley & Sons; 2016. 437 p.
- Moresco RN, Júnior RH, Vargas LCR, Silla LMDR. Association between plasma levels of D-dimer and fibrinogen/fibrin degradation products (FDP) for exclusion of thromboembolic disorders. J Thromb Thrombolysis. 2006 Apr;21(2):199–202. [CrossRef]
- Kim TY, Han SH, Choi R, Hyun C. Evaluation of Plasma D-dimer Concentration in Cats with Hypertrophic Cardiomyopathy. J Vet Clin. 2014 Apr 1;31(2):85–9.
- Reed G, Houng A, Singh S, Wang D. α2-Antiplasmin: New Insights and Opportunities for Ischemic Stroke. Semin Thromb Hemost. 2016 Jul 29;43(02):191–9. [CrossRef]
- Singh S, Saleem S, Reed GL. Alpha2-Antiplasmin: The Devil You Don’t Know in Cerebrovascular and Cardiovascular Disease. Front Cardiovasc Med [Internet]. 2020 Dec 23 [cited 2025 Mar 30];7. Available from: https://www.frontiersin.org/journals/cardiovascular-medicine/articles/10.3389/fcvm.2020.608899/full. [CrossRef]
- Singh S, Houng AK, Reed GL. Venous stasis-induced fibrinolysis prevents thrombosis in mice: role of α2-antiplasmin. Blood. 2019 Sep 19;134(12):970–8. [CrossRef]
- Meltzer ME, Doggen CJM, de Groot PG, Rosendaal FR, Lisman T. Plasma levels of fibrinolytic proteins and the risk of myocardial infarction in men. Blood. 2010 Jul 29;116(4):529–36. [CrossRef]
- Merino A, Hauptman P, Badimon L, Badimon JJ, Cohen M, Fuster V, et al. Echocardiographic “smoke” is produced by an interaction of erythrocytes and plasma proteins modulated by shear forces. J Am Coll Cardiol. 1992 Dec 1;20(7):1661–8. [CrossRef]
- Rastegar R, Harnick DJ, Weidemann P, Fuster V, Coller B, Badimon JJ, et al. Spontaneous echo contrast videodensity isflow-related and is dependent on the relative concentrations of fibrinogen and red blood cells. JACC. 2003 Feb 19;41(4):603–10. [CrossRef]
- Zotz RJ, Müller M, Genth-Zotz S, Darius H. Spontaneous Echo Contrast Caused by Platelet and Leukocyte Aggregates? Stroke. 2001 May;32(5):1127–33. [CrossRef]
- Le Tourneau T, Susen S, Caron C, Millaire A, Maréchaux S, Polge AS, et al. Functional Impairment of Von Willebrand Factor in Hypertrophic Cardiomyopathy. Circulation. 2008 Oct 7;118(15):1550–7.
- Ammash N, Konik EA, McBane RD, Chen D, Tange JI, Grill DE, et al. Left Atrial Blood Stasis and Von Willebrand Factor–ADAMTS13 Homeostasis in Atrial Fibrillation. Arterioscler Thromb Vasc Biol. 2011 Nov;31(11):2760–6. [CrossRef]
- Payne JR, Borgeat K, Connolly DJ, Boswood A, Dennis S, Wagner T, et al. Prognostic Indicators in Cats with Hypertrophic Cardiomyopathy. J Vet Intern Med. 2013 Nov;27(6):1427–36.
- Payne JR, Borgeat K, Brodbelt DC, Connolly DJ, Luis Fuentes V. Risk factors associated with sudden death vs. congestive heart failure or arterial thromboembolism in cats with hypertrophic cardiomyopathy. J Vet Cardiol. 2015 Dec;17:S318–28.
- Hsu A, Kittleson MD, Paling A. Investigation into the use of plasma NT-proBNP concentration to screen for feline hypertrophic cardiomyopathy. J Vet Cardiol. 2009 May;11:S63–70. [CrossRef]
- Bakirel U, Ulgen Saka S, Yildiz K. Feline Arteriyel Tromboembolizm Tanısı ve Prognozunda Kardiyak Biyobelirteçlerin Rolü ve Önemi. Kafkas Univ Vet Fak Derg [Internet]. 2021 [cited 2025 Jan 27]; Available from: http://vetdergikafkas.org/uploads/pdf/pdf_KVFD_2821.pdf.
- Harris AN, Beatty SS, Estrada AH, Winter B, Bohannon M, Sosa I, et al. Investigation of an N-Terminal Prohormone of Brain Natriuretic Peptide Point-of-Care ELISA in Clinically Normal Cats and Cats With Cardiac Disease. J Vet Intern Med. 2017 Jul;31(4):994–9. [CrossRef]
- Lu T, Côté E, Kuo Y, Wu H, Wang W, Hung Y. Point-of-care N-terminal pro B-type natriuretic peptide assay to screen apparently healthy cats for cardiac disease in general practice. J Vet Intern Med. 2021 Jul;35(4):1663–72.
- Hertzsch S, Roos A, Wess G. Evaluation of a sensitive cardiac troponin I assay as a screening test for the diagnosis of hypertrophic cardiomyopathy in cats. J Vet Intern Med. 2019 May;33(3):1242–50. [CrossRef]
- Hori Y, Iguchi M, Heishima Y, Yamashita Y, Nakamura K, Hirakawa A, et al. Diagnostic utility of cardiac troponin I in cats with hypertrophic cardiomyopathy. J Vet Intern Med. 2018;32(3):922–9. [CrossRef]
- Hemdon WE, Kittleson MD, Sanderson K, Drobatz KJ, Clifford CA, Gelzer A, et al. Cardiac Troponin I in Feline Hypertrophic Cardiomyopathy. J Vet Intern Med. 2002;16(5):558–64.
- Park Y, Schoene N, Harris W. Mean platelet volume as an indicator of platelet activation: methodological issues. Platelets. 2002 Jan;13(5–6):301–6. [CrossRef]
- Fries RC, Kadotani S, Stack JP, Kruckman L, Wallace G. Prognostic Value of Neutrophil-to-Lymphocyte Ratio in Cats With Hypertrophic Cardiomyopathy. Front Vet Sci. 2022 Mar 14;9:813524. [CrossRef]
- Naito E, Yuki M, Hirano T, Kainuma D, Aoyama R. Prognostic utility of preoperative neutrophil–lymphocyte ratio in cats with malignant mammary tumors. Res Vet Sci. 2021 Mar;135:349–54. [CrossRef]
- Neumann S. Neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios in dogs and cats with acute pancreatitis. Vet Clin Pathol. 2021 Mar;50(1):45–51.
- Joshua J, Caswell JL, Monné Rodriguez JM, Kipar A, O’Sullivan ML, Wood G, et al. MicroRNA profiling of the feline left heart identifies chamber-specific expression signatures in health and in advanced hypertrophic cardiomyopathy. J Mol Cell Cardiol Plus. 2023 Jun 1;4:100037. [CrossRef]
- Circulating MicroRNAs Identify Early Phenotypic Changes in Sarcomeric Hypertrophic Cardiomyopathy | Circulation: Heart Failure [Internet]. [cited 2025 Apr 3]. Available from: https://www.ahajournals.org/doi/10.1161/CIRCHEARTFAILURE.122.010291. [CrossRef]
- Goggs R, Bacek L, Bianco D, Koenigshof A, Li RHL. Consensus on the Rational Use of Antithrombotics in Veterinary Critical Care (CURATIVE): Domain 2—Defining rational therapeutic usage. J Vet Emerg Crit Care. 2019 Jan;29(1):49–59. [CrossRef]
- Brainard BM, Coleman AE, Kurosawa A, Rush JE, Hogan DF, Brooks MB, et al. Therapy with clopidogrel or rivaroxaban has equivalent impacts on recurrence of thromboembolism and survival in cats following cardiogenic thromboembolism: the SUPERCAT study. J Am Vet Med Assoc. 2024 Dec 18;1–10. [CrossRef]
- Lo ST, Li RHL, Georges CJ, Nguyen N, Chen CK, Stuhlmann C, et al. Synergistic inhibitory effects of clopidogrel and rivaroxaban on platelet function and platelet-dependent thrombin generation in cats. J Vet Intern Med. 2023;37(4):1390–400. [CrossRef]
- Mitropoulou A, Hassdenteufel E, Lin J, Bauer N, Wurtinger G, Vollmar C, et al. Retrospective Evaluation of Intravenous Enoxaparin Administration in Feline Arterial Thromboembolism. Anim Open Access J MDPI. 2022 Aug 4;12(15):1977. [CrossRef]
- Luis Fuentes V, Abbott J, Chetboul V, Côté E, Fox PR, Häggström J, et al. ACVIM consensus statement guidelines for the classification, diagnosis, and management of cardiomyopathies in cats. J Vet Intern Med. 2020 May;34(3):1062–77.
- Ueda Y, Li RHL, Nguyen N, Ontiveros ES, Kovacs SL, Oldach MS, et al. A genetic polymorphism in P2RY1 impacts response to clopidogrel in cats with hypertrophic cardiomyopathy. Sci Rep. 2021 Jun 15;11(1):12522. [CrossRef]
- Weber K, Rostert N, Bauersachs S, Wess G. Serum microRNA profiles in cats with hypertrophic cardiomyopathy. Mol Cell Biochem. 2015 Apr 1;402(1):171–80. [CrossRef]
- Hyatt CE, Brainard BM. Point of Care Assessment of Coagulation. Top Companion Anim Med. 2016 Mar;31(1):11–7. [CrossRef]
- Kornya MR, Abrams-Ogg ACG, Blois SL, Wood RD. Platelet function analyzer-200 closure curve analysis and assessment of flow-obstructed samples. Vet Clin Pathol. 2023 Dec;52(4):576–82. [CrossRef]
- Jandrey KE. Assessment of platelet function. J Vet Emerg Crit Care. 2012 Feb;22(1):81–98.
- Teuber M, Mischke R. Influence of a low dosage of clopidogrel on platelet function in cats as measured by the platelet function analyser PFA-100 and the multiplate analyser. Res Vet Sci. 2016 Dec;109:149–56. [CrossRef]
- Den Toom ML, Van Leeuwen MW, Szatmári V, Teske E. Effects of clopidogrel therapy on whole blood platelet aggregation, the Plateletworks® assay and coagulation parameters in cats with asymptomatic hypertrophic cardiomyopathy: a pilot study. Vet Q. 2017 Jan 1;37(1):8–15.
- Ho KK, Abrams-Ogg AC, Wood RD, O’Sullivan ML, Kirby GM, Blois SL. Assessment of platelet function in healthy cats in response to commonly prescribed antiplatelet drugs using three point-of-care platelet function tests. J Feline Med Surg. 2017 Jun;19(6):638–47. [CrossRef]
- Gouin-Thibault I, Pautas E, Siguret V. Safety Profile of Different Low-Molecular Weight Heparins Used at Therapeutic Dose. Drug Saf. 2005 Apr 1;28(4):333–49. [CrossRef]
- Dixon-Jimenez AC, Brainard BM, Brooks MB, Nie B, Arnold RD, Loper D, et al. Pharmacokinetic and pharmacodynamic evaluation of oral rivaroxaban in healthy adult cats. J Vet Emerg Crit Care San Antonio Tex 2001. 2016 Sep;26(5):619–29. [CrossRef]
- Alwood AJ, Downend AB, Brooks MB, Slensky KA, Fox JA, Simpson SA, et al. Anticoagulant Effects of Low-Molecular-Weight Heparins in Healthy Cats. J Vet Intern Med. 2007;21(3):378–87. [CrossRef]
- McMichael MA, Smith SA. Viscoelastic coagulation testing: technology, applications, and limitations. Vet Clin Pathol. 2011 Jun;40(2):140–53. [CrossRef]
- Hennink I, Peters L, Van Geest G, Adamik KN. Evaluation of a Viscoelastic Coagulation Monitoring System (VCM Vet®) and Its Correlation with Thromboelastometry (ROTEM®) in Diseased and Healthy Dogs. Animals. 2023 Jan 25;13(3):405.
- Bowbrick VA, Mikhailidis DP, Stansby G. Influence of platelet count and activity on thromboelastography parameters. Platelets. 2003 Jun 1;14(4):219. [CrossRef]
- Saxton RA, Sabatini DM. mTOR Signaling in Growth, Metabolism, and Disease. Cell. 2017 Mar 9;168(6):960–76.
- Kaplan JL, Rivas VN, Walker AL, Grubb L, Farrell A, Fitzgerald S, et al. Delayed-release rapamycin halts progression of left ventricular hypertrophy in subclinical feline hypertrophic cardiomyopathy: results of the RAPACAT trial. 2023 Nov 1 [cited 2025 Apr 5]; Available from: https://avmajournals.avma.org/view/journals/javma/261/11/javma.23.04.0187.xml. [CrossRef]
- Rivas VN, Kaplan JL, Kennedy SA, Fitzgerald S, Crofton AE, Farrell A, et al. Multi-Omic, Histopathologic, and Clinicopathologic Effects of Once-Weekly Oral Rapamycin in a Naturally Occurring Feline Model of Hypertrophic Cardiomyopathy: A Pilot Study. Anim Open Access J MDPI. 2023 Oct 12;13(20):3184. [CrossRef]
- Śledź KM, Moore SF, Durrant TN, Blair TA, Hunter RW, Hers I. Rapamycin restrains platelet procoagulant responses via FKBP-mediated protection of mitochondrial integrity. Biochem Pharmacol. 2020 Jul 1;177:113975. [CrossRef]
- Aslan JE, Tormoen GW, Loren CP, Pang J, McCarty OJT. S6K1 and mTOR regulate Rac1-driven platelet activation and aggregation. Blood. 2011 Sep 15;118(11):3129–36. [CrossRef]
- Babinska A, Markell MS, Salifu MO, Akoad M, Ehrlich YH, Kornecki E. Enhancement of human platelet aggregation and secretion induced by rapamycin. Nephrol Dial Transplant Off Publ Eur Dial Transpl Assoc - Eur Ren Assoc. 1998 Dec;13(12):3153–9. [CrossRef]
- Abstracts from the International Veterinary Emergency and Critical Care Symposium and the European Veterinary Emergency and Critical Care Annual Congress 2024. J Vet Emerg Crit Care. 2024;34(S2):S2–47.
- Huang X, Han S, Liu X, Wang T, Xu H, Xia B, et al. Both UFH and NAH alleviate shedding of endothelial glycocalyx and coagulopathy in LPS-induced sepsis. Exp Ther Med. 2020 Feb;19(2):913–22. [CrossRef]
- Alberts MJ. tPA in acute ischemic stroke: United States experience and issues for the future. Neurology [Internet]. 1998 Sep [cited 2025 Jan 23];51(3_suppl_3). Available from: https://www.neurology.org/doi/10.1212/WNL.51.3_Suppl_3.S53. [CrossRef]
- Dewar B, Shamy M. tPA for Acute Ischemic Stroke and Its Controversies: A Review. The Neurohospitalist. 2020 Jan;10(1):5–10. [CrossRef]
- Li S, Gu HQ, Li H, Wang X, Jin A, Guo S, et al. Reteplase versus Alteplase for Acute Ischemic Stroke. N Engl J Med. 2024 Jun 26;390(24):2264–73. [CrossRef]
- Guillaumin J, DeFrancesco TC, Scansen BA, Quinn R, Whelan M, Hanel R, et al. Bilateral lysis of aortic saddle thrombus with early tissue plasminogen activator (BLASTT): a prospective, randomized, placebo-controlled study in feline acute aortic thromboembolism. J Feline Med Surg. 2022 Dec;24(12):e535–45. [CrossRef]
- Sharp CR, Blais MC, Boyd CJ, Brainard BM, Chan DL, de Laforcade A, et al. 2022 Update of the Consensus on the Rational Use of Antithrombotics and Thrombolytics in Veterinary Critical Care (CURATIVE) Domain 6: Defining rational use of thrombolytics. J Vet Emerg Crit Care. 2022;32(4):446–70. [CrossRef]
- E. Moore K, Morris N, Dhupa N, J. Murtaugh R, E. Rush J. Retrospective Study of Streptokinase Administration in 46 Cats with Arterial Thromboembolism. J Vet Emerg Crit Care. 2000 Dec;10(4):245–57.
- Ramsey CC, Riepe RD, Macintire DK, Burney DP. Streptokinase a practical clot buster. In: Proceeding of the 5th International Veterinary Emergency and Critical Care Symposium. 1996. p. 225–8.
- ISIS-3: a randomised comparison of streptokinase vs tissue plasminogen activator vs anistreplase and of aspirin plus heparin vs aspirin alone among 41 299 cases of suspected acute myocardial infarction. The Lancet. 1992 Mar 28;339(8796):753–70.
- null null. An International Randomized Trial Comparing Four Thrombolytic Strategies for Acute Myocardial Infarction. N Engl J Med. 1993 Sep 2;329(10):673–82.
- Whelan MF, O’Toole TE, Chan DL, Rush JE. Retrospective Evaluation of Urokinase Use in Cats with Arterial Thromboembolism; Abstract from the 11th IVECCS. J Vet Emerg Crit Care. 2005;15:S8.
- Vezzosi T, Buralli C, Briganti A, Vannozzi I, Giacomelli E, Talamanca GF, et al. Surgical embolectomy in a cat with cardiogenic aortic thromboembolism. J Vet Cardiol. 2020 Apr 1;28:48–54. [CrossRef]
- Thrombectomy and thrombolysis- the interventional radiology approach - 2011.pdf [Internet]. Google Docs. [cited 2025 May 16]. Available from: https://drive.google.com/file/d/1-k5wf0vhwhDdlK7_SRvnVzobBeauov8G/view?usp=drive_open&usp=embed_facebook.
- Reimer SB, Kittleson MD, Kyles AE. Use of Rheolytic Thrombectomy in the Treatment of Feline Distal Aortic Thromboembolism. J Vet Intern Med. 2006;20(2):290–6. [CrossRef]
- Koyama H, Matsumoto H, Fukushima R uji, Hirose H. Local Intra-Arterial Administration of Urokinase in the Treatment of a Feline Distal Aortic Thromboembolism. J Vet Med Sci. 2010;72(9):1209–11. [CrossRef]
- EVECC 2022 Congress. VIN.com [Internet]. 2022 Jun 23; Available from: https://www.vin.com/doc/?id=11002012.
- Lu J, Qian S, Sun Z. Targeting histone deacetylase in cardiac diseases. Front Physiol. 2024 Jun 24;15:1405569. [CrossRef]
- Kulthinee S, Yano N, Zhuang S, Wang L, Zhao TC. Critical Functions of Histone Deacetylases (HDACs) in Modulating Inflammation Associated with Cardiovascular Diseases. Pathophysiology. 2022 Aug 22;29(3):471–85. [CrossRef]
- Evaluation of the effects of histone deacetylase inhibitors on cells from canine cancer cell lines in: American Journal of Veterinary Research Volume 69 Issue 7 () [Internet]. [cited 2025 May 17]. Available from: https://avmajournals.avma.org/view/journals/ajvr/69/7/ajvr.69.7.938.xml.
- Dias JNR, Aguiar SI, Pereira DM, André AS, Gano L, Correia JDG, et al. The histone deacetylase inhibitor panobinostat is a potent antitumor agent in canine diffuse large B-cell lymphoma. Oncotarget. 2018 Jun 19;9(47):28586–98. [CrossRef]
- Augusto JF, Beauvillain C, Poli C, Paolini L, Tournier I, Pignon P, et al. Clusterin Neutralizes the Inflammatory and Cytotoxic Properties of Extracellular Histones in Sepsis. Am J Respir Crit Care Med. 2023 Jul 15;208(2):176–87. [CrossRef]
| History/Clinical Assessment | Male |
| Breed disposition (Ragdolls, Maine Coon) with mutation of myosin binding protein C gene (MYBPC3) | |
| Previous thrombotic event(s) | |
| Heart murmur | |
| Gallop rhythm | |
| Biomarkers | Elevated troponin (>0.06 ng/ml)* |
| Elevated NT-proBNP (> 99 pmol/l) or POC positive (>200 pmol/)* | |
| Thoracic Radiographs | Cardiomegaly (VHS > 8) |
| LA enlargement | |
| Echocardiogram | LV systolic dysfunction (LV fractional shortening and emptying fraction) |
| LA systolic dysfunction (Low LAA velocity, Low LA fractional shortening) | |
| Spontaneous echocardiographic contrast and/or intracardiac thrombus | |
| LA enlargement (LA:Ao > 1.6) | |
| Electrocardiogram | Arrhythmias: atrial fibrillation, ventricular arrhythmias |
| *No direct association with thrombosis but likely indicates significant heart disease that warrants further investigations LA – left atrial LAA – left atrial appendage LA:Ao – Left atrium to aortic root ratio LV – left ventricular | |
| Viscoelastic Test | Results Interpretation | Pros | Cons | Sample Required |
|---|---|---|---|---|
| TEG |
|
|
|
Citrated or fresh whole blood |
| ROTEM |
|
|
|
Citrated whole blood |
| VCM | Hypercoagulable
|
|
|
Fresh whole blood |
| TEG – Thromboelastography ROTEM – Rotational Thromboelastometry VCM – Viscoelastic monitoring CT – Clotting time CFT – Clot formation time MCF – Maximum clot firmness | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





