1. Introduction
Cotton (
Gossypium hirsutum L.) is an annual herbaceous or perennial shrub plant of the genus Mallow. At the same time, cotton is also one of China’s most important cash crops, and its output ranks first globally [
1]. Owing to its unique geographical and natural conditions, Xinjiang has emerged as a significant-high-quality cotton cultivation region in China [
2]. In 2022, the cotton planting area in Xinjiang amounted to approximately 2,496,900 hectares. However, weeds pose a significant threat to cotton yield and quality improvement, as they compete for sunlight, water, fertilizer, and space with cotton plants. Additionally, weeds serve as intermediate hosts for pest and disease transmission, substantially increasing cotton planting costs (fertilizer and pesticide inputs) and irrigation expenses. Consequently, cotton yield and fiber quality decline [
3]. The perennial yield loss attributable to weeds ranges from 12.5%~14.8% [
4]. Fluridone, chemical name is 1- methyl -3- phenyl -5- (3- trifluoromethylphenyl) -4 (1 H) - pyridone, which is a pyrrolidone soil sealing herbicide widely used in cotton fields in Xinjiang in recent years. Finally, it inhibits photosynthesis and causes plant death. It is often paired with pendimethalin, which can control annual broad-leaved and grassy weeds such as nightshade, gray quinoa, and barnyard grass in cotton fields. Pendimethalin has an effective period of about 60 days and is safe for cotton seedling emergence and growth. [
5,
6]. Fluridone has a long residual period in the soil, which poses a certain potential risk of pesticide damage to crop crops during in subsequent rotations [
7].
Pre-treatment techniques for pesticide residues include methods like QuEChERS (Quick, Easy, Cheap, Effective, Rugged, Safe), Solid Phase Extraction (SPE), Gel Permeation Chromatography (GPC), and Accelerated Solvent Extraction (ASE) [
8,
9]. QuEChERS was introduced at the Fourth European Pesticide Residue Group Conference in 2002. This acronym stands for Quick, Easy, Cheap, Effective, Rugged, and Safe. QuEChERS is widely applied for the detection of multiple pesticide residues by removing impurities through interactions between adsorbents and matrix impurities [
10]. Compared to traditional methods, QuEChERS is widely applied to detect multiple pesticide residues by removing impurities through interactions between adsorbents and matrix impurities. Compared to traditional methods, QuEChERS offers high recovery rates, often exceeding 85% for numerous polar and volatile pesticides. High precision and accuracy were corrected using the internal standard method. Fast analysis and simple operation. Low solvent consumption and minimal environmental impact. Cost-effective and simple experimental equipment [
11].
As a major producer and user of pesticides, China uses pesticides extensively. Detecting pesticide residues in soil is crucial for environmental monitoring. Xu Yihong et al. utilized an improved QuEChERS method to detect toxaphene in soil, exploring different polarity extraction solvents and the influence of graphitized carbon black and PSA on purification and recovery rates. This method boasts good recovery, precision, simplicity, and speed [
12]. Cui Jun et al. applied an enhanced QuEChERS method to soil sample pre-treatment, simplifying and optimizing steps. Direct instrument analysis of the extract allowed for rapid detection of four nitrophenols in soil [
13]. Zhang Hongchao et al. employed the QuEChERS method and UPLC-MS/MS to determine the dissipation dynamics and metabolic patterns of six common pesticides in cucumbers, aiming to provide a scientific basis for their use and guide practical production [
14].
However, there are few reports on the residual analysis of fluridone at home and abroad. In this study, the optimized QuEChERS pretreatment method was selected and combined with HPLC to establish the residual digestion dynamics and final residue levels of fluridone in the cotton field environment, and the risk assessment was carried out. This study provides a scientific basis for the application of fluridone in the process of weeding in cotton fields, provides a basis for the study of subsequent soil environmental behaviour, and provides a reference for the development of analytical methods for fluridone residues in other crops and organisms.
2. Materials and Methods
2.1. Residue Digestion in the Field and Final Residue Testing
The field experiments were carried out in Shihezi City, Xinjiang, China (44.35°N, 86.01°E, altitude 321.4 meters) on April 2023 and April 2024. Shihezi City has a temperate continental climate. In accordance with the Guidelines for Crop Residue. The trial followed the Guidelines for Crop Residue Testing (NY/T 788–2018) issued by the Ministry of Agriculture and Rural Affairs of China and the Standard Operating Procedures for On-site Testing of Pesticide Residues (PVCI, 2021). The cotton cultivar Huiyuan 720 was planted in a randomized complete block design with four replicates. Each plot measured 12 m × 14 m (168 m²), separated by 1.5 m buffer zones to minimize drift interference. Fluridone (42% suspension concentrate) was applied pre-emergence at three rates: 220.5, 441.0, and 661.5 g a.i.·ha⁻¹, using a calibrated backpack sprayer delivering 30 L·ha⁻¹. Adjacent control plots received water with equivalent adjuvant (0.1% Tween 20).
Residual dissolution test: Soil samples were collected at 0.083, 1, 3, 7, 14, 21, and 28 days using—utilizing the five-point sampling method. Subsequent separation was conducted according to the four-point method. Each soil sample, weighing no less than 1 kg, was securely bandaged in a dedicated container and labelled appropriately. The sampling points were evenly distributed to guarantee the representativeness of the collected samples. These samples were stored in a refrigerator at -20℃ in darkened cryovials to detect fluridone residues.
Final residue test: During cotton harvest (September 2023/2024), triplicate samples of soil (0-15 cm depth), cotton leaves, and seeds were collected from randomized 10× 10 m grids using stratified random sampling (NY/T 788-2018). Plant tissues were lyophilized and ground to a particle size of 100 um.
2.2. Collection and Preparation of Samples
Approximately 1 kg of surface soil was randomly collected from multiple points within a depth range of 0 to 10 cm. Post fragment removal allowed the soil to dry naturally. Subsequently, it was ground through a 20-mesh sieve (0.9 mm aperture). Gather around 1.0 kg of cotton plants from various locations, were chop and blended. Additionally, cottonseeds were collected from multiple points. Cotton seeds were harvested after harvesting the cotton plants. After drying at 80℃, the samples were stored at -20℃ until further analysis.
2.3. Sample Preprocessing
Utilize an optimized QuEChERS method for preprocessing field soil, cotton plants, cottonseeds, and blank matrix samples [
15,
16]. Once soil collection is complete, it was air-dried and ground through a 2 mm sieve for subsequent use. Weigh precisely 10.0 g of soil samples, 5.0 g of cotton plant samples, and 5.0 g of cottonseed samples into 50 mL centrifuge tubes using an electronic balance. Add 10 mL of acetonitrile and extract for 5 minutes. Incorporate 2.0 g of NaCl to facilitate the separation of the aqueous and organic phases. Continue vortex mixing for 2 minutes, then centrifuge at 4000 rpm/min for 5 minutes to purify the samples.
2.4. Instrument Test Condition
Chromatographic settings included an injection volume of 2.0 μL, with a mobile phase consisting of acetonitrile and 0.1% formic acid aqueous solution in the 80:20 volume ratio. The flow rate was maintained at 0.3 mL/min, and the column oven temperature was set to 40 °C. The column used is a Sunfire C18-ODS stainless steel column with dimensions of 150mm × 4.6mm. The detection wavelength was set at 310 nm.
2.5. Preparation of Standard Solutions and Matrix-Matched Standard Solutions
2.5.1. Preparation of Standard Solutions
The fluridone standard (10.0 mg) was precisely measured and dissolved in acetonitrile using an ultrasonic cleaner until a final volume of 10 mL was reached, yielding a stock solution with a concentration of 1000 mg/L. The stock solution was stored at -20°C. The stock solution was sequentially diluted with acetonitrile to obtain working solutions with concentrations of 10, 1, 0.5, 0.1, and 0.01 mg/L.
2.5.2. Preparation of Matrix-Matched Standard Solutions
Blank samples of the field soil, cotton plants, and cottonseeds were obtained. These samples were pretreated to extract blank matrix solutions. The blank matrix solutions were used to prepare matrix-matched standard solutions with concentration gradients of 10, 1, 0.5, 0.1, and 0.01 mg/L.
2.5.3. Recovery Rate Experiment with Spiking
Recovery rate experiments were conducted to ascertain the accuracy and precision of the methodology. These experiments involved spiking three distinct concentrations of fluridone standards into blank matrices (comprising field soil, cotton plants, and cottonseeds) devoid of fluridone. Each concentration level was subjected to five parallel treatments. Measurements were then performed in accordance with the methodology established within this study. Subsequently, the average recovery rates and relative standard deviations (RSDs) for each concentration level were computed, as detailed in
Table 1.
2.6. Validation of Analytical Method
The quantitative analytical method has been rigorously validated in compliance with the SANTE guidelines. During this validation process, the analytical method's key parameters were established, including linearity, limit of quantification (LOQ), limit of detection (LOD), matrix effect (ME), precision, and accuracy [
17,
18].
HPLC analysis was conducted on standard solutions, including matrix-matched standards, with five replicates for each concentration. By plotting the mass concentration of fluridone as the x-axis and the corresponding peak area as the y-axis, a matrix-matched standard curve and correlation coefficient (R) for fluridone were obtained. This evaluation assessed the linear relationship between fluridone concentration and peak area. An R² value greater than 0.99 indicates accurate analysis.
The sensitivity of the analytical method was assessed using the limit of detection (LOD) and limit of quantification (LOQ). To determine sensitivity, the lowest concentration was added to selected matrices to meet method specifications. The LOD was defined as the fluridone concentration in the matrix where the signal-to-noise ratio (S/N) reached 3, considering the background noise from the blank matrix. Analogously, the LOQ corresponded to the fluridone concentration with an S/N of 10 [
17,
19,
20].
To assess the matrix effect, a comparison was made between the slope ratio of the solvent standard curve and the matrix-matched standard curve. The formula below was utilized to calculate the matrix effect of the fluridone standard across various matrices:
In the formula: "ME" represents the matrix effect; "A" represents the slope of the solvent (acetonitrile) standard curve; and "B" represents the slope of the matrix-matched standard curve (field soil, cotton plants, cottonseeds).
To ascertain the accuracy and precision of our methodology, recovery experiments were performed by introducing three distinct concentrations of the fluridone standard into pristine matrices (field soil, cotton plants, and cottonseeds) devoid of the fluridone standard. Each concentration was subjected to five parallel treatments. The spiked samples were then analyzed using the methodology established in this study. Subsequently, the average recovery rates and relative standard deviations (RSDs) were computed.
2.7. The Degradation Dynamics of Fluridone in Soil
The formula for computing the recovery rate of fluridone was provided as follows:
In the formula: "R" represents the recovery rate of fluridone; "S2" and "S1" are the peak areas of the matrix and standard sample with the same concentration of fluridone, respectively; "S0" is the peak area of the blank matrix without fluridone.
When fluridone is degraded in the soil, it typically follows a first-order kinetic law. The half-life and kinetic regression equation can be calculated using the following formulas:
In the formula, Ct (mg·kg⁻¹) represents the concentration of fluridone in the soil after (t) days; C0 (mg·kg⁻¹) is the initial concentration of fluridone in the soil; k (d-1) is the degradation rate constant; t: (d-1) represents the incubation time; and T0.5 (d) is the time required for fluridone to degrade to half of its initial concentration, which is also known as the degradation half-life.
2.8. Dietary Risk Assessment
Countries estimate the daily pesticide intake based on formula 5 [
21], while the chronic risk quotient (
RQc) is calculated using formula 6 [
22].
In the formula, NEDI stands for national estimated daily intake, with the unit of μg/ (kg bw·d); STMRi represents supervised trials median residue, with the unit of mg·kg⁻¹; STMR-Pi refers to supervised trials median residue for processed commodity, with the unit of mg·kg⁻¹; Fi denotes the consumption of food agro-product i, with the unit of g/d; bw stands for body weight, with the unit of kg. RQc represents the chronic risk quotient, and ADI stands for acceptable daily intake, with the unit of μg/ (kg bw·d).
When
RQc ≤ 1, it indicates that the chronic risk is acceptable, and the smaller the
RQc, the lower the risk. When
RQc > 1, it indicates that there is an unacceptable chronic risk, and the greater the
RQc, the higher the risk [
22].
3. Results
3.1. Optimization of Testing Conditions
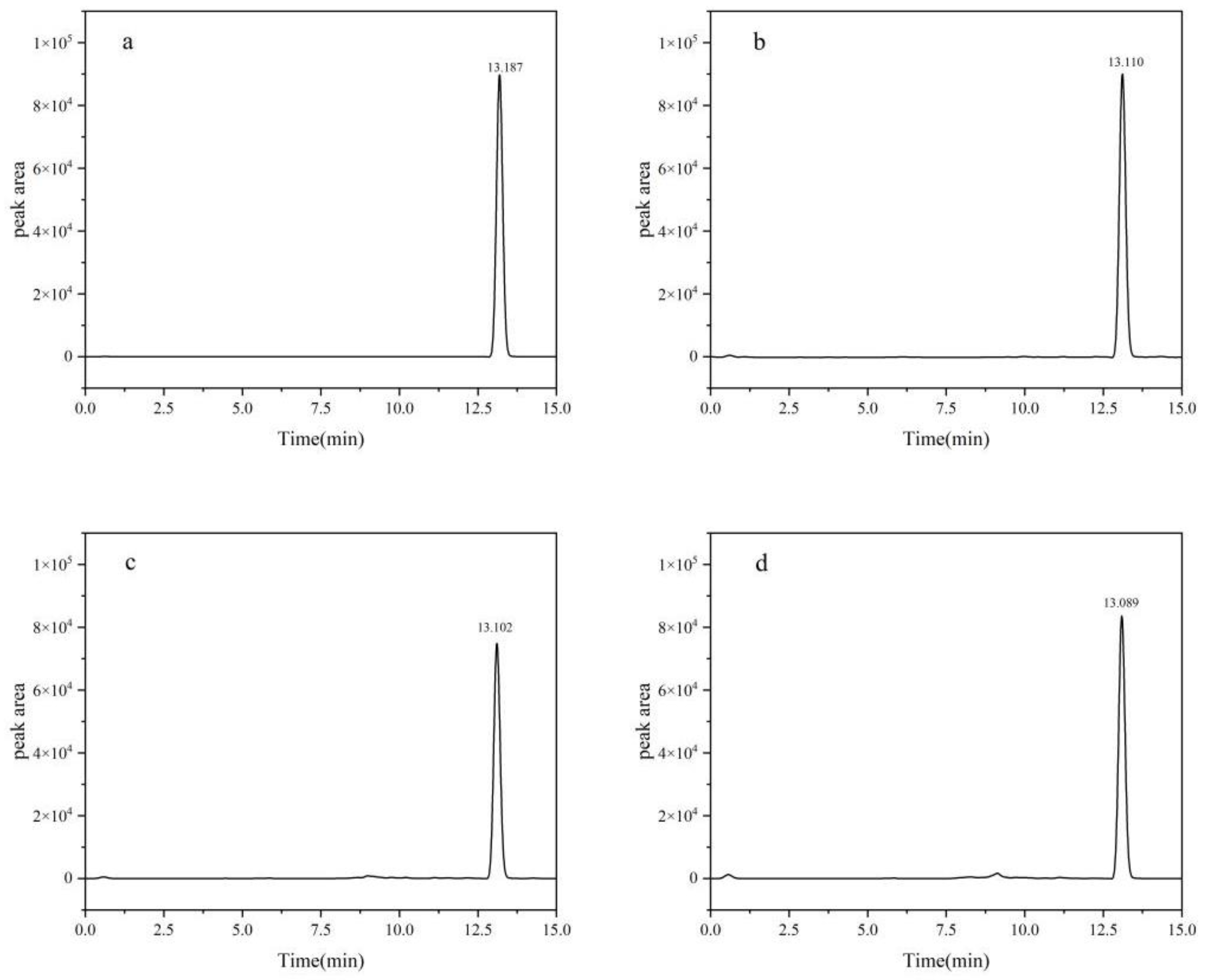
The results indicated that the column oven temperature, ranging from 30 °C to 40°C, exerted minimal influence on both the peak area and retention time of the sample, thus necessitating no separate discussion. A notable trend emerged where minor variations in flow rates led to prolonged retention times for fluridone. When acetonitrile solution was employed as the organic phase, as opposed to a methanol solution, it facilitated superior separation between components and impurities, characterized by fewer stray peaks, enhanced peak areas of active ingredients, and improved peak shapes.
To enhance separation efficiency and peak morphology within an acetonitrile-water mobile phase system, a 0.1% formic acid aqueous solution was substituted for the pure water phase. Subsequent optimization of this mixture's proportion revealed negligible effects on peak shape. To mitigate impurity interference and achieve optimal peak shape, further refinement of the mobile phase ratio and flow rate was conducted. An acetonitrile-0.1% formic acid aqueous solution system, with a volume ratio of 80:20, yielded optimal peak shapes, exhibiting minimal impurity peaks, maximum peak area, moderate retention times, and consistent results across multiple detections at a flow rate of 0.3 mL/min.
The definitive chromatographic conditions were established as follows: an injection volume of 2.0 μL, a mobile phase composed of acetonitrile and 0.1% formic acid aqueous solution in the 80:20 volume ratio, a flow rate of 0.3 mL/min, and a column oven temperature precisely set at 40 ℃.
Figure 1.
HPLC Chromatograms of fluridone: (a) blank; (b) soil; (c) cotton plant; (d) cotton seed.
Figure 1.
HPLC Chromatograms of fluridone: (a) blank; (b) soil; (c) cotton plant; (d) cotton seed.
3.2. Optimization of Preprocessing Methods
3.2.1. Optimization of Extraction Solvents
In the analysis of pesticide residues, the selection of appropriate extraction solvents for the complete recovery of target compounds from diverse matrices constitutes a pivotal step in sample pretreatment [
23,
24]. Among the various solvents employed, acetonitrile, methanol, dichloromethane, and ethyl acetate are commonly utilized in pesticide residue analysis methodologies [
18,
19,
25]. Considering the varying polarities of pesticides and their miscibility with the mobile phase, this study selected four solvents: methanol, acetonitrile, dichloromethane, and ethyl acetate. Appropriate amounts of the mixed pesticide standard solution were added to blank samples, followed by the introduction of each of the four solvents. Under identical processing conditions, addition-recovery experiments were conducted, and each group was repeated three times to ensure accuracy. The extraction efficiencies of the different solvents were then compared. As can be seen from
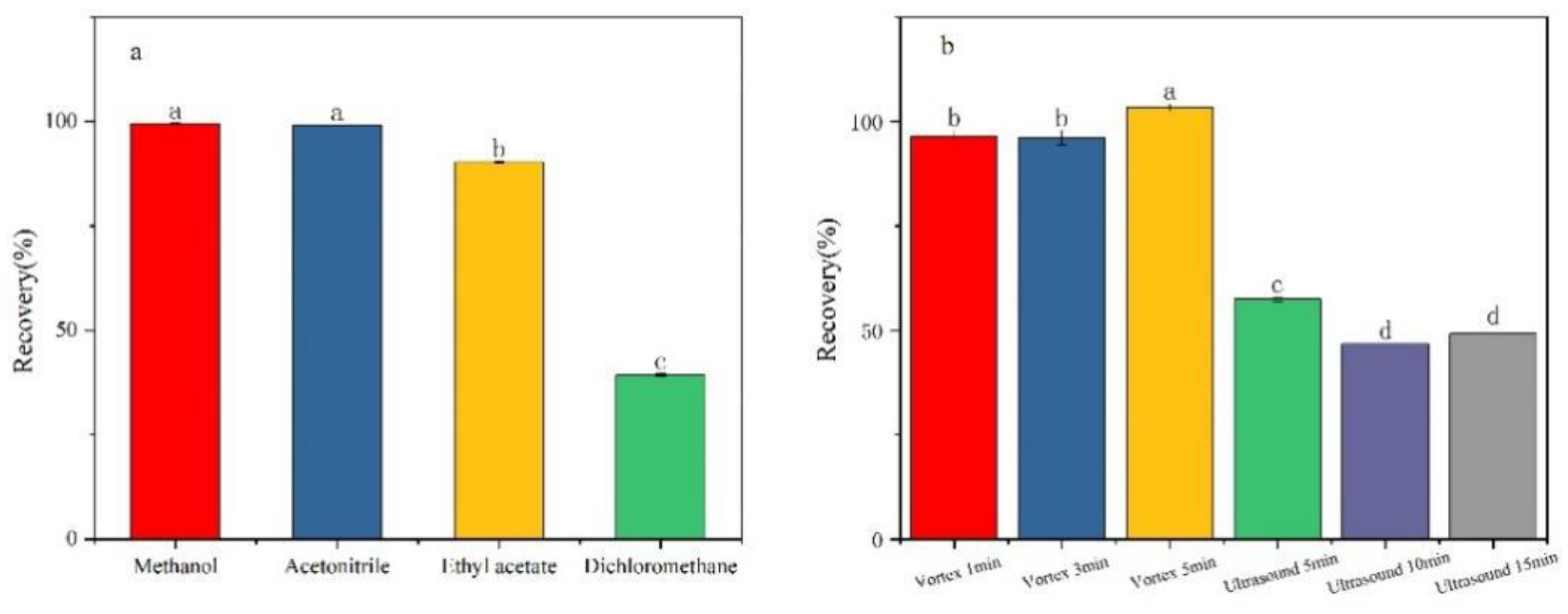
Figure 2(a), the recovery rates of the other three extraction solvents are satisfactory, except for dichloromethane. Considering the practical effectiveness and application cost, this study chose acetonitrile as the extraction solvent.
3.2.2. The Optimization of Extraction Methods
Under identical experimental conditions, the impact of two extraction techniques—ultrasound (5 min, 10 min, 15 min) and vortex mixing (1 min, 3 min, 5 min)—on the recovery rate of fluridone was investigated.
Figure 2(b) demonstrates that vortex mixing extraction yields a significantly higher recovery rate compared to ultrasound extraction. This disparity can be attributed to the inherent characteristics of the two methods. Vortex mixing facilitates rapid and thorough mixing of the sample, ensuring complete dissolution of fluridone. Conversely, ultrasound extraction may not achieve complete dissolution of fluridone within the sample, leading to a lower recovery rate. When vortex mixing was applied for 5 minutes, the recovery rate peaked, thus fulfilling the extraction requirements. Therefore, in this study, vortex mixing for 5 minutes was adopted as the optimal method for extracting fluridone from the sample.
Figure 2.
Pretreatment optimization: (a) extraction solvent; (b) extraction method.
Figure 2.
Pretreatment optimization: (a) extraction solvent; (b) extraction method.
3.2.3. Optimization of Salt
Sodium chloride serves as a potent facilitator in liquid-liquid extraction processes, inducing a salting-out effect that enhances the recovery efficiency of target compounds. Furthermore, anhydrous magnesium sulfate exhibited a remarkable capacity for water binding, thereby substantially reducing the aqueous phase and promoting the preferential distribution of target compounds within the organic layer. The synergistic application of sodium chloride and anhydrous magnesium sulfate not only allows for the precise modulation of the extraction medium's polarity but also optimizes the peak characteristics of the targeted analytes [
19,
26,
27]. Under consistent experimental parameters, the impact of varying NaCl (1.0 g, 2.0 g, 3.0 g) and MgSO
4 (1.0 g, 2.0 g, 3.0 g) concentrations, as well as combined treatments of 0.5 g NaCl + 0.5 g MgSO
4, 1.0 g NaCl + 1.0 g MgSO
4, and 1.5 g NaCl + 1.5 g MgSO
4 on the recovery rate of fluridone was meticulously examined. As illustrated in
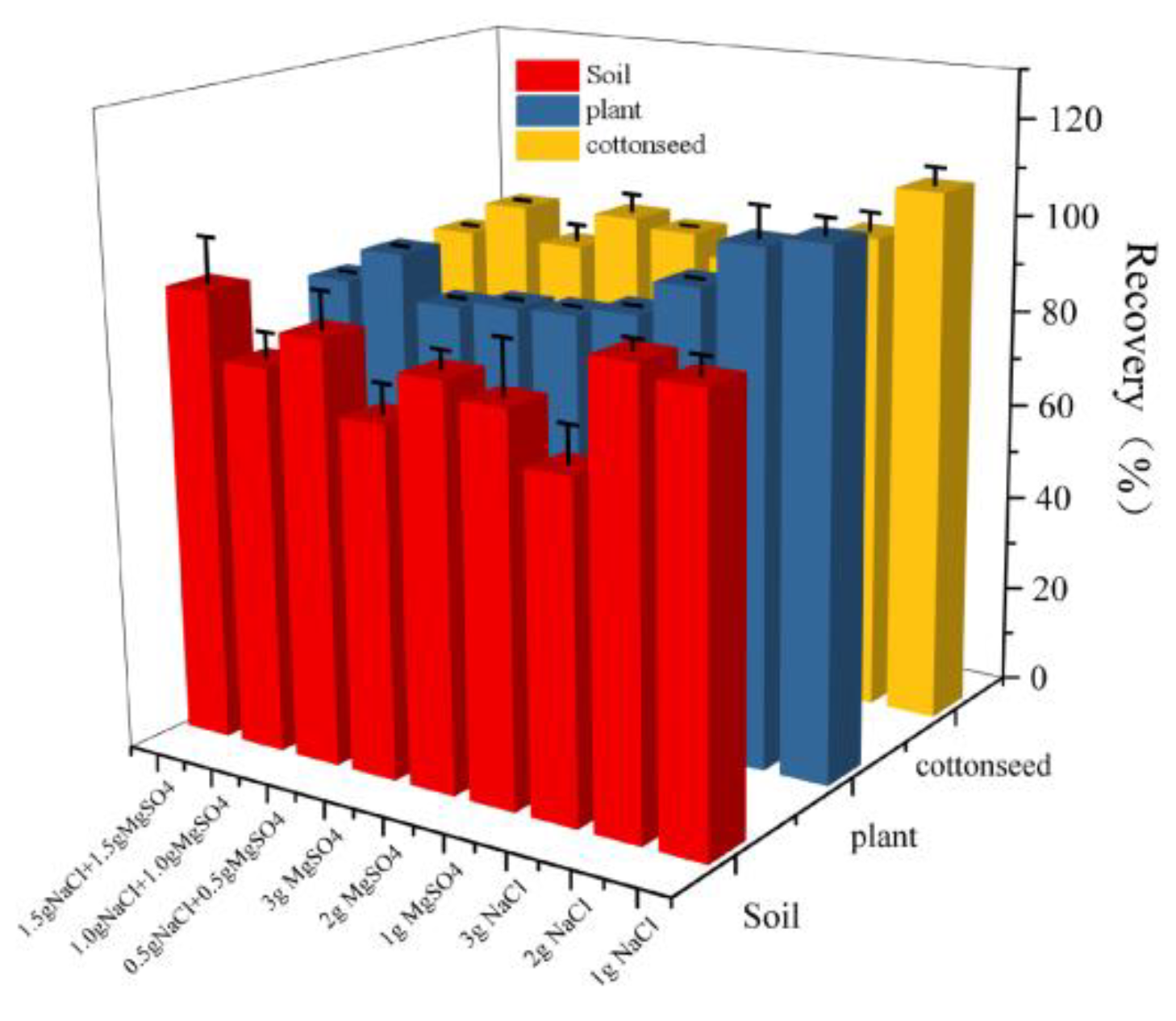
Figure 3, The addition of 2 g of NaCl results in the recovery rate of fluridone in soil, cotton plants, and cottonseeds approaching 100%, and enhances its stability, thus meeting our experimental objectives. Consequently, we opted for the inclusion of 2 g NaCl in the fluridone extraction process from the samples.
Figure 3.
The effects of different salt contents on the recovery rate of fluridone in the soil, cotton plant, and cottonseeds.
Figure 3.
The effects of different salt contents on the recovery rate of fluridone in the soil, cotton plant, and cottonseeds.
3.2.4. Optimization of Purification Conditions
During the extraction of target compounds from cotton field environmental samples using acetonitrile, a substantial quantity of matrix compounds was concurrently extracted. These co-extracted substances have the potential to interfere with target analytes, resulting in significant matrix effects. Post-extraction purification is therefore indispensable.
Graphitized Carbon Black (GCB) is a weakly polar or nonpolar adsorbent. It is mainly used for the removal of compounds through hydrophobic interactions. Examples of such compounds include carotenoids and carotenoid-like substances [
27,
28]. Multi-walled carbon nanotubes (MWCNTs), due to their extensive surface area and distinctive structural characteristics, possess remarkable adsorption capabilities. They can effectively eliminate interfering matrix substances while using a minimal amount of adsorbent [
29,
30]. The results presented in
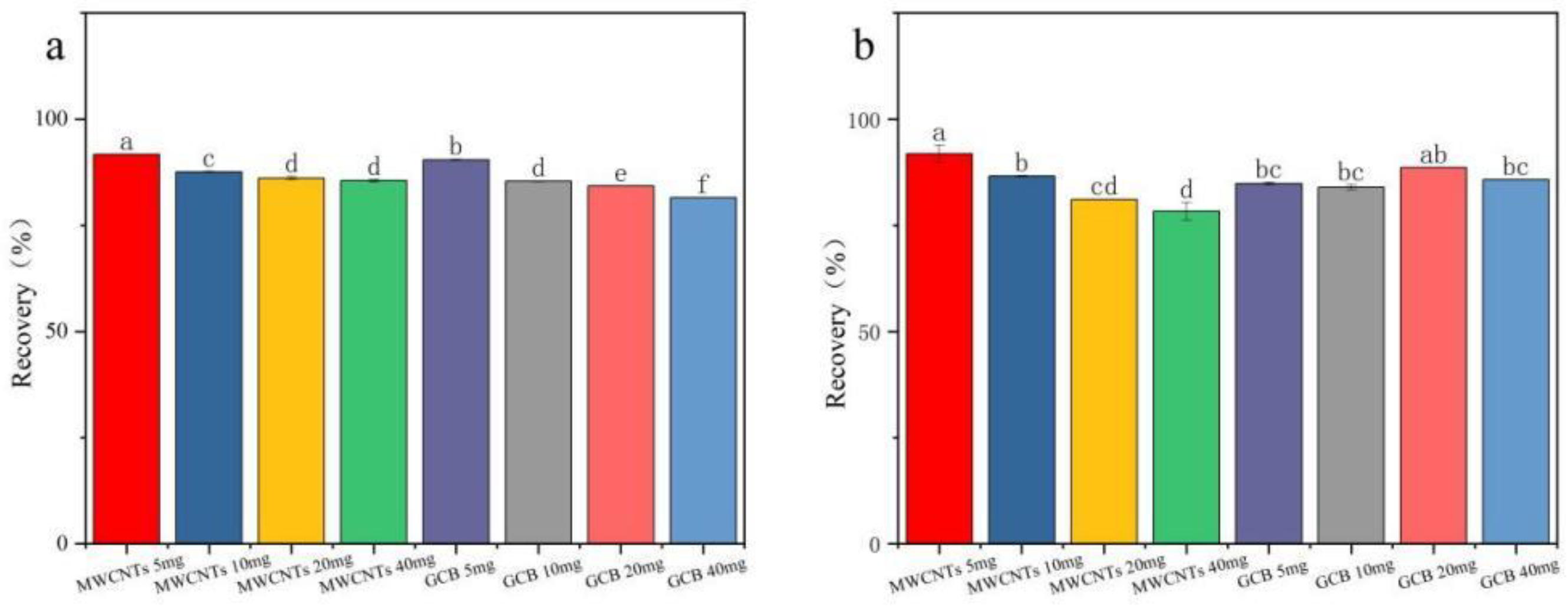
Figure 4 demonstrate that in cotton plants and cottonseed samples, the recovery rate of fluridone was maximized when 5.0 mg of MWCNTs was employed. Nevertheless, as the quantity of MWCNTs increases, the recovery rate of fluridone shows a decline. MWCNTs display superior performancee in removing pigments from the cotton plant and cottonseed matrix, yielding clearer treated solutions. Even with a relatively small amount of MWCNTs, satisfactory recovery rates were achieved. Consequently, MWCNTs (5.0 mg) were selected as the purification agents for this experiment.
3.3. Method Validation
3.3.1. The Linearity, Limit of Detection (LOD), Limit of Quantitation (LOQ), and Matrix Effect of the Method
The standard curves for matrix-matched and pure solvent samples were constructed following the method described in Section "2.2". Subsequently, calculate the matrix effect. The linear regression equation, limit of detection (LOD), limit of quantitation (LOQ), and matrix effect of fluridone in various samples are presented in
Table 2. The results demonstrated a strong linear relationship (R² > 0.99) between the concentration of fluridone and the peak area in field soil, plant, and cottonseed samples within the concentration range of 0.01 to 10 mg/L.
LOD and LOQ are crucial validation parameters for assessing the sensitivity of analytical methods. The LOD of fluridone in the samples ranged from 0.0090 to 0.0108 mg·kg⁻¹, and the LOQ ranged from 0.0030 to 0.0033 mg·kg⁻¹. According to GB2763.1 - 2022, the LOD is lower than the maximum residue limit standard for pesticides in food, which is 0.02 mg·kg⁻¹. Moreover, the LOD and LOQ values obtained by this method are lower than those reported by Luo, Juan et al. in their determination of isocyanamide residues in cabbage and soil samples using high-performance liquid chromatography (HPLC) [
19].
3.3.2. The Accuracy and Precision of This Method
As presented in
Table 3, the average daily recoveries of fluridone in field soil samples spanned from 89.08% to 95.07%. The relative standard deviation of repeatability (RSDr) values fell within the range of 2.06% to 4.57%. Regarding cottonseed samples, the average daily recoveries were in the range of 85.81% to 90.72%, with RSDr values ranging from 0.95% to 2.76%. For cotton plant samples, the daily recoveries were between 85.08% and 89.89%, and the RSDr values were from 0.35% to 0.78%. The daily precision was evaluated by analyzing spiked samples, and the relative standard deviation of repeatability (RSDR) values were found to be in the range of 0.47% to 3.81%. The recoveries of fluridone in all three types of samples were within the 70% - 120% range, and the relative standard deviation (RSD) was less than or equal to 20%. These results comply with the requirements specified in the Chinese Guidelines for Crop Pesticide Residue Testing (GBNY/T 788 - 2018). Thus, this method is suitable for the detection of fluridone and demonstrates a good detection accuracy.
3.4. The Degradation Dynamics of Fluridone in Cotton Field Soil
Pesticides sprayed onto the soil undergo various degradation processes. Pesticide loss is influenced by the pesticide's physicochemical properties, soil characteristics, climatic conditions, and pesticide application methods [
26,
31].
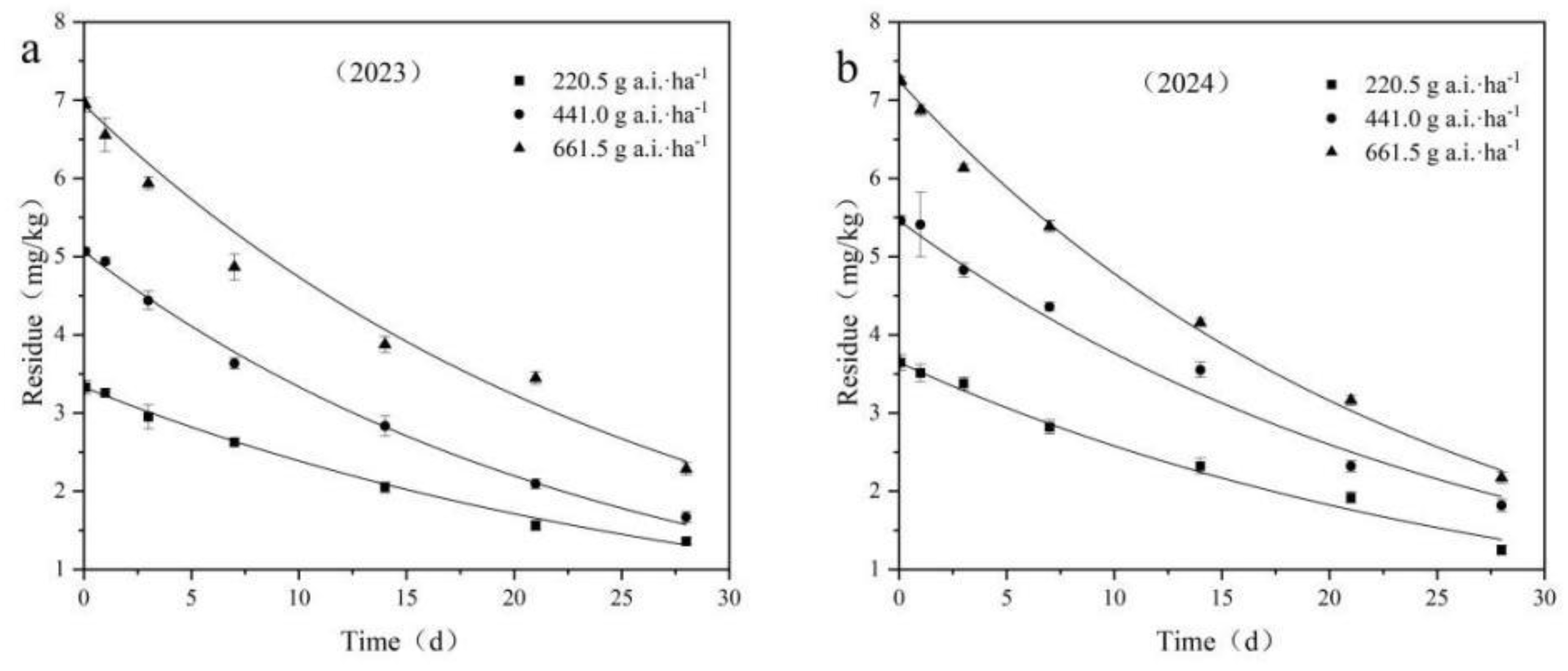
Table 4 presents the kinetic equation parameters for the degradation of fluridone in cotton fields during 2023 - 2024.
Taking the sampling time after application as the abscissa and the residual concentration of fluridone in the samples as the ordinate, dynamic curves of the residual degradation of different fluridone concentrations in field soil were plotted, as depicted in
Figure 5. The residues of fluridone measured 2 hours after application were regarded as the initial deposition.
When the application doses were 220.5 g a.i.·ha⁻¹, 441.0 g a.i.·ha⁻¹, and 661.5 g a.i.·ha⁻¹, the initial deposition in the field ranged from 3.331 mg·kg⁻¹ to 7.247 mg·kg⁻¹, with degradation half - lives ranging from 15.503 days to 21.004 days, respectively.
The results of the fluridone residual dissipation tests indicated that the initial deposition of fluridone increased with the increase in the applied dose, while the half - life decreased as the application dose increased. It is reasonable to assume that various environmental factors, such as soil microorganisms, precipitation levels, temperature, and light intensity, may have an impact on the degradation process of fluridone.
3.5. The Final Residues of Fluridone in Cotton Field Soil, Cotton Plants, and Cottonseeds
Measuring the residue levels at harvest is of crucial significance for assessing the safety of using fluridone to control weeds in cotton fields. The findings revealed that during 2023-2024, the residue levels of fluridone in cotton field soil ranged from 0.032 mg/kg to 0.155 mg/kg. In cotton plants, these levels ranged from 0.215 mg/kg to 0.412 mg/kg. Moreover, in cottonseeds, the residue levels were below the Limit of Quantitation (LOQ). These results suggest that the final residue levels of fluridone in cotton field soil were relatively elevated, presenting a higher risk of environmental pollution. Additionally, the relatively high residue levels in cotton plants imply a potential risk if the plants are used as forage. Nevertheless, it is notable that the final residue levels in cottonseeds are below the LOQ.
Table 5.
Final residues of fluridone in cotton field soil, cotton plants, and cottonseeds.
Table 5.
Final residues of fluridone in cotton field soil, cotton plants, and cottonseeds.
| Year |
Materials |
Dosage
(g a.i.·ha⁻¹) |
Final residue(mg·kg⁻¹) |
| |
|
220.5 |
0.067 |
| |
Soil |
441.0 |
0.077 |
| |
|
661.5 |
0.155 |
| |
|
220.5 |
0.234 |
| 2023 |
Plant |
441.0 |
0.322 |
| |
|
661.5 |
0.412 |
| |
|
220.5 |
<LOQ a
|
| |
Cottonseed |
441.0 |
<LOQ a
|
| |
|
661.5 |
<LOQ a
|
| |
|
220.5 |
0.032 |
| |
Soil |
441.0 |
0.075 |
| |
|
661.5 |
0.134 |
| |
|
220.5 |
0.215 |
| 2024 |
Plant |
441.0 |
0.341 |
| |
|
661.5 |
0.402 |
| |
|
220.5 |
<LOQ a
|
| |
Cottonseed |
441.0 |
<LOQ a
|
| |
|
661.5 |
<LOQ a
|
3.6. Dietary Risk Assessment
Final residue testing not only examines the final residue levels of pesticides applied to crops but also assesses the potential risks associated with dietary intake [
17,
25]. Dietary risk assessment is a crucial element in the evaluation of agricultural product quality and safety and serves as one of the measures to gauge the safety of agricultural products.
Cottonseed, an important byproduct of cotton production, is a significant feed resource. This is attributed to its rich nutritional composition and high yield. Cottonseed is abundant in various nutrients, including protein, fat, and fiber. These nutrients can supply the essential energy and nutrition for animals, and also enhance the production performance and health status of livestock and poultry [
32].
The maximum residue limit for pesticides in food stipulates that the acceptable daily intake (ADI) of fluridone is 0.15 mg·kg⁻¹·bw. In this experiment, the residues in cottonseed were below the limit of quantification (LOQ). When fluridone was applied at rates of 220.5 g a.i.·ha⁻¹, 441.0 g a.i.·ha⁻¹, and 661.5 g a.i.·ha⁻¹, no significant health risks were posed to Chinese consumers through the cottonseed oil extracted from cottonseed.
4. Conclusions
In this study, a relatively straightforward and rapid QuEChERS pretreatment coupled with HPLC method was developed to determine fluridone residues in field soil, cotton plants, and cottonseeds. The validation results demonstrated that the proposed method exhibited excellent linear correlation, accuracy, precision, LOD, LOQ, thereby meeting the requirements for pesticide residue analysis.
The degradation of fluridone in the field adhered to first-order kinetics. Notably, the degradation half-life of fluridone in soil was relatively short. Dietary risk assessment revealed that the residue levels of fluridone in harvested cottonseeds did not pose a significant health risk to Chinese consumers.
At present, certain countries and regions have not yet established maximum residue limits (MRLs) for fluridone, and the understanding of its residues in food and the environment remains limited. Consequently, this study holds great significance for the rational application of fluridone. It offers a reference for the establishment of MRLs and the safe utilization of fluridone. Moreover, it provides a foundation for subsequent investigations of the environmental behaviour of soil.
However, it should be noted that this study might have covered only a limited number of sampling points and a relatively short time period. This limitation could potentially restrict a comprehensive understanding of the degradation dynamics of fluridone. A larger number of sampling points and long-term monitoring are recommended to obtain more accurate and comprehensive data.
In addition, soil type significantly influences the degradation and residue of pesticides. This study might have focused only on specific soil types, thus failing to encompass all possible soil types. Consequently, future research should take into account the impact of different soil types on the degradation dynamics and residues of fluridone.
Furthermore, although this study disclosed the degradation half-life of fluridone in soil, the specific degradation mechanism remains inadequately understood. Further research on the degradation mechanism will enhance our understanding of the behaviour of fluridone in soil and provide a scientific basis for its management.
Author Contributions
Conceptualization, S.W.; methodology, S.W. and R.Y.; investigation, S.W., Y.L. and Z.J.; data curation, S.W. and Y.X.; writing—original draft preparation, S.W. and Y.P.; writing—review and editing, X.H.; G.Z.; C.W.; T.M.; C.W. and D.Y.; funding acquisition D.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Assessment of the Health of Cotton Field Ecosystems and the Safety of Agricultural Products(NK2023AA601)
Data Availability Statement
The data presented in this study are available on request from the corresponding authors.
Acknowledgments
We would also like to thank all reviewers for their valuable comments.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Liu, L.; Y. Chen; Z. Lei. A study on high-quality development of the cotton industry of Xinjiang. Macroeconomic Management, 2021. 10: p. 77-83+90. [CrossRef]
- Xu, F.; Y. Li; S. Ren. Consideration on the application and development of drip irrigation technology under cotton film in Xinjiang. Trans. Chin. Soc. Agric. Eng., 2003. (01)(01): p. 25-27. [CrossRef]
- Cai, X.; P. Lin; Y. Shi; R. Han; Y. Zhang; N. Wu.J. Wang. Effects of Population Density of Bolboschoenus planiculmis on the Absorption and Accumulation of Cotton Nutrients. J. Cotton Sci., 2020(32): p. 143-150. [CrossRef]
- Wei, J.; J. Zhang; D. Ma. Investigation and Study on Law of Succession of Weeds in Cotton Fields in Changji,Xinjiang. China Cotton, 2016. (1): p. 31-33. [CrossRef]
- Zhang, Y. Development of metominostrobin, a fungicide for rice fields. World Pestic., 2002. 24(2): p. 6-12. [CrossRef]
- Zhang, G. Current Status of Application, Development and Prospect of Strobin Fungicides. Pestic. Sci. Adm., 2003. 24(12): p. 30-34. [CrossRef]
- Cahoon, C.W.; A.C. York; D.L. Jordan; R.W. Seagroves; W.J. Everman; K.M. Jennings. Fluridone carryover to rotational crops following application to cotton. J Cotton Sci, 2015. 19: p. 631-340. [CrossRef]
- Wang, L.; Y. Zhou; Y. Li; R. Wang; Z. Lin; D. Lin.S. Zhen. Evaluation of QuEChERS methods for the analysis of 66 organophosphorus pesticide residues in vegetables by liquid chromatography-tandem mass spectrometry. Sepu, 2012. 30: p. 146-153. [CrossRef]
- Zhao, Y.; M. Yu; X. Zhang; L. Sou. Research and Application of Rapid Detection Technology for 10 Kinds of Pesticide Residues in Cowpea [J]. Chin. J. Pestic. Sci., 2023. 25(06): p. 1358-1369. [CrossRef]
- Zhu, B.; S. Jin; C. Tian; F. Hu; X. Xu; J. Luo. Simultaneous Determination of 40 Organophosphorus Pesticides in Tea by Online GPC/GC-MS/MS with Multi-walled Carbon Nanotubes as Dispersive Solid Phase Extraction Sorbent [J]. J. Instrum. Anal., 2018. 37(04): p. 404-410.https://link.cnki.net/urlid/44.1318.TH.20180420.0953.002.
- Schenck, F.J.; J.E. Hobbs. Evaluation of the quick, easy, cheap, effective, rugged, and safe (QuEChERS) approach to pesticide residue analysis. [J] Bull. Environ. Contam. Toxicol., 2004. 73(1): p. 24-30. [CrossRef]
- Xu, Y.; Y. Huang; L. Yu; H. Fu; X. Zhang; m. Zhang. Detection of Toxaphene in Soil by Modified QuEChERS Technique [J] Agrochemicals, 2018. 57(06): p. 435-437. [CrossRef]
- Cui, J. Rapid determination of nitrophenols in soil by improved QuEChERS method combined with HPLC-MS/MS [J] Chem. Res. Appl., 2024. 36(04): p. 905-909. [CrossRef]
- Zhang, H.; D. Chen; Y. Han; J. Chen; X. Dai; Z. Kong.T. Yan. Residues and dissipation dynamics of six typical pesticides and three metabolites in cucumber by QuEChERS-ultra performance liquid chromatography-tandem mass spectrometry [J]. Chin. J. Pestic. Sci., 2022. 24(01): p. 168-176. [CrossRef]
- Attallah, E.R.; M. Hamdy Abdelwahed; M.M. Abo-Aly. Development and validation of multi-residue method for determination of 412 pesticide residues in cotton fiber using GC-MS/MS and LC-MS/MS. J. Text. Inst., 2018. 109(1): p. 46-63. [CrossRef]
- Li, M.; P. Li; L. Wang; M. Feng; L. Han. Determination and Dissipation of Fipronil and Its Metabolites in Peanut and Soil. J. Agric. Food Chem., 2015. 63(18): p. 4435-4443. [CrossRef]
- Dong, Z.; R. Zhou; C. Bian; H. Li; L. Wang; J. Fu; G. Xie; X. Shi; X. Li; Z. Li.B. Li. Persistence, decontamination and dietary risk assessment of propyrisulfuron residue in natural paddy field environment using QuEChERS@UPLC-Q-TOF-MS/MS. Microchem. J., 2022. 181: p. 107832. [CrossRef]
- Zhou, R.; Z. Dong; C. Bian; L. Wang; T. Wu; W. Zhou; Y. Li.B. Li. Residue analysis, dissipation behavior, storage stability and dietary risk assessment of florpyrauxifen-benzyl in natural paddy field environment using UPLC-QTOF-MS/MS. J. Food Compos. Anal., 2022. 114: p. 104781. [CrossRef]
- Luo, J.; C. Bian; L. Rao; W. Zhou; Y. Li; B. Li. Determination of the residue behavior of isocycloseram in Brassica oleracea and soil using the QuEChERS method coupled with HPLC. Food chemistry, 2022. 367: p. 130734. [CrossRef]
- Chang, H.; L. Wang; C. Huang; R. Zhou; T. Wu; B. Li. Residue analysis, dissipation dynamics, and dietary risk assessment of benzovindiflupyr in peanut field environment by LC-MS. J. Food Compos. Anal., 2023. 123: p. 130734. [CrossRef]
- Raymond Bates, J.A. The evaluation of pesticide residues in food: Procedures and problems in setting maximum residues limits. J. Sci. Food Agric., 1979. 30(4): p. 401-416. [CrossRef]
- Zhang, L.; X. Zhang; S. Jiang. Agricultural product quality and safety risk monitoring and assessment. J. Agric. Sci. Technol. , 2013. 15(04): p. 8-13.
- Michelangelo, A.; L.S. J; S. Darinka; S.F. J. Fast and easy multiresidue method employing acetonitrile extraction/partitioning and "dispersive solid-phase extraction" for the determination of pesticide residues in produce. J. AOAC Int., 2003. 86(2): p. 412-431.
- Santana-Mayor, Á.; B. Socas-Rodríguez; A.V. Herrera-Herrera; M.Á. Rodríguez-Delgado. Current trends in QuEChERS method. A versatile procedure for food, environmental and biological analysis. Trends Anal. Chem., 2019. 116: p. 214-235. [CrossRef]
- Wang, L.; C. Bian; Z. Dong; L. Liu; C. Huang; B. Li.Y. Li. Analytical method for the determination of guvermectin residues in rice environment by the QuEChERS method combined with HPLC. J. Food Compos. Anal., 2022. 111: p. 104644. [CrossRef]
- Liu, L.; L. Rao; W. Li; W. Zhou; B. Li; L. Tang. Detection of Glyamifop residues in rice and its environment by the QuEChERS method combined with HPLC–MS. Microchem. J., 2020. 158: p. 105157. [CrossRef]
- Wilkowska, A.; M. Biziuk. Determination of pesticide residues in food matrices using the QuEChERS methodology. Food Chemistry, 2010. 125(3): p. 803-812. [CrossRef]
- Liu, X.; J. Xu; F. Dong; Y. Li; W. Song; Y. Zheng. Residue analysis of four diacylhydrazine insecticides in fruits and vegetables by Quick, Easy, Cheap, Effective, Rugged, and Safe (QuEChERS) method using ultra-performance liquid chromatography coupled to tandem mass spectrometry. Anal. Bioanal. Chem., 2011. 401(3): p. 1051-1058. [CrossRef]
- Pyrzynska, K. Carbon nanotubes as sorbents in the analysis of pesticides. Chemosphere, 2011. 83(11): p. 1407-1413. [CrossRef]
- Zhao, P.; L. Wang; L. Zhou; F. Zhang; S. Kang; C. Pan. Multi-walled carbon nanotubes as alternative reversed-dispersive solid phase extraction materials in pesticide multi-residue analysis with QuEChERS method. J. Chromatogr. A, 2012. 1225: p. 17-25. [CrossRef]
- Saheem, R.; R. Tanveer; G.K. Muzamil. A review of interactions of pesticides within various interfaces of intrinsic and organic residue amended soil environment. Chem. Eng. J. Adv., 2022. 11: p. 100301. [CrossRef]
- Liu, Y.; S. Guan; W. Wang; M. Ren; F. Li. Research progress on nutritional value, virus-free method and application of cottonseed for livestock and poultry feed. China Cotton, 2023. 50(09): p. 35-41. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).