1. Introduction
Osteoarthritis (OA) is a prevalent, multifactorial joint disorder that significantly contributes to pain, functional limitation, and reduced quality of life, particularly among older adults [
1,
2]. As the most common form of arthritis globally, OA affects over 500 million people and represents a leading cause of disability in aging populations [
3,
4]. The disease is primarily characterized by the progressive deterioration of articular cartilage, remodeling of subchondral bone, osteophyte (bone spur) formation, and low-grade synovial inflammation. These pathological changes collectively lead to joint stiffness, decreased range of motion, and chronic pain [
2]. In advanced stages, OA may necessitate surgical intervention, including total joint replacement. The fundamental imbalance between anabolic and catabolic processes in joint tissues underlies disease progression, with chondrocytes—cartilage-resident cells—playing a pivotal role in regulating extracellular matrix turnover [
5,
6]. As OA advances, catabolic signaling predominates, accelerating cartilage breakdown and joint degeneration [
6].
The etiology of OA is multifactorial, encompassing biomechanical, genetic, metabolic, and inflammatory components. Mechanical stress, aging-related tissue degeneration, obesity, and genetic predispositions are well-established risk factors [
7]. In recent years, increasing emphasis has been placed on molecular and biochemical contributors, notably oxidative stress and chronic low-grade inflammation, both of which exacerbate cartilage degradation and synovial dysfunction. These emerging insights have prompted exploration into the roles of systemic factors, including micronutrients and trace elements, in modulating disease onset and progression [
8].
Essential trace elements such as zinc, copper, and selenium play crucial roles in maintaining redox homeostasis, enzymatic function, and immune regulation, which are disrupted in OA [
8,
9]. Zinc is a structural component of over 300 enzymes and plays a vital role in matrix metalloproteinase (MMP) activity, which is directly involved in cartilage breakdown [
10,
11]. Copper is essential for lysyl oxidase-mediated collagen and elastin cross-linking, and it also supports the antioxidant activity of enzymes like superoxide dismutase (SOD) [
8,
12]. Selenium plays a crucial role in human health through its incorporation into selenoproteins, particularly in the form of selenocysteine. There are 25 selenoproteins in humans, many of which are involved in antioxidant defense and redox regulation [
13]. Glutathione peroxidases (GPx) and thioredoxin reductases are key selenoproteins that protect against oxidative stress by reducing hydrogen and lipid hydroperoxides, thus regulating cellular reactive oxygen species (ROS) levels[
14].
Altered levels of zinc, copper, and selenium have been reported in the serum, synovial fluid, and cartilage of OA patients, suggesting a possible link between trace element homeostasis and disease progression [
15,
16]. Both deficiencies and imbalances among these elements may exacerbate oxidative damage, disturb cartilage matrix turnover, and intensify inflammatory signaling—key drivers of OA pathology. To support this review, relevant literature was identified through a broad search of the PubMed, Scopus, and Web of Science databases using combinations of keywords such as “osteoarthritis,” “zinc,” “copper,” “selenium,” “trace elements,” “oxidative stress,” and “cartilage degradation.” Studies were selected based on their relevance to the biological functions and clinical implications of these trace elements in OA. This review synthesizes findings from clinical research, experimental models, and population studies to provide an updated understanding of the emerging roles of zinc, copper, and selenium in OA pathogenesis, diagnosis, and potential management strategies.
2. The Role of Essential Trace Elements in Osteoarthritis: Biological Mechanisms and Clinical Evidence
Essential trace elements are vital micronutrients that, despite being required in minute quantities, play significant roles in maintaining joint health. Their involvement in enzymatic reactions, antioxidant defense systems, immune modulation, and tissue remodeling underscores their importance in maintaining joint health and preventing degenerative conditions such as OA [
8]. Among the numerous trace elements, zinc, copper, and selenium have gained particular attention due to their roles in oxidative balance, connective tissue metabolism, and inflammatory regulation. Deficiencies or imbalances of these elements have been implicated in the pathophysiology of several chronic conditions, including OA.
2.1. Zinc (Zn): Cartilage Regeneration and Inflammatory Modulation
Zinc is an essential trace element that plays an indispensable role in a variety of physiological and biochemical processes in the human body. It serves as a structural, catalytic, and regulatory component for more than 2,700 enzymes, including hydrolases, transferases, oxyreductases, ligases, isomerases, and lyases [
17]. Moreover, Zinc is essential for cellular metabolism, gene expression, signal transduction, immune response, and apoptosis [
18,
19]. As a cofactor in over 300 enzymes, it contributes to critical cellular functions such as DNA synthesis, protein production, and cell division [
20]. These widespread roles highlight the systemic significance of zinc, particularly in tissues with high cellular turnover and metabolic activity.
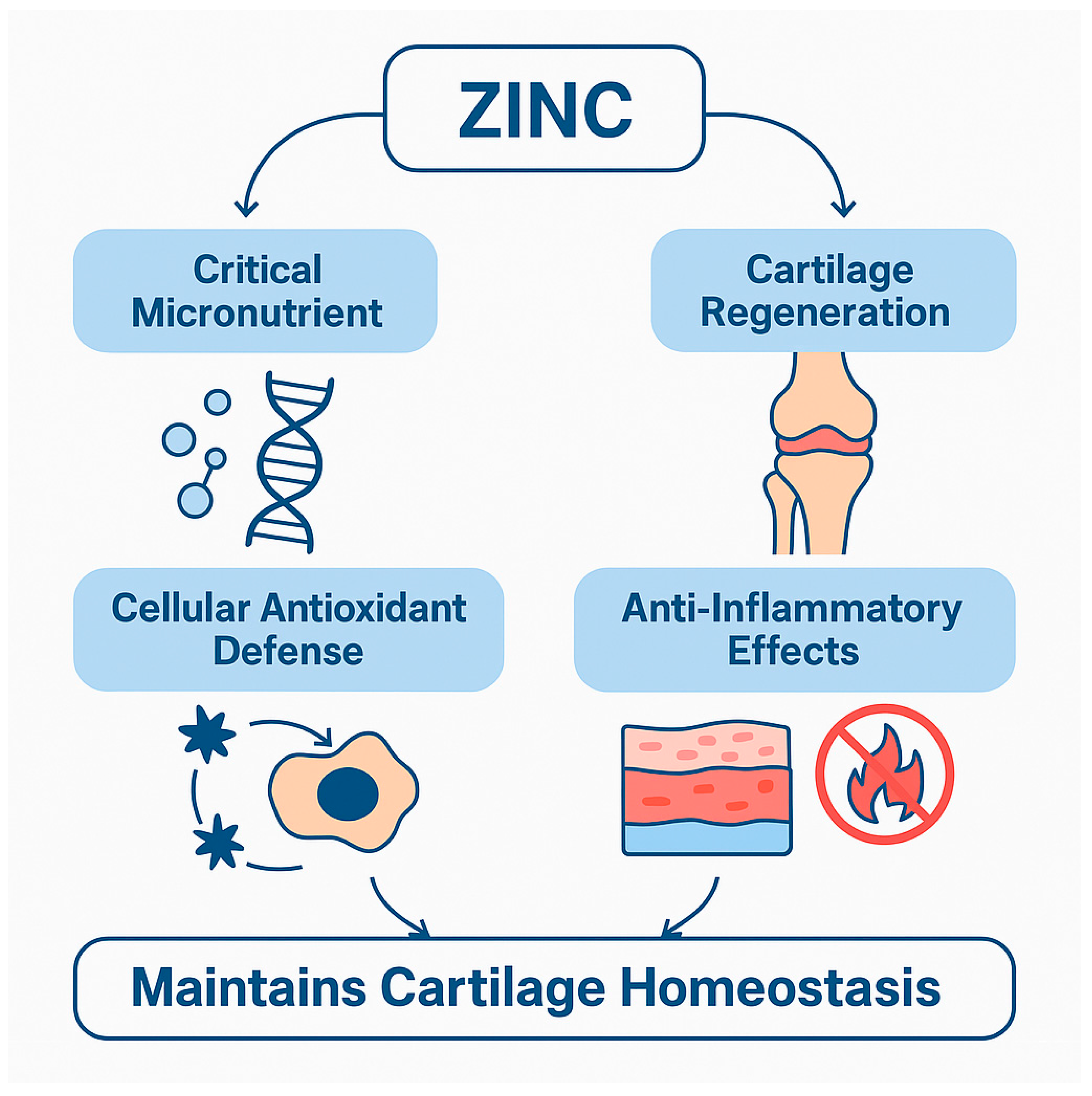
Zinc plays a crucial role in maintaining cartilage homeostasis and regulating immune function, which has direct implications for OA pathogenesis. Zinc ions regulate intracellular signaling pathways in immune cells, with homeostasis maintained by zinc transporters and zinc-binding proteins [
21]. Zinc’s anti-inflammatory and antioxidant properties are well-documented. Zinc deficiency has been linked to the development of a pro-inflammatory phenotype, contributing to cartilage destruction and chondrocyte apoptosis—hallmarks of OA [
22]. The balance between anabolic and catabolic signaling pathways is critical for maintaining cartilage homeostasis, and disturbances in this balance contribute to joint diseases like OA[
23].
One of zinc’s critical roles in joint health is its function in cellular redox homeostasis. As a cofactor in copper/zinc superoxide dismutase (Cu/Zn-SOD), zinc helps catalyze the dismutation of superoxide radicals into molecular oxygen and hydrogen peroxide, mitigating oxidative damage [
24,
25]. This antioxidant activity protects chondrocytes from oxidative stress-induced damage, a key driver of cartilage degradation in OA [
26,
27]. Moreover, in pathological states such as type 2 diabetes, altered zinc homeostasis, and reduced Cu/Zn-SOD activity further link zinc deficiency to joint damage [
28]. Zinc also inhibits NF-κB, a transcription factor involved in pro-inflammatory cytokine production, including TNF-α and IL-1β, thereby counteracting oxidative and inflammatory stressors contributing to OA development [
18,
29].
2.1.1. Clinical and Experimental Evidence
Zinc Supplementation and Chondrocyte Function: Zinc supplementation has demonstrated protective effects by modulating oxidative stress and chondrocyte function. In in vitro and in vivo models, zinc counteracted the damaging effects of monosodium iodoacetate (MIA) via the p-Akt/Nrf2 pathway, enhancing the expression of Nrf2 and phosphorylated Akt [
30,
31]. A moderate zinc dose (1.6 mg/kg/day) effectively prevented OA progression, while higher doses showed no additional benefit [
30]. Additionally, zinc deficiency is associated with impaired cartilage remodeling and increased cellular senescence, further contributing to OA pathophysiology [
30].
Zinc-Based Nanotherapies: Recent advances in zinc-based therapies also show promise. Zinc(II) enhances drug delivery systems, as seen in a Zn-driven nano-assembly delivering metformin and p65 siRNA into cartilage. Zinc’s positive charge improves cartilage retention and penetration. It promotes autophagy via the AMPK/mTOR pathway and suppresses NF-κB signaling, protecting chondrocytes from apoptosis while supporting extracellular matrix (ECM) repair through upregulation of Col2a1 and Acan [
32]. This nano therapy significantly reduced IL-6, TNF-α, and MMP-13, preserved cartilage, and improved chondrocyte viability in OA models.
Zinc Transporters and Catabolic Pathways: However, excessive intracellular zinc can have deleterious effects. In OA cartilage, overexpression of the zinc transporter ZIP8 leads to increased zinc influx, activating metal-responsive transcription factor 1 (MTF1), which induces MMP13 and ADAMTS5—key catabolic enzymes. A positive feedback loop between MTF1 and hypoxia-inducible factor-2α (HIF-2α) further amplifies cartilage degradation. Experimental deletion of ZIP8 or MTF1 reduced OA severity, highlighting this zinc-mediated pathway as a potential therapeutic target [
33].
Population and Genomic Evidence: Epidemiological and genomic studies support zinc’s involvement in OA risk. A large NHANES-based cross-sectional study found that higher daily zinc intake, along with copper and selenium, was significantly associated with increased OA risk, suggesting that excessive intake may be detrimental [
34]. Consistently, Mendelian randomization using GWAS data revealed a strong causal link between elevated serum zinc levels and increased risk of knee and spine OA, likely through promotion of matrix-degrading enzyme activity [
35,
36]. These findings position zinc as a potential biomarker and therapeutic target in OA.
Tissue Zinc Levels and Epigenetic Changes: Conversely, some studies report lower zinc levels in OA patients. Reduced zinc is linked with impaired cartilage integrity and heightened degeneration, emphasizing the importance of maintaining optimal zinc levels for joint health [
30,
37]. In hip OA, patients showed increased zinc content in femoral bone with disease severity, implicating zinc in OA progression and matrix degradation through its role as an MMP cofactor [
38]. A subgroup of hip OA patients exhibited epigenetic and transcriptomic changes, including upregulation of MTF1 and its targets MMP13 and ADAMTS5, supporting the zinc–inflammation–matrix degradation connection [
39].
Zinc Combinations and Biomaterials: In therapeutic development, zinc has shown efficacy in combination treatments. Zinc combined with a probiotic complex and rosavin reduced OA progression in MIA-induced rat models by suppressing proinflammatory cytokines and catabolic gene expression in patient-derived chondrocytes [
40]. Zinc folate (ZnFO)-loaded scaffolds promoted cartilage matrix metabolism and gene expression (e.g., COL2A1, SOX9), with sustained zinc release supporting cell proliferation and differentiation. These scaffolds facilitated cartilage and subchondral bone repair in vivo [
41]. Similarly, ZnO nanoparticle-based biodegradable scaffolds enhanced osteochondral differentiation of mesenchymal stem cells (MSCs), with dose-dependent promotion of chondrogenic and osteogenic markers [
42].
Zinc-Related Genetic Markers: Furthermore, zinc metabolism-related genes (ZMRGs) have been identified in OA. Genes such as MMP2, MMP3, MMP9, and MMP13 were upregulated and associated with disease progression, making them potential biomarkers and therapeutic targets [
43].
The multifaceted role of zinc in osteoarthritis is visually summarized in
Figure 1, which illustrates its contributions to antioxidant defense, inflammation modulation, and cartilage repair. Additionally,
Table 1 consolidates experimental and clinical findings across multiple domains, highlighting key molecular targets and pathways influenced by zinc. Together, these tools provide a comprehensive overview of how zinc homeostasis impacts OA progression and treatment strategies.
2.2. Copper (Cu): Structural, Antioxidant, and Immunomodulatory Roles in OA
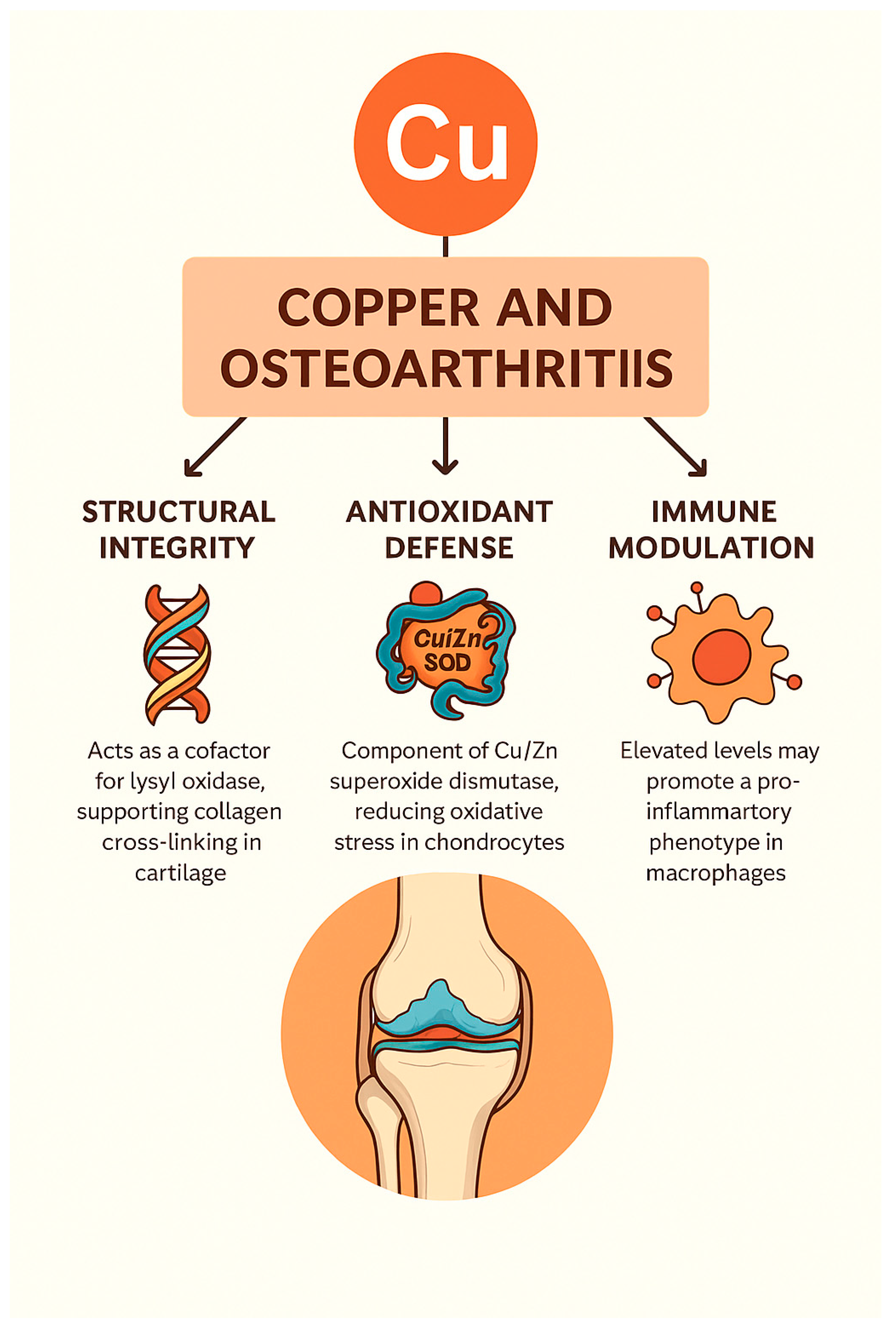
Copper is an indispensable trace element that plays a pivotal role in maintaining the structural integrity of connective tissues, particularly cartilage and bone. One of its primary functions is serving as a cofactor for lysyl oxidase (LOX), an enzyme crucial for the cross-linking of collagen and elastin fibers in the ECM. This cross-linking process is essential for the tensile strength and elasticity of cartilage, attributes that are compromised in osteoarthritic conditions. Studies have demonstrated that dietary copper levels directly influence LOX activity, with deficiencies leading to impaired collagen cross-linking and subsequent weakening of cartilage structure [
44,
45,
46,
47]. Furthermore, copper is involved in the post-translational modification of LOX, facilitating the formation of its active site cofactor, lysyl tyrosylquinone, which is vital for its enzymatic activity[
48].
Beyond its structural roles, copper is integral to the body's antioxidant defense mechanisms. It is a key component of Cu/Zn-SOD, an enzyme that catalyzes the dismutation of superoxide radicals into hydrogen peroxide and molecular oxygen, thereby mitigating oxidative stress within chondrocytes. Oxidative stress is a significant contributor to chondrocyte apoptosis and cartilage degradation in OA. Disruptions in copper homeostasis can lead to decreased Cu/Zn-SOD activity, resulting in increased ROS production and mitochondrial dysfunction. Additionally, copper’s involvement in redox balance plays a role in preventing ferroptosis, a form of iron-dependent cell death associated with lipid peroxidation in chondrocytes [
49].
Copper also plays an important immunomodulatory role. It influences various immune cells, including macrophages, by affecting cytokine production and inflammatory signaling pathways. Elevated copper levels are associated with the promotion of a pro-inflammatory phenotype in macrophages, marked by increased IL-1β and TNF-α production [
50]. Conversely, copper deficiency impairs immune responses, including reduced lymphocyte proliferation and altered acute-phase protein synthesis [
51]. Given that OA is characterized by chronic low-grade inflammation, maintaining optimal copper balance is essential not only for structural and antioxidant defense but also for controlling immune-mediated joint degradation.
2.2.1. Clinical and Experimental Evidence
Serum and Tissue Copper Levels in OA: Numerous studies have explored copper concentrations in biological compartments of OA patients. Elevated serum and plasma copper levels have been linked with inflammatory markers and OA risk [
52,
53]. Higher copper concentrations have also been observed in joint tissues such as femoral heads and menisci in advanced OA stages, indicating localized accumulation during disease progression [
38,
54]. Dietary study has demonstrated a relationship between high copper intake and increased OA risk, although serum levels may not always directly reflect intake due to regulatory imbalances [
34]. Meta-analytic data further reinforce the observation that circulating copper levels are consistently higher in OA patients compared to healthy controls [
55]. However, some research suggests lower copper levels may be associated with OA [
37,
56]. This highlights the complex role of copper in bone and joint health.
The contradictory findings likely reflect variability across populations due to factors like geography, diet, and metabolism. For instance, a study found higher serum copper in picky eaters among children, demonstrating how dietary habits can influence trace element levels[
57]. Additionally, the study showed that high iron intake can lead to copper deficiency, illustrating how nutrient interactions may impact copper status [
58].
Copper Supplementation and Toxicity Risk: Copper-based nanotherapeutics offer novel approaches for OA treatment. Various nanoparticle systems, such as B2M-CuS [
59], CSP@AS-IV [
60], and Cu-indomethacin gels [
61], have demonstrated anti-inflammatory, antioxidant, and regenerative effects in preclinical OA models. Other delivery platforms, including injectable PMs@CuBG microspheres [
62], D-CuS@NR nanoparticles [
63], and MSCs@CuS@CDKN1A systems [
64] have shown effectiveness in modulating immune responses, promoting chondrocyte survival, and enhancing ECM synthesis. These copper-enabled technologies illustrate the potential for safe, localized, and multi-modal OA therapies.
Immunoinflammatory Mechanisms: The immunoregulatory functions of copper extend to modulating macrophage phenotypes and downregulating matrix-degrading enzymes. Studies have shown that copper-incorporated bioactive ceramics can shift macrophage polarization toward an anti-inflammatory profile while suppressing catabolic activity in inflamed cartilage [
65].
Genetic and Molecular Insights: Copper’s influence in OA is also evident at the molecular level. Copper transporter gene variants (e.g., ATP7A, ATP7B) have been implicated in systemic inflammation and mineral regulation. Dysregulation of cuproptosis—a copper-induced cell death pathway—has been linked to OA pathogenesis, with key genes such as FDX1, DLAT, and MTF1 playing central roles [
49,
66]. Mendelian randomization studies provide supportive genetic evidence that higher circulating copper levels may causally contribute to OA susceptibility [
67,
68].
In general,
copper exerts multifaceted effects in the pathophysiology and potential treatment of OA, influencing processes related to structural matrix stability, oxidative stress regulation, immune modulation, and gene-mediated pathways such as cuproptosis. Both copper deficiency and excess have been associated with cartilage degradation, inflammation, and chondrocyte death. Advances in copper-targeted therapeutics—including nanoparticles and bioactive delivery systems—offer promising avenues for restoring joint homeostasis and attenuating disease progression. These mechanistic domains and therapeutic implications are summarized in
Table 2 and visually represented in
Figure 2.
2.3. Selenium (Se): Redox and Immune-Modulatory Roles in Osteoarthritis
Selenium is an essential trace element that exerts its biological effects primarily through its incorporation into a family of selenoproteins, including GPx and thioredoxin reductases (TrxR), which play indispensable roles in redox homeostasis. These selenoenzymes act as potent antioxidants by catalyzing the reduction of hydrogen peroxide and lipid hydroperoxides, thereby protecting cells—including articular chondrocytes—from oxidative damage and apoptosis [
69,
70,
71,
72]. The antioxidant function of selenium is particularly significant in OA joints, where oxidative stress is a known contributor to cartilage degradation and disease progression [
73].
Beyond its antioxidant role, selenium also modulates inflammatory responses through redox-sensitive transcription factors such as NF-κB and AP-1, which regulate the production of pro-inflammatory cytokines like IL-1β, IL-6, and TNF-α [
74,
75]. By mitigating these inflammatory pathways, selenium contributes to a more balanced immune microenvironment within the joint, potentially slowing OA progression. Moreover, selenium is involved in regulating immune cell function, including neutrophil activity and T-cell proliferation, thereby reducing the risk of chronic inflammation and joint tissue damage [
76].
Several clinical and experimental studies have demonstrated that selenium deficiency is associated with increased susceptibility to inflammatory joint diseases, including Kashin-Beck disease and OA [
77,
78]. Low selenium levels in synovial fluid and serum have been correlated with increased oxidative stress markers, elevated inflammatory cytokines, and greater cartilage damage in OA patients [
79]. These findings underscore the therapeutic potential of selenium supplementation or selenoprotein modulation in preserving cartilage integrity and controlling inflammation in OA.
2.3.1. Mechanistic, Therapeutic, and Population-Level Evidence
Antioxidant Signaling and Genetic Regulation: Reduced levels of selenoproteins such as selenoprotein P (SELENOP) and glutathione peroxidase 3 (GPx3) have been identified in OA patients despite comparable serum selenium concentrations. These deficiencies correlate with poorer functional performance and may reflect inflammation-induced suppression of hepatic SELENOP and impaired renal GPx3 synthesis [
80]. Moreover, polymorphisms in selenium-responsive genes, such as GPX1, SELENOS, DIO2, PPARG, SMAD3, ADAM12, and TIMP2, have been linked to increased susceptibility to OA and Kashin–Beck disease by disrupting redox signaling and extracellular matrix (ECM) homeostasis [
81,
82]. These molecular alterations highlight selenium's role in modulating oxidative defense, transcriptional regulation, and genetic risk.
Chondrogenesis, DNA Repair, and Cartilage Anabolism: Selenium deficiency impairs chondrogenesis by downregulating anabolic markers such as SOX9, COL2A1, and aggrecan. In contrast, selenium supplementation restores these gene expressions and promotes cartilage formation and matrix stability. It also enhances DNA repair capacity and shields chondrocytes from oxidative genomic damage, thereby preserving their proliferative and regenerative functions in degenerative joint environments [
82].
Cellular Stress Response and Matrix Preservation: Selenium maintains chondrocyte viability by restoring mitochondrial integrity, reducing ROS accumulation, and rebalancing redox-sensitive signaling pathways (e.g., Nrf2, PI3K/Akt, JNK, Wnt/β-catenin). It also suppresses catabolic and inflammatory mediators such as MMP13, TNF-α, IL-1β, COX-2, and iNOS while supporting type II collagen synthesis[
8,
73,
82,
83,
84,
85]. Notably, selenium influences glycosylation patterns in chondrocytes, suggesting a novel role in post-translational regulation of matrix-associated proteins [
83]. These mechanisms collectively contribute to matrix preservation and suppression of chondrocyte apoptosis.
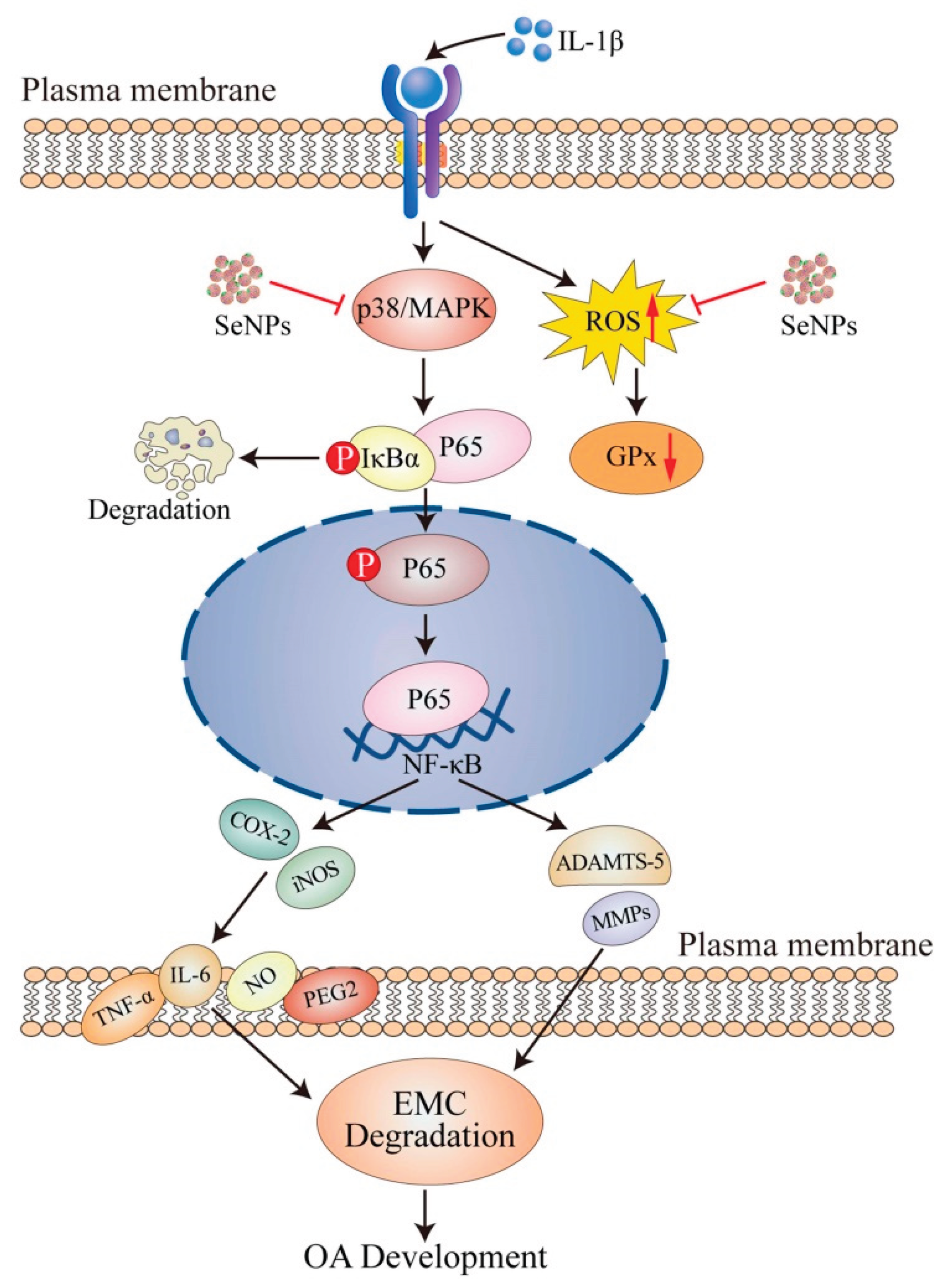
Innovative Nanomedicine Strategies for OA: Recent advances in nanotechnology have enabled the development of selenium-based delivery platforms with enhanced therapeutic efficacy. Selenium nanoparticles (SeNPs) have demonstrated significant anti-inflammatory and chondroprotective effects by downregulating IL-1β–induced pro-inflammatory genes and catabolic enzymes (e.g., MMP13, ADAMTS-5), upregulating ECM components (COL2A1, aggrecan), and modulating NF-κB and MAPK signaling pathways [
86]. Coating SeNPs with polydopamine (PDA-SeNPs) further improved their biocompatibility, antioxidant potential, and chondrogenic activity, while suppressing oxidative stress and canonical Wnt signaling inhibition [
87]. Dual-scale targeted systems—such as HA-SeNPs@AHAMA-HMs—have demonstrated controlled release, enhanced cartilage affinity, and robust selenoprotein reactivation, leading to improved structural outcomes in OA joints [
88]. These nanotherapeutics represent a promising avenue for localized, redox-targeted OA treatment. The therapeutic framework and biological outcomes of SeNP administration in OA are visually summarized in
Figure 3.
Epidemiologic Correlates and Risk Modification: Multiple studies have confirmed the association between selenium status and OA risk. Mendelian randomization has demonstrated a causal, inverse relationship between serum selenium levels and OA incidence, particularly among women [
89]. Cross-sectional analyses from Nigeria and other regions further report significantly lower selenium levels in OA patients, suggesting selenium deficiency contributes to disease vulnerability and may warrant targeted nutritional or therapeutic intervention [
34,
37]. These findings emphasize selenium’s value not only as a therapeutic agent but also as a biomarker for OA risk stratification.
Collectively, selenium exerts multifaceted effects on OA through a range of biological mechanisms. It modulates antioxidant signaling by regulating key selenoproteins such as GPX1 and SELENOP and influences redox-sensitive pathways including NF-κB, PI3K/Akt, and TGF-β. Genetic variations in selenium-regulating genes (e.g., GPX1, SELENOS, PPARG) further implicate selenium in cartilage homeostasis and oxidative defense. Selenium also promotes chondrogenesis and DNA repair by enhancing SOX9, COL2A1, and aggrecan expression, while protecting against genomic instability. At the cellular level, selenium restores mitochondrial function, reduces oxidative stress and inflammation, and supports matrix preservation. Advances in nanomedicine have leveraged selenium’s bioactivity to develop SeNP-based delivery systems that target inflamed joints and promote cartilage repair. Finally, population-based studies and Mendelian randomization analyses support a protective role for selenium against OA risk, particularly among selenium-deficient individuals. These domains are comprehensively summarized in
Table 3.
3. Combined Effects and Interactions
While zinc, copper, and selenium have individually demonstrated significant roles in OA pathogenesis and progression, emerging evidence highlights the importance of examining their interrelated effects. These trace elements are intricately connected through shared involvement in key physiological processes such as redox homeostasis, inflammatory signaling, and ECM remodeling. For instance, zinc and copper are both critical cofactors for the antioxidant enzyme Cu/Zn-SOD, and their ratio is known to influence enzyme stability and activity [
25,
26,
49]. Similarly, selenium is required for the synthesis of GPxs, which function alongside Cu/Zn-SOD in mitigating oxidative stress, particularly in chondrocytes [
70,
72].
The balance among these trace elements appears to be more important than their absolute levels. Differential concentrations of zinc and copper in joint tissues have been observed, suggesting a potential antagonistic relationship [
54]. Elevated zinc-to-copper ratios have also been linked to the radiographic severity of OA, emphasizing the pathological relevance of their relative proportions [
38]. Additionally, selenium’s ability to modulate both NF-κB and Nrf2 signaling pathways enables it to indirectly influence the expression and activity of enzymes regulated by copper and zinc, supporting the concept of a shared regulatory network [
73].
Despite these insights, the mechanistic interplay among these elements remains underexplored in human populations. Most existing studies focus on monotherapies, and few clinical trials have examined multi-element supplementation with controlled dosage and duration. Additionally, factors such as age, comorbidities, dietary patterns, and genetic polymorphisms can influence the bioavailability and metabolism of these trace elements, further complicating their interaction dynamics. Overall, these trace elements likely operate as part of a dynamic micronutrient network that modulates inflammation, oxidative damage, and cartilage metabolism in OA. Future interventions may benefit from a systems biology approach, integrating micronutrient profiling, gene-nutrient interactions, and clinical phenotyping to personalize trace element–based therapies for OA.
4. Challenges and Controversies
While clinical and experimental evidence supports the involvement of zinc, copper, and selenium in OA, several challenges complicate interpretation. Heterogeneity in study design, patient demographics, and measurement techniques often leads to conflicting findings. For example, discrepancies between serum, plasma, and synovial fluid concentrations of trace elements can affect outcome interpretation [
34,
37,
54,
73]. Additionally, biological variables such as age, sex, dietary habits, and comorbidities may influence trace element homeostasis, reducing the generalizability of study results [
38,
52]. The lack of large-scale, randomized controlled trials (RCTs) with long-term follow-up further limits the ability to determine causal relationships and therapeutic efficacy.
These limitations underscore the inherent complexity of micronutrient research in OA and highlight the need for standardized assessment methods and cautious interpretation of current data.
4.1. Conflicting Clinical Evidence
The clinical evidence concerning trace elements such as zinc, copper, and selenium in OA remains inconsistent, presenting a challenge for the identification of reliable biomarkers. Some studies indicate reduced serum zinc levels in OA patients, suggesting a potential deficiency or altered metabolism in these individuals [
34]. Conversely, other research reports elevated zinc concentrations in synovial fluid, which may be linked to the activation of matrix-degrading enzymes contributing to cartilage destruction in OA [
90,
91]. Similar inconsistencies are evident for copper and selenium. Elevated copper levels have been observed in the synovial fluid of OA patients and may reflect underlying inflammatory processes [
92,
93]. Selenium levels also show mixed patterns; while some studies report no significant differences in serum selenium levels between OA and non-arthritis groups, others note decreased levels in inflammatory conditions [
16,
92].
Several factors likely contribute to these conflicting findings. Differences in geographic regions and dietary habits can influence trace element status, leading to variability across populations [
16]. Moreover, methodological inconsistencies, such as small sample sizes, diverse sampling techniques, and the choice of the biological matrix (e.g., serum versus synovial fluid) can significantly affect outcomes and comparability [
92,
93]. While these inconsistencies complicate the development of diagnostic tools, they also highlight the metabolic complexity of trace elements in OA. These findings underscore the urgent need for standardized methodologies and larger, more diverse study populations to clarify the role of trace elements in OA pathogenesis and to support the development of valid biomarkers for diagnosis and prognosis.
4.2. Bioavailability and Absorption Factors
The bioavailability and absorption of trace elements such as zinc, copper, and selenium are regulated by multiple physiological, dietary, and pathological factors, all of which are particularly relevant when considering supplementation strategies for older adults with OA. One key mechanism is competitive inhibition at shared transporters. For instance, zinc and copper utilize overlapping transport pathways, and high zinc intake can inhibit copper absorption by out-competing it at intestinal transport sites [
94,
95]. The copper transporter CRT1 is notably upregulated in states of dietary copper deficiency, further reflecting the dynamic regulation of these elements based on nutritional status [
95].
Additionally, the chemical form of a trace element significantly influences its absorption. Organic forms of selenium, such as selenomethionine and selenocysteine, are more efficiently absorbed than inorganic forms like selenate, largely due to their transport via amino acid carriers [
94,
96]. However, unlike other essential nutrients, these organic selenium compounds lack tight homeostatic control mechanisms, making their supplementation potentially more variable in effect [
95]. Age-related changes in gastrointestinal physiology further complicate absorption. Older adults may experience reduced gastric acid production and altered intestinal permeability, which can negatively impact the uptake of zinc, copper, and selenium [
94]. Moreover, chronic diseases such as diabetes mellitus may impair the metabolism and utilization of these trace elements, altering their bioavailability and increasing the risk of both deficiency and toxicity [
97].
4.3. Safety and Toxicity Concerns
While supplementation can be an effective strategy to correct deficiencies in zinc, copper, and selenium, it must be approached cautiously due to the narrow therapeutic windows associated with these elements. Excessive intake, particularly when administered without consideration of individual absorption capacity and metabolic state, can result in toxicity. For example, chronic overexposure to selenium or copper has been linked to oxidative stress, liver damage, and metabolic disturbances, emphasizing the importance of personalized dosing regimens [
98].
Given the interdependent absorption pathways and variable bioavailability influenced by age, disease, and chemical form, supplementation must be tailored to individual needs. This requires regular monitoring of trace element status and careful selection of dosage and formulation. Particularly in older adults or those with comorbid conditions such as OA, a nuanced understanding of trace element metabolism is essential to avoid adverse effects while maximizing therapeutic benefits.
5. Future Directionss
Despite growing interest in the role of trace elements in OA, critical knowledge gaps remain that hinder the development of evidence-based clinical applications. Bridging these gaps will require interdisciplinary collaboration, standardized methodologies, and a concerted effort to move beyond descriptive studies toward mechanistic and translational research.
Large-Scale Randomized Controlled Trials (RCTs): To establish causal relationships and determine the clinical efficacy of trace element supplementation, robust randomized controlled trials are urgently needed. The current evidence base is constrained by small sample sizes, heterogeneous study designs, short intervention durations, and non-standardized outcome measures. Well-powered, methodologically rigorous RCTs are essential to define optimal dosages, treatment durations, and responsive patient subgroups. Furthermore, trials should integrate mechanistic endpoints to elucidate the biological pathways through which trace elements exert their effects in OA.
Personalized Nutrition and Nutrigenomics: Advances in nutrigenomics and precision medicine offer new opportunities to tailor trace element interventions based on genetic predispositions, metabolic phenotypes, and baseline nutritional status. Interindividual variability in absorption, distribution, and metabolism of zinc, copper, and selenium may influence both disease risk and therapeutic response. Integrating genomic, biochemical, and lifestyle data could optimize supplementation strategies, minimize adverse effects, and enhance patient outcomes through a more individualized approach.
Development of Standardized Biomarkers: A major barrier to progress is the absence of reliable, validated biomarkers for trace element status in OA. Future research should prioritize the identification and validation of sensitive and specific biomarkers—whether in serum, synovial fluid, or cartilage tissue—that can be used for early diagnosis, disease monitoring, and treatment stratification. Biomarker standardization will also be critical for cross-study comparisons and the advancement of personalized interventions.
Investigating Synergistic and Multimodal Therapies: Given the multifactorial nature of OA, future studies should explore the potential of multimodal interventions that combine trace element supplementation with other bioactive compounds, such as vitamin D, omega-3 fatty acids, polyphenols, or glucosamine. Investigating synergistic effects at molecular and clinical levels may yield more comprehensive and durable benefits, potentially modifying disease progression rather than solely alleviating symptoms.
6. Conclusion
Zinc, copper, and selenium are essential trace elements with integral roles in biological processes implicated in OA pathogenesis, including oxidative stress, inflammation, and extracellular matrix degradation. An expanding body of preclinical and observational evidence suggests that imbalances in these micronutrients may influence the onset and progression of OA. However, the clinical translation of these findings remains limited by methodological inconsistencies, inadequate sample sizes, and a lack of mechanistic insight.
Incorporating trace element assessment and targeted supplementation into OA management holds promise as a low-cost, adjunctive therapeutic strategy. However, achieving clinical efficacy will require a shift toward precision-based approaches, supported by robust biomarker development and individualized treatment protocols. Future research must strive to delineate the mechanistic underpinnings of trace element function in joint biology and to evaluate the long-term safety and effectiveness of supplementation strategies through well-designed clinical trials. Advancing our understanding of elemental homeostasis in OA may ultimately facilitate the emergence of nutritionally informed, personalized interventions that enhance both disease management and patient quality of life.
Funding
This research did not receive any external funding.
Conflicts of Interest
The authors declare that they have no competing interests.
References
- Tang, S.; Zhang, C.; Oo, W.M.; Fu, K.; Risberg, M.A.; Bierma-Zeinstra, S.M.; Neogi, T.; Atukorala, I.; Malfait, A.M.; Ding, C., et al. Osteoarthritis. Nat Rev Dis Primers 2025, 11, 10. [CrossRef]
- Tuncay Duruöz, M.; Öz, N.; Gürsoy, D.E.; Hande Gezer, H. Clinical aspects and outcomes in osteoarthritis. Best Pract Res Clin Rheumatol 2023, 37, 101855. [CrossRef]
- Long, H.; Liu, Q.; Yin, H.; Wang, K.; Diao, N.; Zhang, Y.; Lin, J.; Guo, A. Prevalence trends of site-specific osteoarthritis from 1990 to 2019: findings from the global burden of disease study 2019. Arthritis & Rheumatology 2022, 74, 1172-1183.
- Langworthy, M.; Dasa, V.; Spitzer, A.I. Knee osteoarthritis: disease burden, available treatments, and emerging options. Therapeutic Advances in Musculoskeletal Disease 2024, 16, 1759720X241273009.
- Loeser, R.F.; Goldring, S.R.; Scanzello, C.R.; Goldring, M.B. Osteoarthritis: a disease of the joint as an organ. Arthritis and rheumatism 2012, 64, 1697.
- Kapoor, M. Pathogenesis of osteoarthritis. Osteoarthritis: pathogenesis, diagnosis, available treatments, drug safety, regenerative and precision medicine 2015, 1-28.
- Mobasheri, A.; Batt, M. An update on the pathophysiology of osteoarthritis. Ann Phys Rehabil Med 2016, 59, 333-339. [CrossRef]
- Li, G.; Cheng, T.; Yu, X. The Impact of Trace Elements on Osteoarthritis. Front Med (Lausanne) 2021, 8, 771297. [CrossRef]
- Hu, W.; Yao, X.; Li, Y.; Li, J.; Zhang, J.; Zou, Z.; Kang, F.; Dong, S. Injectable hydrogel with selenium nanoparticles delivery for sustained glutathione peroxidase activation and enhanced osteoarthritis therapeutics. Materials Today Bio 2023, 23, 100864. [CrossRef]
- Sanna, A.; Firinu, D.; Zavattari, P.; Valera, P. Zinc Status and Autoimmunity: A Systematic Review and Meta-Analysis. Nutrients 2018, 10. [CrossRef]
- Cuffaro, D.; Gimeno, A.; Bernardoni, B.L.; Di Leo, R.; Pujadas, G.; Garcia-Vallvé, S.; Nencetti, S.; Rossello, A.; Nuti, E. Identification of N-Acyl Hydrazones as New Non-Zinc-Binding MMP-13 Inhibitors by Structure-Based Virtual Screening Studies and Chemical Optimization. Int J Mol Sci 2023, 24. [CrossRef]
- Vallet, S.D.; Ricard-Blum, S. Lysyl oxidases: from enzyme activity to extracellular matrix cross-links. Essays Biochem 2019, 63, 349-364. [CrossRef]
- Handy, D.E.; Joseph, J.; Loscalzo, J. Selenium, a Micronutrient That Modulates Cardiovascular Health via Redox Enzymology. Nutrients 2021, 13. [CrossRef]
- Gamble, S.C.; Wiseman, A.; Goldfarb, P.S. Selenium-dependent glutathione peroxidase and other selenoproteins: Their synthesis and biochemical roles. Journal of Chemical Technology & Biotechnology: International Research in Process, Environmental AND Clean Technology 1997, 68, 123-134.
- Zhou, H.; Zhang, Y.; Tian, T.; Wang, B.; Pan, Y. Meta-analysis of the Relationship Between Zinc and Copper in Patients with Osteoarthritis. Biol Trace Elem Res 2025, 203, 635-645. [CrossRef]
- Yang, W.M.; Lv, J.F.; Wang, Y.Y.; Xu, Y.M.; Lin, J.; Liu, J.; Chen, J.J.; Wang, X.Z. The Daily Intake Levels of Copper, Selenium, and Zinc Are Associated with Osteoarthritis but Not with Rheumatoid Arthritis in a Cross-sectional Study. Biol Trace Elem Res 2023, 201, 5662-5670. [CrossRef]
- Baltaci, A.K.; Yuce, K.; Mogulkoc, R. Zinc Metabolism and Metallothioneins. Biol Trace Elem Res 2018, 183, 22-31. [CrossRef]
- Jarosz, M.; Olbert, M.; Wyszogrodzka, G.; Młyniec, K.; Librowski, T. Antioxidant and anti-inflammatory effects of zinc. Zinc-dependent NF-κB signaling. Inflammopharmacology 2017, 25, 11-24. [CrossRef]
- Yao, G.; Wang, Z.; Xie, R.; Zhanghuang, C.; Yan, B. Trace element zinc metabolism and its relation to tumors. Front Endocrinol (Lausanne) 2024, 15, 1457943. [CrossRef]
- Kiouri, D.P.; Chasapis, C.T.; Mavromoustakos, T.; Spiliopoulou, C.A.; Stefanidou, M.E. Zinc and its binding proteins: essential roles and therapeutic potential. Arch Toxicol 2025, 99, 23-41. [CrossRef]
- Wessels, I.; Maywald, M.; Rink, L. Zinc as a Gatekeeper of Immune Function. Nutrients 2017, 9. [CrossRef]
- Houard, X.; Goldring, M.B.; Berenbaum, F. Homeostatic mechanisms in articular cartilage and role of inflammation in osteoarthritis. Curr Rheumatol Rep 2013, 15, 375. [CrossRef]
- Otsuki, S.; Hanson, S.R.; Miyaki, S.; Grogan, S.P.; Kinoshita, M.; Asahara, H.; Wong, C.H.; Lotz, M.K. Extracellular sulfatases support cartilage homeostasis by regulating BMP and FGF signaling pathways. Proc Natl Acad Sci U S A 2010, 107, 10202-10207. [CrossRef]
- Karmakar, A.; Das, A.K.; Ghosh, N.; Sil, P.C. Chapter2.7 - Superoxide dismutase. In Antioxidants Effects in Health, Nabavi, S.M., Silva, A.S., Eds. Elsevier: 2022; pp. 139-166. [CrossRef]
- Valentine, J.S.; Ellerby, L.M.; Graden, J.A.; Nishida, C.R.; Gralla, E.B. Copper-Zinc Superoxide Dismutase: Mechanistic and Biological Studies. Bioinorganic Chemistry: An Inorganic Perspective of Life 1995, 77-91.
- Marreiro, D.D.; Cruz, K.J.; Morais, J.B.; Beserra, J.B.; Severo, J.S.; de Oliveira, A.R. Zinc and Oxidative Stress: Current Mechanisms. Antioxidants (Basel) 2017, 6. [CrossRef]
- Kloubert, V.; Rink, L. Zinc as a micronutrient and its preventive role of oxidative damage in cells. Food Funct 2015, 6, 3195-3204. [CrossRef]
- Bravo, A.; Araujo, S.; Vargas, M.E.; Mesa, J.; Souki, A.; Bermúdez, V.; Cano, C. Actividad de la enzima antioxidante superóxido dismutasa y niveles de cobre y zinc en pacientes con diabetes mellitus tipo 2. Archivos Venezolanos de Farmacología y Terapéutica 2007, 26, 37-41.
- Prasad, A.S. Zinc is an Antioxidant and Anti-Inflammatory Agent: Its Role in Human Health. Front Nutr 2014, 1, 14. [CrossRef]
- Huang, T.C.; Chang, W.T.; Hu, Y.C.; Hsieh, B.S.; Cheng, H.L.; Yen, J.H.; Chiu, P.R.; Chang, K.L. Zinc Protects Articular Chondrocytes through Changes in Nrf2-Mediated Antioxidants, Cytokines and Matrix Metalloproteinases. Nutrients 2018, 10. [CrossRef]
- Kosik-Bogacka, D.I.; Lanocha-Arendarczyk, N.; Kot, K.; Zietek, P.; Karaczun, M.; Prokopowicz, A.; Kupnicka, P.; Ciosek, Z. Calcium, magnesium, zinc and lead concentrations in the structures forming knee joint in patients with osteoarthritis. Journal of Trace Elements in Medicine and Biology 2018, 50, 409-414. [CrossRef]
- He, H.; Huang, C.; Huang, H.; Lan, N.; Liu, S.; Luo, Y.; Zheng, L.; Liu, G.; Qin, Z.; Zhao, J. Zn2+-driven metformin conjugated with siRNA attenuates osteoarthritis progression by inhibiting NF-κB signaling and activating autophagy. Biomaterials 2025, 319. [CrossRef]
- Lee, M.; Won, Y.; Shin, Y.; Kim, J.H.; Chun, J.S. Reciprocal activation of hypoxia-inducible factor (HIF)-2α and the zinc-ZIP8-MTF1 axis amplifies catabolic signaling in osteoarthritis. Osteoarthritis Cartilage 2016, 24, 134-145. [CrossRef]
- Yang, W.M.; Lv, J.F.; Wang, Y.Y.; Xu, Y.M.; Lin, J.; Liu, J.; Chen, J.J.; Wang, X.Z. The Daily Intake Levels of Copper, Selenium, and Zinc Are Associated with Osteoarthritis but Not with Rheumatoid Arthritis in a Cross-sectional Study. Biological Trace Element Research 2023, 201, 5662-5670. [CrossRef]
- Wei, W.; Qi, X.; Cheng, B.; He, D.; Qin, X.; Zhang, N.; Zhao, Y.; Chu, X.; Shi, S.; Cai, Q., et al. An atlas of causal association between micronutrients and osteoarthritis. Preventive Medicine 2024, 185. [CrossRef]
- Zeng, W.; Hong, E.; Ye, W.; Ma, L.; Cun, D.; Huang, F.; Jiang, Z. Mendelian randomization of serum micronutrients and osteoarthritis risk: focus on zinc. Nutrition Journal 2025, 24. [CrossRef]
- Ajileye, A.S.; Ajileye, A.B.; Emokpae, A.M. Serum Levels of Some Essential Trace Elements in Patients with Osteoarthritis. African Journal of Biomedical Research 2024, 27, 359-366.
- Dąbrowski, M.; Zioła-Frankowska, A.; Frankowski, M.; Kaczmarczyk, J.; Kubaszewski, Ł. Comparison of Bone Tissue Trace Element Content in the Different Radiological Stages of Hip Osteoarthritis. Int J Environ Res Public Health 2021, 18. [CrossRef]
- Rushton, M.D.; Young, D.A.; Loughlin, J.; Reynard, L.N. Differential DNA methylation and expression of inflammatory and zinc transporter genes defines subgroups of osteoarthritic hip patients. Ann Rheum Dis 2015, 74, 1778-1782. [CrossRef]
- Kwon, J.Y.; Lee, S.H.; Jhun, J.; Choi, J.; Jung, K.; Cho, K.H.; Kim, S.J.; Yang, C.W.; Park, S.H.; Cho, M.L. The Combination of Probiotic Complex, Rosavin, and Zinc Improves Pain and Cartilage Destruction in an Osteoarthritis Rat Model. Journal of Medicinal Food 2018, 21, 364-371. [CrossRef]
- Asensio, G.; Benito-Garzón, L.; Ramírez-Jiménez, R.A.; Guadilla, Y.; Gonzalez-Rubio, J.; Abradelo, C.; Parra, J.; Martín-López, M.R.; Aguilar, M.R.; Vázquez-Lasa, B., et al. Biomimetic Gradient Scaffolds Containing Hyaluronic Acid and Sr/Zn Folates for Osteochondral Tissue Engineering. Polymers (Basel) 2021, 14. [CrossRef]
- Khader, A.; Arinzeh, T.L. Biodegradable zinc oxide composite scaffolds promote osteochondral differentiation of mesenchymal stem cells. Biotechnol Bioeng 2020, 117, 194-209. [CrossRef]
- You, X.; Ye, Y.; Lin, S.; Zhang, Z.; Guo, H.; Ye, H. Identification of key genes and immune infiltration in osteoarthritis through analysis of zinc metabolism-related genes. BMC Musculoskeletal Disorders 2024, 25. [CrossRef]
- Ruiz, L.M.; Libedinsky, A.; Elorza, A.A. Role of copper on mitochondrial function and metabolism. Frontiers in molecular biosciences 2021, 8, 711227.
- Medeiros, D.M. Copper, iron, and selenium dietary deficiencies negatively impact skeletal integrity: A review. Exp Biol Med (Maywood) 2016, 241, 1316-1322. [CrossRef]
- Uriu-Adams, J.Y.; Keen, C.L. Copper, oxidative stress, and human health. Mol Aspects Med 2005, 26, 268-298. [CrossRef]
- Lin, W.; Xu, L.; Li, G. Molecular insights into lysyl oxidases in cartilage regeneration and rejuvenation. Frontiers in bioengineering and biotechnology 2020, 8, 359.
- Kagan, H.M.; Li, W. Lysyl oxidase: properties, specificity, and biological roles inside and outside of the cell. J Cell Biochem 2003, 88, 660-672. [CrossRef]
- Yu, Q.; Xiao, Y.; Guan, M.; Zhang, X.; Yu, J.; Han, M.; Li, Z. Copper metabolism in osteoarthritis and its relation to oxidative stress and ferroptosis in chondrocytes. Front Mol Biosci 2024, 11, 1472492. [CrossRef]
- Gaetke, L.M.; Chow, C.K. Copper toxicity, oxidative stress, and antioxidant nutrients. Toxicology 2003, 189, 147-163. [CrossRef]
- Percival, S.S. Copper and immunity. The American Journal of Clinical Nutrition 1998, 67, 1064S-1068S. [CrossRef]
- Guan, T.; Wu, Z.; Xu, C.; Su, G. The association of trace elements with arthritis in US adults: NHANES 2013-2016. JOURNAL OF TRACE ELEMENTS IN MEDICINE AND BIOLOGY 2023, 76. [CrossRef]
- Amerikanou, C.; Valsamidou, E.; Karavoltsos, S.; Tagkouli, D.; Sakellari, A.; Kontou, M.; Houhoula, D.; Kalogeropoulos, N.; Zoumpoulakis, P.; Kaliora, A.C. Circulating Copper Is Associated with Inflammatory Biomarkers in Greek Older Adults with Osteoarthritis. Biological Trace Element Research 2024, 202, 1866-1877. [CrossRef]
- Roczniak, W.; Brodziak-Dopierała, B.; Cipora, E.; Jakóbik-Kolon, A.; Kluczka, J.; Babuśka-Roczniak, M. Factors that Affect the Content of Cadmium, Nickel, Copper and Zinc in Tissues of the Knee Joint. Biol Trace Elem Res 2017, 178, 201-209. [CrossRef]
- Zhou, H.; Zhang, Y.; Tian, T.; Wang, B.; Pan, Y. Meta-analysis of the Relationship Between Zinc and Copper in Patients with Osteoarthritis. BIOLOGICAL TRACE ELEMENT RESEARCH 2025, 203, 635-645. [CrossRef]
- Pasco, J.A.; Anderson, K.B.; Williams, L.J.; Stuart, A.L.; Hyde, N.K.; Holloway-Kew, K.L. Dietary intakes of copper and selenium in association with bone mineral density. Nutrients 2024, 16, 2777.
- Saati, A.A.; Adly, H.M. Assessing the correlation between blood trace element concentrations, picky eating habits, and intelligence quotient in school-aged children. Children 2023, 10, 1249.
- Ha, J.-H.; Doguer, C.; Wang, X.; Flores, S.R.; Collins, J.F. High-iron consumption impairs growth and causes copper-deficiency anemia in weanling Sprague-Dawley rats. Plos one 2016, 11, e0161033.
- Wang, X.; Cai, Y.; Wu, C.; Liang, J.; Tang, K.; Lin, Z.; Chen, L.; Lu, Y.; Wang, Q. Conversion of senescent cartilage into a pro-chondrogenic microenvironment with antibody-functionalized copper sulfate nanoparticles for efficient osteoarthritis therapy. Journal of Nanobiotechnology 2023, 21. [CrossRef]
- Yang, J.; Jiang, H.; Wu, C.; Lin, Y.; Tan, G.; Zhan, J.; Han, L.; Zhu, Y.; Shang, P.; Liu, L., et al. Copper silicate nanoparticle-mediated delivery of astragaloside-IV for osteoarthritis treatment by remodeling the articular cartilage microenvironment. Journal of Controlled Release 2025, 381. [CrossRef]
- Yassin, N.Z.; El-Shenawy, S.M.; Abdel-Rahman, R.F.; Yakoot, M.; Hassan, M.; Helmy, S. Effect of a topical copper indomethacin gel on inflammatory parameters in a rat model of osteoarthritis. Drug Des Devel Ther 2015, 9, 1491-1498. [CrossRef]
- Gao, H.; Ning, E.; Zhang, X.; Shao, Z.; Hu, D.; Bai, L.; Che, H.; Hao, Y. Injectable microspheres filled with copper-containing bioactive glass improve articular cartilage healing by regulating inflammation and recruiting stem cells. Regen Biomater 2025, 12, rbae142. [CrossRef]
- Xue, X.; Liu, H.; Wang, S.; Hu, Y.; Huang, B.; Li, M.; Gao, J.; Wang, X.; Su, J. Neutrophil-erythrocyte hybrid membrane-coated hollow copper sulfide nanoparticles for targeted and photothermal/ anti-inflammatory therapy of osteoarthritis. Composites Part B: Engineering 2022, 237. [CrossRef]
- Liu, H.; Ji, M.; Yang, T.; Zou, S.; Qiu, X.; Zhan, F.; Chen, J.; Yan, F.; Ding, F.; Li, P. Regulation of fibroblast phenotype in osteoarthritis using CDKN1A-loaded copper sulfide nanoparticles delivered by mesenchymal stem cells. American Journal of Physiology - Cell Physiology 2025, 328, C679-C698. [CrossRef]
- Geng, D.; Lin, R.; Wei, P.; Tang, C.; Xu, Y.; Wang, L. The Role of Copper-Induced M2 Macrophage Polarization in Protecting Cartilage Matrix in Osteoarthritis. Medical Science Monitor 2024, 30. [CrossRef]
- Han, J.; Luo, J.; Wang, C.; Kapilevich, L.; Zhang, X.A. Roles and mechanisms of copper homeostasis and cuproptosis in osteoarticular diseases. Biomed Pharmacother 2024, 174, 116570. [CrossRef]
- Luo, H.; Zhang, Y.; Meng, C.; Li, C.; Jia, D.; Xu, Y. The effect of copper and vitamin D on osteoarthritis outcomes: A Mendelian randomization study. Medicine (United States) 2024, 103, e39828. [CrossRef]
- Zhou, J.; Liu, C.; Sun, Y.; Francis, M.; Ryu, M.S.; Grider, A.; Ye, K. Genetically predicted circulating levels of copper and zinc are associated with osteoarthritis but not with rheumatoid arthritis. Osteoarthritis and Cartilage 2021, 29, 1029-1035. [CrossRef]
- Sies, H. The Concept of Oxidative Stress After 30 Years. In Biochemistry of Oxidative Stress: Physiopathology and Clinical Aspects, Gelpi, R.J., Boveris, A., Poderoso, J.J., Eds. Springer International Publishing: Cham, 2016; pp. 3-11. [CrossRef]
- Rayman, M.P. Selenium and human health. Lancet 2012, 379, 1256-1268. [CrossRef]
- Prasad, B.; Akanksha, A.; Kaur, P.S.; Gupta, S. Understanding selenoproteins: Structural insights, biological functions and transformative applications in therapeutics. Process Biochemistry 2025, 150, 148-160. [CrossRef]
- Zhang, Y.; Roh, Y.J.; Han, S.J.; Park, I.; Lee, H.M.; Ok, Y.S.; Lee, B.C.; Lee, S.R. Role of Selenoproteins in Redox Regulation of Signaling and the Antioxidant System: A Review. Antioxidants (Basel) 2020, 9. [CrossRef]
- Cheng, H.L.; Yen, C.C.; Huang, L.W.; Hu, Y.C.; Huang, T.C.; Hsieh, B.S.; Chang, K.L. Selenium Lessens Osteoarthritis by Protecting Articular Chondrocytes from Oxidative Damage through Nrf2 and NF-κB Pathways. Int J Mol Sci 2024, 25. [CrossRef]
- Huang, Z.; Rose, A.H.; Hoffmann, P.R. The role of selenium in inflammation and immunity: from molecular mechanisms to therapeutic opportunities. Antioxid Redox Signal 2012, 16, 705-743. [CrossRef]
- Li, Y.; Zhu, S.; Luo, J.; Tong, Y.; Zheng, Y.; Ji, L.; He, Z.; Jing, Q.; Huang, J.; Zhang, Y., et al. The Protective Effect of Selenium Nanoparticles in Osteoarthritis: In vitro and in vivo Studies. Drug Des Devel Ther 2023, 17, 1515-1529. [CrossRef]
- Hoffmann, P.R.; Berry, M.J. The influence of selenium on immune responses. Mol Nutr Food Res 2008, 52, 1273-1280. [CrossRef]
- Deng, H.; Liu, H.; Yang, Z.; Bao, M.; Lin, X.; Han, J.; Qu, C. Progress of Selenium Deficiency in the Pathogenesis of Arthropathies and Selenium Supplement for Their Treatment. Biological Trace Element Research 2022, 200, 4238-4249. [CrossRef]
- Wang, N.; Xie, M.; Lei, G.; Zeng, C.; Yang, T.; Yang, Z.; Wang, Y.; Li, J.; Wei, J.; Tian, J., et al. A Cross-Sectional Study of Association between Plasma Selenium Levels and the Prevalence of Osteoarthritis: Data from the Xiangya Osteoarthritis Study. The journal of nutrition, health & aging 2022, 26, 197-202. [CrossRef]
- Wahl, L.; Chillon, T.S.; Seemann, P.; Ohrndorf, S.; Ochwadt, R.; Becker, W.; Schomburg, L.; Hoff, P. AB0522 SELENIUM STATUS IN PATIENTS WITH RHEUMATOID ARTHRITIS, PSORIATIC ARTHRITIS AND JUVENILE IDIOPATHIC ARTHRITIS. Annals of the Rheumatic Diseases 2024, 83, 1535. [CrossRef]
- Wahl, L.; Samson Chillon, T.; Seemann, P.; Ohrndorf, S.; Ochwadt, R.; Becker, W.; Schomburg, L.; Hoff, P. Serum selenium, selenoprotein P and glutathione peroxidase 3 in rheumatoid, psoriatic, juvenile idiopathic arthritis, and osteoarthritis. Journal of Nutritional Biochemistry 2025, 135. [CrossRef]
- Ning, Y.; Hu, M.; Diao, J.; Gong, Y.; Huang, R.; Chen, S.; Zhang, F.; Liu, Y.; Chen, F.; Zhang, P., et al. Genetic Variants and Protein Alterations of Selenium- and T-2 Toxin-Responsive Genes Are Associated With Chondrocytic Damage in Endemic Osteoarthropathy. Frontiers in Genetics 2022, 12. [CrossRef]
- Kang, D.; Lee, J.; Wu, C.; Guo, X.; Lee, B.J.; Chun, J.S.; Kim, J.H. The role of selenium metabolism and selenoproteins in cartilage homeostasis and arthropathies. Exp Mol Med 2020, 52, 1198-1208. [CrossRef]
- Wang, S.; Geng, L.; Zhao, G.; Meng, P.; Yuan, L.; Guo, X. Effectiveness of Selenium on Chondrocyte Glycoprotein Glycosylation Which Play Important Roles in the Pathogenesis of an Endemic Osteoarthritis, Kashin–Beck Disease. Biological Trace Element Research 2022, 200, 1531-1537. [CrossRef]
- Deng, H.; Liu, H.; Yang, Z.; Bao, M.; Lin, X.; Han, J.; Qu, C. Progress of Selenium Deficiency in the Pathogenesis of Arthropathies and Selenium Supplement for Their Treatment. Biol Trace Elem Res 2022, 200, 4238-4249. [CrossRef]
- Hu, W.; Yao, X.; Li, Y.; Li, J.; Zhang, J.; Zou, Z.; Kang, F.; Dong, S. Injectable hydrogel with selenium nanoparticles delivery for sustained glutathione peroxidase activation and enhanced osteoarthritis therapeutics. Materials Today Bio 2023, 23. [CrossRef]
- Li, Y.; Zhu, S.; Luo, J.; Tong, Y.; Zheng, Y.; Ji, L.; He, Z.; Jing, Q.; Huang, J.; Zhang, Y., et al. The Protective Effect of Selenium Nanoparticles in Osteoarthritis: In vitro and in vivo Studies. Drug Design, Development and Therapy 2023, 17, 1515-1529. [CrossRef]
- Park, K.C.; Choi, J.; Choi, S.; Lee, G.; An, H.J.; Yun, H.; Lee, S. Therapeutic potential of Polydopamine-Coated selenium nanoparticles in Osteoarthritis treatment. International Journal of Pharmaceutics 2025, 675. [CrossRef]
- Liu, J.; Liu, J.; Liu, S.; Xiao, P.; Du, C.; Zhan, J.; Chen, Z.; Chen, L.; Li, K.; Huang, W., et al. Cascade targeting selenium nanoparticles-loaded hydrogel microspheres for multifaceted antioxidant defense in osteoarthritis. Biomaterials 2025, 318. [CrossRef]
- Qu, Z.; Yang, F.; Hong, J.; Wang, W.; Li, S.; Jiang, G.; Yan, S. Causal relationship of serum nutritional factors with osteoarthritis: A Mendelian randomization study. Rheumatology (United Kingdom) 2021, 60, 2383-2390. [CrossRef]
- Kraus, V.B. The zinc link. Nature 2014, 507, 441-442. [CrossRef]
- Buneaux, F.; Buneaux, J.J.; Fabiani, P.; Galmiche, P. [Zinc and enzymes in the synovial fluid and blood in various types of rheumatism]. Rev Rhum Mal Osteoartic 1978, 45, 699-701.
- Yazar, M.; Sarban, S.; Kocyigit, A.; Isikan, U.E. Synovial fluid and plasma selenium, copper, zinc, and iron concentrations in patients with rheumatoid arthritis and osteoarthritis. Biol Trace Elem Res 2005, 106, 123-132. [CrossRef]
- Peretz, A.; Neve, J.; Vertongen, F.; Famaey, J.P. Synovial Fluid Copper, Zinc and Selenium in Relation to Inflammatory Parameters in Rheumatic Diseases. In Biology of Copper Complexes, Sorenson, J.R.J., Ed. Humana Press: Totowa, NJ, 1987; pp. 583-589. [CrossRef]
- Struniolo, G.C.; D'Inca, R.; Lecis, P.E.; Naccarato, R. Factors influencing the absorption of trace elements in healthy conditions and in gastrointestinal diseases. Ital J Gastroenterol 1994, 26, 247-260.
- Cousins, R.J.; Liuzzi, J.P. Chapter 61 - Trace Metal Absorption and Transport. In Physiology of the Gastrointestinal Tract (Sixth Edition), Said, H.M., Ed. Academic Press: 2018. pp. 1485-1498. [CrossRef]
- do Nascimento da Silva, E.; Cadore, S. Bioavailability Assessment of Copper, Iron, Manganese, Molybdenum, Selenium, and Zinc from Selenium-Enriched Lettuce. J Food Sci 2019, 84, 2840-2846. [CrossRef]
- Quilliot, D.; Dousset, B.; Guerci, B.; Dubois, F.; Drouin, P.; Ziegler, O. Evidence that diabetes mellitus favors impaired metabolism of zinc, copper, and selenium in chronic pancreatitis. Pancreas 2001, 22, 299-306. [CrossRef]
- Pattan, V.; Chang Villacreses, M.M.; Karnchanasorn, R.; Chiu, K.C.; Samoa, R. Daily Intake and Serum Levels of Copper, Selenium and Zinc According to Glucose Metabolism: Cross-Sectional and Comparative Study. Nutrients 2021, 13. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).