Submitted:
27 May 2025
Posted:
28 May 2025
Read the latest preprint version here
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Patients
- HC group: Individuals with no history of epilepsy or other known chronic neurological or inflammatory diseases.
- SFE group: Patients with a confirmed diagnosis of epilepsy, who exhibited adequate seizure control (absence of seizures in the past 12 months) under pharmacological treatment.
- DRE group: Patients with a confirmed diagnosis of DRE, defined as those who failed to achieve adequate seizure control despite treatment with at least two appropriate antiseizure medications (ASM), used at tolerated doses, for an adequate period (according to the International League Against Epilepsy - ILAE guidelines) [16].
2.3. Patients and Sampling
- ●
- HC group:
- ■
- Inclusion: Absence of a diagnosis of epilepsy, neurological diseases, chronic inflammatory, autoimmune, or active infectious diseases.
- ■
- Exclusion: First-degree family history of epilepsy, presence of any significant medical condition that could affect neuroinflammation biomarkers, chronic use of immunomodulatory or anti-inflammatory drugs.
- ●
- SFE group:
- ■
- Inclusion: Confirmed diagnosis of epilepsy according to ILAE criteria, absence of epileptic seizures in the past 12 months.
- ■
- Exclusion: Presence of progressive epileptic syndromes, evidence of underlying active encephalopathy, significant neurological or inflammatory comorbidities unrelated to epilepsy, recent changes in antiepileptic medication.
- ●
- DRE group:
- ■
- Inclusion: Confirmed diagnosis of DRE according to the ILAE definition, current treatment with at least two antiepileptic drugs.
- ■
- Exclusion: Presence of progressive epileptic syndromes, evidence of underlying active encephalopathy, significant neurological or inflammatory comorbidities unrelated to epilepsy.
2.4. Sample Collection and Biomarker Determination
- Pro-inflammatory biomarkers (20): MIP-1α (Macrophage Inflammatory Protein 1-alpha), IL-1β (Interleukin-1 beta), IP-10 (Interferon gamma-induced Protein 10 or CXCL10), IL-8 (Interleukin-8), IL-12 (Interleukin-12), IL-17A (Interleukin-17A), IL-33 (Interleukin-33), IFN-γ (Interferon gamma), GM-CSF (Granulocyte-Macrophage Colony-Stimulating Factor), TNF-α (Tumor Necrosis Factor-alpha), MIP-1β (Macrophage Inflammatory Protein 1-beta), IFN-α (Interferon-alfa), MCP-1 (Monocyte Chemoattractant Protein-1), P-Selectin (CD62P), IL-1α (Interleukin-1 alfa), ICAM-1 (Intercellular Adhesion Molecule-1), E-Selectin (CD62E), sTNF-RII (soluble tumor necrosis factor receptor TNF-RII), TLR4 (Toll-Like Receptor 4), HMGB1 (High Mobility Group Box 1),
- Anti-inflammatory biomarkers (3): IL-4 (Interleukin-4), IL-10 (Interleukin-10), and IL-13 (Interleukin-13).
- Dual function (2): IL-33, sTNF-RII (depending on the context).
2.5. Demographic and Clinical Data
2.6. Statistical Analysis
- Descriptive Analysis: Descriptive statistics (mean, standard deviation for continuous variables; frequencies and percentages for categorical variables) were calculated to characterize the three study populations. Normality tests (e.g., Shapiro-Wilk) were used to determine the distribution of continuous variables.
- Univariate Analysis: The concentrations of each of the 24 determinations of biomarkers were compared between the three groups (HC, SFE, DRE) using appropriate statistical tests. For continuous variables with normal distribution, analysis of variance (ANOVA) followed by post-hoc tests (Bonferroni) for pairwise comparisons was used. For continuous variables without normal distribution, the Kruskal-Wallis test followed by Dunn's post-hoc tests with Bonferroni correction was used. For categorical variables, the chi-square test or Fisher's exact test was used, as appropriate.
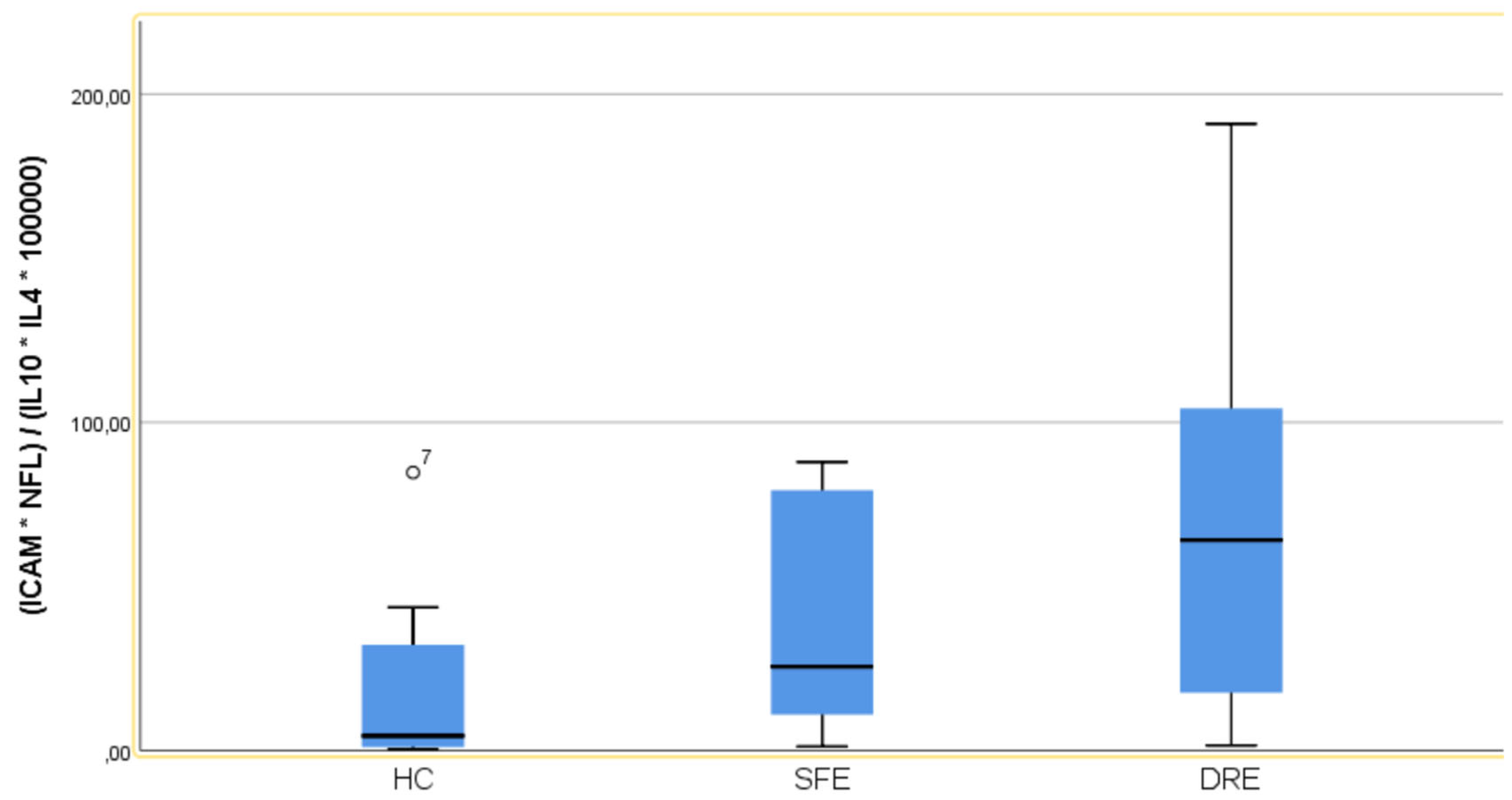
- Index Generation: Molecules that achieved statistical significance in the multivariate analysis, as well as those with results approaching significance, were selected. The index was designed to encompass both pro-inflammatory and anti-inflammatory factors.
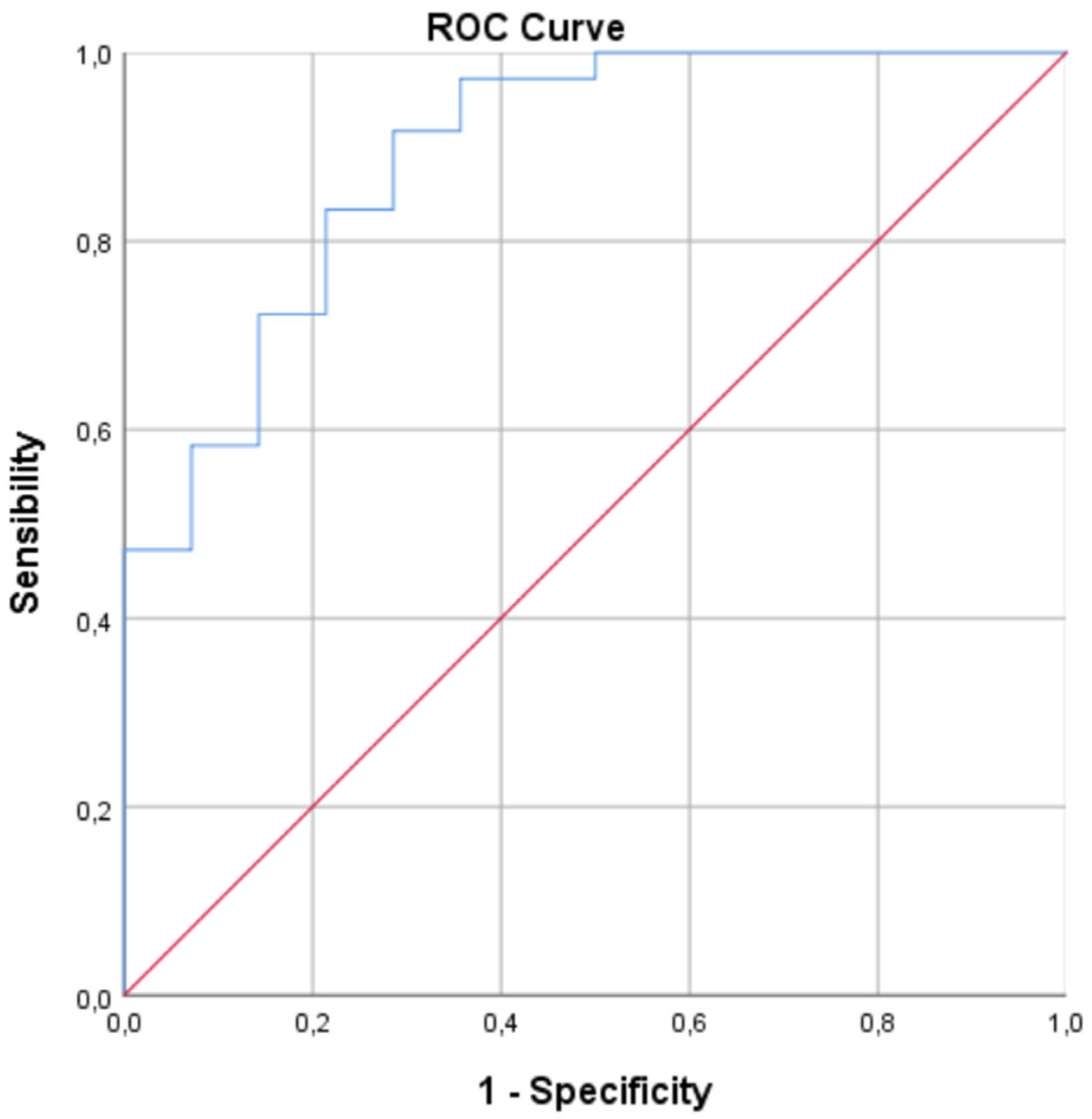
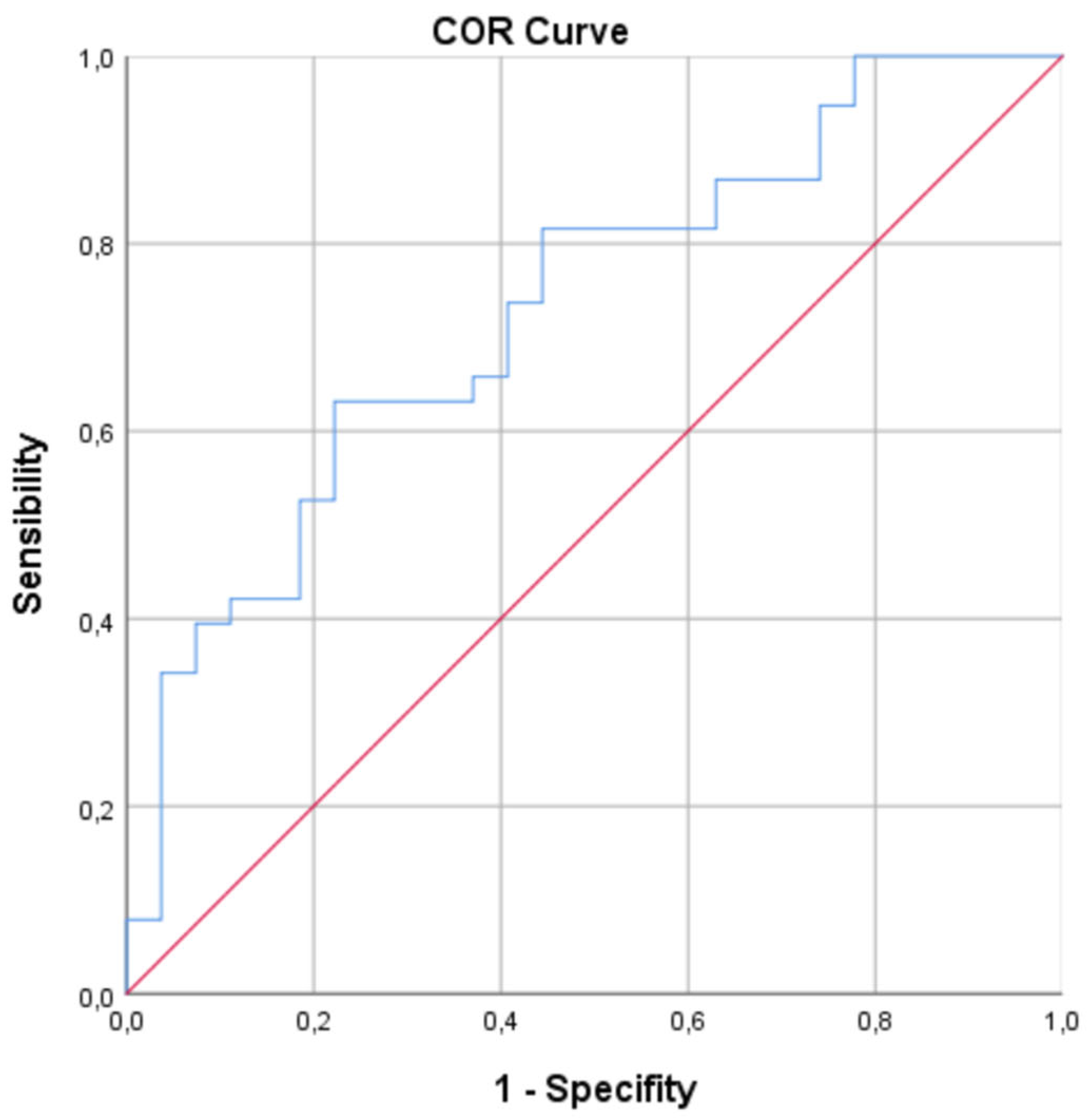
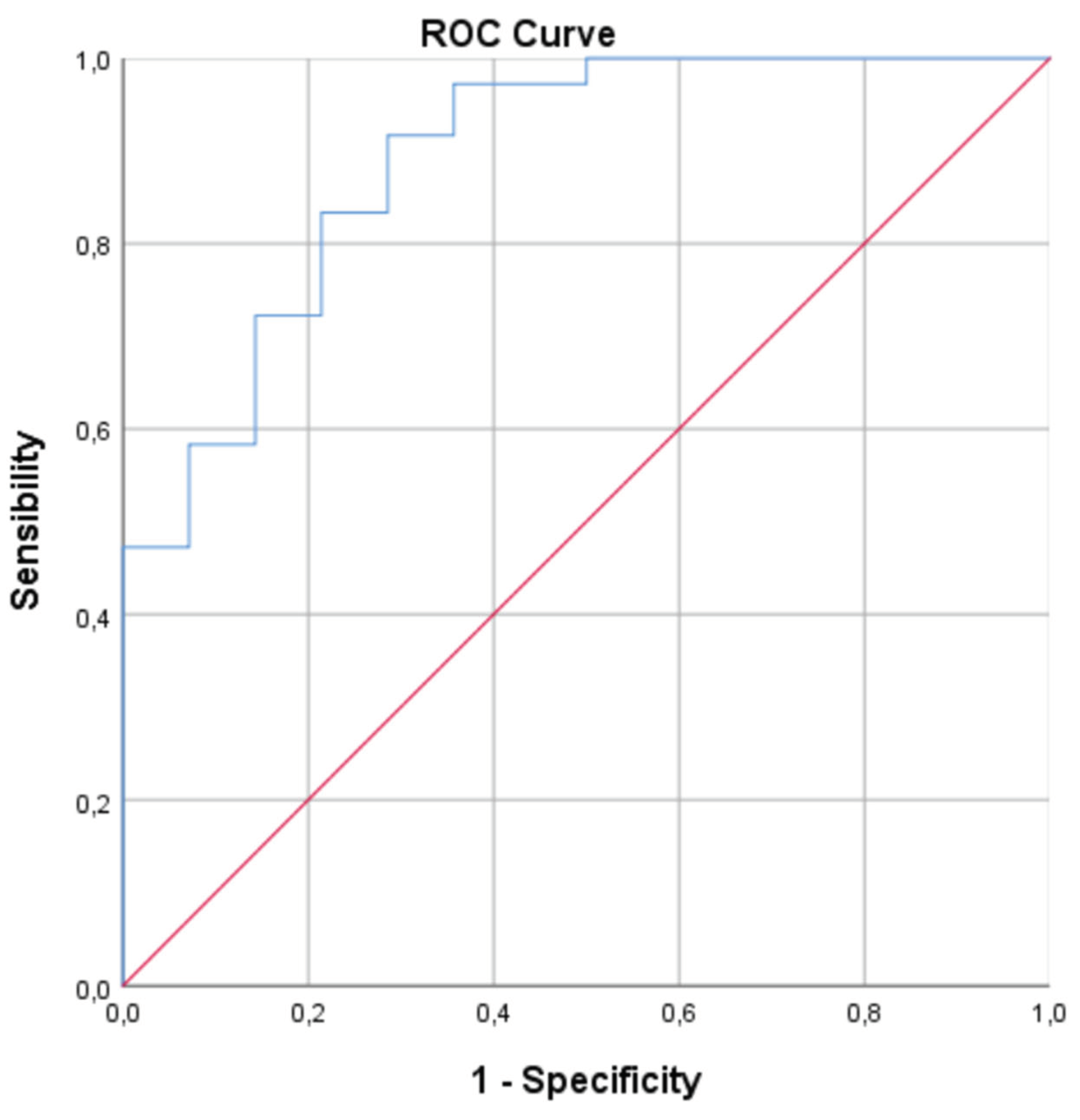
- ROC Curve Analysis: To evaluate the diagnostic potential of biomarkers that showed significant differences in the univariate and/or multivariate analysis to discriminate between groups, Receiver Operating Characteristic (ROC) curve analyses were performed. The area under the curve (AUC) with its 95% CI, sensitivity, and specificity for different cut-off points were calculated. The optimal cut-off point was determined using the Youden's index.
- Multivariate Analysis: Multinomial logistic regression models were constructed to identify which biomarkers were independently associated with membership in the epilepsy groups (SFE and DRE) compared to the healthy control group, adjusting for potential confounding variables.
3. Reslts:
3.1. Descriptive Analysis
3.2. Univariate Analysis
3.4. ROC Curve Analysis
3.5. Multivariate Analysis
4. Discusion
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| DRE | Drug-Resistant epilepsy |
| SFE | Seizure-free epilepsy |
| HC | Healthy control |
| IDREI | Inflammatory Drug-Resistant Epilepsy Index |
| AUC | Area under the curve |
| ROC | Receiver Operating Characteristic |
| CI | Confidence interval |
| IQR | Interquartile range |
References
- Serrano-Castro, P.J.; Mauri-Llerda, J.A.; Hernández-Ramos, F.J.; Sánchez-Alvarez, J.C.; Parejo-Carbonell, B.; Quiroga-Subirana, P.; Vázquez-Gutierrez, F.; Santos-Lasaosa, S.; Mendez-Lucena, C.; Redondo-Verge, L.; et al. Adult Prevalence of Epilepsy in Spain: EPIBERIA, a Population-Based Study. Sci. World J. 2015, 2015, 602710. [Google Scholar] [CrossRef] [PubMed]
- Sander, JW. The epidemiology of epilepsy revisited. Curr Opin Neurol. 2003 Apr;16(2):165–70.
- García-martín G, Serrano-castro PJ. Epidemiología de la epilepsia en España y Latinoamérica. 2018;67(July):249–62.
- Forsgren, L.; Beghi, E.; Õun, A.; Sillanpää, M. The epidemiology of epilepsy in Europe – a systematic review. Eur. J. Neurol. 2005, 12, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Scheffer IE, Berkovic S, Capovilla G, Connolly MB, French J, Guilhoto L, et al. ILAE classification of the epilepsies: Position paper of the ILAE Commission for Classification and Terminology. Epilepsia. 2017 Apr;58(4):512–21.
- Aguilar-Castillo, M.J.; Cabezudo-García, P.; Ciano-Petersen, N.L.; García-Martin, G.; Marín-Gracia, M.; Estivill-Torrús, G.; Serrano-Castro, P.J. Immune Mechanism of Epileptogenesis and Related Therapeutic Strategies. Biomedicines 2022, 10, 716. [Google Scholar] [CrossRef]
- Myers, K.A.; Scheffer, I.E. Precision Medicine Approaches for Infantile-Onset Developmental and Epileptic Encephalopathies. Annu. Rev. Pharmacol. Toxicol. 2022, 62, 641–662. [Google Scholar] [CrossRef]
- Yu, J.Y.; Pearl, P.L. Metabolic Causes of Epileptic Encephalopathy. Epilepsy Res. Treat. 2013, 2013, 1–20. [Google Scholar] [CrossRef]
- Matin, N.; Tabatabaie, O.; Falsaperla, R.; Lubrano, R.; Pavone, P.; Mahmood, F.; Gullotta, M.; Serra, A.; Di Mauro, P.; Cocuzza, S.; et al. Epilepsy and innate immune system: A possible immunogenic predisposition and related therapeutic implications. Hum. Vaccines Immunother. 2015, 11, 2021–2029. [Google Scholar] [CrossRef] [PubMed]
- Terrone, G.; Balosso, S.; Pauletti, A.; Ravizza, T.; Vezzani, A. Inflammation and reactive oxygen species as disease modifiers in epilepsy. Neuropharmacology 2020, 167, 107742. [Google Scholar] [CrossRef] [PubMed]
- Webster, K.M.; Sun, M.; Crack, P.; O’brien, T.J.; Shultz, S.R.; Semple, B.D. Inflammation in epileptogenesis after traumatic brain injury. J. Neuroinflammation 2017, 14, 1–17. [Google Scholar] [CrossRef]
- Lucas, S.; Rothwell, N.J.; Gibson, R.M. The role of inflammation in CNS injury and disease. Br. J. Pharmacol. 2006, 147, S232–S240. [Google Scholar] [CrossRef]
- Brodie, M.J. Diagnosing and predicting refractory epilepsy. Acta Neurol. Scand. 2005, 112, 36–39. [Google Scholar] [CrossRef]
- Kwan P, Brodie M. EARLY IDENTIFICATION OF REFRACTORY EPILEPSY. N Engl J Med. 2000 [cited 2013 Apr 14]. ;342:314–9.
- Aguilar-Castillo, M.J.; Cabezudo-García, P.; García-Martín, G.; Lopez-Moreno, Y.; Estivill-Torrús, G.; Ciano-Petersen, N.L.; Oliver-Martos, B.; Narváez-Pelaez, M.; Serrano-Castro, P.J. A Systematic Review of the Predictive and Diagnostic Uses of Neuroinflammation Biomarkers for Epileptogenesis. Int. J. Mol. Sci. 2024, 25, 6488. [Google Scholar] [CrossRef] [PubMed]
- Asadi-Pooya, A.A.; Farazdaghi, M. Definition of drug-resistant epilepsy: A reappraisal based on epilepsy types. Acta Neurol. Scand. 2022, 145, 627–632. [Google Scholar] [CrossRef]
- Miller, M.R.; Landis, H.E.; Miller, R.E.; Tizabi, Y. Intercellular Adhesion Molecule 1 (ICAM-1): An Inflammatory Regulator with Potential Implications in Ferroptosis and Parkinson’s Disease. Cells 2024, 13, 1554. [Google Scholar] [CrossRef]
- Vignarajah, M.; Wood, A.J.T.; Nelmes, E.; Subburayalu, J.; Herre, J.; Nourshargh, S.; Summers, C.; Chilvers, E.R.; Farahi, N. Regulation of ICAM-1 in human neutrophils. J. Leukoc. Biol. 2024, 116, 901–908. [Google Scholar] [CrossRef] [PubMed]
- Mizee M, van Doorn R, Prat A, de Vries HE. Blood-Brain Barrier Disruption in Multiple Sclerosis. The Blood-Brain Barrier in Health and Disease: Volume 2: Pathophysiology and Pathology. 2015; pp 1–22.
- Khaboushan, A.S.; Yazdanpanah, N.; Rezaei, N. Neuroinflammation and Proinflammatory Cytokines in Epileptogenesis. Mol. Neurobiol. 2022, 59, 1724–1743. [Google Scholar] [CrossRef]
- Vezzani A, French J, Bartfai T, Baram TZ. The role of inflammation in epilepsy. Nat Rev Neurol. 2011 Jan;7(1):31–40.
- Bronisz, E.; Cudna, A.; Wierzbicka, A.; Kurkowska-Jastrzębska, I. Blood-Brain Barrier-Associated Proteins Are Elevated in Serum of Epilepsy Patients. Cells 2023, 12, 368. [Google Scholar] [CrossRef]
- Saadatnia, M.; Farhang, A.; Javanmard, S.H.; Mehvari, J.; Zare, M. Inflammation and endothelium response in epileptic patients: A case-control study. Adv. Biomed. Res. 2016, 5, 131–131. [Google Scholar] [CrossRef] [PubMed]
- Fabene, P.F.; Mora, G.N.; Martinello, M.; Rossi, B.; Merigo, F.; Ottoboni, L.; Bach, S.; Angiari, S.; Benati, D.; Chakir, A.; et al. A role for leukocyte-endothelial adhesion mechanisms in epilepsy. Nat. Med. 2008, 14, 1377–1383. [Google Scholar] [CrossRef]
- Khalil, M.; Teunissen, C.E.; Lehmann, S.; Otto, M.; Piehl, F.; Ziemssen, T.; Bittner, S.; Sormani, M.P.; Gattringer, T.; Abu-Rumeileh, S.; et al. Neurofilaments as biomarkers in neurological disorders — towards clinical application. Nat. Rev. Neurol. 2024, 20, 269–287. [Google Scholar] [CrossRef]
- Kahn, O.I.; Dominguez, S.L.; Glock, C.; Hayne, M.; Vito, S.; Ghosh, A.S.; Adrian, M.; Burgess, B.L.; Meilandt, W.J.; Friedman, B.A.; et al. Secreted neurofilament light chain after neuronal damage induces myeloid cell activation and neuroinflammation. Cell Rep. 2025, 44, 115382. [Google Scholar] [CrossRef]
- Foiadelli, T.; Santangelo, A.; Costagliola, G.; Costa, E.; Scacciati, M.; Riva, A.; Volpedo, G.; Smaldone, M.; Bonuccelli, A.; Clemente, A.M.; et al. Neuroinflammation and status epilepticus: a narrative review unraveling a complex interplay. Front. Pediatr. 2023, 11, 1251914. [Google Scholar] [CrossRef] [PubMed]
- Dingledine R, Varvel NH, Ravizza T, Vezzani A. Neuroinflammation in Epilepsy. In: Vezzani A, Scharfman HE, editors. Jasper’s Basic Mechanisms of the Epilepsies. Oxford University PressNew York; 2024; pp 611–32.
- Sun, Y.; Ma, J.; Li, D.; Li, P.; Zhou, X.; Li, Y.; He, Z.; Qin, L.; Liang, L.; Luo, X. Interleukin-10 inhibits interleukin-1β production and inflammasome activation of microglia in epileptic seizures. J. Neuroinflammation 2019, 16, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhu, L.; Lu, D.; Wu, Z.; Han, Y.; Xu, P.; Chang, L.; Wu, Q. Interleukin 4 Affects Epilepsy by Regulating Glial Cells: Potential and Possible Mechanism. Front. Mol. Neurosci. 2020, 13. [Google Scholar] [CrossRef] [PubMed]
| HC (n=16) | SFE (n=14) | DRE (n=38) | p | ||
|---|---|---|---|---|---|
| Sex (%) | Male | 31,25 (5/16) | 42,85 (6/14) | 39,47 (15/38) | 0,78 |
| Female | 68,75 (11/16) | 57,15 (8/14) | 60,52 (23/38) | ||
| Age (+/-SD) | 47,44 (19,94) | 54,40 (15,70) | 52,08 (14,22) | 0,46 | |
| Clinical onset (+/- SD) | N/A | 37,21 (21,18) | 27,89 (15,35) | 0,09 | |
| Duration of epilepsy (+-SD) | N/A | 16,29 (15,71) | 23,39 (13,44) | 0,11 | |
| Type of seizures (%) | Focal Seizures | N/A | 78,57 (11/14) | 42,10 (16/38) | 0,147 |
| Focal and Bilteral tonico-clonic seizures | N/A | 21,42 (3/14) | 57,89 (22/38) | ||
| MRI findings (%) | Normal | N/A | 78,57 (11/14) | 52,63 (20/38) | 0,083 |
| Abnormal | N/A | 21,42 (3/14) | 47,36 (18/38) | ||
| EEG findings (%) | Focal IED | N/A | 42,85 (6/14) | 65,79 (25/38) | 0,007 |
| Multifocal IED | N/A | 7,14 (1/14) | 23,68 (9/38) | ||
| No IED | N/A | 50 (7/14) | 10,52 (4/38) | ||
| Etiology (%) | Hippocampal sclerosis | N/A | 21,42 (3/14) | 28,9 (11/38) | 0,082 |
| Focal Cortical Dysplasia | N/A | 0 (0/14) | 10,52 (4/38) | ||
| Gliotic lession | N/A | 0 (0/14) | 5,26 (2/38) | ||
| Encephalocele | N/A | 0 (0/14) | 2,63 (1/38) | ||
| Non lessional | N/A | 78,57 (11/14) | 52,63 (20/38) | ||
| Seizure frequency | Daily | N/A | 0 (0/14) | 26,31 (10/38) | <0,001 |
| Weekly | N/A | 0 (0/14) | 36,84 (14/38) | ||
| Monthly | N/A | 0 (0/14) | 21,05 (8/38) | ||
| Annual | N/A | 0 (0/14) | 15,78 (6/38) | ||
| Concurrent ASMs (+-SD) | N/A | 1,57 (0,94) | 2,55 (0,86) | 0,002 | |
| Mechanism of action of ASM (%) | Sodium channel blokers | N/A | 71,42 (10/14) | 89,47 (34/38) | 0,081 |
| Gabaergic | N/A | 28,57 (4/14) | 36,84 (14/38) | ||
| SV2A | N/A | 28,57 (4/14) | 55,26 (21/38) | ||
| Mean (SD) | Median (IQR) | p | |||||
|---|---|---|---|---|---|---|---|
| HC (n=16) | SFE (n=14) | DRE (n=38) | HC (n=16) | SFE (n=14) | DRE (n=38) | ||
| Plasmatic biomarkers | |||||||
| MIP-1a (CCL3) | 2,37 (3,16) | 6,20 (12,92) | 3,83 (5,87) | 1,12 (2,00) | 2,62 (4,27) | 1,73 (2,39) | 0,318 |
| IL-1b | 9,82 (8,51) | 5,27 (2,78) | 6,98 (4,50) | 6,42 (8,53) | 4,02 (4,22) | 5,85 (6,78) | 0,220 |
| IL4 | 12,37 (9,42) | 7,64 (2,76) | 8,50 (4,15) | 9,11 (7,90) | 7,71 (4,15) | 8,61 (5,48) | 0,169 |
| IP-10 (CXCL10) | 2,28 (1,57) | 3,62 (2,33) | 3,10 (1,99) | 1,89 (2,11) | 3,08 (4,48) | 2,39 (3,50) | 0,161 |
| IL8 (CXCL8) | 1,40 (0,24) | 1,10 (0,53) | 1,24 (0,54) | 1,40 (0,41) | 1,21 (1,18) | 1,23 (0,54) | 0,294 |
| IL10 | 1,97 (1,42) | 1,12 (0,63) | 1,43 (0,90) | 1,55 (1,65) | 0,97 (0,97) | 1,31 (1,34) | 0,116 |
| IL12 | 40,60 (29,96) | 20,72 (14,24) | 28,59 (21,69) | 39,53 (37,46) | 13,43 (27,46) | 27,38 (33,89) | 0,095 |
| IL13 | 9,42 (5,65) | 9,55 (4,79) | 8,73 (4,99) | 6,08 (7,15) | 11,29 (8,46) | 7,24 (6,60) | 0,636 |
| IL17A | 6,94 (3,34) | 7.06 (3,15) | 6,98 (2,75) | 6,40 (4,44) | 6,52 (5,19) | 6,19 (2,15) | 0,971 |
| IL33 | 4,73 (2,85) | 3,53 (1,15) | 4,42 (2,87) | 3,90 (4,06) | 3,50 (1,89) | 3,49 (3,35) | 0,663 |
| IFN-g | 2,87 (3,91) | 6,64 (5,51) | 5,41 (5,79) | 1,05 (1,59) | 8,02 (10,73) | 1,50 (33,56) | 0,181 |
| GM-CSF | 54,20 (31,67) | 34,50 (24,57) | 42,47 (31,49) | 48,88 (38,58) | 23,16 (48,54) | 36,50 (43,18) | 0,160 |
| TNF-a | 13,81 (6,24) | 14,80 (6,84) | 15,65 (6,84) | 12,76 (9,02) | 13,32 (9,00) | 14,99 (8,80) | 0,504 |
| MIP1-b | 11,96 (4,60) | 12,04 (4,63) | 14,45 (11,56) | 10,50 (8,63) | 10,02 (7,02) | 11,00 (5,90) | 0,375 |
| IFN-a | 1,03 (0,84) | 1,42 (0,80) | 1,20 (0,73) | 0,72 (1,25) | 1,32 (1,28) | 0,95 (1,06) | 0,422 |
| MCP-1 | 10,34 (6,08) | 17,95 (11,08) | 13,96 (8,26) | 9,64 (8,14) | 18,34 (17,40) | 11,42 (11,49) | 0,145 |
| P-SELECTINE | 16005,10 (39258,50) | 17186,03 (9084,97) | 23688,20 (46499,29) | 10260,24 (12498,80) | 20237,49 (18099,64) | 13435,29 (14714,05) | 0,538 |
| IL1-a | 0,57 (0,37) | 0,41 (0,24) | 0,68 (1,14) | 0,48 (0,54) | 0,32 (0,34) | 0,50 (0,31) | 0,285 |
| ICAM-1 | 35193,99 (118445,28) | 50128,29 (34555,28) | 351519,10 (983829,16) | 33781,59 (92659,30) | 43290,99 (40665,20) | 67975,41 (170840,08) | 0,035 |
| E-SELECTINE | 5442,02 (3718,05) | 6659,13 (4264,43) | 6268,81 (4044,53) | 5913,69 (5589,29) | 6946,73 (7445,26) | 5560,47 (7085,13) | 0,809 |
| rsTNF-RII | 2083,04 (789,64) | 2370,70 (476,90) | 2368,34 (767.92) | 1719,94 (845,10) | 2424,01 (759,34) | 2420,43 (1089,06) | 0,271 |
| TRL4 | 2323,72 (1404,43) | 2777,24 (3053,35) | 2421,90 (2070,78) | 2043,52 (1963,93) | 1705,35 (1236,20) | 1883,44 (1384,76) | 0,804 |
| HMGB | 9919,10 (11223,93) | 9385,59 (7822,42) | 6870,22 (3727, | 6586,39 (7592,98) | 6635,64 (4578,25) | 6121,22 (3891,71) | 0,442 |
| CSF biomarker | |||||||
| NfL | 391,61 (317,45) | 683,09 (693,19) | 596,82 (617,01) | 236,10 (343,15) | 398,99 (501,51) | 462,77 (415,50) | 0,145 |
| Median (IQR) | p | |||
| HC (n=16) | SFE (n=14) | DRE (n=38) | 0.002 | |
| IDREI | 4,46 (31,80) | 25,53 (70,62) | 64,10 (87,58) | |
| Variable | Tolerance | VIF |
| IL4 | 0,611 | 1,636 |
| IL10 | 0,603 | 1,657 |
| ICAM-1 | 0,981 | 1,020 |
| NfL | 0,997 | 1,003 |
| Chi-square | P | |
| Concomitant ASM | 3,357 | 0,067 |
| Clinical onsed | 0,756 | 0,385 |
| IL10 | 1,304 | 0,254 |
| ICAM-1 | 5,047 | 0,025 |
| NfL | 0,530 | 0,467 |
| IL4 | 1,026 | 0,311 |
| EEG findings | 1,692 | 0,429 |
| Type of seizures | 0,014 | 0,906 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





