1. Introduction
The treatment strategy for ulcerative colitis (UC) has evolved based on the treat-to-target approach, which emphasizes achieving predefined therapeutic goals to improve long-term patient outcomes [
1]. In recent years, the number of therapeutic agents for UC with distinct mechanisms of action has expanded rapidly, reflecting advances in the understanding of UC pathophysiology and immune modulation. Consequently, there are now various treatment options available for active UC. This expansion in therapeutic choices has led to a more complex decision-making process for both patients and clinicians when selecting the most appropriate treatment strategy [
2,
3]. Among the immunological pathways involved in UC, interleukin-23 (IL-23) has been recognized as playing a key role in disease pathogenesis, particularly through its interactions with neutrophils [
4]. IL-23 exerts its effects by activating Th17 cells, which in turn activate neutrophils. Neutrophilic infiltration into the colonic mucosal epithelium plays a critical role in tissue damage and inflammation in UC, making it a crucial histopathological marker for disease activity assessment [
4,
5].
The Geboes score Is a widely used histological grading system for UC, and Its Grade 3 specifically focuses on the presence and extent of neutrophilic infiltration in the colonic mucosal epithelium [
6]. We have been exploring methods to identify patients with active UC who have IL-23 activation. However, one of the major challenges associated with the Geboes score is interobserver variability among pathologists, which leads to inconsistencies in histological assessment, particularly in grades other than Grade 3 and 4 [
7]. Furthermore, the original description of the Geboes score did not indicate how to specifically evaluate Geboes score Grade 3 [
6]. This study aimed to develop a simplified and clearly defined scoring method based on the definition of Geboes score Grade 3, with the goal of enhancing consistency and reproducibility in histological assessment among pathologists. Additionally, we have attempted to prospectively identify active UC patients with Grades 3.0, 3.1, 3.2 or 3.3 using this scoring method.
2. Materials and Methods
2.1. Patients and Study Design
This was a prospective study conducted at Showa Inan General Hospital (Komagane, Japan) between February 2024 and January 2025. A total of 30 patients with active UC who underwent colonoscopy with histopathological examination were included. Among them, some patients had recurrent UC, while others were newly diagnosed with UC in this study. Patients were diagnosed with UC based on standard diagnostic criteria combining endoscopic and histopathological findings [
6,
8,
9]. Patients with indeterminate colitis or Crohn’s disease were excluded. Blood tests and colonoscopy with histology were performed before the enrollment of this study and we assessed the clinical activity (CAI and numeric rating scale), endoscopic activity, and pathological activity. The CAI used in this study was the Rachmilewitz Clinical Activity Index [
8].
The Ethics Ie of Showa Inan General Hospital reviewed and approved the study protocol (No. 2023-7). All patients provided their written informed consent for participation when their enrollment in the study was scheduled. The study adhered to the tenets of the Declaration of Helsinki.
2.2. Endoscopic Evaluation
Colonoscopy findings were assessed using MES [
9] as follows: MES0, normal or inactive disease; MES1, mild disease (erythema, decreased vascular pattern, mild friability); MES2, moderate disease (marked erythema, absent vascular pattern, friability, erosions); MES3, severe disease (spontaneous bleeding, ulceration). Biopsies were obtained from the most inflamed area identified during colonoscopy, corresponding to the site used for MES evaluation.
2.3. Histopathological Evaluation
A total of 95 hematoxylin and eosin (H&E)-stained slides were analyzed. Multiple slides per patient were evaluated to ensure comprehensive sampling of the colonic mucosa. Two clinicians independently assessed the histological samples: one was an experienced pathologist, and the other was a gastroenterologist with experience in histopathological evaluation. Histological assessment was mainly performed using virtual slides. In cases where the interpretation was unclear, glass slides were reviewed for confirmation. The virtual slide scanning system used in this study was NanoZoomerS210® (Hamamatsu Photonics, Shizuoka, Japan). The Geboes score Grade 3 was used to evaluate the presence and extent of neutrophilic infiltration in the colonic mucosal epithelium in active UC patients [
6].
2.4. Komagane Evaluation Method of Geboes Score Grade 3
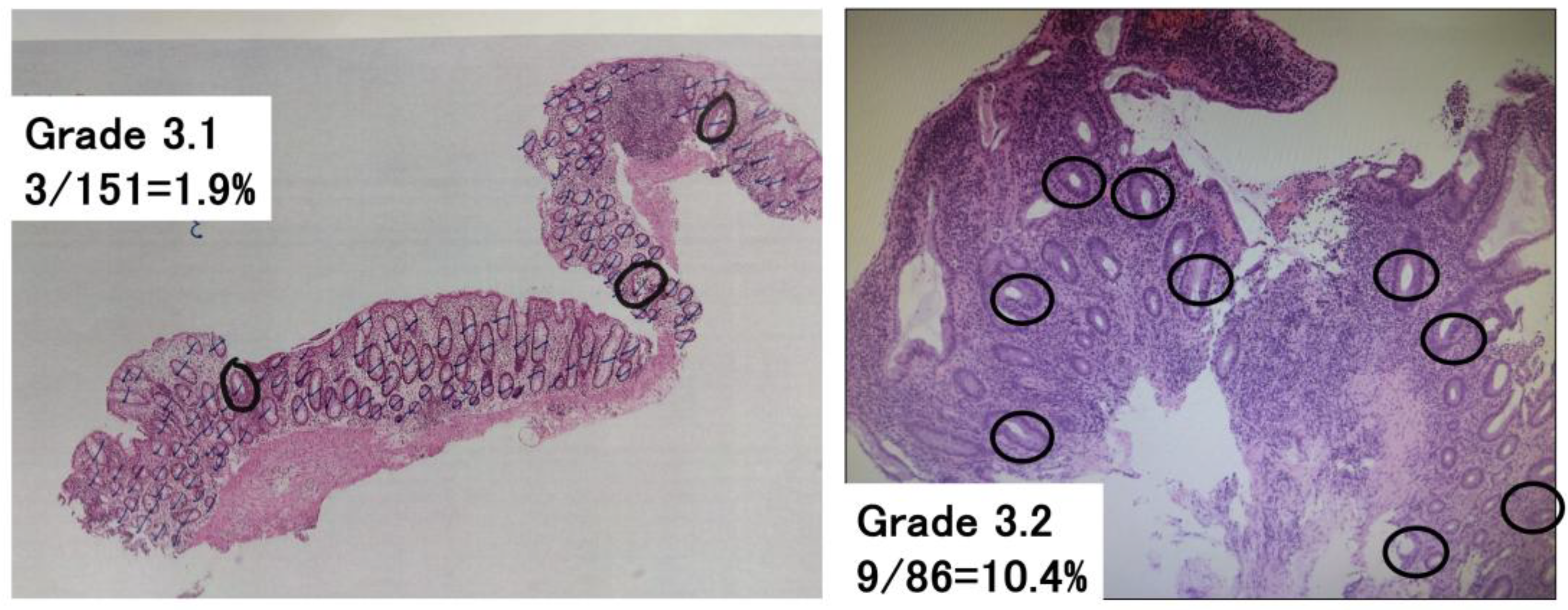
To assess Geboes score Grade 3, which evaluates neutrophilic infiltration into the epithelium, the following methodology was newly developed (
Figure 1): The total number of crypts in a glass slide was counted, and then the number of crypts with neutrophilic infiltration was counted. Crypts with neutrophilic infiltration were defined as those containing at least two neutrophils containing cytoplasmic neutral pink granules within the crypt lumen. The number of crypts with neutrophilic infiltration/the total number of crypts in a glass slide was calculated and used to classify patients with Grade 3 into groups with Geboes score Grade 3.0, 3.1, 3.2 or 3.3. Both cross-sectional and longitudinally sectioned crypts were counted, while the superficial epithelium was excluded from this analysis. Based on the original Geboes scoring system, Grade 3 was classified as follows:
No Infiltration: Grade 3.0
<5% of crypts involved: Grade 3.1
<50% of crypts involved: Grade 3.2
>50% of crypts involved: Grade 3.3
2.5. Raters
Two raters evaluated Komagane evaluation method of Geboes score Grade 3. The raters had different durations of experience in performing and interpreting histological evaluations (28 years for the expert rater, and 6 months for the beginner rater). Both raters had extensive training (including written definitions, visual depictions, and verbal explanations) regarding the reliable and consistent use of the Komagane evaluation method for Geboes score Grade 3.
2.6. Reliability Testing
The intra-rater test–retest reliability, the inter-rater reliability, and the construct validity were evaluated for the Komagane evaluation method for Geboes score Grade 3 obtained for all glass slides by the same two raters, 4 weeks apart, and with the order of slide presentations randomized.
2.7. Statistical Analysis
Data are presented as means and standard deviations or median (25th and 75th percentiles). Statistical tests to compare the results of two groups were as follows: the χ2-test (with Yates’ correction for continuity where appropriate) was used for the comparisons of categorical data. Fisher’s exact test was used when the numbers were small. For parametric data, Student’s t-test was used when two means were compared. For nonparametric data, the Mann–Whitney rank sum test was used when two medians were compared. A two-sided p-value <0.05 was considered statistically significant. Statistical analyses were conducted using EZR (Jichi Medical University, Saitama, Japan).
Kappa statistics were used to assess intra- and inter-rater reliability and construct validity. To assess intra-rater reliability, 30 paired ratings were made, with a weighted Kappa [
10] calculated to account for the level of disagreement, with comparisons made on the same slide four weeks apart. A similar approach was used to examine construct validity by comparing the initial scores with those of the criterion standard provided by the same two raters. Inter-rater reliability was determined using a multi-rater Kappa statistic [
10], which measures the degree of agreement between raters for each of the four categories (Grades 3.0, 3.1, 3.2, and 3.3). A weighted average of the category-specific agreements was then calculated, with weights based on the number of ratings in each category, yielding the Kappa value.
3. Results
3.1. Patients’ Clinical Characteristics
The clinical features of the 30 patients with active UC are summarized in
Table 1. There were 20 men and 10 women, with a median age of 46 years (IQR: 37–57 years) and a median illness duration of 0 years (IQR: 0–2 years). The types of disease extension were total colitis in 18 patients, left-sided colitis in 7 patients and proctitis in 5 patients. Among the 30 patients, 11 (37%) had a pre-existing diagnosis of UC, while 19 (63%) were newly diagnosed with UC based on the findings of this examination.
At study enrollment, the median scores of clinical activities were as follows: clinical activity index (CAI), 8 points; numeric rating scale (for bowel urgency), 6 points. The distribution of Mayo endoscopic subscore (MES) was MES 1, 11 patients (37%); MES 2, 18 (60%); and MES 3, 1 (3%). Among the 11 patients with a pre-existing diagnosis of UC, 5 received 5-aminosalicylic acid (5-ASA) monotherapy, 1 received sulfasalazine monotherapy, 1 received a combination therapy of prednisolone and 5-ASA, and 1 received a combination of Janus kinase inhibitor and 5-ASA. The remaining 3 patients had no medication related to UC.
3.2. The Distribution of Geboes Score Grade 3
The intra-rater reliability and the construct validity were both 100% for the two raters based on the results of the Komagane evaluation method for Geboes score Grade 3 in 30 patients. The inter-rater reliability for the Komagane evaluation method was 0.85 (Kappa value). Evaluation of Geboes score Grade 3 revealed the following distribution: 6 patients (20%) had Grade 3.0, 7 (23%) had Grade 3.1, 16 (53%) had Grade 3.2, and 1 (3%) had Grade 3.3 (
Table 2).
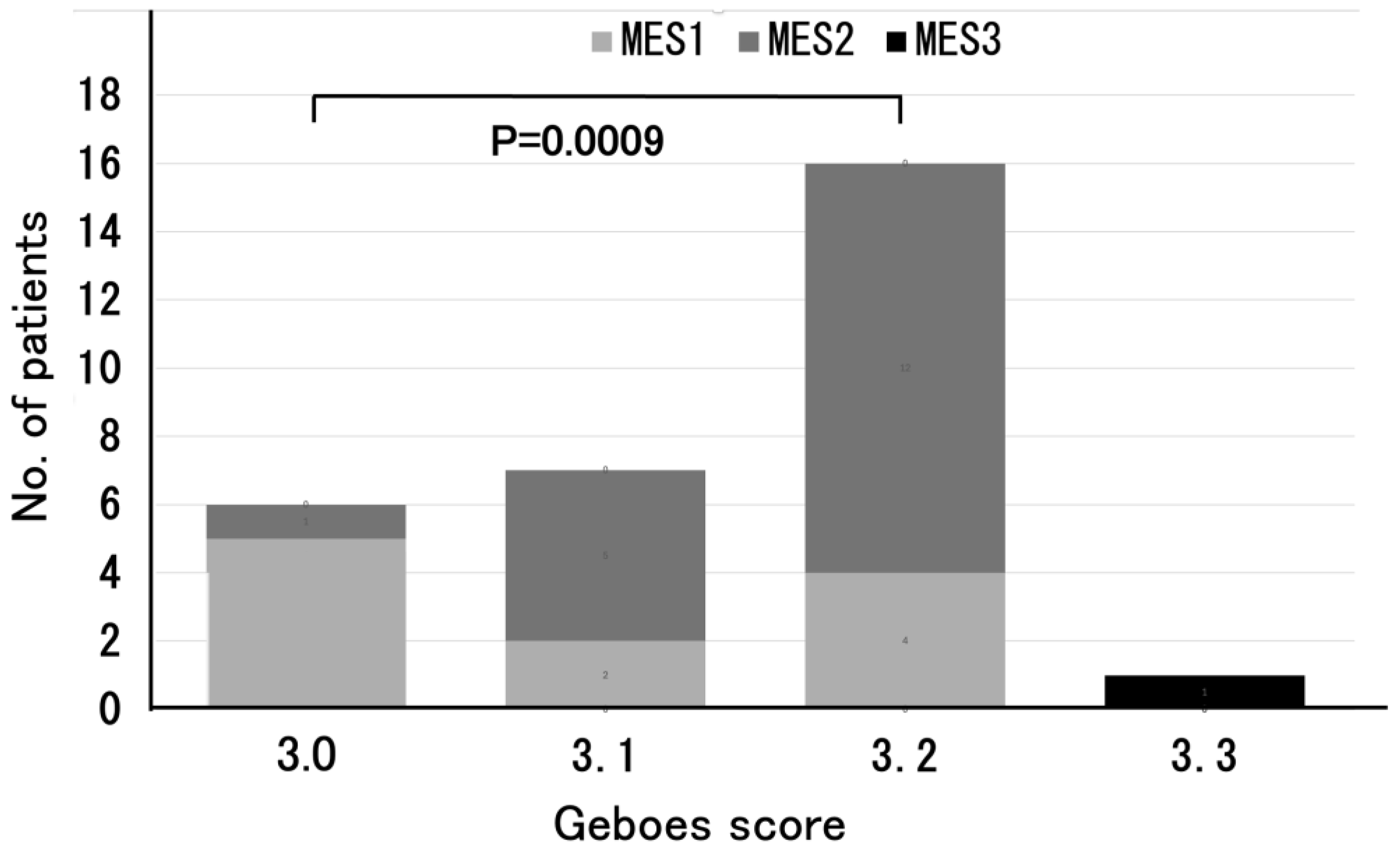
In addition, among the 6 patients classified into the Grade 3.0 group, the majority (83%) had an MES of 1. The 7 patients with Grade 3.1 had an MES of 2 in 5 (71%), and 12 out of the 16 patients with Grade 3.2 (75%) had an MES of 2. A significant difference in MES was observed between the Grade 3.0 and Grade 3.2 group (
p = 0.0009) (
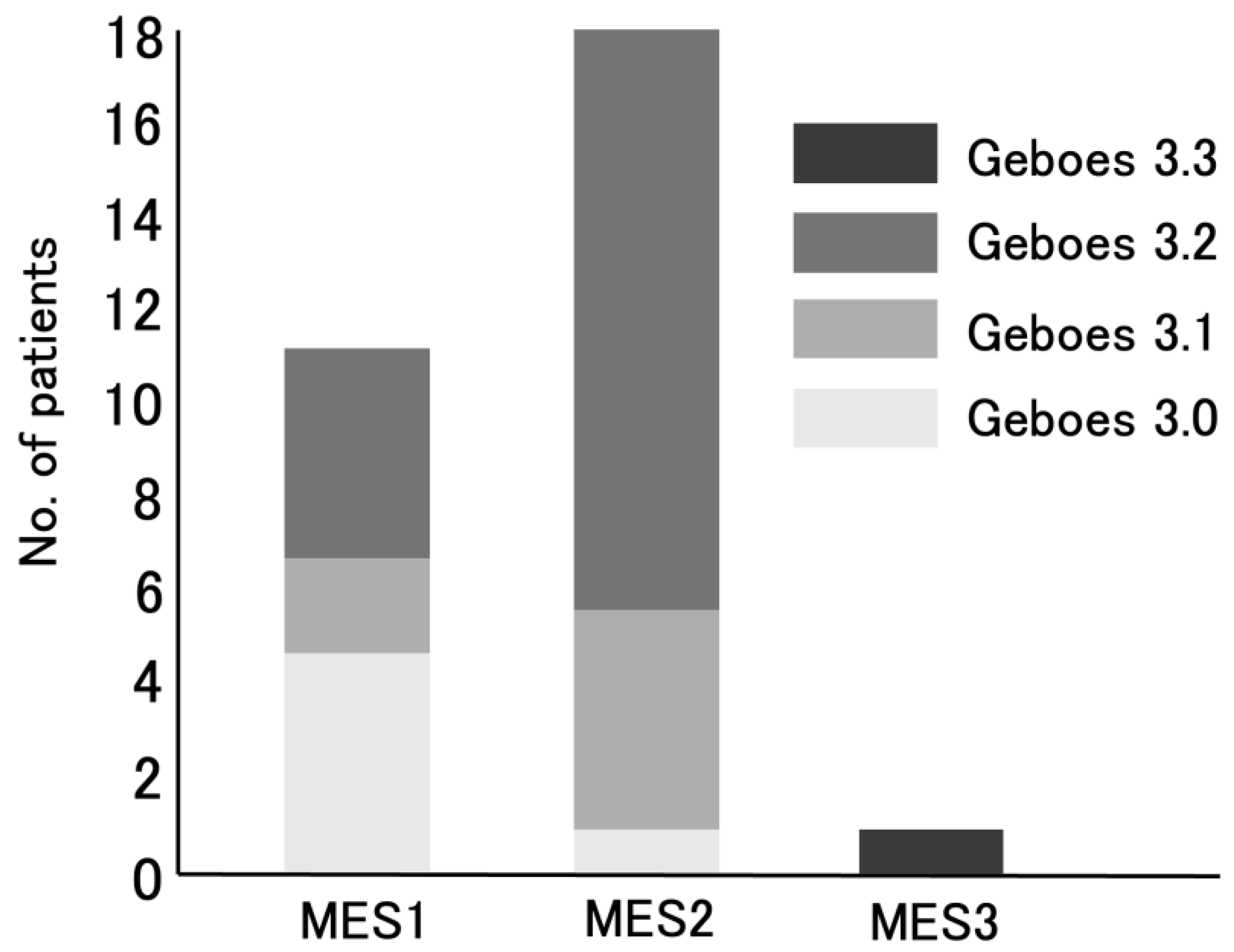
Figure 2). Of the 18 UC patients with MES 2, 5 (28%) had Grade 3.1 and 12 (67%) had Grade 3.2 (
Figure 3).
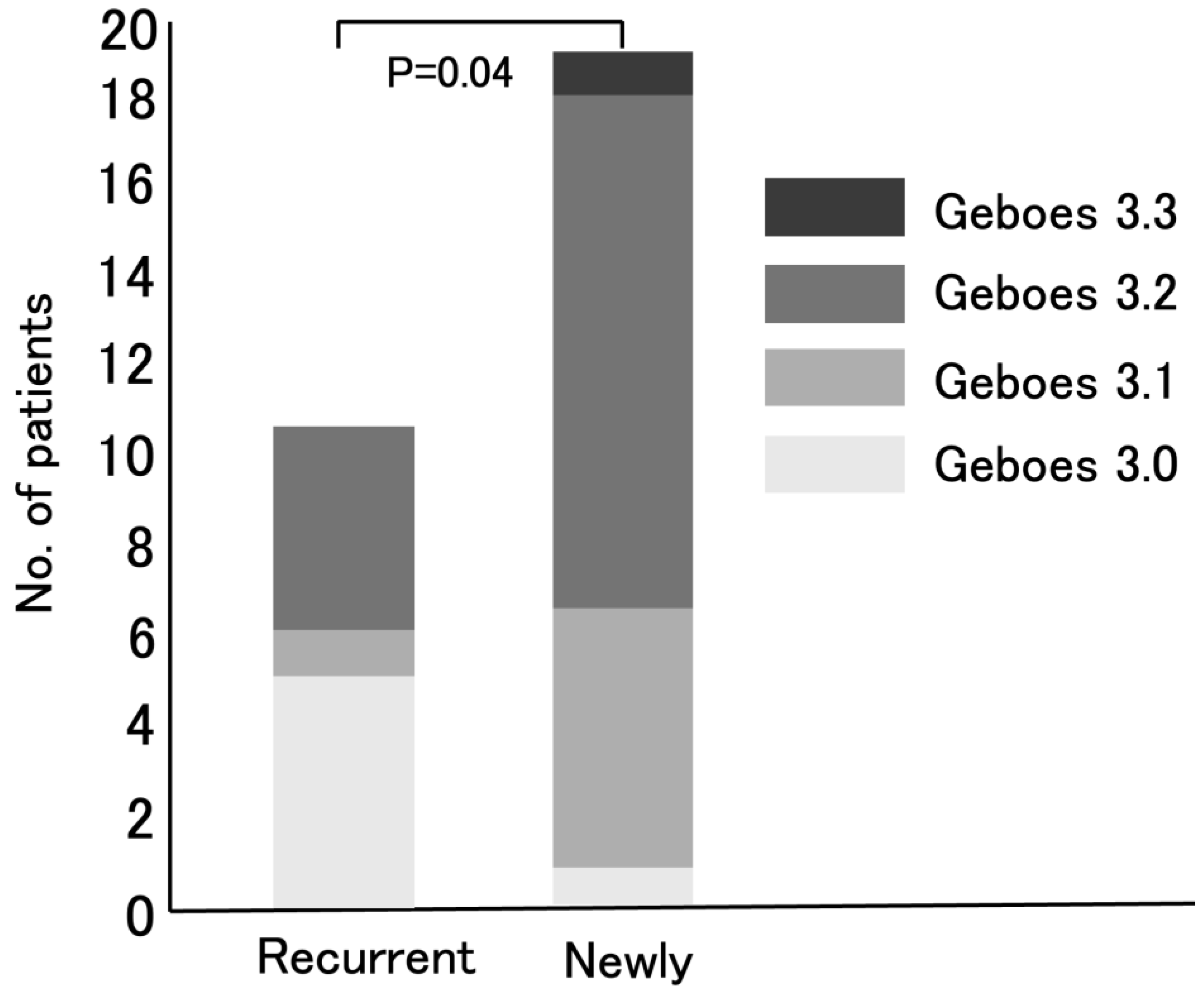
The distribution of Geboes score Grade 3 was then compared between patients with recurrent UC and newly diagnosed UC patients (
Figure 4). Baseline characteristics (age, sex, disease duration and CAI) were similar between recurrent UC and newly diagnosed UC patients. In particular, MES scores were similar between the two groups (
p=0.099). The significant difference in Geboes Grade 3 scores is therefore unlikely to be due to these factors. Among the 11 recurrent UC patients, 5 (45%), 1 (9%), and 5 (45%) were classified into the Grade 3.0, Grade 3.1, and Grade 3.2 groups, respectively. In contrast, among the 19 newly diagnosed patients, 1 (5%), 6 (32%), 11 (58%), and 1(5%) were classified as having Grade 3.0, Grade 3.1, Grade 3.2, and Grade 3.3 epithelial neutrophilic infiltration, respectively. There was a significant difference in neutrophilic infiltration between the two groups (
p = 0.04).
4. Discussion
In this study we presented a simple and reproducible method for the evaluation of Geboes score Grade 3. High neutrophilic infiltration within crypts, corresponding to Grade 3.2 or 3.3, was observed in approximately 60% of the enrolled UC patients. Additionally, endoscopic mucosal activity was correlated with the histological findings, suggesting that more severe mucosal inflammation was associated with increased neutrophilic infiltration in the epithelium. Our study improves diagnostic accuracy in UC by developing a reproducible subclassification of Geboes score Grade 3, with potential future implications for prognostication - particularly in identifying IL-23 antagonist responders - although the current focus remains on refining the consistency of histopathological assessment. To our knowledge, there are currently no published studies demonstrating a direct correlation between Geboes score Grade 3 and IL-23 levels. Our study is exploratory in this regard and aims to provide a histological framework that may facilitate such future investigations.
The relationship between Geboes score Grade 3 and MES is particularly noteworthy. Most of the patients with Grade 3.0 had an MES of 1, whereas those with Grades 3.1 and 3.2 more frequently had an MES of 2. As shown in
Figure 2, there was a significant difference in MES between the Grade 3.0 and Grade 3.2 groups (
p = 0.0009), reinforcing previous reports in which the Geboes score and MES were positively correlated, with greater mucosal inflammation reflecting more severe histological inflammation [
11]. These findings support the idea that MES, commonly used for evaluating endoscopic activity, may complement histological assessment for a more comprehensive evaluation of UC disease activity.
From an immunological perspective, the role of Th17 in chronic inflammation is well established. Th17-driven cytokine production mobilizes and activates neutrophils, contributing to sustained inflammation [
4,
5]. In active UC, inflammatory cytokine profiles evolve over time, with Th1-associated cytokines being predominant in the early phase and Th2-related cytokines in the late phase. On the other hand, Th17-associated cytokines are elevated in both the early and late phases, suggesting their continuous involvement [
4]. Based on the evaluation of Geboes score Grade 3 the activation of Th17 in UC patients can be predicted. Notably, newly diagnosed UC patients exhibited significantly higher Grade 3 scores than recurrent UC patients (
p = 0.04) (
Figure 4). This may mean that the number of active UC patients in whom IL-23 exerts its effects by activating Th17 cells, resulting in high neutrophil infiltration in the colonic mucosal epithelium, has been increasing in Japan. In other words, the number of active UC patients likely to respond effectively to IL-23 antagonists may be growing.
MES scores were similar between newly diagnosed and relapsed UC patients (p=0.099). The significant difference in Geboes Grade 3 scores is therefore unlikely to be due to MES. As 73% of patients were untreated prior to the study and only 2/30 were on immunosuppressants (1 prednisolone, 1 Janus kinase inhibitor), systemic drug effects are unlikely. These findings suggest that the differences in inflammation between the two groups are due to intrinsic disease biology.
To ensure a straightforward and reproducible assessment of Geboes score Grade 3, a simplified methodology was developed in this study. Although relatively high interobserver variability has been reported for Grades 3 and 4 [
7], the original description of the Geboes score lacked detailed criteria for counting crypts and neutrophilic infiltration [
6]. As the inter-rater reliability for this Komagane evaluation method was 0.85 (Kappa value), we believe that we have developed an accessible and reproducible method. Therefore, we expect that even non-expert pathologists could obtain reliable results using the Komagane evaluation method for Geboes score Grade 3, thereby improving their identification of UC patients who are likely to respond effectively to IL-23 antagonists in routine clinical practice.
The evaluation of Geboes score Grade 3 was primarily conducted using virtual slides, which provided efficient and accurate histological assessment. In most cases, virtual slides alone were sufficient for histological evaluation. In ambiguous cases, standard glass slides were reviewed to confirm the presence of neutrophils. The use of virtual slides improved workflow efficiency and facilitated in-depth discussions between the two independent reviewers, highlighting the utility of this approach for Geboes score assessment.
In our study, the term “Th17 activation” specifically refers to IL-23-driven pathogenic Th17 activity associated with epithelial neutrophilic infiltration (Grades 3.2/3.3) [
12]. While non-pathogenic Th17 cells may coexist in the mucosa, their IL-10-mediated immunosuppressive functions are unlikely to drive the neutrophilic infiltration central to our scoring system [
13]. While our focus on Grade 3 identifies epithelial phase Th17 activity, Grade 2B lamina propria neutrophils may represent earlier IL-23/Th17 signaling that precedes epithelial invasion. Our study focused only on Grade 3 to clarify the reproducibility of the association between neutrophil infiltration and IL-23 levels in UC patients.
This study has several limitations. It was conducted at a single hospital in Japan with a small number of patients (n=30). The small sample size and use of MES as the main comparator limit the strength of our conclusions. Future research should compare the Komagane method with other histological scoring systems and biomarkers. Although we tried to reduce selection bias by including all consecutive patients with active UC over one year, the results may not be widely applicable. Therefore, this study should be considered preliminary. Another limitation is that we did not collect clinical outcome data for patients treated with IL-23 antagonists. Future studies should use this scoring method in treatment trials to see if it can predict drug response. Larger, prospective studies with longer follow-up are needed to confirm our findings and to evaluate the benefits of combining histological and endoscopic assessments in UC.
5. Conclusions
We have developed a simple and reproducible method for the evaluation of Geboes score Grade 3. Applying this method to a group of 30 patients, we found that approximately 60% were classified as having Grade 3.2 epithelial neutrophilic inflammation or greater based on the reproducible scoring method. This method may help to identify UC patients who are likely to respond effectively to IL-23 antagonists.
Author Contributions
Study concept and design; S.U., I.H., A.H., data acquisition; S.U., T.T., A.H., data analysis and interpretation; S.U., I.H., A.H., drafting of the manuscript; S.H., I.H., A.H., critical revision of the manuscript for important intellectual content; S.U., I.H., K.H., A.H., statistical analysis; S.U., A.H., obtained funding; A.H. All authors reviewed the manuscript.
Institutional Review Board Statement
The study was conducted in accord with the latest version of the Helsinki Declaration and was approved by the Showa Inan General Hospital’s Ethics Committee on July 24, 2023. In addition, we confirmed that all examinations in this study were performed in accordance with relevant guidelines/regulations.
Informed Consent Statement
All subjects gave written informed consent when the enrollment of this study was scheduled. The study was registered at
www.clinicaltrials.gov (NCT 06372613). All authors had access to the study data and reviewed and approved the final manuscript.
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.
Conflicts of Interest
The authors declare no competing interests.
References
- Turner, D.; Ricciuto, A.; Lewis, A.; D’Amico, F.; Dhaliwal, J.; Griffiths, AM.; Bettenworth, D.; Sandborn, W.J.; Sands, B.E.; Reinisch, W.; et al. International Organization for the Study of IBD.STRIDE-II: an update on the selecting therapeutic targets in inflammatory bowel disease (STRIDE) initiative of the international organization for the study of IBD (IOIBD): determining therapeutic goals for treat-to-target strategies in IBD. Gastroenterology 2021, 160, 1570–1583. [Google Scholar] [PubMed]
- Sleiman, J.; Bassi, M.; Tsipotis, E.; Charabaty, A. Medical treatment options for ulcerative colitis. Clin. Colon. Rectal. Surg. 2022, 35, 428–436. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Loftus, E.V. Jr.; Limketkai, B.N.; Haydek, J.P.; Agrawal, M.; Scott, F.I.; Ananthakrishnan, A.N. AGA Clinical Guidelines Committee. Electronic address: clinicalpractice@gastro.org. AGA living clinical practice guideline on pharmacological management of moderate-to-severe ulcerative colitis. Gastroenterology, 2024; 167, 1307–1343. [Google Scholar]
- Nakase, H.; Sato, N.; Mizuno, N.; Ikawa, Y. The influence of cytokines on the complex pathology of ulcerative colitis. Autoimmun. Rev. 2022, 21, 103017. [Google Scholar] [CrossRef] [PubMed]
- Allocca, M.; Furfaro, F.; Fiorino, G.; Gilardi, D.; D’Alessio, S.; Danese, S. Can IL-23 be a good target for ulcerative colitis? Best Pract. Res. Clin. Gastroenterol. 2018, 32-33, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Geboes, K.; Riddell, R.; Ost, A.; Jensfelt, B.; Persson, T.; Löfberg, R. A reproducible grading scale for histological assessment of inflammation in ulcerative colitis. Gut 2000, 47, 404–409. [Google Scholar] [CrossRef] [PubMed]
- Bressenot, A.; Salleron, J.; Bastien, C.; Danese, S.; Boulagnon-Rombi, C.; Peyrin-Biroulet, L. Comparing histological activity indexes in UC. Gut 2015, 64, 1412–1418. [Google Scholar] [CrossRef] [PubMed]
- Rachmilewitz, D. Coated mesalazine (5-aminosalicylic acid) versus sulphasalazine in the treatment of active ulcerative colitis: a randomised trial. BMJ. 1989, 298, 82–86. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, K.W.; Tremaine, W.J.; Ilstrup, D.M. Coated oral 5-aminosalicylic acid therapy for mildly to moderately active ulcerative colitis. A randomized study. N. Engl. J. Med. 1987, 317, 1625–1629. [Google Scholar] [CrossRef] [PubMed]
- Kundel, H.L.; Polansky, M. Measurement of observer agreement. Radiology 2003, 228, 303–308. [Google Scholar] [CrossRef] [PubMed]
- Lemmens, B.; Arijs, I.; Van Assche, G.; Sagaert, X.; Geboes, K.; Ferrante, M.; Rutgeerts, P.; Vermeire, S.; De Hertogh, G. Correlation between the endoscopic and histologic score in assessing the activity of ulcerative colitis. Inflamm. Bowel Dis. 2013, 19, 1194–1201. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Yang, W.; Tang, B.; Wang, X.; Zhang, Q.; Li, W.; Li, L. The protective and pathogenic role of Th17 cell plasticity and function in the tumor microenvironment. Front Immunol. 2023, 14, 1192303. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Tian, J.; Wang, S. Insight into non-pathogenic Th17 cells in autoimmune diseases. Front Immunol. 2018, 9, 1112. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).