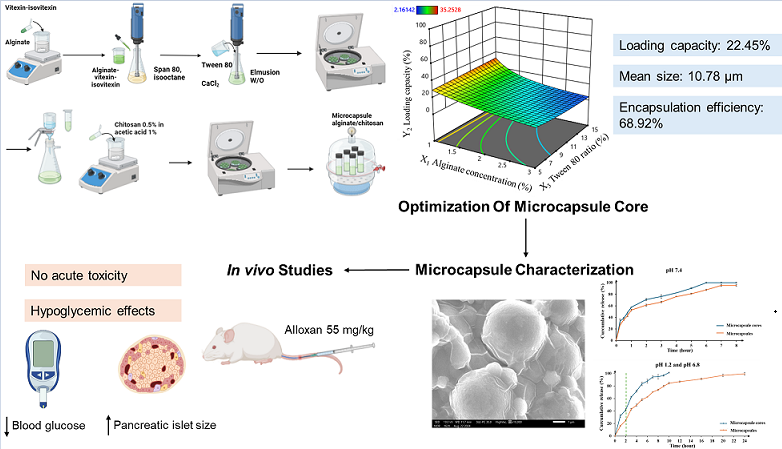
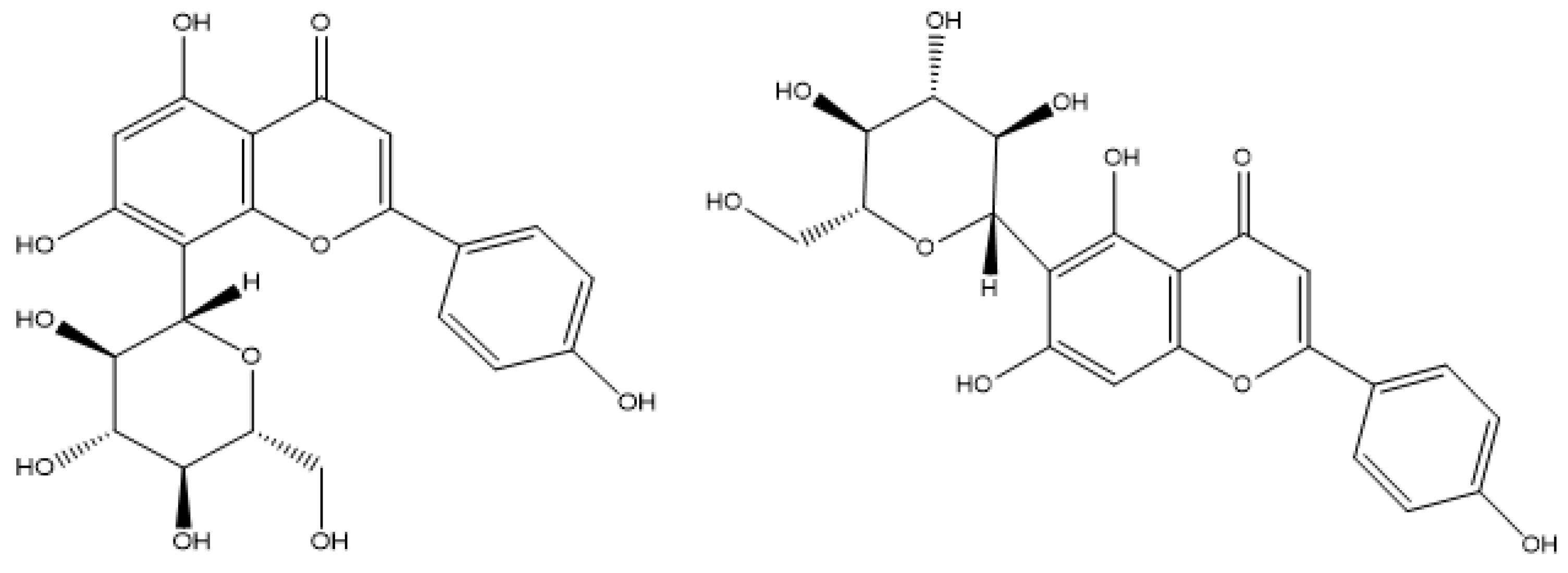
This study focused on developing an alginate-chitosan microsphere drug delivery system. Both polymers exhibit biocompatibility, low toxicity, and have been extensively investigated for their influence on microsphere structure, making them suitable for pharmaceutical applications. A two-stage alginate-chitosan microencapsulation technique was employed to precisely control the morphology and size of the microspheres through a combination of emulsion and ion gelation processes. Subsequently, a coating process was applied to optimize the release kinetics of the encapsulated active ingredients. The active ingredients selected for this study were vitexin and isovitexin, poorly water-soluble flavonoids extracted and purified from medicinal herbs. Despite the recognized pharmacological potential of these compounds, limited research has been conducted on their encapsulation within alginate-chitosan microsphere systems. The optimization of the alginate-chitosan microsphere formulation presented in this study is distinct from previously published research, offering a unique contribution to the field of drug delivery.
4.1. Optimization of Alginate Cores Formulation
The preparation process for the alginate cores was inspired by the work of Meixia Jin et al. [
55] and utilized Design Expert v.13.0.5.0 software. Design of Experiment (DoE) methodologies were employed to ensure a systematic and efficient approach to experimental design and analysis. maximizing the study’s impact [
56]. An I-optimal response surface design was selected due to its suitability for models incorporating both quantitative and qualitative factors, as well as for scenarios involving varying levels of independent variables. This design prioritizes predictive accuracy, offering advantages over other algorithms such as D-optimal or G-optimal [
57,
58]. A quadratic regression model was employed as the highest-order model to comprehensively assess the interactions between independent variables and their impact on response variables.
As demonstrated by Alshora et al. [
59], particle size played a crucial role in enhancing the solubility of active ingredients by increasing their surface area. In the context of alginate microspheres, particle size significantly influenced both drug loading capacity and release rate [
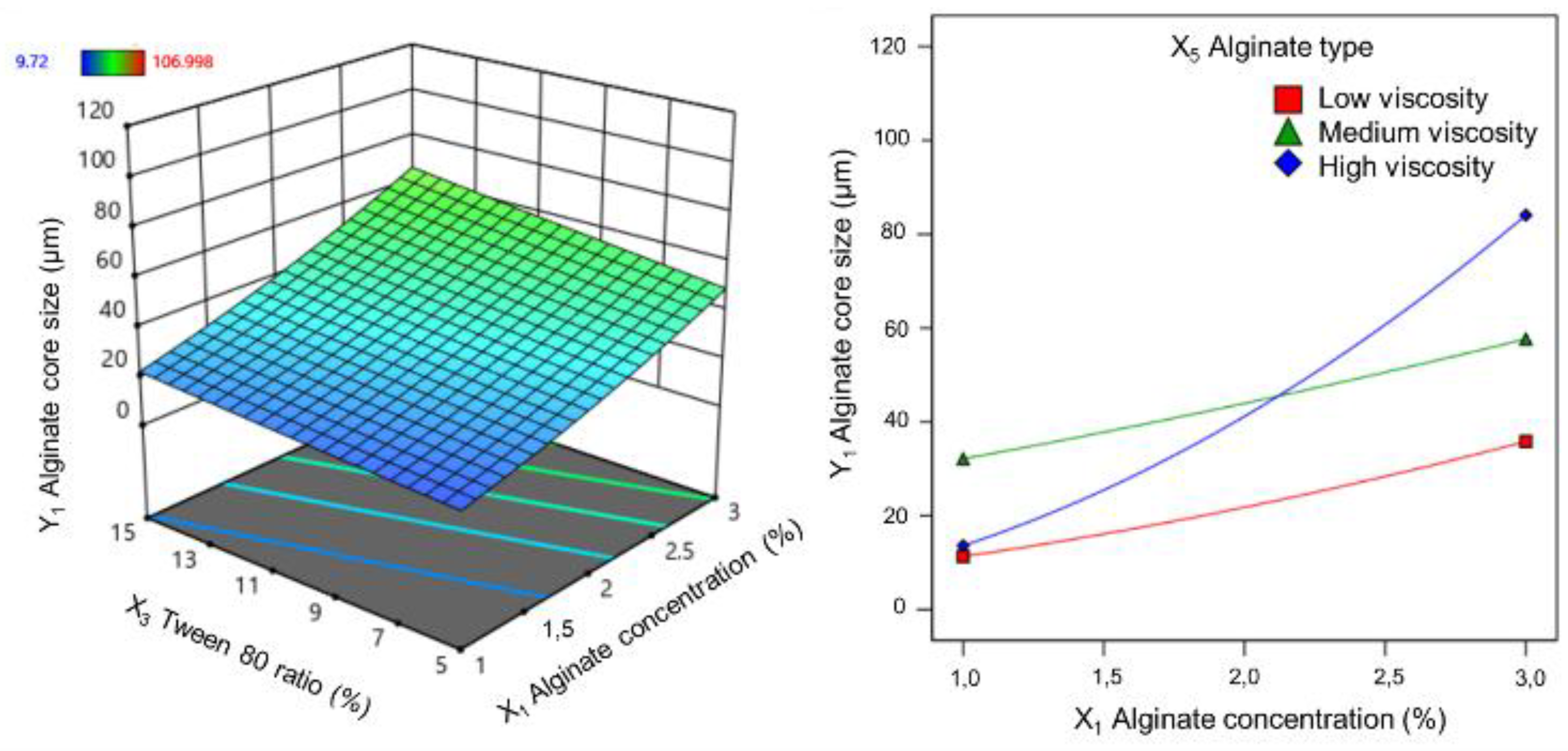
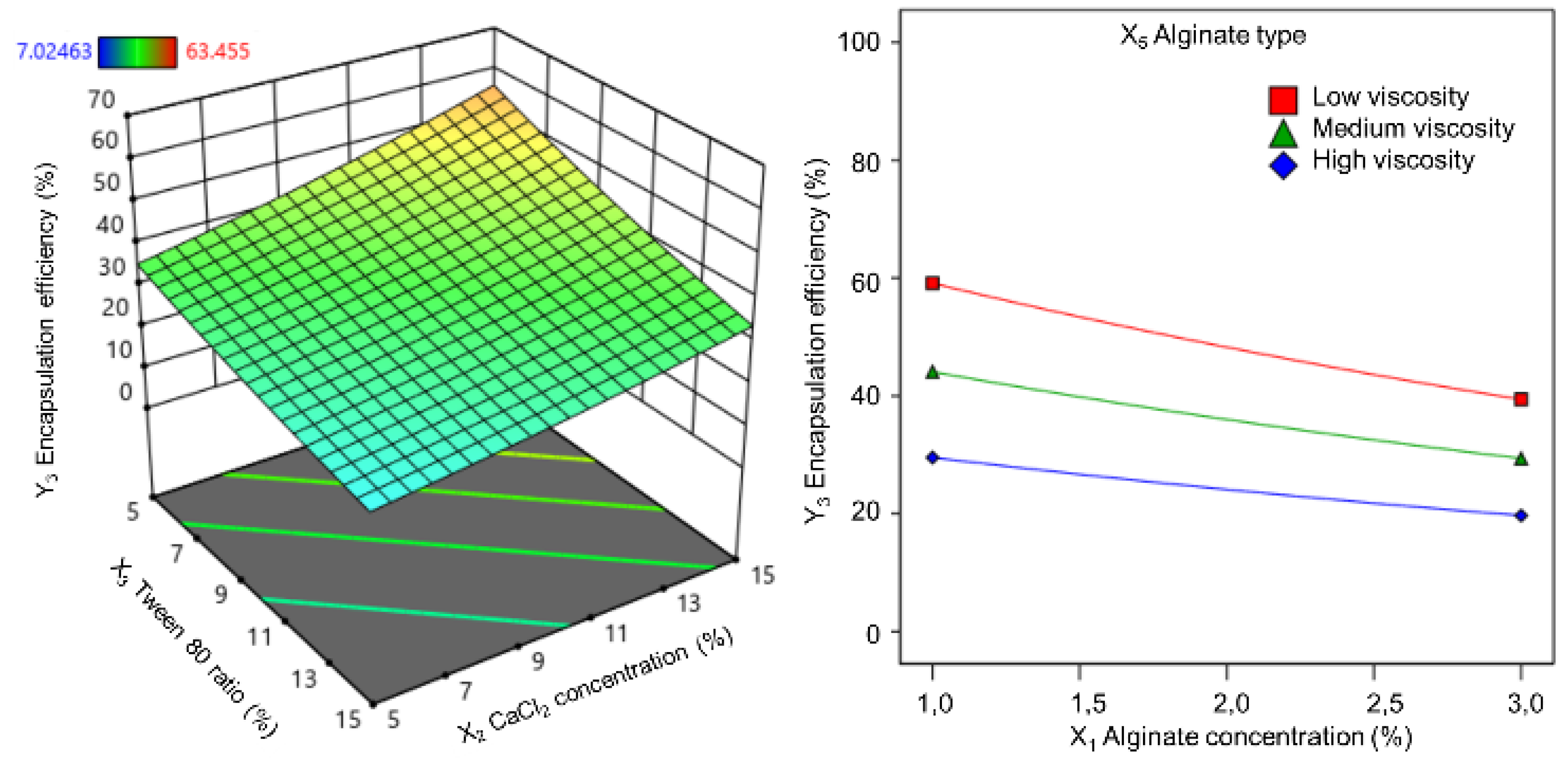
60]. Moreover, a uniform particle size distribution reflected the consistency and efficiency of the microencapsulation process. Alginate concentration was widely recognized as a primary factor affecting the physicochemical properties of alginate microspheres, including morphology, size, and encapsulation efficiency [
61]. The findings of this study aligned with this established knowledge, confirming the influence of both alginate concentration and type on alginate core size. Previous studies have reported similar trends. Mokhtari et al. [
62] investigated the impact of alginate concentration on the properties of nanoparticles prepared by the internal gelation emulsion method, demonstrating a significant boost in particle size from 512 to 4303 nm as the alginate concentration was increased from 0.5 to 1.0% (w/v). Similarly, Shukla et al. [
63] also reported a proportional relationship between alginate concentration (3 to 5%, w/v) and microsphere size (417.8 to 453.9 µm) produced by the ionized emulsion method. These findings suggested that alginate polymer concentration exerted a substantial influence on the size and distibuition polydispersity index of nanoparticles formed through emulsion techniques. This effect can be attributed to the interaction between the carboxylate functional groups (COO
-) of the alginate chains and calcium ions. Higher alginate concentrations lead to an increased number of COO
- groups and the formation of multiple alginate layers around calcium cations, resulting in the formation of larger particles. Higher alginate concentrations also had resulted in increased solution viscosity and decreased shear stress, leading to larger emulsion droplets. This explained the influence of alginate viscosity on alginate core size. Additionally, the interaction between alginate type and concentration had significantly impacted core size. High-viscosity alginates exhibited a more pronounced increase in core size with increasing concentration compared to that of low and medium viscosity alginates.
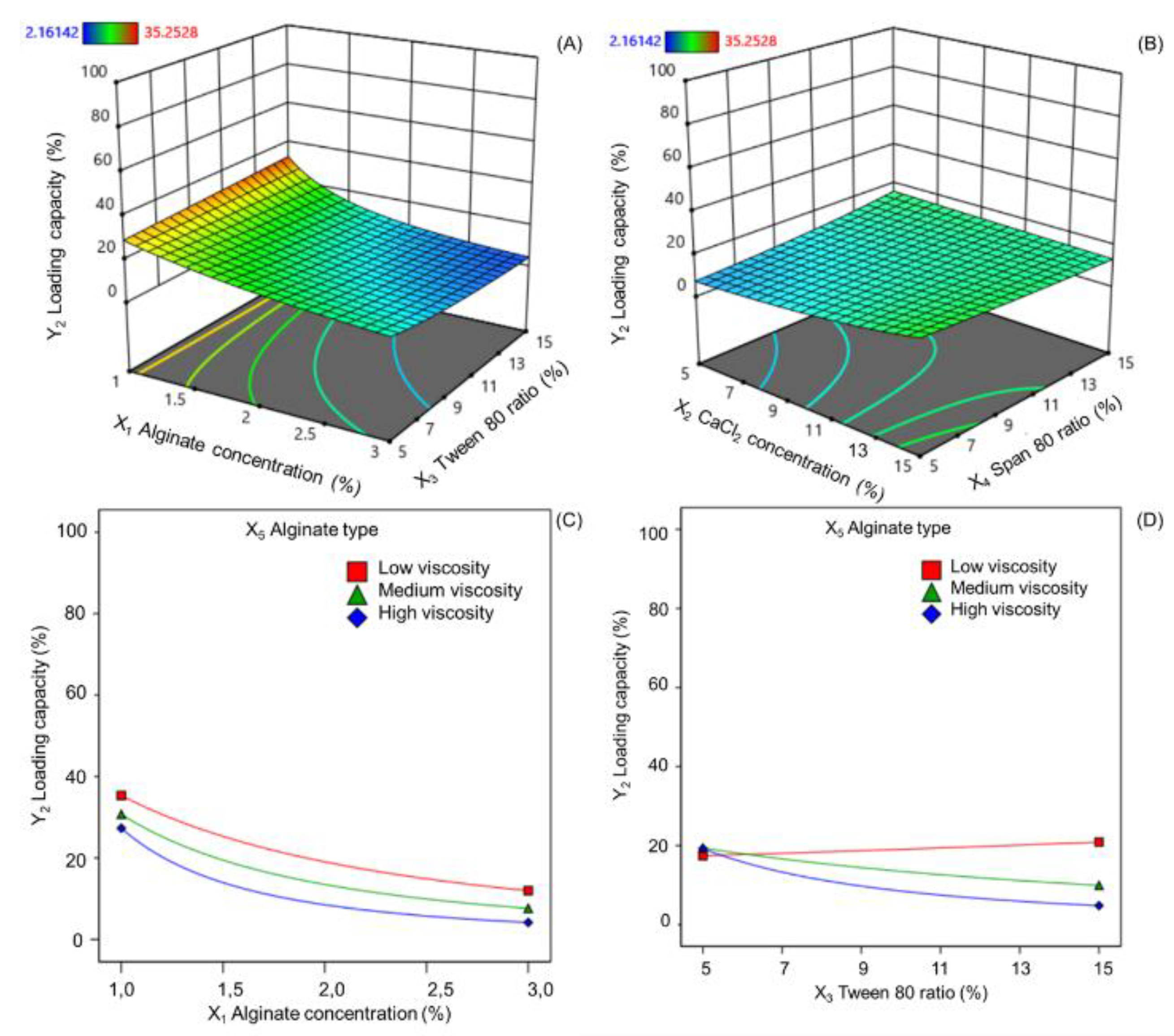
Alginate concentration significantly impacts the quality indicators of alginate cores, particularly the loading capacity and encapsulation efficiency of the active substance. In this study, increasing alginate concentration primarily reduced the loading ratio without significantly affecting encapsulation efficiency. This finding aligns with previous research by Kalalo et al. [
64] demonstrated that higher alginate concentrations increase solution viscosity, leading to thicker emulsion droplets and reduced quercetin loading. Similarly, Silva et al. [
65] observed no significant change in insulin encapsulation efficiency with increasing alginate concentration. Essifi et al. [
66] further elucidated this phenomenon. They reported that higher alginate concentrations increase encapsulation efficiency while reducing loading capacity. This is attributed to the formation of denser microsphere structures with more cross-links between calcium ions and alginate chains. Additionally, the interaction between alginate concentration and Tween 80 ratio influences vitexin-isovitexin loading. Independently, increasing Tween 80 tends to slightly decrease loading. However, at low alginate concentrations, increasing Tween 80 can paradoxically increase active ingredient loading.
Alginate type significantly influenced both the loading capacity and encapsulation efficiency. Low-viscosity alginate yielded alginate cores with higher loading and entrapment than medium- and high-viscosity alginates. Combining low-viscosity alginate with a lower concentration further optimized these parameters. Additionally, the interaction between Tween 80 ratio and alginate type was significant. Although low-viscosity alginate was minimally affected by the Tween 80 ratio, medium- and high-viscosity alginates exhibited decreased loadings with increasing Tween 80 content. This is attributed to the increased polymer chain self-interaction, reduced available space for active ingredient incorporation, and potential loss due to decreased particle surface tension [
67].
Calcium chloride, a critical cross-linking agent in alginate-based emulsion gelation, forms a stable three-dimensional “egg-box” network [
68]. While the calcium chloride concentration did not influence the loading rate in this study, it exhibited a positive correlation with the encapsulation efficiency. This aligns with previous studies by Mokhtari et al. [
62] and Hu et al. [
69], who demonstrated an increased encapsulation efficiency of phenolic extracts and curcumin, respectively, at higher calcium chloride concentrations. This effect is attributed to the formation of a dense network of cross-links on the microsphere surface.
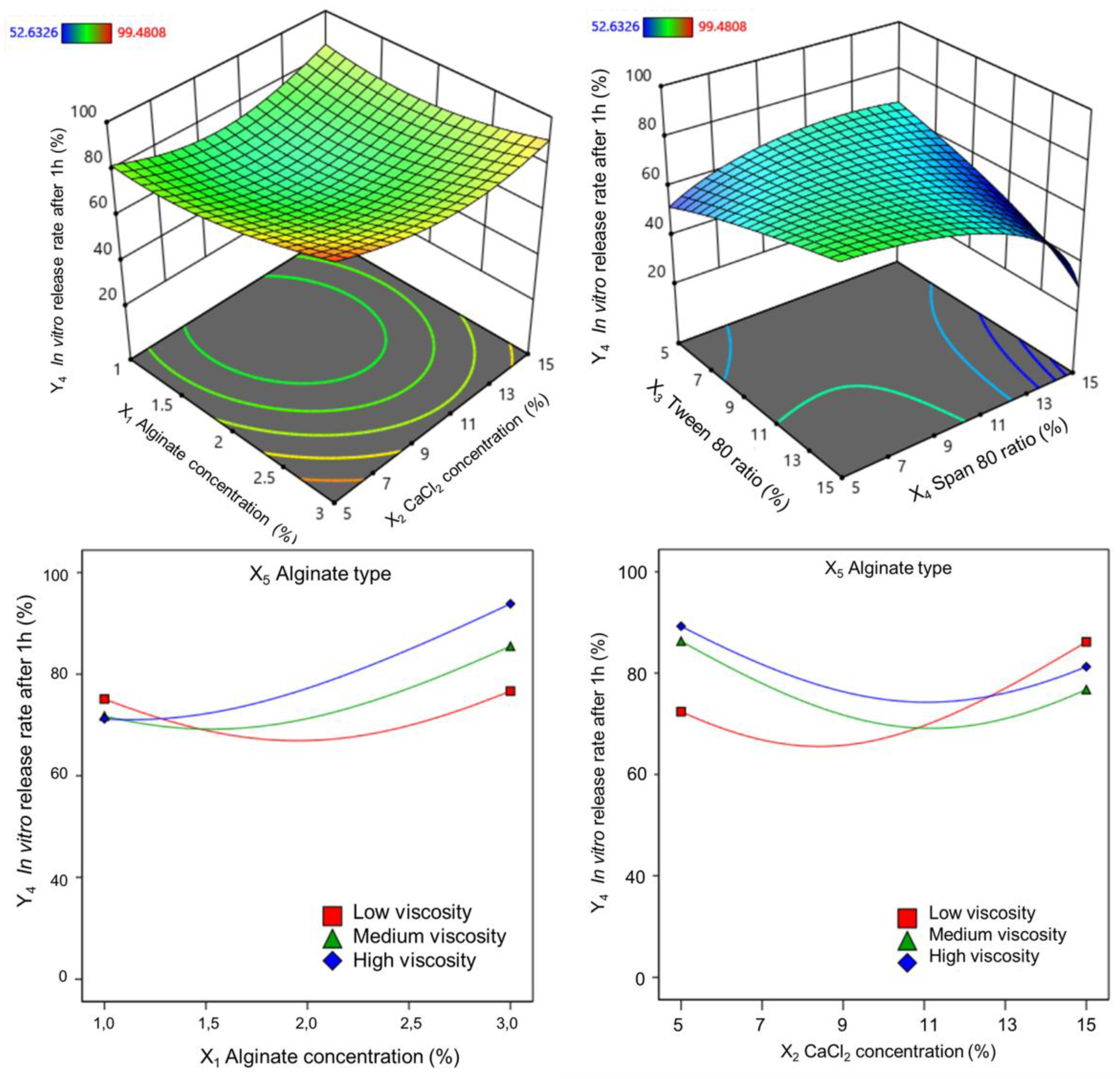
Alginate concentration significantly influenced the initial in vitro release rate. Increasing alginate concentration from 1% to 2% slightly decreased the in vitro release rate, while further increasing to 3% led to an increased the in vitro release rate. This trend is consistent with previous findings [
70], which suggest that higher alginate concentrations can lead to larger alginate cores with more active molecules closer to the surface, resulting in a rapid initial release. Similarly, increasing calcium chloride concentration can also impact the in vitro release rate. This is likely due to the formation of a more porous particle structure, facilitating faster release (brust release). Alginate type, which influences solution viscosity, also affects the in vitro release rates. Higher viscosity alginate resulted in higher the in vitro release rate. This is consistent with the general understanding that ion-gelated alginate particles often exhibit rapid initial release due to their porous structure [
71].
Tween 80 and Span 80 were selected as the emulsifiers to stabilize the emulsion and reduce the interfacial tension between the oil and water phases. Tween 80 facilitates calcium-ion diffusion into the dispersed phase, promoting cross-linking and microsphere solidification. Span 80 primarily stabilizes emulsion droplets by adsorbing them onto their surfaces, preventing agglomeration and enhancing the alginate core structure. Among the formulation parameters, alginate concentration, Tween 80 ratio, and alginate type had the most significant effects on microsphere quality. In contrast, the calcium chloride concentration and Span 80 ratio had less pronounced effects on the final alginate core properties.
4.2. Characterization of the Microspheres
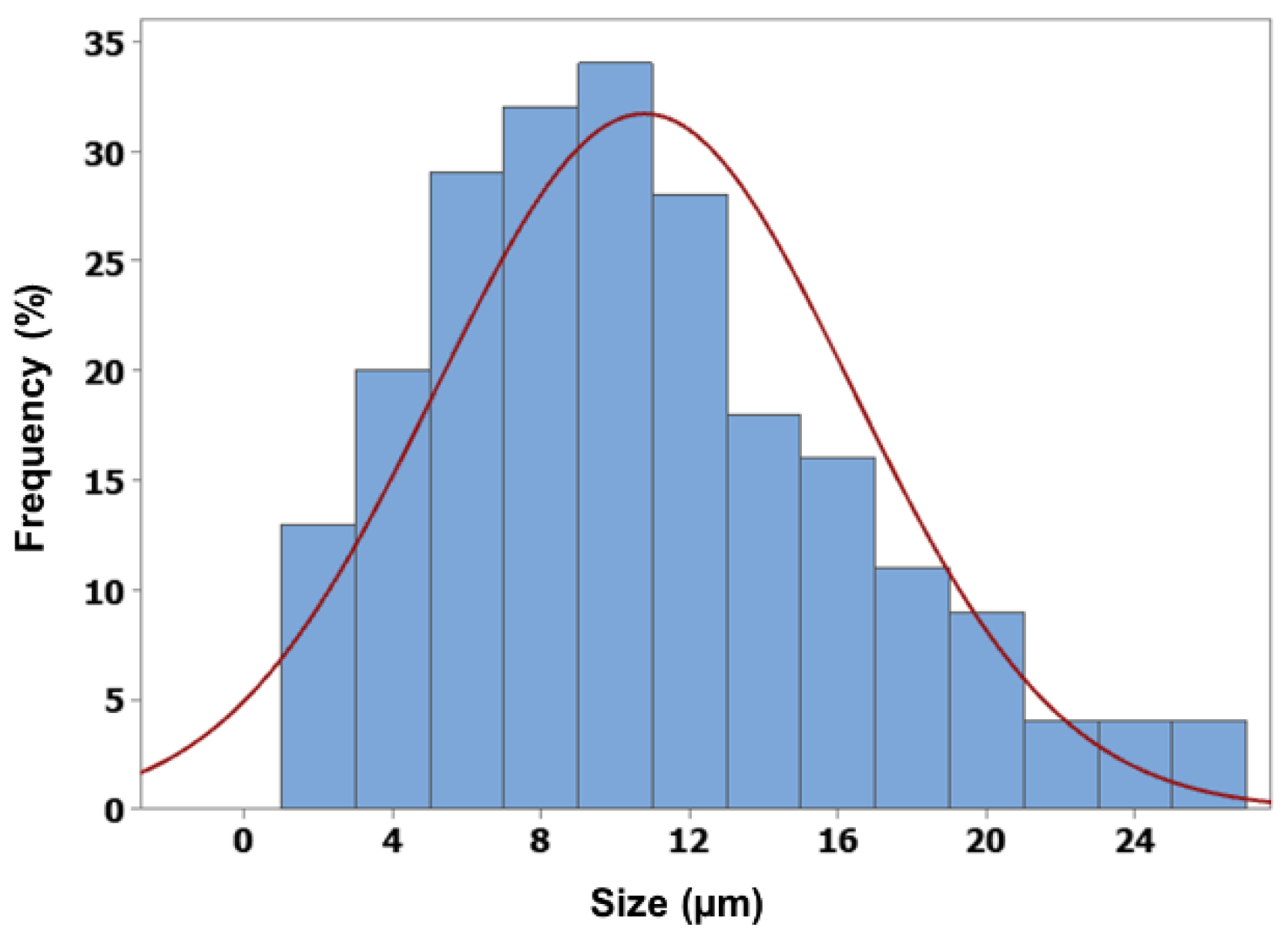
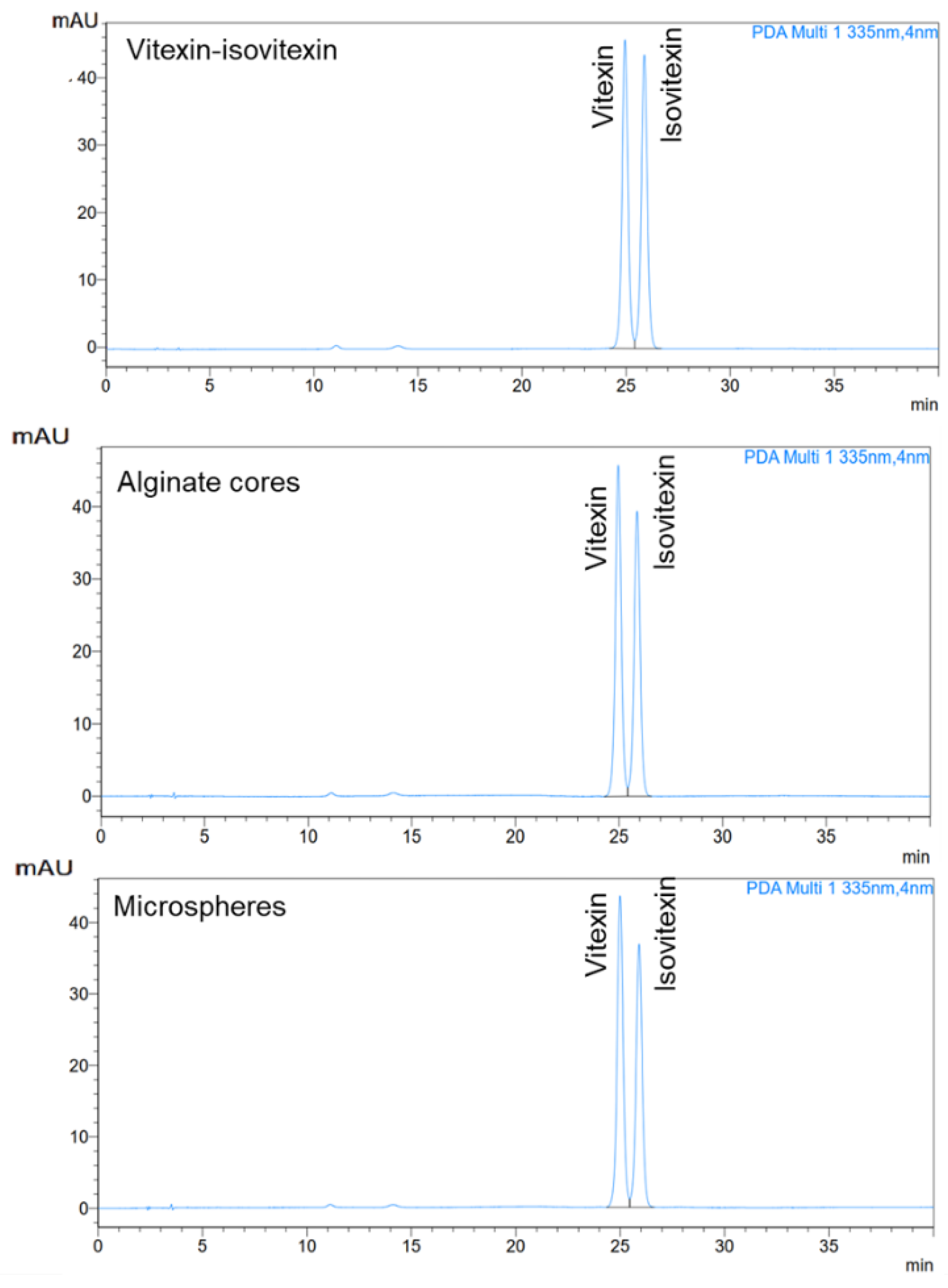
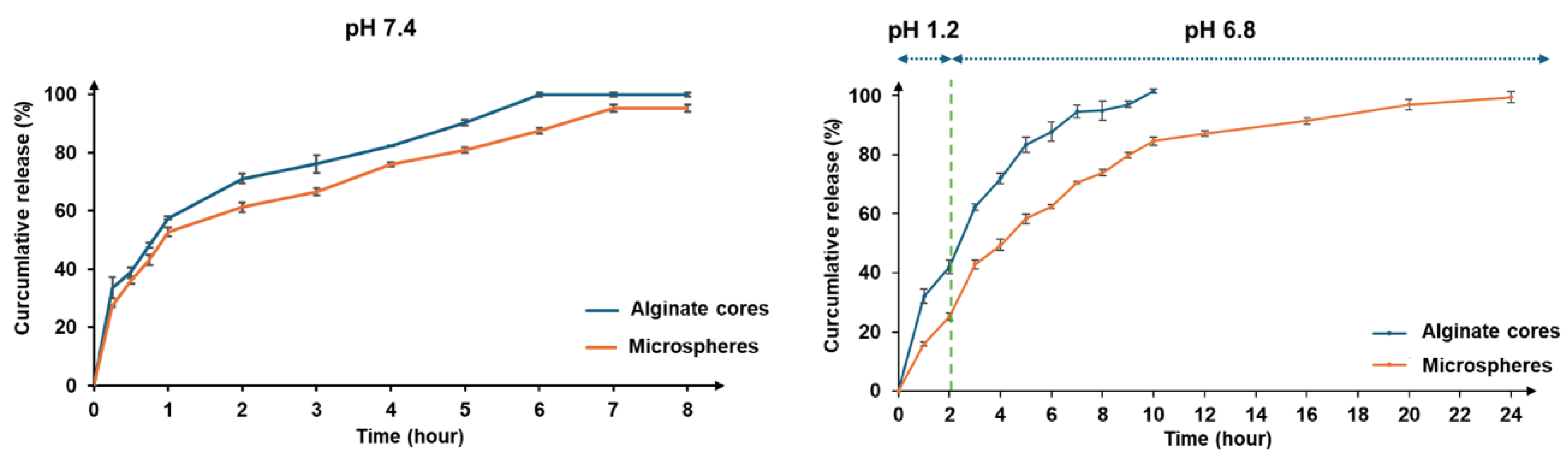
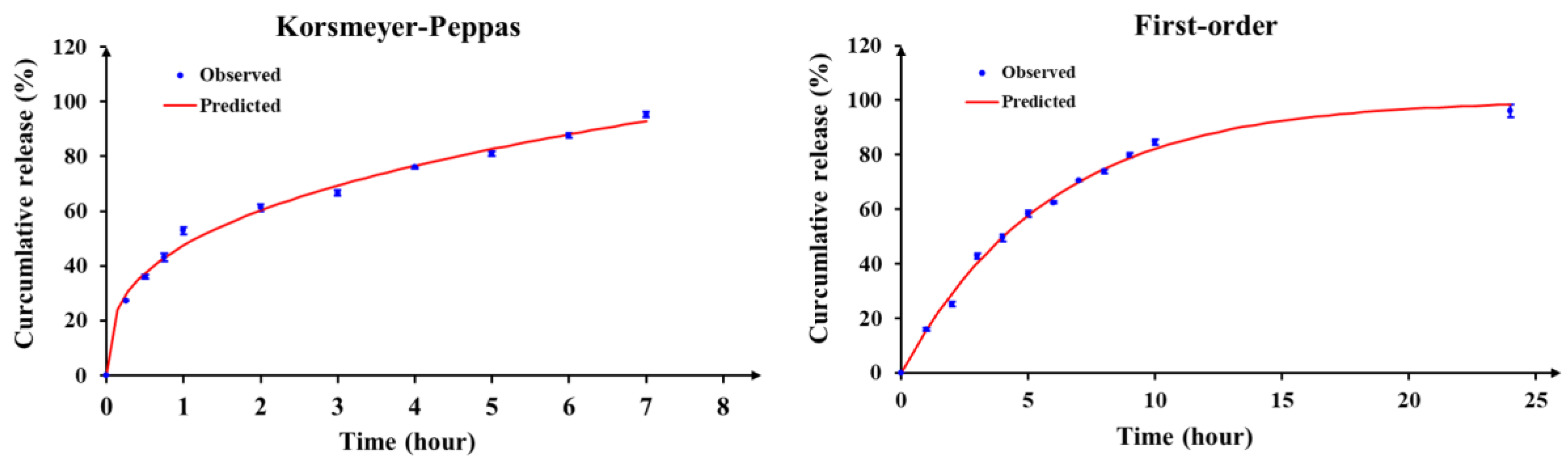
The ion gelation process successfully produced spherical microcapsules with micron-sized dimensions. Alginate-chitosan microcapsules exhibited controlled release of vitexin-isovitexin in pH 7.4, following a diffusion-controlled mechanism as described by the Korsmeyer-Peppas model. The chitosan coating formed a polyelectrolyte complex, slowing down ion exchange and contributing to the controlled release. Additionally, the higher solubility of vitexin-isovitexin in alkaline pH accelerated release. Two-stage release experiments simulating digestive pH further highlighted the effectiveness of the polyelectrolyte complex. The microcapsule core exhibited rapid release, while the alginate-chitosan microcapsule demonstrated a more controlled release profile, with approximately 25% release at pH 1.2 and 96% release at pH 6.8. This behavior aligns with first-order kinetics and a diffusion-controlled mechanism. These findings are consistent with previous studies on alginate-chitosan microspheres loaded with various active ingredients, such as lidocaine [
72], galactagogue extract [
73], nifedipine [
74], and cannabis extract [
75]. These studies also reported controlled release profiles and mechanisms similar to those observed in the current research. The prolonged release of the active ingredient from the microspheres was attributed to changes in the properties of both alginate and chitosan polymers. While a simple mechanical coating of chitosan might have led to rapid dissolution at pH 1.2, the formation of a polyelectrolyte complex between alginate and chitosan resulted in a more controlled release profile. Sæther et al. [
76] described the mechanism of this composite film formation, which involves dipole interactions and hydrogen bonding between the carboxyl groups of alginate and the amine groups of chitosan. This complexation reduces the porosity of alginate and enhances its mechanical strength, leading to a controlled release of active ingredients in a dynamic pH environment.
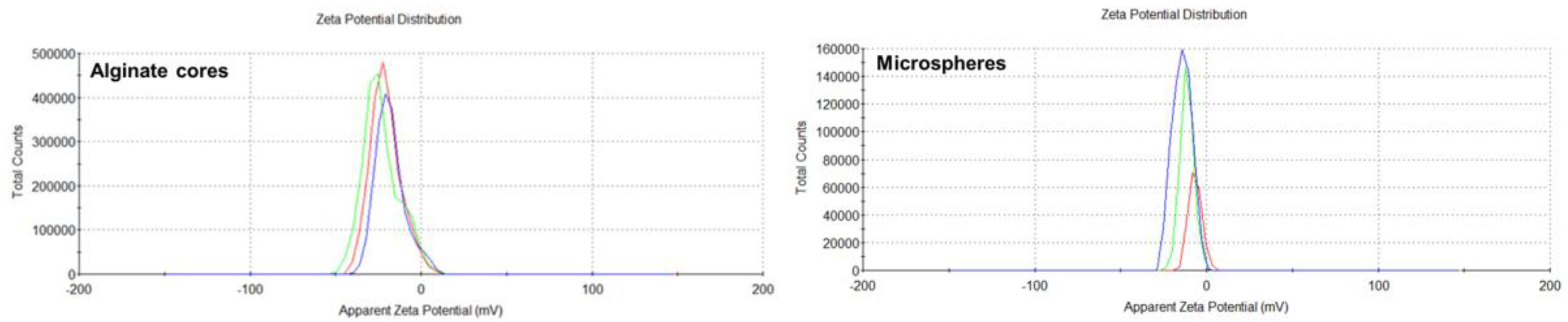
The negative zeta potential of the alginate cores, as observed in this study, is consistent with the properties of alginate polymers. This negative charge is attributed to the presence of carboxyl groups in the alginate structure, which ionize to form negatively charged carboxylate groups. This negative charge contributes to the stability of the microspheres in solution by repelling each other and preventing aggregation. Previous studies have also reported negative zeta potentials for alginate-based nanoparticles. For example, Zimet et al. [
77] observed zeta potentials in the range of -38.7 to -33.22 mV for Nisaplin-containing alginate nanoparticles, while Bhunchu et al. [
78] reported values between -27.8 and -19.8 mV for alginate nanoparticles. These findings further corroborate the negative surface charge of alginate-based particles. The significant decrease in zeta potential from -20.7 mV to -6.93 mV upon chitosan coating indicates a substantial reduction in the negative surface charge of the microspheres. This reduction is attributed to the interaction between the negatively charged carboxyl groups (COO
-) of alginate and the positively charged amino groups (NH
3+) of chitosan. This interaction results in the formation of a polyelectrolyte complex, which effectively neutralizes the negative charge on the microsphere surface. This complexation has implications for the stability, release properties, and biocompatibility of the microspheres.
Thermal analysis revealed that the first endothermic peak at 106 °C corresponded to the loss of water from the alginate polymer, while the second peak at 262 °C represented a combination of the melting peak of vitexin-isovitexin (257 °C) and the crystallization peak of alginate (239 °C). The upward trend in the baseline further confirmed the presence of alginate in the core. Similarly, the alginate-chitosan microspheres exhibited two endothermic peaks. The first peak corresponded to the melting of both alginate and chitosan polymers, while the second peak required less energy compared to the alginate cores, likely due to the presence of chitosan. Chitosan, with a crystallization onset at 276 °C, reduced the overall energy absorption of the microspheres. Thermal analysis confirmed the presence of alginate, chitosan, and vitexin-isovitexin in the final microsphere structure.
4.3. Acute Toxicity and Hypoglycemic Effects of Vitexin-Isovitexin-Loaded Microspheres
This result resonated with the study of Choo et al. [
11] that vitexin and isovitexin at the dose of 2 g/kg showed safety on both normoglycemic and induced diabetic rat. According to the Globally Harmonized System (GHS) of Classification and Labelling of Chemicals, isovitexin and vitexin can be classified into Category 5, which is considered relatively safe following acute exposure.
Vitexin and isovitexin have long been proved to possess hypoglycemic effects through several mechanisms, including antioxidant activity as flavones [
9], α-glucosidase [
11], and aldose reductase [
14,
15,
16] inhibition. Several studies have been conducted, both in vitro and in vivo, to confirm the anti-diabetes capacity of these two compounds [
2,
11,
12,
17]. Despite their enormous potential as antidiabetic agents, vitexin and isovitexin have been investigated separately, and there has not been any research carried out on their combination, let alone on the microsphere form. Therefore, it is important to mention that this is the first study ever to evaluate the bioactivity of vitexin and isovitexin as a combination, especially the hypoglycemic effect.
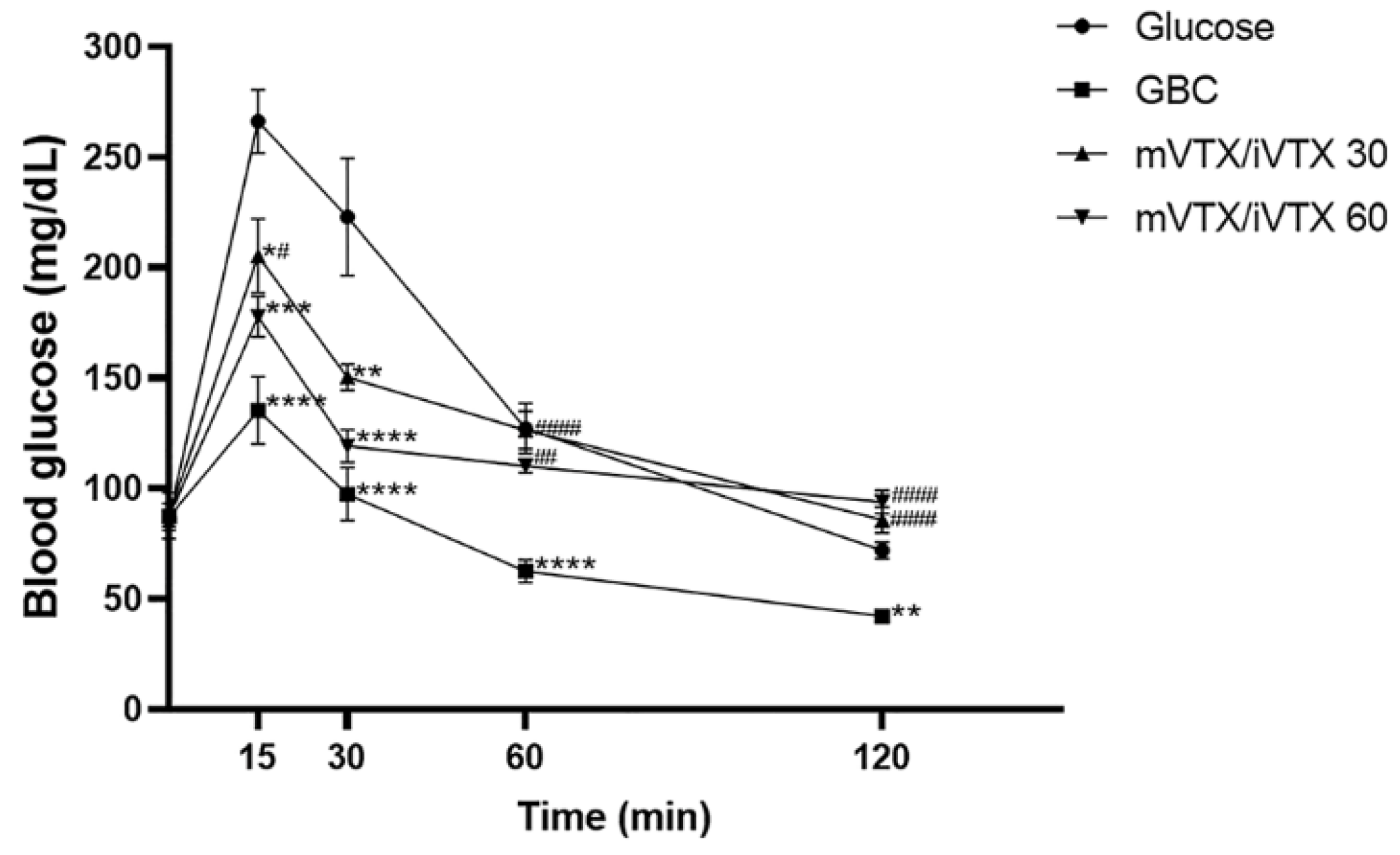
Mice receiving vitexin-isovitexin microspheres at both doses (30 and 60 mg/kg) showed a clear decrease in blood glucose levels and AUC after 21 days. The hypoglycemic effects of this preparation may be attributed to the pharmacological activities of vitexin and isovitexin themselves [
2], combining with the bioavailability-enhancing actions of the microspheres. This result is consistent with those of various studies that support the antidiabetic effects of vitexin and isovitexin [
2,
11,
12,
17]. In addition, the blood glucose-modulating activity of the microsphere form appeared to be dose-dependent, as a higher dose of 60 mg/kg resulted in a greater improvement in blood glucose than the 30 mg/kg dose. Our findings are consistent with those of other studies, which reported that vitexin and isovitexin reduced postprandial blood glucose levels in a dose-dependent manner [
11,
17].
Glucose tolerance tests have long been important tools for identifying impairments in glucose homeostasis [
79,
80]. Moreover, as the need for novel antidiabetic medications has become increasingly strong, glucose tolerance tests, especially oral administration, are being utilized to study the effectiveness of new treatments for elevated blood glucose levels [
81]. OGTT evaluates the glucose uptake in peripheral tissues and a rise in glucose concentration during OGTT implies a reduction in insulin response as well as the insulin-secreting ability of pancreatic β-cells [
82]. Our results demonstrate that the microsphere form of the vitexin-isovitexin combination alleviated glucose intolerance. These findings are in agreement with those of another study by Choo et al. [
11], who also examined the influence of vitexin and isovitexin as separate compounds on glucose tolerance. However, the glucose tolerance test in the study by Choo et al. was performed on both normoglycemic and diabetic rodents, whereas our study only investigated non-diabetic mice. Therefore, the effects of vitexin-isovitexin-loaded microspheres on glucose tolerance should also be tested in animal models of diabetes.
The lack of statistically significant differences in HbA1c, insulin levels, HOMA-IR, and HOMA-β between the treated groups and the ALX group suggests that, under the conditions of this study, the vitexin-isovitexin microspheres did not significantly impact these specific parameters. HbA1c or glycated hemoglobin is a biochemical index used to monitor blood glucose levels [
82], and HbA1c tests are commonly performed at 3-month intervals in humans. In mouse models, HbA1c concentration is mostly evaluated after an experimental period of over 40 days [
44,
83,
84]. However, in the alloxan-induced hyperglycemia model, HbA1c levels should be measured at an earlier time point of 14-21 days considering the high mortality rate of this model [
44]. In this study, mice treated with vitexin-isovitexin-loaded microspheres showed a decrease in HbA1c levels compared with those of untreated mice. Vitexin has been shown to reduce HbA1c levels, as stated in a study by Gayathri et al. [
85], which was conducted on high-fat diet-streptozotocin-induced diabetic rats. Regarding isovitexin or the combination of these two compounds, the determination of their effects on HbA1c remains limited. There was no significant difference in insulin levels between groups. However, the untreated group still showed a slight decrease in insulin levels compared with the control group. In contrast, the treatment groups displayed a rising tendency in insulin levels compared to the ALX group, although the differences were not statistically significant. The increase in insulin levels, although not remarkable, corresponded with a decline in blood glucose levels (
Table 9), as described above.
HOMA-IR and HOMA-β indices are widely used tools for assessing insulin sensitivity and pancreatic β-cell function, as they are calculated from fasting glucose and insulin levels [
52,
86,
87]. Alloxan, a commonly used diabetogenic agent, induces hyperglycemia through two primary mechanisms. First, it acts as a glucose analog, selectively accumulating in β-cells through the GLUT2 glucose transporter, where it provokes reactive oxygen species (ROS) formation, leading to β-cell deterioration. Second, as a thiol reagent, alloxan inhibits glucose-induced insulin production by targeting the enzyme glucokinase in β-cells [
88]. This β-cell damage restricts insulin secretion, resulting in a decreased HOMA-β value. Our findings align with these mechanisms, as the HOMA-β index in the ALX group was lower than in the control group (
Table 12). The moderate increase in HOMA-β observed in the treatment groups may reflect partial regeneration of β-cells, which also corresponds with the slight rise in insulin levels noted earlier (
Table 11). Various in vitro studies have demonstrated that vitexin protects pancreatic β-cells through mechanisms such as improving insulin signaling [
89] and reducing ROS and lipid peroxidation [
90]. Regarding the HOMA-IR index, administration of alloxan reduced insulin sensitivity, leading to an increase in this score (
Table 12), as confirmed by previous studies [
40,
44]. In contrast, treatment with vitexin-isovitexin-loaded microspheres resulted in a slight decrease in HOMA-IR, indicating their capacity to enhance insulin sensitivity and promote peripheral glucose utilization. This impact on HOMA-IR may contribute to the hypoglycemic mechanisms of vitexin-isovitexin-loaded microspheres alongside β-cell-stimulating [
89] and α-glucosidase inhibitory (
Supplementary Figure S1) effects of vitexin/isovitexin. Future studies should investigate the effects of the microspheres over longer treatment durations, with varying doses, and in different animal models to better understand their impact on insulin dynamics and long-term glycemic control.
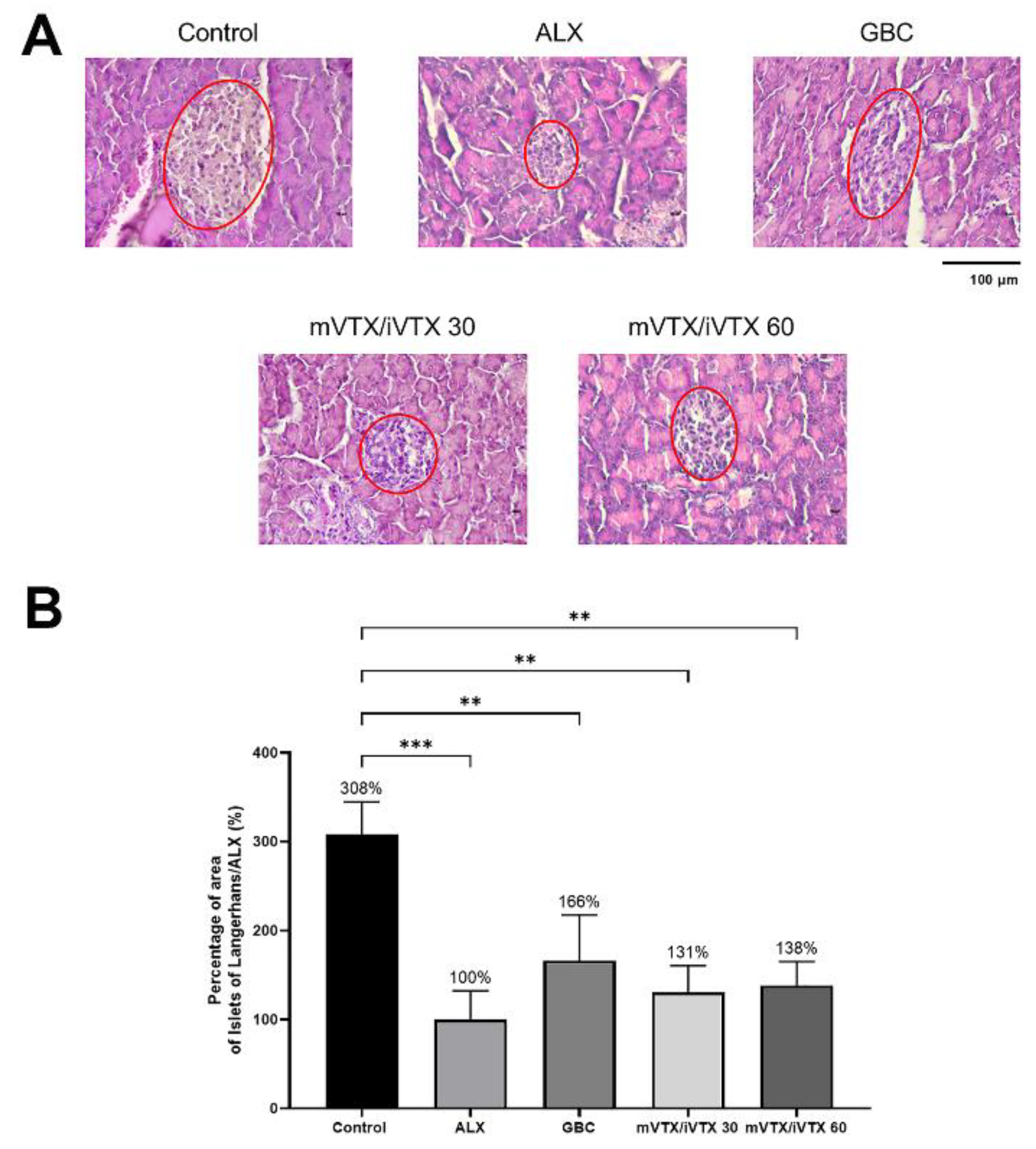
Vitexin has been proved to exert protective effect on pancreatic β-cells [
89], which might lead to islet size enhancement. Moreover, the H&E staining results are in line with the research of Wang et al. [
91], which showed the effects of vitexin on improving deteriorated islets. Similar findings have been reported, with vitexin promoting islet regeneration in streptozotocin-induced diabetic models [
92]. To the best of our knowledge, this is the first study that evaluates the positive influence of vitexin and isovitexin combination in microspheres on islet size, which was previously diminished by alloxan. These results suggest that treatment with vitexin-isovitexin-loaded microspheres mitigated the detrimental effects of alloxan on the Islets of Langerhans. This may further contribute to the underlying mechanisms of vitexin-isovitexin for reducing blood glucose levels.