Introduction
Chronic obstructive pulmonary disease (COPD) is a respiratory disorder characterized by irreversible and progressive airflow limitation[
1]. COPD is the most common chronic respiratory disease in older adults and the third leading cause of death worldwide[
2,
3]. Patients with COPD commonly present with dyspnea, chronic cough, and sputum production; their lung function declines, which is routinely assessed by spirometry. Although COPD has different clinical forms and levels of airway obstruction severity, many patients experience exacerbations that lead to hospitalization[
4], and the disease significantly decreases the health status of patients[
5]. The current recommendations of the American Thoracic Society/European Respiratory Society (ATS/ESR) present a new system for the evaluation of lung function impairment severity that does not use the percentage of predicted FEV1 values but focuses on z-score values[
6].
The Global Initiative for Chronic Obstructive Lung Disease (GOLD) recommends maintenance therapy with different types of bronchodilators depending on the disease severity phenotype (short-acting bronchodilators, long-acting beta agonists [LABAs], or long-acting muscarinic antagonists [LAMAs]). For patients with repeated exacerbations, combination treatment with inhaled corticosteroids (ICS) such as ICS/LABA or ICS/LABA/LAMA should be considered[
4].
The efficacy of ICS/LABA in COPD treatment has been widely studied since many years. It has been shown that these drugs’ combination reduces the exacerbation rate, improves health status and spirometric characteristics of lung function of COPD patients[
7,
8,
9,
10,
11]. However, there are a small number of real-life longitudinal (> 12 months) observational studies aimed at evaluating the effectiveness of ICS/LABAs in COPD populations. In particular, there is a lack of studies on Polish COPD patients[
12]. Moreover, clinical studies applying new ATS/ERS recommendations for the evaluation of lung function impairment severity have not yet been conducted.
The purpose of this observational, non-interventional study was to assess the frequency of COPD exacerbations and hospitalizations due to these exacerbations and to evaluate changes in lung function for 2 years observation period in patients treated with ICS/LABA in everyday medical practice in Poland. Additionally, the risk of COPD exacerbations was assessed using the new strategy of pulmonary functional test interpretation recommended by the ATS/ERS in 2022.
Materials and Methods
Study Design and Participants
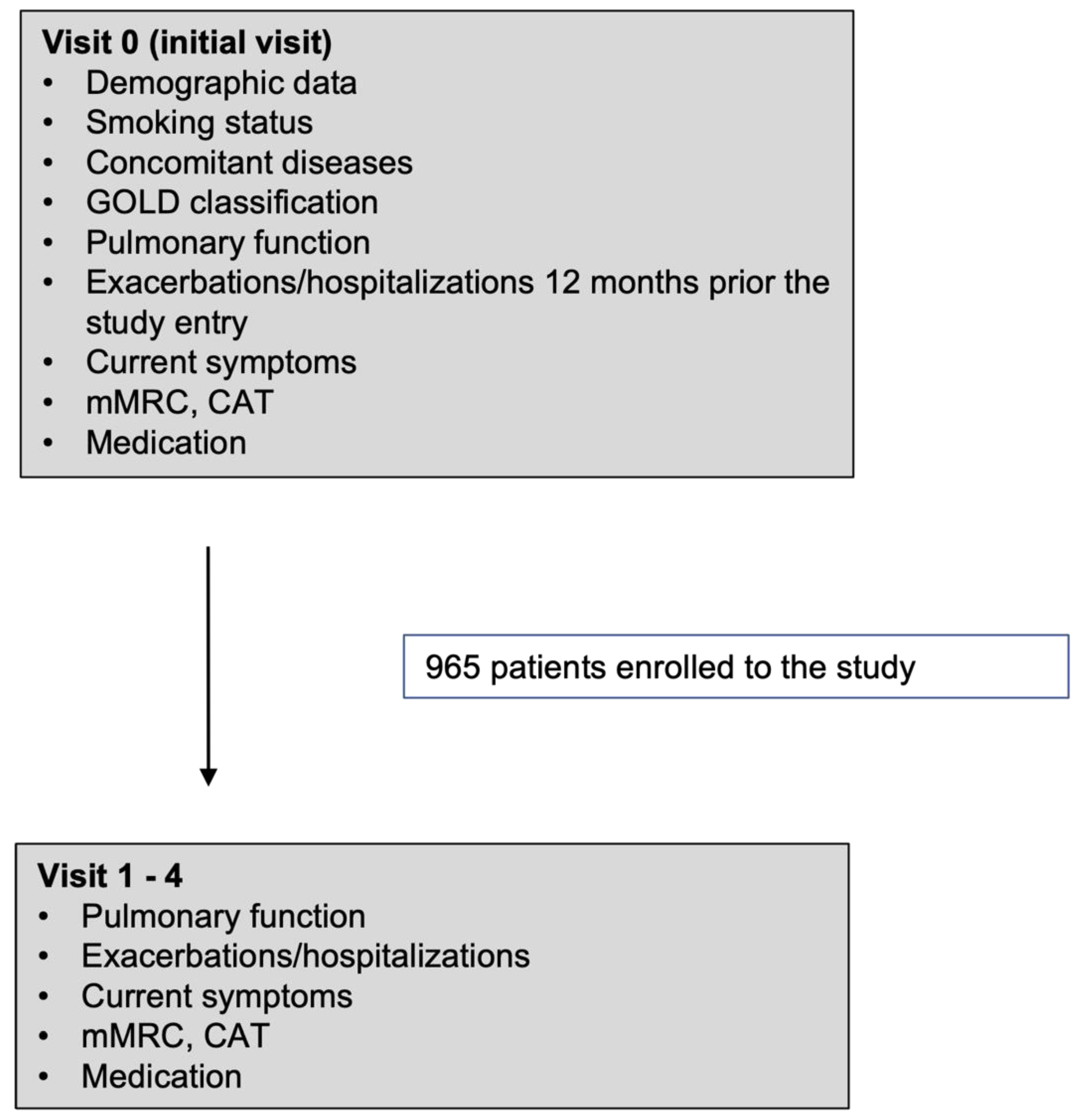
This open, prospective, multicenter, non-interventional observational study enrolled 965 patients who were diagnosed with COPD for at least 12 months before enrolment and were treated with ICS/LABA for at least 6 months prior to enrolment. The study lasted 24 months (2015 – 2017) , during which patients had five visits (visits 0 – 4) to the treating physician.
The study was performed in accordance with the Declaration of Helsinki, the International Conference on Harmonization Harmonized Tripartite Guideline for Good Clinical Practice, and local regulations. This is a prospective observational, non-interventional, real-life study, so according to Polish law, the Local Ethical Committee does not have to approve the study protocol. Additionally, in this type of study, the informed consent of the participants was not required at the time of conducting observation.
Patients were excluded from this study if any of the following criteria were present: (i) had bronchial asthma, (ii) had a contraindication to treatment with an ICS/LABA combination, (iii) were pregnant or lactating, and (iv) were currently participating in another clinical trial. If any of the abovementioned criteria were met, the patient was excluded from the study.
Endpoints and Measurements of the Study
Patients were enrolled in the study at visit 0 (the initial visit). Six months after the initial visit, visit 1 was scheduled. Subsequent visits (Visit 2-4) were performed every 6 months (+/- 14 days). Patient data collected during the initial and subsequent visits are presented in the flow chart (
Figure 1).
The primary endpoints of the study were a change of pulmonary function parameter FEV1 and the frequency of COPD exacerbations and hospitalizations due to exacerbations. Secondary endpoints included assessment of GOLD category distribution, type of comorbidities, and degree of stability of COPD treatments. Study variables were following: FEV1 and FVC (expressed in absolute values, % predicted and z-scores) applying Global Lung Function Initiative (GLI) equations[
13], number of exacerbation and hospitalization, demographic data of patients and data on: (i) smoking habits (ii) concomitant diseases, (iii) symptoms currently occurring, (iv) level of dyspnea’s severity assessed by modified Medical Research Council (mMRC) scale, (v) COPD Assessment Test (CAT) score, (vi) current treatment, (vii) treatment with antidepressants, (viii) classification to GOLD 2017-2022 groups, (ix) adverse drug reactions.
In this study, it was assumed that the FEV1/FVC ratio in obstructive patients should be ≤ 0.7. Additionally, patients were categorized as ‘decliner’ or ‘non-decliner’ depending on the occurrence of FEV1 loss greater than 100 ml during the study. Based on GOLD and new ATS/ERS recommendations [
6], patients were divided into four categories based on spirometry values:
non-severe; ATS/ERS-non-severe (FEV1> 50% pred. and z-score>-4);
GOLD-non-severe: ATS/ERS-severe (FEV1> 50% pred. and z-score<-4);
GOLD-severe - ATS/ERS-non-severe (FEV1< 50% pred. and z-score>-4);
GOLD-severe: ATS/ERS-severe (FEV1<50% pred. and z-score<-4).
Statistical Analysis
Descriptive statistics were provided for all the variables. Multivariate logistic regression was used to assess predictors of COPD exacerbation. results of multivariate regression analysis are presented as odds ratios with respective 95% confidence intervals (CI). Poisson regression analysis was used to assess the predictors of pulmonary function. The results of the Poisson regression are presented as β coefficients and respective p-values. The results were considered statistically significant at p<0.05. All calculations and analyses were performed using R 3.5 statistical software and MedCalc® Statistical Software version 20.218 (MedCalc Software Ltd, Ostend, Belgium;
https://www.medcalc.org; 2023).
Discussion
This non-interventional observational study aimed to assess the frequency of COPD exacerbations and hospitalizations due to these exacerbations and to evaluate the changes in lung function for 2 years observation period in patients treated with ICS/LABA in everyday medical practice in Poland. Patient-related outcomes, such as symptoms, smoking habits, and type of treatment were also recorded during this study. Moreover, in this publication, the risk of COPD exacerbations was assessed using a new strategy of pulmonary functional test interpretation recommended by the ATS/ERS in 2022[
6].
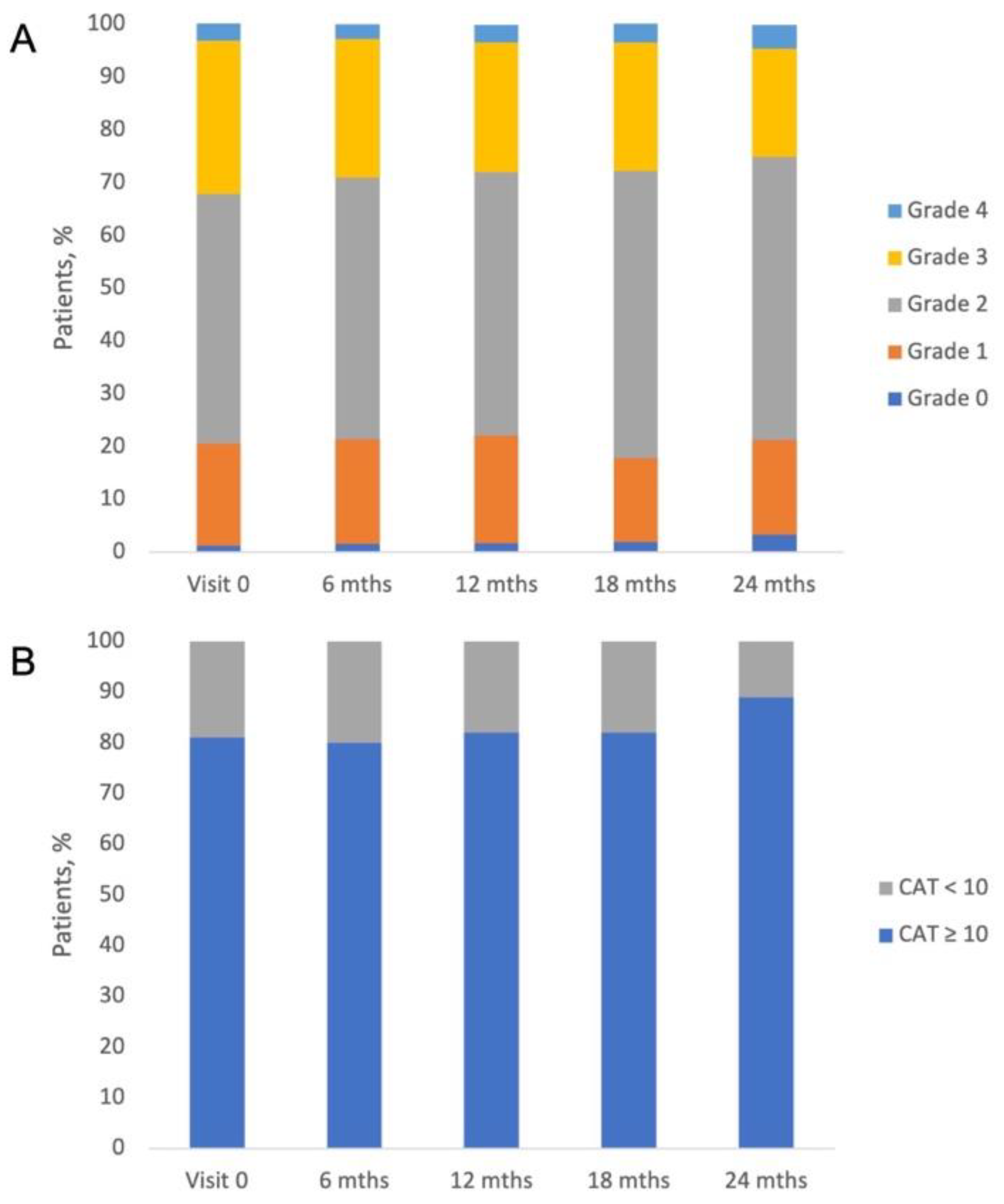
The results of our study demonstrated that the lung function of study patients measured by FEV1 did not change during the study (
Table 4). The proportion of patients with mild, moderate, severe, and very severe airflow limitations was generally stable throughout the study. At initial visit patients had mild airflow limitation accounted for 2.5%, with moderate airflow limitation for 25.1%, with severe – for 59.0% and with very severe – and 13.4% (
Table 4). Comparable distribution of airflow limitation severity categories can be found in other real-life studies where approximately 10% of patients are categorized with very severe airflow limitation and the patients with mild category are the less numerous (approximately 2-3%)[
14,
15,
16]. An interesting observation is that the decliners were mainly in the group with milder airflow limitation, which agrees with observations from large clinical trials[
17].
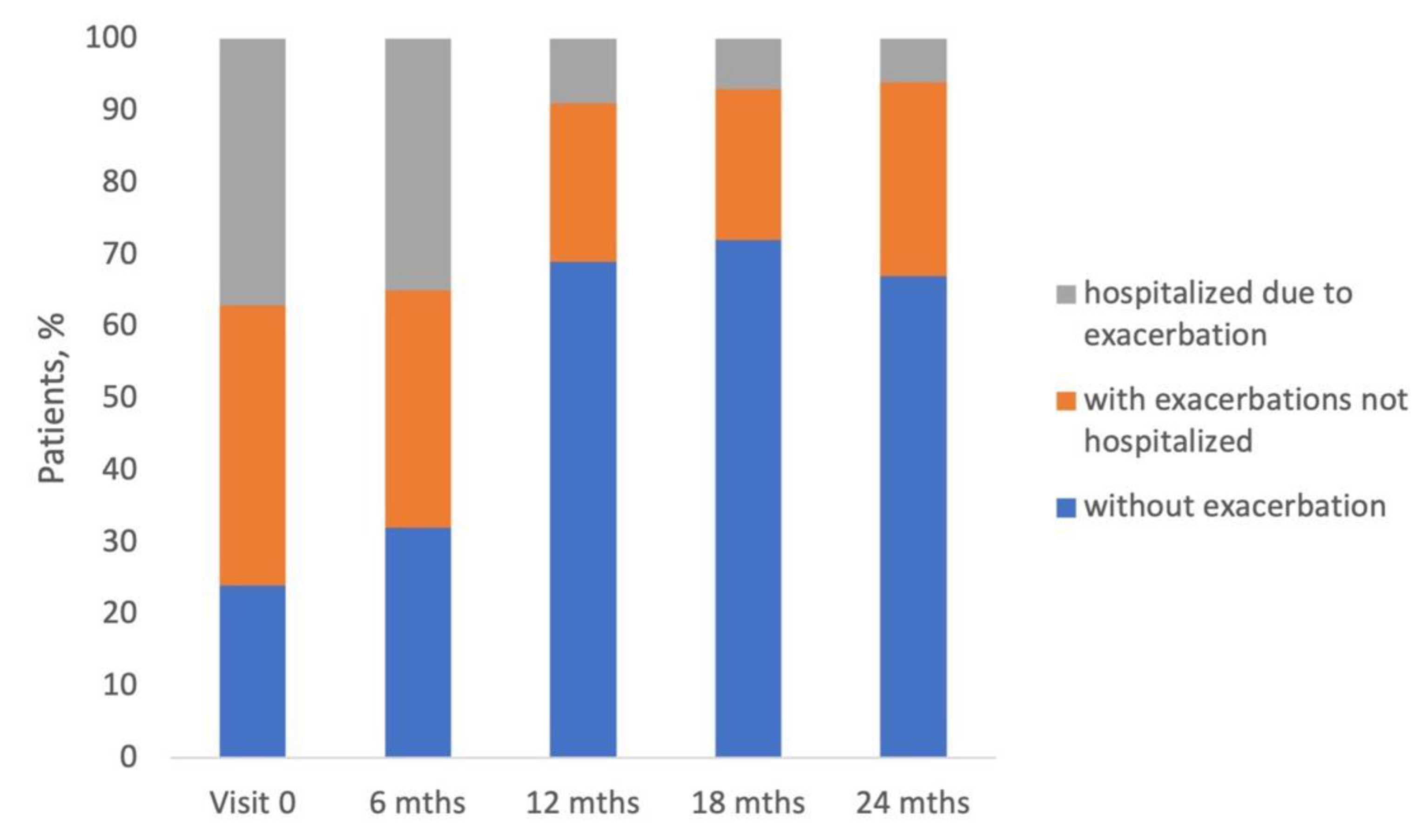
Above 75% of patients enrolled in the study experienced exacerbation of COPD, and almost half of them were hospitalized due to exacerbation in 12-months period preceding the study entry (
Figure 2). This proportion is relatively high and shows that COPD leads to a significant medical and financial burden on the Polish healthcare system. Interestingly, 330 patients were defined as 'decliners' (the decrease of FEV1 greater than 100 ml was observed during the study conduct), but the rate of exacerbation in this group was similar to that in 'non-decliners' (
Table 3).
A wide range of exacerbating COPD patient proportions can be found in other real-life studies. In Bulgarian 1-year prospective observational study, the percentage of patients with exacerbations in 12-months period before the study was similar, accounting for approximately 75%[
18]. Two large observational studies conducted in Central and Eastern European countries, Switzerland and Israel, also demonstrated comparable proportions of exacerbating COPD in similar periods [
14,
15]. The POPE study, another observational study conducted in Central and Eastern European countries, demonstrated much lower percentage of exacerbating patients accounting for 37%)[
19]. The German study DACCORD showed that only about 20-25% of COPD patients had exacerbation in 6-months period prior to the study entry[
16,
20]. Another German non-interventional study on 3653 COPD patients reported a 35% frequency of exacerbations in the 2-years period preceding the study entry[
21].
The categorization of patients by two systems of lung function impairment severity evaluation (GOLD and ATS/ERS) showed that there were no patients categorized simultaneously as GOLD-non-severe and ATS/ERS-severe (FEV1> 50% pred. and z-score <-4) (
Figure 3). Patients categorized by the two systems as non-severe statistically significantly more frequently were decliners (
Table 5). Patients classified into different severity lung function groups (non-severe and severe, independent of the method of classification GOLD or ATS/ERS) had similar exacerbation risk, demonstrating that the new ATS/ERS 2022 strategy for evaluation of lung function impairment severity seems inappropriate for the assessment of exacerbation risk.
The classic risk factors for COPD are male sex and tobacco smoking. According to meta-analysis performed in 2018, the prevalence of COPD among women is 6.16% and among men – 9.23%[
22]. In our study men accounted for 67% of enrolled patients and were also more numerous in 'decliners' comparing with non-decliners' (74% vs 64%, p=0.004). Tobacco smoke is the most common cause of COPD, and smoking cessation is recommended for all smoking COPD patients[
23]. In the present study smokers accounted for 35.8%, whereas ex-smokers for 55.2% (
Table 1), which indicates that the vast majority of patients enrolled in our study were exposed to the tobacco smoke in some period of their lives. These results are generally in line with data from other real-life studies showing that at least 75% of studied COPD patients were current or ex-smokers[
14,
15,
16,
18,
19,
20].
In our study, the number of exacerbations and hospitalizations reported by patients at each visit decreased. Other real-life studies have also shown that during the study course, patients usually improve their state, especially in the first 6 months of the study[
15,
20]. It was observed that shortly after enrolment in the study, patients adhered to the treatment very well and therefore experienced significant health improvement. Other patient-related outcomes, such as severity of dyspnea measured by mMRC score (grade 2 in half of patients) and CAT (median score from 15 to 17 points) were generally stable throughout the study (
Figure 2). According to the GOLD 2017-2022 strategy of classification, we identified the following distribution in the study groups A/B/C/D: 6.7%/41.5%/1.4%,/50.4%, respectively. During this study, data on concomitant diseases and medications were collected. Hypertension was the most frequent concomitant disease reported by the study patients, followed by ischemic heart disease, heart failure, diabetes, and hyperlipidemia (
Table 1). The high prevalence of cardiovascular diseases in COPD patients has been reported in other real-life studies[
14,
15,
16,
21]. Other important comorbidities in COPD are depression and anxiety, which are associated with poor prognosis and the risk of exacerbations[
24,
25]. It has been demonstrated that the prevalence of depression in COPD patients is approximately 25% [
26]. In our study, depression and anxiety disorders were diagnosed in approximately 7% of patients, which may suggest the underdiagnosis of these disorders in Polish COPD population. The medication taken during the study was stable (
Table 2), and there were three combinations of ICS/LABA: extrafine beclomethasone/formoterol, fluticasone propionate/salmeterol, or budesonide/formoterol. Data on the impact of ICS/LABA combination on patient state were not gathered in this study; however, it is known that these three combinations are comparable in terms of their effectiveness and safety, and there is no evidence for the superiority of either ICS/LABA combination in the published literature[
27,
28,
29,
30,
31,
32].
This study has several strengths and limitations. The strong point of this study is its observational, real-world design and long duration, enabling collection of patient-related outcomes and data describing everyday life of ICS/LABA-treated COPD patients in Poland. This study is the first to analyze the use of ICS/LABA fixed-dose combination in the Polish COPD population and utilized the new ATS/ERS 2022 recommendation for the evaluation of lung function impairment severity[
6]. The main limitation of the study was the gradual reduction of participants from visit to visit (from 922 at Visit 1 to 151 at Visit 4). The small number of patients at visit 4 impedes proper statistical analysis and weakens the conclusions drawn from the obtained results. However, the reduction in the number of participants is a normal phenomenon in observational studies because, with time, patients lose their interest in participation in the study.