Submitted:
20 May 2025
Posted:
21 May 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
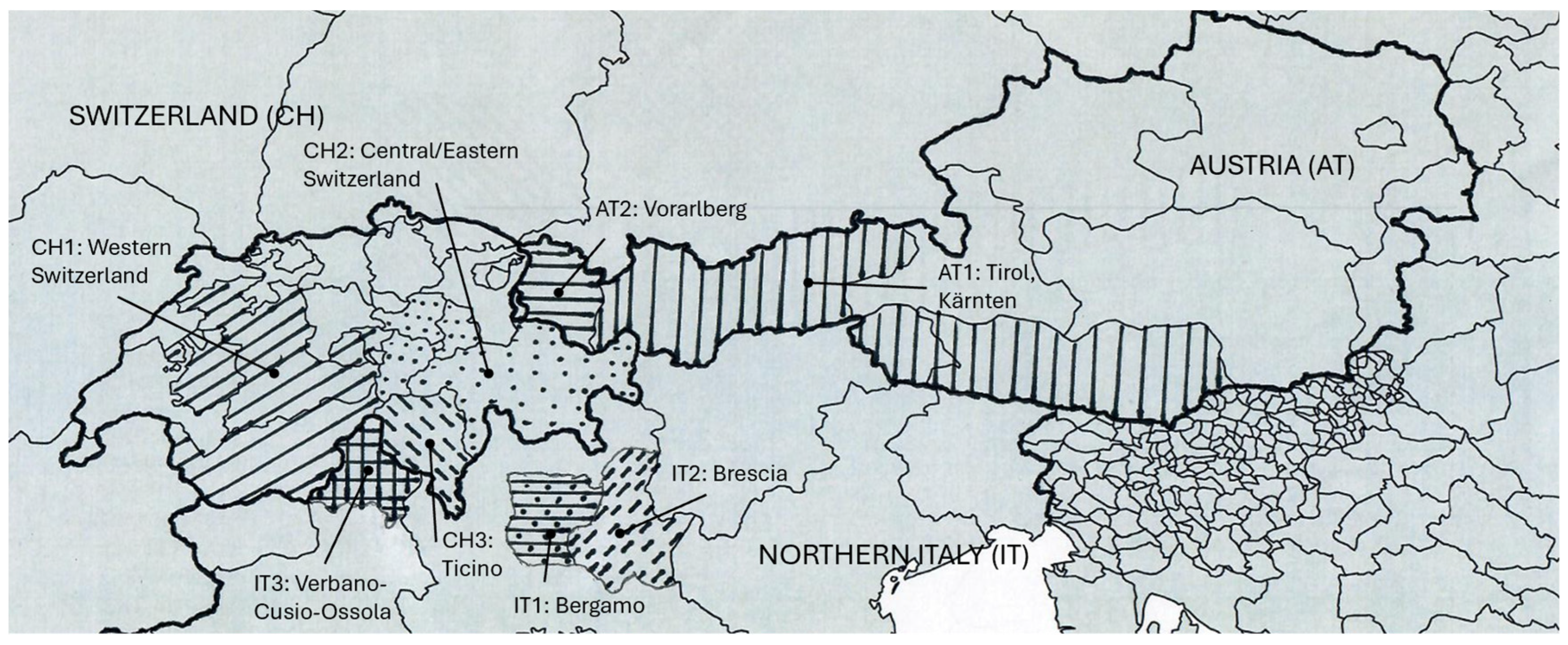
2.1. Sampling and Sample Collection
2.2. Collection of Data on Milk Processing and the Manufacturing Conditions for Cheese
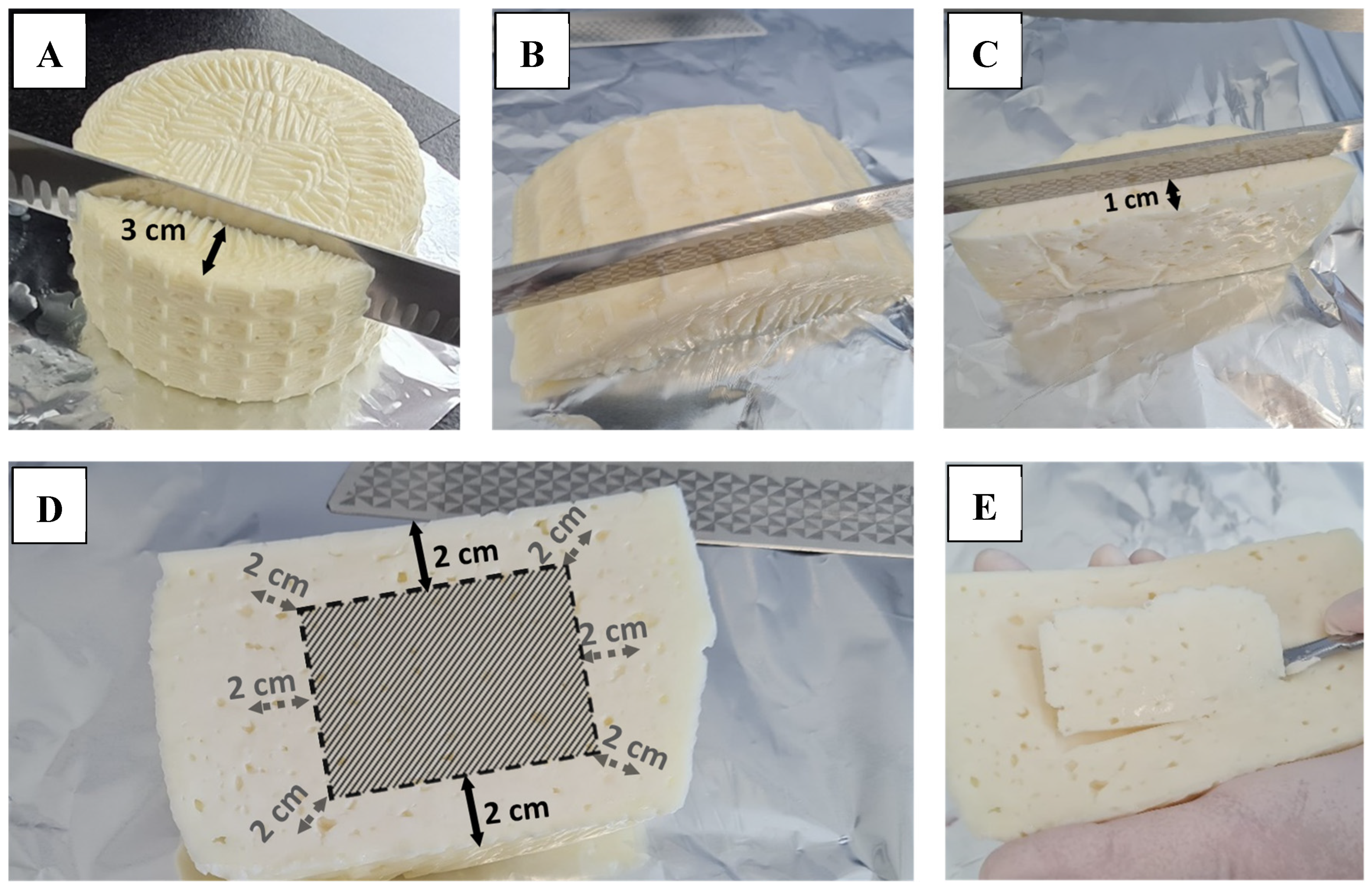
2.3. Enumeration and Isolation of CPS
2.4. CPS Strains Identification
2.5. CPS Genotyping
2.5. SE Analysis in Cheese Samples
2.6. Statistical Analysis
3. Results
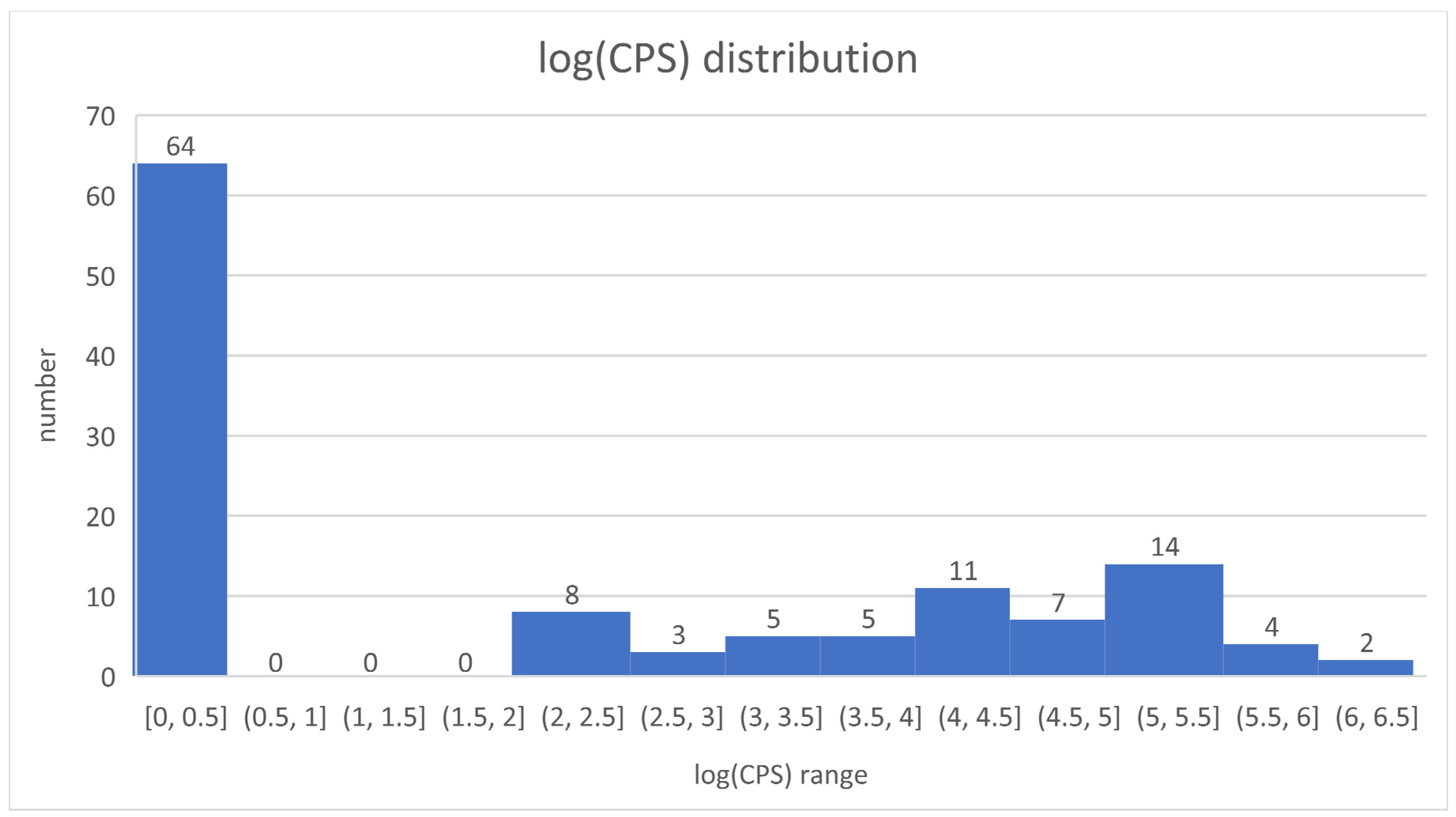
3.1. General Findings
3.2. Differences Between Countries and Regions
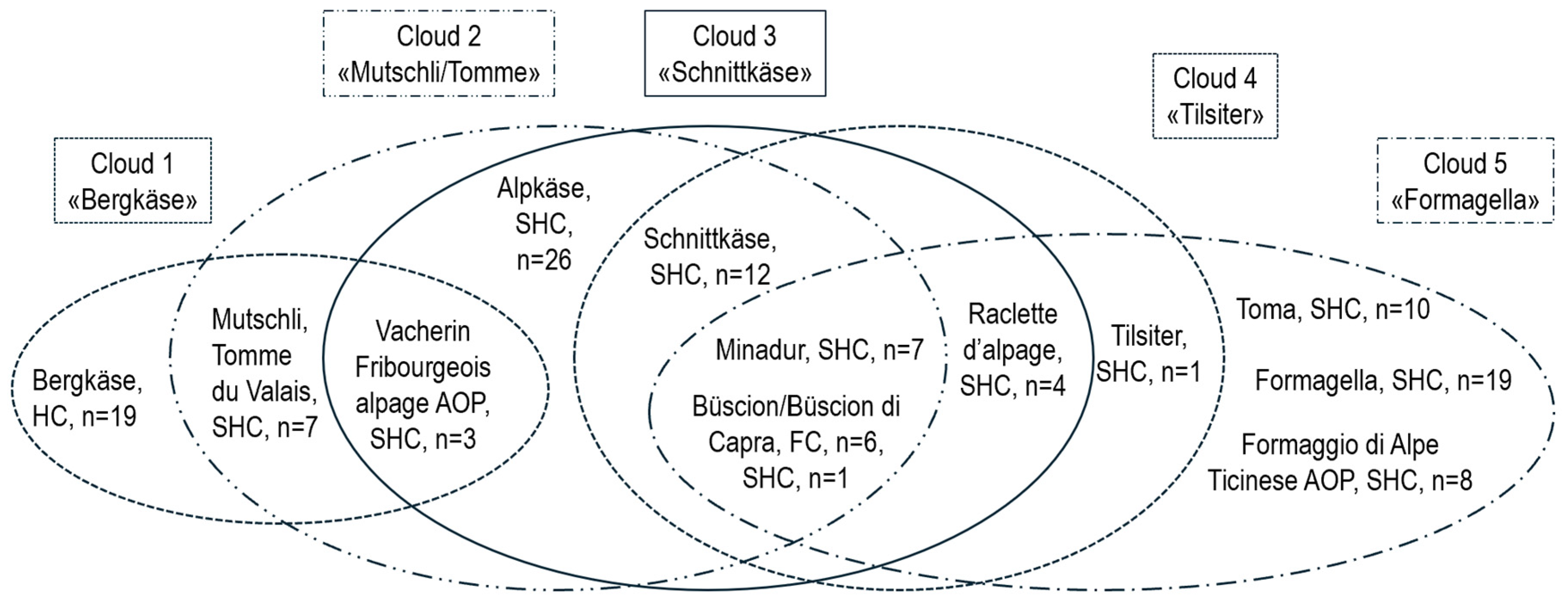
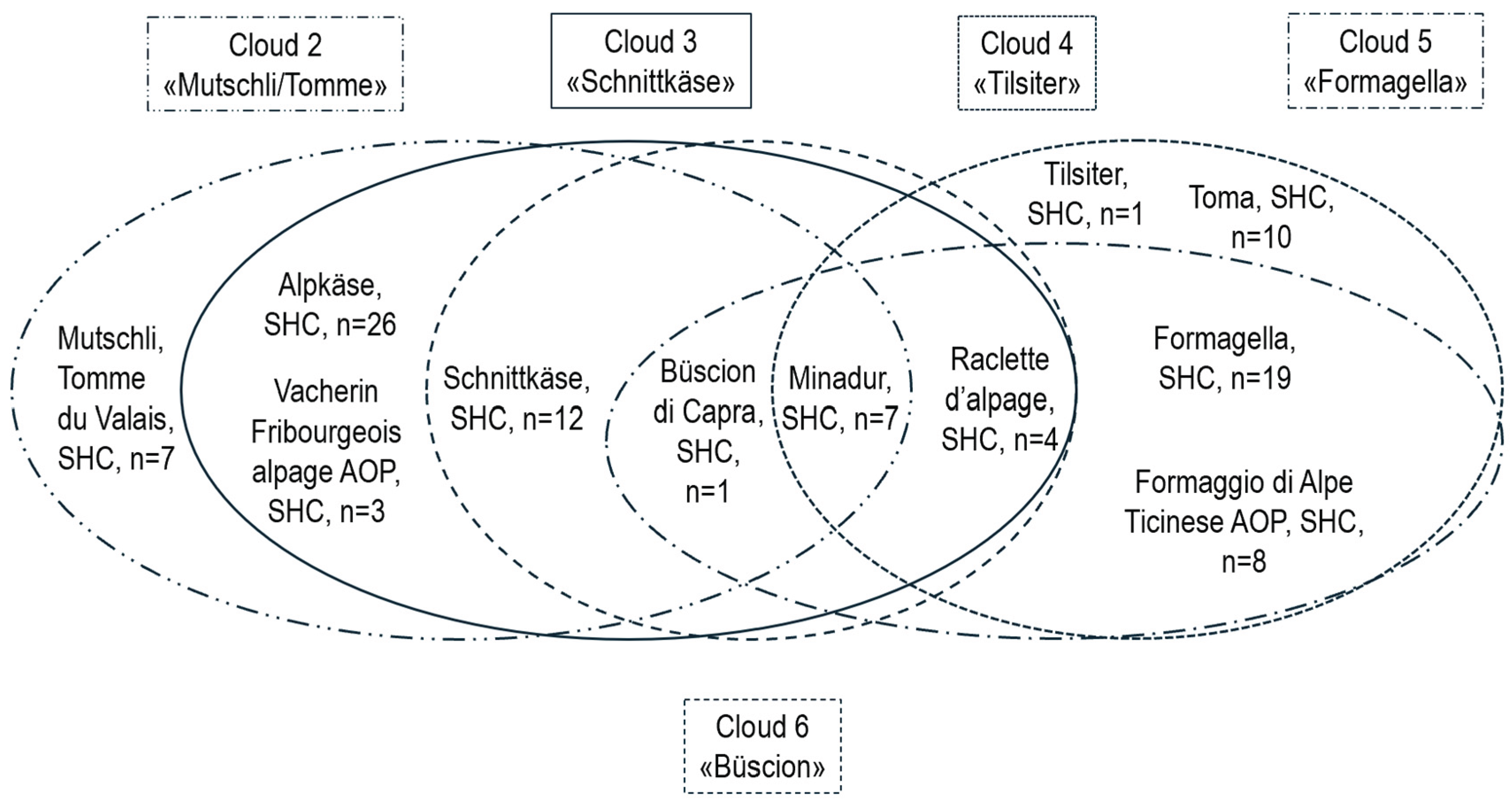
3.3. Differences Between Types of Raw and Thermized Milk and Cheese Varieties
3.4. Differences Between Types of Culture
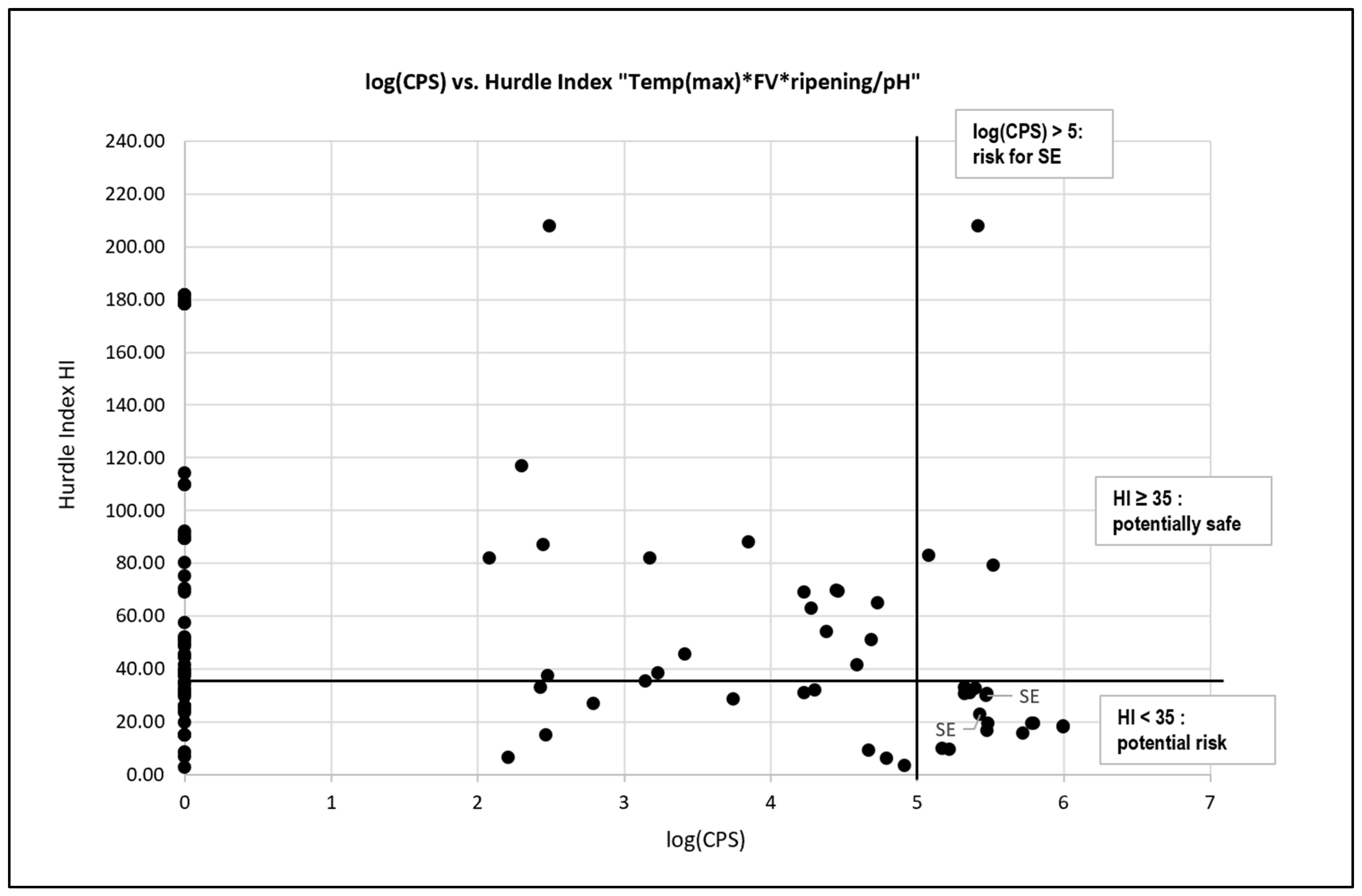
3.5. Calculation of Risk Groups Based on a Selected Number of Hurdle Indices
| HI |
log(CPS) [-] |
FV [-] |
max. temp. [°C] |
ripening [w] |
pH [-] |
sample no. | comment |
| 6.06 | 4.972 | 1 | 28.00 | 1 | 4.62 | 79 | a) |
| 6.56 | 2.206 | 1 | 28.00 | 1 | 4.27 | 78 | a) |
| 9.59 | 5.223 | 0.1 | 37.00 | 14 | 5.40 | 4 | b) |
| 9.77 | 5.170 | 0.1 | 37.00 | 14 | 5.30 | 1 | b) |
| 15.76 | 5.724 | 0.5 | 39.00 | 4 | 4.95 | 15 | b) |
| 22.70 | 5.431 | 1 | 37.00 | 4 | 6.52 | 135 | c) |
| 29.52 | 0 | 1 | 62.00 | 2 | 4.20 | 84 | d) |
| 29.86 | >5.477 | 1 | 43.60 | 4 | 5.84 | 13 | c) |
| 30.71 | >5.477 | 1 | 41.00 | 4 | 5.34 | 11 | e) |
| 34.44 | 0 | 1 | 62.00 | 3 | 5.40 | 83 | d) |
| 34.72 | 0 | 1 | 38.00 | 5.5 | 6.02 | 129 | f) |
| 35 | 0 | 1 | 49 | 4 | 5.6 | ||
| 57.27 | 0 | 1 | 45.10 | 8 | 6.3 | 43 | g) |
| 79.00 | 5.516 | 1 | 39.50 | 12 | 6 | 103 | h) |
| 83.02 | 5.079 | 1 | 55.00 | 8 | 5.30 | 80 | i) |
| 114.17 | 0 | 1 | 42.00 | 14 | 5.15 | 34 | j) |
| 207.69 | 2.493 | 1 | 45.00 | 24 | 5.20 | 86 | k) |
| 207.69 | 5.415 | 1 | 45.00 | 24 | 5.20 | 87 | k) |
4. Discussion
4.1. Prevalence of S. aureus and the Detection of SE in Cheese Samples
4.2. Factors Influencing the Growth of S. aureus and the Production of SEs
4.3. Hurdle Index as a tool for Risk Assessment
| Parameter / Guideline | [47] | [66] | [44] | [65] | |
|---|---|---|---|---|---|
| milk quality of individual dairy animal (either CMT or SCC) | CMT | - | inconspicuous | inconspicuous | inconspicuous |
| SSC | - | <150,000/ml | <150,000/ml | <200,000/ml | |
| frequency | - | regular testing, e.g. every month | regular testing, e.g. 1..2x/month | regular testing, e.g. every 14 days | |
| Scalding temperature [°C] | HC | ≥48 | ≥52 | 50..57 | 50..53 |
| SHC | - | typically <46, eventually thermization | 40..48 | - | |
| FC | - | only with thermized milk | only with pasteurized milk | - | |
| Acidification rate pH [-] | HC | <6.2 (after 2 h) | <6.2 (after 2 h) | <6.2 (after 2 h) | |
| SHC | <6.0 (after 2 h) | ≤5.4 (before salting) | <6.0 (after 2 h) | ||
| FC | <5.0 | <4.5 (after 2 h) | <5.0 (after cutting) | ||
| Ripening time [d] | HC | - | 120 | >120 | - |
| SHC | >60d | >60d | - | - | |
| FC | - | - | - | - | |
5. Conclusion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Seegers, H.; Fourichon, C.; Beaudeau, F. Production effects related to mastitis and mastitis economics in dairy cattle herds. Veterinary Research 2003, 34, 475–491. [Google Scholar] [CrossRef] [PubMed]
- Ruegg, P.L. J. A 100-Year Review: Mastitis detection, management, and prevention. Journal of Dairy Science 2017, 100, 10381–10397. [Google Scholar] [CrossRef]
- Johler, S.; Macori, G.; Bellio, A.; Acutis, P.L.; Gallina, S.; Decastelli, L. Characterization of Staphylococcus aureus isolated along the raw milk cheese production process in artisan dairies in Italy. Journal of Dairy Science 2018, 101, 2915–2920. [Google Scholar] [CrossRef]
- Leuenberger, A.; Sartori, C.; Boss, R.; Resch, G.; Oechslin, F.; Steiner, A.; Moreillon, P.; Graber, H.U. Genotypes of Staphylococcus aureus: On-farm epidemiology and the consequences for prevention of intramammary infections. Journal of Dairy Science 2019, 102, 3295–3309. [Google Scholar] [CrossRef] [PubMed]
- Fournier, C.; Kuhnert, P.; Frey, J.; Miserez, R.; Kirchhofer, M.; Kaufmann, T.; Steiner, A.; Graber, H.U. Bovine Staphylococcus aureus: association of virulence genes, genotypes and clinical outcome. Research in Veterinary Science 2008, 85, 439–448. [Google Scholar] [CrossRef] [PubMed]
- Schwendimann, L.; Berger, T.; Graber, H.U.; Meier, S.; Hummerjohann, J.; Jakob, E. Effect of scalding temperature on growth of Staphylococcus aureus and formation of staphylococcal enterotoxin during the production of alpine cheese in a laboratory cheesemaking model. Journal of Food Protection 2020, 83, 1822–1828. [Google Scholar] [CrossRef]
- Cosandey, A.; Boss, R.; Luini, M.; Artursson, K.; Bardiau, M.; Breitenwieser, F.; Hehenberger, E.; Lam, T.; Mansfeld, M.; Michel, A.; Mösslacher, G.; Naskova, J.; Nelson, S.; Podpečan, O.; Raemy, A.; Ryan, E.; Salat, O.; Zangerl, P.; Steiner, A.; Graber, H.U. Staphylococcus aureus genotype B and other genotypes isolated from cow milk in European countries. Journal of Dairy Science 2016, 99, 529–540. [Google Scholar] [CrossRef]
- Monistero, V.; Graber, H.U.; Pollera, C.; Cremonesi, P.; Castiglioni, B.; Bottini, E.; Ceballos-Marquez, A.; Lasso-Rojas, L.; Kroemker, V.; Wente, N.; Petzer, I.M.; Santisteban, C.; Runyan, J.; Veiga Dos Santos, M.; Alves, B.G.; Piccinini, R.; Bronzo, V.; Abbassi, M.S.; Said, M.B.; Moroni, P. Staphylococcus aureus Isolates from Bovine Mastitis in Eight Countries: Genotypes, Detection of Genes Encoding Different Toxins and Other Virulence Genes. Toxins 2018, 10, 247. [Google Scholar] [CrossRef]
- Graber, H.U.; Naskova, J.; Studer, E.; Kaufmann, T.; Kirchhofer, M.; Brechbuhl, M.; Schaeren, W.; Steiner, A.; Fournier, C. Mastitis-related subtypes of bovine Staphylococcus aureus are characterized by different clinical properties. Journal of Dairy Science 2009, 92, 1442–1451. [Google Scholar] [CrossRef]
- van den Borne, B.H.P.; Graber, H.U.; Voelk, V.; Sartori, C.; Steiner, A.; Haerdi-Landerer, M.C.; Bodmer, M. A longitudinal study on transmission of Staphylococcus aureus genotype B in Swiss communal dairy herds. Preventive Veterinary Medicine 2017, 136, 65–68. [Google Scholar] [CrossRef]
- Monistero, V.; Hossain, D.; Fusar Poli, S.; de Medeiros, E.S.; Cremonesi, P.; Castiglioni, B.; Biscarini, F.; Graber, H.U.; Mochettaz, G.; Ganio, S.; Gazzola, A.; Addis, M.F.; Roullet, C.; Barberio, A.; Deotto, S.; Biasio, L.; Ulloa, F.; Galanti, D.; Bronzo, V.; Moroni, P. Prevalence of Variant GTRI Staphylococcus aureus Isolated from Dairy Cow Milk Samples in the Alpine Grazing System of the Aosta Valley and Its Association with AMR and Virulence Profiles. Antibiotics 2025, 14, 348. [Google Scholar] [CrossRef] [PubMed]
- Sartori, C.; Boss, R.; Bodmer, M.; Leuenberger, A.; Ivanovic, I.; Graber, H.U. Sanitation of Staphylococcus aureus genotype B-positive dairy herds: A field study. Journal of Dairy Science 2018, 101, 6897–6914. [Google Scholar] [CrossRef]
- Sesso, L.; Vanzetti, T.; Weber, J.; Romanò, A.; Bodmer, M.; Vaccani, M.; Riva Scettrini, P.; Sartori, C.; Bacciarini, L.N.; Struchen, R.; Steiner, A.; Ivanovic, I.; Graber, H.U. District-wide herd sanitation and eradication of intramammary Staphylococcus aureus genotype B infection in dairy herds in Ticino, Switzerland. Journal of Dairy Science 2024, 107, 8299–8312. [Google Scholar] [CrossRef]
- De Buyser, M.L.; Dufour, B.; Maire, M.; Lafarge, V. Implication of milk and milk products in food-borne diseases in France and in different industrialised countries. International Journal Food Microbiology 2001, 67, 1–17. [Google Scholar] [CrossRef]
- Bergdoll, M.S. Staphylococcus aureus. In Foodborne Bacterial Pathogens; Doyle, M.P., Ed.; Marcel Dekker: New York, USA, 1989; pp. 463–524. [Google Scholar]
- Asao, T.; Kumeda, Y.; Kawai, T.; Shibata, T.; Oda, H.; Haruki, K.; Nakazawa, H.; Kozaki, S. An extensive outbreak of staphylococcal food poisoning due to low-fat milk in Japan: estimation of enterotoxin A in the incriminated milk and powdered skim milk. Epidemiology & Infection 2003, 130, 33–40. [Google Scholar] [CrossRef]
- Fung, D.Y.; Steinberg, D.H.; Miller, R.D.; Kurantnick, M.J.; Murphy, T.F. Thermal inactivation of staphylococcal enterotoxin B and C. Applied Microbiology 1973, 26, 938–942. https://journals.asm.org/doi/10.1128/am.26.6.938-942.1973. [CrossRef]
- Hennekinne, J.A.; De Buyser, M.L.; Dragacci, S. Staphylococcus aureus and its food poisoning toxins: characterization and outbreak investigation. FEMS Microbiology Reviews 2012, 36, 815–836. [Google Scholar] [CrossRef]
- Lina, G.; Bohach, G.A.; Nair, S.P.; Hiramatsu, K.; Jouvin-Marche, E.; Mariuzza, R. Standard nomenclature for the superantigens expressed by Staphylococcus. The Journal of Infectious Diseases 2004, 189, 2334–2336. [Google Scholar] [CrossRef]
- EFSA and ECDC (European Food Safety Authority and European Centre for Disease Prevention and Control). The European Union One Health 2023 Zoonoses Report. EFSA Journal 2024, 22, e9106. [Google Scholar] [CrossRef]
- Ikeda, T.; Tamate, N.; Yamaguchi, K.; Makino, S. Mass outbreak of food poisoning disease caused by small amounts of staphylococcal enterotoxins A and H. Applied Environmental Microbiology 2005, 71, 2793–2795. [Google Scholar] [CrossRef] [PubMed]
- Benkerroum, N. Staphylococcal enterotoxins and enterotoxin-like toxins with special reference to dairy products: An overview. Critical Reviews in Food Science and Nutrition 2018, 58, 1943–1970. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhu, A.; Tang, J.; Tang, C.; Chen, J. Comparative Effects of Food Preservatives on the Production of Staphylococcal Enterotoxin I from Staphylococcus aureus Isolate. Journal of Food Quality 2017, 15. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhu, A.; Tang, J.; Tang, C.; Chen, J. Identification and measurement of staphylococcal enterotoxin M from Staphylococcus aureus isolate associated with staphylococcal food poisoning. Letters in Applied Microbiology 2017, 65, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Graber, H.U. Genotyping of Staphylococcus aureus by Ribosomal Spacer PCR (RS-PCR). Journal of Visualized Experiments 2016, 117, 54623. [Google Scholar] [CrossRef]
- Hummerjohann, J.; Naskova, J.; Baumgartner, A.; Graber, H.U. Enterotoxin-producing Staphylococcus aureus genotype B as a major contaminant in Swiss raw milk cheese. Journal of Dairy Science 2014, 97, 1305–1312. [Google Scholar] [CrossRef]
- Johler, S.; Weder, D.; Bridy, C.; Huguenin, M.C.; Robert, L.; Hummerjohann, J.; Stephan, R. Outbreak of staphylococcal food poisoning among children and staff at a Swiss boarding school due to soft cheese made from raw milk. Journal of Dairy Science 2015, 98, 2944–2948. [Google Scholar] [CrossRef] [PubMed]
- Kummel, J.; Stessl, B.; Gonano, M.; Walcher, G.; Bereuter, O.; Fricker, M.; Grunert, T.; Wagner, M.; Ehling-Schulz, M. Staphylococcus aureus Entrance into the Dairy Chain: Tracking S. aureus from Dairy Cow to Cheese. Frontiers in Microbiology 2016, 13, 1603. [Google Scholar] [CrossRef] [PubMed]
- Ciupescu, L.M.; Auvray, F.; Nicorescu, I.M.; Meheut, T.; Ciupescu, V.; Lardeux, A.L.; Tanasuica, R.; Hennekinne, J.A. Characterization of Staphylococcus aureus strains and evidence for the involvement of non-classical enterotoxin genes in food poisoning outbreaks. FEMS Microbiology Letters 2018, 365, 13. [Google Scholar] [CrossRef]
- Jørgensen, H.J.; Mørk, T.; Høgåsen, H.R.; Rørvik, L.M. Enterotoxigenic Staphylococcus aureus in bulk milk in Norway. Journal of Applied Microbiology 2005, 99, 158–166. [Google Scholar] [CrossRef]
- Johler, S.; Giannini, P.; Jermini, M.; Hummerjohann, J.; Baumgartner, A.; Stephan, R. Further evidence for staphylococcal food poisoning outbreaks caused by egc-encoded enterotoxins. Toxins 2015, 7, 997–1004. [Google Scholar] [CrossRef]
- Umeda, K.; Nakamura, H.; Yamamoto, K.; Nishina, N.; Yasufuku, K.; Hirai, Y.; Hirayama, T.; Goto, K.; Hase, A.; Ogasawara, J. Molecular and epidemiological characterization of staphylococcal foodborne outbreak of Staphylococcus aureus harboring seg, sei, sem, sen, seo, and selu genes without production of classical enterotoxins. International Journal of Food Microbiology 2017, 256, 30–35. [Google Scholar] [CrossRef]
- Duquenne, M.; Derzelle, S.; Fleurot, I.; Aigle, M.; Darrigo, C.; Hennekinne, J.A.; Mutel, I.; Bouix, M.; Deperrois-Lafarge, V.; Delacroix-Buchet, A. Milk maturation temperature and time are key technological parameters to limit staphylococcal enterotoxin production during uncooked semi-hard cheese manufacture. Food Control 2016, 59, 118–127. [Google Scholar] [CrossRef]
- Jakobsen, R.A.; Heggebo, R.; Sunde, E.B.; Skjervheim, M. Staphylococcus aureus and Listeria monocytogenes in Norwegian raw milk cheese production. Food Microbiology 2011, 28, 492–496. [Google Scholar] [CrossRef] [PubMed]
- Walcher, G.; Gonano, M.; Kümmel, J.; Barker, G.C.; Lebl, K.; Bereuter, O.; Ehling-Schulz, M.; Wagner, M.; Stessl, B. Staphylococcus aureus reservoirs during traditional Austrian raw milk cheese production. Journal of Dairy Research 2014, 81, 462–470. [Google Scholar] [CrossRef]
- EC. Commission Regulation No 178/2002 laying down the general principles and requirements of food law, establishing the European Food Safety Authority and laying down procedures in matters of food safety. Official Journal of the European Union 2002, 31, 1–24. https://eur-lex.europa.eu/eli/reg/2002/178/oj/eng.
- Tiberti, R.; Rogora, M.; Tartari, G.; Callieri, C. Ecological impact of transhumance on the trophic state of alpine lakes in Gran Paradiso National Park. Knowledge and Management of Aquatic Ecosystems 2014, 415, 17. [Google Scholar] [CrossRef]
- UNESCO. Transhumance – sheep drive in the Ötztal alps. Knowledge concerning nature and the universe in Tyrol, inscriebed 2011. https://www.unesco.at/en/culture/intangible-cultural-heritage/national-inventory/news-1/article/transhumance-migratory-herding-of-sheep-in-the-oetztal-alps (accessed on 31 August 2023).
- Primas, M. Bronzezeit zwischen Elbe und Po. Strukturwandel in Zentraleuropa 2200-800 V. Chr. Universitätsforschungen zur Prähistorischen Archäologie, - UPA, Band 150. XII. Habelt, Bonn, Germany, 2008 267 pp. https://www.antikmakler.de/bv8241.
- Glick, F.; Poschlod, P. The origin of alpine farming: A review of archeological, linguistical and archaeobotanical studies in the Alps. The Holocene 2019, 29, 1503–1511. [Google Scholar] [CrossRef]
- Garde, L.; Dimanche, M.; Lasseur, J. Permanence and changes in pastoral farming in the Southern Alps. Journal of Alpine Research | Revue de géographie alpine 2014, 102-2. [Google Scholar] [CrossRef]
- Jurt, C.; Häberli, I.; Rossier, R. Transhumance farming in Swiss mountains: Adaptation to a changing environment. Mountain Research and Development 2015, 35, 57–65. [Google Scholar] [CrossRef]
- Previtali, F. Mountain Anthroscapes, the Case of the Italian Alps. In Sustainable Land Management; Kapur, S., Eswaran, H., Eds.; Blum, W.E.H. Springer: Berlin Heidelberg, Germany, 2011; pp. 143–161. [Google Scholar]
- Saha, S.; Amalfitano, N.; Sturaro, E.; Schiavon, S.; Tagliapietra, F.; Bittante, G.; Carafa, I.; Franciosi, E.; Gallo, L. Effects of Summer Transhumance of Dairy Cows to Alpine Pastures on Body Condition, Milk Yield and Composition, and Cheese Making Efficiency. Animals 2019, 9, 4–192. [Google Scholar] [CrossRef]
- Mulet-Cabero, A.-I.; Torres-Gonzalez, M.; Geurts, J.; Rosales, A.; Farhang, B.; Marmonier, C.; Ulleberg, E.K.; Hocking, E.; Neiderer, I.; Gandolfi, I.; Anderson, L.; Brader, L.; Vermaak, M.; Cameron, M.; Christensen, M.M.; Haryono, R.; Peters, S. The Dairy Matrix: Its Importance, Definition, and Current Application in the Context of Nutrition and Health. Nutrients 2024, 16, 2908. [Google Scholar] [CrossRef]
- Jakob, E.: Menéndez Gonzàlez, S. Leitlinie für die gute Verfahrenspraxis bei der Milchgewinnung und -verarbeitung in Sömmerungsbetrieben. Schweizerischer Alpwirtschaftlicher Verband SAV, Berne, Switzerland, 2015, 127 pp. https://www.alpwirtschaft.ch/sav-branchenleitlinie/.
- BMG. Leitlinie für eine gute Hygienepraxis und die Anwendung der Grundsätze des HACCP bei der Milchverarbeitung auf Almen. BMG-75220/0054-IV/B/10/2005 2005, 19.12.2005. https://www.verbrauchergesundheit.gv.at/Lebensmittel/buch/hygieneleitlinien/LL_Milchverarbeitung_auf_Almen.pdf?a2d5pz.
- Leistner, L. Basic aspects of food preservation by hurdle technology. International Journal of Food Microbiology 2000, 55, 181–186. [Google Scholar] [CrossRef]
- Herzog, F.; Seidl, I. Swiss alpine summer farming: current status and future development under climate change. The Rangeland Journal 2018, 40, 501–511. [Google Scholar] [CrossRef]
- De Respinis, S.; Caminada, A.; Pianta, E.; Buetti-Dinh, A.; Riva Scettrini, P.; Petrini, L.; Tonolla, M.; Petrini, O. Fungal communities on alpine cheese rinds in Southern Switzerland. Botanical Studies 2023, 64, 6. [Google Scholar] [CrossRef]
- EC. Commission Regulation No 853/2004 laying down specific hygiene rules for food of animal origin. Official Journal of the European Union 2004, 39, 55–205. https://eur-lex.europa.eu/eli/reg/2004/853/oj/eng.
- Bachmann, H.-P.; Spahr, U. The fate of potentially pathogenic bacteria in Swiss hard and semihard cheeses made from raw milk. Journal of Dairy Science 1995, 78, 476–483. https://www.journalofdairyscience.org/article/S0022-0302(95)76657-7/pdf. [CrossRef]
- Schmitt, M.; Schuler-Schmid, U.; Schmidt-Lorenz, W. Temperature limits of growth, TNase and enterotoxin production of Staphylococcus aureus strains isolated from foods. International Journal of Food Microbiology 1990, 11, 1–19. [Google Scholar] [CrossRef]
- Troller, J.A.; Stinson, J.V. Influence of water activity on growth and enterotoxin formation by Staphylococcus aureus in foods. Journal of Food Science 1975, 40, 802–804. [Google Scholar] [CrossRef]
- Hennekinne, J.-A. Chapter 7 - Staphylococcus aureus as a Leading Cause of Foodborne Outbreaks Worldwide. In Staphylococcus aureus; Fetsch, A., Ed.: Academic Press, Elsevier: Amsterdam, The Netherlands, 2018; pp. 129–146. ISBN 9780128096710. [Google Scholar] [CrossRef]
- Le Marc, Y.; Valík, L.; Medveďová, A. Modelling the effect of the starter culture on the growth of Staphylococcus aureus in milk. International Journal of Food Microbiology 2009, 129, 306–311. [Google Scholar] [CrossRef]
- Jameson, J.E. A discussion of the dynamics of salmonella enrichment. Journal of Hygiene 1962, 60, 193–207. [Google Scholar] [CrossRef]
- Smith, J.L.; Buchanan, R.L.; Palumbo, S.A. Effect of Food Environment on Staphylococcal Enterotoxin Synthesis: A Review. Journal of Food Protection 1983, 46, 545–555. [Google Scholar] [CrossRef]
- Genigeorgis, C.A. Present state of knowledge on staphylococcal intoxication. International Journal of Food Microbiology 1989, 9, 327–360. [Google Scholar] [CrossRef]
- Taitini, S.R. Influence of Food Environments on Growth of Staphylococcus aureus and Production of Various Enterotoxins. Journal of Milk and Food Technology 1973, 36, 559–563. https://www.sciencedirect.com/science/article/pii/S0362028X23065407. [CrossRef]
- Mullan, W.M.A. Use of starter concentrates in fermented dairy product manufacture. 2006. Available online: https://www.dairyscience.info/index.php/cheese-starters/108-starter-concentrates.html (accessed on 13 April 2025).
- Pretto, A.N.; Reck, C.; Menin, A.; Sant’Anna, V. Kinetic modeling of inactivation of foodborne bacterial pathogens in serrano artisanal cheese during ripening. Brazilian Journal of Food Technology 2021, 24, e2019322. [Google Scholar] [CrossRef]
- Romanò, A.; Gazzola, A.; Bianchini, V.; Cortimiglia, C.; Maisano, A.M.; Cremonesi, P.; Graber, H.U.; Vezzoli, F.; Luini, M. Staphylococcus aureus From Goats Are Genetically Heterogeneous and Distinct to Bovine Ones. Frontiers in Veterinary Science 2020, 7. https://www.frontiersin.org/journals/veterinary-science/articles/10.3389/fvets.2020.00628. [CrossRef]
- FACE. European Guide for Good Hygiene Practices in the production of artisanal cheese and dairy products Target: - Farmhouse and Artisan producers. FACE Network, 2016. Available online: https://food.ec.europa.eu/system/files/2017-12/biosafety_fh_guidance_artisanal-cheese-and-dairy-products_en.pdf (accessed on 19 April 2025).
- Matlschweiger, C. Hygiene in der Milchverarbeitung auf der Alm. Ländliches Fortbildungs Institut LFI, Schauflergasse 6, 1015 Vienna, Austria, 2022, 31 pp. https://www.almwirtschaft.com/wp-content/uploads/2022/11/Hygiene-in-der-Milchverarbeitung-auf-der-Alm.pdf.
- Anonymous. Milchprodukte von der Alp – schmackhaft und sicher! Empfehlung für Alp-Berater. ALP forum 2012, Nr. 92. https://www.agroscope.admin.ch/agroscope/de/home/publikationen/suchen/reihen-bis-2013/alp-forum.html.
- Lindquist, R. Estimation of Staphylococcus aureus Growth Parameters from Turbidity Data: Characterization of Strain Variation and Comparison of Methods. Applied and Environmental Microbiology 2006, 72, 4862–4870. https://journals.asm.org/doi/10.1128/aem.00251-06. [CrossRef]
- Cattaneo, S.; Masotti, F.; Silvetti, T.; Susca, A.; Tringali, S.; Tamburini, A.; Brasca, M. La stagionatura in miniera quale strategia di tipizzazione del formaggio. Il Latte 2023, 98, 54–61. https://iris.cnr.it/handle/20.500.14243/461482.
- Zeppa, G.; Alessandria, V.; Scursatone, B.; Rantsiou, K.; Cocolin, L.S. Approccio multidisciplinare per la selezione di ceppi autoctoni per la produzione di Toma piemontese artigianale. SCIENZA E TECNICA LATTIERO-CASEARIA 2011, 62, 353–359. [Google Scholar]
| 1 | Phosphatase-positive milk. |
| 2 | See also the region groups in Table S2a. |
| 3 | The temperatures given in Table S2a exceed, in some cases, those specified in Regulation (EC) No. 853/2004 [51]. However, it is also specified in Regulation (EC) No. 853/2004 that food business operators need not comply with the temperature requirements laid down in points 2 and 3 if the milk meets the criteria provided for in Part III and either (a) the milk is processed within two hours of milking or (b) a higher temperature is necessary for technological reasons related to the manufacture of certain dairy products and the competent authority so authorises. |
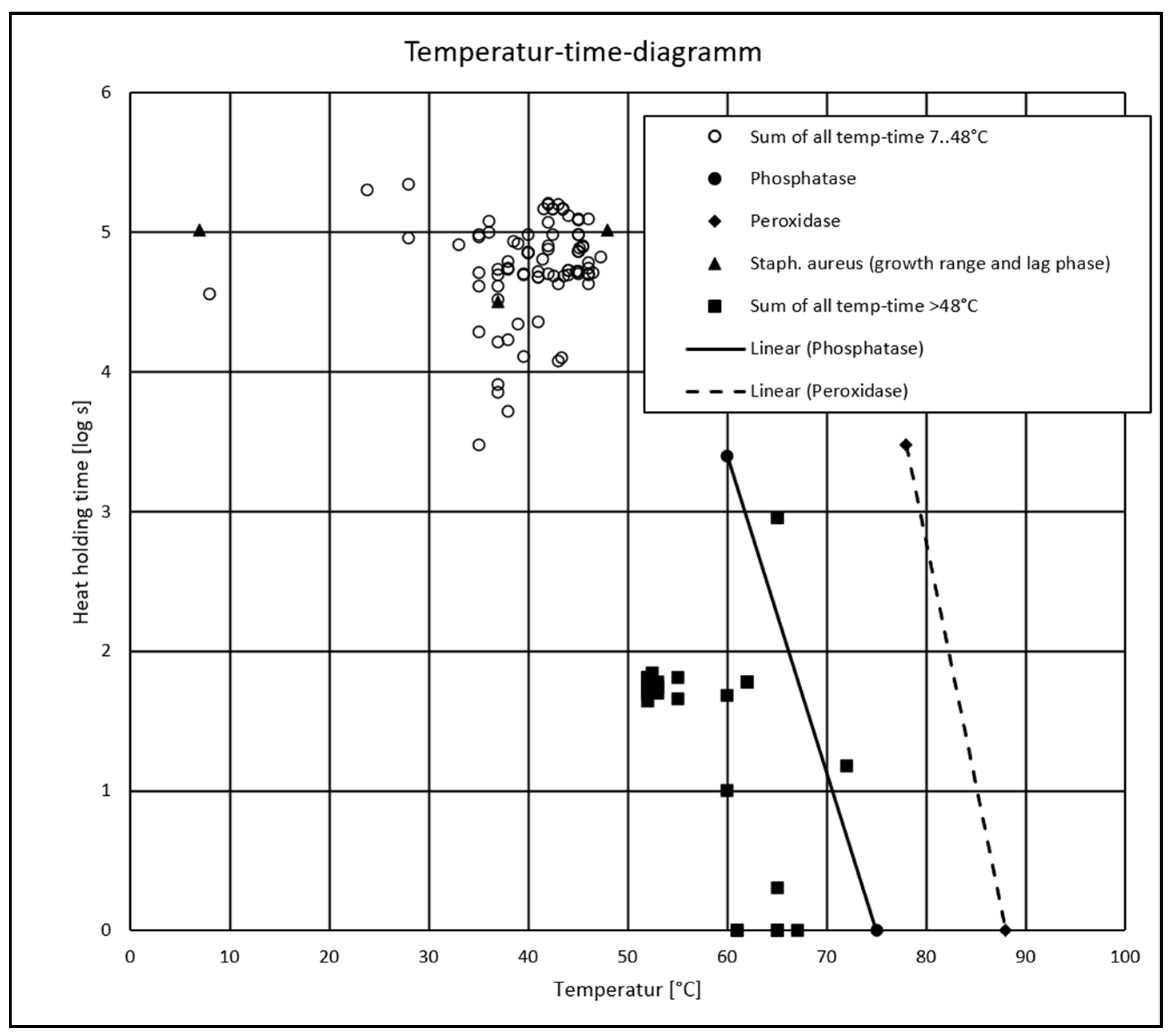
| 4 | To estimate the critical range of S. aureus growth (temperature-time), the data obtained from Taitini [60] (S. aureus grows between 7 and 48 °C, with temperature being optimal at around 37 °C) and Lindquist [67] (the mean lag times ranged from 8. 8 to 19.5 h for strain S30 and from 12.2 to 28.7 h for strain S119) are used. There was minimum growth from 7 °C and maximal growth up to 48 °C, with the largest lag time of 28.7 h, and optimal growth at 37 °C, with the smallest lag time of 8.8 h. The growth of S. aureus can be expected from these timepoints on at the latest if no other limiting factors are present. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





