1. Introduction
Transition metal nitrides have attracted significant research attention as potential high-energy density materials (HEDMs).[
1,
2,
3,
4,
5] Copper nitrides, in particular, have been investigated as HEDMs up to 50 GPa, where the CuN
2 phase has been previously observed.[
6] However, the limitations of powder X-ray diffraction, such as difficulties in accurately determining oxidation states and N–N bond lengths, have led researchers to rely on density functional theory (DFT) calculations to infer these properties.[
6] In contrast, single-crystal X-ray diffraction provides superior precision in resolving crystal structures and atomic positions, especially for compounds synthesized under high- pressure conditions.[
7,
8] The N-N bond length in polynitrides significantly influences related physical properties such as metallicity and bulk modulus.[
2,
7] Therefore, single-crystal X-ray diffraction of the CuN
2 phase is essential for elucidating its crystal chemistry under high-pressure conditions.
2. Results
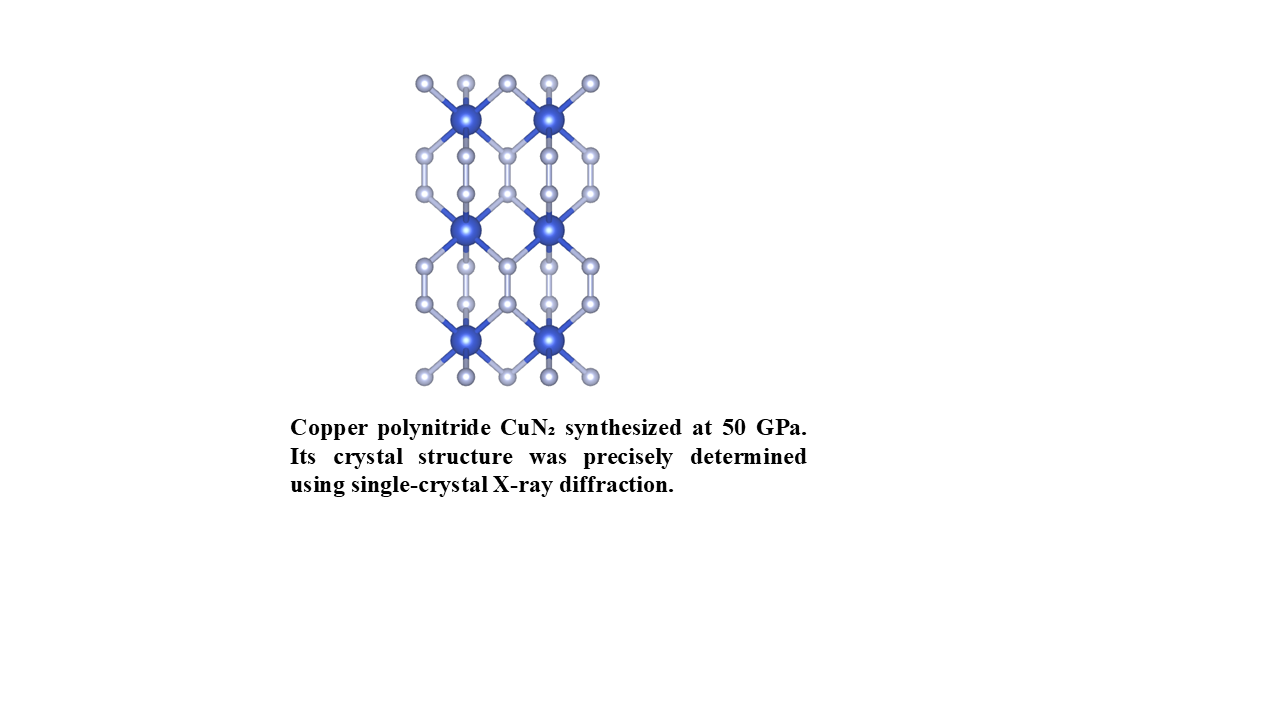
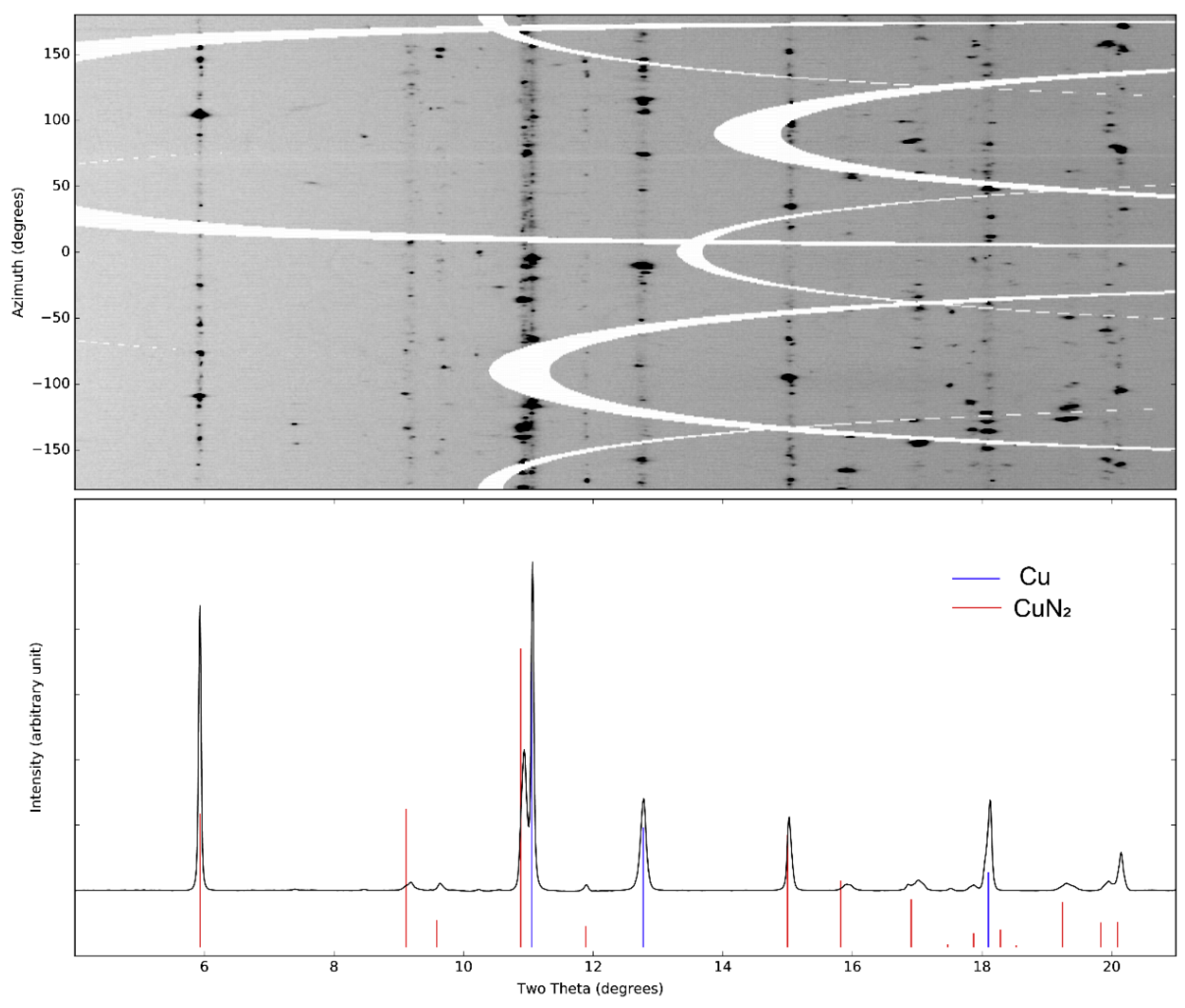
Using powder diffraction, the crystal structure of CuN₂ was indexed to a hexagonal phase with lattice parameters a = 2.70(1) Å and c = 7.21(1) Å at 50 GPa as determined with UNITCELL.[
9] These results are based on X-ray diffraction data presented in
Figure 1. The starting materials, copper and nitrogen, were also identified in the diffraction patterns.[
10] The refined crystal structure of CuN
2, resolved using single-crystal X-ray diffraction, is shown in
Figure 2. The refined crystallographic data for CuN₂ at 50 GPa are presented in
Table 1.
3. Discussion
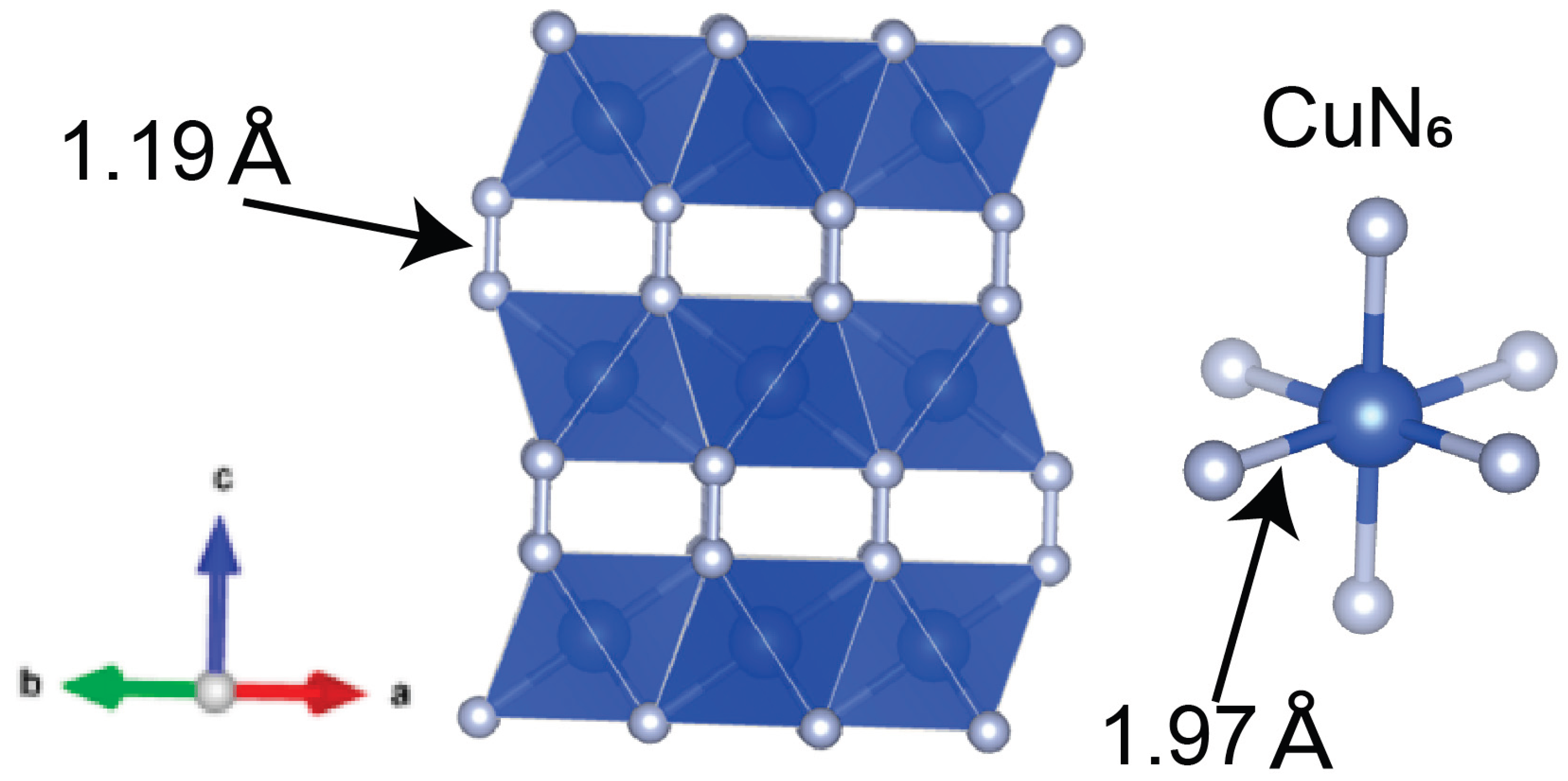
Our single-crystal X-ray diffraction results are generally consistent with previous powder X-ray diffraction studies conducted at similar pressures.[
6] We confirmed the crystal structure and atomic bonding in the CuN₂ phase. However, unlike powder diffraction, single-crystal X-ray diffraction allows for precise determination of bond distances. The N–N bond length was determined to be 1.19(1) Å, suggesting the presence of a (N-N)
2- unit.[
2,
7] This value closely matches DFT calculations, which report an N–N bond length of 1.197 Å.[
6] Additionally, the Cu–N bond distance was determined to be 1.97(1) Å. No evidence of a Jahn–Teller distortion was observed, indicating that the oxidation state of copper in CuN₂ is likely not +2. Given the critical role of N–N bonding in determining the oxidation state of copper and the physical properties of polynitrides—such as metallicity and bulk modulus—we estimated the copper oxidation state using an empirical equation.[
2] The equation relates N–N bond length (BL) to the formal charge (FC) of the N₂ⁿ⁻ unit: BL = 0.074(1) Å · FC + 1.104 Å. Using the measured N–N bond length of 1.19(1) Å for CuN₂ at 50 GPa, we calculate the formal charge of the N–N unit to be approximately –1.2(1). This value is consistent with DFT calculations, which estimate the copper oxidation state to lie between +1 and +2.[
6]
4. Materials and Methods
Diamond anvil cell experiments: A rhenium (Re) gasket was indented, and a center hole was drilled to form a sample chamber. The indented gasket was placed between two opposing diamond anvils. Copper metal was loaded into the sample chamber, and high-purity nitrogen gas was then introduced using the gas-loading system at the Earth and Planetary Laboratory (EPL), Carnegie Science. The DAC was then compressed to target pressures for subsequent laser heating.
Laser heating experiments were conducted at EPL. In a typical experiment, an infrared laser was directed onto the sample, and X-ray diffraction data were collected after laser heating. The X-ray wavelength used was 0.3738 Å at beamline ID27 of the European Synchrotron Radiation Facility (ESRF).[
11] Two-dimensional diffraction images were integrated into one-dimensional patterns using Dioptas[
12] software. We used 1s exposure time for collecting powder X-ray diffraction patterns. A 1-second exposure time was used for collecting powder X-ray diffraction patterns. The resulting diffraction patterns were plotted using Peakpo.[
13]
Single-crystal X-ray diffraction data were acquired from selected spots after laser heating. The diamond anvil cell was rotated up to ±30° to collect single-crystal diffraction data. Orthoenstatite crystals were used to calibrate the single-crystal diffraction setup. The orthoenstatite, with the composition (Mg
1.93Fe
0.06)(Si
1.93Al
0.06)O
6 crystallizes in the orthorhombic space group,
Pbca with lattice parameters a = 8.812(1) Å, b = 5.183(1) Å, and c = 18.239(1) Å. Data integration and reduction were performed using CrysAlisPro.[
14,
15] The crystal structure was solved using Olex2 with the intrinsic phasing method.[
15,
16] The atomic position of Cu was resolved, and electron density maps were examined for residual electron density, which was interpreted as potential nitrogen positions. The structure was refined iteratively until the calculated and observed single-crystal X-ray diffraction patterns were in agreement. A typical weighted R-factor (Rwp) of less than 10% was achieved, indicating a reliable structural fit.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org.
Author Contributions
Conceptualization, HC, AFG and MFM.; methodology, GG and MM.; software, AFG.; validation HC, AFG and MFM.; formal analysis, HC.; investigation, HC.; resources, MFM.; data curation, AFG.; writing—original draft preparation, HC.; writing—review and editing, AFG.; visualization, HC.; supervision, AFG.; project administration, AFG and MFM.; funding acquisition, AFG and MFM. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Science Foundation under grants DMR-2200670, CHE-2302437 (PIs: Mohammad F. Mahmood and Alexander F. Goncharov).
Data Availability Statement
Deposition Numbers 2444907 contain the
supplementary crystallographic data for this paper. These data can be obtained free of charge via the joint Cambridge Crystallographic Data Centre (CCDC) and Fachinformationszentrum Karlsruhe.
Acknowledgments
This work was supported by the National Science Foundation under grants DMR-2200670, CHE-2302437 (PIs: Mohammad F. Mahmood and Alexander F. Goncharov) and Carnegie Science. We acknowledge the European Synchrotron Radiation Facility (ESRF), beamline ID27, for the provision of synchrotron radiation facilities under proposal numbers MA5485, CH6476, CH6817.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Bykov, M.; Bykova, E.; Aprilis, G.; Glazyrin, K.; Koemets, E.; Chuvashova, I.; Kupenko, I.; McCammon, C.; Mezouar, M.; Prakapenka, V.; et al. Fe-N System at High Pressure Reveals a Compound Featuring Polymeric Nitrogen Chains. Nat. Commun. 2018, 9, 2756. [Google Scholar] [CrossRef] [PubMed]
- Aslandukov, A.; Aslandukova, A.; Laniel, D.; Koemets, I.; Fedotenko, T.; Yuan, L.; Steinle-Neumann, G.; Glazyrin, K.; Hanfland, M.; Dubrovinsky, L.; et al. High-Pressure Yttrium Nitride, Y5N14, Featuring Three Distinct Types of Nitrogen Dimers. J. Phys. Chem. C 2021, 125, 18077–18084. [Google Scholar] [CrossRef]
- Laniel, D.; Dewaele, A.; Garbarino, G. High Pressure and High Temperature Synthesis of the Iron Pernitride FeN2. Inorg. Chem. 2018, 57, 6245–6251. [Google Scholar] [CrossRef]
- Laniel, D.; Winkler, B.; Koemets, E.; Fedotenko, T.; Bykov, M.; Bykova, E.; Dubrovinsky, L.; Dubrovinskaia, N. Synthesis of Magnesium-Nitrogen Salts of Polynitrogen Anions. Nat Commun 2019, 10, 4515. [Google Scholar] [CrossRef] [PubMed]
- Niwa, K.; Terabe, T.; Kato, D.; Takayama, S.; Kato, M.; Soda, K.; Hasegawa, M. Highly Coordinated Iron and Cobalt Nitrides Synthesized at High Pressures and High Temperatures. Inorg. Chem. 2017, 56, 6410–6418. [Google Scholar] [CrossRef] [PubMed]
- Binns, J.; Donnelly, M.-E.; Peña-Alvarez, M.; Wang, M.; Gregoryanz, E.; Hermann, A.; Dalladay-Simpson, P.; Howie, R.T. Direct Reaction between Copper and Nitrogen at High Pressures and Temperatures. J. Phys. Chem. Lett. 2019, 10, 1109–1114. [Google Scholar] [CrossRef] [PubMed]
- Laniel, D.; Winkler, B.; Fedotenko, T.; Aslandukova, A.; Aslandukov, A.; Vogel, S.; Meier, T.; Bykov, M.; Chariton, S.; Glazyrin, K.; et al. High-Pressure Na3(N2)4, Ca3(N2)4, Sr3(N2)4, and Ba(N2)3 Featuring Nitrogen Dimers with Noninteger Charges and Anion-Driven Metallicity. Phys. Rev. Materials 2022, 6, 023402. [Google Scholar] [CrossRef]
- Aslandukov, A.; Trybel, F.; Aslandukova, A.; Laniel, D.; Fedotenko, T.; Khandarkhaeva, S.; Aprilis, G.; Giacobbe, C.; Lawrence Bright, E.; Abrikosov, I.A.; et al. Anionic N18 Macrocycles and a Polynitrogen Double Helix in Novel Yttrium Polynitrides YN6 and Y2N11 at 100 GPa. Angew. Chem. Int. Ed. 2022, 61, e202207469. [Google Scholar] [CrossRef] [PubMed]
- Holland, T.J.B.; Redfern, S.A.T. UNITCELL : A Nonlinear Least-Squares Program for Cell-Parameter Refinement and Implementing Regression and Deletion Diagnostics. J Appl Crystallogr 1997, 30, 84–84. [Google Scholar] [CrossRef]
- Laniel, D.; Trybel, F.; Aslandukov, A.; Spender, J.; Ranieri, U.; Fedotenko, T.; Glazyrin, K.; Bright, E.L.; Chariton, S.; Prakapenka, V.B.; et al. Structure Determination of ζ-N2 from Single-Crystal X-Ray Diffraction and Theoretical Suggestion for the Formation of Amorphous Nitrogen. Nat Commun 2023, 14, 6207. [Google Scholar] [CrossRef] [PubMed]
- Garbarino, G.; Hanfland, M.E.; Gallego-Parra, S.; Rosa, A.D.; Mezouar, M.; Duran, D.; Martel, K.; Papillon, E.; Roth, T.; Got, P.; et al. Extreme Conditions X-Ray Diffraction and Imaging Beamline ID15B on the ESRF Extremely Brilliant Source. High Pressure Research 2024, 44, 199–216. [Google Scholar] [CrossRef]
- Prescher, C.; Prakapenka, V.B. DIOPTAS: A Program for Reduction of Two-Dimensional X-Ray Diffraction Data and Data Exploration. High Pressure Research 2015, 35, 223–230. [Google Scholar] [CrossRef]
- Shim, S.-H. PeakPo - A Python Software for X-Ray Diffraction Analysis at High Pressure and High Temperature; 2017;
- Oxford Diffraction Ltd. Yarnton, England 2009.
- Sheldrick, G.M. A Short History of SHELX. Acta Cryst A 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. SHELXT – Integrated Space-Group and Crystal-Structure Determination. Acta Crystallogr A Found Adv 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).