The National Essential Medicine System is the foundation of China's pharmaceutical supply chain and healthcare security framework and an important part of the medical and health services [
1,
2]. Since the implementation of the National Essential Medicines List in 2009, the implementation of the essential medicines system in medical and health institutions was completed in 2011, establishing the initial framework of the national essential medicines system [
3,
4]. In 2020, all medical institutions across the nationwide country began to fully implement the National Essential Medicine System [
5,
6]. National essential medicines are medicines that meet basic medical and health care needs, are in appropriate dosage forms, are reasonably priced, are guaranteed in supply, and the public can obtain urgently needed medical treatment drugs openly, fairly and impartially. With the increase in the variety of drugs and the introduction of the 2018 version of the National Essential Medicines List policy, the needs of the people have been met, enhanced medication safety protocols and management of essential medicines have been improved [
1], and the use and service of national essential medicines have been promoted. With the optimization and adjustment of the National Essential Medicines List, The National Essential Medicines List is an important basis for the allocation and use of medicines by medical and health institutions at all levels in the city [
5,
6,
7]. The list has effectively changed the reform of medical and health care and guaranteed the medical needs of the people (patients' medication needs), and actively alleviated the problems of "difficulty and high cost in accessing medical care" [
6]. At present, it is necessary to strengthen the management of the use of national essential medicines in medical institutions, increase the usage volume and proportion of national essential medicines [
1], and actively explore comparative analysis in aspects such as the types of national essential medicines, drug categories, drug names, drug dosage forms, specification distribution, prescription qualification requirements and usage restrictions [
5,
6]. We should conduct in-depth research on the efficacy, variety, distribution of dosage forms and the current situation of use in relevant departments of traditional Chinese patent medicine prescriptions [
8], and continuously incorporate the use of national essential medicines as an important part of prescription evaluation [
1]. At the same time, training the prescriptions (physicians, pharmacists) and managers on the use of drugs and the application of prescriptions is of great practical significance for confusing drug varieties and the selection [
1,
6], production and circulation, bidding and procurement, drug use, payment and reimbursement, and supervision and tracking of national essential drugs.
To understand the impact of the 2018 version of the National Essential Medicines List policy on the use of essential medicines in tertiary public medical institutions, and to provide reference for promoting the further implementation of the national essential medicines system and related policies, This study investigated and analyzed the changes in the specific varieties of national essential medicines and the differences in the overall use of national essential medicines in a certain tertiary public medical institution in Zunyi City at different times.
1. Data and Methods
1.1. Sources of Data and Methods of Investigation
In this study, the Hospital information System (HIS) and Excel 2020 software were used to calculate the specific varieties of national essential medicines equipped in a certain tertiary public medical institution in Zunyi City at different times. According to the implementation of the 2018 version of the Essential Medicine list, the drug procurement data were calculated from November 2018 to December 2023. The specific varieties equipped with national essential medicines were counted in two parts: Western medicine and Chinese patent medicine, using SPSS 22.0 software.
1.2. Survey Items and Indicators
The overall use of national essential medicines is reflected by the following five prescription indicators: The proportion of essential drug varieties (the number of national essential drug varieties/the total number of drug procurement varieties × 100%), the proportion of essential drugs in centralized procurement drugs (the number of essential drug varieties/the number of centralized procurement drug varieties × 100%), the prescription rate of essential drugs (the number of prescriptions including essential drugs/the total number of prescriptions × 100%), and the prescription rate of all essential drugs (the number of prescriptions of all essential drugs/the total number of prescriptions ×1) 00% and the proportion of medical insurance drugs =(the number of drug types in the medical insurance directory/the number of drug procurement varieties × 100%).
2. Research Results
2.1. Changes in the Specific Varieties of Drugs Included Since the Implementation of the 2018 Essential Medicines List
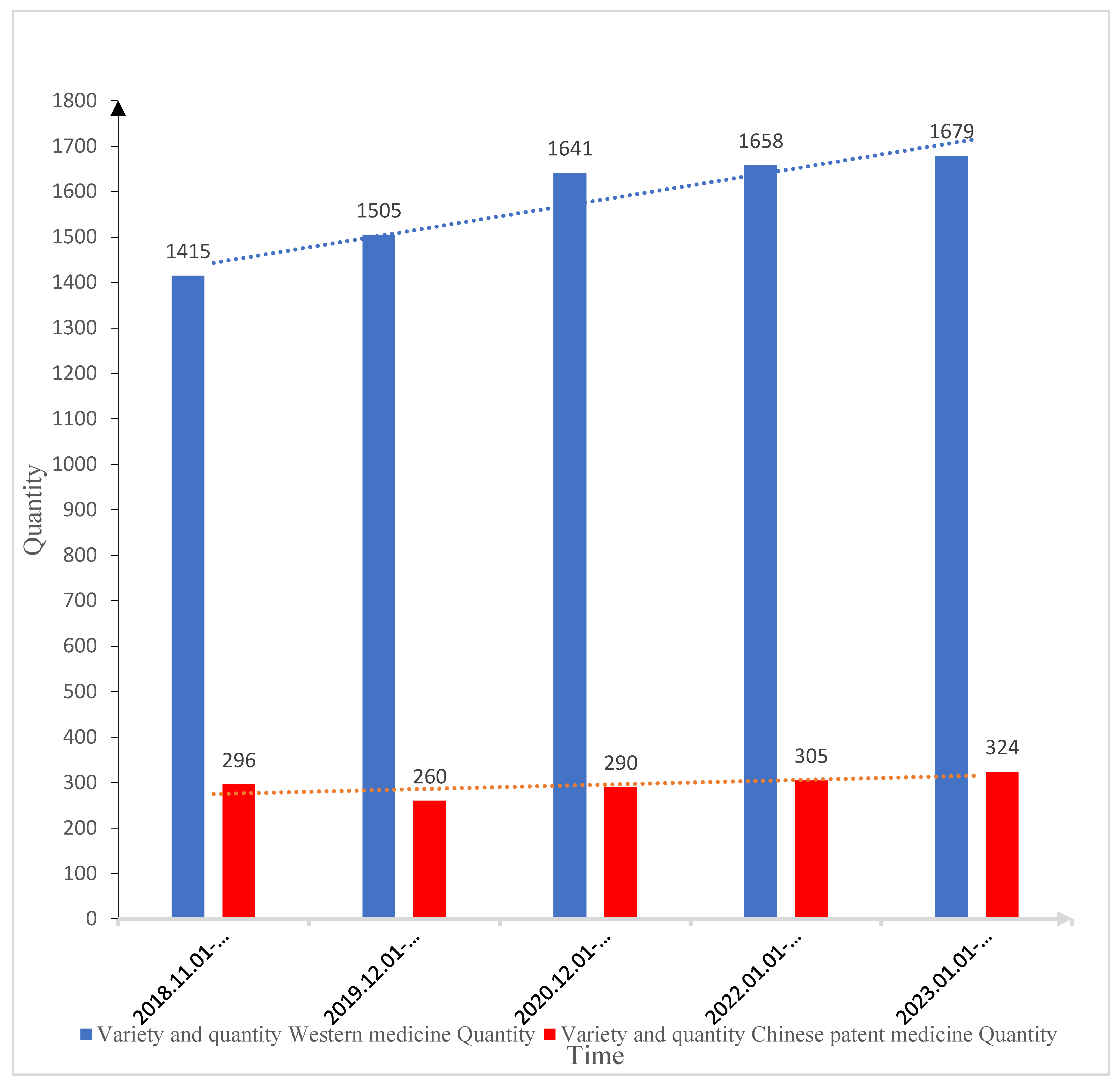
It was found through analysis that the number of varieties of Western medicine and Chinese patent medicine used in this medical institution has increased year by year (the number of Western medicine varieties has increased from 1,415 to 1,679 in the past five years, and the number of Chinese patent medicine varieties has increased from 296 to 324), the number of Western medicine varieties has increased by 264, and the number of Chinese patent medicine varieties has increased by 28 (
Figure 1 and
Table 1).
2.2. Analysis Results of Drugs Used in the National Essential Medicines List
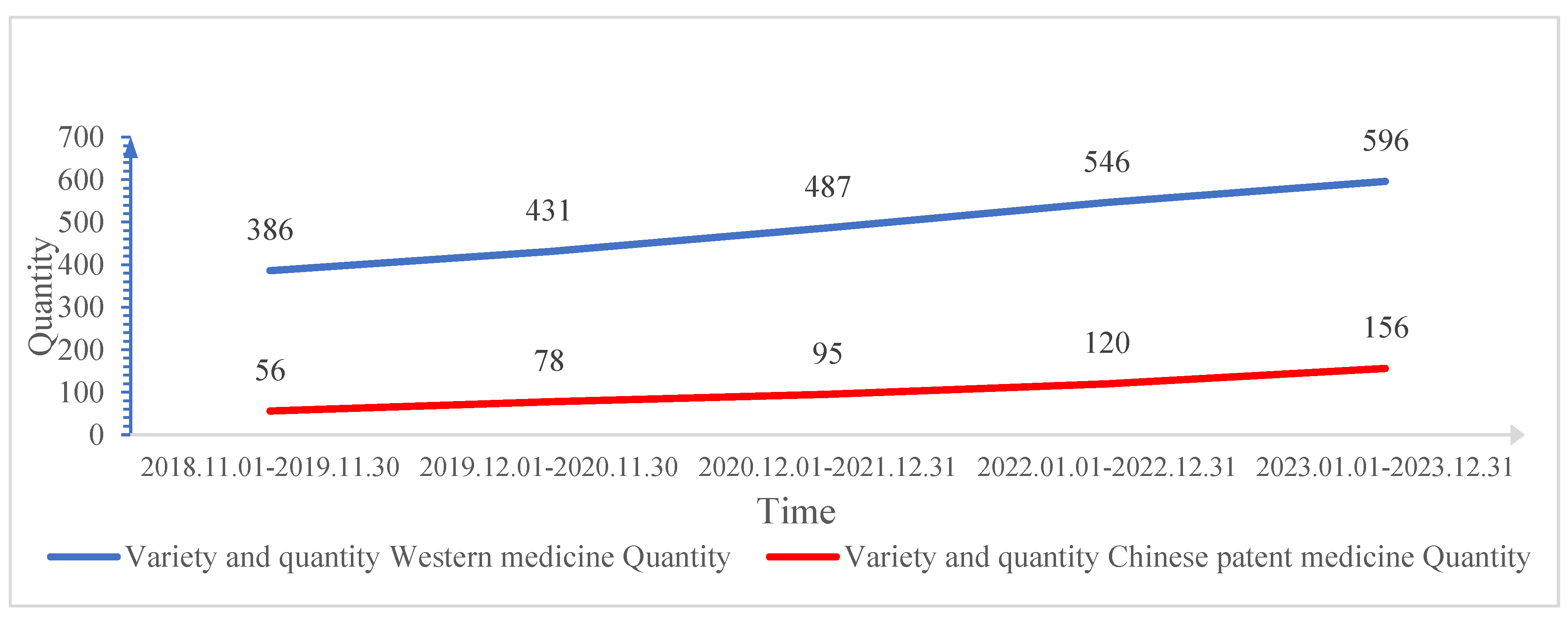
Through analysis, it was found that in the National Essential Medicines List used by this hospital, the number of basic Chinese and Western medicines has been increasing year by year. The number of Western medicine varieties has developed from 386 to 596, and the number of Chinese patent medicine varieties has developed from 56 to 156. Among them, the number of Western medicine varieties has increased by 210, and the number of Chinese patent medicine varieties has increased by 100. Among them, the percentage increase in the number of Western medicines each year was lower than the increase in the number of Chinese patent medicines each year (
Figure 2 and
Table 2).
2.3. Analysis Results of the Increase in the Number of Drugs in the Medical Insurance Directory
Through the analysis, it was found that the number and growth rate of Chinese and Western medicine varieties used in the medical insurance directory have increased year by year. The number of Chinese and Western medicine varieties in the medical insurance directory has grown from 1,393 to 1,662, and the number of Chinese patent medicine varieties has grown from 291 to 318. Among them, the number of Western medicine varieties has increased by 269, and the number of Chinese patent medicine varieties has increased by 27. The increase in the number of Western medicines and Chinese patent medicines in the last two years was relatively consistent. Among them, the number of Western medicines that increased each year in the first two years was significantly higher than the number that increased each year in the last two years; Except for the period from December 1, 2019 to November 30, 2020 when the number of medicines on the medical insurance list was negative, the number of Chinese patent medicines increased relatively steadily in other years. From December 1, 2020 to December 31, 2021, the growth rates of Western medicine and Chinese patent medicine were at their highest levels during these years, both above 9 percent, with Chinese patent medicine reaching 11.63 percent (
Table 3).
2.4. Analysis Results of the Proportion of Essential Medicines, Western Medicines and Chinese Patent Medicines
Through the analysis of the number of national essential medicines used by the medical institution, it was found that the overall proportion of essential medicines, the proportion of essential medicines of Western medicines, the proportion of essential medicines of Chinese patent medicines, and the proportion of each drug variety all showed an increasing trend year by year. The proportion of essential medicines rose from 25.83% to 37.54%, and the proportion of essential medicines of Western medicines rose from 27.28% to 35.50%. The proportion of Chinese patent medicines increased from 18.92% to 48.15%; Except for the 18.92% share of Chinese patent medicines from November 1, 2018 to November 30, 2019, both the overall share of Chinese patent medicines and the share of Chinese patent medicines increased above 25.83%, but the share and growth rate varied among different drug varieties. Among them, the share of Chinese patent medicines was as high as 35.50%. Chinese patent medicines accounted for 48.15 percent (about 50 percent of Chinese patent medicines) (
Table 4).
2.5. Analysis Results of the Proportion of Medical Insurance Drugs, Western Medicine Medical Insurance Drugs, and Chinese Patent Medicine Medical Insurance Drugs
By analyzing the number of drugs in the medical insurance directory, it was found that the proportion of medical insurance drugs, Western medicine medical insurance drugs and Chinese patent medicine medical insurance drugs, each variety and each year remained above 98%, which was basically in line with the national designated medical insurance management policy (
Table 4).
2.6. Analysis Results of the Increase in the Number of Varieties and Proportion of Essential Medicines in National Centralized Procurement Drugs
Through the analysis, it was found that among the drugs under national centralized procurement, the number of essential drugs increased year by year. The number of essential drugs of Western medicine increased from 0 to 298, and the number of varieties of Western medicine in centralized procurement increased from 0 to 314. The number of Chinese patent medicine base drugs increased from 0 to 14, and the number of Chinese patent medicine centralized procurement increased from 0 to 27. Among them, the number of Western medicine base drugs and centralized procurement varieties increased by 298 and 314 respectively; The number of Chinese patent medicine base drugs and centralized procurement varieties increased by 14 and 27 respectively; In terms of the number of Western medicine varieties and the proportion of essential medicines in centralized procurement, the number of varieties and the proportion of essential medicines in centralized procurement have been increasing year by year, while the proportion of essential medicines in centralized procurement has been increasing year by year. The proportion in the first four years (2018-2021) was less than that in the last two years (2022-2023), approaching 95% from January 1, 2023 to December 31, 2023. The number of Chinese patent medicine varieties and the proportion of essential medicines in centralized procurement have been increasing year by year, and the proportion of essential medicines in centralized procurement has been increasing year by year, with the proportion remaining above 50% in the last two years (2022-2023). The proportion of Chinese and Western medicine varieties and the proportion of essential medicines in centralized procurement has been increasing year by year. The proportion of essential medicines in centralized procurement in the first four years is less than that in the last two years (2022-2023), and it is close to over 90% from January 1, 2023 to December 31, 2023 (
Table 5).
2.7. Analysis of the Total Number of Prescriptions in the Entire Hospital
It was found through analysis that, except for the period from December 1, 2019 to November 30, 2020 (which was the peak period of the COVID-19 outbreak and the lockdown period), the total number of prescriptions in the hospital was relatively the lowest and negatively increased. The total number of prescriptions, the number of prescriptions containing essential drugs (1-4), the number of prescriptions all containing essential drugs, and the number of prescriptions containing essential drugs) and their growth rates increased year by year in the remaining years. The total number of prescriptions increased from 2,470,317 between December 1, 2019 and November 30, 2020 to 3,258,654; The number of prescriptions containing essential drugs (1-4) increased from 1,104,890 to 1,562,431; The number of prescriptions consisting entirely of essential drugs increased from 548,084 to 902,584; The number of prescriptions containing essential drugs increased from 1,652,974 to 2,4650,15 (
Table 6).
2.8. Analysis Results of Prescription Rates for Essential Drugs and All-Essential Drug Prescription Rates
Through the analysis, it was found that the prescription rates containing essential drugs (1-4), all essential drugs, and essential drugs all showed an increasing trend year by year. Among them, the prescription rate containing essential drugs (1-4) was above 44.55%, and the all-essential drugs prescription rate remained above 20.76%. The prescription rate of essential drugs remained above 65.31% (the proportion rose from 65.31% to 75.65%), with the highest reaching above 75.65% (
Table 6).
3. Discussions
With the implementation of the National Essential Medicines List, the varieties of national essential medicines have also changed. Since the implementation of the National Essential Medicines List in 2009, medical institutions have a new basis for judging the allocation and use of medicines. The results of this study are largely in line with the major policies implemented by the state, namely, the number and variety of essential drugs in the national centralized procurement drugs, the number and variety of drugs in the medical insurance list and the growth rate, the prescription rate of all essential drugs and the prescription rate of essential drugs have all increased year by year.
In 2011, the system of essential medicines was implemented in medical and health institutions, and a national system of essential medicines was established to adapt to the changing needs of society [
3]. In 2018, the basicNational Essential Medicine System was improved, the reform of the medical and health care system was deepened, and the development of the drug supply guarantee system and basic public services in the medical and health care field was further advanced [
1], which played an important role in improving the drug supply guarantee system, ensuring the basic medication of the people and reducing the medication burden of patients. In 2020, the National Essential Medicine System was fully implemented in all medical institutions across the country [
5,
6].
In the continuous update of the national system and the list of essential medicines, the number of essential medicines and the categories of medicines have shown a continuous increase or decrease. In 2009, there were only 307 varieties of drugs on the national essential medicines list [
6,
9]; Xue Huiying et al. compared the 2012 version with the 2018 version of the National Essential Medicines List and found that the 2018 version added three ingredient drugs (chemical drugs, biological products, and Chinese patent medicines), and added 165 new drug varieties (including 100 chemical drugs and biological products, and 65 new Chinese patent medicines [
6]; In 2018, the number of drugs in the National Essential Medicines List increased from 520 in 2012 (317 in chemical drugs and biological products and 203 in Chinese patent medicines) to 685 (417 in chemical drugs and biological products and 268 in Chinese patent medicines) [
7,
10]. With the increase in the variety of medicines and the introduction of the 2018 version of the National Essential Medicines List policy, the number of essential medicines has grown year by year, meeting the basic needs of the people and actively improving the safe use and management level of essential medicines [
1,
11].
With the joint efforts of various departments, the National Healthcare Security Administration has actively promoted the adjustment of the medical insurance drug list through a regular, standardized and scientific adjustment mechanism [
12,
13], adopted comprehensive measures to reduce the burden of medical treatment for insured people [
14], and focused on meeting the basic drug needs of the general public. In 2024, the National Healthcare Security Administration and the Ministry of Human Resources and Social Security issued the "National Basic Medical Insurance, Work-related Injury Insurance and Maternity Insurance Drug Catalogue (2024)", adding a total of 91 drugs in this adjustment. Among them, 26 drugs for tumors (including 4 rare diseases), 15 drugs for chronic diseases such as diabetes (including 2 rare diseases), 13 drugs for rare diseases, 7 anti-infection-fighting drugs, 11 Chinese patent medicines, 4 drugs for mental illness, and 21 drugs in other fields were removed. 43 zombie drugs were removed. After this adjustment, the total number of drugs in the list will increase to 3,159. Among them, there are 1,765 Western medicines, 1,394 Chinese patent medicines and 892 Chinese herbal decoctions. The level of protection in areas such as tumors, chronic diseases, rare diseases and children's medicines has been significantly improved [
13,
14]. More new and good medicines have been included in the medical insurance directory, and the level of drug security for insured people has been continuously improved, effectively addressing the problem of accessing, using and reimbursing drugs for insured patients [
14]. Actively promoting the centralized procurement of drugs and medical consumables organized by the state has effectively facilitated the completion of procurement tasks by medical institutions and prevented the exclusivity of some medical staff to use expensive or special drugs. That is, the investigation and research on the use of drugs in this tertiary hospital shows that the hospital's use of essential drugs is highly consistent with the national policy of the "Essential Drugs Catalogue", actively responds to the national policy guidelines, steadily promotes the development of the hospital and improves the medical environment for the people.
4. Suggestions
4.1. Narrow the Gap in Quality and Efficacy Between Generic and Original Varieties
Narrowing the gap between generic drugs and original drugs in terms of quality and efficacy will increase patients' trust in generic drugs and the credibility of the system, thereby effectively promoting the implementation effect of the essential medicine system.
To enhance the consistency evaluation of generic drugs, First, expand the scope of evaluation, giving priority to varieties with high clinical usage, good efficacy, low price and high patient dependence, while ensuring that generic drugs are consistent with or even better than the original drug base in terms of quality and efficacy. Second, introduce international certification evaluation standards (such as WHO precertification, FDA standards) to promote the alignment of domestic generic drug quality with international and domestic standards.
For the strict supervision of enterprises at the source production end, First, strengthen the screening of raw materials (excipients), dynamic monitoring and spot checks of production processes, conduct random and irregular batch and classified clinical efficacy comparison studies with original drugs to identify quality and efficacy gaps, encourage medical institutions to carry out research and innovation between generic drugs and original drugs, and actively disclose data to enhance credibility and public trust; Second, establish enterprise quality credit files and evaluations, implement red and black list quality assessment and evaluation, revoke the qualification for the production and supply of essential drugs for enterprises that fail to meet the standards, and put the enterprises and legal persons on the blacklist and cut off all business supply.
Encourage and support technological innovation. First, support generic drug enterprises in technological upgrading, enhance the R&D innovation and production level of generic drugs, and encourage enterprises to develop highly challenging generic drugs (such as complex dosage forms and biosimilars). Second, provide subsidies or tax breaks for research and development and patents; Third, support the balance between patent protection for original drugs and reasonable competition for generic drugs.
Establish a quality traceability system, build an information circulation platform (a supply chain management platform for essential drugs), establish a quality traceability system for generic drugs through information technology means, ensure the traceability of the entire process of drugs from production to use, strictly implement the responsibility system, "whoever is responsible bears the responsibility", and enhance the credibility of society and the credibility of the people.
4.2. Opinions and Suggestions on Ensuring a Sound Supply Mechanism Under the National Essential Medicine System
Insufficient supply or unstable supply chain of essential medicines in some areas, especially in remote areas and primary medical institutions, may cause patients to be unable to obtain the medicines they need in a timely manner, resulting in shortages.
Improve supply chain management by building an information circulation platform (essential medicine supply chain management platform), optimizing the reserve and distribution system, and monitoring drug inventory and demand in real time to ensure timely supply and replenishment of drugs and price fluctuations. Establish essential medicine reserve depots in key areas of each province (military system) to deal with shortages of medicines caused by emergencies (emergencies or natural disasters); Implement "graded warnings" (red, yellow, green levels) for drugs that are truly in short supply or scarce, formulate emergency plans for the supply of essential drugs, and activate emergency production or import plans in advance to ensure the continuity of drug supply in time.
Optimize the procurement mechanism by reducing drug costs through centralized procurement, volume-based procurement, joint procurement, etc., and providing special subsidies; Second, policy incentives to encourage more drug manufacturers to participate in the production and supply of essential medicines; Third, encourage third-party logistics companies to cooperate with medical institutions to achieve precise delivery and accurate support in remote areas (with a focus on areas with poor transportation) to ensure the stability of drug supply. Ensure the supply of essential medicines will be included in the assessment of corporate social responsibility, and enterprises that fail to fulfill their obligations will be placed on the list of untrustworthy entities or on the red and black list.
4.3. Suggestions and Opinions on the Incentive Mechanism for the Use of Drugs Under the National Essential Medicine System
Some medical institutions and doctors have a low enthusiasm for the use of essential medicines or a preference for expensive drugs, resulting in a low utilization rate of essential medicines.
First, increase the frequency of training, intensify the online learning and examination of essential drug knowledge for medical staff, and enhance their understanding and usage ability of essential drugs. Second, establish a reward mechanism by setting up honors such as "Model of Rational Use of Essential Medicines" and linking them to professional title promotion, research project approval, etc. Rewards should be given to medical institutions and doctors with high utilization rates of essential drugs to set up role models, promote experiences and change previous drug use habits. Third, establish a performance assessment mechanism, incorporate the use of essential medicines into the performance assessment system of medical institutions and doctors, and encourage them to give priority to the use of essential medicines; The use rate of essential medicines will be included in the performance assessment of hospital directors and linked to hospital ratings. Fourth, strengthen the support of medical insurance policies, doctor behavior guidance, patient-oriented policies, encourage patients to use essential drugs through the inclination of medical insurance reimbursement policies, embed the priority recommendation function of essential drugs in the prescription system, and provide review prompts for the use of expensive drugs beyond the scope; Increase the proportion of medical insurance reimbursement for essential drugs to reduce the financial burden on patients. Fifth, strengthen information-based management. Through information-based means, monitor the use of essential drugs in real time, identify problems promptly and make improvements. For medical institutions that meet the standards for the proportion of essential drugs used, the total budget for medical insurance will be increased or financial rewards will be given. At the same time, the government's rewards for medical institutions must be implemented.
4.4. Opinions and Suggestions on How the National Essential Medicine System Can Meet the Needs of Basic Medicines in Clinical Practice
The core of the problem is the lag in the update of the essential medicine list, which fails to adapt to changes in clinical medication needs (new diseases and special populations) in a timely manner, resulting in some urgently needed drugs not being included in the list.
First, scientifically and dynamically adjust the list, optimize the structure of drugs in the essential medicine list, update and dynamically adjust the mechanism regularly based on clinical needs and changes in the drug market, and quickly and scientifically incorporate them in combination with real-time changes in the disease spectrum (urgently needed drugs, children's drugs and rare disease drugs) to ensure coverage of the needs of common, frequently-occurring and major diseases. At the same time, the proportion of long-acting and compound preparations in the list is optimized, and the list is scientifically adjusted based on pharmacoeconomic evaluation and evidence-based medical evidence. Second, we will strengthen the scientific argumentation of new drugs, special drugs and generic drugs, and fully listen to the opinions of clinical and pharmaceutical experts during the adjustment of the list. Under the premise of ensuring safety and efficacy, new drugs and special drugs will be included in the essential medicine list in a timely manner, with temporary additions and flexible response to public health emergencies. Third, strengthen demand research, conduct extensive research to understand the actual medication needs of primary medical institutions and patients, and ensure the practicality of the list.
4.5. Suggestions and Opinions on the Promotion and Training of the National Essential Medicines System
Some medical staff and the public have insufficient knowledge of the essential medicines system, which may lead to unsatisfactory implementation of the system; Strengthen public awareness through various channels such as media (TikTok, WeChat, etc.) and community activities, produce easy-to-understand science popularization short videos and brochures to popularize knowledge about the essential medicine system to the public, and focus on promoting the efficacy and price advantages of generic drugs to increase public acceptance, awareness and awareness. Establish public feedback channels (such as hotlines, online platforms or APP Internet platforms), regularly collect demands and optimize policy information and policy interpretations related to the essential medicine system to increase publicity coverage; At the same time, support functions such as drug list query, priority promotion, clinical comparison of drug efficacy, differences and advantages and disadvantages, etc. reminders. Regularly train medical staff on the essential medicines system to enhance their understanding and application of the system.
4.6. Strengthen the Supervision of National Medical Security Work
In order to prevent the occurrence of private interests and the deterioration of corruption, there is an urgent need to enhance the balance of interests, supervision and control methods among various fields.
First, strengthen the supervision of medical insurance funds, improve the coordinated use of regional medical insurance funds, ensure the efficiency of medical insurance fund utilization, insurance bureaus should manage the "money for medical treatment" and "money for saving lives" of the people, identify the disadvantages and gaps in this field, find out the blind spots or deficits in this field or industry, and truly solve the problems of "expensive and difficult medical treatment" for the people.
Second, strengthen the supervision of medical insurance funds to prevent violations. Organize special inspections and inspections in key areas to look for violations such as repeated charges, excessive charges, cross-charges and over-treatment by designated medical insurance institutions, and whether they provide convenience for insured people to resell drugs or obtain other illegal benefits. There is an urgent need to promote vigorous reform of the medical security system, including medical expenses that are not covered by medical insurance in medical insurance or changing the settlement items. Starting from January 2025, designated medical institutions will issue "dual-channel" prescriptions for drug delivery through the medical insurance electronic prescription center, and designated retail pharmacies will obtain prescriptions and settle drug deliveries online to address illegal and irregular issues [
13] such as false prescriptions and fraud in the process of medical insurance drug delivery services.
Third, we will promote the centralized volume-based procurement of drugs and medical consumables organized by the state. For those who illegally interfere, provide convenience or interfere in the bidding and procurement, and take advantage of the convenience of the work to provide information such as drugs, medical consumables and drug usage by medical production and operation enterprises, as well as clinical doctors' intentional tendency to use non-centralized procurement drugs or non-medical insurance drugs, The act of accepting kickbacks from pharmaceutical companies to seek improper benefits will be dealt with according to law. Actively search for the completion of centralized procurement tasks, and at the same time make public the ranking in the province. The medical system should establish positive incentive and restraint systems and improve compensation mechanisms, incorporate the usage ratio of national strategic drugs and prescription essential drugs into the personal salary and benefits of doctors, increase or expand the usage ratio of essential drugs and centralized procurement drugs, and ensure reasonable income for doctors.
References
- Bai Jingwei. Ge Yanfeng: China's medical reform goes against the basic laws of the development of health services [J]. Health Vision, 2005,13 (8) : 27-28.
- Zhang M, Zou K, Liu Z, et al. Availability of essential medicines, progress and regional distribution in China: a systematic review and meta-analysis [J]. Front Public Health, 2023, 11(1149838).
- Chen Ming, Yan Junfeng, Tong Rongsheng, et al. Research progress on the essential medicine system [J]. Chinese Pharmacy, 2013, 24 (20) : 1913-1917.
- Zhao Y W, Wu J Y, Wang H, et al. A Cross-sectional Study Assessing Predictors of Essential Medicines Prescribing Behavior Based on Information-motivation-behavioral Skills Model among County Hospitals in Anhui, China [J]. Chin Med J (Engl), 2015, 128(21): 2887-95.
- Yan Jiaqing, Liu Min, Zhang Yuan, Ma Yinglin, Le Kaidi, Shen Xin, Cao Xiuping, Li Guohui. Comparison and analysis of anti-tumor drugs in the National Essential Medicines List (2018) and the WHO Model List of Essential Medicines (2017) [J]. Chinese Journal of Pharmacy,2019,54(22):1901-1906.
- Xue Huiying, Li Juan. Interpretation of the 2018 Edition of the National Essential Medicines List [J]. Medical Review,2019,38(01):1-8.
- Chen Yun, Wu Yanli, Zheng Yuling. A hospital national essential drug use directory status of proprietary Chinese medicine using analysis [J]. Journal of traditional Chinese medicine management journal, 2022, 30 (5) : 112-114.
- Zhang Taozhi, Yin Xili, Li Meng, Wang Yanwen, Liu Wei. Chinese Pharmaceutical Affairs, 2020, 34(12):1359-1365.
- Li X, Liu M, Lin J, et al. A questionnaire-based study to comprehensively assess the status quo of rare disease patients and care-givers in China [J]. Orphanet J Rare Dis, 2021, 16(1): 327.
- He J, Tang M, Ye Z, et al. China issues the National Essential Medicines List (2018 edition): Background, differences from previous editions, and potential issues [J]. Biosci Trends, 2018, 12(5): 445-9. [CrossRef]
- Tao W, Zeng Z, Dang H, et al. Towards universal health coverage: achievements and challenges of 10 years of healthcare reform in China [J]. BMJ Glob Health, 2020, 5(3): e002087. [CrossRef]
- Wei N, Wang Z, LiX, et al. Improved staffing policies and practices in healthcare based on a conceptual model [J]. Front Public Health, 2024, 12(1431017). [CrossRef]
- Liu Min. The adjustment of the medical insurance directory focuses on implementation [J]. Chinese Hospital Directors, Jan 1, 2025,27-30.
- Fu Tian yi, Di Yun. Our city fully implements the new version of the medical insurance drug list [N]. Huaibei Daily, February 12,2025, Page 002,1-1.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).