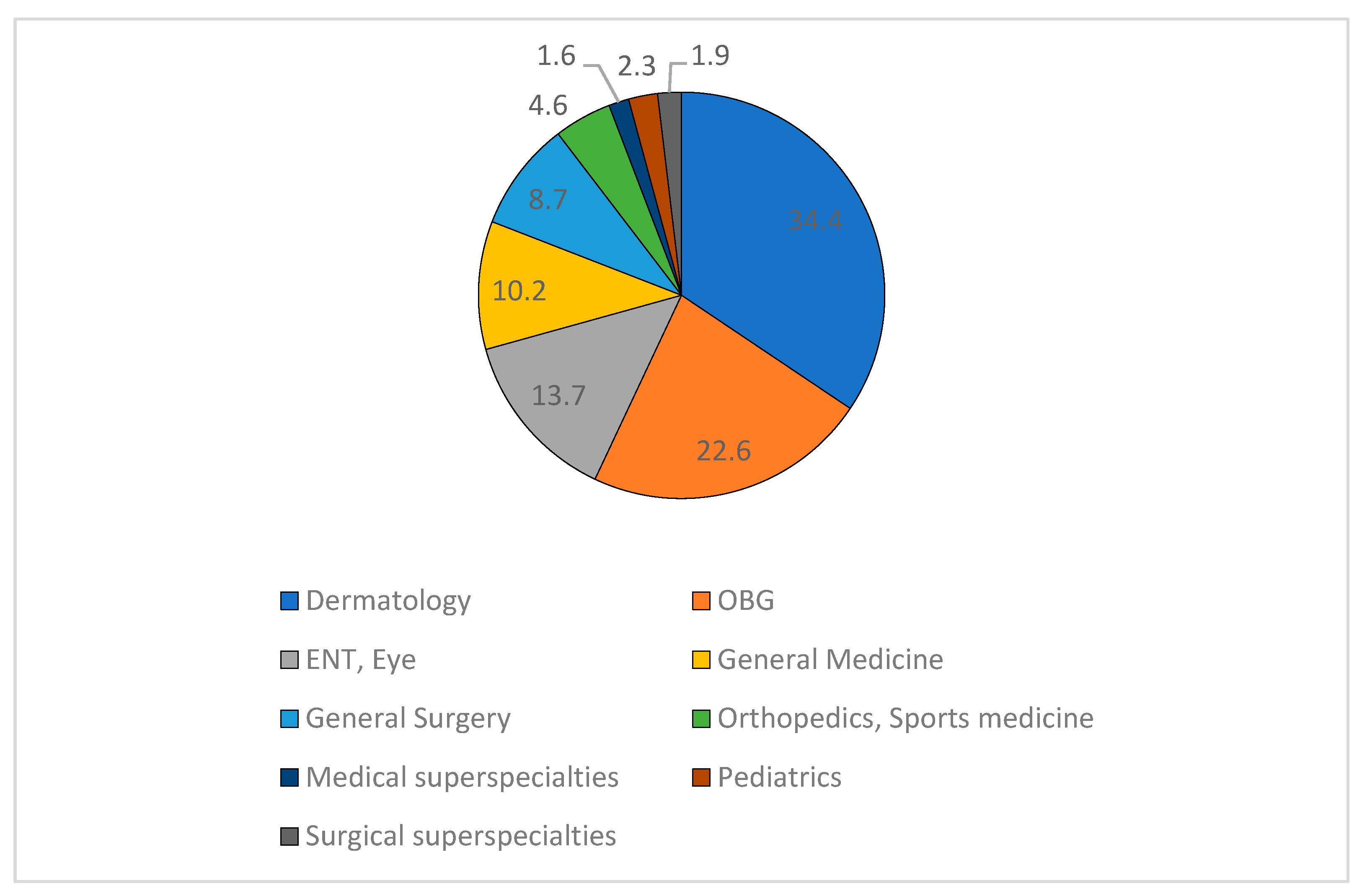1. Introduction
Rational use of medicines (RUM) is crucial in attaining optimal medical and healthcare for patients and the community as a whole. According to WHO, “
Rational use of drugs requires that patients receive medications appropriate to their clinical needs, in doses that meet their own individual requirements for an adequate period of time, at the lowest cost to them and their community” [
1]. Irrational prescribing of drugs is a serious global issue. WHO estimates an approximately more than fifty percent incidence of overuse, underuse and misuse of medicines worldwide which in turn leads to wastage of scarce resources and extensive health hazards [
1]. Numerous factors through different phases of medicine use cycle contribute to irrational drug use, some of these being inadequate knowledge and skills among prescribers and patients, prescribing driven by misconceptions, gaps in drug supply system, weak control and regulations over prescriptions, inappropriate drug promotion strategies by pharmaceutical companies, biased and poor information about medicines, polypharmacy, self-medication, over-the-counter availability of drugs etc [
2,
3]. Irrational use of drugs is linked to many untoward consequences such as worsening quality and higher cost of drug therapy, increased incidence of undesirable effects (e.g. adverse effects, antimicrobial resistance) and untenable psycho-social impacts on patients [
4,
5].
A regular and timely evaluation of the rational use of medicines is very essential to take desired actions. In 1993, WHO in collaboration with the International Network of Rational Use of Drugs (INRUD) formulated a manual that defines core and complementary drug use indicators for investigating drug use in healthcare facilities at national, regional or local levels in a standardized manner [
6,
7]; a set of recommended optimum values for each core indicator have also been defined [
8]. The core drug use indicators, further divided into prescribing, patient care and facility indicators help in identifying general prescribing and quality of care problems at health care facilities and are recommended for inclusion in drug use studies [
6]. Such measures or indicators empower the concerned stakeholders including healthcare planners, managers and researchers to compare data across different areas or at different times and take necessary remedial actions [
3]. Previous studies from developing countries including India have identified significant gaps in prescribing from the WHO/INRUD recommendations [
9,
10,
11,
12,
13,
14,
15,
16,
17,
18,
19,
20,
21,
22,
23,
24,
25,
26,
27].
The present study was conducted with an aim to provide insight into out-patient prescription of drugs in our tertiary care hospital to (1) assess the WHO/INRUD core prescribing indicators, (2) evaluate the completeness of prescriptions, and (3) identify the targets for promoting rational use of medicines and delivering quality patient care in terms of prescriptions.
2. Materials and Methods
Study design and setting
This was a cross sectional observational study conducted by the department of Pharmacology, Postgraduate Institute of Medical Sciences (PGIMS), Rohtak, Haryana, North India. Located approximately 70 kms from the national capital, New Delhi, our public tertiary care hospital is a major centre for provision of specialised healthcare services not only to the people of state of Haryana, but also to the adjacent states of Punjab, Rajasthan, Delhi and western Uttar Pradesh. The hospital’s outpatient department (OPD) comprising of various specialty and super-specialty clinics witnesses a daily OPD throughput of nearly 10,000-12,000 patients.
Data collection approach and tools
Paper-based hand written prescriptions from various clinical departments were collected from the hospital’s OPD pharmacy from January to June 2024. In the pharmacy, there are separate queues for various groups as for general public, females, elderly and medical staff/ physically handicapped/ policemen. Similar number of patients from each queue were approached on a particular day using systematic random sampling to ensure uniform representation from all groups. The prescriptions thus collected were screened for potential eligibility in the study. First encounter prescriptions i.e. prescriptions of new cases were included while prescriptions of follow up cases and those with illegible or difficult to understand handwriting by the study investigators were excluded. From the included prescriptions, information pertinent to study objectives was extracted using standardised pre-tested data collection form.
Study objectives
The study objectives included the evaluation of prescriptions for:
1. WHO/ INRUD core prescribing indicators. These included:
(i) Average number of drugs per prescription/encounter: Total number of drugs prescribed/ Number of prescriptions assessed (WHO recommended value: 1.6-1.8);
(ii) Percentage of drugs prescribed by generic name: (Number of drugs prescribed by generic names/ Total number of drugs prescribed) x 100 (WHO recommended value: 100%);
(iii) Percentage of encounters resulting in prescription of an antibiotic: (Number of prescriptions with an antibiotic prescribed/ Total number of prescriptions assessed) x 100 (WHO recommended value: 20 – 26.8%);
(iv) Percentage of encounters resulting in prescription of an injection: (Number of prescriptions with an injection prescribed/ Total number of prescriptions assessed) x 100 (WHO recommended value: 13.4 – 24.1%); and
(v) Percentage of drugs prescribed from essential medicines list (EML): (Number of medicines included in EML/ Total number of medicines prescribed) x 100 (WHO recommended value: 100%);
2. Index of rational drug prescribing (IRDP). This is the sum of index values of all prescribing indicators calculated as per the index system developed by Zhang and Zhi for the comprehensive assessment and comparison of different healthcare systems [
28].
For indices of non-polypharmacy, antibiotic and safe injection use, following formula is used:
Index value = WHO optimal value
Observed value
The indices of generic prescribing and essential medicines are calculated as:
Index value = Observed value
WHO optimal value
The optimal values for all the indicators are set as 1, and values close to 1 indicate rational use. Hence, the value of IRDP ranges from 0 to 5.
3. Completeness of prescriptions: This was assessed on the basis of presence of following parameters:
General details: patient demographic details (name, age, sex and address), unique patient ID, date of prescription and diagnosis (4 points);
Treatment details: name of medicine, dosage form, strength of formulation,
dosage regimen/ frequency, duration of treatment, and advisory instructions (such as before/after food, at bedtime etc.) (6 points); and
Each parameter was scored as 1 and prescriptions were allotted a completeness score ranging from 0 to 11 on the basis of number of parameters present. The percentage of compliance for each parameter was also calculated.
Sample size calculation
As per the WHO recommendation, a minimum of 600 prescriptions need to be analysed for evaluating core prescribing indicators6. Hence, sufficient number of prescriptions were collected in order to have at least 600 prescriptions available for analysis.
Statistical analysis
Data were entered into Microsoft Excel and analysed primarily using descriptive statistics, including frequency distributions (percentages) and means with standard deviations (SD). No specific hypothesis testing was conducted.
Ethical and administrative approvals
The study was conducted after obtaining administrative approval from the office of Medical Superintendent of the hospital (PGIMS/Misc/23/8944-47) and ethical approval from Biomedical Research Ethics Committee (vide letter no. BREC/23/497 dated 17.10.23). Written informed consent was obtained from the patients prior to collection of prescriptions. Adequate measures were taken to ensure data confidentiality.
3. Results
Out of 844 prescriptions collected, 220 were follow-up cases and 17 were illegible; hence 607 prescriptions were included in the analysis. The prescriptions coming from various specialty and super-specialty departments were collected from outpatient pharmacy for the study (
Figure 1).
A total of 1837 drugs were prescribed in 607 prescriptions assessed with a mean (SD) of 3.03 (1.51) drugs (range: 1 to 9). 93 (15.3%) prescriptions had five or more drugs prescribed. Of the 1837 drugs, 1378 (75%) were prescribed as generic names. 125 prescriptions (20.59%) had an antibiotic prescribed. Metronidazole (31; 24.8%), amoxicillin-clavulanic acid (29; 23.2%) and cefixime (26; 20.8%) were among the frequently prescribed antibiotics. Injectables were given in 7 (1.15%) prescriptions. Regarding essential medicines, of the total 1837 drugs prescribed, 1018 (55.4%) were mentioned in National List of Essential Medicines (NLEM) 2022 while 934 (50.8%) were included in Haryana state essential medicines list (2013-2014). A relatively good proportion of drugs were, however, prescribed from the hospital formulary [1596 (86.9%)]. The index of rational drug prescribing (IRDP) calculated by adding all the indices of prescribing indicators was 3.86 (
Table 1).
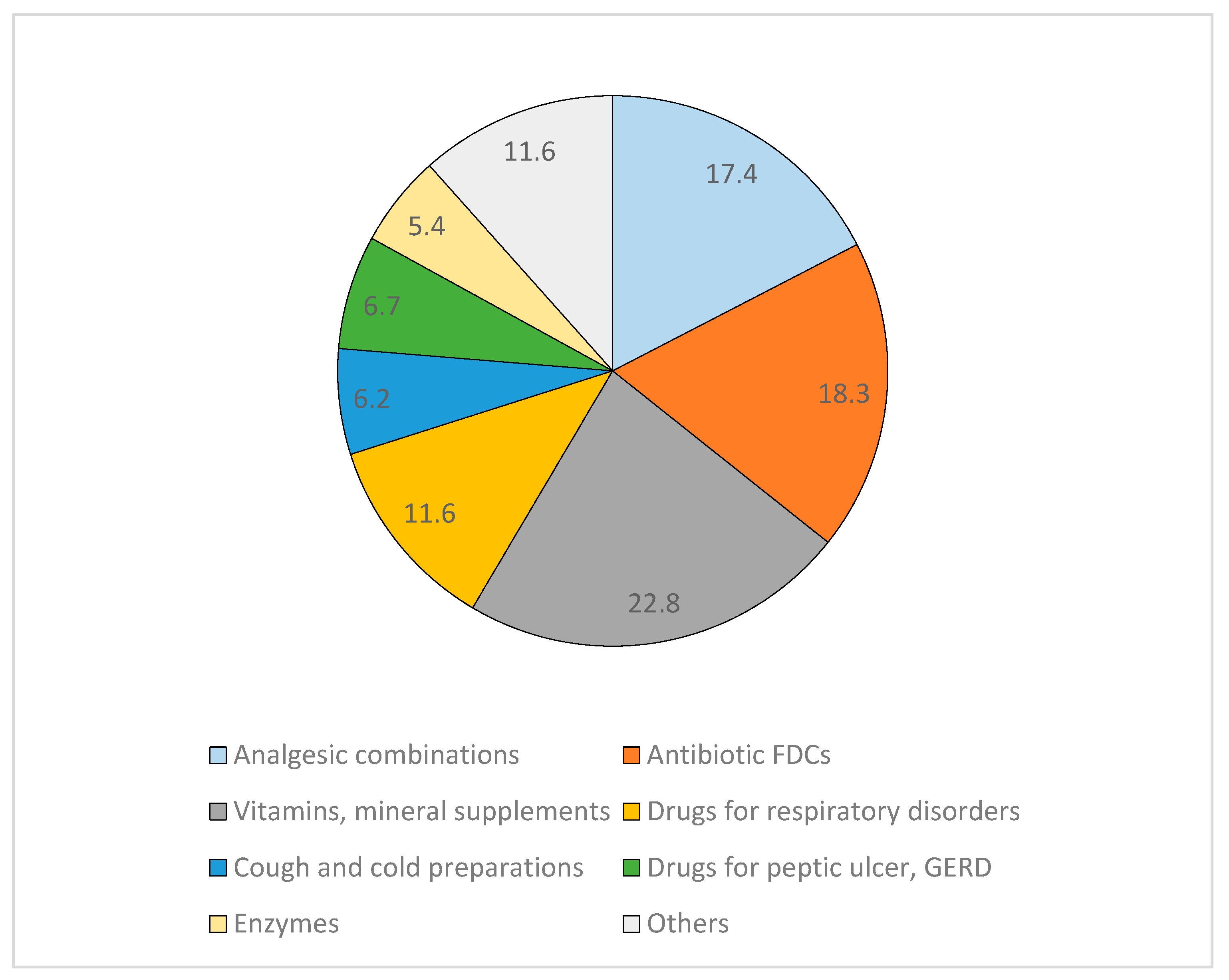
24.2% (147/607) prescriptions had at least one FDC prescribed with a total of 224 FDC prescriptions (12.2% of total 1837 prescriptions). Vitamins/ mineral supplements, nonsteroidal anti-inflammatory drugs (NSAIDs) and antibiotic combinations were among the most commonly prescribed FDCs (
Figure 2).
Topical preparations were given in 291 out of total 607 prescriptions (47.9%) and mainly included antifungals, anti-acne preparations, antibiotic ear/ eye drops and analgesics. The strength of the topical preparation was mentioned in 44.8% (130/291).
Completeness of prescriptions
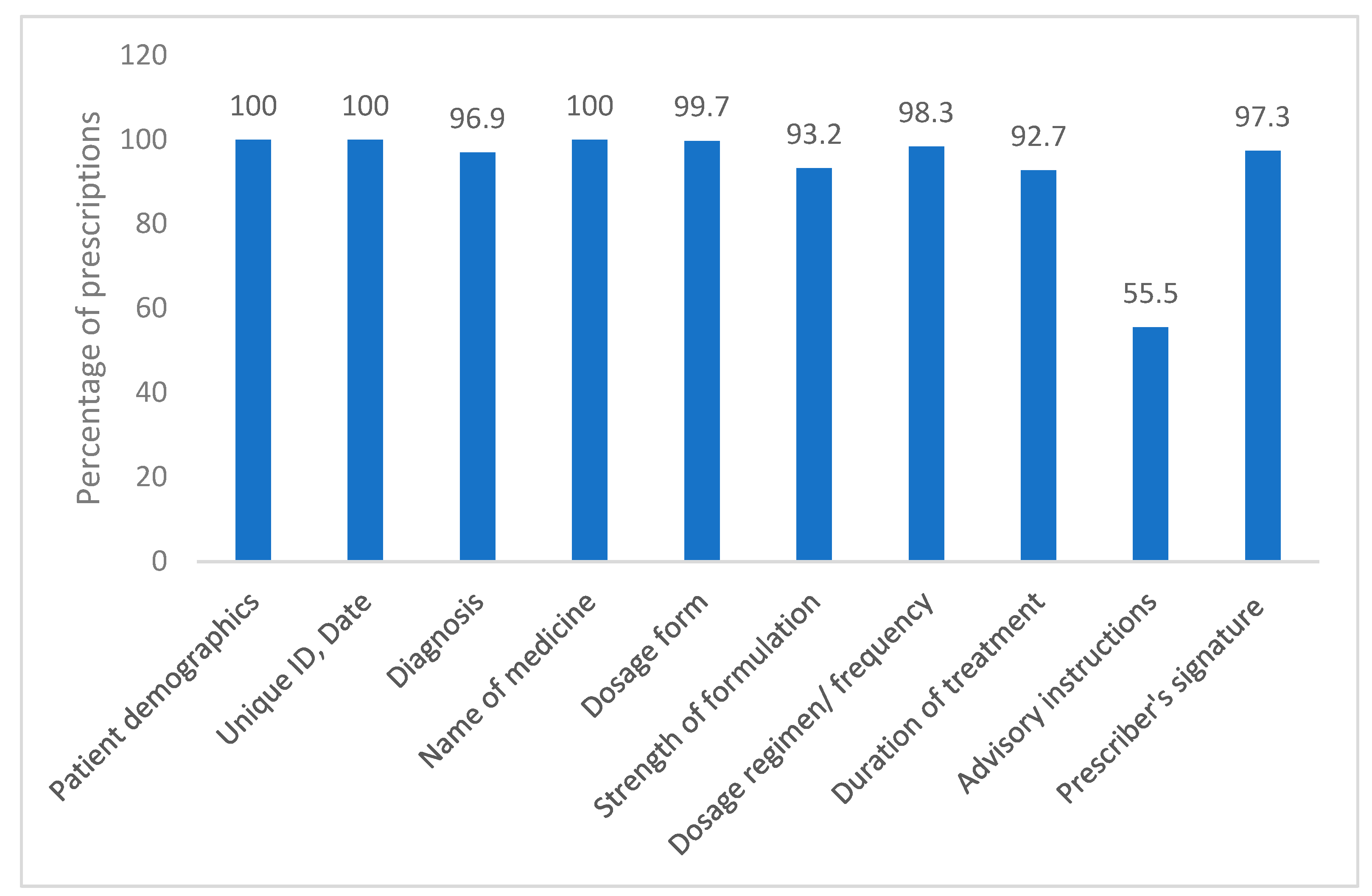
The mean (SD) completeness score of the prescriptions was 10.33 (0.8) (range 5 to 11). All the prescriptions mentioned patients’ demographic details and names of medicines. Majority of the prescriptions contained diagnosis, treatment dosage form and regimen. The strength of formulation and duration of treatment were given in 93.2% and 92.7% prescriptions, respectively. More than half of the prescriptions mentioned special advisory instructions. 97.3% prescriptions had prescribers’ signatures on them while 72.16% had date written by prescriber (
Figure 3). Drug names were written in capital letters in approximately 90% (544) prescriptions. None of the prescriptions had the registration number of physicians mentioned in them.
4. Discussion
This cross-sectional study analysed a total of 607 individual out-patient prescriptions, a number based on WHO recommendations and large enough to draw conclusions regarding compliance to WHO/INRUD core prescribing indicators. Data from the study is expected to throw light on prevailing prescription practices in our public tertiary care hospital and define targets to encourage rational use of medicines in hospital and community as a whole. Our findings will also serve as a source of baseline information for regular monitoring of prescribing practices in our hospital. Similar published studies from other parts of India were also reviewed for comparison purposes.
Table 2 provides a comprehensive comparison of the summary findings on WHO indicators from the present study with those from other parts of the country.
An average of 3.03 drugs per encounter were prescribed in our study with a range of 1 to 9 indicating polypharmacy as against the WHO proposed optimal value of 1.6 to 1.8 drugs per encounter. This finding was congruent to drug use patterns in tertiary care hospitals from other parts of India [
16,
17,
18,
19,
20]. Increasing prevalence of non-communicable diseases with aging population and associated comorbidities are possible contributing factors towards prescription of greater number of medicines per encounter in ours and other tertiary care hospitals in India. Moreover, majority of the prescribing doctors in such hospitals hold master’s degrees who have been speculated to have tendency to prescribe a greater number of drugs than their counterparts with bachelor’s degrees [
29]. Of note, few studies from secondary care hospitals also report similar data in this regard reflecting widespread prevalence of polypharmacy among outpatient departments of different levels of hospital care in our country [
21,
22,
26]. Besides being associated with adverse consequences such as decreased adherence, enhanced risk of drug interactions and adverse drug reactions, polypharmacy imposes unnecessary financial burden on patients as well as healthcare system. Polypharmacy has also been identified as the single most important predictor of prescription errors; for every additional drug prescribed, the risk of prescription error increases by 14% [
30]. Data from other developing and lower-middle income countries (LMICs) also demonstrate similar prescription patterns on average number of medicines per encounter
13-15,23,24. In contrast, few studies from Eritrea and Ethipoia report this indicator falling within the frame of WHO standards in outpatient settings [
9,
10,
11,
12] which may partly be attributed to variation in study sites and prevailing prescribing practices. Hence, training the prescribers for desired but at the same time limited prescribing is a key area of intervention towards achieving the goal of rational and quality use of medicines. Additionally, implementation of local evidence-based and optimised policies to curtail polypharmacy is a pressing priority.
WHO clearly enforces prescribing of drugs by their generic names due to lower cost and reasonably good accessibility and adherence compared to brand names
6. Generic drug prescribing was seen in a much greater proportion of prescriptions in our study (75%) when compared to other studies in India where it ranged from nil [
19] to 55.4% [
16]. Studies from Pakistan [
13], Sri Lanka [
14], Tanzania [
15] and Kenya [
24] also reported poor compliance to the WHO recommended standards of 100% generic name prescribing. Difference in study settings may possibly account for such variation as generic prescribing is found to be better in public over private healthcare settings. Lack of trust among prescribers on the quality of generic medicines and trying to prevent pharmacists from dispensing high-cost branded medicines are among other cited reasons for prescribers falling back on brand name prescribing. In this direction, national level strategies are needed to strongly advocate generic prescribing.
Regarding the encounters with injections, we observed a high degree of conformity with WHO standards, a finding in agreement with studies from other parts of India and other developing countries which can be explained by the study samples representing outpatient department prescriptions. Lower rate of injections is encouraging as it helps to avoid unwarranted hazards associated with use of injectable preparations such as increased likelihood of healthcare associated infections, financial implications, complications due to non-sterile technique etc. Hence, it is crucial to maintain consistency in the injection use rate to recommended standards by means of conducting periodic and judicious reviews of prescribing patterns.
In our study, the usage of antibiotics (20.6%) was within the WHO optimal values (20-26.8%) and showed agreement with some other studies from India [
16,
18,
20]. On the contrary, data from studies conducted in some other parts of India and LMICs is alarming with antibiotic usage exceeding the WHO standards. Irrational antibiotic prescription is a global issue contributing towards the emergence and spread of antimicrobial resistance. Adequate measures need to be implemented at regional, national and global levels to promote their optimal use.
In our survey, a relatively lesser proportion of the drugs were prescribed from national 55.4%) and state (50.8%) essential medicine lists. There is huge variation in figures regarding this as per the published reports from India (ranging from 9 to 100%). Data from other LMICs is however contrasting with most of them resorting to essential medicines. Lack of knowledge of essential medicines may be one reason for this. Limited availability of essential medicines, reported in our earlier survey [
31], is another potential factor driving physicians to fall over to non-essential medicines which may be less effective or safe and/or more expensive leading to poor treatment outcomes and increased healthcare costs [
32]. In this direction, periodic revision of essential medicine lists in line with emerging needs should be emphasized. Additionally, formulation and implementation of standard treatment guidelines is a key step towards enhancing the quality of prescriptions and achieving RUM. Also, prescribers should be sensitized regarding the importance of essential medicines in optimizing cost effective prescriptions.
IRPD, as a useful index for district or region wise comparisons, may guide policy makers in prioritizing and designing improvement strategies. An IRDP of 3.86 was calculated in our study which is quite comparable to reported indices from neighbouring LMICs, Sri Lanka (3.58)
14 and Pakistan (3.38 to 4.27) [
13]. Some differences in the IRDP across studies from other parts of India (not calculated in any study) may be assumed considering the values of various WHO indicators in these. Such variations in IRDP may be attributed to numerous factors for example poor penetration and adoption of evidence-based treatment guidelines in comparatively resource limited areas may translate to greater prescribing of antibiotics and injectables and lesser generic and essential drug prescribing.
In the present study, 2% (17/844) prescriptions were illegible which was lesser than that reported by other researchers from India (Sunny et al [
20]: 9%, Dhanya et al [
18]: 3.4%, Ahsan et al [
19]: 8.16%). There were inconsistencies in the reporting of completeness parameters for prescriptions across different studies. Patient demographics, date and unique ID were present in all prescriptions as these are printed on barcode at the time of registration which was similar to other studies from India. Diagnosis was, however, mentioned to a lesser extent in some studies (Singh et al [
22]: 64.2%; Shelat et al [
25] 34%; Mulkalwar et al [
17]: 57.2%; Ahsan et al [
19]: 56%) than ours. Majority of our prescriptions were complete with respect to treatment related details (name of medicine, dosage form, strength of formulation, dosage regimen/ frequency, duration of treatment) compared to few studies reporting insufficient details in their prescriptions. For example, dosage was mentioned in 10% [
25] and 61.2% [
16] prescriptions in two studies while incorrect dosages were mentioned in 9% prescriptions in the study by Ahsan et al. Duration of treatment was documented in 20% and 87% prescriptions in the studies by Shelat eat al [
25] and Ahsan et al [
19], respectively. In one study [
20], a relatively good proportion of prescriptions (84%) had drug names mentioned in capital letters, which was in alignment with our findings (90%); however this did not hold true for prescriptions from some other studies (Meenakshi et al [
16]:17.3%; Mercy et al [
21]:56.77%; Ahsan et al [
19]: 0%). Very few studies reported registration numbers of physicians mentioned in their prescriptions (Meenakshi et al [
16]: 79.7%; Mercy et al [
21]: 46.77%; Mulkalwar et al [
17]: 88%).
A limitation of the present study is that although the results indicate the areas which are lacking in terms of RUM but the reasons leading to irrational prescribing were not looked upon and revealed. Also, the indicators do not indicate whether the prescribed medicines are in harmony with diagnosis and standard treatment guidelines. Hence, further studies need to be conducted to explore the reasons for irrational use of medicines and define improvement strategies accordingly.
5. Conclusions
Prescription audit data from outpatient pharmacy of our tertiary care hospital in North India reflected conformance to WHO optimal values with respect to the number of antibiotics and injections prescribed per prescription. On the contrary, there were deviations from the recommended values for other WHO/INRUD indicators with high incidence of polypharmacy, brand name and non-essential drug prescribing. Variable adherence to WHO standards have been observed in previous studies from India with some reporting high incidence of antibiotics and injection use as well which may partly be explained by differences in study settings (public versus private), prescribers’ qualifications and prevailing prescribing practices. Based on our findings and comprehensive evaluation of data from other parts of country, sensitization and periodic training of prescribers on rational drug use is an important target for intervention. Indian Council of Medical Research (ICMR) has taken a pivotal step in this direction by launching “ICMR- National Virtual Centre Clinical Pharmacology (NvCCP) Prescribing Skills course for Indian Medical Graduates [
33]. Further, there is a continuous need to formulate, enforce and periodically revise the standard treatment guidelines. There must be strong political commitments to ensure availability of essential medicines in a sustainable manner as a key step towards promoting their prescribing.
Author Contributions
Conceptualization, N.M. and R.M.; methodology, N.V., S.V., and N.M.; validation, N.V. and S.V.; formal analysis, N.V., S.V., N.M., and N.B.; investigation, N.V., S.V., and N.B.; resources, N.M., and R.M.; data curation, N.V., S.V., and N.B.; writing—original draft preparation, N.V., and N.M.; writing—review and editing, N.V., S.V., N.M., R.M., and N.B.; visualization, N.M., and R.M.; supervision, N.M., and R.M.; project administration, N.M., and R.M.; funding acquisition, Not applicable. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board (or Ethics Committee) of PGIMS, Rohtak (vide letter no. BREC/23/497 dated 17.10.23).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
All the relevant data are contained within the article.
Acknowledgments
The authors thank the Medical Superintendent of hospital for granting permission to collect data for the study.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| RUM |
Rational use of medicines |
| WHO |
World Health Organisation |
| INRUD |
International Network of Rational Use of Drugs |
| NLEM |
National List of Essential Medicines |
| IRDP |
Index of rational drug prescribing |
| OPD |
Outpatient department |
| EML |
Essential Medicines List |
| FDC |
Fixed dose combination |
| LMICs |
Lower-middle income countries |
| NvCCP |
National Virtual Centre Clinical Pharmacology |
| ICMR |
Indian Council of Medical Research |
References
- World Health Organization. Promoting rational use of medicines. Accessed December 22, 2024. https://www.who.int/activities/promoting-rational-use-of-medicines.
- Ofori-Asenso, R.; Agyeman, A. Irrational Use of Medicines-A summary of key concepts. Pharmacy. 2016, 4, 35. [Google Scholar] [CrossRef] [PubMed]
- Mao, W.; Vu, H.; Xie, Z.; Chen, W.; Tang, S. Systematic review on irrational use of medicines in China and Vietnam. PLoS ONE. 2015, 10, e0117710. [Google Scholar] [CrossRef] [PubMed]
- Pan American Health Organization. Irrational Use of Medicines. Published 2010. Accessed January 3, 2025. https://www3.paho.org/hq/dmdocuments/2010/3_IrrationalSG.pdf.
- Wiedenmayer, K.; Summers, R.S.; Mackie, C.A.; Gous, A.G.; Everard, M.; Tromp, D. The pursuit of responsible use of medicines: Sharing and learning from country experiences. ResearchGate. Accessed January 3, 2025. https://www.researchgate.net/publication/269640048_The_pursuit_of_responsible_use_of_medicines_Sharing_and_learning_from_country_experiences.
- World Health Organization. How to Investigate Drug Use in Health Facilities: Selected Drug Use Indicators. Geneva, Switzerland: World Health Organization; 1993. Accessed January 3, 2025. https://www.who.int/publications/i/item/who-dap-93.1.
- World Health Organization. Introduction to Drug Utilization Research. Geneva, Switzerland: World Health Organization; 2003. Accessed January 3, 2025. https://www.who.int/publications/i/item/8280820396.
- Isah, A.; Laing, R.; Quick, J. , et al. The development of reference values for the WHO health facility core prescribing indicators. West Afr J Pharmacol Drug Res. [CrossRef]
- Siele, S.M.; Abdu, N.; Ghebrehiwet, M.; Hamed, M.R.; Tesfamariam, E.H. Drug prescribing and dispensing practices in regional and national referral hospitals of Eritrea: Evaluation with WHO/INRUD core drug use indicators. PLoS ONE. 2022, 17, e0272936. [Google Scholar] [CrossRef] [PubMed]
- Amaha, N.D.; Weldemariam, D.G.; Abdu, N.; Tesfamariam, E.H. Prescribing practices using WHO prescribing indicators and factors associated with antibiotic prescribing in six community pharmacies in Asmara, Eritrea: a cross-sectional study. Antimicrob Resist Infect Control. 2019, 8, 62. [Google Scholar] [CrossRef] [PubMed]
- Gebramariam, E.T.; Ahmed, M. Evaluation of rational medicine use based on WHO core drug use indicators in public hospitals in West Shoa Zone, Oromia, Ethiopia. Adv Pharmacoepidemiol Drug Saf. 2019, 8, 225. [Google Scholar] [CrossRef]
- Sisay, M.; Mengistu, G.; Molla, B.; Amare, F.; Gabriel, T. Evaluation of rational drug use based on World Health Organization core drug use indicators in selected public hospitals of eastern Ethiopia: a cross-sectional study. BMC Health Serv Res. 2017, 17, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Atif, M.; Sarwar, M.R.; Azeem, M.; Naz, M.; Amir, S.; Nazir, K. Assessment of core drug use indicators using WHO/INRUD methodology at primary healthcare centers in Bahawalpur, Pakistan. BMC Health Serv Res. 2016, 16, 684. [Google Scholar] [CrossRef] [PubMed]
- Galappatthy, P.; Ranasinghe, P.; Liyanage, C.K.; Wijayabandara, M.S.; Mythily, S.; Jayakody, R.L. WHO/INRUD core drug use indicators and commonly prescribed medicines: a national survey from Sri Lanka. BMC Pharmacol Toxicol. 2021, 22, 67. [Google Scholar] [CrossRef] [PubMed]
- Kilipamwambu, A.; Bwire, G.M.; Myemba, D.T.; Njiro, B.J.; Majigo, M.V. WHO/INRUD core prescribing indicators and antibiotic utilization patterns among primary health care facilities in Ilala district, Tanzania. JAC Antimicrob Resist. 2021, 3, dlab049. [Google Scholar] [CrossRef] [PubMed]
- Meenakshi, R.; Selvaraj, N.; Anandabaskar, N.; Dhamodharan, A.; Badrinath, A.K.; Rajamohammad, M.A. Prescription audit of a teaching hospital in South India using World Health Organization core prescribing indicators – a cross-sectional study. Perspect Clin Res, 1: 13. [CrossRef]
- Mulkalwar, S.; Patel, A.; David, S.; Pabari, K.; Math, P.; Tilak, A.V. Prescription audit for WHO prescribing indicators and prescription errors in a tertiary care teaching hospital. Med J DY Patil Vidyapeeth, 2: 17. [CrossRef]
- Dhanya, T.H.; Sanalkumar, K.B.; Andrews, M.A. Prescription auditing based on the World Health Organization (WHO) prescribing indicators in outpatient department of a teaching hospital in Kerala. Asian J Pharm Clin Res. 2021, 14, 41305. [Google Scholar] [CrossRef]
- Ahsan, M.; Shaifali, I.; Mallick, A.K.; Singh, H.K.; Verma, S.; Shekhar, A. Prescription auditing based on World Health Organization (WHO) prescribing indicators in a teaching hospital in North India. Int J Med Res Rev, 1: 4, 1847. [Google Scholar] [CrossRef]
- Sunny, D.; Roy, K.; Benny, S.S.; Mathew, D.C.; Naik, G.J.; Gauthaman, K. Prescription audit in an outpatient pharmacy of a tertiary care teaching hospital—a prospective study. J Young Pharm. 2019, 11, 417–420, Accessed January 3, 2025. https://www.academia.edu/51624556/Prescription_Audit_in_an_Outpatient_Pharmacy_of_a_Tertiary_Care_Teaching_Hospital_A_Prospective_Study. [Google Scholar] [CrossRef]
- Mercy, M.; Antony, L.J. A prescription audit using the WHO core drug use indicators in a rural health training center of Pondicherry. CHRISMED J Health Res. 2022, 9, 183–7. [Google Scholar] [CrossRef]
- Singh, T.; Banerjee, B.; Garg, S.; Sharma, S. A prescription audit using the World Health Organization-recommended core drug use indicators in a rural hospital of Delhi. J Educ Health Promot. 2019, 8, 37. [Google Scholar] [CrossRef] [PubMed]
- Galappatthy, P.; Ranasinghe, P.; Liyanage, C.K. , et al. Core prescribing indicators and the most commonly prescribed medicines in a tertiary health care setting in a developing country. Adv Pharmacol Pharm Sci, 1: 2021, 2021. [Google Scholar] [CrossRef]
- Nyabuti, A.O.; Okalebo, F.A.; Guantai, E.M. Examination of WHO/INRUD core drug use indicators at public primary healthcare centers in Kisii County, Kenya. Adv Pharmacol Pharm Sci, 1: 2020, 2020. [Google Scholar] [CrossRef]
- Shelat, P.R. Analysis of outdoor patients’ prescriptions according to World Health Organization (WHO) prescribing indicators among private hospitals in western India. J Clin Diagn Res. 2015, 9, FC01–FC04. [Google Scholar] [CrossRef] [PubMed]
- Potharaju, H.; Kabra, S. Prescription audit of outpatient attendees of secondary level government hospitals in Maharashtra. Indian J Pharmacol. 2011, 43, 150. [Google Scholar] [CrossRef] [PubMed]
- Hazra, A.; Tripathi, S.K.; Alam, M.S. Prescribing and dispensing activities at the health facilities of a non-governmental organization. Natl Med J India. 2000, 13, 177–82. [Google Scholar] [PubMed]
- Zhang Y, Zhi M. Index system, appraising method for comprehensive appraisal. J North Jiaotong Univ. 1995;19:393-400. Accessed January 3, 2025. https://www.scirp.org/reference/referencespapers?referenceid=2435385.
- Kun Y. Analysis of factors affecting physicians’ prescribing conduct. Published 2002. Accessed January 3, 2025. https://www.semanticscholar.org/paper/Analysis-of-factors-affecting-physicians'-conduct-Kun/ec8dab27292099fc12d919aa772d1a30e0cdaec4.
- Seden, K.; Kirkham, J.J.; Kennedy, T. , et al. Cross-sectional study of prescribing errors in patients admitted to nine hospitals across North West England. BMJ Open. 2013, 3, e002036. [Google Scholar] [CrossRef] [PubMed]
- Mittal, N.; Mittal, R.; Singh, S.; Godara, S. The availability of essential antimicrobials in public and private sector facilities: a cross-sectional survey in a district of North India. Antibiotics. 2024, 13, 131. [Google Scholar] [CrossRef] [PubMed]
- Shafiq, N.; Pandey, A.K.; Malhotra, S. , et al. Shortage of essential antimicrobials: a major challenge to global health security. BMJ Global Health. 2021, 6, e006961. [Google Scholar] [CrossRef] [PubMed]
- ICMR-SPH. RUD. ICMR-SPH. Published August 10, 2022. Accessed January 3, 2025. https://nie.gov.in/icmr_sph/RUD.html.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).