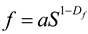1. Introduction
Rare earths are often referred to as the “mother of new materials” and “industrial gold,” and are widely used in cutting-edge technological fields such as optics, electronic information, aerospace, and nuclear industries [
1,
2]. Ionic rare earths, also known as weathered crust elution rare earths, are rich in medium and heavy rare earth elements and are predominantly found in southern regions such as Jiangxi, Fujian, and Guangxi [
3]. As an important strategic resource in China, ionic rare earths hold substantial economic value. In ionic rare earth deposits, rare earths are adsorbed onto clay minerals in the form of hydrated cations or hydroxylated cations, with characteristics such as a complete distribution, high content of heavy rare earth elements, low radioactivity contrast, high comprehensive utilization value, and simple extraction processes [
4,
5]. The mining of ionic rare earths has gone through three generations of mining processes: pool leaching, heap leaching, and in-situ leaching. In-situ leaching, known for its high efficiency, environmental friendliness, and low cost, has been widely adopted in areas with favorable geological conditions [
6,
7,
8,
9]. The in-situ leaching process involves injecting leaching agents into the ore body through a well network from the mountain top, without disturbing surface soil or vegetation. After sufficient ion exchange reactions between the leaching agent and the rare earths, the rare earth ions on the clay surface are desorbed by the cations in the leaching agent, forming rare earth leachate, which then flows into a collection pool at the mountain’s base through the collection tunnel [
10,
11,
12,
13]. In-situ leaching requires the injection of large volumes of leaching agent, placing the ore body in a complex environment of prolonged liquid injection, high saturation, enhanced chemical reactions, and combined self-weight and additional stress. This process gradually weakens the inter-particle structure and cohesion, resulting in a continuous decrease in the shear strength of the ore body [
14,
15]. During this process, the bond between mineral particles in the leached area is weakened, particle rearrangement and framework displacement occur, leading to changes in the effective stress of the ore body. These alterations significantly affect the pore structure of the ore body, decreasing the stability of the rock-soil mass and increasing the risk of geological disasters such as landslides [
16,
17]. This study examines the impact of different leaching actions on the shear strength properties of ionic rare earth ores and, through microscopic structural characterization, provides a theoretical basis for slope stability analysis and the development of safe mining plans in the in-situ leaching process of ionic rare earth deposits.
In the context of the safe and efficient mining of ionic rare earths, many scholars have made valuable scientific contributions and research advancements. Chen [
18] conducted shear tests on ionic rare earth ores after leaching with 3% (NH
4)
2SO
4. The results showed that after leaching, the cohesion of the mineral samples decreased, and the cohesion of each layer of the ore samples was lower than that of the pre-leaching samples. The internal friction angle was greater than that of the pre-leaching samples, and the internal friction angle increased progressively from the top to the bottom of the ore layers. Chen et al. [
19] conducted direct shear tests on rare earth ore samples subjected to varying leaching durations using a simulated leaching apparatus, and analyzed the influence of leaching agents on the shear strength of the ore body. The study elucidated the underlying mechanisms by which leaching time affects cohesion and internal friction angle. Huang et al. [
20], using triaxial compression tests, analyzed the effects of different concentrations of (NH
4)
2SO
4 leaching agents on the shear strength of the samples during the leaching process. The infiltration of the leaching agent increased soil porosity, and intense chemical reactions occurred between (NH
4)
2SO
4 and the rare earth ores during the leaching process, altering the internal structure of the ores and weakening the strength of the ionic rare earth ore body. Peng et al. [
21] conducted zeta potential tests, particle size distribution tests, and direct shear tests to reveal the effects of different concentrations of (NH
4)
2SO
4 leaching agents on the thickness of the double electric layer, particle size distribution, and shear strength parameters of the ion-adsorbed rare earth ore samples.
Wang et al. [
22] conducted leaching tests and nuclear magnetic resonance (NMR) tests to investigate the chemical and physical effects of (NH
4)
2SO
4 leaching during ion exchange and infiltration. These effects alter the stability of fine particles, causing them to migrate and leading to changes in the pore structure. At low confining pressures (50 kPa and 100 kPa), the shear behavior of the samples transitioned from strain hardening to strain softening, and the peak strength of the rare earth samples increased. Zhou et al. [
23] carried out triaxial tests on rare earth ore bodies at different leaching times, developed a constitutive model for the stress-strain behavior of the rare earth samples, and analyzed the pore structure changes in the ore body using NMR technology. Yin et al. [
24,
25] performed direct shear tests on ionic rare earth ore samples with varying porosity ratios and developed a model linking porosity ratio to shear strength indicators, revealing the influence mechanism of porosity ratio on the cohesion and internal friction angle of the samples. Guo et al. [
26] utilized digital image analysis to study the microstructural evolution of pore size, distribution, and shape at different depths before and after leaching in rare earth ore samples. Liu et al. [
27] conducted direct shear tests and X-ray diffraction (XRD) analysis under varying normal stresses to examine the effect of particle size on the shear strength of rare earth ore samples, revealing the influence mechanism of particle size on cohesion and internal friction angle. Zhang et al. [
28] performed laboratory leaching simulation experiments to compare and analyze the changes in Zeta potential during leaching with different concentrations of leaching agents. They explored the transformation of the pore structure during the leaching process and found that the pure water leaching process had minimal impact on the ore body’s pore structure, whereas the MgSO
4 solution leaching process induced significant changes in the ore body’s pore structure. Currently, research on the strength change laws of ionic rare earth ore bodies under various leaching conditions remains incomplete, and there is limited research on how different leaching agents and their concentrations affect the strength characteristics of ionic rare earths. Therefore, studying the variations in ore body strength characteristics under different leaching conditions is crucial for understanding the intrinsic mechanisms behind shear strength changes in ionic rare earths.
This study focuses on ionic rare earth ores from southern China and utilizes the GDS stress-path triaxial apparatus to conduct triaxial shear tests. It aims to investigate the variations in shear strength of ionic rare earths after leaching with different types of leaching agents and varying concentrations of MgSO4. Scanning electron microscope (SEM) analysis was performed on the ore samples before and after treatment with different concentrations of leaching agents, investigating the effects of different leaching actions on the microstructure of ionic rare earths. The findings provide a theoretical foundation for understanding the macroscopic strength characteristics of ionic rare earths, offer theoretical support for enhancing rare earth extraction efficiency, and provide theoretical guidance for landslide prevention and safe mining practices.
2. Experimental Materials and Methods
2.1. Test Samples
The soil samples used in the experiment were collected from a rare earth mining area in Longyan City, Fujian Province. Sampling was performed manually around the injection wells, with samples taken from depths of 1 to 3 meters below the surface. In accordance with relevant standards, basic physical properties, including density, moisture content, liquid limit, and plastic limit, of the undisturbed soil were measured, as shown in
Table 1. The particle size distribution curve is presented in
Figure 1. Based on the soil engineering classification standard, the plasticity index (IP) is 12.6 (≥7) and the liquid limit (WL) is 41.83 (<50%). Judging by the liquid limit and plasticity index, this soil is classified as low liquid limit silty clay.
The mineral composition of the soil samples was determined using an X-ray diffractometer (XRD). X-ray fluorescence (XRF) uses X-rays to excite characteristic radiation emitted by elements in the mineral samples, enabling the analysis of their elemental composition and concentration. The primary elemental composition of the soil samples, as determined in the experiment, is presented in
Table 2.
2.2. Experimental Equipment
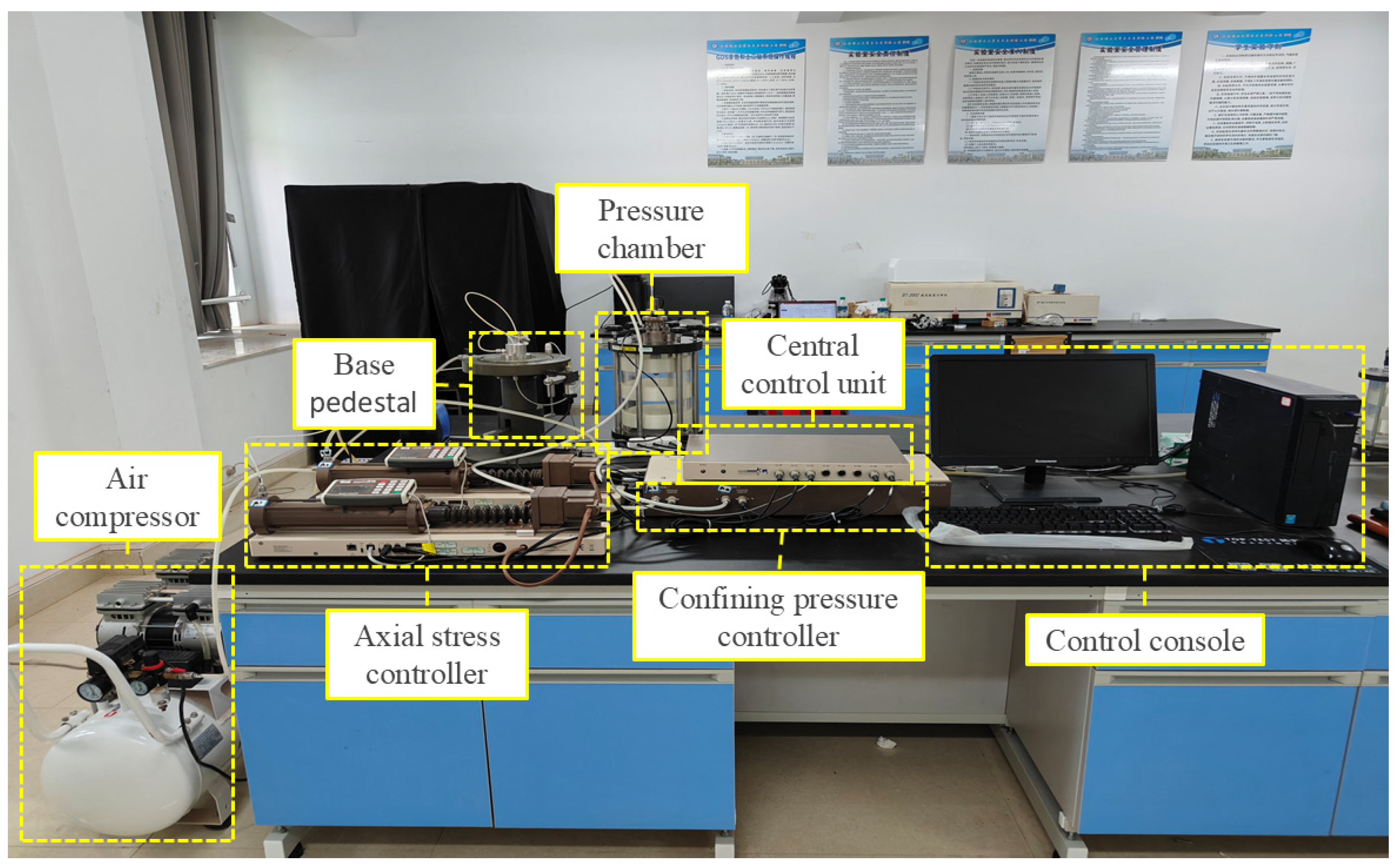
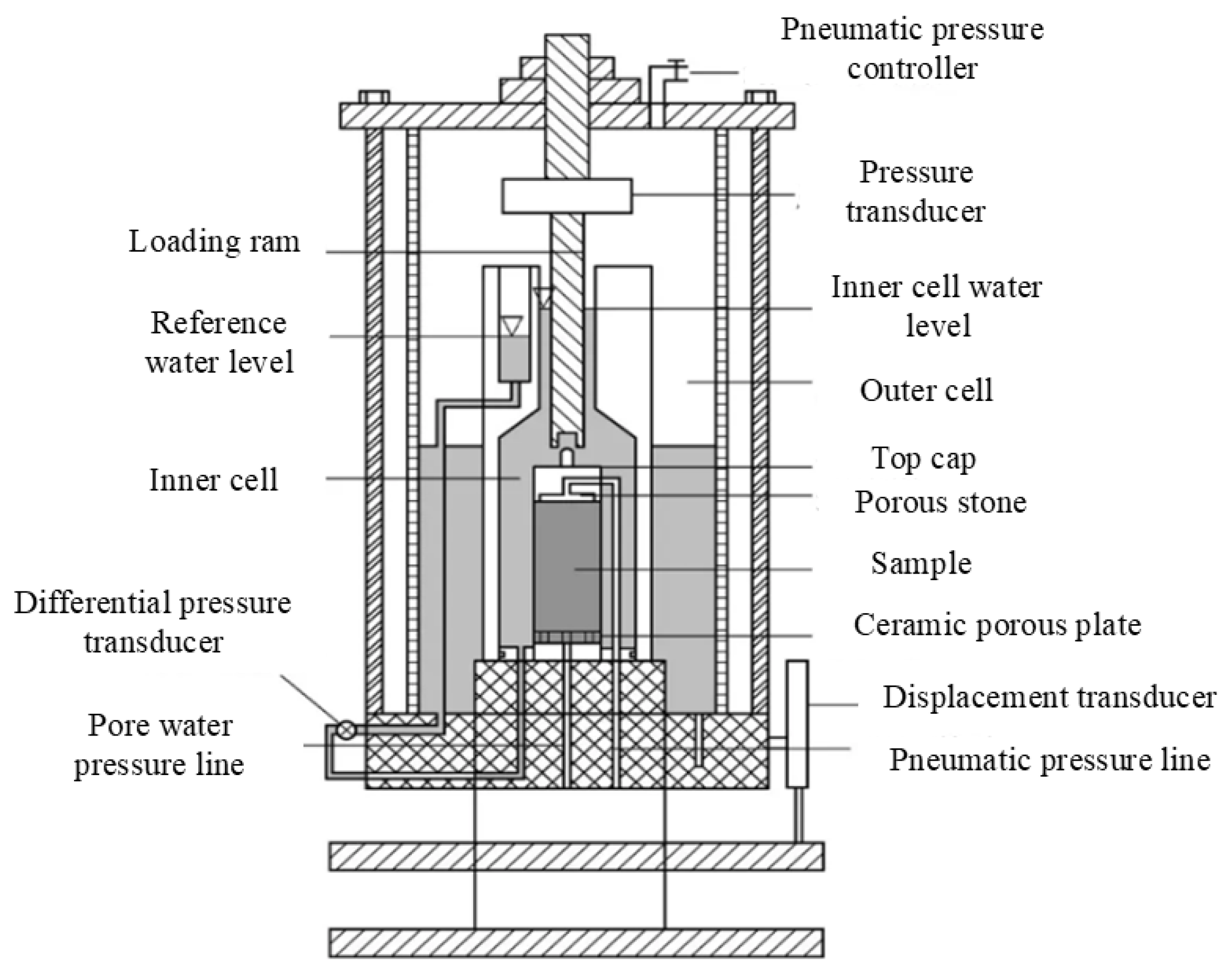
In this experiment, triaxial shear tests were conducted using the GDS Stress-Path Triaxial Testing System. A photograph of the device is shown in
Figure 2, and a schematic of the pressure chamber is shown in
Figure 3. Developed by Geomaterials Instruments Technology Ltd. (Europe and America), this geotechnical testing device is primarily used for conducting triaxial shear tests and is designed to investigate the mechanical properties and behavior of mineral samples under complex stress paths.
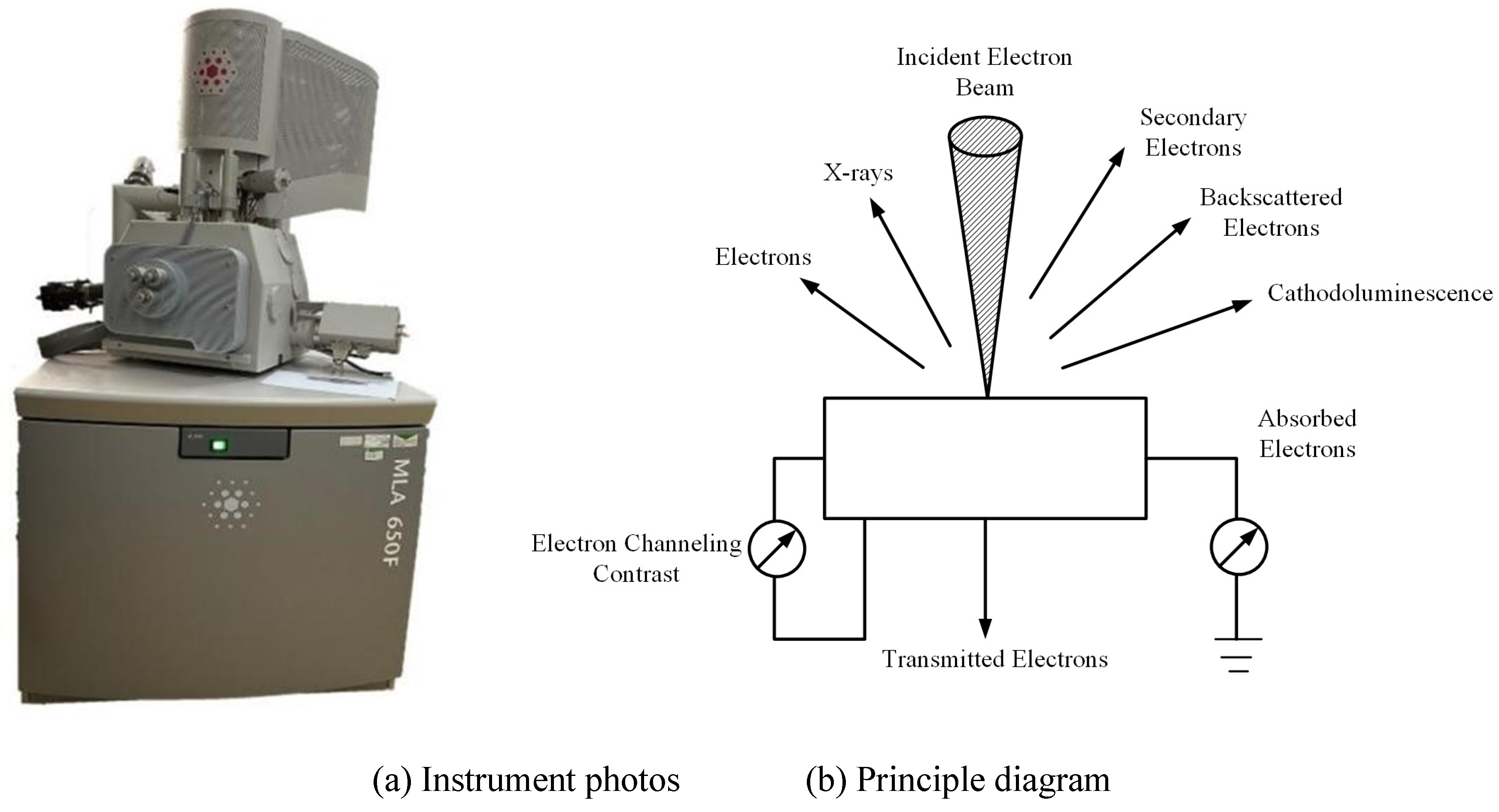
In this study, scanning electron microscopy (SEM) was conducted using the MLA650F field emission scanning electron microscope to analyze the microstructural pore characteristics of the samples. The instrument and its operating principle are illustrated in
Figure 4.
Prior to SEM testing, the sample was mounted on a copper stub using conductive adhesive, and loose particles were removed to enhance image clarity. As the sample consisted of dried powdered rare earth material, gold sputter coating was performed using a specimen coater to improve electrical conductivity. After coating, the sample was placed in the observation chamber of the electron microscope, where the chamber was evacuated and high voltage was applied. During observation, well-preserved and representative microstructural regions were selected and examined progressively from low to high magnification. SEM images of the selected areas were captured and saved.
2.3. Experimental Design
This study focuses on examining the influence of different types and concentrations of leaching agents on the shear strength of ionic rare earths. Triaxial shear tests were conducted under confining pressures of 50 kPa, 100 kPa, and 150 kPa. The leaching test conditions were designed as follows:
(1) Leaching agent types: Pure water, 6% (NH4)2SO4, 6% MgSO4, and 6% Al2(SO4)3 solutions were selected as leaching agents, with a leaching duration of 48 hours.
(2) Leaching agent concentrations: MgSO4 solutions with concentrations of 3%, 6%, and 9% were used, also with a leaching duration of 48 hours.
The detailed experimental conditions are listed in
Table 3.
After completing the triaxial shear tests, the samples were removed from the apparatus, immediately wrapped in plastic film, and stored in a test cabinet. The pure water condition was used as the control group, while the 6% (NH4)2SO4, 6% MgSO4, and 6% Al2(SO4)3 conditions served as test groups for different leaching agent types. The 3%, 6%, and 9% MgSO4 conditions were designated as test groups for varying leaching agent concentrations. Each condition was tested under confining pressures of 50 kPa, 100 kPa, and 150 kPa, resulting in a total of 18 samples. Prior to SEM analysis, all samples were retrieved, and soil was taken from the central part of each specimen, placed in a container, and oven-dried at 105°C for 12 hours to remove internal moisture. After drying, the mineral samples from each condition were placed into labeled sealed bags and submitted for testing.
4. Microscopic Mechanism Analysis
4.1. SEM Morphological Analysis Under Different MgSO4 Concentrations
The original scanning electron microscopy (SEM) images were grayscale and required post-processing for pseudo-coloring. Image coloration was based on a grayscale threshold, with higher values corresponding to lighter shades. The optical threshold was determined using PCAS image processing software to generate binarized images, where black regions indicate ore particles and white regions correspond to pores. Particle–pore boundaries in the SEM images were delineated using a pore segmentation method [
30,
31]. To ensure clear visualization of particle morphology within the observation plane, SEM imaging was performed from low to high magnification, and images at 8000× magnification were ultimately selected for comparative analysis.
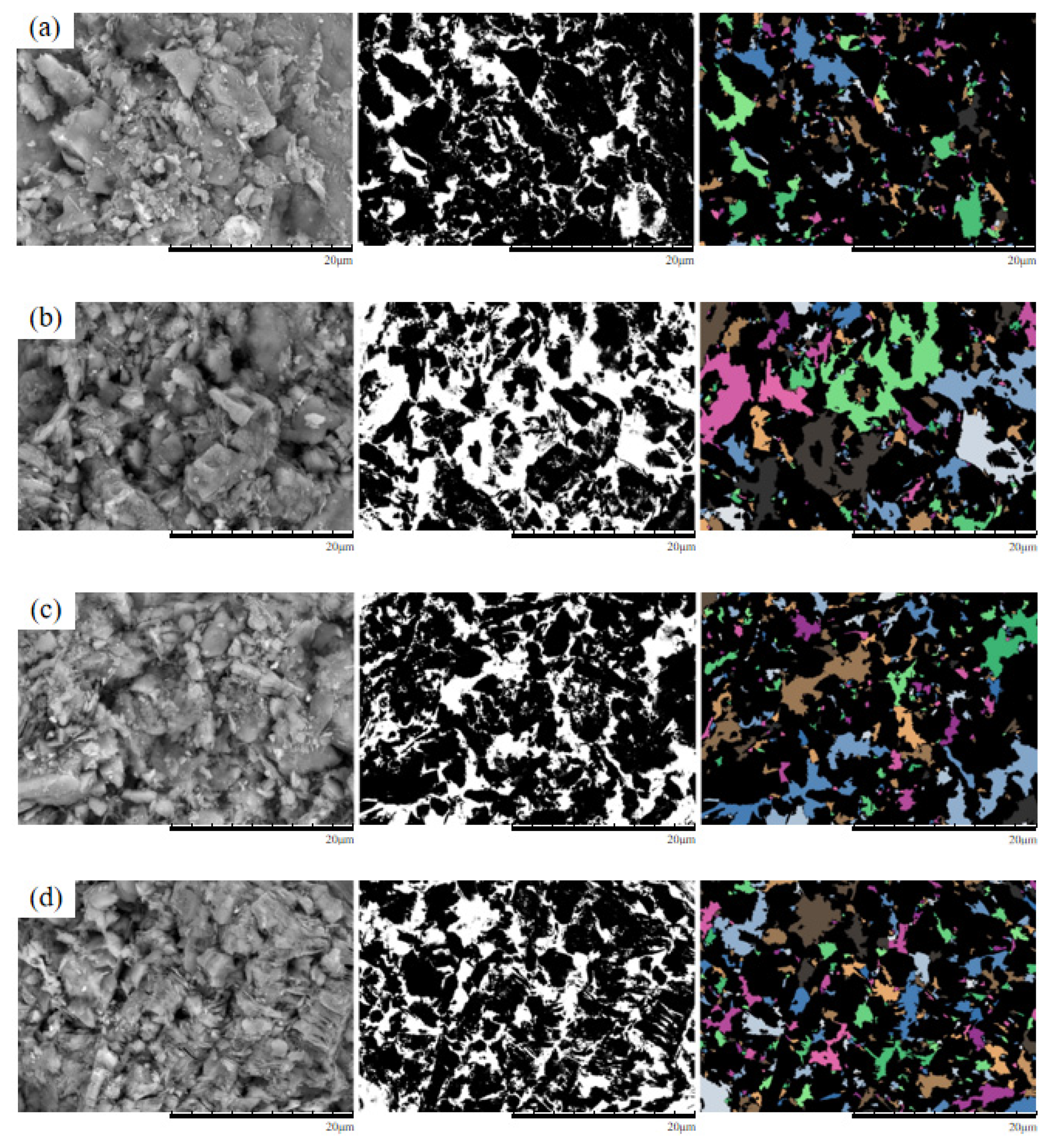
To investigate the structural evolution of ore sample microstructures under different leaching agent concentrations, four experimental conditions—pure water (0%), 3% MgSO
4, 6% MgSO
4, and 9% MgSO
4—were established at confining pressures of 50 kPa, 100 kPa, and 150 kPa. SEM analyses were conducted under these leaching conditions to assess the influence of MgSO
4 concentration on microstructural changes in the samples. At a confining pressure of 50 kPa, the original and processed SEM images for samples treated with different MgSO
4 concentrations are shown in
Figure 11.
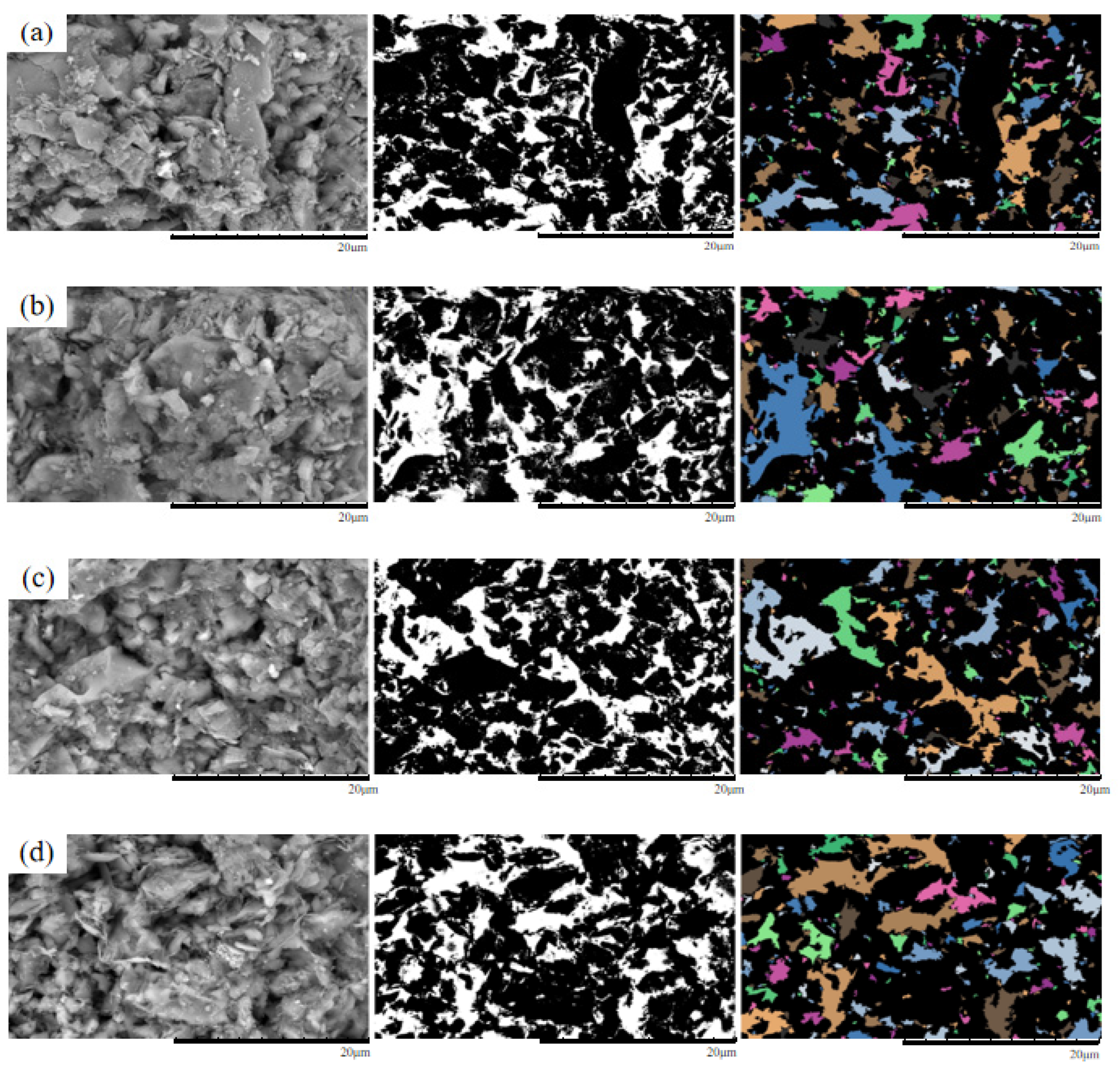
As shown in the figure, under a confining pressure of 50 kPa, the ore samples exhibit relatively concentrated distributions of large pores, predominantly angular in shape. Under the 3% MgSO4 condition, the pore distribution remains concentrated, with primarily angular pores indicative of an initially fragmented particle structure. At 6% MgSO4, the number of pores is reduced compared to other samples, with a more dispersed distribution pattern and a combination of angular and elongated morphologies. In the 9% MgSO4 condition, pores are more widely dispersed and predominantly elongated, with increased pore interconnectivity. Comparative analysis indicates that with increasing MgSO4 concentration, the pore distribution shifts from concentrated to dispersed, and pore morphology evolves from a predominantly angular form to a composite structure characterized by both angular and elongated features.
Under a confining pressure of 100 kPa, the SEM images and processed images of ionic rare earth samples subjected to different MgSO
4 concentrations are shown in
Figure 12.
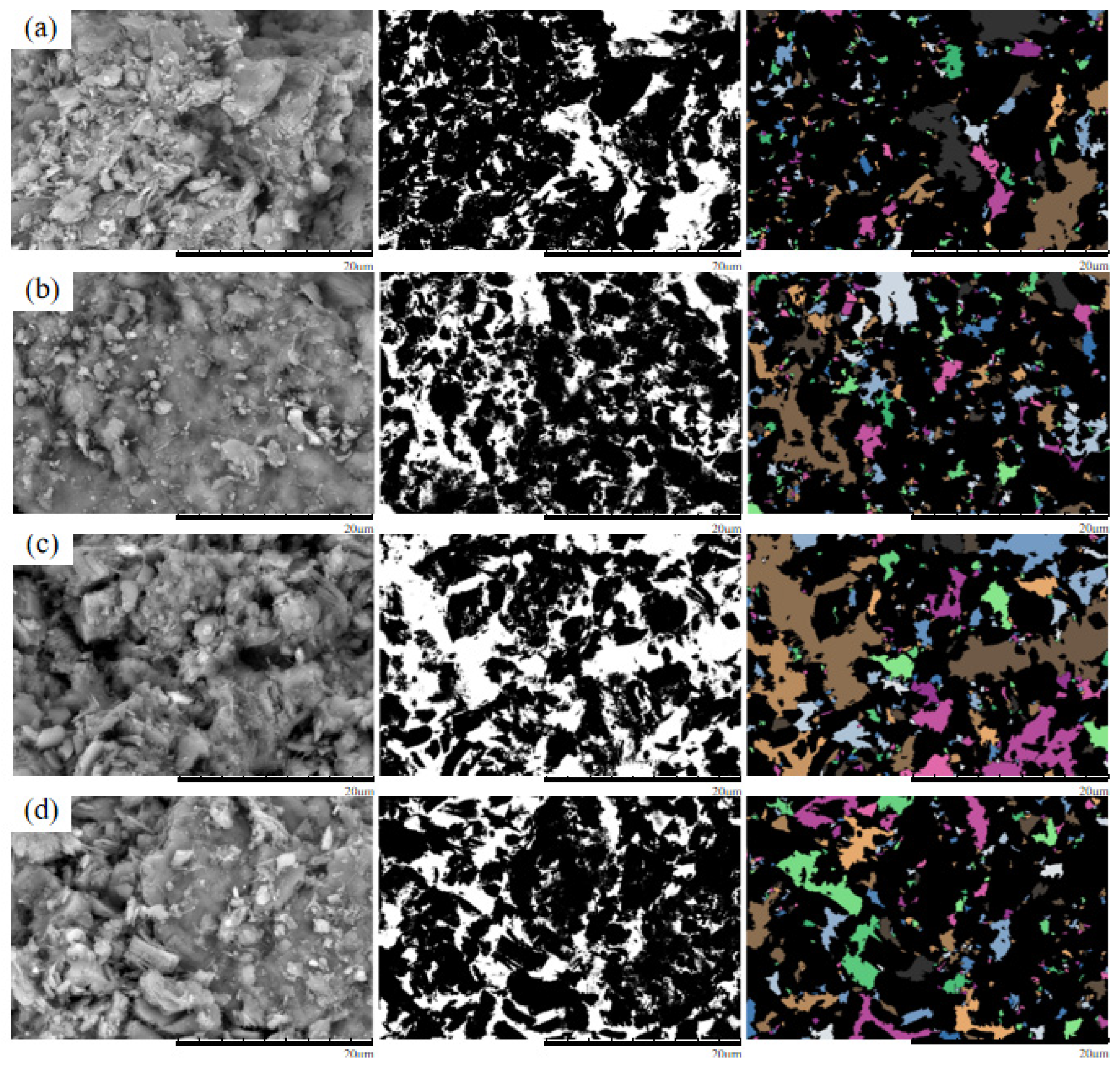
As shown in the figure, under a confining pressure of 100 kPa, the ore sample treated with 3% MgSO4 exhibits predominantly angular pores with uniform spatial distribution. At 6% MgSO4, both the number and average area of pores increase; small pores coalesce into larger ones, and pore shapes become more complex, primarily block-shaped. Under 9% MgSO4, the pore quantity increases markedly, average pore area further enlarges, and the morphology becomes more irregular, with most pores exhibiting block-shaped and elongated forms. This suggests that at 3% concentration, secondary fragmentation of particles dominates, resulting in finer pore structures. In contrast, at higher concentrations (6% and 9%), the synergistic effect of crystallization-induced compaction and confining pressure promotes the development of a stable particle skeleton, leading to increased pore area.
Under a confining pressure of 150 kPa, the SEM images and processed images of ionic rare earth samples subjected to different MgSO
4 concentrations are shown in
Figure 13.
As shown in the figure, at a confining pressure of 150 kPa, the sample treated with 3% MgSO4 exhibits predominantly angular pores, with multiple pores coalescing into large macropores. The soil particles appear relatively disaggregated. Under the 6% MgSO4 condition, the number of pores increases, the average pore area decreases, and the pore distribution becomes more uniform; the soil particles appear more fragmented. In the 9% MgSO4 condition, the number of pores decreases, the average pore area further diminishes, and the distribution becomes more concentrated.
4.2. Quantitative Analysis of Microscopic Pore Structure
A quantitative analysis was conducted to investigate the evolution of the microscopic pore structure of the ore samples before and after leaching, focusing on three key aspects: pore proportion, pore distribution, and pore morphology.
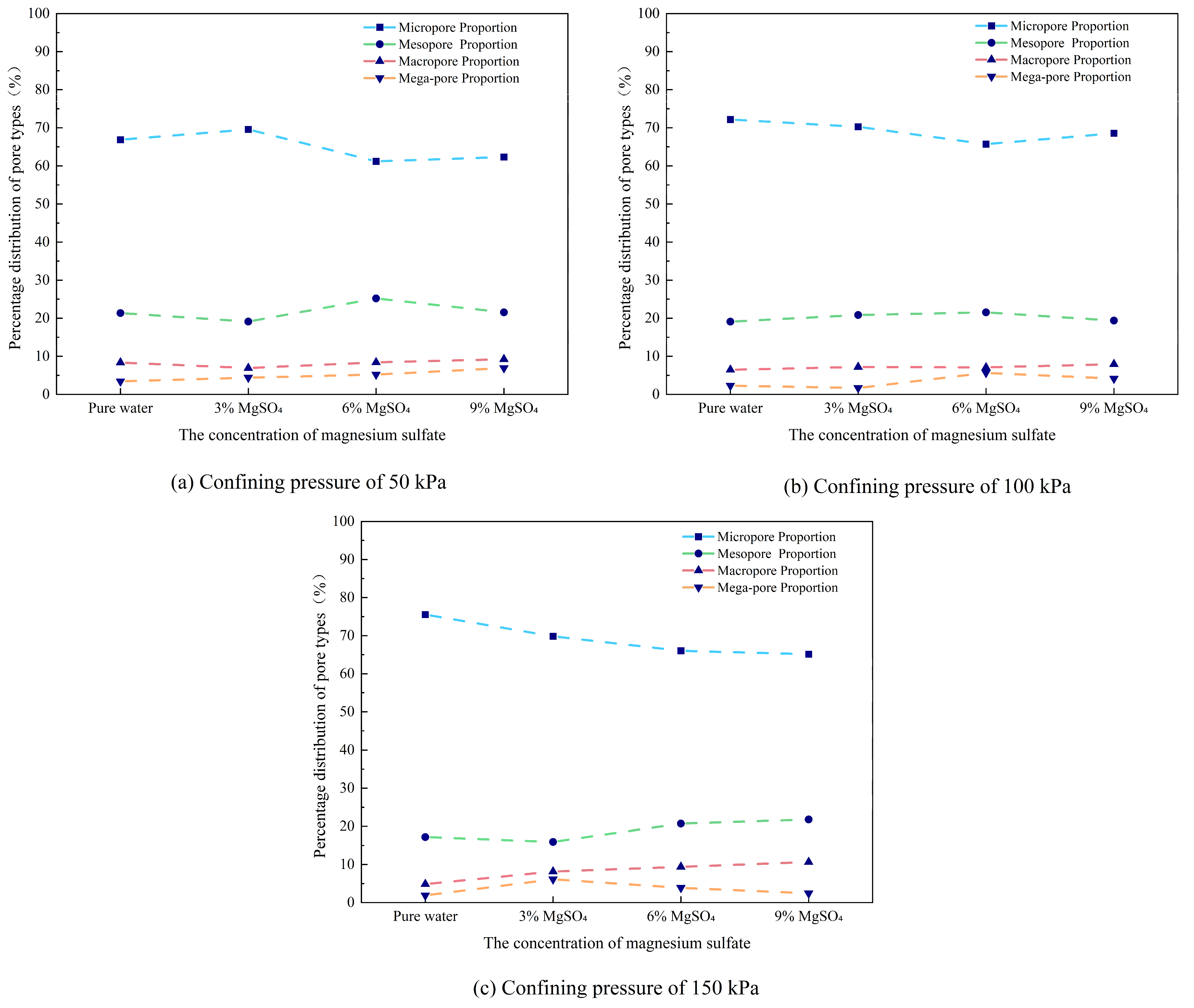
(1) Pore Proportion
In this study, pore sizes were classified as follows: micropores (<4 μm), small pores (4–16 μm), medium pores (16–64 μm), large pores (64–256 μm), and macropores (>256 μm). SEM images were processed at a consistent magnification of 8000× using images of identical dimensions (1536 × 1103 pixels). According to the scale bar, each image corresponds to a real area of 51.2 μm × 36.8 μm, yielding a resolution of 30 pixels/μm. Based on this resolution, the corresponding pixel-based classification is as follows: micropores (<120 pixels), small pores (120–480 pixels), medium pores (480–1920 pixels), large pores (1920–7680 pixels), and macropores (>7680 pixels). The proportions of each pore size category and the total number of pores for the ore samples under different test conditions are summarized in
Table 5. The variation in pore structure with MgSO
4 concentration is shown in
Figure 14.
Under a confining pressure of 50 kPa, the proportions of large and macropores gradually increase with rising MgSO4 concentration, although the overall variation remains modest. Medium pores exhibit a non-linear trend, initially decreasing, then increasing, and subsequently decreasing again. Small pores show a complementary pattern, with an initial increase followed by a decrease and a subsequent rise.
Under a confining pressure of 100 kPa, the proportion of large pores remains relatively stable across the concentration gradient. Macropores show minor fluctuations with a slight upward trend, while medium pores vary within a narrow range. In contrast, the proportion of small pores changes more significantly, displaying a decrease followed by an increase.
Under a confining pressure of 150 kPa, the macropore proportion first increases and then decreases, with more pronounced changes than those observed under lower confining pressures. Large pores consistently increase with concentration. Medium pores follow a similar trend as in the other conditions—first decreasing, then increasing, and decreasing again. The proportion of small pores shows a clear downward trend.
Under different conditions, the proportion of pores at each scale evolves through a synergistic regulation mechanism driven by the combined effects of MgSO4 concentration and confining pressure. At low confining pressure, the variation in pore size distribution is mainly governed by the ion exchange reaction involving Mg²⁺, which leads to the breaking of hydrogen bonds between clay mineral layers and promotes the initiation of microcracks. Meanwhile, the dynamic balance between cementation and dispersion causes fluctuations in the proportions of medium and small pores. At high confining pressure, the influence of MgSO4 on pore size distribution becomes more pronounced. The increase in confining pressure allows the leaching agent to react more fully with the sample, promoting the merging of small pores into medium pores, while macropores are decomposed into multiple large or medium pores during the exchange process.
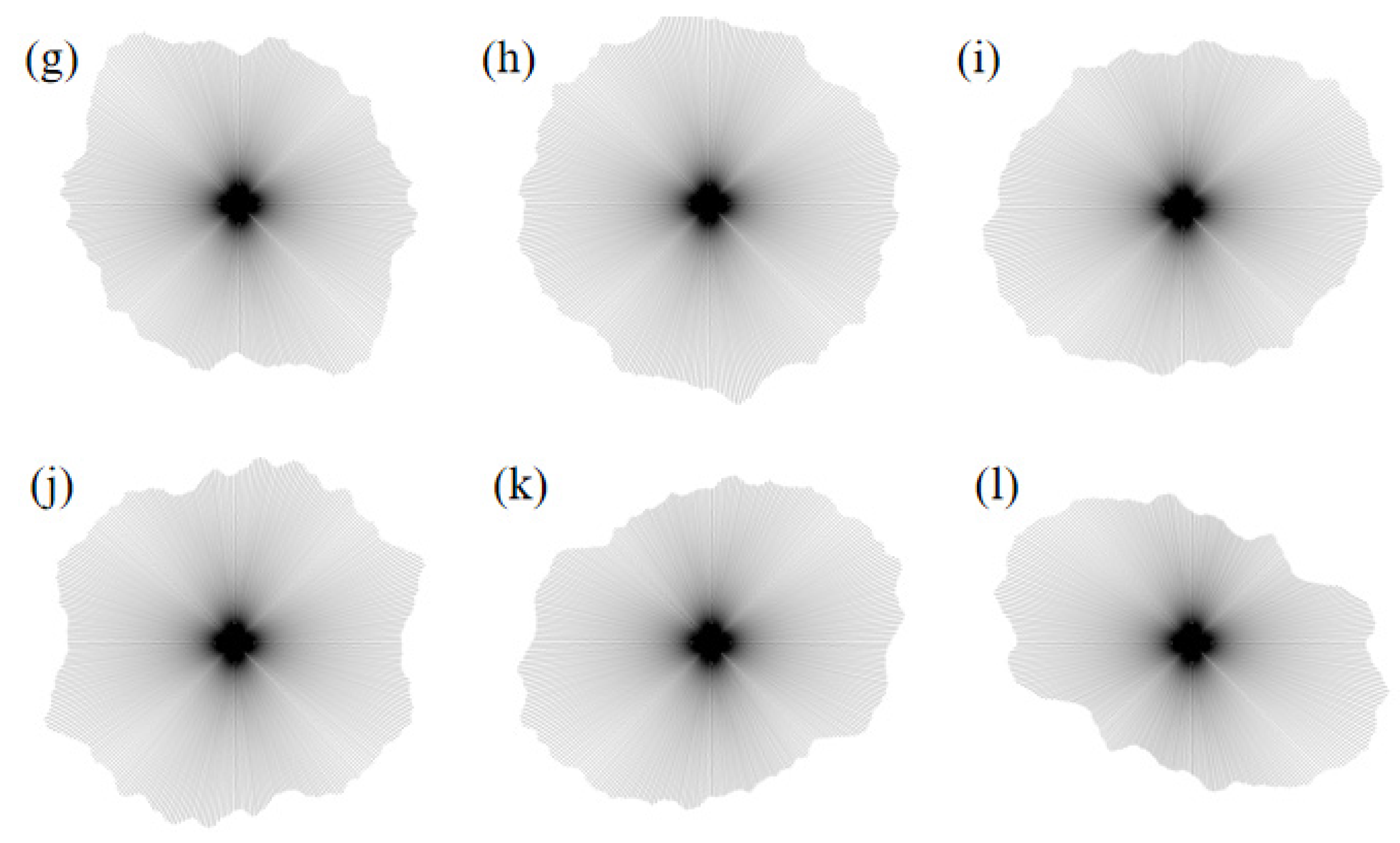
(2) Pore Distribution Rose Diagram
The pore distribution rose diagram, also known as a “polar plot,” is a graphical representation in polar coordinates that illustrates the relationship between pore distribution and characteristics such as pore size, shape, and orientation. Each sector corresponds to a specific range of pore characteristics, and the size and height of each sector indicate the number and proportion of pores within that range. The rose diagram enables a more intuitive observation and analysis of pore distribution patterns and effectively reflects the consistency and diversity of different pore types within the sample. The rose diagrams of pore distribution, drawn based on SEM images of the samples after testing, are shown in
Figure 15.
As shown in the figure, under a confining pressure of 50 kPa, the rose diagram for the pure water condition reveals a well-developed and relatively concentrated pore space, indicating that the material remains loosely structured and has not undergone significant compaction at this stress level. With 3% MgSO4, the pore distribution becomes more uniform, and the number of pores decreases. At 6% concentration, the distribution trend continues toward homogenization, reflected in the diagram by fewer sectors and a denser overall structure. At 9%, the pore morphology becomes more intricate and tightly packed, suggesting a pronounced compaction effect. This trend is attributed to the presence of MgSO4, which facilitates partial compaction of the pore structure. The MgSO4 solution interacts with soil particles, enhancing interparticle bonding. At higher concentrations, the intensified ion exchange promotes particle aggregation, thereby reinforcing the structural integrity of the granular skeleton.
Under a confining pressure of 100 kPa, the pore distribution under the 3% MgSO4 condition becomes further homogenized, accompanied by a noticeable reduction in pore size, indicating that this concentration effectively facilitates pore compaction. At 6% MgSO4, the pore structure exhibits increased stability, characterized by finer and more uniformly distributed pores—likely resulting from the enhanced chemical interactions of MgSO4 at this concentration. At 9%, the pores demonstrate a higher degree of compaction, with the emergence of more diverse and complex pore configurations, suggesting that increased MgSO4 concentration leads to further refinement and densification of the pore structure.
Under a confining pressure of 150 kPa, the pore structure in the pure water condition appears dense, indicating a high level of compaction and structural stability. In the 3% MgSO4 condition, the pore structure remains relatively compact, with further enhancement suggesting improved compressive resistance, likely due to hydration effects and increased interparticle affinity. At a concentration of 6%, the pore structure becomes more uniform and exhibits a more complex morphology compared to previous cases, suggesting that higher MgSO4 concentration effectively promotes structural cohesion and reinforcement. Under the 9% MgSO4 condition, the changes in pore structure are most evident, resulting in a denser and more tightly packed configuration. With increasing MgSO4 concentration, the rose diagrams under high confining pressure show significant changes, reflecting the compaction and reorganization of the pore network. These transformations are driven not only by chemical interactions but also by physical effects such as particle rearrangement and stress-induced densification.
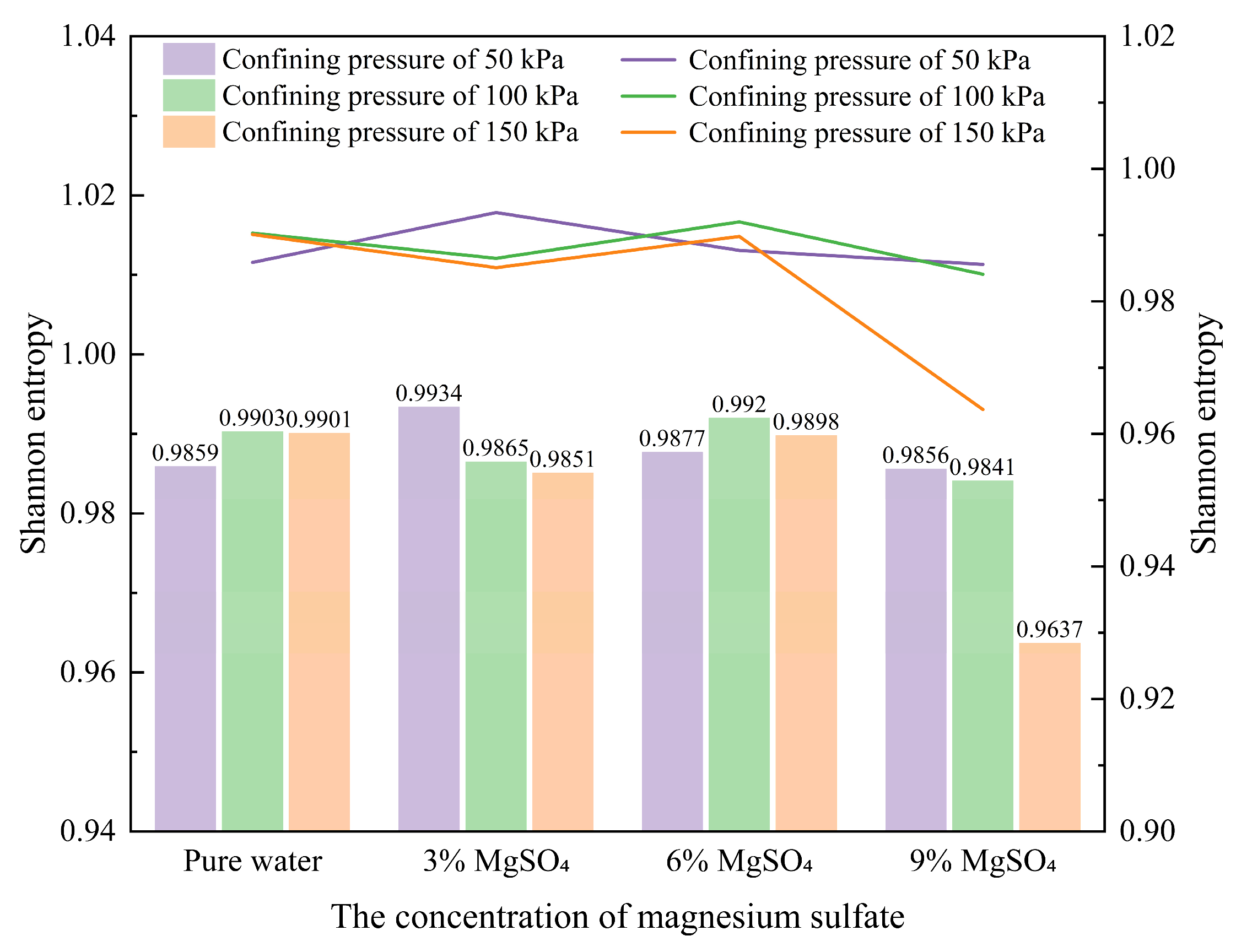
(1) Probability Entropy
Probability entropy is a metric used to measure the uncertainty or degree of disorder in a system. In information theory, entropy can be understood as the average uncertainty or information content. In the context of data analysis, it can be used to quantify the diversity, distribution patterns, or structural complexity of a sample. For the analysis of pore distribution in ionic rare earth samples, entropy can be applied to quantitatively evaluate the uniformity and distribution characteristics of the pores. From a microscopic perspective, this study employs probability entropy to reveal the variation patterns in pore distribution. The calculation formula is shown in Equation (1).
 |
(1) |
In the equation, the value of
Hi ranges from 0 to 1, where a higher value indicates a more disordered arrangement of particles and pore units. When
Hi =0, all pores are oriented in the same direction; when
Hi =1, the pore orientations are entirely divergent.
Pi represents the proportion of pores falling within a specific angular interval, and
n is the number of equal intervals dividing the 0°–180° range—for example, if the interval is 10°, then
n=18. The calculated probability entropy variations for different regions are illustrated in
Figure 16.
As illustrated in the figure, under a confining pressure of 50 kPa, the sample treated with 3% MgSO4 exhibits the highest probability entropy, indicating the greatest degree of disorder in its pore structure. As the MgSO4 concentration increases to 6% and 9%, the entropy values exhibit a slight decline, reflecting a more uniform and orderly distribution of microscopic pores within the ore samples.
Under a confining pressure of 100 kPa, the entropy value under the 3% MgSO4 condition is lower than that of the pure water condition, indicating a more ordered pore structure. As the MgSO4 concentration increases, the probability entropy initially rises and then falls, with the highest entropy observed at 6%, suggesting that this concentration results in the most heterogeneous and disordered pore distribution, exerting the greatest influence on the sample’s microstructural evolution. The entropy values for 3% and 9% MgSO4 are relatively close, indicating that both insufficient and excessive MgSO4 concentrations can induce particle aggregation and pore compaction, thereby increasing structural heterogeneity.
Under a confining pressure of 150 kPa, the probability entropy for the pure water condition remains comparable to that observed at 100 kPa, while the entropy value for the 3% MgSO4 condition exhibits a more pronounced decrease. With increasing MgSO4 concentration, the probability entropy initially rises and then drops sharply, indicating that under high confining pressure, the uniformity of the pore structure is more sensitive to changes in leaching agent concentration. The entropy reaches its lowest value at 9% MgSO4, suggesting that at this concentration, pore structure diversity and uniformity are significantly reduced, reflecting intensified particle aggregation and an overall trend of structural compaction.
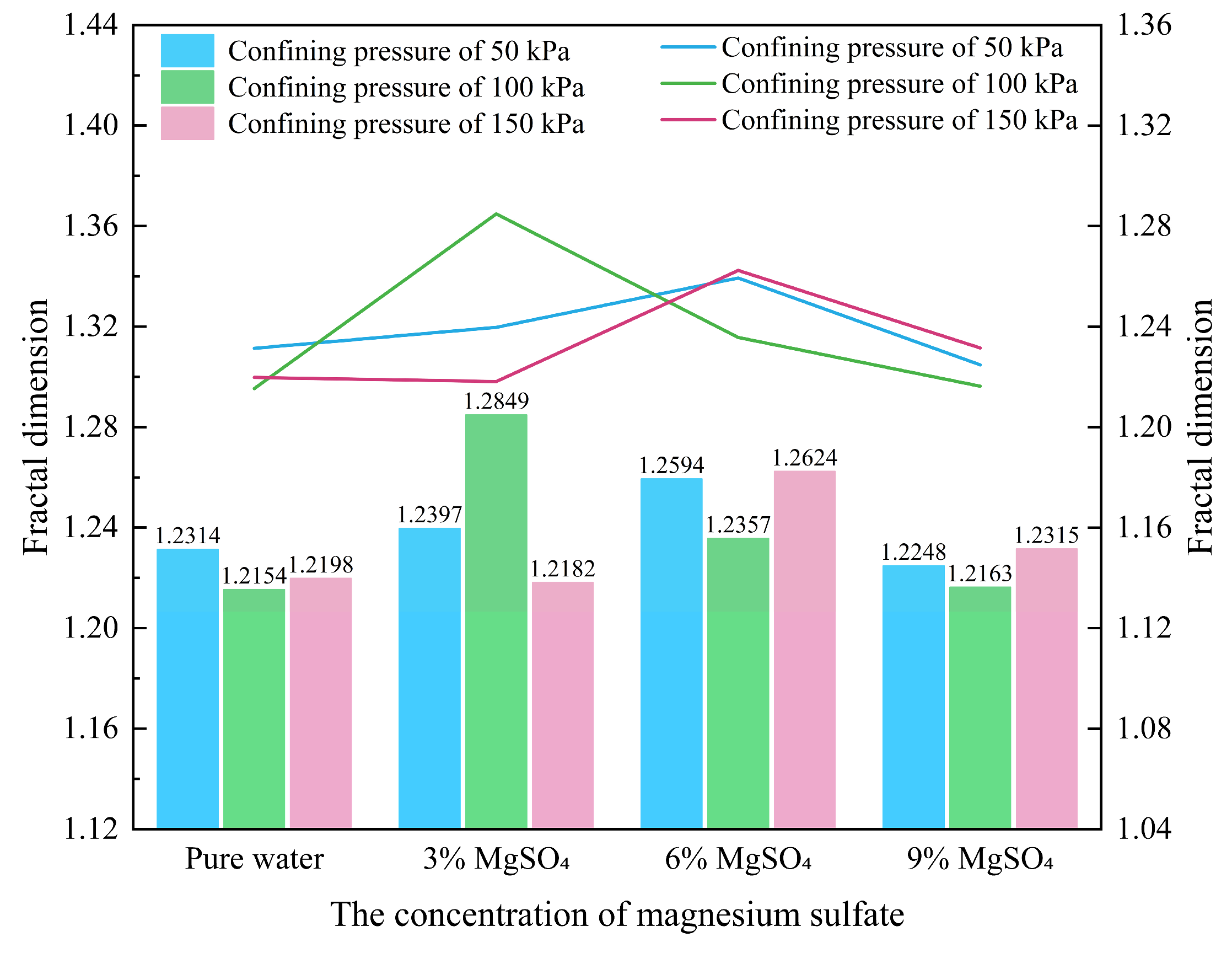
(2) Fractal Dimension
Fractal dimension is a powerful tool for understanding and analyzing complex structures, offering a more refined geometric characterization than traditional Euclidean dimensions. It reflects the space-filling capacity of irregular shapes. SEM observations of rare earth ore samples reveal that the microscopic pore structures exhibit pronounced fractal characteristics. Accordingly, fractal theory is applied to quantitatively investigate variations in pore morphology. The fractal dimension of pore shape is derived from the relationship between pore area and shape factor, expressed by the following equation:
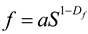 |
(2) |
In the equation,
Df denotes the fractal dimension of the pore shape, which takes values in the range of 1~2, higher values correspond to simpler pore structures.
a is a constant, and
S represents the pore area. Based on this relationship, the calculated fractal dimensions of pore shapes in the rare earth ore samples are presented in
Figure 17.
It can be observed that under a confining pressure of 50 kPa, the fractal dimension of the pore structure exhibits a slight initial increase followed by a marked decrease as MgSO4 concentration increases. Under 100 kPa, the fractal dimension rises sharply at first and then declines. At 150 kPa, the fractal dimension shows a more complex trend—first decreasing, then increasing, and finally decreasing again with increasing MgSO4 concentration.
In the early stages of leaching, the pore structures in the upper sections of the ore body are more fragmented. As particle migration progresses during the leaching process, the middle and lower sections of the ore body become structurally compensated. With increasing MgSO4 concentration, pore geometries tend to simplify, and the post-leaching pore structures are overall simpler than those prior to leaching. This trend aligns with the observed variation in the average pore shape factor following leaching.
Figure 1.
Curve of particle size distribution of the soil samples.
Figure 1.
Curve of particle size distribution of the soil samples.
Figure 2.
GDS stress path triaxial apparatus.
Figure 2.
GDS stress path triaxial apparatus.
Figure 3.
GDS stress path triaxial pressure chamber diagram.
Figure 3.
GDS stress path triaxial pressure chamber diagram.
Figure 4.
MLA650F Field Emission Scanning Electron Microscope and Schematic of Its Working Principle.
Figure 4.
MLA650F Field Emission Scanning Electron Microscope and Schematic of Its Working Principle.
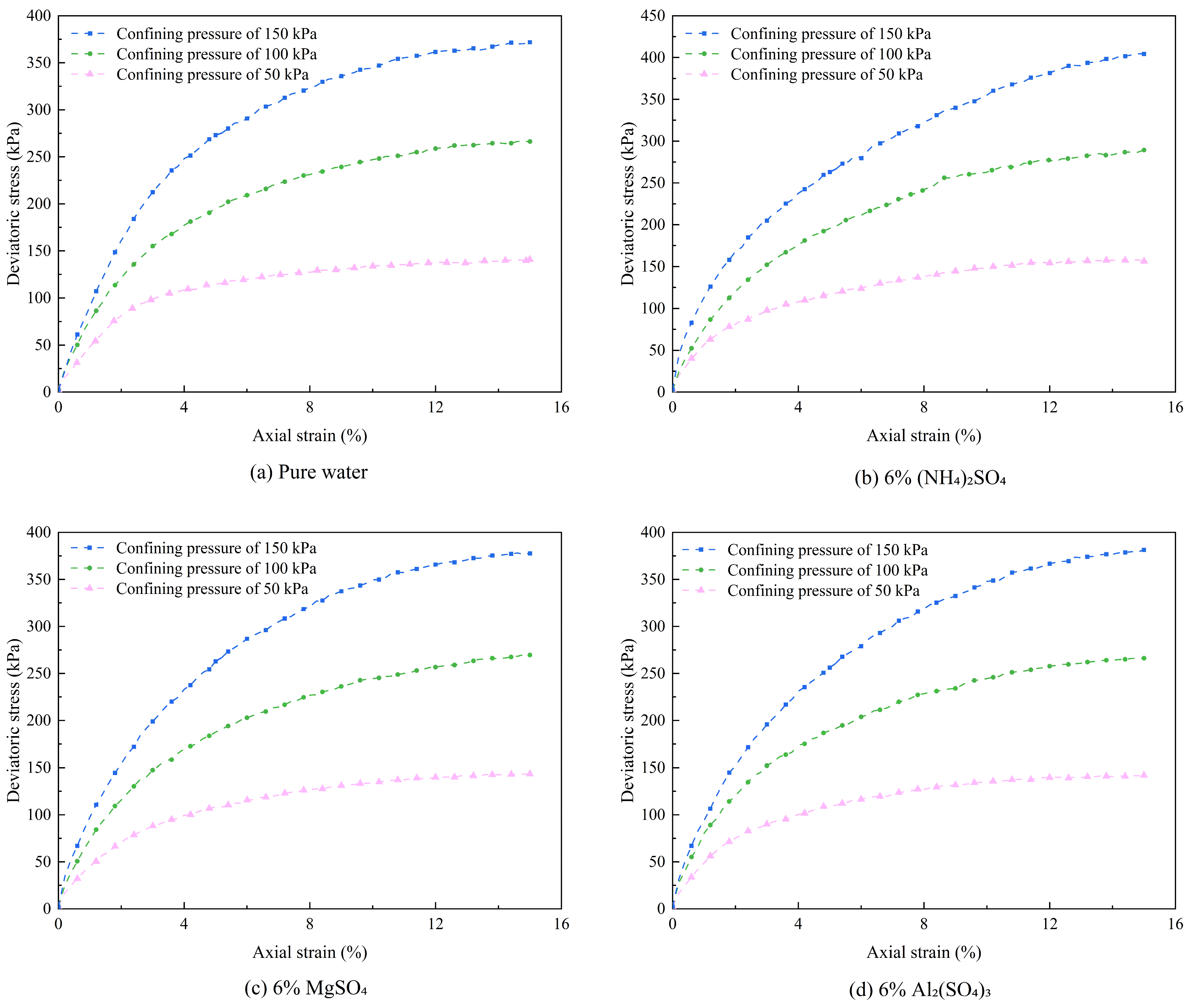
Figure 5.
Stress-strain curves under different leaching agents.
Figure 5.
Stress-strain curves under different leaching agents.
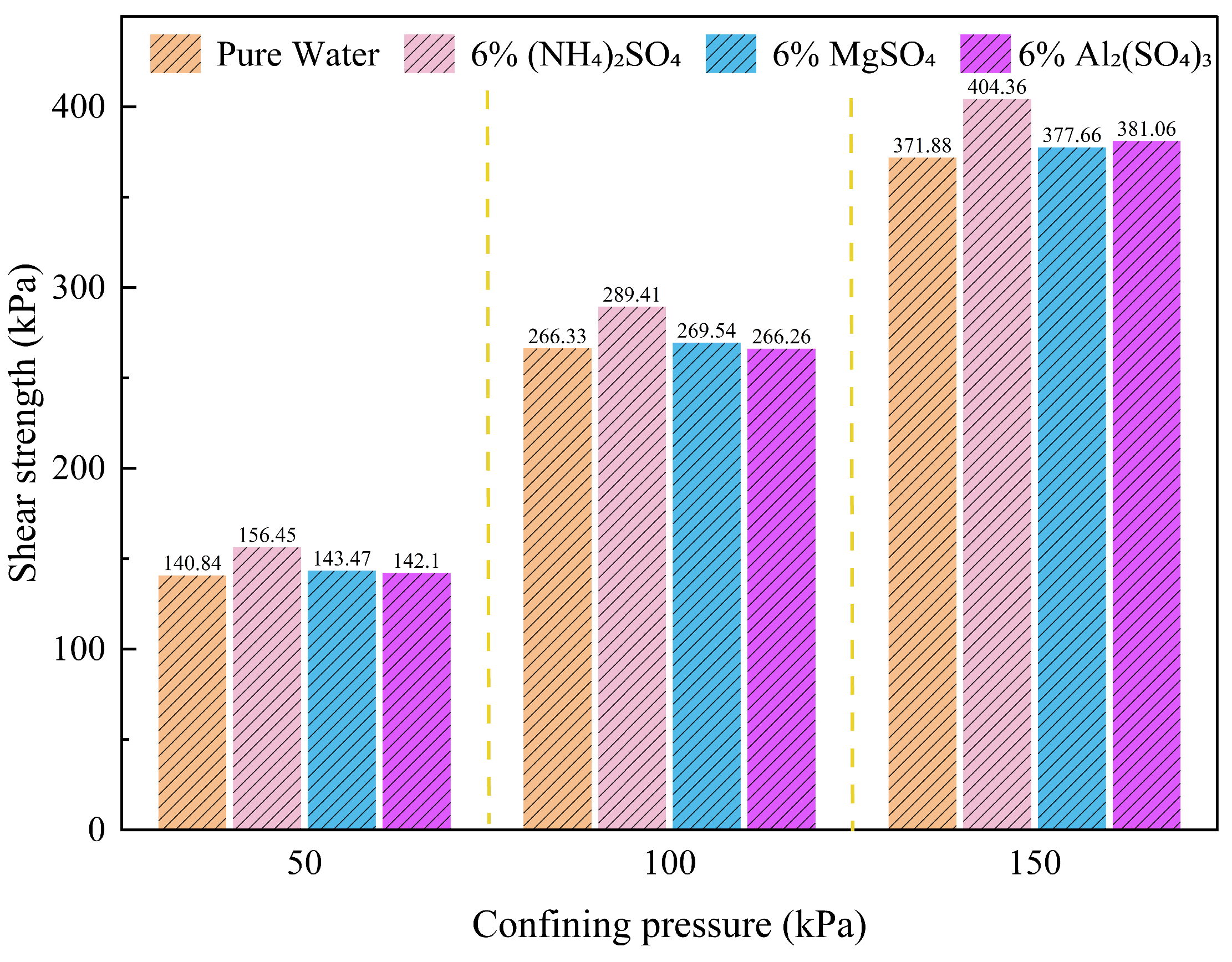
Figure 6.
Shear strength of ionic rare earth soils under different leaching agents.
Figure 6.
Shear strength of ionic rare earth soils under different leaching agents.
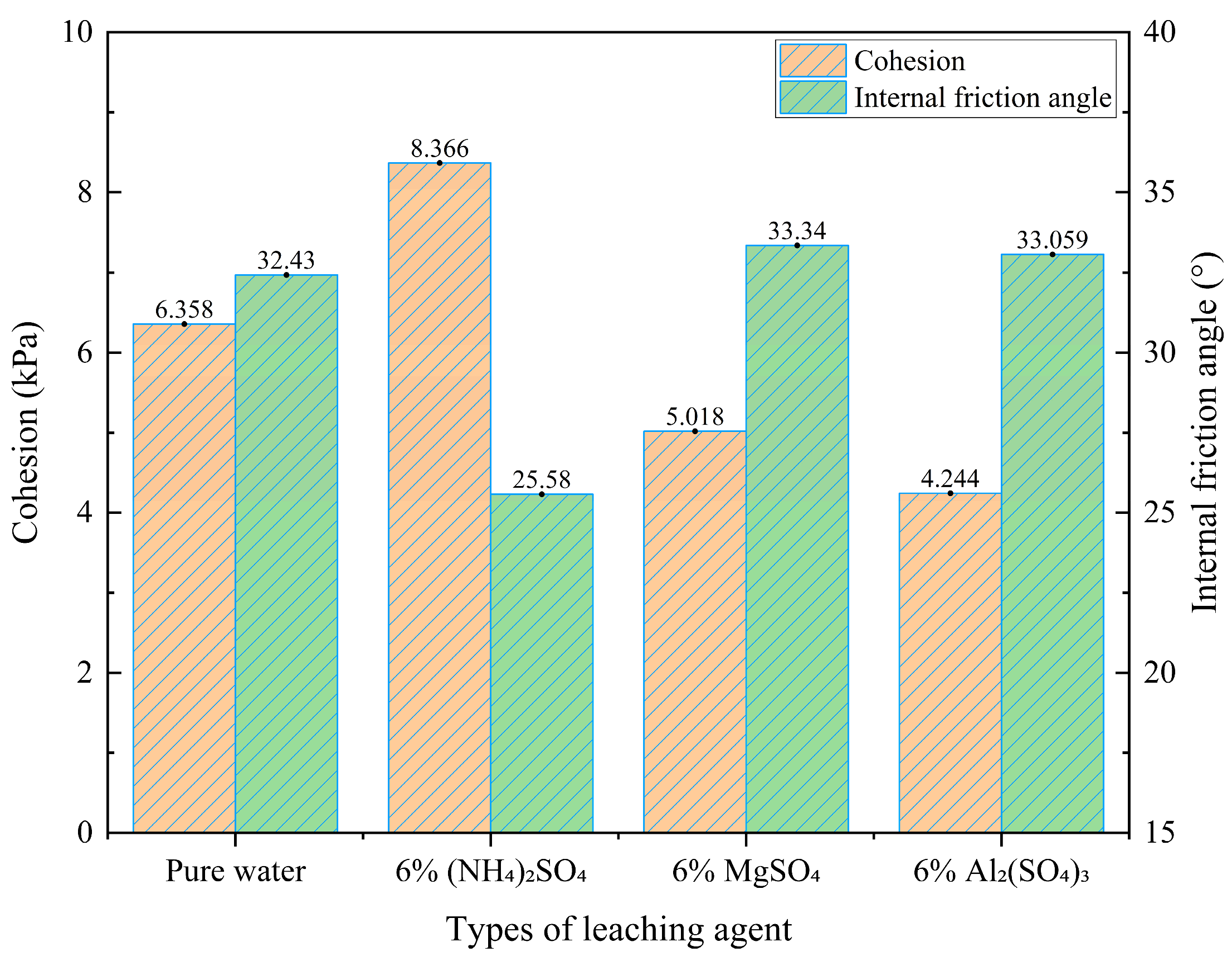
Figure 7.
Cohesion and internal friction angle under different leaching agents.
Figure 7.
Cohesion and internal friction angle under different leaching agents.
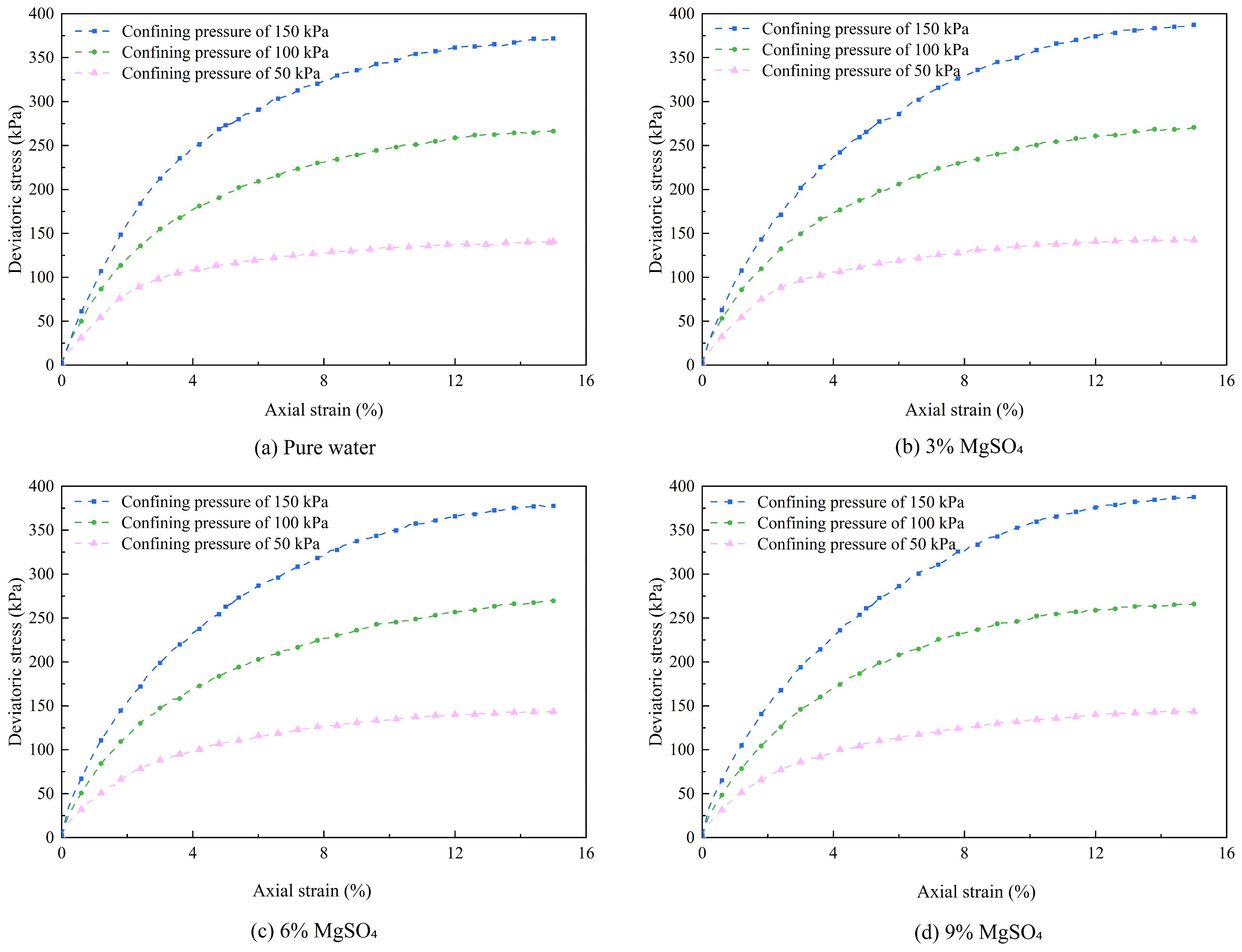
Figure 8.
Stress-strain curves under different magnesium sulfate concentrations.
Figure 8.
Stress-strain curves under different magnesium sulfate concentrations.
Figure 9.
Shear strength under different magnesium sulfate concentrations.
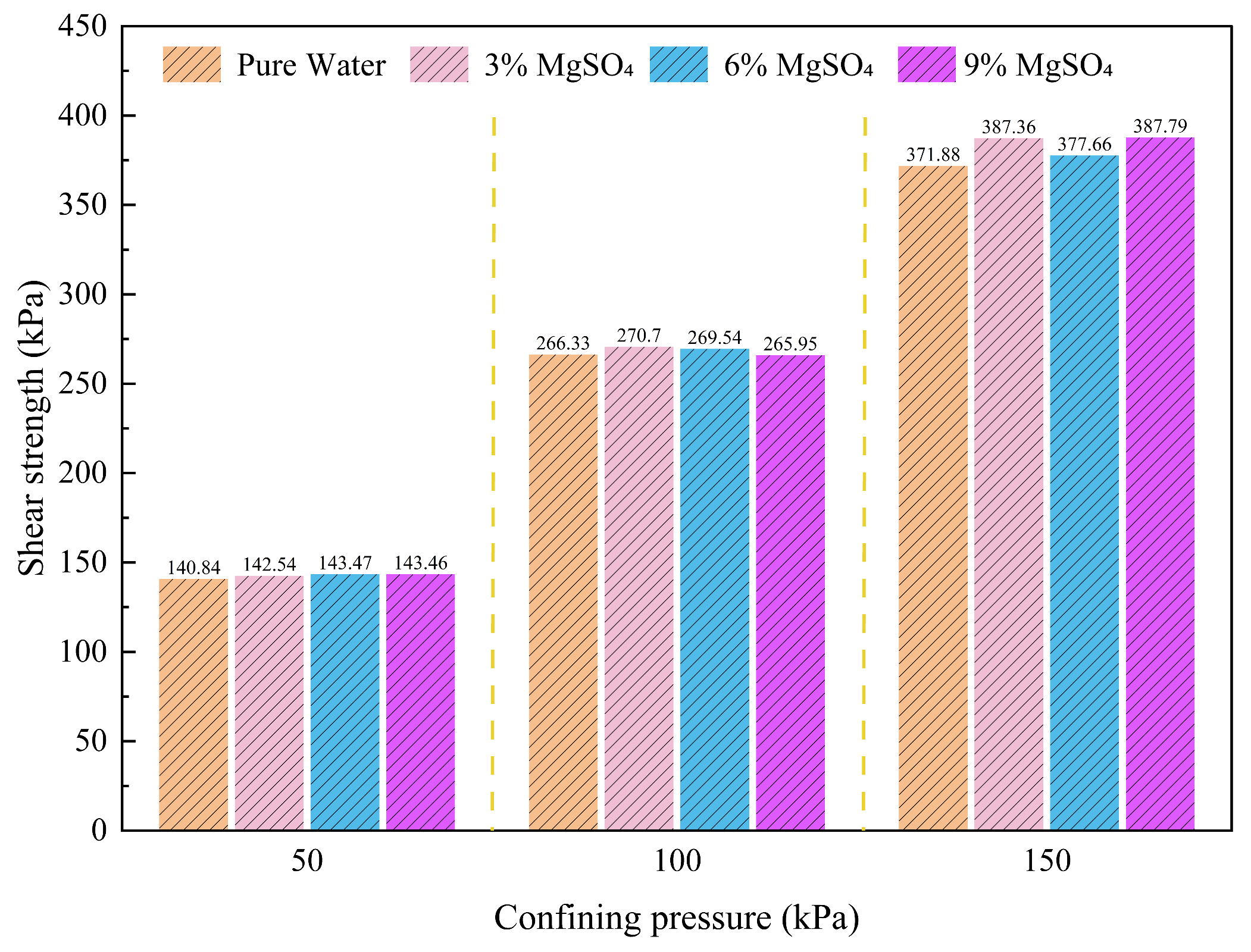
Figure 9.
Shear strength under different magnesium sulfate concentrations.
Figure 10.
Cohesion and internal friction angle under different magnesium sulfate concentrations.
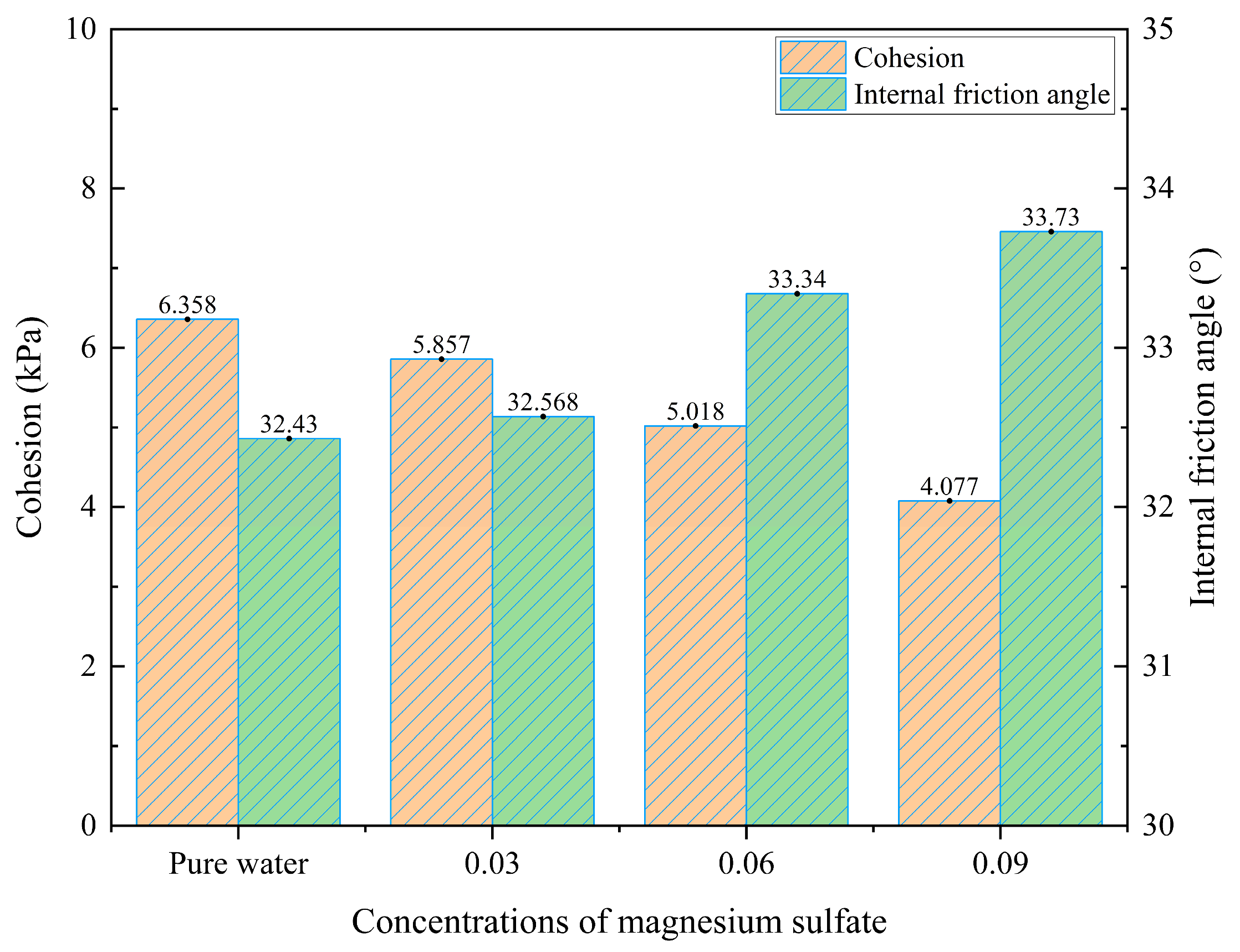
Figure 10.
Cohesion and internal friction angle under different magnesium sulfate concentrations.
Figure 11.
Original and processed SEM images of specimens under 50 kPa confining pressure.(a) Pure water;(b) 3% MgSO4;(c) 6% MgSO4;(d) 9% MgSO4.
Figure 11.
Original and processed SEM images of specimens under 50 kPa confining pressure.(a) Pure water;(b) 3% MgSO4;(c) 6% MgSO4;(d) 9% MgSO4.
Figure 12.
Original and processed SEM images of specimens under 100 kPa confining pressure (a) Pure water;(b) 3% MgSO4;(c) 6% MgSO4;(d) 9% MgSO4.
Figure 12.
Original and processed SEM images of specimens under 100 kPa confining pressure (a) Pure water;(b) 3% MgSO4;(c) 6% MgSO4;(d) 9% MgSO4.
Figure 13.
Original and processed SEM images of specimens under 150 kPa confining pressure (a) Pure water;(b) 3% MgSO4;(c) 6% MgSO4;(d) 9% MgSO4.
Figure 13.
Original and processed SEM images of specimens under 150 kPa confining pressure (a) Pure water;(b) 3% MgSO4;(c) 6% MgSO4;(d) 9% MgSO4.
Figure 14.
Pore size distribution under different confining pressure.
Figure 14.
Pore size distribution under different confining pressure.
Figure 15.
Polar histogram under different magnesium sulfate concentrations (a) Pure water 50 kPa (b) Pure water 100 kPa (c) Pure water 150 kPa (d) 3%MgSO4 50 kPa (e) 3%MgSO4 100 kPa (f) 3%MgSO4 150 kPa (g) 6%MgSO4 50 kPa (h) 6%MgSO4 100 kPa (i) 6%MgSO4 150 kPa (j) 9%MgSO4 50 kPa (k) 9%MgSO4 100 kPa (l) 9%MgSO4 150 kPa.
Figure 15.
Polar histogram under different magnesium sulfate concentrations (a) Pure water 50 kPa (b) Pure water 100 kPa (c) Pure water 150 kPa (d) 3%MgSO4 50 kPa (e) 3%MgSO4 100 kPa (f) 3%MgSO4 150 kPa (g) 6%MgSO4 50 kPa (h) 6%MgSO4 100 kPa (i) 6%MgSO4 150 kPa (j) 9%MgSO4 50 kPa (k) 9%MgSO4 100 kPa (l) 9%MgSO4 150 kPa.
Figure 16.
Probability entropy under different magnesium sulfate concentrations.
Figure 16.
Probability entropy under different magnesium sulfate concentrations.
Figure 17.
Fractal dimension variation under different magnesium sulfate concentrations.
Figure 17.
Fractal dimension variation under different magnesium sulfate concentrations.
Table 1.
Physical indexes of the original rare earth ore sample.
Table 1.
Physical indexes of the original rare earth ore sample.
Physical
indexes |
Water
content/% |
Density
(g/cm3) |
Dry density
(g/cm3) |
Liquid limit
(WP)/% |
Plastic limit
(WL)/% |
Plastic index
(IP) |
Original
rare earth
|
18.03 |
1.77 |
1.49 |
41.83 |
29.23 |
12.6 |
Table 2.
Composition analysis of soil samples.
Table 2.
Composition analysis of soil samples.
| Element |
SiO2 |
Al2O3 |
Fe2O3 |
K2O |
MnO |
MgO |
| Content(%) |
20.201 |
18.069 |
3.916 |
1.453 |
0.046 |
0.161 |
| Element |
PbO |
Rb2O |
CaO |
ZnO |
Y2O3 |
|
| Content(%) |
0.015 |
0.014 |
0.009 |
0.007 |
0.001 |
|
Table 3.
Design of test conditions.
Table 3.
Design of test conditions.
| Soil number |
Leaching conditions |
Confining pressure |
| A1 |
Pure water |
50 kPa, 100 kPa, 150 kPa |
| B1 |
6%(NH4)2SO4
|
| B2 |
6%MgSO4
|
| B3 |
6%Al2(SO4)3
|
| C1 |
3%MgSO4
|
| C2 |
6%MgSO4
|
| C3 |
9%MgSO4
|
Table 5.
Pore proportion and total number of pores in the SEM images.
Table 5.
Pore proportion and total number of pores in the SEM images.
| Testing condition |
Confining pressure |
Micropore proportion |
Mesopore proportion |
Macropore proportion |
Mega-pore proportion |
Total porosity |
| Pure water |
50kPa |
66.87% |
21.36% |
8.36% |
3.41% |
323 |
| 100kPa |
72.17% |
19.1% |
6.47% |
2.27% |
309 |
| 150kPa |
75.54% |
17.2% |
4.84% |
1.88% |
372 |
| 3%MgSO4
|
50kPa |
69.59% |
19.12% |
6.9% |
4.39% |
319 |
| 100kPa |
70.26% |
20.86% |
7.19% |
1.68% |
417 |
| 150kPa |
69.83% |
15.93% |
8.14% |
6.1% |
295 |
| 6%MgSO4
|
50kPa |
61.2% |
25.2% |
8.4% |
5.2% |
250 |
| 100kPa |
65.72% |
21.55% |
7.07% |
5.65% |
283 |
| 150kPa |
66.02% |
20.71% |
9.39% |
3.88% |
309 |
| 9%MgSO4
|
50kPa |
62.31% |
21.54% |
9.23% |
6.92% |
260 |
| 100kPa |
68.57% |
19.37% |
7.94% |
4.13% |
315 |
| 150kPa |
65.13% |
21.79% |
10.65% |
2.42% |
413 |