Submitted:
13 May 2025
Posted:
13 May 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. SLC4A11 Gene Expression, Resulting Protein Isoforms and Cellular Localization
2.1. Transcript Variants and Isoforms of the Protein
2.2. SLC4A11 mRNA Detection
2.3. SLC4A11 Protein Detection
2.4. SLC4A11 Expression in Pathologies
2.5. Structural Characteristics of the SLC4A11 Protein
3. Functional Features of the SLC4A11 Protein
3.1. Transport Function
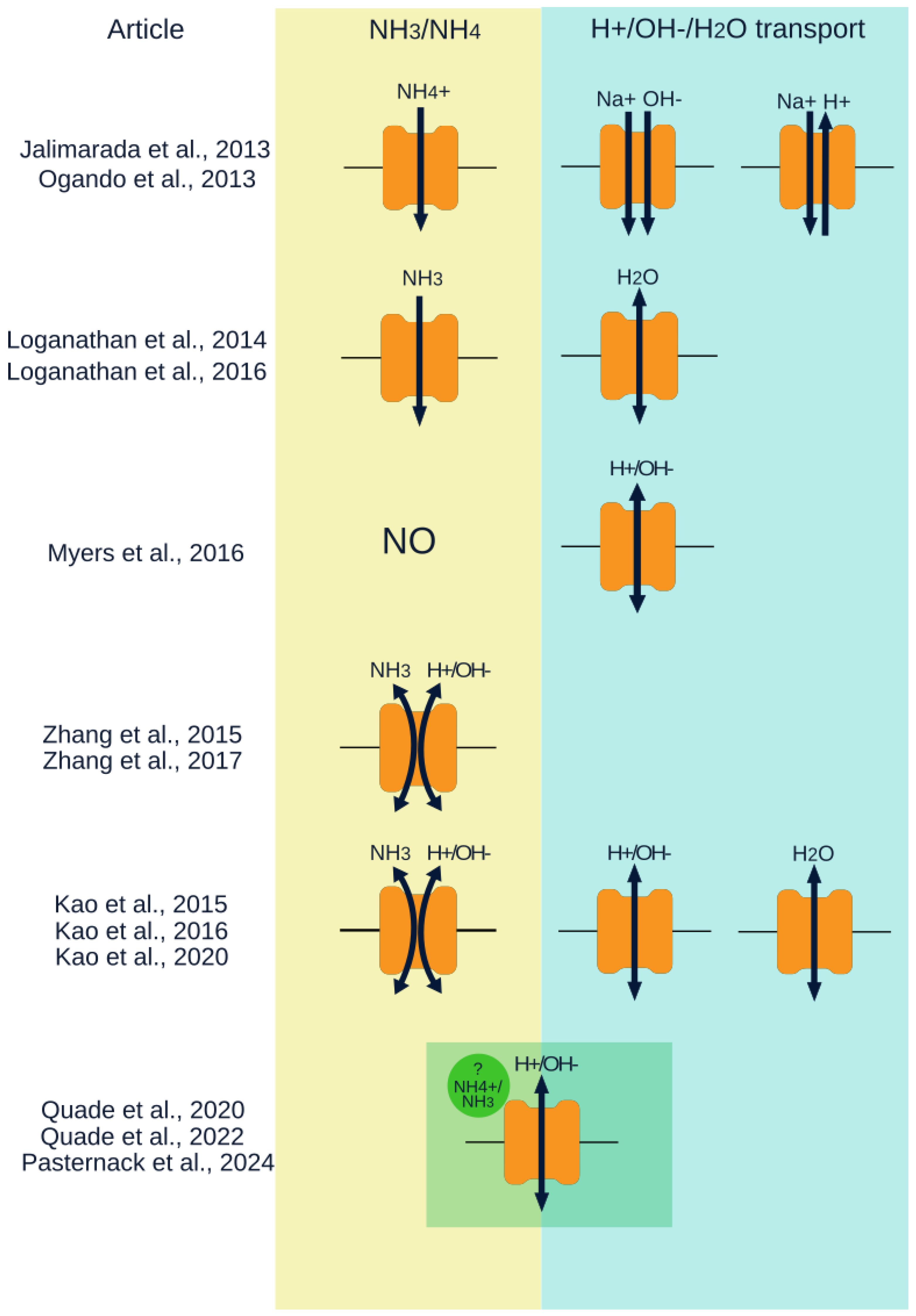
3.1.1. Initial works
4.2. NH3 and pH Regulated H+/OH- Transpor
3.1.2. Water Transport
3.1.3. SLC4A11 and Its Function in Lactate-Mediated Corneal Endothelial Pump Activity
3.2. SLC4A11 and Oxidative Stress
3.3. Role in Cell Adhesion, Effects on Cell Proliferation and Viability
3.4. Association of SLC4A11 with Regulation of Cellular Processes
4. SLC4A11 Functions in Pathologies
4.1. The Role of SLC4A11 Variants in FECD
4.2. Mouse Models of CHED
4.3. Functional Impacts of SLC4A11 Gene Mutations
4.4. Role of SLC4A11 in Cancer
Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SLC4A11 | Solute carrier family 4 member 11 |
| BTR1 | Bicarbonate transporter-related protein-1 |
| NaBC1 | Na+-coupled borate cotransporter 1 |
| CHED | Congenital hereditary endothelial dystrophy |
| FECD | Fuchs corneal endothelial dystrophy |
| RT-PCR | Reverse transcription polymerase chain reaction |
| IHC | Immunohistochemistry |
| AQP1 | Aquaporin 1 |
| IF | Immunofluorescence |
| SAGE | Serial analysis of gene expression |
| EST | Expressed sequence tag |
| pHi | intracellular pH |
| pHe | external pH |
| NHE | Na+/H+ exchanger |
| BCEC | Bovine corneal endothelial cells |
| MCEC | Mouse corneal endothelial cell |
| AGE | Advanced glycosylation end-products |
| ARE | Antioxidant response element |
| tBHQ | Tert-butylhydroquinone |
| KO | Knockout |
| ER | Endoplasmic reticulum |
| ROS | Reactive oxygen species |
| BafA1 | Bafilomycin A1 |
| TMD | Transmembrane domain |
| NTD | N-terminal cytoplasmic domain |
| HCC | Hepatocellular carcinoma |
| CSC | Cancer stem cells |
| mHCC | mouse HCC |
References
- Parker, M.D.; Ourmozdi, E.P.; Tanner, M.J. Human BTR1, a New Bicarbonate Transporter Superfamily Member and Human AE4 from Kidney. Biochemical and biophysical research communications 2001, 282, 1103–1109. [Google Scholar] [CrossRef] [PubMed]
- Park, M.; Li, Q.; Shcheynikov, N.; Zeng, W.; Muallem, S. NaBC1 Is a Ubiquitous Electrogenic Na+-Coupled Borate Transporter Essential for Cellular Boron Homeostasis and Cell Growth and Proliferation. Molecular cell 2004, 16, 331–341. [Google Scholar] [CrossRef] [PubMed]
- Vithana, E.N.; Morgan, P.; Sundaresan, P.; Ebenezer, N.D.; Tan, D.T.; Mohamed, M.D.; Anand, S.; Khine, K.O.; Venkataraman, D.; Yong, V.H.; et al. Mutations in Sodium-Borate Cotransporter SLC4A11 Cause Recessive Congenital Hereditary Endothelial Dystrophy (CHED2). Nature genetics 2006, 38, 755–757. [Google Scholar] [CrossRef] [PubMed]
- Jiao, X.; Sultana, A.; Garg, P.; Ramamurthy, B.; Vemuganti, G.K.; Gangopadhyay, N.; Hejtmancik, J.F.; Kannabiran, C. Autosomal Recessive Corneal Endothelial Dystrophy (CHED2) Is Associated with Mutations in SLC4A11. Journal of medical genetics 2007, 44, 64–68. [Google Scholar] [CrossRef]
- Desir, J.; Moya, G.; Reish, O.; Van Regemorter, N.; Deconinck, H.; David, K.L.; Meire, F.M.; Abramowicz, M.J. Borate Transporter SLC4A11 Mutations Cause Both Harboyan Syndrome and Non-Syndromic Corneal Endothelial Dystrophy. Journal of medical genetics 2007, 44, 322–326. [Google Scholar] [CrossRef]
- Siddiqui, S.; Zenteno, J.C.; Rice, A.; Chacón-Camacho, O.; Naylor, S.G.; Rivera-de la Parra, D.; Spokes, D.M.; James, N.; Toomes, C.; Inglehearn, C.F.; et al. Congenital Hereditary Endothelial Dystrophy Caused by SLC4A11 Mutations Progresses to Harboyan Syndrome. Cornea 2014, 33, 247–251. [Google Scholar] [CrossRef]
- Firasat, S.; Khan, W.A.; Sughra, U.; Nousheen; Kaul, H.; Naz, S.; Noreen, B.; Gul, R.; Afshan, K. SLC4A11 Mutations Causative of Congenital Hereditary Endothelial Dystrophy (CHED) Progressing to Harboyan Syndrome in Consanguineous Pakistani Families. Molecular Biology Reports 2021, 48, 7467–7476. [Google Scholar] [CrossRef]
- Vithana, E.N.; Morgan, P.E.; Ramprasad, V.; Tan, D.T.; Yong, V.H.; Venkataraman, D.; Venkatraman, A.; Yam, G.H.; Nagasamy, S.; Law, R.W.; et al. SLC4A11 Mutations in Fuchs Endothelial Corneal Dystrophy. Human molecular genetics 2008, 17, 656–666. [Google Scholar] [CrossRef]
- Riazuddin, S.A.; Vithana, E.N.; Seet, L.-F.; Liu, Y.; Al-Saif, A.; Koh, L.W.; Heng, Y.M.; Aung, T.; Meadows, D.N.; Eghrari, A.O.; et al. Missense Mutations in the Sodium Borate Cotransporter SLC4A11 Cause Late-Onset Fuchs Corneal Dystrophy a. Human mutation 2010, 31, 1261–1268. [Google Scholar] [CrossRef]
- Tsedilina, T.R.; Sharova, E.; Iakovets, V.; Skorodumova, L.O. Systematic Review of SLC4A11, ZEB1, LOXHD1, and AGBL1 Variants in the Development of Fuchs’ Endothelial Corneal Dystrophy. Frontiers in Medicine 2023, 10, 1153122. [Google Scholar] [CrossRef]
- Qin, L.; Li, T.; Liu, Y. High SLC4A11 Expression Is an Independent Predictor for Poor Overall Survival in Grade 3/4 Serous Ovarian Cancer. PLoS One 2017, 12, e0187385. [Google Scholar] [CrossRef]
- Bonanno, J.A.; Shyam, R.; Choi, M.; Ogando, D.G. The h+ Transporter SLC4A11: Roles in Metabolism, Oxidative Stress and Mitochondrial Uncoupling. Cells 2022, 11, 197. [Google Scholar] [CrossRef] [PubMed]
- Damkier, H.H.; Nielsen, S.; Praetorius, J. Molecular Expression of SLC4-Derived Na+-Dependent Anion Transporters in Selected Human Tissues. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology 2007, 293, R2136–R2146. [Google Scholar] [CrossRef]
- Malhotra, D.; Loganathan, S.K.; Chiu, A.M.; Lukowski, C.M.; Casey, J.R. Human Corneal Expression of SLC4A11, a Gene Mutated in Endothelial Corneal Dystrophies. Scientific reports 2019, 9, 9681. [Google Scholar] [CrossRef]
- Kao, L.; Azimov, R.; Shao, X.M.; Frausto, R.F.; Abuladze, N.; Newman, D.; Aldave, A.J.; Kurtz, I. Multifunctional Ion Transport Properties of Human SLC4A11: Comparison of the SLC4A11-b and SLC4A11-c Variants. American Journal of Physiology-Cell Physiology 2016, 311, C820–C830. [Google Scholar] [CrossRef]
- Ogando, D.G.; Jalimarada, S.S.; Zhang, W.; Vithana, E.N.; Bonanno, J.A. SLC4A11 Is an EIPA-Sensitive Na+ Permeable pHi Regulator. American Journal of Physiology-Cell Physiology 2013, 305, C716–C727. [Google Scholar] [CrossRef]
- Jalimarada, S.S.; Ogando, D.G.; Vithana, E.N.; Bonanno, J.A. Ion Transport Function of SLC4A11 in Corneal Endothelium. Investigative ophthalmology & visual science 2013, 54, 4330–4340. [Google Scholar]
- Myers, E.J.; Marshall, A.; Jennings, M.L.; Parker, M.D. Mouse Slc4a11 Expressed in Xenopus Oocytes Is an Ideally Selective h+/OH- Conductance Pathway That Is Stimulated by Rises in Intracellular and Extracellular pH. American Journal of Physiology-Cell Physiology 2016, 311, C945–C959. [Google Scholar] [CrossRef] [PubMed]
- Loganathan, S.K.; Schneider, H.-P.; Morgan, P.E.; Deitmer, J.W.; Casey, J.R. Functional Assessment of SLC4A11, an Integral Membrane Protein Mutated in Corneal Dystrophies. American Journal of Physiology-Cell Physiology 2016, 311, C735–C748. [Google Scholar] [CrossRef]
- Zhang, W.; Ogando, D.G.; Bonanno, J.A.; Obukhov, A.G. Human SLC4A11 Is a Novel NH3/h+ Co-Transporter. Journal of Biological Chemistry 2015, 290, 16894–16905. [Google Scholar] [CrossRef]
- Kao, L.; Azimov, R.; Shao, X.M.; Abuladze, N.; Newman, D.; Zhekova, H.; Noskov, S.; Pushkin, A.; Kurtz, I. SLC4A11 Function: Evidence for h+ (OH-) and NH3-h+ Transport. American Journal of Physiology-Cell Physiology 2020, 318, C392–C405. [Google Scholar] [CrossRef] [PubMed]
- Ogando, D.G.; Choi, M.; Shyam, R.; Li, S.; Bonanno, J.A. Ammonia Sensitive SLC4A11 Mitochondrial Uncoupling Reduces Glutamine Induced Oxidative Stress. Redox biology 2019, 26, 101260. [Google Scholar] [CrossRef]
- Malhotra, D.; Jung, M.; Noskov, S.; Zimmermann, R.; Casey, J.R. SLC4A11 Extracellular Loop 3 in Corneal Endothelial Cell Adhesion, FECD Pathology and Therapeutics. Investigative Ophthalmology & Visual Science 2018, 59, 4433–4433. [Google Scholar]
- Malhotra, D.; Jung, M.; Fecher-Trost, C.; Lovatt, M.; Peh, G.S.; Noskov, S.; Mehta, J.S.; Zimmermann, R.; Casey, J.R. Defective Cell Adhesion Function of Solute Transporter, SLC4A11, in Endothelial Corneal Dystrophies. Human Molecular Genetics 2020, 29, 97–116. [Google Scholar] [CrossRef]
- Kao, L.; Azimov, R.; Abuladze, N.; Newman, D.; Kurtz, I. Human SLC4A11-c Functions as a DIDS-Stimulatable h+ (OH-) Permeation Pathway: Partial Correction of R109H Mutant Transport. American Journal of Physiology-Cell Physiology 2015, 308, C176–C188. [Google Scholar] [CrossRef] [PubMed]
- O’Leary, N.A.; Wright, M.W.; Brister, J.R.; Ciufo, S.; Haddad, D.; McVeigh, R.; Rajput, B.; Robbertse, B.; Smith-White, B.; Ako-Adjei, D.; et al. Reference Sequence (RefSeq) Database at NCBI: Current Status, Taxonomic Expansion, and Functional Annotation. Nucleic acids research 2016, 44, D733–D745. [Google Scholar] [CrossRef]
- Uhlén, M.; Fagerberg, L.; Hallström, B.M.; Lindskog, C.; Oksvold, P.; Mardinoglu, A.; Sivertsson, Å.; Kampf, C.; Sjöstedt, E.; Asplund, A.; et al. Tissue-Based Map of the Human Proteome. Science 2015, 347, 1260419. [Google Scholar] [CrossRef] [PubMed]
- Kotova, E.S.; Sharova, E.I.; Kovaleva, P.A.; Malyugin, B.E.; Antonova, O.P.; Belodedova, A.V.; Tkachenko, I.S.; Doludin, Y.V.; Garmanova, T.N.; Skorodumova, L.O. High Expression of the Underexplored SLC4A11 Protein-Coding Transcript Is Specific to the Corneal Endothelium. Scientific Reports, in review. 2025. [Google Scholar]
- Hemadevi, B.; Veitia, R.A.; Srinivasan, M.; Arunkumar, J.; Prajna, N.V.; Lesaffre, C.; Sundaresan, P. Identification of Mutations in the SLC4A11 Gene in Patients with Recessive Congenital Hereditary Endothelial Dystrophy. Archives of Ophthalmology 2008, 126, 700–708. [Google Scholar] [CrossRef]
- Guha, S.; Roy, S. Enhanced Expression of SLC4A11 by Tert-Butylhydroquinone Is Mediated by Direct Binding of Nrf2 to the Promoter of SLC4A11. Free Radical Biology and Medicine 2021, 167, 299–306. [Google Scholar] [CrossRef]
- Uhlén, M.; Fagerberg, L.; Hallström, B.M.; Lindskog, C.; Oksvold, P.; Mardinoglu, A.; Sivertsson, Å.; Kampf, C.; Sjöstedt, E.; Asplund, A.; et al. Human Protein Atlas Available online:. Available online: https://www.proteinatlas.org/ENSG00000088836-SLC4A11/tissue#rna_expression (accessed on 11 April 2025).
- Nakagawa, T.; Tokuda, Y.; Nakano, M.; Komori, Y.; Hanada, N.; Tourtas, T.; Schlötzer-Schrehardt, U.; Kruse, F.; Tashiro, K.; Koizumi, N.; et al. RNA-Seq–Based Transcriptome Analysis of Corneal Endothelial Cells Derived from Patients with Fuchs Endothelial Corneal Dystrophy. Scientific reports 2023, 13, 8647. [Google Scholar] [CrossRef]
- Consortium, T.G. The GTEx Portal Available online:. Available online: https://gtexportal.org/home/ (accessed on 7 May 2025).
- Gee, M.T. Expression of SLC4A11, Claudin 4 and Other Cell Membrane Transporters in the Mammalian Renal Medulla and Their Physiological Implications. 2019.
- Vilas, G.L.; Loganathan, S.K.; Liu, J.; Riau, A.K.; Young, J.D.; Mehta, J.S.; Vithana, E.N.; Casey, J.R. Transmembrane Water-Flux Through SLC4A11: A Route Defective in Genetic Corneal Diseases. Human molecular genetics 2013, 22, 4579–4590. [Google Scholar] [CrossRef] [PubMed]
- Chung, D.D.; Chen, A.C.; Choo, C.H.; Zhang, W.; Williams, D.; Griffis, C.G.; Bonezzi, P.; Jatavallabhula, K.; Sampath, A.P.; Aldave, A.J. Investigation of the Functional Impact of CHED-and FECD4-Associated SLC4A11 Mutations in Human Corneal Endothelial Cells. Plos one 2024, 19, e0296928. [Google Scholar] [CrossRef] [PubMed]
- Lopez, I.A.; Rosenblatt, M.I.; Kim, C.; Galbraith, G.C.; Jones, S.M.; Kao, L.; Newman, D.; Liu, W.; Yeh, S.; Pushkin, A.; et al. Slc4a11 Gene Disruption in Mice: Cellular Targets of Sensorineuronal Abnormalities. Journal of Biological Chemistry 2009, 284, 26882–26896. [Google Scholar] [CrossRef] [PubMed]
- Gröger, N.; Fröhlich, H.; Maier, H.; Olbrich, A.; Kostin, S.; Braun, T.; Boettger, T. SLC4A11 Prevents Osmotic Imbalance Leading to Corneal Endothelial Dystrophy, Deafness, and Polyuria. Journal of Biological Chemistry 2010, 285, 14467–14474. [Google Scholar] [CrossRef]
- Yang, N.-Y.; Mukaibo, T.; Gao, X.; Kurtz, I.; Melvin, J.E. Slc4a11 Disruption Causes Duct Cell Loss and Impairs NaCl Reabsorption in Female Mouse Submandibular Glands. Physiological Reports 2019, 7, e14232. [Google Scholar] [CrossRef]
- Gottsch, J.D.; Bowers, A.L.; Margulies, E.H.; Seitzman, G.D.; Kim, S.W.; Saha, S.; Jun, A.S.; Stark, W.J.; Liu, S.H. Serial Analysis of Gene Expression in the Corneal Endothelium of Fuchs’ Dystrophy. Investigative ophthalmology & visual science 2003, 44, 594–599. [Google Scholar]
- Wieben, E.D.; Baratz, K.H.; Aleff, R.A.; Kalari, K.R.; Tang, X.; Maguire, L.J.; Patel, S.V.; Fautsch, M.P. Gene Expression and Missplicing in the Corneal Endothelium of Patients with a TCF4 Trinucleotide Repeat Expansion Without Fuchs’ Endothelial Corneal Dystrophy. Investigative ophthalmology & visual science 2019, 60, 3636–3643. [Google Scholar]
- De Roo, A.-K.; Wouters, J.; Govaere, O.; Foets, B.; Oord, J.J. van den Identification of Circulating Fibrocytes and Dendritic Derivatives in Corneal Endothelium of Patients with Fuchs’ Dystrophy. Investigative Ophthalmology & Visual Science 2017, 58, 670–681. [Google Scholar]
- Alka, K.; Casey, J.R. Molecular Phenotype of SLC4A11 Missense Mutants: Setting the Stage for Personalized Medicine in Corneal Dystrophies. Human mutation 2018, 39, 676–690. [Google Scholar] [CrossRef]
- Wang, Y.; Cheng, C.; Lu, Y.; Lian, Z.; Liu, Q.; Xu, Y.; Li, Y.; Li, H.; Zhang, L.; Jiang, X.; et al. β-Catenin Activation Reprograms Ammonia Metabolism to Promote Senescence Resistance in Hepatocellular Carcinoma. Cancer Research 2024, 84, 1643–1658. [Google Scholar] [CrossRef]
- Sun, M.; Qi, Y.; Jin, Q.; Zhu, H.; Wang, Y.; Jiang, X. Increased SLC4A11 Expression Is Associated with Poor Prognosis of Gastric Cancer. Blood&Genomics 2020, 4, 61–70. [Google Scholar]
- Shinto, E.; Yoshida, Y.; Kajiwara, Y.; Okamoto, K.; Mochizuki, S.; Yamadera, M.; Shiraishi, T.; Nagata, K.; Tsuda, H.; Hase, K.; et al. Clinical Significance of a Gene Signature Generated from Tumor Budding Grade in Colon Cancer. Annals of surgical oncology 2020, 27, 4044–4054. [Google Scholar] [CrossRef]
- Yamadera, M.; Shinto, E.; Nagata, K.; Shiraishi, T.; Kajiwara, Y.; Mochizuki, S.; Okamoto, K.; Kishi, Y.; Ueno, H. Proposal for a Tumor Budding Predictive Score Derived from Endoscopic Biopsy Samples in Colorectal Cancer. International Journal of Clinical Oncology 2022, 27, 756–764. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, J.; Chen, L.-M. Structure and Function of SLC4 Family HCO 3-Transporters. Frontiers in physiology 2015, 6, 355. [Google Scholar] [CrossRef]
- Vilas, G.L.; Morgan, P.E.; Loganathan, S.K.; Quon, A.; Casey, J.R. A Biochemical Framework for SLC4A11, the Plasma Membrane Protein Defective in Corneal Dystrophies. Biochemistry 2011, 50, 2157–2169. [Google Scholar] [CrossRef] [PubMed]
- Vilas, G.L.; Loganathan, S.K.; Quon, A.; Sundaresan, P.; Vithana, E.N.; Casey, J. Oligomerization of SLC4A11 Protein and the Severity of FECD and CHED2 Corneal Dystrophies Caused by SLC4A11 Mutations. Human mutation 2012, 33, 419–428. [Google Scholar] [CrossRef]
- Loganathan, S.K.; Lukowski, C.M.; Casey, J.R. The Cytoplasmic Domain Is Essential for Transport Function of the Integral Membrane Transport Protein SLC4A11. American Journal of Physiology-Cell Physiology 2016, 310, C161–C174. [Google Scholar] [CrossRef]
- Romero, M.F.; Fulton, C.M.; Boron, W.F. The SLC4 Family of HCO 3- Transporters. Pflügers Archiv 2004, 447, 495–509. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Ogando, D.G.; Kim, E.T.; Choi, M.-J.; Li, H.; Tenessen, J.M.; Bonanno, J.A. Conditionally Immortal Slc4a11-/- Mouse Corneal Endothelial Cell Line Recapitulates Disrupted Glutaminolysis Seen in Slc4a11-/- Mouse Model. Investigative ophthalmology & visual science 2017, 58, 3723–3731. [Google Scholar]
- Quade, B.N.; Marshall, A.; Parker, M.D. pH Dependence of the Slc4a11-Mediated h+ Conductance Is Influenced by Intracellular Lysine Residues and Modified by Disease-Linked Mutations. American Journal of Physiology-Cell Physiology 2020, 319, C359–C370. [Google Scholar] [CrossRef]
- Quade, B.N.; Marshall, A.; Parker, M.D. Corneal Dystrophy Mutations R125H and R804H Disable SLC4A11 by Altering the Extracellular pH Dependence of the Intracellular pK That Governs h+ (OH-) Transport. American Journal of Physiology-Cell Physiology 2022, 323, C990–C1002. [Google Scholar] [CrossRef] [PubMed]
- Pasternack, R.A.; Quade, B.N.; Marshall, A.; Parker, M.D. NH3/NH4+ Allosterically Activates SLC4A11 by Causing an Acidic Shift in the Intracellular pK That Governs h+ (OH-) Conductance. Frontiers in Physiology 2024, 15, 1440720. [Google Scholar] [CrossRef]
- Lu, Y.; Zuo, P.; Chen, H.; Shan, H.; Wang, W.; Dai, Z.; Xu, H.; Chen, Y.; Liang, L.; Ding, D.; et al. Structural Insights into the Conformational Changes of BTR1/SLC4A11 in Complex with PIP2. Nature Communications 2023, 14, 6157. [Google Scholar] [CrossRef] [PubMed]
- Soumittra, N.; Loganathan, S.K.; Madhavan, D.; Ramprasad, V.L.; Arokiasamy, T.; Sumathi, S.; Karthiyayini, T.; Rachapalli, S.R.; Kumaramanickavel, G.; Casey, J.R.; et al. Biosynthetic and Functional Defects in Newly Identified SLC4A11 Mutants and Absence of COL8A2 Mutations in Fuchs Endothelial Corneal Dystrophy. Journal of human genetics 2014, 59, 444–453. [Google Scholar] [CrossRef]
- Riley, M.V.; Winkler, B.S.; Peters, M.I.; Czajkowski, C.A. Relationship Between Fluid Transport and in Situ Inhibition of Na (+)-k+ Adenosine Triphosphatase in Corneal Endothelium. Investigative ophthalmology & visual science 1994, 35, 560–567. [Google Scholar]
- Riley, M. Glucose and Oxygen Utilization by the Rabbit Cornea. Experimental eye research 1969, 8, 193–200. [Google Scholar] [CrossRef]
- Hogan, M.J. Histology of the Human Eye: An Atlas and Textbook. (No Title) 1971, 187.
- Leung, B.K.; Bonanno, J.A.; Radke, C.J. Oxygen-Deficient Metabolism and Corneal Edema. Progress in retinal and eye research 2011, 30, 471–492. [Google Scholar] [CrossRef] [PubMed]
- Chhabra, M.; Prausnitz, J.M.; Radke, C.J. Modeling Corneal Metabolism and Oxygen Transport During Contact Lens Wear. Optometry and Vision Science 2009, 86, 454–466. [Google Scholar] [CrossRef]
- Bonanno, J.; Polse, K. Corneal Acidosis During Contact Lens Wear: Effects of Hypoxia and CO2. Investigative ophthalmology & visual science 1987, 28, 1514–1520. [Google Scholar]
- Pang, K.; Lennikov, A.; Yang, M. Hypoxia Adaptation in the Cornea: Current Animal Models and Underlying Mechanisms. Animal Models and Experimental Medicine 2021, 4, 300–310. [Google Scholar] [CrossRef]
- Klyce, S. Stromal Lactate Accumulation Can Account for Corneal Oedema Osmotically Following Epithelial Hypoxia in the Rabbit. The Journal of physiology 1981, 321, 49–64. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Bonanno, J.A. Lactate-h+ Transport Is a Significant Component of the in Vivo Corneal Endothelial Pump. Investigative Ophthalmology & Visual Science 2012, 53, 2020–2029. [Google Scholar]
- Li, S.; Kim, E.; Ogando, D.G.; Bonanno, J.A. Corneal Endothelial Pump Coupling to Lactic Acid Efflux in the Rabbit and Mouse. Investigative ophthalmology & visual science 2020, 61, 7–7. [Google Scholar]
- Li, S.; Kim, E.; Bonanno, J.A. Fluid Transport by the Cornea Endothelium Is Dependent on Buffering Lactic Acid Efflux. American Journal of Physiology-Cell Physiology 2016, 311, C116–C126. [Google Scholar] [CrossRef] [PubMed]
- Kirk, áP.; Wilson, M.; Heddle, C.; Brown, M.; Barclay, A.; Halestrap, A. CD147 Is Tightly Associated with Lactate Transporters MCT1 and MCT4 and Facilitates Their Cell Surface Expression. The EMBO journal 2000. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Nguyen, T.T.; Bonanno, J.A. CD147 Required for Corneal Endothelial Lactate Transport. Investigative Ophthalmology & Visual Science 2014, 55, 4673–4681. [Google Scholar]
- Nguyen, T.T.; Bonanno, J.A. Bicarbonate, NBCe1, NHE, and Carbonic Anhydrase Activity Enhance Lactate-h+ Transport in Bovine Corneal Endothelium. Investigative ophthalmology & visual science 2011, 52, 8086–8093. [Google Scholar]
- Ogando, D.G.; Bonanno, J.A. RNA Sequencing Uncovers Alterations in Corneal Endothelial Metabolism, Pump and Barrier Functions of Slc4a11 KO Mice. Experimental eye research 2022, 214, 108884. [Google Scholar] [CrossRef]
- Ogando, D.G.; Shyam, R.; Kim, E.T.; Wang, Y.-C.; Liu, C.-Y.; Bonanno, J.A. Inducible Slc4a11 Knockout Triggers Corneal Edema Through Perturbation of Corneal Endothelial Pump. Investigative Ophthalmology & Visual Science 2021, 62, 28–28. [Google Scholar]
- Ogando, D.G.; Kim, E.T.; Li, S.; Bonanno, J.A. Corneal Edema in Inducible Slc4a11 Knockout Is Initiated by Mitochondrial Superoxide Induced Src Kinase Activation. Cells 2023, 12, 1528. [Google Scholar] [CrossRef]
- Shyam, R.; Ogando, D.G.; Kim, E.T.; Murugan, S.; Choi, M.; Bonanno, J.A. Rescue of the Congenital Hereditary Endothelial Dystrophy Mouse Model by Adeno-Associated Virus–Mediated Slc4a11 Replacement. Ophthalmology science 2022, 2, 100084. [Google Scholar] [CrossRef]
- Nehrke, K. H (OH), h (OH), h (OH): A Holiday Perspective. Focus on “Mouse Slc4a11 Expressed in Xenopus Oocytes Is an Ideally Selective h+/OH- Conductance Pathway That Is Stimulated by Rises in Intracellular and Extracellular pH. American Journal of Physiology-Cell Physiology 2016. [Google Scholar] [CrossRef] [PubMed]
- Buddi, R.; Lin, B.; Atilano, S.R.; Zorapapel, N.C.; Kenney, M.C.; Brown, D.J. Evidence of Oxidative Stress in Human Corneal Diseases. Journal of Histochemistry & Cytochemistry 2002, 50, 341–351. [Google Scholar]
- Wang, Z.; Handa, J.T.; Green, W.R.; Stark, W.J.; Weinberg, R.S.; Jun, A.S. Advanced Glycation End Products and Receptors in Fuchs’ Dystrophy Corneas Undergoing Descemet’s Stripping with Endothelial Keratoplasty. Ophthalmology 2007, 114, 1453–1460. [Google Scholar] [CrossRef] [PubMed]
- Czarny, P.; Seda, A.; Wielgorski, M.; Binczyk, E.; Markiewicz, B.; Kasprzak, E.; Jimenez-Garcia, M.; Grabska-Liberek, I.; Pawlowska, E.; Blasiak, J.; et al. Mutagenesis of Mitochondrial DNA in Fuchs Endothelial Corneal Dystrophy. Mutation Research/Fundamental and Molecular Mechanisms of Mutagenesis 2014, 760, 42–47. [Google Scholar] [CrossRef]
- Bitar, M.S.; Liu, C.; Ziaei, A.; Chen, Y.; Schmedt, T.; Jurkunas, U.V. Decline in DJ-1 and Decreased Nuclear Translocation of Nrf2 in Fuchs Endothelial Corneal Dystrophy. Investigative ophthalmology & visual science 2012, 53, 5806–5813. [Google Scholar]
- Roy, S.; Praneetha, D.; Vendra, V.P.R. Mutations in the Corneal Endothelial Dystrophy–Associated Gene SLC4A11 Render the Cells More Vulnerable to Oxidative Insults. Cornea 2015, 34, 668–674. [Google Scholar] [CrossRef]
- Guha, S.; Chaurasia, S.; Ramachandran, C.; Roy, S. SLC4A11 Depletion Impairs NRF2 Mediated Antioxidant Signaling and Increases Reactive Oxygen Species in Human Corneal Endothelial Cells During Oxidative Stress. Scientific reports 2017, 7, 4074. [Google Scholar] [CrossRef]
- Zhang, W.; Li, H.; Ogando, D.G.; Li, S.; Feng, M.; Price, F.W.; Tennessen, J.M.; Bonanno, J.A. Glutaminolysis Is Essential for Energy Production and Ion Transport in Human Corneal Endothelium. EBioMedicine 2017, 16, 292–301. [Google Scholar] [CrossRef]
- Zhong, J.; Dong, J.; Ruan, W.; Duan, X. Potential Theranostic Roles of SLC4 Molecules in Human Diseases. International Journal of Molecular Sciences 2023, 24, 15166. [Google Scholar] [CrossRef]
- Shyam, R.; Ogando, D.G.; Choi, M.; Liton, P.B.; Bonanno, J.A. Mitochondrial ROS Induced Lysosomal Dysfunction and Autophagy Impairment in an Animal Model of Congenital Hereditary Endothelial Dystrophy. Investigative Ophthalmology & Visual Science 2021, 62, 15–15. [Google Scholar]
- Shyam, R.; Ogando, D.G.; Bonanno, J.A. Mitochondrial ROS in Slc4a11 KO Corneal Endothelial Cells Lead to ER Stress. Frontiers in Cell and Developmental Biology 2022, 10, 878395. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.; Bonanno, J.A. Mitochondrial Targeting of the Ammonia-Sensitive Uncoupler SLC4A11 by the Chaperone-Mediated Carrier Pathway in Corneal Endothelium. Investigative Ophthalmology & Visual Science 2021, 62, 4–4. [Google Scholar]
- Ehlers, N.; Módis, L.; Møller-Pedersen, T. A Morphological and Functional Study of Congenital Hereditary Endothelial Dystrophy. Acta Ophthalmologica Scandinavica 1998, 76, 314–318. [Google Scholar] [CrossRef] [PubMed]
- Han, S.B.; Ang, H.-P.; Poh, R.; Chaurasia, S.S.; Peh, G.; Liu, J.; Tan, D.T.; Vithana, E.N.; Mehta, J.S. Mice with a Targeted Disruption of Slc4a11 Model the Progressive Corneal Changes of Congenital Hereditary Endothelial Dystrophy. Investigative Ophthalmology & Visual Science 2013, 54, 6179–6189. [Google Scholar]
- Charpentier, P.; Santerre, K.; Proulx, S. Role of SLC4A11 and Its Third Extracellular Loop in the Adhesion of Corneal Endothelial Cells to Descemet Membrane. Investigative Ophthalmology & Visual Science 2023, 64, 626–626. [Google Scholar]
- Hirokawa, N.; Heuser, J.E. Quick-Freeze, Deep-Etch Visualization of the Cytoskeleton Beneath Surface Differentiations of Intestinal Epithelial Cells. The Journal of cell biology 1981, 91, 399–409. [Google Scholar] [CrossRef]
- Yonemura, S. Cadherin–Actin Interactions at Adherens Junctions. Current opinion in cell biology 2011, 23, 515–522. [Google Scholar] [CrossRef]
- Takeichi, M. Dynamic Contacts: Rearranging Adherens Junctions to Drive Epithelial Remodelling. Nature reviews Molecular cell biology 2014, 15, 397–410. [Google Scholar] [CrossRef]
- Alvarez, B.V.; Piché, M.; Aizouki, C.; Rahman, F.; Derry, J.M.; Brunette, I.; Casey, J.R. Altered Gene Expression in Slc4a11-/- Mouse Cornea Highlights SLC4A11 Roles. Scientific Reports 2021, 11, 20885. [Google Scholar] [CrossRef]
- Zhang, W.; Frausto, R.; Chung, D.D.; Griffis, C.G.; Kao, L.; Chen, A.; Azimov, R.; Sampath, A.P.; Kurtz, I.; Aldave, A.J. Energy Shortage in Human and Mouse Models of SLC4A11-Associated Corneal Endothelial Dystrophies. Investigative Ophthalmology & Visual Science 2020, 61, 39–39. [Google Scholar]
- Roblek, M.; Bicher, J.; Gogh, M. van; György, A.; Seeböck, R.; Szulc, B.; Damme, M.; Olczak, M.; Borsig, L.; Siekhaus, D.E. The Solute Carrier MFSD1 Decreases the Activation Status of β1 Integrin and Thus Tumor Metastasis. Frontiers in oncology 2022, 12, 777634. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; DeBose-Boyd, R.A. Regulation of Cholesterol and Fatty Acid Synthesis. Cold Spring Harbor perspectives in biology 2011, 3, a004754. [Google Scholar] [CrossRef] [PubMed]
- Agostini, F.; Pereyra, L.; Dale, J.; Yambire, K.F.; Maglioni, S.; Schiavi, A.; Ventura, N.; Milosevic, I.; Raimundo, N. Upregulation of Cholesterol Synthesis by Lysosomal Defects Requires a Functional Mitochondrial Respiratory Chain. Journal of Biological Chemistry 2024, 300. [Google Scholar] [CrossRef]
- Weiss, J.S.; Rapuano, C.J.; Seitz, B.; Busin, M.; Kivelä, T.T.; Bouheraoua, N.; Bredrup, C.; Nischal, K.K.; Chawla, H.; Borderie, V.; et al. IC3D Classification of Corneal Dystrophies—Edition 3. Cornea 2024, 466–527. [Google Scholar] [CrossRef] [PubMed]
- Liskova, P.; Dudakova, L.; Tesar, V.; Bednarova, V.; Kidorova, J.; Jirsova, K.; Davidson, A.E.; Hardcastle, A.J. Detailed Assessment of Renal Function in a Proband with Harboyan Syndrome Caused by a Novel Homozygous SLC4A11 Nonsense Mutation. Ophthalmic research 2015, 53, 30–35. [Google Scholar] [CrossRef]
- Gupta, R.; Kumawat, B.L.; Paliwal, P.; Tandon, R.; Sharma, N.; Sen, S.; Kashyap, S.; Nag, T.C.; Vajpayee, R.B.; Sharma, A. Association of ZEB1 and TCF4 Rs613872 Changes with Late Onset Fuchs Endothelial Corneal Dystrophy in Patients from Northern India. Molecular Vision 2015, 21, 1252. [Google Scholar]
- Hemadevi, B.; Srinivasan, M.; Arunkumar, J.; Prajna, N.V.; Sundaresan, P. Genetic Analysis of Patients with Fuchs Endothelial Corneal Dystrophy in India. BMC ophthalmology 2010, 10, 1–6. [Google Scholar] [CrossRef]
- Minear, M.A.; Li, Y.-J.; Rimmler, J.; Balajonda, E.; Watson, S.; Allingham, R.R.; Hauser, M.A.; Klintworth, G.K.; Afshari, N.A.; Gregory, S.G. Genetic Screen of African Americans with Fuchs Endothelial Corneal Dystrophy. Molecular vision 2013, 19, 2508. [Google Scholar]
- Tang, H.; Zhang, W.; Yan, X.-M.; Wang, L.-P.; Dong, H.; Shou, T.; Lei, H.; Guo, Q. Analysis of SLC4A11, ZEB1, LOXHD1, COL8A2 and TCF4 Gene Sequences in a Multi-Generational Family with Late-Onset Fuchs Corneal Dystrophy. International Journal of Molecular Medicine 2016, 37, 1487–1500. [Google Scholar] [CrossRef]
- Chaurasia, S.; Ramappa, M.; Annapurna, M.; Kannabiran, C. Coexistence of Congenital Hereditary Endothelial Dystrophy and Fuchs Endothelial Corneal Dystrophy Associated with SLC4A11 Mutations in Affected Families. Cornea 2020, 39, 354–357. [Google Scholar] [CrossRef] [PubMed]
- Okumura, N.; Hayashi, R.; Nakano, M.; Tashiro, K.; Yoshii, K.; Aleff, R.; Butz, M.; Highsmith, E.W.; Wieben, E.D.; Fautsch, M.P.; et al. Association of Rs613872 and Trinucleotide Repeat Expansion in the TCF4 Gene of German Patients with Fuchs Endothelial Corneal Dystrophy. Cornea 2019, 38, 799–805. [Google Scholar] [CrossRef] [PubMed]
- Skorodumova, L.O.; Belodedova, A.V.; Antonova, O.P.; Sharova, E.I.; Akopian, T.A.; Selezneva, O.V.; Kostryukova, E.S.; Malyugin, B.E. CTG18. 1 Expansion Is the Best Classifier of Late-Onset Fuchs’ Corneal Dystrophy Among 10 Biomarkers in a Cohort from the European Part of Russia. Investigative Ophthalmology & Visual Science 2018, 59, 4748–4754. [Google Scholar]
- Igo Jr, R.P.; Kopplin, L.J.; Joseph, P.; Truitt, B.; Fondran, J.; Bardenstein, D.; Aldave, A.J.; Croasdale, C.R.; Price, M.O.; Rosenwasser, M.; et al. Differing Roles for TCF4 and COL8A2 in Central Corneal Thickness and Fuchs Endothelial Corneal Dystrophy. 2012.
- Ng, K.H.; Subrayan, V.; Ramachandran, V.; Ismail, F. Screening of Single Nucleotide Polymorphisms Among Fuchs’ Endothelial Corneal Dystrophy Subjects in Malaysia. Egyptian Journal of Medical Human Genetics 2021, 22, 1–11. [Google Scholar] [CrossRef]
- Kim, J.; Ko, J.M.; Tchah, H. Fuchs Endothelial Corneal Dystrophy in a Heterozygous Carrier of Congenital Hereditary Endothelial Dystrophy Type 2 with a Novel Mutation in SLC4A11. Ophthalmic Genetics 2015, 36, 284–286. [Google Scholar] [CrossRef] [PubMed]
- Fautsch, M.P.; Wieben, E.D.; Baratz, K.H.; Bhattacharyya, N.; Sadan, A.N.; Hafford-Tear, N.J.; Tuft, S.J.; Davidson, A.E. TCF4-Mediated Fuchs Endothelial Corneal Dystrophy: Insights into a Common Trinucleotide Repeat-Associated Disease. Progress in retinal and eye research 2021, 81, 100883. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Sadan, A.N.; Bhattacharyya, N.; Zarouchlioti, C.; Szabo, A.; Abreu Costa, M.; Hafford-Tear, N.J.; Kladny, A.-M.S.; Dudakova, L.; Ciosi, M.; et al. Genetic and Demographic Determinants of Fuchs Endothelial Corneal Dystrophy Risk and Severity. JAMA Ophthalmology 2025, 143, 338–347. [Google Scholar] [CrossRef]
- Weiss, J.S.; Afshari, N.A. Corneal Guttae Alone Do Not Make a Diagnosis of Fuchs’ Endothelial Corneal Dystrophy. American journal of ophthalmology 2024, 264, x–xii. [Google Scholar] [CrossRef]
- Patel, S.V.; Baratz, K.H. Comment on: Corneal Guttae Alone Do Not Make a Diagnosis of Fuchs’ Endothelial Corneal Dystrophy. American journal of ophthalmology 2024, 267, 304–305. [Google Scholar] [CrossRef]
- Weiss, J.S.; Afshari, N. Reply to Comment on: Corneal Guttae Alone Do Not Make a Diagnosis of Fuchs’ Endothelial Corneal Dystrophy. American Journal of Ophthalmology 2024, 267, 306–307. [Google Scholar] [CrossRef]
- Goar, E.L. Dystrophy of the Corneal Endothelium (Cornea Guttata), with Report of a Histologic Examination. Transactions of the American Ophthalmological Society 1933, 31, 48. [Google Scholar]
- Lorenzetti, D.; Uotila, M.; Parikh, N.; Kaufman, H. Central Cornea Guttata: Incidence in the General Population. American journal of ophthalmology 1967, 64, 1155–1158. [Google Scholar] [CrossRef] [PubMed]
- Krachmer, J.H.; Purcell, J.J.; Young, C.W.; Bucher, K.D. Corneal Endothelial Dystrophy: A Study of 64 Families. Archives of ophthalmology 1978, 96, 2036–2039. [Google Scholar] [CrossRef] [PubMed]
- Magan, T.; Hammersmith, K.M.; Viaene, A.N.; Kumar, P.; Eagle Jr, R.C.; Milman, T. Harboyan Syndrome: A Novel SLC4A11 Variant with Unique Genotype–Phenotype Correlation. Cornea 2022, 41, 1053–1057. [Google Scholar] [CrossRef]
- Tananuvat, N.; Tananuvat, R.; Chartapisak, W.; Mahanupab, P.; Hokierti, C.; Srikummool, M.; Kampuansai, J.; Intachai, W.; Olsen, B.; Ketudat Cairns, J.R.; et al. Harboyan Syndrome: Novel SLC4A11 Mutation, Clinical Manifestations, and Outcome of Corneal Transplantation. Journal of Human Genetics 2021, 66, 193–203. [Google Scholar] [CrossRef] [PubMed]
- Abramowicz, M.; Albuquerque-Silva, J.; Zanen, A. Corneal Dystrophy and Perceptive Deafness (Harboyan Syndrome): CDPD1 Maps to 20p13. Journal of medical genetics 2002, 39, 110–112. [Google Scholar] [CrossRef]
- Li, S.; Hundal, K.S.; Chen, X.; Choi, M.; Ogando, D.G.; Obukhov, A.G.; Bonanno, J.A. R125H, W240S, C386R, and V507I SLC4A11 Mutations Associated with Corneal Endothelial Dystrophy Affect the Transporter Function but Not Trafficking in PS120 Cells. Experimental eye research 2019, 180, 86–91. [Google Scholar] [CrossRef]
- Zahra, A.; Hall, M.; Chatterjee, J.; Sisu, C.; Karteris, E. In Silico Study to Predict the Structural and Functional Consequences of SNPs on Biomarkers of Ovarian Cancer (OC) and BPA Exposure-Associated OC. International Journal of Molecular Sciences 2022, 23, 1725. [Google Scholar] [CrossRef]
- QI, Y.; Liu, S.; Sun, M.; LI, P.; Zhu, H.; Kuai, X. Correlation Between the Expression of Sodium Borate Transporter Member 11 of Solute Carrier Family 4 Protein and Tumor Protein P53 Protein and Clinicopathological Features and Prognosis in Gastric Carcinoma. Chinese Journal of Digestion 2018, 811–817. [Google Scholar]
- Elaimy, A.L.; El-Derany, M.O.; James, J.; Wang, Z.; Pearson, A.N.; Holcomb, E.A.; Huber, A.K.; Gijón, M.; Bell, H.N.; Sanghvi, V.R.; et al. SLC4A11 Mediates Ammonia Import and Promotes Cancer Stemness in Hepatocellular Carcinoma. JCI insight 2024, 9, e184826. [Google Scholar] [CrossRef]
- Ye, Y.; Yu, B.; Wang, H.; Yi, F. Glutamine Metabolic Reprogramming in Hepatocellular Carcinoma. Frontiers in Molecular Biosciences 2023, 10, 1242059. [Google Scholar] [CrossRef] [PubMed]
- Warburg, O.; Wind, F.; Negelein, E. The Metabolism of Tumors in the Body. The Journal of general physiology 1927, 8, 519. [Google Scholar] [CrossRef] [PubMed]
- Andersen, A.P.; Samsøe-Petersen, J.; Oernbo, E.K.; Boedtkjer, E.; Moreira, J.M.; Kveiborg, M.; Pedersen, S.F. The Net Acid Extruders NHE1, NBCn1 and MCT4 Promote Mammary Tumor Growth Through Distinct but Overlapping Mechanisms. International journal of cancer 2018, 142, 2529–2542. [Google Scholar] [CrossRef] [PubMed]
- Michl, J.; Monterisi, S.; White, B.; Blaszczak, W.; Hulikova, A.; Abdullayeva, G.; Bridges, E.; Yin, Z.; Bodmer, W.F.; Swietach, P. Acid-Adapted Cancer Cells Alkalinize Their Cytoplasm by Degrading the Acid-Loading Membrane Transporter Anion Exchanger 2, SLC4A2. Cell Reports 2023, 42. [Google Scholar] [CrossRef]
- Gorbatenko, A.; Olesen, C.W.; Boedtkjer, E.; Pedersen, S.F. Regulation and Roles of Bicarbonate Transporters in Cancer. Frontiers in physiology 2014, 5, 130. [Google Scholar] [CrossRef]
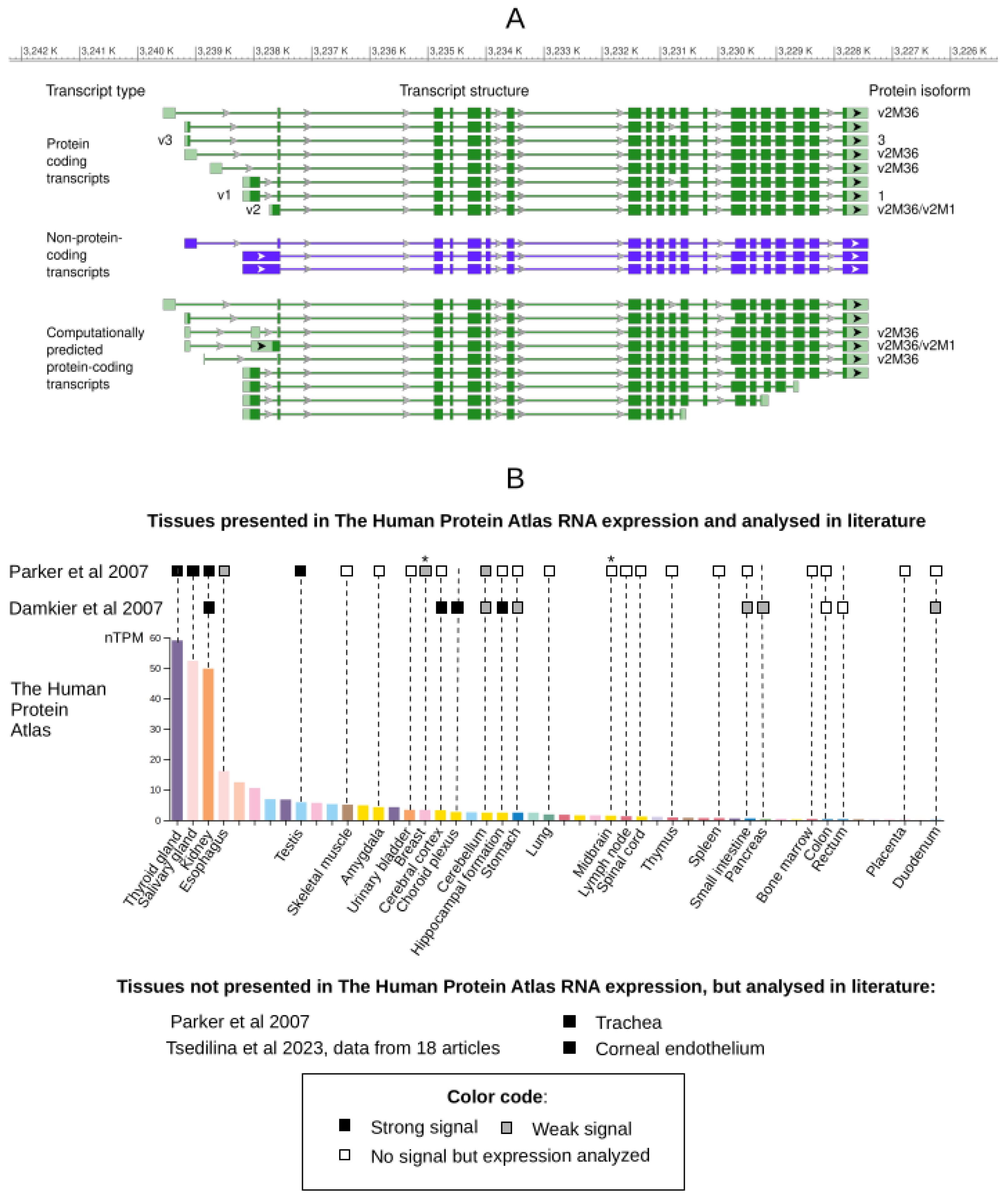
| Isoform names [14,25] | Isoform length (aar) |
RefSeq isoform name and Protein ID in consistence with publications [14,25] |
RefSeq mRNA name and ID in consistence with publications [14,25] |
Other RefSeq SLC4A11 isoforms with 100% amino acid sequence identity (isoform name: Protein ID/mRNA ID) |
Tissue, from which the corresponding transcript was isolated | First mention |
|---|---|---|---|---|---|---|
| SLC4A11-A, SLC4A11-v1 | 918 | isoform 1 (NP_ 001167561) |
transcript variant 1 (NM_ 001174090) |
- | brain | [25] |
| SLC4A11-B, SLC4A11-v2-M1 | 891 | isoform 2 (NP_ 114423) |
transcript variant 2 (NM_032034) | isoform X2: XP_047296496/ XM_047440540 |
kidney | [1] |
| SLC4A11-v2-M36 | 856 | - | transcript variant 2 (NM_032034) |
isoform 5: NP_001387206/ NM_001400277, NP_001387207/ NM_001400278, NP_001387208/ NM_001400279, isoform X4: XP_016883585/ XM_017028096, XP_016883583/ XM_017028094 |
corneal endothelial cells | [14] |
| SLC4A11-C, SLC4A11-v3 | 875 | isoform 3 (NP_ 001167560) |
transcript variant 3 (NM_ 001174089) |
- | corneal endothelial cells | [25] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





