1. Introduction
Radioactive elements such as cesium, iodine, strontium, plutonium, barium, cobalt, lanthanum, yttrium, and tellurium have been detected around the Fukushima Daiichi nuclear power plant (Fukushima, Japan), which was damaged by 9.0-magnitude earthquake during a tsunami event.
Removal of radioactive elements from contaminated sources is crucial for environmental control. However, research efforts have been primarily focused on studying microorganisms, including bacteria [1-4], actinomycetes [5-7], fungi [5, 8-11], and yeasts [4, 12], for removing uranium.
In this study, we investigated the removal and recovery of uranium from aqueous systems using microorganisms isolated from uranium mines. We identified some strains of bacteria with extremely high uranium removal efficiencies [13]; thus, microbial biomass may be considered as an adsorbing agent for the removal and recovery of uranium and heavy metals present in aqueous systems around the Fukushima Daiichi nuclear power plant.
We screened various species and strains of uranium-absorbing bacteria, actinomycetes, fungi, and yeasts [14]. The basic features?coexisting cations, cell amounts, and the adsorption time?that affect the uranium adsorption capacity of Arthrobacter nicotianae cells (which adsorb the largest amount of uranium) were also investigated.
This paper presents the results of removing cobalt, strontium, and cesium, detected around Fukushima, from a solution (containing each metal ion) using A. nicotianae cells
2. Materials and Methods
2.1.Cultures of Microorganisms
The microorganisms were grown in a medium containing 3 g/L of meat extract, 5 g/L of peptone, and 5 g/L of NaCl in deionized water. The cultures of microorganisms, maintained on agar slants, were grown in 300 mL of the medium in a 500-mL flask with continuous shaking (120 rpm) at 30 °C. To obtain a sufficient amount of resting microorganisms after separation from the growth medium, the cultures were grown for 72 h.
The cells were collected via centrifugation, washed thoroughly with deionized water, and used in the subsequent removal experiments.
2.2. Effect of pH on Metal-Ion Removal Using A. nicotianae Cells
The metals were supplied as nitrates. The pH of the solution was adjusted to a desired value (pH = 1.0-8.0 for cesium; pH = 1.0–5.0 for cobalt and strontium) with 0.1 M HNO3. The resting cells (15 mg of dry weight basis) were suspended in 100 mL solutions containing 75 µM of each metal for 1 h at 30 °C. The microorganisms were collected by filtration through a nitrocellulose membrane filter (pore size: 0.2 µm). Control studies confirmed that free metals were not adsorbed onto the filter.
The amount of each metal removed from the cells was determined by measuring the difference between the initial and final metal contents in the filtrate using an atomic absorption analysis quantometer (AA-6300, Shimadzu Corporation, Kyoto, Japan).
2.3. Effect of External Cesium, Cobalt, and Strontium Concentration on Their Removal Using A. nicotianae Cells
The resting cells (15 mg of dry weight basis) were suspended in a 100 mL solution (pH 5) containing 20–150 μM for 1 h at 30 °C. The amount of each metal remaining in the cell-free filtrate was measured, as described above.
2.4.Cell Amount Dependence on the Removal of Each Metal Using A. nicotianae Cells
The resting cells (5–75 mg of dry weight basis) were suspended in a 100 mL solution (pH 5) containing 75 μM of each metal for 1 h at 30 °C. The amount of each metal remaining in the cell-free filtrate was measured, as described above.
2.5. Time Course of the Removal of Each Metal Using A. nicotianae Cells
The resting cells (15 mg of dry weight basis) were suspended in a 100 mL solution (pH 5.8) containing 150 μM of strontium or cobalt, or 37.6 μM of cesium, at 30 °C for 5 min to 24 h. the amount of strontium remaining in the cell-free filtrate was measured, as described above.
3. Results and Discussion
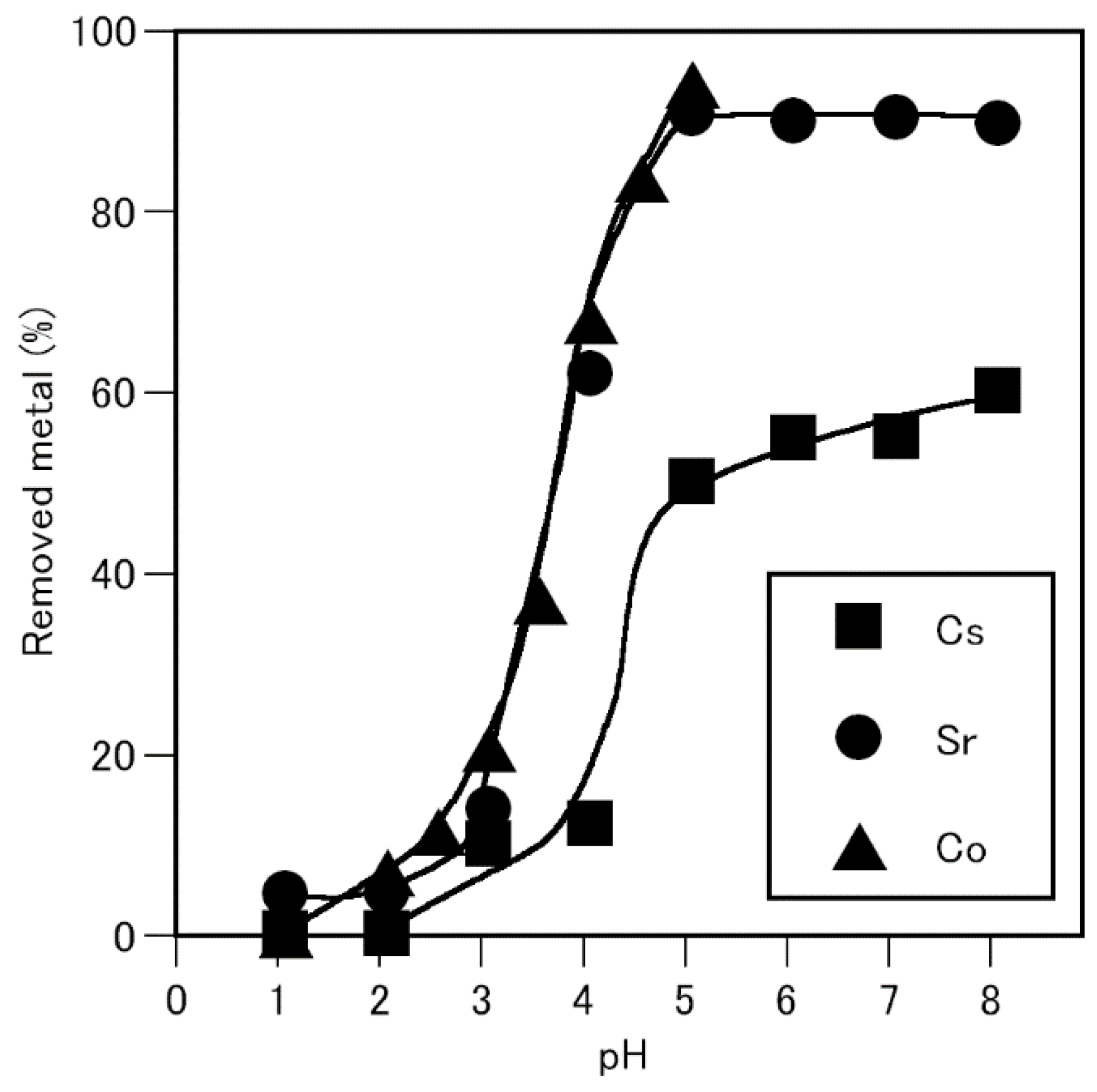
3.1. Effect of pH on the Removal of Cobalt, Strontium, and Cesium Using A. nicotianae Cells
The effect of pH on the removal of cobalt, strontium, and cesium from aqueous solutions using A.nicotianae cells were examined. Strontium and cesium removal was examined at pH 1-8, whereas; cobalt rmoval was examined at pH 1-5, because the hydroxide precipitate was produced at pH 6. The amount of metal removed increased with increasing solution pH (
Figure1). Evidently, 94% and 90% of cobalt and strontium were removed at pH 5.0, whereas only 60% of cesium was removed at pH 8.0, indicating that removing cobalt and strontium was easier than removing cesium.
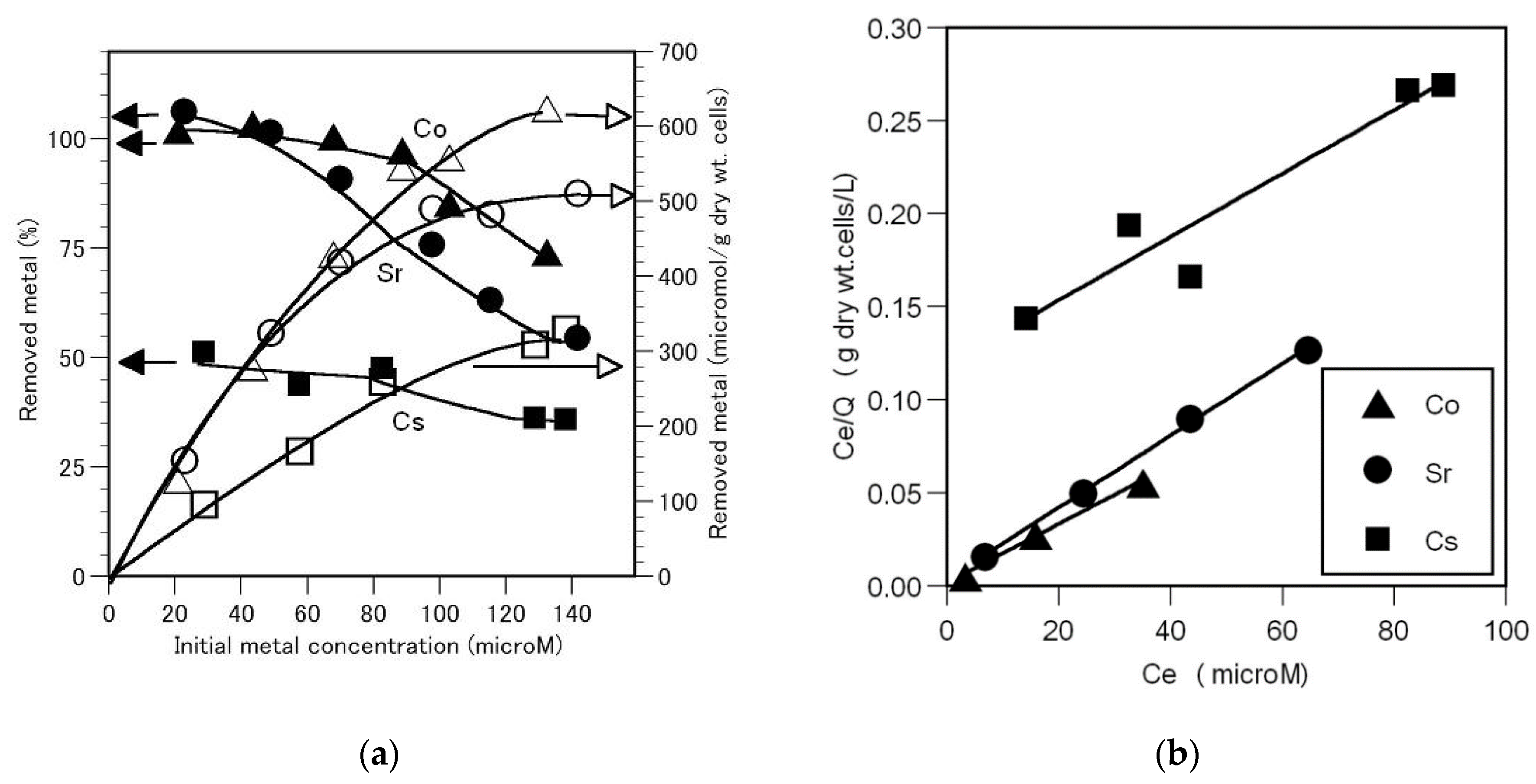
3.2. Effect of Externl Metal Concentration on the Removal of Cobalt, Strontium, and Cesium
Next, the effect of external metal concentration of the removal of cobalt, strontium and cesium was examined. The removed metal amount (μmol/g dry wt. cells) increased with increasing external metal concentration, whereas the total amount of metal removed (%) decreased (
Figure 2(a)). Under these experimental conditions, cobalt and strontium were completely removed from the solution, which finally retains only <66.5 and <47,6 μM of cobalt and strontium, respectively. However, the total amount of cesium removed from a solution containing 27.4 μM of cesium was 51.0 %. The maximum amounts of cobalt, strontium and cesium removed were 624, 510, and 328 μmol/g dry wt. cells, respectively. Accordingly, the order of relative removal degree of each metal ion was cobalt > strontium >> cesium, which confirmed that compared to strontium and cesium, cobalt was removed more readily by the A. nicotianae cells.
The relationship between the residual cobalt, srrontium, and cesium concentrations in the solution and the amount of each metal removed is shown in Figure 3B. Evidently, the removal of cobalt or strontium using the A.nicotianae cells is reflected by the Langmuir isotherm, Ce/Q = mCe, where Q indicates the amount of each metal removed (μmol/g dry wt. cells), Ce is the residual metal amount in the solution (μM) and m is a constant. The maximum amounts of cobalt and strontium removed, estimated from the reciprocal of the line slope shown in figure 2(b), are 635 and 517 μmol/g dry wt. cells, respectively. According to the Langmuir isotherm, the relative degree of maximum removal of each metal is cobalt > strontium.
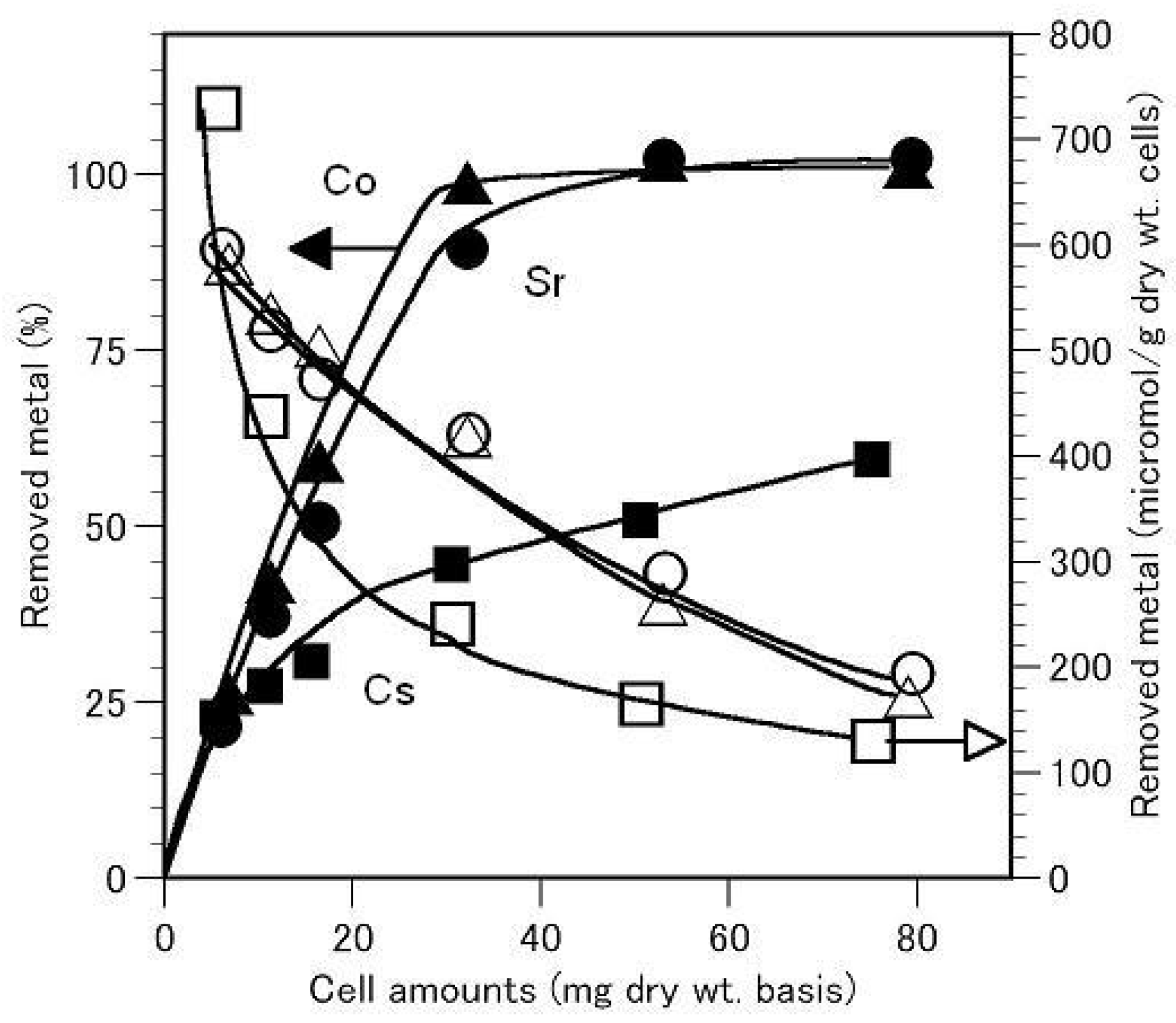
3.3. Effect of Cell Amount on the Removal of Cobalt, Strontium, and Cesium
The effct of the A. nicotianae cell amount on the removal of cobalt, strontium, and cesium was examined (
Figure 3). The total amount of each metal removed increased with the cell amount, whereas the relative amount of each metal removed by the cell (μmol/g dry wt. cells) decreased. Under this experimental condition, large amounts of cobalt and strontium (52.2 and 52.t mg of dry weight basis of the cells) were readily removed. However, a total of 59.3% of cesium was removed when 74.6 mg of the A. nicotianae cells was used. The maximum amounts of cobalt, strontium, and cesium removed using 6.0, 5.3, and 5.0 mg of dry weight cells, were 582, 594, and 728 μmol/g dry wt. cells, respectively.
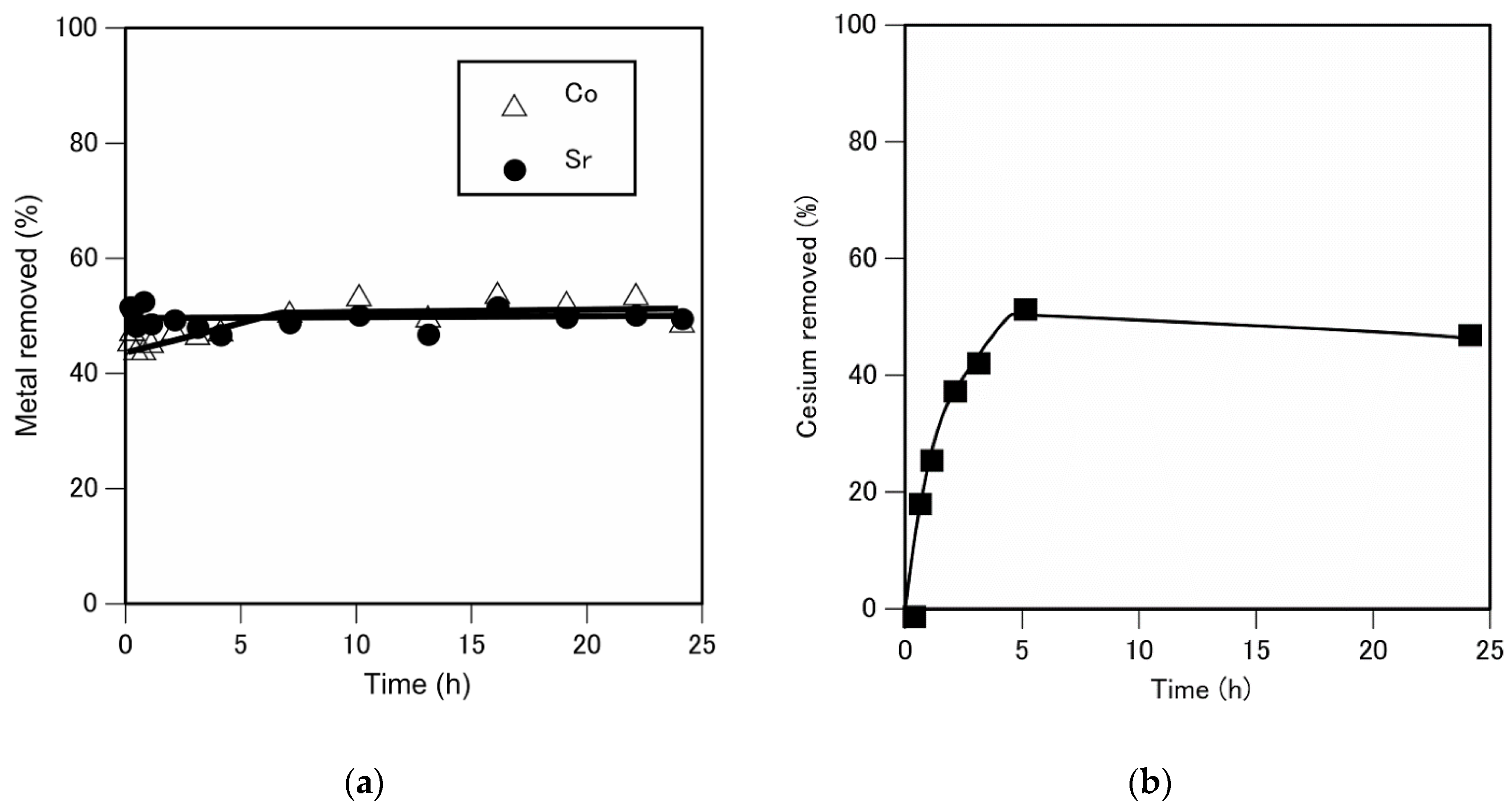
3.4. Time Course of Cobalt, Strontium, and Cesium Removal Using A. nicotianae Cells
Next, we determined the time course of cobalt, strontium, and cesium removal using
A. nicotianae cells (
Figure 4(a) and 4(b)).
Figure 4(a) shows that the amounts of cobalt and strontium removed using the
A. nicotianae cells increase rapidly during the first 5 min following the supply of cobalt and strontium, respectively. In contrast, the amount of cesium removed gradually increases and reaches equilibrium within 5 h.
4. Conclusions
This study was focused on the assessment of cobalt, strontium, and cesium ion removals from aqueous solutions using microorganisms, viz. A. nicotianae, exhibiting a strong ability to remove metal cations. The cobalt, strontium, and cesium removal efficiency of A. nicotianae was affected by the solution pH, metal concentration, and cell amount. The metal- ion content (μmol/g dry wt. cells) removed increased with increasing solution pH (1-5) and that also increased with external metal-ion concentration, whereas the total amount of metal ions removed (%) decreased. The experimental data of the amount of metal removed (μmol/g dry wt. cells) were well-fitted by a Langmuir isotherm. Conversely, the total amount of metal ions removed (%) increased with increasing cell amount, whereas the amount of each metal ion removed (μmol/g dry wt. cells) decreased. A. nicotianae, facilitated fast strontium removal, and the amount of rapidly reached equilibrium within 5 min. The removed amounts of cobalt and strontium were higher than that of cesium.
References
- Andres, Y.; Maccordick, H. J.; Hubert, J.-C. Adsorption of several actinide (Th, U) and lanthanide (La, Eu, Yb) ions by Mycobacterium smegmatis, Appl. Microbiol. Biotechnol. 1993, 39, 413-417. [CrossRef]
- Hu, M. Z. -C.; Norman, J. M.; Faison, B. D.; Reeves, M. E. Biosorption of uranium by Pseudomonas aeruginosa strain CSU: characterization and comparison studies, Biotechnol. Bioeng. 1996, 51, 237-247.
- Marques A. M.; Roca, X.; Simon-Pujol, M. D.; Congregado, F. Uranium accumulation by Pseudomonas sp. EPS-5028, Appl. Microbiol. Biotechnol. 1991, 35, 406-410.
- Strandberg, G. W.; Shumate II, S. E.; Parrott, J. R. Microbial cells as biosorbents for heavy metals: accumulation of uranium by Saccharomyces cerevisiae and Pseudomonas aeruginosa, Appl. Env. Microbiol. 1981, 41, 237-245. [CrossRef]
- Byerley, J. J.; Scharer, J. M.; Charles, A. M. Uranium (VI) biosorption from process solutions, Chem. Eng. J. 1987, 36, B49-B59.
- Friiss, N.; Myers-Keith P. Biosorption of uranium and lead by Streptomyces longwoodensis, Biotechnol. Bioeng. 1986, 28, 21-28. [CrossRef] [PubMed]
- Author 1, A.B. Title of Thesis. Level of Thesis, Degree-Granting University, Location of University, Date of Completion.
- Golab, Z.; Orlowwska, B.; Smith, R. W. Biosorption of lead and uranium by Streptomyces sp, Water, Air, and Soil Pollut. 1991, 60, 99-106.
- Galun, M.; Keller, P.; Malki, D.; Fedelstein, H.; Galun, E. Siegel, S.; Siegel, B. Recovery of uranium (VI) from solution using precultured Penicillium biomass, Water, Air, and Soil Pollut. 1983a, 20, 221-232.
- Galun, M.; Keller, P.; Malki, D.; Feldstein, H.; Galun, E.; Siegel, S. M.; Siegel, B. Z. Removal of uranium (VI) from solution by fungal biomass and fungal wall-related biopolymers, Science, 1983b, 219, 285-286.
- Tsezos, M.; Volesky, B. Biosorption of uranium and thorium, Biotechnol. Bioeng. 1981, 23, 583-604. [CrossRef]
- White, C., Gadds, G. M. (1990) : Biosorption of radionuclides by fungal biomass, J. Chem. Technol. Biotechnol. 49, 331-343.
- Shumate, S. E. II; Strandberg, G. W.; Parrott, J. R. Jr. Biological removal of metal ions from aqueous process streams, Biotechnol. Bioeng. Symp. 1978, 8, 13-20.
- Sakaguchi, T.; Tsuruta, T.; Nakajima, A. Removal of uranium by using microorganisms isolated from uranium mines, In Proceedings of the Technical Solutions for Pollution Prevention in the Mining and Mineral Processing Industries, Engineering Foundation Conference, Palm Coast, FL, USA, 21-25 Janually 1995, pp.183-191.
- Tsuruta, T. Removal and recovery of uranyl ion using various microorganisms, J. Biosci. Bioeng., 2002, 94, 23-28.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).