Submitted:
13 May 2025
Posted:
15 May 2025
You are already at the latest version
Abstract
Keywords:
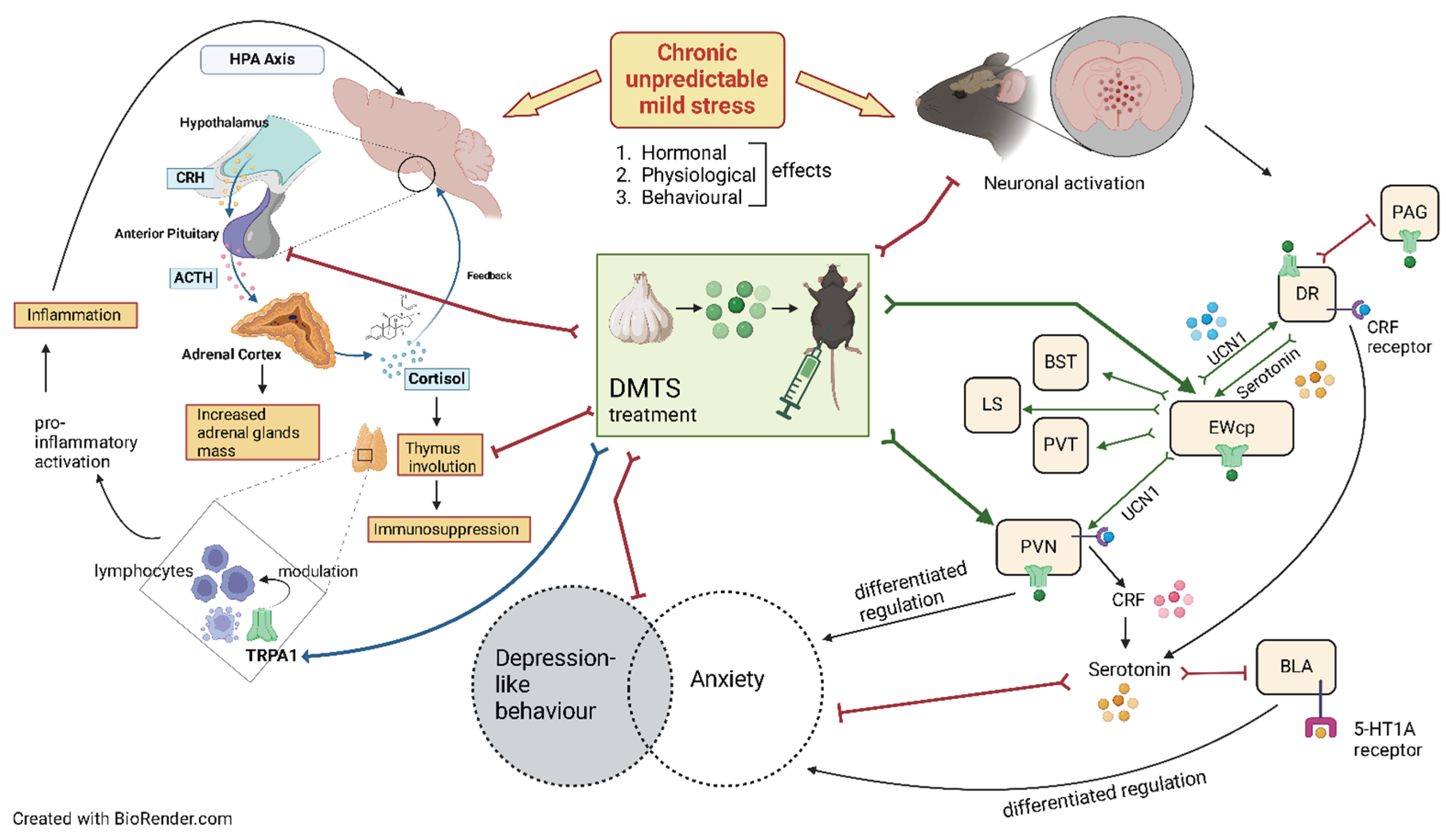
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Chemicals
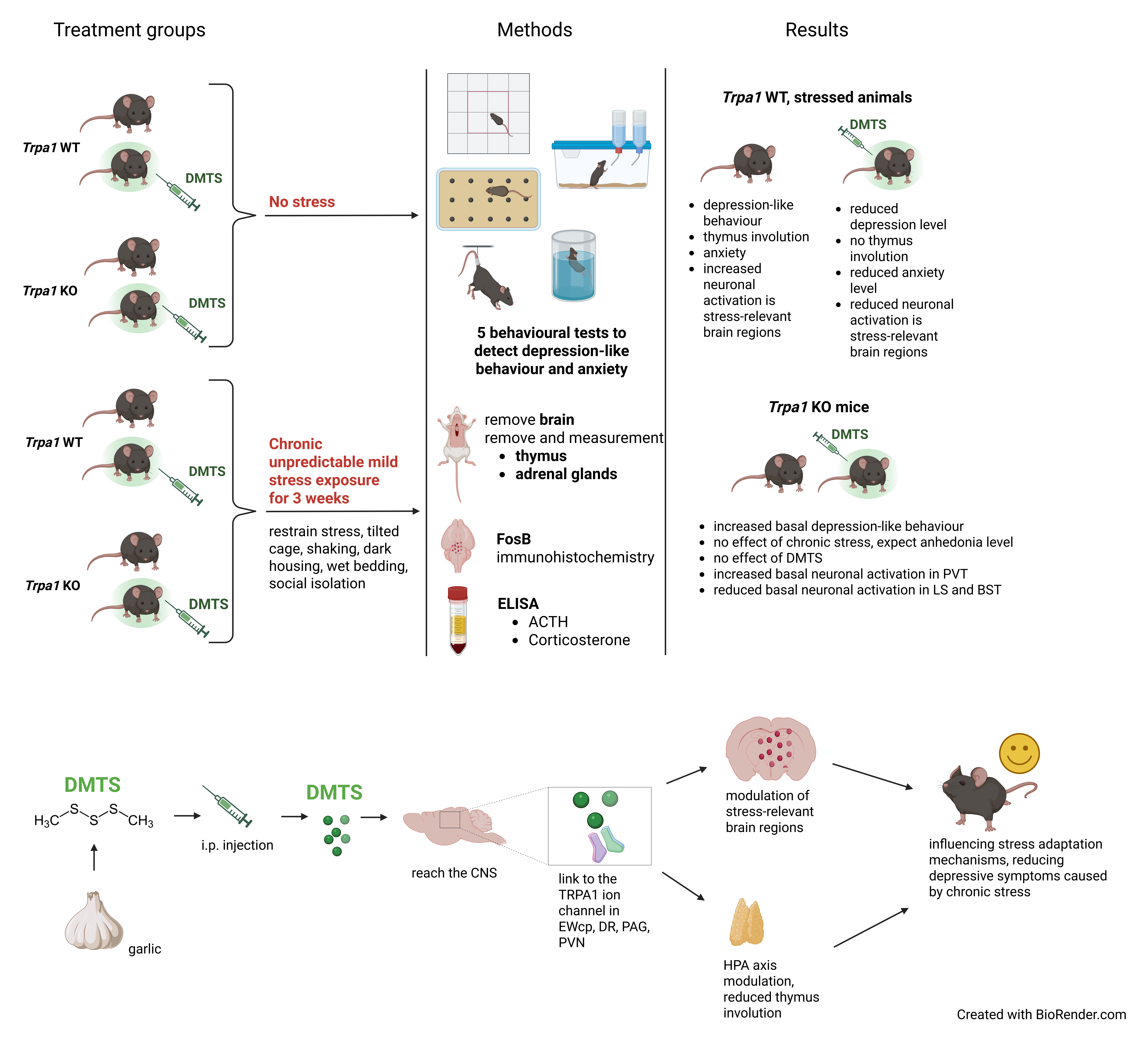
2.3. Experimental Design
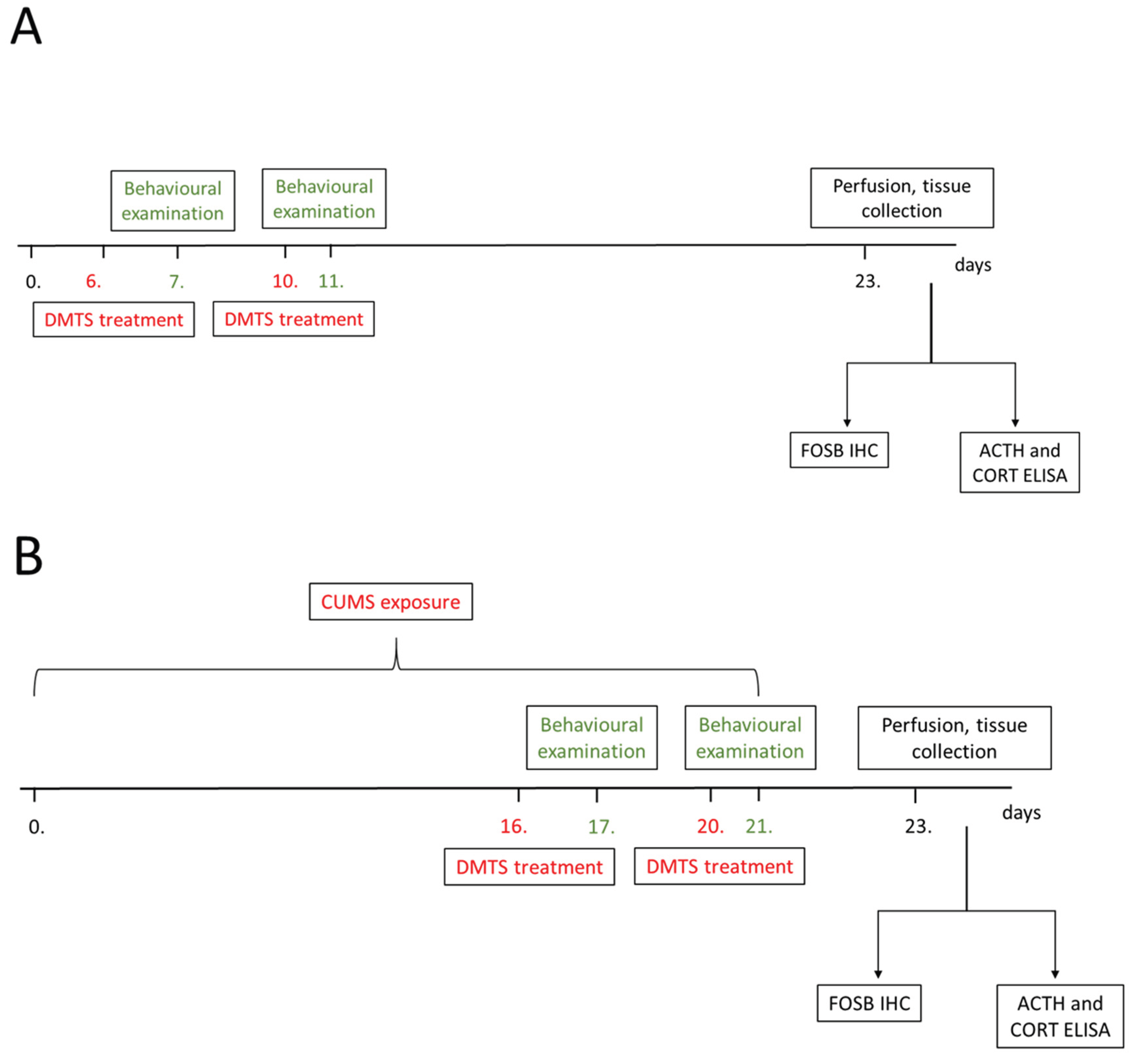
2.3.1. Dose Determination and Treatment Groups
2.3.2. Experimental Schedule
2.4. CUMS Paradigm
2.5. Behavioural Tests
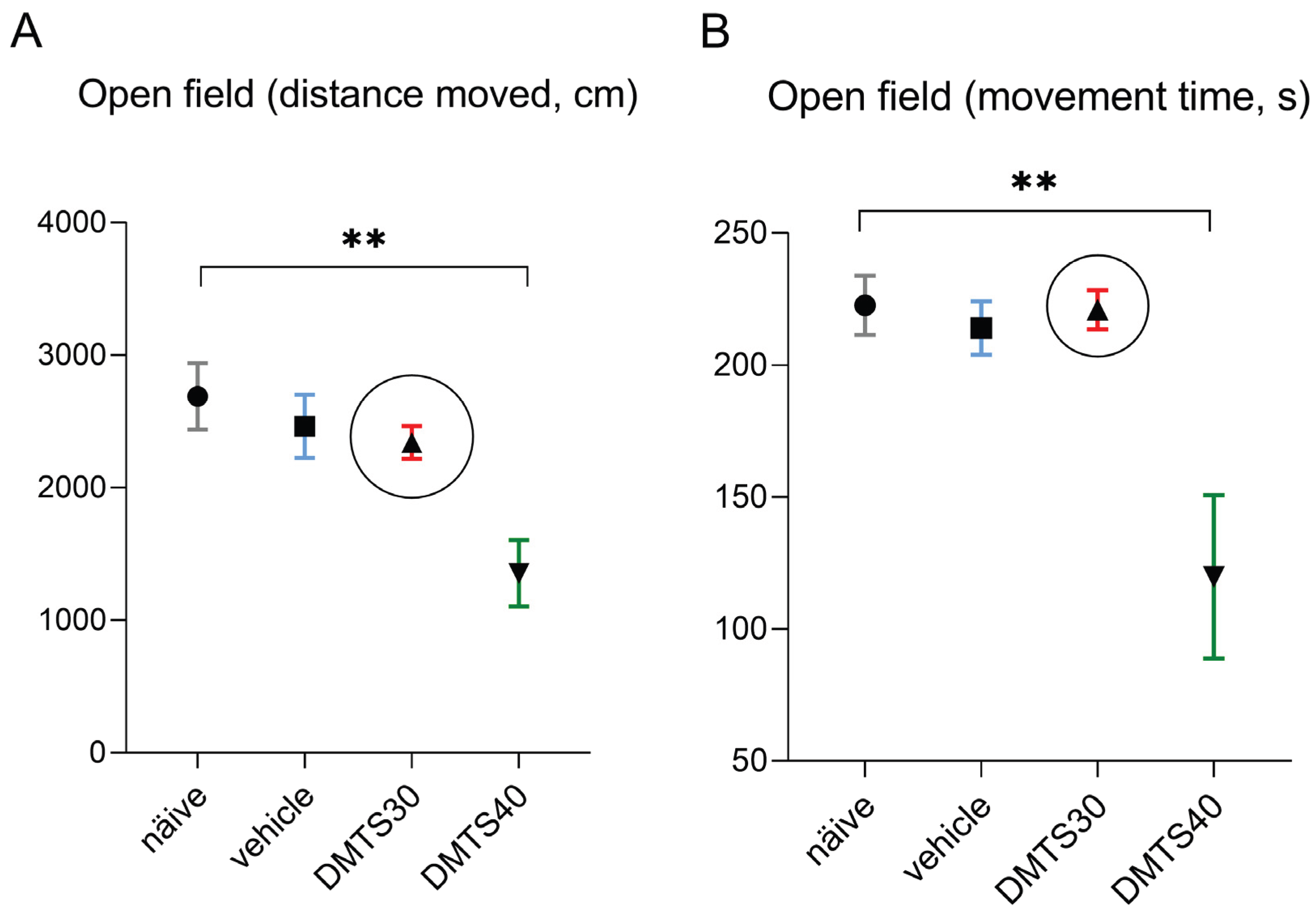
2.5.1. Open Field Test
2.5.2. Marble Burying Test
2.5.3. Sucrose Preference Test
2.5.4. Forced Swim Test and Tail Suspension Test
2.6. Termination
2.6.1. Perfusion and Tissue Collection
2.6.2. Decapitation and Blood Sample Collection
2.7. FOSB Immunohistology
2.8. Microscopy and Digital Imaging
2.9. Enzyme-Linked Immunosorbent Assay
2.7. Evaluation Methods
2.7.1. Noldus EthoVision XT 15
2.7.2. ImageJ
2.8. Statistics
3. Results
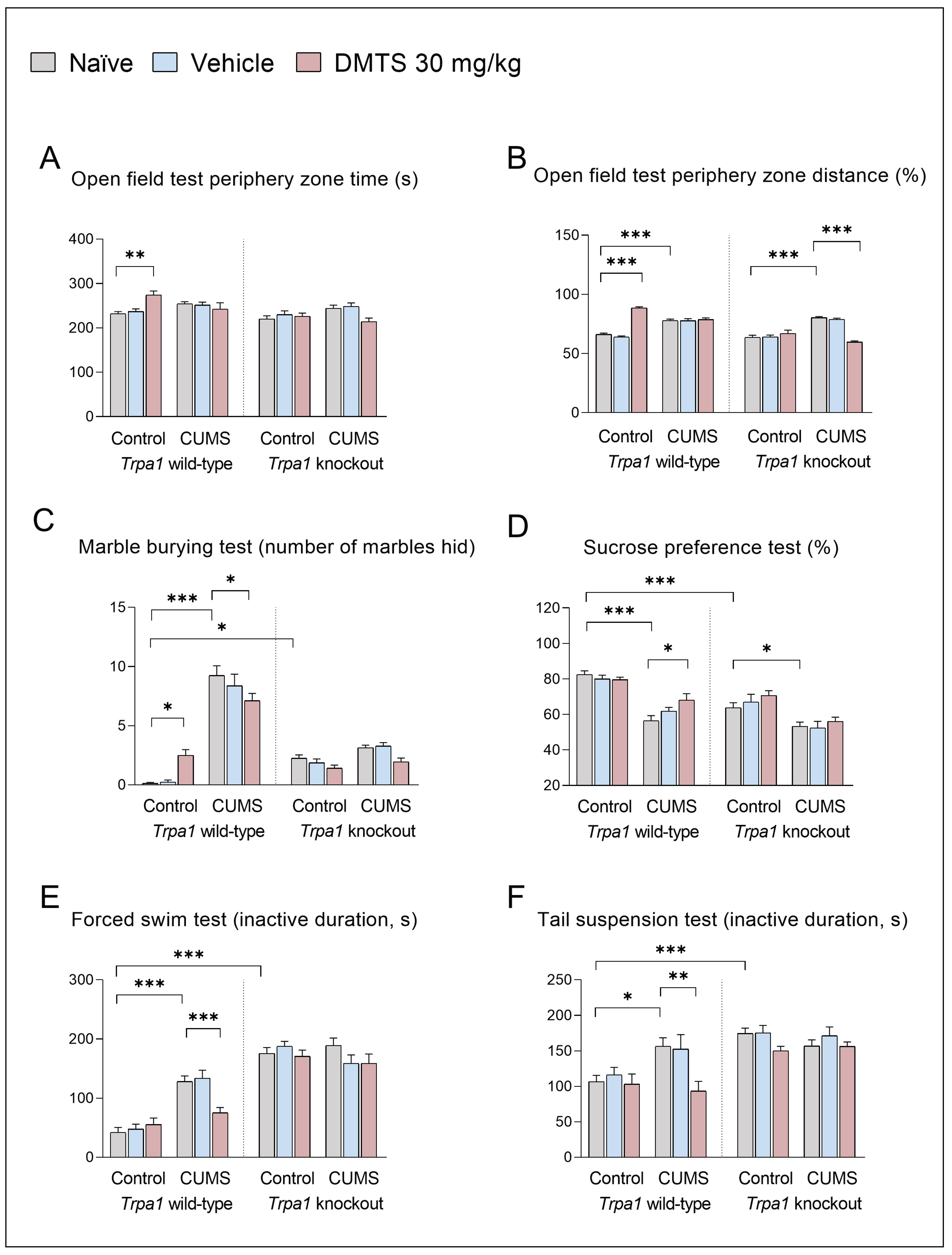
3.1. Finding the Suitable Dose of DMTS Using Open Field Test
3.2. Validity of the Model
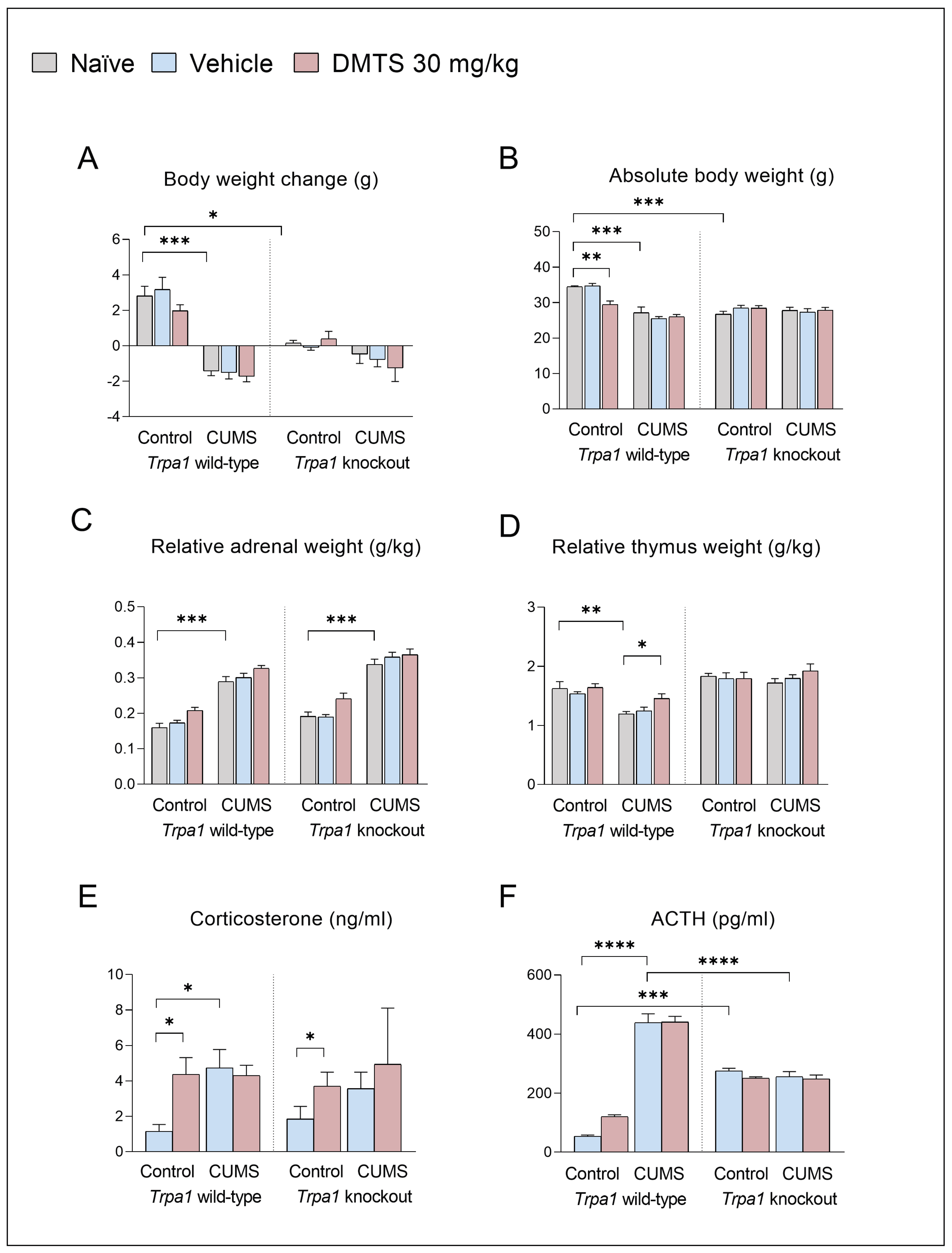
3.2.1. Endocrine and Physical Parameters
3.2.1.2. Adrenal Gland and Thymus Weight
3.2.1.3. CORT and ACTH Serum Concentration
3.2.2. Behavioural Tests
3.2.2.1. Tests to Detect Anxiety Level
3.2.2.2. Tests to Detect Anhedonia and Depression-like Behaviour
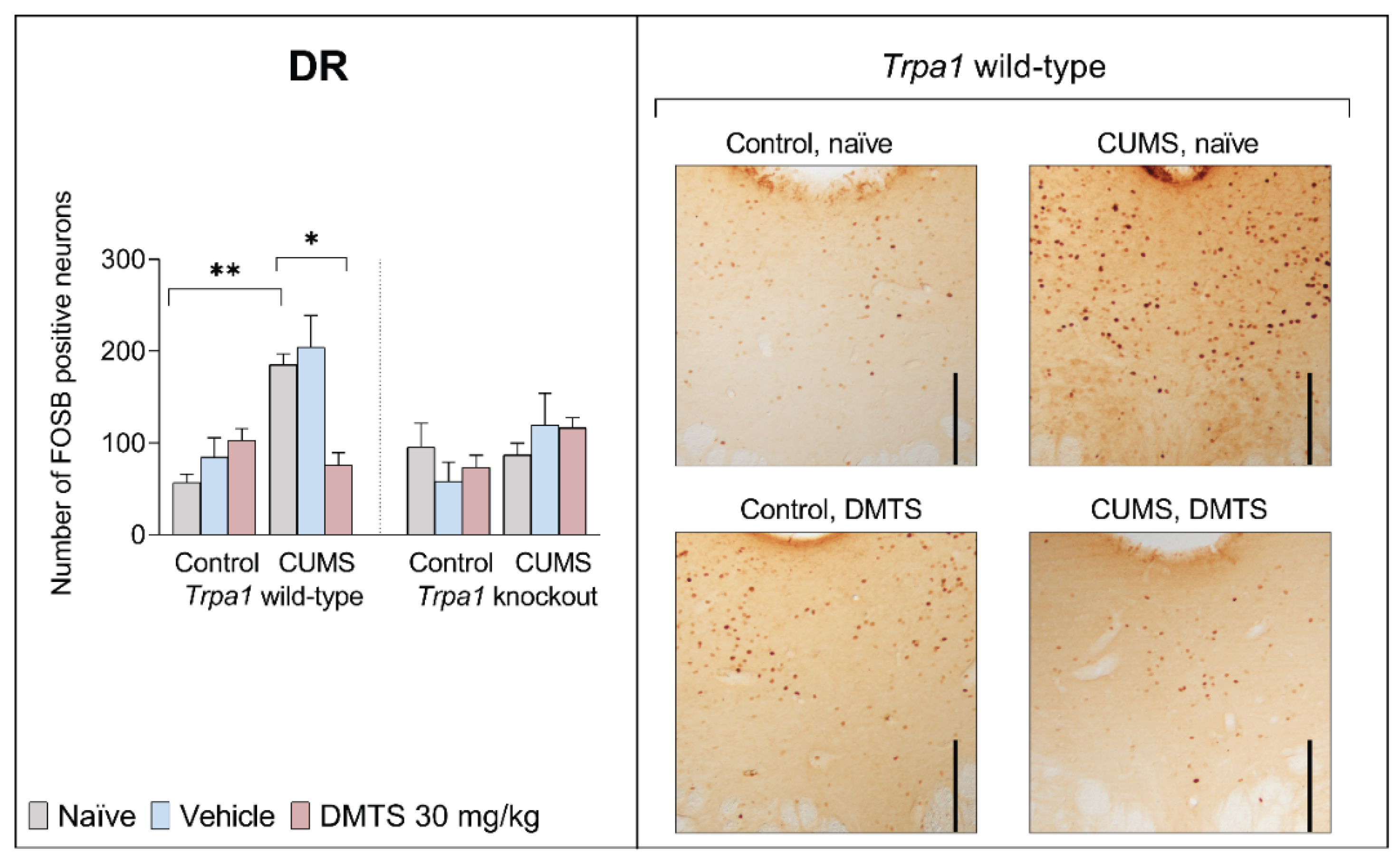
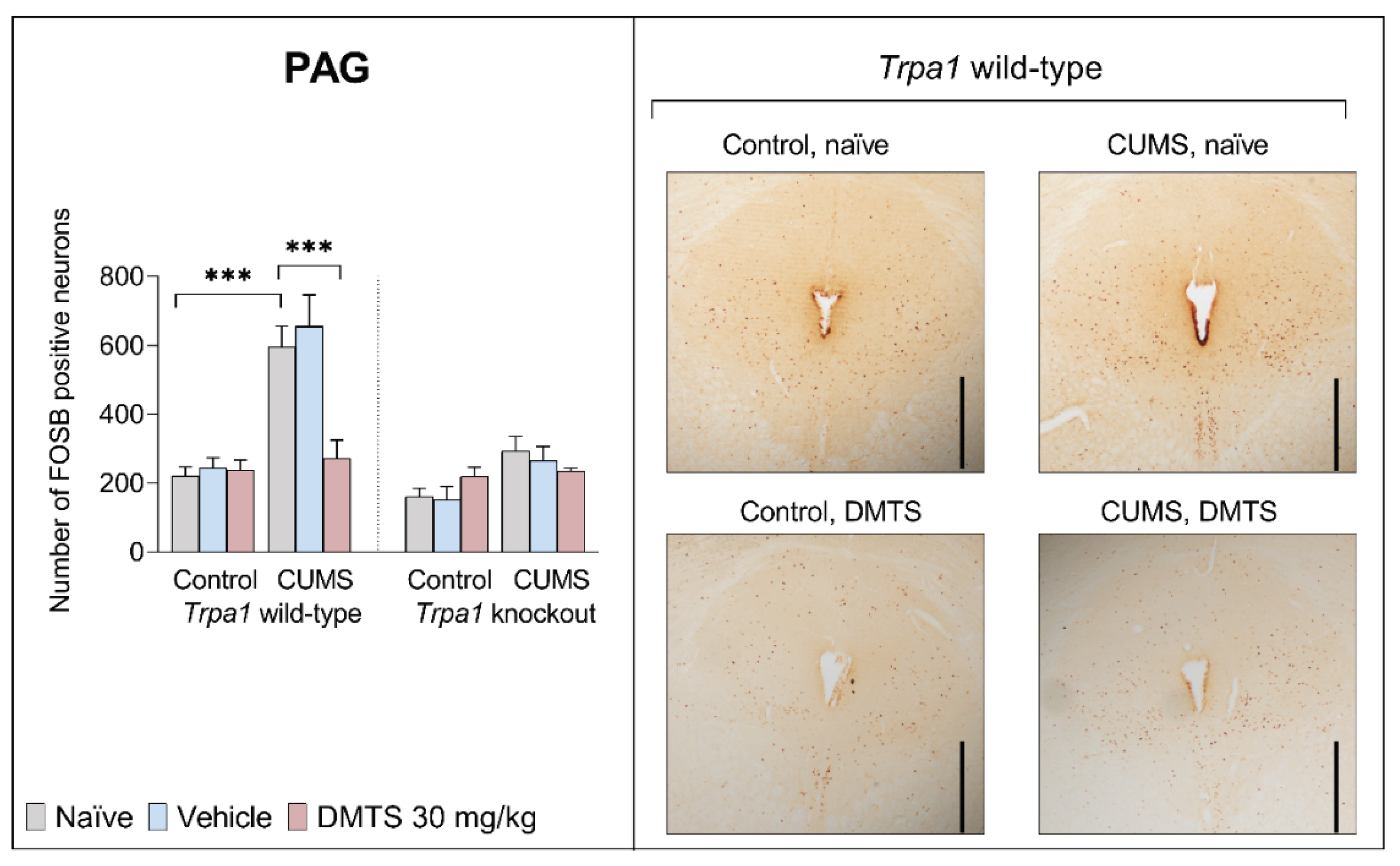
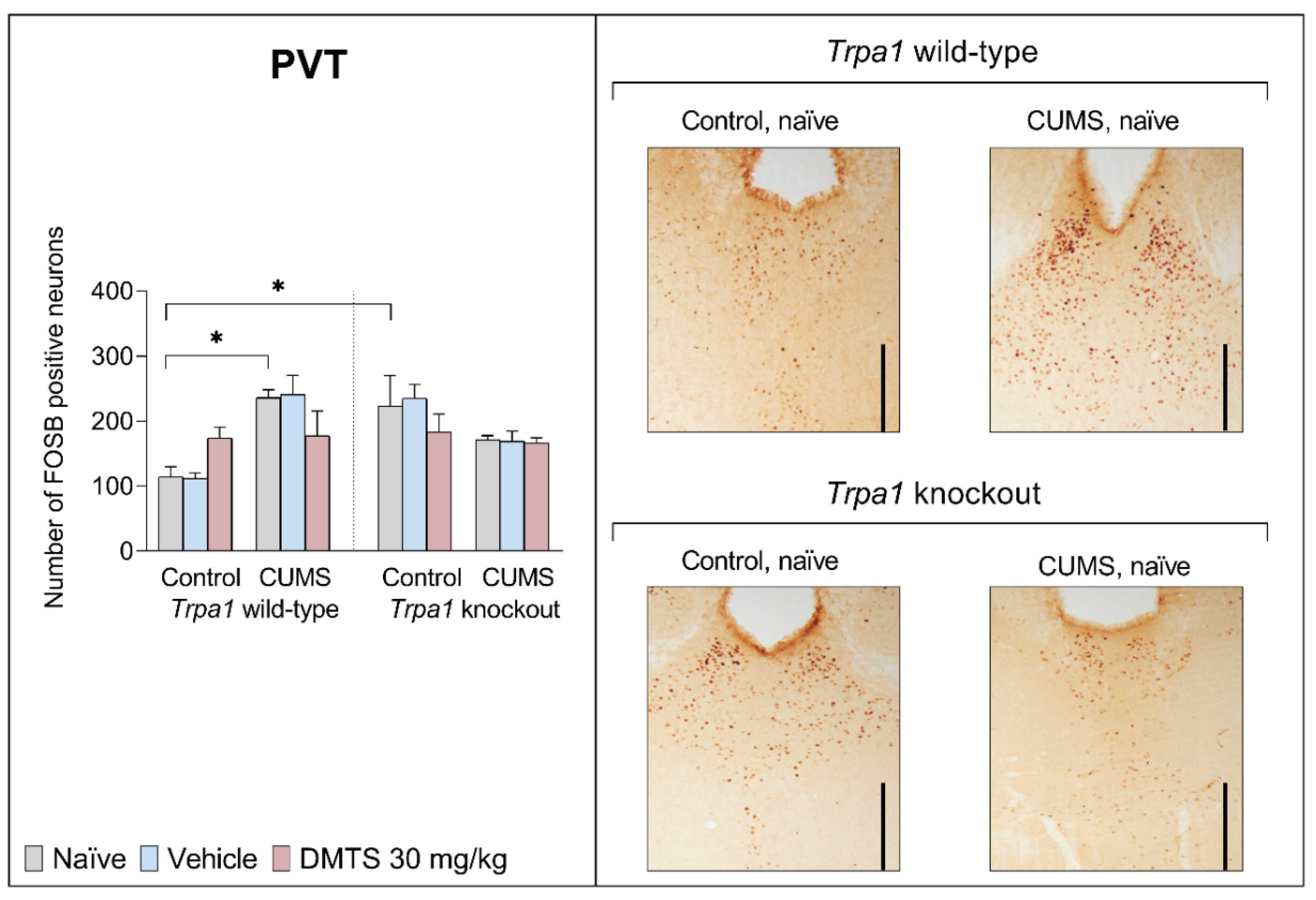
3.3. Pattern of FOSB Neuronal Activation in Stress-Related Brain Areas
3.3.1. Centrally Projecting Edinger-Westphal Nucleus
3.3.2. Dorsal Raphe Nucleus
3.3.3. Periaqueductal Gray Matter
3.3.4. Paraventricular Nucleus of the Thalamus
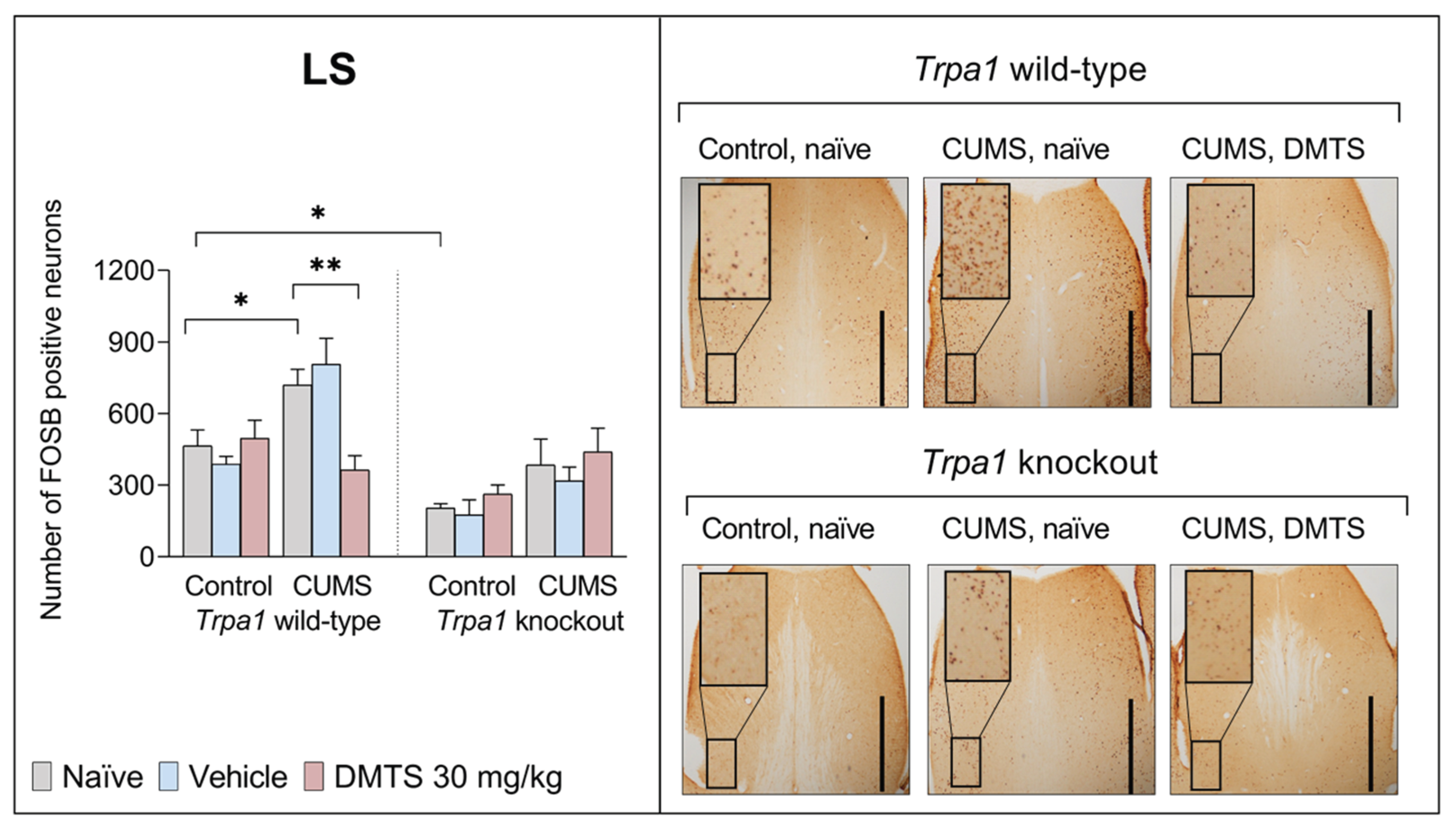
3.3.5. Lateral Septal Nucleus
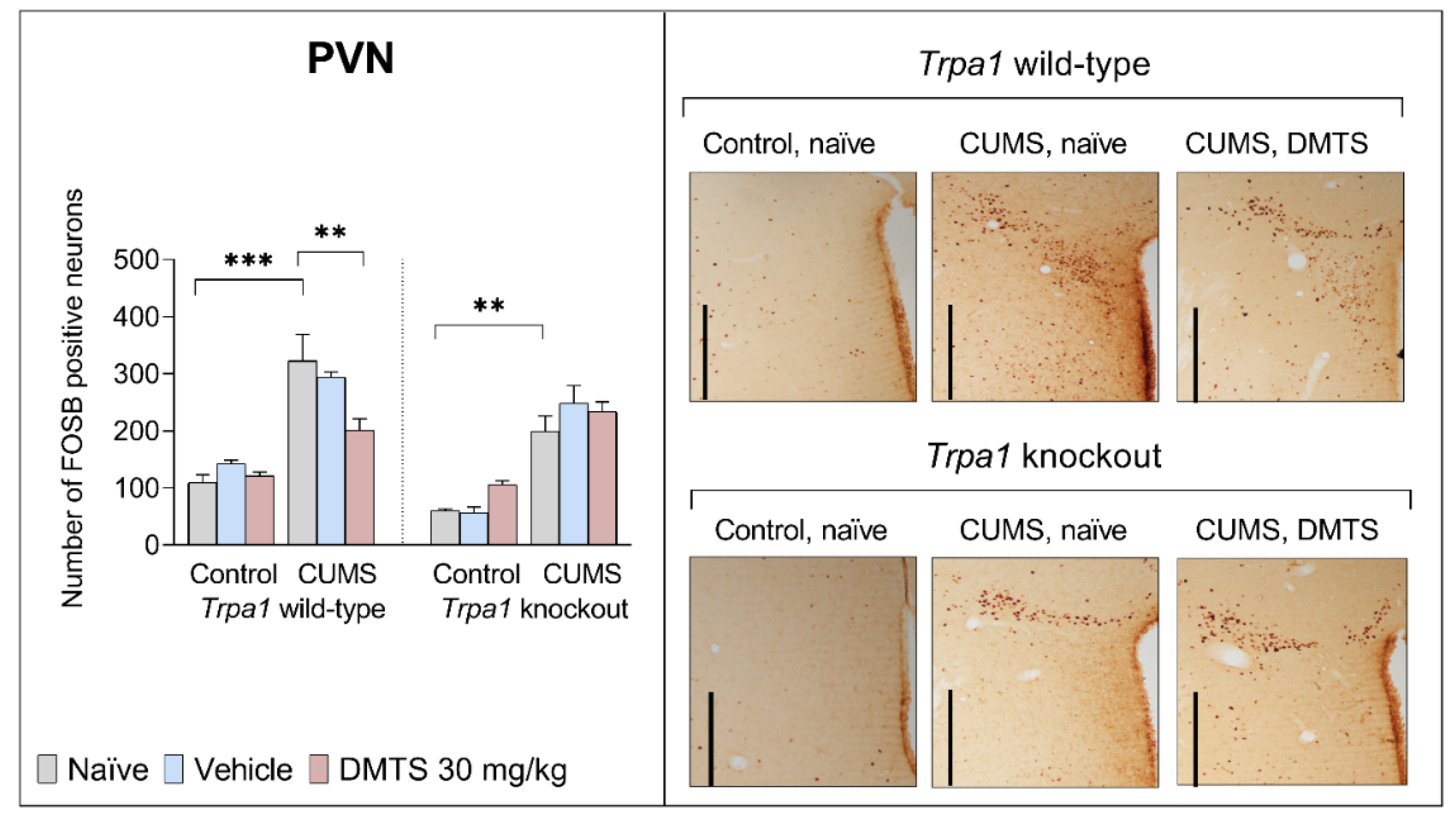
3.3.6. Paraventricular Nucleus of the Hypothalamus
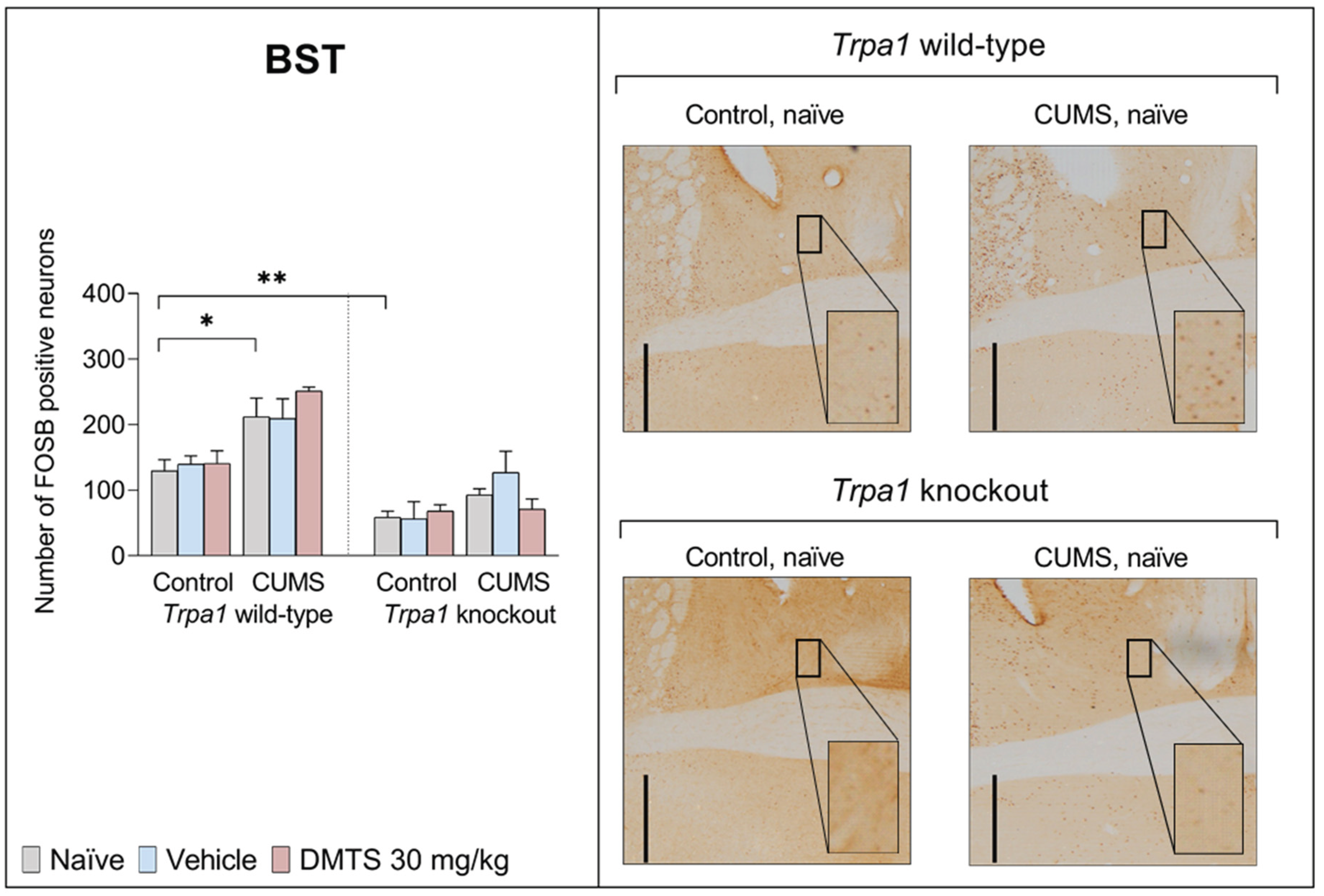
3.3.7. Bed Nucleus of the Stria Terminalis
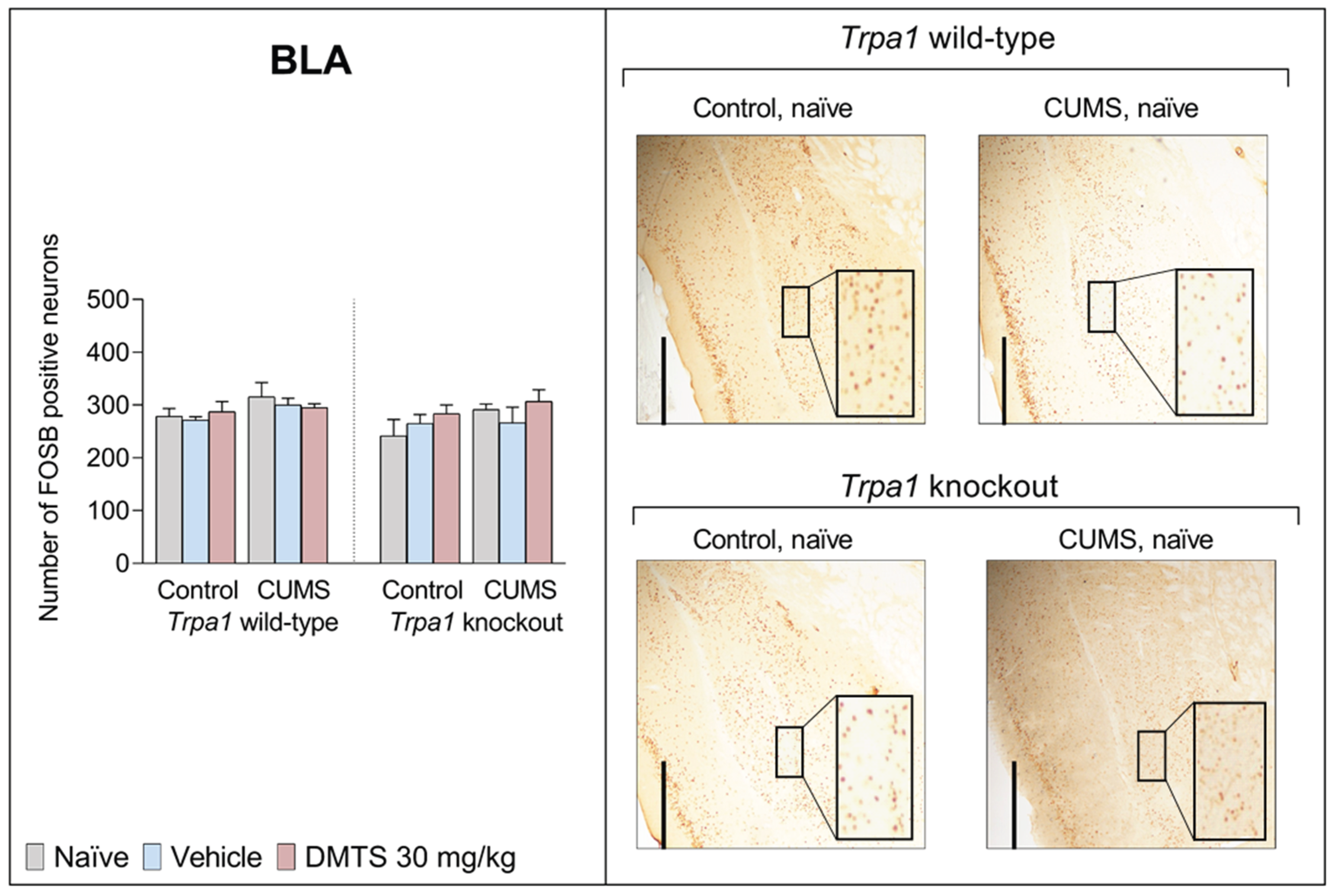
3.3.8. Basolateral Amygdala
4. Discussion
4.1. Validity of the Model
4.2. Normal Stress Response Requires TRPA1 Ion Channel, But the Impact of DMTS Is Independent of the TRPA1 on Physical and Endocrine Parameters
4.3. Lack of TRPA1 Abolished the Inhibitory Effect of DMTS on CUMS-Induced Depression-like Behaviour of Mice
4.4. Effect of DMTS Treatment on Neuronal Activation Depends on Stress Exposure and the Presence of Functional Trpa1
- Trpa1 deficiency affects/modifies the response to chronic stress, both at the level of behaviour and neuronal activation.
- CUMS leads to anxiety and depression-like behaviour, dysregulation of the HPA axis and activation of brain areas important in stress adaptation in a TRPA1-dependent manner.
- DMTS alleviates depression-like behaviour in Trpa1 WT mice.
- Lack of TRPA1 reverses the antidepressant-like action of DMTS in chronic stress-exposed mice according to the SPT test.
- DMTS alleviates thymus involution caused by dysregulation of the HPA axis due to chronic stress.
- The action of DMTS on anxiety and depression-like behaviour may be differentially and independently regulated.
- The absence of TRPA1 ion channel mitigates the inhibitory effect of DMTS on chronic stress-induced neuronal activation in PVN.
5. Conclusions
6. Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 5-HT | Serotonin |
| ACTH | Adrenocorticotropic hormone |
| ANOVA | Analysis of variance |
| BLA | Basolateral amygdala |
| BST | Bed nucleus of the stria terminalis |
| CeA | Central amygdala |
| CHO | Chinese hamster ovary |
| CORT | Corticosterone |
| CNS | Central nervous system |
| CRF | Corticotropin-releasing factor |
| CUMS | Chronic unpredictable mild stress |
| DAB | 3,3′-Diaminobenzidine |
| DARK | Dark room |
| DMTS | Dimethyl trisulfide |
| DR | Dorsal raphe nucleus |
| ELISA | Enzyme-linked immunosorbent assay |
| ER | Endoplasmic reticulum |
| EWcp | Centrally projecting Edinger-Westphal nucleus |
| FTS | Forced swim test |
| GH | Group holding |
| H2O2 | Hydrogen peroxide |
| H2S | Hydrogen sulfide |
| HPA | Hypothalamic-pituitary-adrenal |
| IHC | Immunohistochemistry |
| i.p. | Intraperitoneal |
| ISOL | Social isolation |
| KO | Knockout |
| LS | Lateral septum |
| MBT | Marble burying test |
| MeA | Medial amygdala |
| mPFC | Medial prefrontal cortex |
| OFT | Open field test |
| PACAP | Pituitary adenylate cyclase-activating polypeptide |
| PAG | Periaqueductal gray matter |
| PBS | Phosphate-buffered saline |
| POLY | Inorganic polysulfide |
| PTSD | Post-traumatic stress disorder |
| PVN | Paraventricular nucleus of the hypothalamus |
| PVT | Paraventricular nucleus of the thalamus |
| REST | Restraint stress |
| SHAKE | Shaker stress |
| SPT | Sucrose preference test |
| TILT | Tilted cage |
| TRPA1 | Transient receptor potential ankyrin 1 |
| TST | Tail suspension test |
| UCN1 | Urocortin 1 |
| WET | Wet bedding |
| WM | Body weight measurements |
| WT | Wild type |
References
- Shi, L.; Lin, Y.; Jiao, Y.; Herr, S.A.; Tang, J.; Rogers, E.; Chen, Z.; Shi, R. Acrolein scavenger dimercaprol offers neuroprotection in an animal model of Parkinson’s disease: implication of acrolein and TRPA1. Transl. Neurodegener. 2021, 10, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Kormos, V.; Kecskés, A.; Farkas, J.; Gaszner, T.; Csernus, V.; Alomari, A.; Hegedüs, D.; Renner, É.; Palkovits, M.; Zelena, D.; et al. Peptidergic neurons of the Edinger–Westphal nucleus express TRPA1 ion channel that is downregulated both upon chronic variable mild stress in male mice and in humans who died by suicide. J. Psychiatry Neurosci. 2022, 47, E162–E175. [Google Scholar] [CrossRef] [PubMed]
- Al-Omari, A.; Kecskés, M.; Gaszner, B.; Biró-Sütő, T.; Fazekas, B.; Berta, G.; Kuzma, M.; Pintér, E.; Kormos, V. Functionally active TRPA1 ion channel is downregulated in peptidergic neurons of the Edinger-Westphal nucleus upon acute alcohol exposure. Front. Cell Dev. Biol. 2023, 10, 1046559. [Google Scholar] [CrossRef] [PubMed]
- Konkoly, J.; Kormos, V.; Gaszner, B.; Correia, P.; Berta, G.; Biró-Sütő, T.; Zelena, D.; Pintér, E. Transient receptor potential ankyrin 1 ion channel expressed by the Edinger-Westphal nucleus contributes to stress adaptation in murine model of posttraumatic stress disorder. Front. Cell Dev. Biol. 2022, 10, 1059073. [Google Scholar] [CrossRef]
- Talavera, K.; Startek, J.B.; Alvarez-Collazo, J.; Boonen, B.; Alpizar, Y.A.; Sanchez, A.; Naert, R.; Nilius, B. Mammalian Transient Receptor Potential TRPA1 Channels: From Structure to Disease. Physiol. Rev. 2020, 100, 725–803. [Google Scholar] [CrossRef]
- De Silva, D.; Lee, S.; Duke, A.; Angalakurthi, S.; Chou, C.-E.; Ebrahimpour, A.; Thompson, D.E.; Petrikovics, I. Intravascular Residence Time Determination for the Cyanide Antidote Dimethyl Trisulfide in Rat by Using Liquid-Liquid Extraction Coupled with High Performance Liquid Chromatography. J. Anal. Methods Chem. 2016, 2016, 1–6. [Google Scholar] [CrossRef]
- Kiss, L.; Bocsik, A.; Walter, F.R.; Ross, J.; Brown, D.; A Mendenhall, B.; Crews, S.R.; Lowry, J.; Coronado, V.; E Thompson, D.; et al. From the Cover: In Vitro and In Vivo Blood-Brain Barrier Penetration Studies with the Novel Cyanide Antidote Candidate Dimethyl Trisulfide in Mice. Toxicol. Sci. 2017, 160, 398–407. [Google Scholar] [CrossRef]
- Kiss, L.; Holmes, S.; Chou, C.-E.; Dong, X.; Ross, J.; Brown, D.; Mendenhall, B.; Coronado, V.; De Silva, D.; Rockwood, G.A.; et al. Method development for detecting the novel cyanide antidote dimethyl trisulfide from blood and brain, and its interaction with blood. J. Chromatogr. B 2017, 1044-1045, 149–157. [Google Scholar] [CrossRef]
- Liu, S.-Y.; Li, D.; Zeng, H.-Y.; Kan, L.-Y.; Zou, W.; Zhang, P.; Gu, H.-F.; Tang, X.-Q. Hydrogen Sulfide Inhibits Chronic Unpredictable Mild Stress-Induced Depressive-Like Behavior by Upregulation of Sirt-1: Involvement in Suppression of Hippocampal Endoplasmic Reticulum Stress. Int. J. Neuropsychopharmacol. 2017, 20, 867–876. [Google Scholar] [CrossRef]
- Wei, L.; Kan, L.-Y.; Zeng, H.-Y.; Tang, Y.-Y.; Huang, H.-L.; Xie, M.; Zou, W.; Wang, C.-Y.; Zhang, P.; Tang, X.-Q. BDNF/TrkB Pathway Mediates the Antidepressant-Like Role of H2S in CUMS-Exposed Rats by Inhibition of Hippocampal ER Stress. NeuroMolecular Med. 2018, 20, 252–261. [Google Scholar] [CrossRef]
- Cortese-Krott, M.M.; Kuhnle, G.G.C.; Dyson, A.; Fernandez, B.O.; Grman, M.; Dumond, J.F.; Barrow, M.P.; McLeod, G.; Nakagawa, H.; Ondrias, K.; et al. Key bioactive reaction products of the NO/H2S interaction are S/N-hybrid species, polysulfides, and nitroxyl. Proc. Natl. Acad. Sci. USA 2015, 112, E4651–E4660. [Google Scholar] [CrossRef] [PubMed]
- Cortese-Krott, M.M.; Pullmann, D.; Feelisch, M. Nitrosopersulfide (SSNO − ) targets the Keap-1/Nrf2 redox system. Pharmacol. Res. 2016, 113, 490–499. [Google Scholar] [CrossRef] [PubMed]
- Greiner, R.; Pálinkás, Z.; Bäsell, K.; Becher, D.; Antelmann, H.; Nagy, P.; Dick, T.P. Polysulfides Link H2S to Protein Thiol Oxidation. Antioxidants Redox Signal. 2013, 19, 1749–1765. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Wu, L. Trend in H2S Biology and Medicine Research—A Bibliometric Analysis. Molecules 2017, 22, 2087. [Google Scholar] [CrossRef]
- Pozsgai, G.; Payrits, M.; Sághy, É.; Sebestyén-Bátai, R.; Steen, E.; Szőke, É.; Sándor, Z.; Solymár, M.; Garami, A.; Orvos, P.; et al. Analgesic effect of dimethyl trisulfide in mice is mediated by TRPA1 and sst 4 receptors. Nitric Oxide 2017, 65, 10–21. [Google Scholar] [CrossRef]
- Dombi, Á.; Sánta, C.; Bátai, I.Z.; Kormos, V.; Kecskés, A.; Tékus, V.; Pohóczky, K.; Bölcskei, K.; Pintér, E.; Pozsgai, G. Dimethyl Trisulfide Diminishes Traumatic Neuropathic Pain Acting on TRPA1 Receptors in Mice. Int. J. Mol. Sci. 2021, 22, 3363. [Google Scholar] [CrossRef]
- Kimura, Y.; Mikami, Y.; Osumi, K.; Tsugane, M.; Oka, J.; Kimura, H. Polysulfides are possible H 2 S-derived signaling molecules in rat brain. FASEB J. 2013, 27, 2451–2457. [Google Scholar] [CrossRef]
- Bátai, I.Z.; Horváth, Á.; Pintér, E.; Helyes, Z.; Pozsgai, G. Role of Transient Receptor Potential Ankyrin 1 Ion Channel and Somatostatin sst4 Receptor in the Antinociceptive and Anti-inflammatory Effects of Sodium Polysulfide and Dimethyl Trisulfide. Front. Endocrinol. 2018, 9, 55. [Google Scholar] [CrossRef]
- Voineskos, D.; Daskalakis, Z.J.; Blumberger, D.M. Management of Treatment-Resistant Depression: Challenges and Strategies. Neuropsychiatr. Dis. Treat. 2020, ume 16, 221–234. [Google Scholar] [CrossRef]
- Kormos, V.; Gáspár, L.; Kovács, L.Á.; Farkas, J.; Gaszner, T.; Csernus, V.; Balogh, A.; Hashimoto, H.; Reglődi, D.; Helyes, Z.; et al. Reduced response to chronic mild stress in PACAP mutant mice is associated with blunted FosB expression in limbic forebrain and brainstem centers. Neuroscience 2016, 330, 335–358. [Google Scholar] [CrossRef]
- Ruilian, L.; Honglin, Q.; Jun, X.; Jianxin, L.; Qingyun, B.; Yilin, C.; Haifeng, M. H2S-mediated aerobic exercise antagonizes the hippocampal inflammatory response in CUMS-depressed mice. J. Affect. Disord. 2021, 283, 410–419. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.-L.; Xie, B.; Zhang, C.; Xu, K.-L.; Niu, Y.-Y.; Tang, X.-Q.; Zhang, P.; Zou, W.; Hu, B.; Tian, Y. Antidepressant-like and anxiolytic-like effects of hydrogen sulfide in behavioral models of depression and anxiety. Behav. Pharmacol. 2013, 24, 590–597. [Google Scholar] [CrossRef]
- Kedia, S.; Chattarji, S. Marble burying as a test of the delayed anxiogenic effects of acute immobilisation stress in mice. J. Neurosci. Methods 2014, 233, 150–154. [Google Scholar] [CrossRef] [PubMed]
- Njung'E, K.; Handley, S.L. Evaluation of marble-burying behavior as a model of anxiety. Pharmacol. Biochem. Behav. 1991, 38, 63–67. [Google Scholar] [CrossRef] [PubMed]
- Cohen, I.L.; Gardner, J.M.; Karmel, B.Z.; Kim, S.-Y. Rating scale measures are associated with Noldus EthoVision-XT video tracking of behaviors of children on the autism spectrum. Mol. Autism 2014, 5, 15–15. [Google Scholar] [CrossRef]
- S.S. Shapiro, M.B. Wilk, An Analysis of Variance Test for Normality (Complete Samples), (2024).
- Göntér, K.; Dombi, Á.; Kormos, V.; Pintér, E.; Pozsgai, G. Examination of the Effect of Dimethyl Trisulfide in Acute Stress Mouse Model with the Potential Involvement of the TRPA1 Ion Channel. Int. J. Mol. Sci. 2024, 25, 7701. [Google Scholar] [CrossRef]
- Willner, P. The chronic mild stress (CMS) model of depression: History, evaluation and usage. Neurobiol. Stress 2017, 6, 78–93. [Google Scholar] [CrossRef]
- Boleij, H.; Willems, J.; Leijten, M.; Klooster, J.V.; Lesscher, H.; Kirchhoff, S.; Lavrijsen, M.; Arndt, S.S.; Ohl, F. Chronic social stress does not affect behavioural habituation in male CD1 mice. Behav. Brain Res. 2014, 273, 34–44. [Google Scholar] [CrossRef]
- Harris, R.B.S. Chronic and acute effects of stress on energy balance: are there appropriate animal models? Am. J. Physiol. Integr. Comp. Physiol. 2015, 308, R250–R265. [Google Scholar] [CrossRef]
- Rabasa, C.; Pastor-Ciurana, J.; Delgado-Morales, R.; Gómez-Román, A.; Carrasco, J.; Gagliano, H.; García-Gutiérrez, M.S.; Manzanares, J.; Armario, A. Evidence against a critical role of CB1 receptors in adaptation of the hypothalamic–pituitary–adrenal axis and other consequences of daily repeated stress. Eur. Neuropsychopharmacol. 2015, 25, 1248–1259. [Google Scholar] [CrossRef]
- Farkas, J.; Kovács, L.Á.; Gáspár, L.; Nafz, A.; Gaszner, T.; Ujvári, B.; Kormos, V.; Csernus, V.; Hashimoto, H.; Reglődi, D.; et al. Construct and face validity of a new model for the three-hit theory of depression using PACAP mutant mice on CD1 background. Neuroscience 2017, 354, 11–29. [Google Scholar] [CrossRef] [PubMed]
- Spina, M.; Merlo-Pich, E.; Chan, R.K.W.; Basso, A.M.; Rivier, J.; Vale, W.; Koob, G.F. Appetite-Suppressing Effects of Urocortin, a CRF-Related Neuropeptide. Science 1996, 273, 1561–1564. [Google Scholar] [CrossRef] [PubMed]
- Landini, L.; de Araujo, D.S.M.; Titiz, M.; Geppetti, P.; Nassini, R.; De Logu, F. TRPA1 Role in Inflammatory Disorders: What Is Known So Far? Int. J. Mol. Sci. 2022, 23, 4529. [Google Scholar] [CrossRef] [PubMed]
- Naert, R.; López-Requena, A.; Talavera, K. TRPA1 Expression and Pathophysiology in Immune Cells. Int. J. Mol. Sci. 2021, 22, 11460. [Google Scholar] [CrossRef]
- Szabó, K.; Kemény, Á.; Balázs, N.; Khanfar, E.; Sándor, Z.; Boldizsár, F.; Gyulai, R.; Najbauer, J.; Pintér, E.; Berki, T. Presence of TRPA1 Modifies CD4+/CD8+ T Lymphocyte Ratio and Activation. Pharmaceuticals 2022, 15, 57. [Google Scholar] [CrossRef]
- Szabó, K.; Makkai, G.; Konkoly, J.; Kormos, V.; Gaszner, B.; Berki, T.; Pintér, E. TRPA1 Covalent Ligand JT010 Modifies T Lymphocyte Activation. Biomolecules 2024, 14, 632. [Google Scholar] [CrossRef]
- Russo, D.; Tringali, G.; Ragazzoni, E.; Maggiano, N.; Menini, E.; Vairano, M.; Preziosi, P.; Navarra, P. Evidence That Hydrogen Sulphide Can Modulate Hypothalamo-Pituitary-Adrenal Axis Function: In Vitro and In Vivo Studies in the Rat. J. Neuroendocr. 2000, 12, 225–233. [Google Scholar] [CrossRef]
- Hassan, E.; Kahilo, K.; Kamal, T.; El-Neweshy, M.; Hassan, M. Protective effect of diallyl sulfide against lead-mediated oxidative damage, apoptosis and down-regulation of CYP19 gene expression in rat testes. Life Sci. 2019, 226, 193–201. [Google Scholar] [CrossRef]
- Wang, H.; Shi, X.; Qiu, M.; Lv, S.; Liu, H. Hydrogen Sulfide Plays an Important Protective Role through Influencing Endoplasmic Reticulum Stress in Diseases. Int. J. Biol. Sci. 2020, 16, 264–271. [Google Scholar] [CrossRef]
- Sodhi, R.K.; Singh, R.; Bansal, Y.; Bishnoi, M.; Parhar, I.; Kuhad, A.; Soga, T. Intersections in Neuropsychiatric and Metabolic Disorders: Possible Role of TRPA1 Channels. Front. Endocrinol. 2021, 12. [Google Scholar] [CrossRef]
- Renard, C.E.; Dailly, E.; David, D.J.; Hascoet, M.; Bourin, M. Monoamine metabolism changes following the mouse forced swimming test but not the tail suspension test. Fundam. Clin. Pharmacol. 2003, 17, 449–455. [Google Scholar] [CrossRef] [PubMed]
- Júnior, E.D.D.S.; Da Silva, A.V.; Da Silva, K.R.; Haemmerle, C.A.; Batagello, D.S.; Da Silva, J.M.; Lima, L.B.; Da Silva, R.J.; Diniz, G.B.; Sita, L.V.; et al. The centrally projecting Edinger–Westphal nucleus—I: Efferents in the rat brain. J. Chem. Neuroanat. 2015, 68, 22–38. [Google Scholar] [CrossRef] [PubMed]
- van der Doelen, R.H.; Robroch, B.; Arnoldussen, I.A.; Schulpen, M.; Homberg, J.R.; Kozicz, T. Serotonin and urocortin 1 in the dorsal raphe and Edinger–Westphal nuclei after early life stress in serotonin transporter knockout rats. Neuroscience 2017, 340, 345–358. [Google Scholar] [CrossRef] [PubMed]
- Waselus, M.; Nazzaro, C.; Valentino, R.J.; Van Bockstaele, E.J. Stress-Induced Redistribution of Corticotropin-Releasing Factor Receptor Subtypes in the Dorsal Raphe Nucleus. Biol. Psychiatry 2009, 66, 76–83. [Google Scholar] [CrossRef]
- Craske, M.G.; Meuret, A.E.; Ritz, T.; Treanor, M.; Dour, H.J. Treatment for Anhedonia: A Neuroscience Driven Approach. Depression Anxiety 2016, 33, 927–938. [Google Scholar] [CrossRef]
- Yakovleva, O.; Bogatova, K.; Mukhtarova, R.; Yakovlev, A.; Shakhmatova, V.; Gerasimova, E.; Ziyatdinova, G.; Hermann, A.; Sitdikova, G. Hydrogen Sulfide Alleviates Anxiety, Motor, and Cognitive Dysfunctions in Rats with Maternal Hyperhomocysteinemia via Mitigation of Oxidative Stress. Biomolecules 2020, 10, 995. [Google Scholar] [CrossRef]
- Chen, M.; Pritchard, C.; Fortune, D.; Kodi, P.; Grados, M. Hydrogen sulfide: a target to modulate oxidative stress and neuroplasticity for the treatment of pathological anxiety. Expert Rev. Neurother. 2019, 20, 109–121. [Google Scholar] [CrossRef]
- Prenoveau, J.M.; Zinbarg, R.E.; Craske, M.G.; Mineka, S.; Griffith, J.W.; Epstein, A.M. Testing a hierarchical model of anxiety and depression in adolescents: A tri-level model. J. Anxiety Disord. 2010, 24, 334–344. [Google Scholar] [CrossRef]
- Vose, L.R.; Stanton, P.K. Synaptic Plasticity, Metaplasticity and Depression. Curr. Neuropharmacol. 2017, 15, 71–86. [Google Scholar] [CrossRef]
- Sha, Z.; Xu, J.; Li, N.; Li, O. Regulatory Molecules of Synaptic Plasticity in Anxiety Disorder. Int. J. Gen. Med. 2023, ume 16, 2877–2886. [Google Scholar] [CrossRef]
- Duman, R.S.; Aghajanian, G.K.; Sanacora, G.; Krystal, J.H. Synaptic plasticity and depression: new insights from stress and rapid-acting antidepressants. Nat. Med. 2016, 22, 238–249. [Google Scholar] [CrossRef] [PubMed]
- Nestler, E.J. ∆FosB: A transcriptional regulator of stress and antidepressant responses. Eur. J. Pharmacol. 2015, 753, 66–72. [Google Scholar] [CrossRef] [PubMed]
- E.J. Nestler, M.B. Kelz, J. Chen, DFosB: a molecular mediator of long-term neural and behavioral plasticity, (1999).
- Perrotti, L.I.; Hadeishi, Y.; Ulery, P.G.; Barrot, M.; Monteggia, L.; Duman, R.S.; Nestler, E.J. Induction of ΔFosB in Reward-Related Brain Structures after Chronic Stress. J. Neurosci. 2004, 24, 10594–10602. [Google Scholar] [CrossRef] [PubMed]
- Sterrenburg, L.; Gaszner, B.; Boerrigter, J.; Santbergen, L.; Bramini, M.; Elliott, E.; Chen, A.; Peeters, B.W.M.M.; Roubos, E.W.; Kozicz, T. Chronic Stress Induces Sex-Specific Alterations in Methylation and Expression of Corticotropin-Releasing Factor Gene in the Rat. PLOS ONE 2011, 6, e28128. [Google Scholar] [CrossRef]
- Vialou, V.; Thibault, M.; Kaska, S.; Cooper, S.; Gajewski, P.; Eagle, A.; Mazei-Robison, M.; Nestler, E.J.; Robison, A. Differential induction of FosB isoforms throughout the brain by fluoxetine and chronic stress. Neuropharmacology 2015, 99, 28–37. [Google Scholar] [CrossRef]
- Virk, H.S.; Rekas, M.Z.; Biddle, M.S.; Wright, A.K.A.; Sousa, J.; Weston, C.A.; Chachi, L.; Roach, K.M.; Bradding, P. Validation of antibodies for the specific detection of human TRPA1. Sci. Rep. 2019, 9, 1–9. [Google Scholar] [CrossRef]
- Patil, M.; Jeske, N.; Akopian, A. Transient receptor potential V1 regulates activation and modulation of transient receptor potential A1 by Ca2+. Neuroscience 2010, 171, 1109–1119. [Google Scholar] [CrossRef]
- Rojas-Galvan, N.S.; Ciotu, C.I.; Heber, S.; Fischer, M.J. Correlation of TRPA1 RNAscope and Agonist Responses. J. Histochem. Cytochem. 2024, 72, 275–287. [Google Scholar] [CrossRef]
- Kozicz, T.; Tilburg-Ouwens, D.; Faludi, G.; Palkovits, M.; Roubos, E. Gender-related urocortin 1 and brain-derived neurotrophic factor expression in the adult human midbrain of suicide victims with major depression. Neuroscience 2008, 152, 1015–1023. [Google Scholar] [CrossRef]
- Bouwknecht, J.A.; Spiga, F.; Staub, D.R.; Hale, M.W.; Shekhar, A.; Lowry, C.A. Differential effects of exposure to low-light or high-light open-field on anxiety-related behaviors: Relationship to c-Fos expression in serotonergic and non-serotonergic neurons in the dorsal raphe nucleus. Brain Res. Bull. 2007, 72, 32–43. [Google Scholar] [CrossRef]
- Kozicz, T. The missing link; the significance of urocortin 1/urocortin 2 in the modulation of the dorsal raphe serotoninergic system. Mol. Psychiatry 2009, 15, 340–341. [Google Scholar] [CrossRef] [PubMed]
- Neufeld-Cohen, A.; Tsoory, M.M.; Evans, A.K.; Getselter, D.; Gil, S.; Lowry, C.A.; Vale, W.W.; Chen, A. A triple urocortin knockout mouse model reveals an essential role for urocortins in stress recovery. Proc. Natl. Acad. Sci. 2010, 107, 19020–19025. [Google Scholar] [CrossRef] [PubMed]
- Bittencourt, J.C.; Vaughan, J.; Arias, C.; Rissman, R.A.; Vale, W.W.; Sawchenko, P.E. Urocortin Expression in Rat Brain: Evidence Against a Pervasive Relationship of Urocortin-Containing Projections With Targets Bearing Type 2 CRF Receptors. J. Comp. Neurol. 1999, 415, 285–312. [Google Scholar] [CrossRef]
- Kozicz, T. On the role of urocortin 1 in the non-preganglionic Edinger–Westphal nucleus in stress adaptation. Gen. Comp. Endocrinol. 2007, 153, 235–240. [Google Scholar] [CrossRef]
- de Bortoli, V.C.; Yamashita, P.S.d.M.; Zangrossi, H. 5-HT1A and 5-HT2A receptor control of a panic-like defensive response in the rat dorsomedial hypothalamic nucleus. J. Psychopharmacol. 2013, 27, 1116–1123. [Google Scholar] [CrossRef]
- Zuniga, A.; E Ryabinin, A. Involvement of Centrally Projecting Edinger–Westphal Nucleus Neuropeptides in Actions of Addictive Drugs. Brain Sci. 2020, 10, 67. [Google Scholar] [CrossRef]
- Kirouac, G.J. Placing the paraventricular nucleus of the thalamus within the brain circuits that control behavior. Neurosci. Biobehav. Rev. 2015, 56, 315–329. [Google Scholar] [CrossRef]
- Ma, J.; du Hoffmann, J.; Kindel, M.; Beas, B.S.; Chudasama, Y.; Penzo, M.A. Divergent projections of the paraventricular nucleus of the thalamus mediate the selection of passive and active defensive behaviors. Nat. Neurosci. 2021, 24, 1429–1440. [Google Scholar] [CrossRef]
- Singewald, G.M.; Rjabokon, A.; Singewald, N.; Ebner, K. The Modulatory Role of the Lateral Septum on Neuroendocrine and Behavioral Stress Responses. Neuropsychopharmacology 2010, 36, 793–804. [Google Scholar] [CrossRef]
- Hammack, S.E.; Cheung, J.; Rhodes, K.M.; Schutz, K.C.; Falls, W.A.; Braas, K.M.; May, V. Chronic stress increases pituitary adenylate cyclase-activating peptide (PACAP) and brain-derived neurotrophic factor (BDNF) mRNA expression in the bed nucleus of the stria terminalis (BNST): Roles for PACAP in anxiety-like behavior. Psychoneuroendocrinology 2009, 34, 833–843. [Google Scholar] [CrossRef]
- Kiss, A.; Aguilera, G. Regulation of the hypothalamic pituitary adrenal axis during chronic stress: responses to repeated intraperitoneal hypertonic saline injection. Brain Res. 1993, 630, 262–270. [Google Scholar] [CrossRef] [PubMed]
- Verkuyl, J.M.; Hemby, S.E.; Joëls, M. Chronic stress attenuates GABAergic inhibition and alters gene expression of parvocellular neurons in rat hypothalamus. Eur. J. Neurosci. 2004, 20, 1665–1673. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, D.R.; Cullinan, W.E.; Herman, J.P. Organization and regulation of paraventricular nucleus glutamate signaling systems: N-methyl-D-aspartate receptors. J. Comp. Neurol. 2005, 484, 43–56. [Google Scholar] [CrossRef] [PubMed]
- Flak, J.N.; Ostrander, M.M.; Tasker, J.G.; Herman, J.P. Chronic stress-induced neurotransmitter plasticity in the PVN. J. Comp. Neurol. 2009, 517, 156–165. [Google Scholar] [CrossRef]
- Crestani, C.C.; Alves, F.H.; Gomes, F.V.; Resstel, L.B.; Correa, F.M.; Herman, J.P. Mechanisms in the Bed Nucleus of the Stria Terminalis Involved in Control of Autonomic and Neuroendocrine Functions: A Review. Curr. Neuropharmacol. 2013, 11, 141–159. [Google Scholar] [CrossRef]
- Herman, J.P.; Figueiredo, H.; Mueller, N.K.; Ulrich-Lai, Y.; Ostrander, M.M.; Choi, D.C.; Cullinan, W.E. Central mechanisms of stress integration: hierarchical circuitry controlling hypothalamo–pituitary–adrenocortical responsiveness. Front. Neuroendocr. 2003, 24, 151–180. [Google Scholar] [CrossRef]
- V. Grinevich, A. Fournier, G. Pelletier, Effects of pituitary adenylate cyclase-activating polypeptide Ž PACAP. on corticotropin-releasing hormone Ž CRH. gene expression in the rat hypothalamic paraventricular nucleus, (1997).
- Choi, D.C.; Evanson, N.K.; Furay, A.R.; Ulrich-Lai, Y.M.; Ostrander, M.M.; Herman, J.P. The Anteroventral Bed Nucleus of the Stria Terminalis Differentially Regulates Hypothalamic-Pituitary-Adrenocortical Axis Responses to Acute and Chronic Stress. Endocrinology 2007, 149, 818–826. [Google Scholar] [CrossRef]
- Deji, C.; Yan, P.; Ji, Y.; Yan, X.; Feng, Y.; Liu, J.; Liu, Y.; Wei, S.; Zhu, Y.; Lai, J. The Basolateral Amygdala to Ventral Hippocampus Circuit Controls Anxiety-Like Behaviors Induced by Morphine Withdrawal. Front. Cell. Neurosci. 2022, 16, 894886. [Google Scholar] [CrossRef]
- Kovács, L.Á.; Füredi, N.; Ujvári, B.; Golgol, A.; Gaszner, B. Age-Dependent FOSB/ΔFOSB Response to Acute and Chronic Stress in the Extended Amygdala, Hypothalamic Paraventricular, Habenular, Centrally-Projecting Edinger-Westphal, and Dorsal Raphe Nuclei in Male Rats. Front. Aging Neurosci. 2022, 14, 862098. [Google Scholar] [CrossRef]
- Sun, H.-J.; Wu, Z.-Y.; Nie, X.-W.; Bian, J.-S. Role of Hydrogen Sulfide and Polysulfides in Neurological Diseases: Focus on Protein S-Persulfidation. Curr. Neuropharmacol. 2021, 19, 868–884. [Google Scholar] [CrossRef]
- Kimura, H. Physiological role of hydrogen sulfide and polysulfide in the central nervous system. Neurochem. Int. 2013, 63, 492–497. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Bian, J.-S. Hydrogen Sulfide: A Neuromodulator and Neuroprotectant in the Central Nervous System. ACS Chem. Neurosci. 2014, 5, 876–883. [Google Scholar] [CrossRef] [PubMed]
- Kimura, H. Hydrogen Sulfide (H2S) and Polysulfide (H2Sn) Signaling: The First 25 Years. Biomolecules 2021, 11, 896. [Google Scholar] [CrossRef] [PubMed]
- De Silva, D.; Lee, S.; Duke, A.; Angalakurthi, S.; Chou, C.-E.; Ebrahimpour, A.; Thompson, D.E.; Petrikovics, I. Intravascular Residence Time Determination for the Cyanide Antidote Dimethyl Trisulfide in Rat by Using Liquid-Liquid Extraction Coupled with High Performance Liquid Chromatography. J. Anal. Methods Chem. 2016, 2016, 1–6. [Google Scholar] [CrossRef]
- Dong, X.; Kiss, L.; Petrikovics, I.; Thompson, D.E. Reaction of Dimethyl Trisulfide with Hemoglobin. Chem. Res. Toxicol. 2017, 30, 1661–1663. [Google Scholar] [CrossRef]
- Manandhar, E.; Maslamani, N.; Petrikovics, I.; Rockwood, G.A.; Logue, B.A. Determination of dimethyl trisulfide in rabbit blood using stir bar sorptive extraction gas chromatography-mass spectrometry. J. Chromatogr. A 2016, 1461, 10–17. [Google Scholar] [CrossRef]
- Petrikovics, I.; Kiss, L.; Chou, C.-E.; Ebrahimpour, A.; Kovács, K.; Kiss, M.; Logue, B.; Chan, A.; Manage, A.B.W.; Budai, M.; et al. Antidotal efficacies of the cyanide antidote candidate dimethyl trisulfide alone and in combination with cobinamide derivatives. Toxicol. Mech. Methods 2019, 29, 438–444. [Google Scholar] [CrossRef]
- A Rockwood, G.; E Thompson, D.; Petrikovics, I. Dimethyl trisulfide. Toxicol. Ind. Heal. 2016, 32, 2009–2016. [Google Scholar] [CrossRef]
- Warnakula, I.K.; Ebrahimpour, A.; Li, S.Y.; Ralalage, R.D.G.; Hewa-Rahinduwage, C.C.; Kiss, M.; Rios, C.T.; Kelley, K.D.; Whiteman, A.C.; Thompson, D.E.; et al. Evaluation of the Long-Term Storage Stability of the Cyanide Antidote: Dimethyl Trisulfide and Degradation Product Identification. ACS Omega 2020, 5, 27171–27179. [Google Scholar] [CrossRef]
- Konkoly, J.; Kormos, V.; Gaszner, B.; Sándor, Z.; Kecskés, A.; Alomari, A.; Szilágyi, A.; Szilágyi, B.; Zelena, D.; Pintér, E. The Role of TRPA1 Channels in the Central Processing of Odours Contributing to the Behavioural Responses of Mice. Pharmaceuticals 2021, 14, 1336. [Google Scholar] [CrossRef]
- Olah, E.; Rumbus, Z.; Kormos, V.; Tekus, V.; Pakai, E.; Wilson, H.V.; Fekete, K.; Solymar, M.; Kelava, L.; Keringer, P.; et al. The Hypothermic Effect of Hydrogen Sulfide Is Mediated by the Transient Receptor Potential Ankyrin-1 Channel in Mice. Pharmaceuticals 2021, 14, 992. [Google Scholar] [CrossRef] [PubMed]
- Cano, G.; Hernan, S.L.; Sved, A.F. Centrally Projecting Edinger-Westphal Nucleus in the Control of Sympathetic Outflow and Energy Homeostasis. Brain Sci. 2021, 11, 1005. [Google Scholar] [CrossRef] [PubMed]
- Donner, N.; Handa, R. Estrogen receptor beta regulates the expression of tryptophan-hydroxylase 2 mRNA within serotonergic neurons of the rat dorsal raphe nuclei. Neuroscience 2009, 163, 705–718. [Google Scholar] [CrossRef]
- Leite, C.; Madeira, M.D.; Sá, S.I. Effects of sex steroids and estrogen receptor agonists on the expression of estrogen receptor alpha in the principal division of the bed nucleus of the stria terminalis of female rats. Brain Res. 2014, 1582, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Menon, R.; Süß, T.; Oliveira, V.E.d.M.; Neumann, I.D.; Bludau, A. Neurobiology of the lateral septum: regulation of social behavior. Trends Neurosci. 2022, 45, 27–40. [Google Scholar] [CrossRef]
- Sheng, Z.; Kawano, J.; Yanai, A.; Fujinaga, R.; Tanaka, M.; Watanabe, Y.; Shinoda, K. Expression of estrogen receptors (α, β) and androgen receptor in serotonin neurons of the rat and mouse dorsal raphe nuclei; sex and species differences. Neurosci. Res. 2004, 49, 185–196. [Google Scholar] [CrossRef]
- Stern, J.; Zhang, W. Preautonomic neurons in the paraventricular nucleus of the hypothalamus contain estrogen receptor β. Brain Res. 2003, 975, 99–109. [Google Scholar] [CrossRef]
- Tian, Z.; Wang, Y.; Zhang, N.; Guo, Y.-Y.; Feng, B.; Liu, S.-B.; Zhao, M.-G. Estrogen receptor GPR30 exerts anxiolytic effects by maintaining the balance between GABAergic and glutamatergic transmission in the basolateral amygdala of ovariectomized mice after stress. Psychoneuroendocrinology 2013, 38, 2218–2233. [Google Scholar] [CrossRef]

| Trpa1 WT | |||||
| Non-stressed | CUMS | ||||
| naïve | vehicle-treated | DMTS30-treated | naïve | vehicle-treated | DMTS30-treated |
| 8 | 16 | 16 | 8 | 16 | 16 |
| Trpa1 KO | |||||
| Non-stressed | CUMS | ||||
| naïve | vehicle-treated | DMTS30-treated | naïve | vehicle-treated | DMTS30-treated |
| 8 | 16 | 16 | 8 | 16 | 16 |
| No. of days | Mid-day stressors | Overnight stressors |
| 1. | WM, REST | WET |
| 2. | DARK | ISOL |
| 3. | TILT | GH |
| 4. | DARK | WET |
| 5. | SHAKE | GH |
| 6. | TILT | ISOL |
| 7. | REST | GH |
| 8. | TS, TILT | WET |
| 9. | SHAKE | ISOL |
| 10. | REST | WET |
| 11. | TILT | GH |
| 12. | SHAKE | WET |
| 13. | DARK | GH |
| 14. | TILT | WET |
| 15. | WM, REST | GH |
| 16. | TILT | WET |
| 17. | REST | GH |
| 18. | DARK | WET |
| 19. | TILT | GH |
| 20. | DARK | WET |
| 21. | REST | ISOL |
| Main effects | Interactions | |||||||
| Variable | Treatment | Genotype | CUMS | Treatment x genotype | CUMS x treatment | CUMS x genotype | CUMS x treatment x genotype | |
| MBT, number of marbles hid | F2,149 | 0.9901 | 57.66 | 186.3 | 2.325 | 8.423 | 110.6 | 5.556 |
| p | 0.374 | <0.0001 | <0.0001 | 0.1013 | 0.0003 | <0.0001 | 0.0047 | |
| OFT, periphery zone time | F2,72 | 0.1927 | 13.57 | 1.654 | 4.723 | 45583 | 0.7645 | 0.3862 |
| p | 0.8252 | 0.0004 | 0.204 | 0.0118 | 0.0002 | 0.3859 | 0.6815 | |
| OFT, periphery zone distance | F2,72 | 3.657 | 30498 | 46.64 | 93.32 | 61.24 | 2.189 | 0.3012 |
| p | 0.0333 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | 0.1455 | 0.7413 | |
| FST, activity | F2,89 | 3.923 | 148.1 | 18.73 | 0.7528 | 1.389 | 53.56 | 5.322 |
| p | 0.0233 | <0.0001 | <0.0001 | 0.474 | 0.2559 | <0.0001 | 0.007 | |
| TST, activity | F2,73 | 5.814 | 45322 | 1.963 | 0.9288 | 0.8436 | 4.864 | 4.135 |
| p | 0.0045 | <0.0001 | 0.1678 | 0.3997 | 0.4366 | 0.0323 | 0.0222 | |
| SPT, sucrose preference | F2,78 | 3.172 | 13516 | 31079 | 0.02048 | 45304 | 2.555 | 3.496 |
| p | 0.0474 | <0.0001 | <0.0001 | 0.9797 | 0.3292 | 0.1146 | 0.0359 | |
| Serum ACTH | F 1,16 | 0.7709 | 0.3015 | 243.3 | 5.494 | 1.222 | 275.1 | 3.534 |
| p | 0.3929 | 0.5905 | <0.0001 | 0.0323 | 0.2854 | <0.0001 | 0.0785 | |
| Serum corticosterone | F1,30 | 4.197 | 0.03008 | 4.84 | 0.02317 | 1.975 | 0.03587 | 1.174 |
| p | 0.0493 | 0.8635 | 0.0357 | 0.88 | 0.1702 | 0.8511 | 0.2872 | |
| Relative adrenal weight | F1,56 | 10.87 | 25.35 | 415.3 | 0.009887 | 1.105 | 2.79 | 0.5117 |
| p | <0.0001 | <0.0001 | <0.0001 | 0.9902 | 0.3383 | 0.1004 | 0.6023 | |
| Relative thymus weight | F2,121 | 2.248 | 55.79 | 9.346 | 0.3015 | 2.179 | 10.35 | 0.01058 |
| p | 0.11 | <0.0001 | 0.0028 | 0.7402 | 0.1176 | 0.0017 | 0.9895 | |
| Change in body weight | F2,107 | 15.02 | 14.43 | 45.22 | 2.162 | 0.969 | 13.44 | 0.7456 |
| p | 0.1215 | 0.0002 | <0.0001 | 0.1201 | 0.3833 | 0.0004 | 0.4773 | |
| Body weight | F2,77 | 2.326 | 13.49 | 50.72 | 5.797 | 3.747 | 43.66 | 3.461 |
| p | 0.1045 | 0.0004 | <0.0001 | 0.0045 | 0.028 | <0.0001 | 0.0364 | |
| Main effects | Interactions | ||||||||
| Variable | Treatment | Genotype | CUMS | Treatment x genotype | CUMS x treatment | CUMS x genotype | CUMS x treatment x genotype | ||
| EWcp | F2,27 | 2.708 | 14.12 | 12.71 | 11.09 | 1.611 | 22.19 | 3.491 | |
| p | 0.0848 | 0.0008 | 0.0014 | 0.0003 | 0.2182 | <0.0001 | 0.0448 | ||
| LS | F2,27 | 0.5911 | 16.68 | 36.4 | 3.381 | 4.289 | 0.02364 | 4.091 | |
| p | 0.5616 | 0.0004 | <0.0001 | 0.0509 | 0.0255 | 0.8791 | 0.0296 | ||
| PAG | F2,27 | 4.024 | 58.24 | 29.59 | 10.78 | 5.261 | 15.65 | 45378 | |
| p | 0.0311 | <0.0001 | <0.0001 | 0.0005 | 0.0128 | 0.0006 | 0.0555 | ||
| DR | F2,27 | 1.434 | 20.61 | 5.002 | 4.278 | 2.267 | 3.268 | 6.678 | |
| p | 0.2559 | 0.0002 | 0.0338 | 0.0276 | 0.123 | 0.085 | 0.0057 | ||
| PVN | F2,27 | 0.2366 | 166 | 16.27 | 5.995 | 4.281 | 0.03658 | 3.206 | |
| p | 0.7911 | <0.0001 | 0.0005 | 0.0077 | 0.0257 | 0.8499 | 0.0583 | ||
| PVT | F2,27 | 0.4047 | 1.945 | 1.356 | 0.8528 | 0.4163 | 20.27 | 3.702 | |
| p | 0.6717 | 0.1759 | 0.2557 | 0.4387 | 0.6642 | 0.0001 | 0.0397 | ||
| BLA | F2,27 | 1.146 | 3.03 | 2.553 | 0.7379 | 1.137 | 0.04659 | 0.4684 | |
| p | 0.3347 | 0.0945 | 0.1232 | 0.4887 | 0.3375 | 0.8309 | 0.6316 | ||
| BST | F2,27 | 0.2597 | 39.09 | 62.11 | 0.1842 | 1.029 | 6.838 | 2.529 | |
| p | 0.7733 | <0.0001 | <0.0001 | 0.833 | 0.3715 | 0.0158 | 0.1026 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





