Submitted:
12 May 2025
Posted:
13 May 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
Background
2. Materials and Methods
Implementation Approach
Quality Improvement Approach
Co-Creation and Co-Production Approach
3. Results
Knowledge Creation cycle
Knowledge-to-Action Cycle
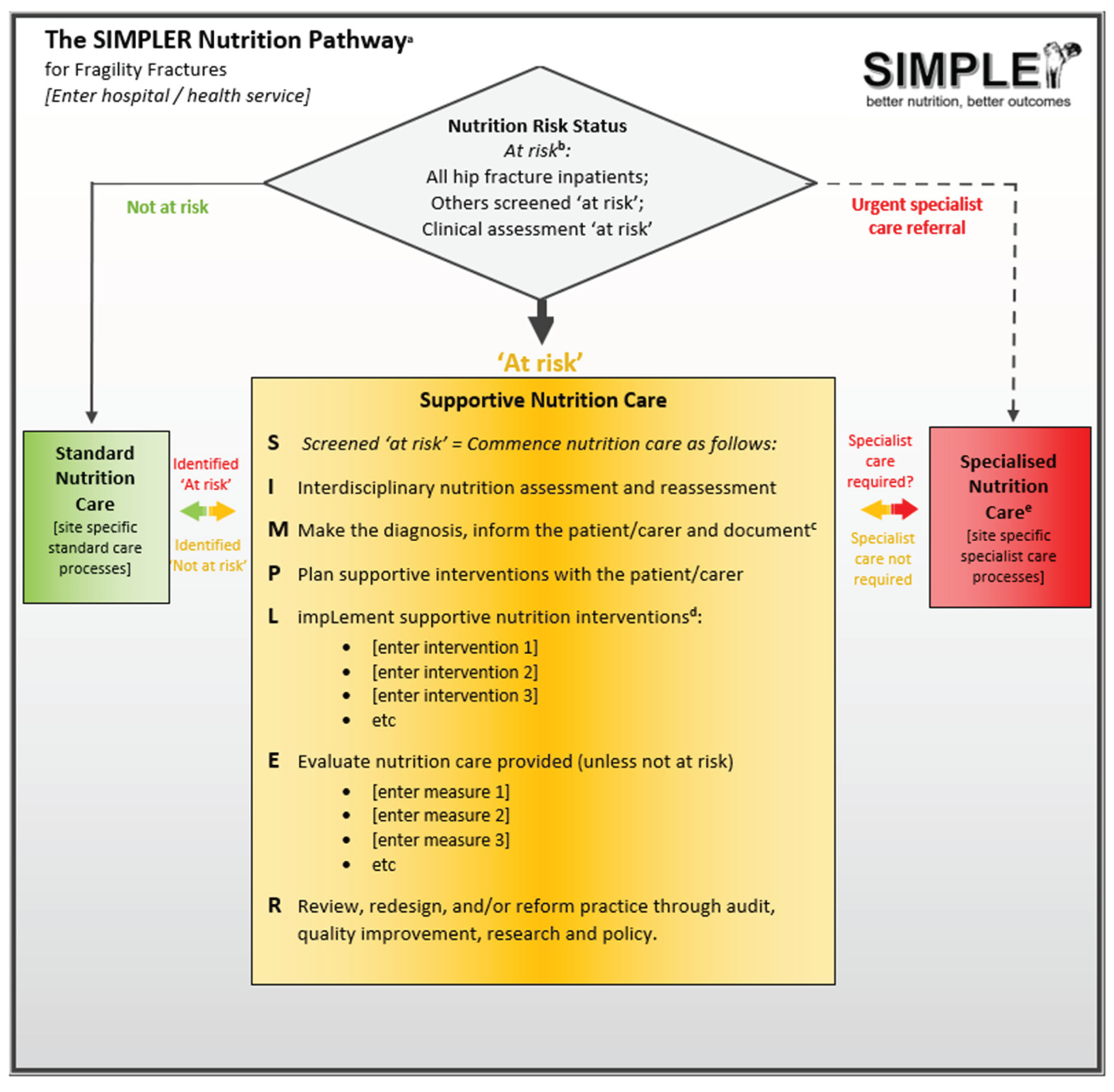
Introducing SIMPLER
The SIMPLER Protocol and Toolkit
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MDPI | Multidisciplinary Digital Publishing Institute |
| DOAJ | Directory of open access journals |
| FFN | Fragility Fracture Network |
| KTA | Knowledge-To-Action (KTA) |
| PDSA | Plan-Do-Study-Act |
| RE-AIM | Reach, Effectiveness, Adoption, Implementation, Maintenance |
References
- World Health Organization. Fragility fractures. Available online: https://www.who.int/news-room/fact-sheets/detail/fragility-fractures (accessed on 2 May 2025).
- Chiavarini, M.; Ricciotti, G.M.; Genga, A.; Faggi, M.I.; Rinaldi, A.; Toscano, O.D.; D’Errico, M.M.; Barbadoro, P. Malnutrition-Related Health Outcomes in Older Adults with Hip Fractures: A Systematic Review and Meta-Analysis. Nutrients 2024, 16, 1069. [Google Scholar] [CrossRef]
- Inoue, T.; Maeda, K.; Nagano, A.; Shimizu, A.; Ueshima, J.; Murotani, K.; Sato, K.; Tsubaki, A. Undernutrition, Sarcopenia, and Frailty in Fragility Hip Fracture: Advanced Strategies for Improving Clinical Outcomes. Nutrients 2020, 12. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Momosaki, R.; Yasufuku, Y.; Nakamura, N.; Maeda, K. Nutritional Therapy in Older Patients With Hip Fractures Undergoing Rehabilitation: A Systematic Review and Meta-Analysis. Journal of the American Medical Directors Association 2020, 21, 1364–1364.e1366. [Google Scholar] [CrossRef]
- Lai, W.Y.; Chiu, Y.C.; Lu, K.C.; Huang, I.T.; Tsai, P.S.; Huang, C.J. Beneficial effects of preoperative oral nutrition supplements on postoperative outcomes in geriatric hip fracture patients: A PRISMA-compliant systematic review and meta-analysis of randomized controlled studies. Medicine (Baltimore) 2021, 100, e27755. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Zhang, J.H.; Duckworth, A.D.; Clement, N.D. Effect of oral nutritional supplementation on outcomes in older adults with hip fractures and factors influencing compliance. Bone Joint J 2023, 105-b, 1149–1158. [Google Scholar] [CrossRef]
- Volkert, D.; Beck, A.M.; Cederholm, T.; Cereda, E.; Cruz-Jentoft, A.; Goisser, S.; de Groot, L.; Großhauser, F.; Kiesswetter, E.; Norman, K.; et al. Management of Malnutrition in Older Patients-Current Approaches, Evidence and Open Questions. Journal of clinical medicine 2019, 8. [Google Scholar] [CrossRef] [PubMed]
- Australian Commission on Safety and Quality in Healthcare. Hip Fracture Clinical Care Standard. Available online: https://www.safetyandquality.gov.au/standards/clinical-care-standards/hip-fracture-care-clinical-care-standard (accessed on 23 January 2025).
- Scottish Government. Scottish standards of care for hip fracture patients. 2024.
- Foo, M.X.E.; Wong, G.J.Y.; Lew, C.C.H. A systematic review of the malnutrition prevalence in hospitalized hip fracture patients and its associated outcomes. JPEN. Journal of parenteral and enteral nutrition 2021, 45, 1141–1152. [Google Scholar] [CrossRef]
- Bell, J.J.; Bauer, J.D.; Capra, S.; Pulle, R.C. Multidisciplinary, multi-modal nutritional care in acute hip fracture inpatients - results of a pragmatic intervention. Clinical Nutrition 2014, 33, 1101–1107. [Google Scholar] [CrossRef]
- Malafarina, V.; Reginster, J.-Y.; Cabrerizo, S.; Bruyère, O.; Kanis, J.A.; Martinez, J.A.; Zulet, M.A. Nutritional Status and Nutritional Treatment Are Related to Outcomes and Mortality in Older Adults with Hip Fracture. Nutrients 2018, 10, 555. [Google Scholar] [CrossRef]
- Bell, J.J.; Pulle, R.C.; Crouch, A.M.; Kuys, S.S.; Ferrier, R.L.; Whitehouse, S.L. Impact of malnutrition on 12-month mortality following acute hip fracture. ANZ J Surg 2016, 86, 157–161. [Google Scholar] [CrossRef] [PubMed]
- Nikkel, L.E.; Fox, E.J.; Black, K.P.; Davis, C.; Andersen, L.; Hollenbeak, C.S. Impact of comorbidities on hospitalization costs following hip fracture. J Bone Joint Surg Am 2012, 94, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Bell, J.J.; Pulle, R.C.; Lee, H.B.; Ferrier, R.; Crouch, A.; Whitehouse, S.L. Diagnosis of overweight or obese malnutrition spells DOOM for hip fracture patients: A prospective audit. Clinical nutrition (Edinburgh, Scotland) 2021, 40, 1905–1910. [Google Scholar] [CrossRef]
- Bell, J.J.; Bauer, J.D.; Capra, S.; Pulle, R.C. Quick and easy is not without cost: implications of poorly performing nutrition screening tools in hip fracture. Journal of the American Geriatrics Society 2014, 62, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Bell, J.J.; Bauer, J.D.; Capra, S.; Pulle, R.C. Concurrent and predictive evaluation of malnutrition diagnostic measures in hip fracture inpatients: a diagnostic accuracy study. European journal of clinical nutrition 2014, 68, 358–362. [Google Scholar] [CrossRef]
- Volkert, D.; Beck, A.M.; Cederholm, T.; Cruz-Jentoft, A.; Goisser, S.; Hooper, L.; Kiesswetter, E.; Maggio, M.; Raynaud-Simon, A.; Sieber, C.C.; et al. ESPEN guideline on clinical nutrition and hydration in geriatrics. Clinical Nutrition 2019, 38, 10–47. [Google Scholar] [CrossRef]
- Volkert, D.; Beck, A.M.; Cederholm, T.; Cruz-Jentoft, A.; Hooper, L.; Kiesswetter, E.; Maggio, M.; Raynaud-Simon, A.; Sieber, C.; Sobotka, L.; et al. ESPEN practical guideline: Clinical nutrition and hydration in geriatrics. Clinical nutrition (Edinburgh, Scotland) 2022, 41, 958–989. [Google Scholar] [CrossRef]
- Correa-Pérez, A.; Abraha, I.; Cherubini, A.; Collinson, A.; Dardevet, D.; de Groot, L.; de van der Schueren, M.A.E.; Hebestreit, A.; Hickson, M.; Jaramillo-Hidalgo, J.; et al. Efficacy of non-pharmacological interventions to treat malnutrition in older persons: A systematic review and meta-analysis. The SENATOR project ONTOP series and MaNuEL knowledge hub project. Ageing research reviews 2019, 49, 27–48. [Google Scholar] [CrossRef]
- Avenell, A.; Smith, T.O.; Curtain, J.P.; Mak, J.C.; Myint, P.K. Nutritional supplementation for hip fracture aftercare in older people. Cochrane Database Syst Rev 2016, 11, CD001880. [Google Scholar] [CrossRef]
- Bell, J.J.; Young, A.; Hill, J.; Banks, M.; Comans, T.; Barnes, R.; Keller, H.H. Rationale and developmental methodology for the SIMPLE approach: A Systematised, Interdisciplinary Malnutrition Pathway for impLementation and Evaluation in hospitals. Nutrition & dietetics: the journal of the Dietitians Association of Australia 2018, 75, 226–234. [Google Scholar] [CrossRef]
- Bell, J.J.; Young, A.M.; Hill, J.M.; Banks, M.D.; Comans, T.A.; Barnes, R.; Keller, H.H. Systematised, Interdisciplinary Malnutrition Program for impLementation and Evaluation delivers improved hospital nutrition care processes and patient reported experiences – An implementation study. Nutrition & Dietetics 2021, 78, 466–475. [Google Scholar] [CrossRef]
- Neaves, B.; Bell, J.J.; McCray, S. Impact of room service on nutritional intake, plate and production waste, meal quality and patient satisfaction and meal costs: A single site pre-post evaluation. Nutrition & Dietetics 2022, 79, 187–196. [Google Scholar] [CrossRef]
- Geirsdóttir, Ó.G.; Bell, J.J. Interdisciplinary Nutritional Management and Care for Older Adults: An Evidence-Based Practical Guide for Nurses; Springer International Publishing: Cham, 2021; p. 271. [Google Scholar]
- Swan, W.I.; Vivanti, A.; Hakel-Smith, N.A.; Hotson, B.; Orrevall, Y.; Trostler, N.; Beck Howarter, K.; Papoutsakis, C. Nutrition Care Process and Model Update: Toward Realizing People-Centered Care and Outcomes Management. Journal of the Academy of Nutrition and Dietetics 2017, 117, 2003–2014. [Google Scholar] [CrossRef]
- Olofsson, B.; Stenvall, M.; Lundstrom, M.; Svensson, O.; Gustafson, Y. Malnutrition in hip fracture patients: an intervention study. Journal of Clinical Nursing 2007, 16, 2027–2038. [Google Scholar] [CrossRef]
- Hoekstra, J.C.; Goosen, J.H.M.; de Wolf, G.S.; Verheyen, C.C.P.M. Effectiveness of multidisciplinary nutritional care on nutritional intake, nutritional status and quality of life in patients with hip fractures: a controlled prospective cohort study. Clinical Nutrition 2011, 30, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Graham, I.D.; Logan, J.; Harrison, M.B.; Straus, S.E.; Tetroe, J.; Caswell, W.; Robinson, N. Lost in knowledge translation: time for a map? J Contin Educ Health Prof 2006, 26, 13–24. [Google Scholar] [CrossRef]
- Institute for Healthcare Improvement. How to Improve: Model for Improvement. Available online: https://www.ihi.org/resources/Pages/HowtoImprove/default.aspx (accessed on 12 April 2024).
- Langley GL, M.R. , Nolan KM, Nolan TW, Norman CL, Provost LP.,. Improvement Guide: A Practical Approach to Enhancing Organizational Performance (2nd edition); Jossey-Bass Publishers: San Francisco, 2009. [Google Scholar]
- Greenhalgh, T.; Jackson, C.; Shaw, S.; Janamian, T. Achieving Research Impact Through Co-creation in Community-Based Health Services: Literature Review and Case Study. Milbank Q 2016, 94, 392–429. [Google Scholar] [CrossRef]
- Robert, G.; Locock, L.; Williams, O.; Cornwell, J.; Donetto, S.; Goodrich, J. Co-Producing and Co-Designing. 2022. [Google Scholar] [CrossRef]
- Chew, S.; Armstrong, N.; Martin, G.P. Understanding knowledge brokerage and its transformative potential: a Bourdieusian perspective. Evidence & Policy 2022, 18, 25–42. [Google Scholar] [CrossRef]
- Young, A.; Keller, H.; Barnes, R.; Bell, J. Clinicians as novice facilitators: a SIMPLE case study. Journal of Health Organization and Management 2019, 33, 78–92. [Google Scholar] [CrossRef]
- Participation., I.A.f.P. Public Participation Spectrum. Available online: https://iap2.org.au/resources/spectrum/ (accessed on 25 January).
- Bell, J.J.; Mitchell, R.J.; Harris, I.A.; Seymour, H.; Armstrong, E.; Harris, R.; Fleming, S.; Hurring, S.; Close, J. Oral Nutritional Supplementation in Older Adults with a Hip Fracture-Findings from a Bi-National Clinical Audit. Healthcare (Basel) 2024, 12. [Google Scholar] [CrossRef]
- ANZ Hip Fracture Registry. ANZ HFR Nutrition Sprint Audit. Available online: https://anzhfr.org/sprintaudits/ (accessed on day month year).
- Australian and New Zealand Hip Fracture Registry. Preoperative Fasting Sprint Audit. Available online: https://anzhfr.org/sprintaudits/ (accessed on 23 January 2025).
- Bell, J.; Turabi, R.; Olsen, S.U.; Sheehan, K.J.; Geirsdóttir, Ó.G. Interdisciplinary Oral Nutrition Support and Supplementation After Hip Fracture Surgery in Older Adult Inpatients: A Global Cross-Sectional Survey (ONS-STUDY). Nutrients 2025, 17, 240. [Google Scholar] [CrossRef] [PubMed]
- Fragility Fracture Network SIMPLER Implementation Steering Committee. SIMPLER Nutrition Measures and Actions recommended for implementation - consensus from the Fragility Fracture Network (Global) Implementation Steering Committee. 2024.
- Fragility Fracture Network SiMPLER Site Champions Network. Core SIMPLER Nutrition Care Measures and Actions: consensus from the Fragility Fracture Network SiMPLER Site Champions Network (unpublished data). 2024.
- World Health Organisation. Countries. Available online: https://www.who.int/countries (accessed on 21 March 2025).
- Keller, H.; Laur, C.; Atkins, M.; Bernier, P.; Butterworth, D.; Davidson, B.; Hotson, B.; Nasser, R.; Laporte, M.; Marcell, C. Update on the Integrated Nutrition Pathway for Acute Care (INPAC): post implementation tailoring and toolkit to support practice improvements. Nutrition journal 2018, 17, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Keller, H.H.; Valaitis, R.; Laur, C.V.; McNicholl, T.; Xu, Y.; Dubin, J.A.; Curtis, L.; Obiorah, S.; Ray, S.; Bernier, P.; et al. Multi-site implementation of nutrition screening and diagnosis in medical care units: Success of the More-2-Eat project. Clinical nutrition (Edinburgh, Scotland) 2019, 38, 897–905. [Google Scholar] [CrossRef] [PubMed]
- Writing Group of the Nutrition Care Process/Standardized Language Committee. Nutrition care process and model part I: the 2008 update. J Am Diet Assoc 2008, 108, 1113–1117. [Google Scholar] [CrossRef]
- Elia, M. Screening for malnutrition: A Multidisciplinary Responsibility. Development and Use of the Malnutrition Universal Screening Tool (MUST) for Adults; BAPEN: Redditch, 2003. [Google Scholar]
- Isenring, E.A.; Bauer, J.D.; Banks, M.; Gaskill, D. The Malnutrition Screening Tool is a useful tool for identifying malnutrition risk in residential aged care. Journal of Human Nutrition & Dietetics 2009, 22, 545–550. [Google Scholar] [CrossRef]
- Cederholm, T.; Barazzoni, R.; Austin, P.; Ballmer, P.; Biolo, G.; Bischoff, S.C.; Compher, C.; Correia, I.; Higashiguchi, T.; Holst, M.; et al. ESPEN guidelines on definitions and terminology of clinical nutrition. Clinical Nutrition 2017, 36, 49–64. [Google Scholar] [CrossRef]
- The Prince Charles Hospital - Metro North Hospital and Health Service. SIMPLER Toolkit. Available online: https://tpch.qld.libguides.com/simpler (accessed on 23 January 2025).
- Kaiser, M.J.; Bauer, J.M.; Ramsch, C.; Uter, W.; Guigoz, Y.; Cederholm, T.; Thomas, D.R.; Anthony, P.; Charlton, K.E.; Maggio, M.; et al. Validation of the Mini Nutritional Assessment short-form (MNA-SF): a practical tool for identification of nutritional status. J Nutr Health Aging 2009, 13, 782–788. [Google Scholar] [CrossRef]
- Ferguson, M.; Capra, S.; Bauer, J.; Banks, M. Development of a valid and reliable malnutrition screening tool for adult acute hospital patients. Nutrition 1999, 15, 458–464. [Google Scholar] [CrossRef]
- Cederholm, T.; Jensen, G.L.; Correia, M.; Gonzalez, M.C.; Fukushima, R.; Higashiguchi, T.; Baptista, G.; Barazzoni, R.; Blaauw, R.; Coats, A.; et al. GLIM criteria for the diagnosis of malnutrition - A consensus report from the global clinical nutrition community. Clinical nutrition (Edinburgh, Scotland) 2019, 38, 1–9. [Google Scholar] [CrossRef]
- World Health Organisation. International Classification of Diseases 11th Revision. Available online: https://icd.who.int/en (accessed on 23 July 2024).
- Detsky, A.S.; McLaughlin, J.R.; Baker, J.P.; Johnston, N.; Whittaker, S.; Mendelson, R.A.; Jeejeebhoy, K.N. What is subjective global assessment of nutritional status? Jpen: Journal of Parenteral & Enteral Nutrition 1987, 11, 8–13. [Google Scholar]
- Nestle. MNA(R) Forms. Available online: https://www.mna-elderly.com/mna-forms (accessed on 23 June 2024).
- Bell, J.J.; Geirsdóttir, Ó.G.; Hertz, K.; Santy-Tomlinson, J.; Skúladóttir, S.S.; Eleuteri, S.; Johansen, A. Nutritional Care of the Older Patient with Fragility Fracture: Opportunities for Systematised, Interdisciplinary Approaches Across Acute Care, Rehabilitation and Secondary Prevention Settings. In Orthogeriatrics; Springer: Cham, 2020; pp. 311–329. [Google Scholar]
- Rushton, A.; Young, A.; Keller, H.; Bauer, J.; Bell, J. Delegation Opportunities for Malnutrition Care Activities to Dietitian Assistants—Findings of a Multi-Site Survey. Healthcare 2021, 9, 446. [Google Scholar] [CrossRef] [PubMed]
- Rushton, A.; Elmas, K.; Bauer, J.; Bell, J.J. Identifying Low Value Malnutrition Care Activities for De-Implementation and Systematised, Interdisciplinary Alternatives—A Multi-Site, Nominal Group Technique Approach. Nutrients 2021, 13, 2063. [Google Scholar] [CrossRef]
- ten Cate, D.; Ettema, R.G.A.; Huisman-de Waal, G.; Bell, J.J.; Verbrugge, R.; Schoonhoven, L.; Schuurmans, M.J.; Group, t.B.C.R. Interventions to prevent and treat malnutrition in older adults to be carried out by nurses: A systematic review. Journal of Clinical Nursing 2020, 29, 1883–1902. [Google Scholar] [CrossRef]
- Rushton, A.; Edwards, A.; Bauer, J.; Bell, J.J. Dietitian assistant opportunities within the nutrition care process for patients with or at risk of malnutrition: a systematic review. Nutrition & Dietetics 2021, 78, 69–85. [Google Scholar] [CrossRef]
- Bell, J.J.; Rushton, A.; Elmas, K.; Banks, M.D.; Barnes, R.; Young, A.M. Are Malnourished Inpatients Treated by Dietitians Active Participants in Their Nutrition Care? Findings of an Exploratory Study of Patient-Reported Measures across Nine Australian Hospitals. Healthcare 2023, 11. [Google Scholar] [CrossRef]
- Gomes, K.; Bell, J.; Desbrow, B.; Roberts, S. Lost in Transition: Insights from a Retrospective Chart Audit on Nutrition Care Practices for Older Australians with Malnutrition Transitioning from Hospital to Home. Nutrients 2024, 16, 2796. [Google Scholar] [CrossRef]
- Keller, H.H.; Xu, Y.; Dubin, J.A.; Curtis, L.; Laur, C.V.; Bell, J. Improving the standard of nutrition care in hospital: Mealtime barriers reduced with implementation of the Integrated Nutrition Pathway for Acute Care. Clinical nutrition ESPEN 2018, 28, 74–79. [Google Scholar] [CrossRef]
- Roberts, S.; Marshall, A.P.; Bromiley, L.; Hopper, Z.; Byrnes, J.; Ball, L.; Collins, P.F.; Kelly, J. Patient-Led, Technology-Assisted Malnutrition Risk Screening in Hospital: A Feasibility Study. Nutrients 2024, 16. [Google Scholar] [CrossRef] [PubMed]
- Bell, J. Nutrition Screening and Assessment in Hip Fracture. In Handbook of Famine, Starvation, and Nutrient Deprivation: From Biology to Policy; Preedy, V., Patel, V.B., Eds.; Springer International Publishing: Cham, 2018; pp. 1–22. [Google Scholar]
- Arkley, J.; Dixon, J.; Wilson, F.; Charlton, K.; Ollivere, B.J.; Eardley, W. Assessment of Nutrition and Supplementation in Patients With Hip Fractures. Geriatr Orthop Surg Rehabil 2019, 10, 2151459319879804–2151459319879804. [Google Scholar] [CrossRef]
- Holst, M.; Beck, A.M. Nutritional Assessment, Diagnosis, and Treatment in Geriatrics. In Interdisciplinary Nutritional Management and Care for Older Adults: An Evidence-Based Practical Guide for Nurses; Geirsdóttir, Ó.G., Bell, J.J., Eds.; Springer International Publishing: Cham, 2021; pp. 31–50. [Google Scholar]
- Norman, K.; Haß, U.; Pirlich, M. Malnutrition in Older Adults-Recent Advances and Remaining Challenges. Nutrients 2021, 13. [Google Scholar] [CrossRef]
- Bellanti, F.; Lo Buglio, A.; Quiete, S.; Vendemiale, G. Malnutrition in Hospitalized Old Patients: Screening and Diagnosis, Clinical Outcomes, and Management. Nutrients 2022, 14. [Google Scholar] [CrossRef] [PubMed]
- Dent, E.; Wright, O.R.L.; Woo, J.; Hoogendijk, E.O. Malnutrition in older adults. Lancet (London, England) 2023. [Google Scholar] [CrossRef]
- Bell, J.J.; Bauer, J.; Capra, S.; Pulle, C.R. Barriers to nutritional intake in patients with acute hip fracture: time to treat malnutrition as a disease and food as a medicine? Can J Physiol Pharmacol 2013, 91, 489–495. [Google Scholar] [CrossRef]
- Ng, W.L.; Collins, P.F.; Hickling, D.F.; Bell, J.J. Evaluating the concurrent validity of body mass index (BMI) in the identification of malnutrition in older hospital inpatients. Clinical Nutrition 2019, 38, 2417–2422. [Google Scholar] [CrossRef] [PubMed]
- American Dietetic Association. International dietetics and nutritional terminology (IDNT) Reference Manual. Standardized language for the Nutrition Care Process; 2010; Volume 3.
- Bell, J. Nutrition in orthopaedics; Hickson, M., Smith, S., Eds.; British Dietetic Association: UK, 2017; in press. [Google Scholar]
- Academy of Nutrition and Dietetics. Nutrition Care Process Terminology (NCPT): Reference Manual; Academy of Nutrition and Dietetics: Chicago, IL, 2021. [Google Scholar]
- Barry, M.J.; Edgman-Levitan, S. Shared Decision Making — The Pinnacle of Patient-Centered Care. New England Journal of Medicine 2012, 366, 780–781. [Google Scholar] [CrossRef]
- Truglio-Londrigan, M.; Slyer, J.T. Shared Decision-Making for Nursing Practice: An Integrative Review. Open Nurs J 2018, 12, 1–14. [Google Scholar] [CrossRef]
- Boeykens, D.; Boeckxstaens, P.; De Sutter, A.; Lahousse, L.; Pype, P.; De Vriendt, P.; Van de Velde, D.; on behalf of the Primary Care, A. Goal-oriented care for patients with chronic conditions or multimorbidity in primary care: A scoping review and concept analysis. PLOS ONE 2022, 17, e0262843. [Google Scholar] [CrossRef]
- Kang, E.; Kim, M.Y.; Lipsey, K.L.; Foster, E.R. Person-Centered Goal Setting: A Systematic Review of Intervention Components and Level of Active Engagement in Rehabilitation Goal-Setting Interventions. Archives of Physical Medicine and Rehabilitation 2022, 103, 121–130.e123. [Google Scholar] [CrossRef]
- Crawford, L.; Maxwell, J.; Colquhoun, H.; Kingsnorth, S.; Fehlings, D.; Zarshenas, S.; McFarland, S.; Fayed, N. Facilitators and barriers to patient-centred goal-setting in rehabilitation: A scoping review. Clinical Rehabilitation 2022, 36, 1694–1704. [Google Scholar] [CrossRef]
- Australian and New Zealand College of Anaesthetists. PG07 Guideline on pre-anaesthesia consultation and patient preparation 2024. Available online: https://www.anzca.edu.au/getattachment/d2c8053c-7e76-410e-93ce-3f9a56ffd881/PG07-Guideline-on-pre-anaesthesia-consultation-and-patient-preparation (accessed on 23 January 2025).
- Bell, J.J.; Bauer, J.D.; Capra, S. The Malnutrition Screening Tool versus objective measures to detect malnutrition in hip fracture. Journal of Human Nutrition & Dietetics 2013, 26, 519–526. [Google Scholar] [CrossRef]
- Volkert, D.; Kiesswetter, E.; Cederholm, T.; Donini, L.M.; Eglseer, D.; Norman, K.; Schneider, S.M.; Ströbele-Benschop, N.; Torbahn, G.; Wirth, R.; et al. Development of a Model on Determinants of Malnutrition in Aged Persons: A MaNuEL Project. Gerontol Geriatr Med 2019, 5, 2333721419858438. [Google Scholar] [CrossRef] [PubMed]
- Druml, C.; Ballmer, P.E.; Druml, W.; Oehmichen, F.; Shenkin, A.; Singer, P.; Soeters, P.; Weimann, A.; Bischoff, S.C. ESPEN guideline on ethical aspects of artificial nutrition and hydration. Clinical nutrition (Edinburgh, Scotland) 2016, 35, 545–556. [Google Scholar] [CrossRef]
- Australian Commission on Safety and Quality in Healthcare. Comprehensive Care Standard. Available online: https://www.safetyandquality.gov.au/standards/nsqhs-standards/comprehensive-care-standard#background-to-this-standard (accessed on 23 January 2025).
- Australian and New Zealand Hip Fracture Registry. Data Dictionary Master, Version 17, October 2024. Available online: https://anzhfr.org/wp-content/uploads/sites/1164/2024/11/ANZHFR-Data-Dictionary-v17_October-2024-1.pdf (accessed on 23 January 2025).
- Cass, A.R.; Charlton, K.E. Prevalence of hospital-acquired malnutrition and modifiable determinants of nutritional deterioration during inpatient admissions: A systematic review of the evidence. Journal of human nutrition and dietetics: the official journal of the British Dietetic Association 2022, 35, 1043–1058. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, K.R.; Wolfe, R.R.; Ferrando, A.A. Pre- and Post-Surgical Nutrition for Preservation of Muscle Mass, Strength, and Functionality Following Orthopedic Surgery. Nutrients 2021, 13. [Google Scholar] [CrossRef] [PubMed]
- Weimann, A.; Braga, M.; Carli, F.; Higashiguchi, T.; Hübner, M.; Klek, S.; Laviano, A.; Ljungqvist, O.; Lobo, D.N.; Martindale, R.; et al. ESPEN guideline: Clinical nutrition in surgery. Clinical Nutrition 2017. [Google Scholar] [CrossRef]
- Rasmussen, N.M.L.; Belqaid, K.; Lugnet, K.; Nielsen, A.L.; Rasmussen, H.H.; Beck, A.M. Effectiveness of multidisciplinary nutritional support in older hospitalised patients: A systematic review and meta-analyses. Clinical nutrition ESPEN 2018, 27, 44–52. [Google Scholar] [CrossRef]
- Schuetz, P.; Sulo, S.; Walzer, S.; Vollmer, L.; Brunton, C.; Kaegi-Braun, N.; Stanga, Z.; Mueller, B.; Gomes, F. Cost savings associated with nutritional support in medical inpatients: an economic model based on data from a systematic review of randomised trials. BMJ Open 2021, 11, e046402. [Google Scholar] [CrossRef]
- Australian Healthcare; Hospitals Association. Experience-based Co-design Toolkit. (accessed on 23 June 2024).
- Meloncelli, N.; Young, A.; Christoffersen, A.; Rushton, A.; Zhelnov, P.; Wilkinson, S.A.; Scott, A.M.; de Jersey, S. Co-designing nutrition interventions with consumers: A scoping review. Journal of human nutrition and dietetics: the official journal of the British Dietetic Association 2022. [Google Scholar] [CrossRef]
- Agency for Clinical Innovation. Co-Design Toolkit. Available online: https://aci.health.nsw.gov.au/projects/co-design/library-of-related-resources (accessed on 21 March 2025).
- Elia, M.; Stratton, R.J. Considerations for screening tool selection and role of predictive and concurrent validity. Current opinion in clinical nutrition and metabolic care 2011, 14, 425–433. [Google Scholar] [CrossRef]
- Proctor, E.K.; Powell, B.J.; McMillen, J.C. Implementation strategies: recommendations for specifying and reporting. Implementation Science 2013, 8, 139. [Google Scholar] [CrossRef]
- Bowen, D.J.; Kreuter, M.; Spring, B.; Cofta-Woerpel, L.; Linnan, L.; Weiner, D.; Bakken, S.; Kaplan, C.P.; Squiers, L.; Fabrizio, C.; et al. How we design feasibility studies. Am J Prev Med 2009, 36, 452–457. [Google Scholar] [CrossRef] [PubMed]
- Skivington, K.; Matthews, L.; Simpson, S.A.; Craig, P.; Baird, J.; Blazeby, J.M.; Boyd, K.A.; Craig, N.; French, D.P.; McIntosh, E.; et al. A new framework for developing and evaluating complex interventions: update of Medical Research Council guidance. BMJ 2021, 374, n2061. [Google Scholar] [CrossRef]
- Proctor, E.; Silmere, H.; Raghavan, R.; Hovmand, P.; Aarons, G.; Bunger, A.; Griffey, R.; Hensley, M. Outcomes for implementation research: conceptual distinctions, measurement challenges, and research agenda. Adm Policy Ment Health 2011, 38, 65–76. [Google Scholar] [CrossRef]
- Greenhalgh, T.; Wherton, J.; Papoutsi, C.; Lynch, J.; Hughes, G.; A’Court, C.; Hinder, S.; Fahy, N.; Procter, R.; Shaw, S. Beyond Adoption: A New Framework for Theorizing and Evaluating Nonadoption, Abandonment, and Challenges to the Scale-Up, Spread, and Sustainability of Health and Care Technologies. J Med Internet Res 2017, 19, e367. [Google Scholar] [CrossRef]
- Laur, C.; Valaitis, R.; Bell, J.; Keller, H. Changing nutrition care practices in hospital: a thematic analysis of hospital staff perspectives. BMC Health Serv Res 2017, 17, 498. [Google Scholar] [CrossRef]
- Laur, C.; Bell, J.; Valaitis, R.; Ray, S.; Keller, H. The Sustain and Spread Framework: strategies for sustaining and spreading nutrition care improvements in acute care based on thematic analysis from the More-2-Eat study. BMC health services research 2018, 18, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Albers, B.; Metz, A.; Burke, K. Implementation support practitioners – a proposal for consolidating a diverse evidence base. BMC Health Services Research 2020, 20, 368. [Google Scholar] [CrossRef]
- Wang, Y.; Wong, E.L.-Y.; Nilsen, P.; Chung, V.C.-h.; Tian, Y.; Yeoh, E.-K. A scoping review of implementation science theories, models, and frameworks — an appraisal of purpose, characteristics, usability, applicability, and testability. Implementation Science 2023, 18, 43. [Google Scholar] [CrossRef] [PubMed]
- Braithwaite, J. Changing how we think about healthcare improvement. Bmj 2018, 361, k2014. [Google Scholar] [CrossRef]
- Braithwaite, J.; Churruca, K.; Long, J.C.; Ellis, L.A.; Herkes, J. When complexity science meets implementation science: a theoretical and empirical analysis of systems change. BMC Med 2018, 16, 63. [Google Scholar] [CrossRef]
- Fragility Fracture Network. FFN Board Members. Available online: https://fragilityfracturenetwork.org/board-members/ (accessed on day month year).
- Curran, G.M.; Bauer, M.; Mittman, B.; Pyne, J.M.; Stetler, C. Effectiveness-implementation hybrid designs: combining elements of clinical effectiveness and implementation research to enhance public health impact. Med Care 2012, 50, 217–226. [Google Scholar] [CrossRef] [PubMed]
- French Simon, D.; Green Sally, E.; O’connor Denise, A.; McKenzie Joanne, E.; Francis Jill, J.; Michie, S.; Buchbinder, R.; Schattner, P.; Spike, N.; Grimshaw Jeremy, M. Developing theory-informed behaviour change interventions to implement evidence into practice: a systematic approach using the Theoretical Domains Framework. Implementation Science 2012, 7, 38. [Google Scholar] [CrossRef] [PubMed]
- National Health and Medical Research Council, N. Ethical Considerations in Quality Assurance and Evaluation Activities. Available online: https://www.nhmrc.gov.au/sites/default/files/documents/attachments/ethical-considerations-in-quality-assurance-and-evaluation-activites.pdf (accessed on 28 April).
| Process | Region: n |
|---|---|
| Semi-structured healthcare professional interviews (number of interview sets) | Global: 2 |
| Interprofessional workshops (number of workshops) | Global: 2 Asia Pacific: 4 Europe: 3 North America: 1 |
| Interprofessional focus group meetings (number of meetings) | Global: 4 North America: 1 Europe: 3 Asia-Pacific: 1 |
| Site support meetings (number of meetings) | Asia Pacific: 6 Europe: 14 South America: 2 |
| Webinars (number of webinars) | Asia Pacific: 3 Europe: 3 |
| Plenary / keynote / symposia /platform presentations (number of presentations) |
Global: 4 Asia-Pacific 10 Europe: 12 North America: 2 South America: 1 |
| Web-based surveys, polls, and consensus processes (number conducted) |
Global: 5 Asia Pacific: 1 |
| Patient level audits | Asia Pacific: 2 |
| YouTubes, PodCasts | Global:6 AsiaPacific: 7 |
| Web-based and face to face Education Modules and Lectures (number conducted) |
Global: 1 Europe: 1 Asia-Pacific: 1 |
| Strategic meeting presentations (FFN Executive / Board, Regionalisation, Education, Scientific Committees; Special Interest Advisory Boards (number of presentations) | Global: 6 Europe: 4 Asia-Pacific: 3 |
| Improvement opportunities | Evidence-informed rationale |
|---|---|
| 1. Avoid unnecessary, prolonged or repeat fasting | Unnecessary, prolonged, or repeated fasting is harmful and should be avoided [8,9,19,41,42,82]. |
| 2. Offer information about nutrition [risk] statusa | Up to one in two hip fracture patients are malnourished on admission to hospital; hip fracture patients rarely meet post operative nutritional requirements in the absence of early, interdisciplinary, multicomponent interventions [8,11,18,19,25,57]. Evidence suggests malnutrition screening tools have limited criterion validity in hip fracture; therefore all hip fracture patients should be treated as ‘at risk’ of malnutrition, and offered information about their nutrition risk status, until a systematic nutrition assessment is performed by a trained person [8,9,16,18,19,41,42,83]. This assessment should apply a tool validated for the purposes of diagnosing protein / energy malnutrition, as well as identifying ongoing nutrition risk factors, for example inadequate low intake, high requirements, or nutrient availability issues [51,53,55,84]. Following assessment, patients (or carers where appropriate) should be offered diagnostic advice regarding whether they are malnourished or remain at risk of malnutrition [18,19,85]. |
| 3. Offer information about nutrition interventionsa | Interdisciplinary, multicomponent interventions should be offered to all hip fracture patient unless assessed ‘not at risk’ or not in line with patient treatment preferences [8,9,18,19,41,42,86]. This should include provision of information or education to support informed consent, shared decision-making regarding treatment choices, and adherence to interdisciplinary, multicomponent interventions [85,86]. |
| 4. Offer high quality, high protein / energy food and fluids, with regular intake assessmenta |
All hip fracture inpatients, unless assessed as well-nourished and not at risk of malnutrition, should be offered high quality, appropriately textured, high protein / energy food and fluids, fortified food, additional snacks and/or finger foods to support adequate dietary intake [7,8,9,18,19,25,57]. Consumption of these should be assessed to support corresponding adjustment of interventions [18,19]. |
| 5. Offer oral nutritional supplements,* with regular intake assessmenta | All hip fracture inpatients, unless assessed as well-nourished and not at risk of malnutrition, should be offered oral nutritional supplements, in combination with dietary information/counselling and food fortification, to improved patient and healthcare outcomes [4,5,6,18,19]. Intake of these should be regularly assessed [18,19]. |
| 6. Offer malnutrition [risk] status and treatment plan to be provided to preferred post-hospital health care providerb | Ongoing nutrition care should be offered to all inpatients who remain at risk of malnutrition or malnourished at time of discharge from hospital [18,19]. Where consent is provided, a referral should be made to the patients preferred health care provider which includes their nutrition status and treatment plan [86]. |
| Core measure | Audit Source: medical record, bed chart, and/or discharge documentation |
Patient / carerc reported measure Source: Standardized PREM collected by designated person |
Treating cliniciand estimate example Source: Clinician survey (paper and/or electronic versions) |
|---|---|---|---|
| Unnecessary, prolonged or repeat fasting? [8,9,18,19,39,82,88,89,90] | Fasted for more than about 6 hours before surgery, or fasted more than once? (No; Yes or not documented) |
Were your fasted for more than about 6 hours before your surgery, or fasted more than once? (No; Yes or not documented) |
What percentage of all hip fracture patients you have cared for in the past month are fasted for more than about 6 hours before surgery, or fasted more than once? 0-25 | 25-50 | 50-75 | 75-100 |
| Awareness of nutrition [risk] status? a [7,8,9,18,19,23,26,41,42,46,86] | Documented nutrition assessment and provision of malnutrition [risk] assessment to patient / caregiver? a (Yes; No or not documented) |
Anybody who is 65 or older who has had hip fracture surgery should have a nutritional assessment. Have you been provided with the results of your nutritional assessment? (Yes; No or don’t know) |
What percentage of all hip fracture patients you have cared for in the past month have had a nutrition [risk] assessment and are aware of their nutrition [risk] status? 0-25 | 25-50 | 50-75 | 75-100 |
| Provided with information / education about nutrition? a [7,8,18,19,23,26,41,42,46,59,60,61,91] | Documented provision of information / education about nutrition? a (Yes; No or not documented) |
Have you received any information or education about nutrition since you have been in hospital? (Yes; No or don’t know) | What percentage of all hip fracture patients you have cared for in the past month are provided with information / education about nutrition? 0-25 | 25-50 | 50-75 | 75-100 |
| Provided high protein / energy food and fluids and intake is regularly assessed? a[18,19,23,41,42,60,91] |
Documented evidence of provision of high protein / energy food and fluid choices and assessment of food and fluid intake? a (Yes; No or not documented) |
Are you receiving high quality, high protein food and fluid choices, and has anybody asked you how much you have been eating? (Yes; No or don’t know) |
What percentage of all hip fracture patients you have cared for in the past month have received high protein/energy foods and have had their intake assessed within 72 hours of surgery? 0-25 | 25-50 | 50-75 | 75-100 |
| Provided with oral nutritional supplements* and intake is regularly assessed? a [4,5,6,18,19,41,42,60,91,92] |
Documented evidence of provision of supplements and assessment of supplement intake? a (Yes; No or not documented) |
Are you receiving oral nutrition supplements and have you been asked about your intake of these? (Yes; No or don’t know) |
What percentage of all hip fracture inpatients you have cared for in the past month have been provided oral nutritional supplements and have had their intake of these assessed within 72 hours of surgery and weekly thereafter? 0-25 | 25-50 | 50-75 | 75-100 |
| Malnutrition [risk] status and nutrition plan provided to a post-hospital health care professional? b [8,18,19,23,41,42,86] |
Evidence of malnutrition [risk] status and nutrition plan in hospital discharge summary / discharge letter or other discharge documentation? (Yes; No or not documented) |
Has anybody asked you if they can give your nutrition diagnosis and plan to your preferred post-hospital health care provider? (Yes; No or don’t know) |
What percentage of malnourished (or still at risk) hip fracture patients have had their malnutrition (risk) status documented in their medical discharge summary and have a nutrition treatment plan included in their discharge paperwork? 0-25 | 25-50 | 50-75 | 75-100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





