1. Introduction
The introduction of biosimilars is a cost-effective strategy to provide an alternative for a reference product [
1]. Integration of biosimilars into the clinical practice in the Kingdom of Saudi Arabia (KSA) is befitting Saudi Vision 2030. KSA is the largest biosimilar market in the Middle East and Africa [
2]. A local simulation study evaluated the cost efficiency and expanded access to care by switching from reference filgrastim and pegfilgrastim to biosimilar filgrastim in 4000 patients in the country. Biosimilar conversion from reference to biosimilar filgrastim enabled expanded access to ado-trastuzumab emtansine ranging from 61 patients to 191 patients with locally advanced her2neu positive breast cancer in adjuvant settings [
3].
The Saudi Food and Drug Authority (SFDA) has approved many biosimilars for monoclonal antibodies such as rituximab, trastuzumab and bevacizumab. The rituximab biosimilar was implemented only partially in our organization and mainly in in-patient regimens for treating malignant and non-malignant conditions. Subcutaneous (SC) rituximab was kept in the formulary since there is a major advantage with SC rituximab regarding ease and convenience of administration. Therefore, our organization decided to limit IV biosimilar rituximab use to B-cell acute lymphocytic leukemia, salvage regimens for lymphomas, chronic lymphocytic leukemia, first cycle for lymphoma patients aiming for SC rituximab in subsequent cycles in out-patient setting and all non-malignant conditions [
1,
2].
Rituximab is an anti-CD20 monoclonal antibody that is highly effective in treating B-cell malignancies. It has also shown efficacy in autoimmune disorders like rheumatoid arthritis and granulomatosis with polyangiitis. Rituximab’s mechanism of action involves the depletion of B-cells through complement-dependent cytotoxicity (CDC) and antibody-dependent cell-mediated cytotoxicity (ADCC) [
4].
The originator rituximab (MabThera) was first approved by the Food and Drug Administration (FDA) in 1997, followed by the European Medicines Agency (EMA) in 1998, and the Saudi Food and Drug Authority (SFDA) in 2008. Later, with the expiration of Rituximab’s patent, Truxima (rituximab-abbs) became the first biosimilar, receiving FDA approval on November 28, 2018 [
5]. This was followed by the approval of Ruxience (rituximab-pvvr) in 2019 and Riabni (rituximab-arrx) in 2020 [
6,
7].
Several studies have demonstrated that rituximab biosimilars offer equivalent efficacy and safety compared to the originator rituximab [
8,
9]. In REFLECT trial, Riximyo (a rituximab biosimilar), combined with R-CHOP in CD20-positive DLBCL. An 94.7% overall response rate (ORR) was achieved in this trial; 65% of patients achieved complete response (CR) and 30 percent partial response (PR). The one-year progression-free survival rate was 84.9%, while 78% successful PFS was obtained after two years. The safety data indicated that adverse events were suffered by 84.6% of patients. Serious adverse events (SAEs) occurred in 37 percent of cases [
8]. Similarly, in the ASSIST-FL study, patients with untreated advanced follicular lymphoma (FL) received rituximab biosimilar Rixathon (GP2013) and CVP (cyclophosphamide, vincristine, prednisone) [
9]. The study established that Rixathon was equally safe and effective as the originator rituximab. In the test group, the ORR was 87.1% for Rixathon, compared to 87.5% in the originator, meeting its primary endpoint of equivalence. In addition, the safety data pointed to an also similar profile across both groups [
9].
Rituximab is a key therapy used in the treatment of B-cell lymphomas and can be administered either intravenously (IV) or subcutaneously (SC). Although both formulations have shown similar efficacy, there are important differences in pharmacokinetics, safety, route of administration, and resource use [
9]. Clinical trials have shown that SC rituximab is non-inferior to IV rituximab in terms of efficacy in follicular lymphoma SABRINA trial and in diffuse large B-cell lymphoma (DLBCL) MabEase trial [
10,
11]. Pharmacokinetically, SC rituximab achieves higher and more sustained serum trough levels due to its slower absorption, whereas IV administration results in a rapid peak concentration but similar overall drug exposure [
12,
13,
14]. The SABRINA trial showed that SC rituximab was non-inferior to IV rituximab in follicular lymphoma patients, with an overall response rate (ORR) of 84.9% with SC and 84.4% with IV. The progression-free survival (PFS) was similar in both groups [
10]. In contrast, The MabEase study showed that in diffuse large B-cell lymphoma (DLBCL) setting, SC rituximab was equally efficacious as IV rituximab with ORRs of 90.5% and 84.4%, respectively. Response rates for complete responses were also similar [
11].
Safety profiles between the two formulations are comparable, though IV rituximab is associated with a higher incidence of infusion-related reactions (IRRs), particularly during the first infusion, necessitating premedication, extended infusion time, and monitoring. In contrast, SC administration offers a significant advantage in terms of patient convenience, as it is administered over 5–7 minutes, thereby reducing bedtime and healthcare resource utilization [
15,
16].
Building trust in biosimilars is a vital component in this paradigm shift. Real-world clinical data will be an important next step in instilling trust in healthcare providers. King Abdulaziz Medical City Jeddah (KAMC-J) is currently doing many real-world evidence studies in extrapolated indications on the use of oncology biosimilars. One of our published studies in the extrapolated indication [
17], and preliminary data of many other unpublished studies is reassuring the comparability of efficacy and safety of oncology biosimilars in extrapolated indications of oncology biosimilars.
Infusion-related reactions (IRRs) are expected after rituximab administration and can be life-threatening; thus, it is recommended to give the patient one full IV dose before transitioning to the SC formulation. At the Ministry of National Guards Health Affairs (MNGHA), an initial IV rituximab biosimilar is used, and if no severe IRR are reported, subsequent cycles are administered using SC rituximab per institutional guidelines. There is currently no safety data available for this switch; however, many centers in UK and Canada have already adopted this practice based on the extrapolation and switchability principles of biosimilar indications which are approved by international regulators [
18]. MNGHA had also approved this practice based on our institutional guidelines [
19].
This study aims to evaluate the real-world safety and efficacy of rituximab biosimilar (Truxima) compared to the originator, as well as the IV-to-SC combined strategy of rituximab biosimilars in patients with B-cell lymphoma, focusing on IRRs. While clinical trials support the IV-to-SC switch for originator rituximab, real-world data on biosimilars are lacking.
2. Materials and Methods
2.1. Study Design and Patient Population
A retrospective observational study was conducted at Princess Nourah Oncology Center (PNOC), King Abdulaziz Medical City in Jeddah, Saudi Arabia. Following approval from the Institutional Review Board (IRB No. IRB/1539/23), electronic medical records were reviewed for eligible patients treated between October 2022 and June 2023. The objective was to assess the safety of IV rituximab biosimilar during the first cycle, followed by the administration of SC rituximab in the second cycle. Eligible patients were adults (≥18 years) diagnosed with follicular lymphoma, low-grade lymphoma, or diffuse large B-cell lymphoma (DLBCL). They must have received IV rituximab biosimilar (Truxima) during the first treatment cycle, followed by SC rituximab (Mabthera) in subsequent cycles at PNOC. Patients were excluded if they had incomplete medical records, received only IV rituximab (Truxima), or were under 18 years of age.
2.2. Study Outcomes and Data Collection
The primary endpoint was the safety of IV Rituximab Biosimilar (Truxima-Biosimilar), assessed by the proportion of patients who developed IRR after the first cycle. The severity of IRR was graded using the Common Terminology Criteria for Adverse Events Version 5 (CTCAE.V5), which categorizes reactions into five grades [
20]. Grade 1 indicates a mild, transient reaction that does not necessitate infusion interruption. Grade 2 refers to a reaction that requires symptomatic treatment or temporary interruption of infusion, with rapid symptom resolution. Grade 3 includes a prolonged reaction that does not respond quickly to treatment and may require hospitalization. Grade 4 involves life-threatening symptoms requiring immediate medical intervention, while Grade 5 corresponds to death resulting from the reaction. Data were collected retrospectively from the patient’s electronic medical records and entered into a pre-designed Excel sheet in a de-identified manner. The secondary endpoint was the effectiveness of Rituximab biosimilar, assessed through the overall response rate (ORR) based on positron emission tomography/computed tomography (PET/CT) findings. Complete Response (CR) was defined as a Deauville score of 1, 2, or 3; partial response (PR) as a score of 4; and progressive disease (PD) as a score of 5. The ORR was calculated as the combined proportion of patients achieving CR and PR.
2.3. Statistical Analysis
Continuous variables were reported as the mean with standard deviation (SD), while categorical data was presented as frequencies and percentages. Data were entered into Microsoft Office Excel and analyzed using GraphPad Prism software (version 10.0).
2.3. The Use of generative artificial intelligence (GenAI)
During the preparation of this manuscript, the authors used ChatGPT 4.0 Mini to paraphrase the text and enhance readability. In addition, the authors reviewed and edited the content as needed and take full responsibility for the content of the publication.
3. Results
3.1. Baseline Characteristics
A total of 81 patients were initially screened. After excluding duplicate records, 71 unique patients remained, of whom 35 met the eligibility criteria and were included in the final analysis. The majority were male (63%), with a mean age of 55 ± 18 years and an average weight of 73 ± 18 kg. Laboratory parameters relevant to the risk of infusion-related reactions included a mean white blood cell (WBC) count of 6 ± 3 × 10⁹/L and an absolute neutrophil count (ANC) of 4 ± 3 × 10⁹/L. Electrolyte levels, including potassium and calcium, were within normal ranges, with mean values of 4 ± 1 mmol/L and 2 ± 0.1 mmol/L, respectively. Common comorbidities included hypertension (43%), diabetes mellitus (31%), and cardiovascular disease (20%), while 45% of patients were medically free. The most frequent lymphoma diagnosis was diffuse large B-cell lymphoma (63%), followed by follicular lymphoma (20%). The most administered treatment regimen was R-CHOP (63%) followed by R-B (20%). The baseline characteristics of the patients are presented in
Table 1.
3.2. The Incidence of IRR
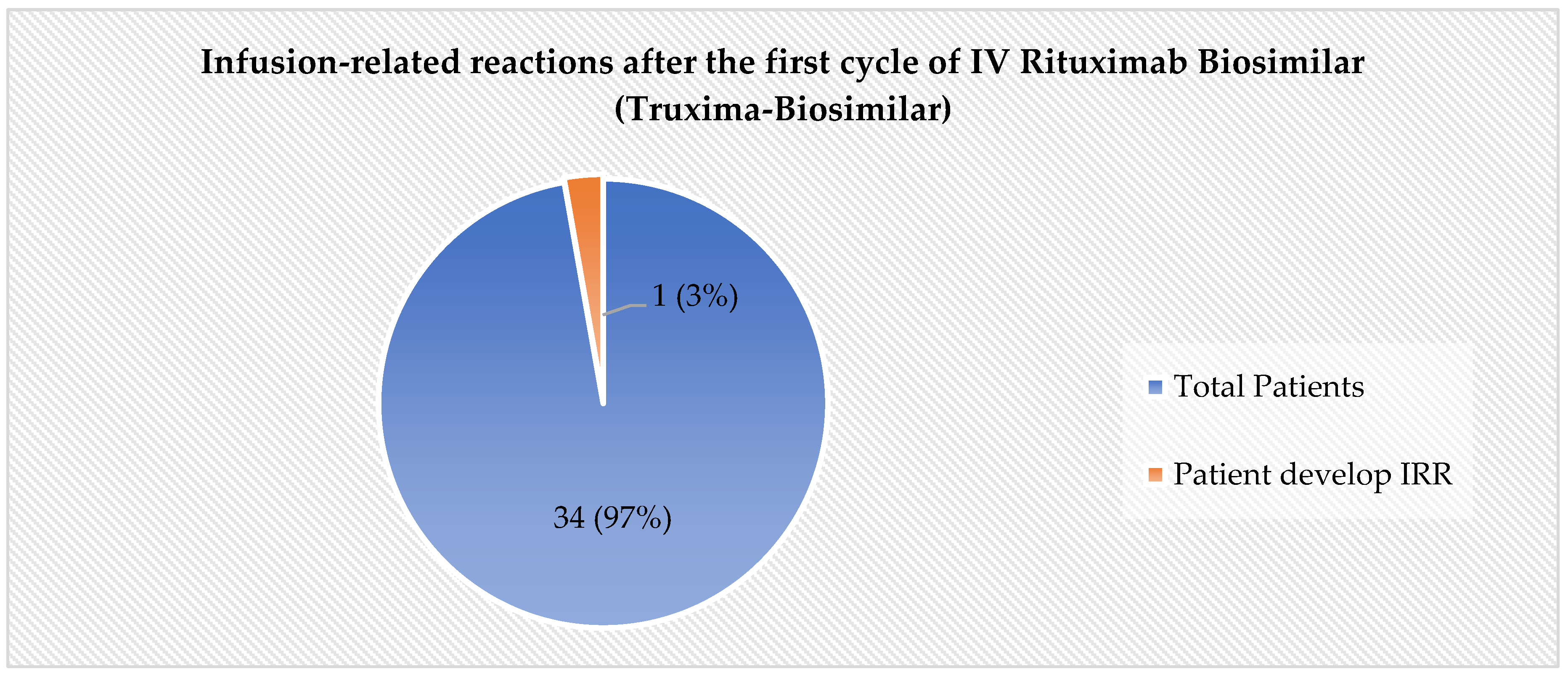
In the assessment of the primary safety endpoint, the incidence of IRRs was found to be low. Among the 35 patients included in the final analysis, only one patient (3%) experienced Grade 1 IRR as per CTCAE.V5 during the first cycle of intravenous rituximab biosimilar (Truxima)
Figure 1. Patient who developed grade 1 IRR had eventually completed the intravenous infusion of Rituximab (Biosimilar) and successfully transitioned to Subcutaneous Rituximab from the subsequent cycle.
3.3. Effectiveness Outcomes
The effectiveness of the rituximab biosimilar was evaluated in 33 patients with available PET/CT results at the end of therapy. Among these, 79% (n = 26) achieved CR, while 6% (n = 2) had PR. In contrast, PD was observed in 15% of patients (n = 5). The ORR was 85% (n = 28), indicating a favorable treatment response in this cohort. The treatment response is summarized in
Table 2.
4. Discussion
Rituximab remains a cornerstone treatment for non-Hodgkin’s lymphoma, chronic lymphocytic leukemia, and even rheumatoid arthritis. It was the first monoclonal antibody approved by the FDA as an anticancer agent. The introduction of biosimilars, such as Truxima, offers a cost-effective alternative to the originator product, potentially increasing access to this critical therapy [
6,
21].
It is recommended to give the patient one full IV dose before transitioning to the SC formulation. At the MNGHA, an initial IV rituximab biosimilar is used, and if no severe IRR are reported, subsequent cycles are given using SC rituximab per institutional guidelines. There is currently no safety data available for this switch; however. The KAMC-J has been the first institution to retrospectively evaluate the safety of this practice and to design and complete a real-world evidence study on this practice for B-cell lymphoma. Results of this study demonstrated that only one out of 34 patients developed IRR; however, it was grade 1 as per Common Terminology Criteria for Adverse Events v5.0, and the patient was able to complete the IV rituximab infusion in the first cycle. This study provides the first evidence that the transition from IV rituximab biosimilar to SC rituximab (MabThera) is well tolerated and safe practice and is recommended to be implemented in other institutions.
The findings of our study indicate that the transition from IV to SC Rituximab is well tolerated and safe. Only one patient (2.8%) out of 35 patients developed an IRR, which was classified as grade 1 according to the CTCAE.V5. This patient was able to complete the IV infusion successfully and subsequently transitioned to SC administration without further complications. This low incidence of IRRs is consistent with the safety profile observed in clinical trials of Rituximab biosimilars [
8,
9].
Several studies have demonstrated the equivalent efficacy and safety of Rituximab biosimilars compared to the originator product. For instance, the REFLECT trial reported a 94.7% ORR with Riximyo (a Rituximab biosimilar) in combination with R-CHOP for CD20-positive diffuse large B-cell lymphoma (DLBCL) [
8]. Similarly, the ASSIST-FL study found that Rixathon (another Rituximab biosimilar) was equally effective and safe as the originator Rituximab in patients with advanced follicular lymphoma [
9].
In our study, the secondary endpoint analysis showed that 79% of patients achieved a CR, defined as a Deauville score of 1, 2, or 3. This high response rate further supports the efficacy of Rituximab biosimilars in real-world settings. The transition from IV to SC administration offers several advantages, including reduced infusion times and improved patient convenience, without compromising safety or efficacy.
The safety profiles of IV and SC Rituximab are comparable, although IV administration is associated with a higher incidence of IRRs, particularly during the first infusion. On the other hand, SC administration may cause local injection-site reactions, such as pain or erythema [
22,
23]. The SC formulation also is more convenient, taking only a few minutes compared to over an hour for IV, thus reducing bed time and healthcare resource use [
9].
One of the strengths of our study is that it provides real-world evidence on the safety and efficacy of Rituximab biosimilars, which is essential for informing clinical practice. However, our study has some limitations. The sample size was relatively small, and the study was conducted at a single center. Therefore, larger, prospective, multicenter studies are needed to confirm our findings and to explore the potential cost savings associated with the use of Rituximab biosimilars.
5. Conclusions
In conclusion, our study demonstrates that the transition from IV Rituximab biosimilar to SC Rituximab Mabthera is a well-tolerated and safe practice. This approach can potentially improve patient convenience and reduce healthcare resource utilization. We recommend that this practice be implemented on a larger scale and in other institutions to further validate our findings and to explore the potential benefits of Rituximab biosimilars in clinical practice.
Author Contributions
Conceptualization, T.A., M.A.K., A.A., M.B.A., M.A.A., A.A. M.A. and A.A.; methodology, T.A., M.A.K., A.A., M.B.A., M.A.A. and M.A.; software, T.A., M.A.K., A.A., M.B.A., M.A.A.; validation, T.A., M.A.K., A.A., M.B.A., M.A.A. and M.A.; formal analysis, T.A., M.A.K., A.A., M.B.A., M.A.A.; investigation, T.A., M.A.K., A.A., M.B.A., M.A.A; resources, T.A., and M.A.K.; data curation, T.A., M.A.K, A.A., M.A.A; writing—original draft preparation, T.A., M.A.K., A.A., M.B.A., M.A.A; writing—review and editing, T.A., M.A.K., A.A., M.B.A., M.A.A., M.A., A.A. M.A. and A.A.; visualization, T.A., M.A.K., M.A.A.; supervision, M.A.K; project administration, M.A.K., M.A., A.A., M.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Ethical approval was received from King Abdullah International Medical Research Center, Jeddah, Saudi Arabia (IRB No. IRB/1539/23).
Informed Consent Statement
Data were collected retrospectively; therefore, informed consent was waived, as it was considered exempt.
Data Availability Statement
The data are available upon reasonable request from the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Khan MA, Aseeri MA, Alshamrani MA, Alnatsheh AH, Alhamdan HS. Emerging Role of Biosimilars in Oncology-Hematology in Saudi Arabia: A Practical Perspective. Global Journal on Quality and Safety in Healthcare [Internet]. 2019 Feb 1 [cited 2025 Apr 4];3(1):22. Available from: https://pmc.ncbi.nlm.nih.gov/articles/PMC10335781/.
- Almutairi AR, Al-Samil AM, Alsayyari A, Yousef CC, Khan MA, Alhamdan HS, et al. The landscape of biosimilars in Saudi Arabia: preparing for the next decade. Expert Opin Biol Ther [Internet]. 2023 [cited 2025 Apr 6];23(8):679–88. Available from: https://pubmed.ncbi.nlm.nih.gov/37503858/.
- Yousef CC, Khan MA, Almodaimegh H, Alshamrani M, Al-Foheidi M, AlAbdalkarim H, et al. Cost-efficiency analysis of conversion to biosimilar filgrastim for supportive cancer care and resultant expanded access analysis to supportive care and early-stage HER2+ breast cancer treatment in Saudi Arabia: simulation study. J Med Econ [Internet]. 2023 [cited 2025 Apr 4];26(1):394–402. Available from: https://pubmed.ncbi.nlm.nih.gov/36815700/.
- Salles G, Barrett M, Foà R, Maurer J, O’Brien S, Valente N, et al. Rituximab in B-Cell Hematologic Malignancies: A Review of 20 Years of Clinical Experience. Adv Ther [Internet]. 2017 Oct 1 [cited 2025 Apr 4];34(10):2232–73. Available from: https://pubmed.ncbi.nlm.nih.gov/28983798/.
- FDA approves Truxima as biosimilar to Rituxan for non-Hodgkin’s lymphoma | FDA [Internet]. [cited 2025 Apr 6]. Available from: https://www.fda.gov/drugs/fda-approves-truxima-biosimilar-rituxan-non-hodgkins-lymphoma.
- RUXIENCETM (rituximab-pvvr) Prescribing Information [Internet]. [cited 2025 Apr 6]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/761103s000lbl.pdf.
- RIABNITM (rituximab-arrx) Prescribing Information [Internet]. [cited 2025 Apr 6]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/761140s000lbl.pdf.
- Welslau M, Kubuschok B, Topaly J, Otremba B, Wolff T, Bryn G. REFLECT: prospective multicenter non-interventional study evaluating the effectiveness and safety of Sandoz rituximab (SDZ-RTX; Rixathon®) in combination with CHOP for the treatment of patients with previously untreated CD20-positive diffuse large B-cell lymphoma. Ther Adv Hematol [Internet]. 2023 Jan 1 [cited 2025 Apr 4];14. Available from: https://pubmed.ncbi.nlm.nih.gov/37492394/.
- Jurczak W, Moreira I, Kanakasetty GB, Munhoz E, Echeveste MA, Giri P, et al. Rituximab biosimilar and reference rituximab in patients with previously untreated advanced follicular lymphoma (ASSIST-FL): primary results from a confirmatory phase 3, double-blind, randomised, controlled study. Lancet Haematol [Internet]. 2017 Aug 1 [cited 2025 Apr 4];4(8):e350–61. Available from: https://pubmed.ncbi.nlm.nih.gov/28712941/.
- Davies A, Merli F, Mihaljević B, Mercadal S, Siritanaratkul N, Solal-Céligny P, et al. Efficacy and safety of subcutaneous rituximab versus intravenous rituximab for first-line treatment of follicular lymphoma (SABRINA): a randomised, open-label, phase 3 trial. Lancet Haematol [Internet]. 2017 Jun 1 [cited 2025 Apr 6];4(6):e272–82. Available from: https://pubmed.ncbi.nlm.nih.gov/28476440/.
- Lugtenburg P, Avivi I, Berenschot H, Ilhan O, Marolleau JP, Nagler A, et al. Efficacy and safety of subcutaneous and intravenous rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone in first-line diffuse large B-cell lymphoma: the randomized MabEase study. Haematologica [Internet]. 2017 Oct 27 [cited 2025 Apr 6];102(11):1913–22. Available from: https://pubmed.ncbi.nlm.nih.gov/28935843/.
- MabThera summary of product characteristics [Internet]. [cited 2025 Apr 6]. Available from: https://www.ema.europa.eu/en/documents/product-information/mabthera-epar-product-information_en.pdf.
- Hill SL, Davies A. Subcutaneous Rituximab with Recombinant Human Hyaluronidase in the Treatment of Non-Hodgkin Lymphoma and Chronic Lymphocytic Leukemia. Future Oncology [Internet]. 2018 Jul 1 [cited 2025 Apr 6];14(17):1691–9. Available from: https://www.tandfonline.com/doi/abs/10.2217/fon-2017-0574.
- Assouline S, Buccheri V, Delmer A, Gaidano G, Trneny M, Berthillon N, et al. Pharmacokinetics, safety, and efficacy of subcutaneous versus intravenous rituximab plus chemotherapy as treatment for chronic lymphocytic leukaemia (SAWYER): a phase 1b, open-label, randomised controlled non-inferiority trial. Lancet Haematol [Internet]. 2016 Mar 1 [cited 2025 Apr 6];3(3):e128–38. Available from: https://www.thelancet.com/action/showFullText?pii=S2352302616000041.
- Davies A, Berge C, Boehnke A, Dadabhoy A, Lugtenburg P, Rule S, et al. Subcutaneous Rituximab for the Treatment of B-Cell Hematologic Malignancies: A Review of the Scientific Rationale and Clinical Development. Adv Ther [Internet]. 2017 Oct 1 [cited 2025 Apr 6];34(10):2210. Available from: https://pmc.ncbi.nlm.nih.gov/articles/PMC5656720/.
- Si T, Ma X, Zhu W, Zhou Y. Clinical efficacy and safety of subcutaneous rituximab in non-Hodgkin lymphoma: a systematic literature review and meta-analysis. Hematology [Internet]. 2023 Dec 31 [cited 2025 Apr 6];28(1). Available from: https://www.tandfonline.com/doi/abs/10.1080/16078454.2023.2284047.
- Islami MM, Khan MA, Aseeri MA, Alshamrani MA, Alnatsheh A, Alamoudi S, et al. Comparison of Biosimilar Filgrastim with Innovator Fligrastim for Peripheral Blood Stem Cells Mobilization, Collection of CD34+ Stem Cells, and Engraftment in Patients Undergoing Autologous and Allogeneic Stem Cell Transplantation: A Single-Center Experience. Ann Transplant [Internet]. 2023 [cited 2025 Apr 6];28. Available from: https://pubmed.ncbi.nlm.nih.gov/36864713/.
- Biosimilar Product Regulatory Review and Approval [Internet]. [cited 2025 Apr 7]. Available from: https://www.fda.gov/files/drugs/published/Biosimilar-Product-Regulatory-Review-and-Approval.pdf.
- Ismail S, Abu Esba L, Khan M, Al-Abdulkarim H, Modimagh H, Yousef C. An Institutional Guide for Formulary Decisions of Biosimilars. Hosp Pharm [Internet]. 2022 Feb 1 [cited 2025 Apr 6];58(1):38. Available from: https://pmc.ncbi.nlm.nih.gov/articles/PMC9837324/.
- Common Terminology Criteria for Adverse Events (CTCAE) | Protocol Development | CTEP [Internet]. [cited 2025 Apr 6]. Available from: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/ctc.htm#ctc_50.
- Ogura M, Sancho JM, Cho SG, Nakazawa H, Suzumiya J, Tumyan G, et al. Efficacy, pharmacokinetics, and safety of the biosimilar CT-P10 in comparison with rituximab in patients with previously untreated low-tumour-burden follicular lymphoma: a randomised, double-blind, parallel-group, phase 3 trial. Lancet Haematol [Internet]. 2018 Nov 1 [cited 2025 Apr 6];5(11):e543–53. Available from: https://pubmed.ncbi.nlm.nih.gov/30389036/.
- Lenz HJ. Management and preparedness for infusion and hypersensitivity reactions. Oncologist [Internet]. 2007 May 1 [cited 2025 Apr 8];12(5):601–9. Available from: https://pubmed.ncbi.nlm.nih.gov/17522249/.
- Kasi PM, Tawbi HA, Oddis C V., Kulkarni HS. Clinical review: Serious adverse events associated with the use of rituximab - a critical care perspective. Crit Care [Internet]. 2012 Aug 31 [cited 2025 Apr 8];16(4). Available from: https://pubmed.ncbi.nlm.nih.gov/22967460/.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).