Submitted:
12 May 2025
Posted:
12 May 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
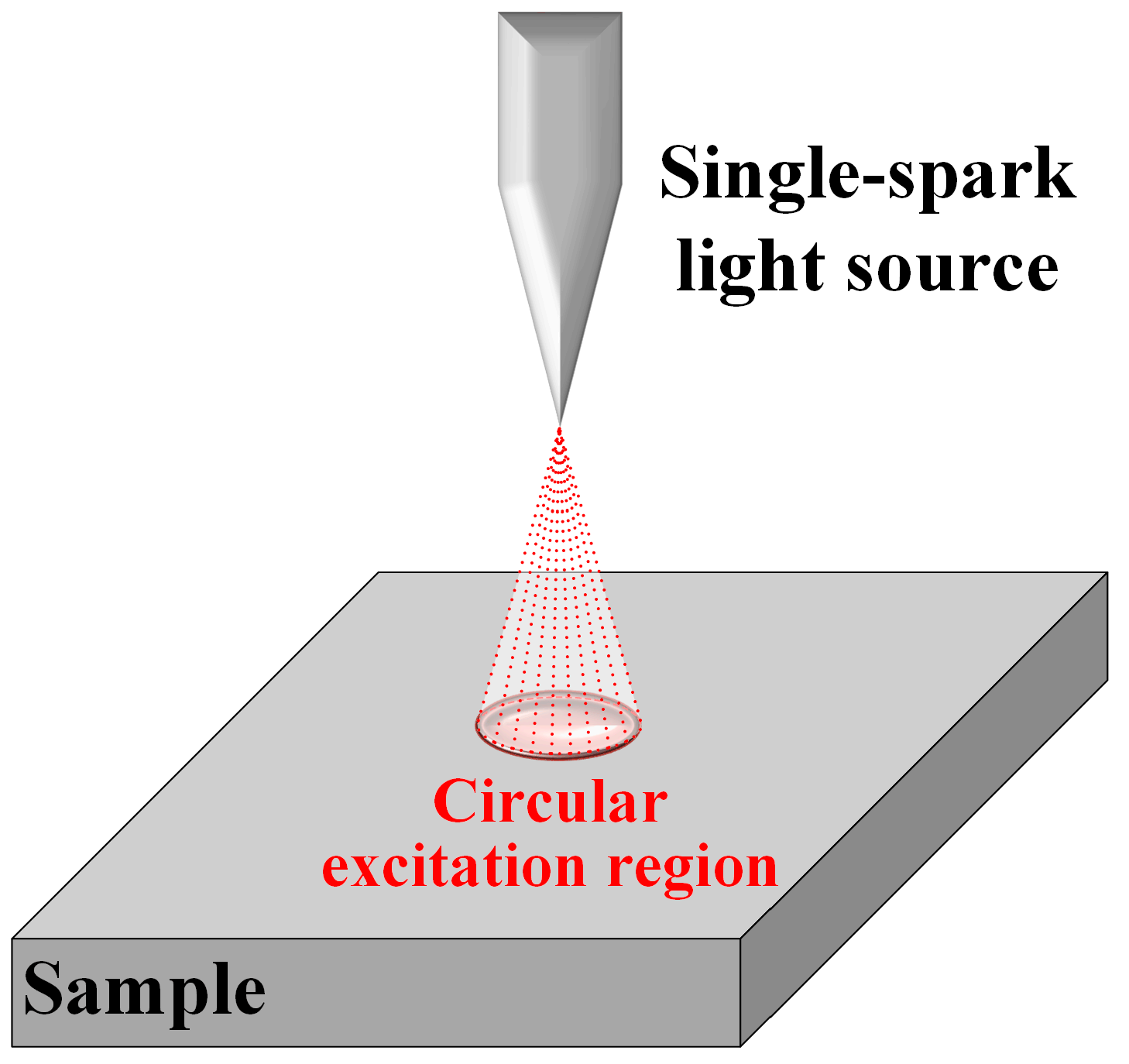
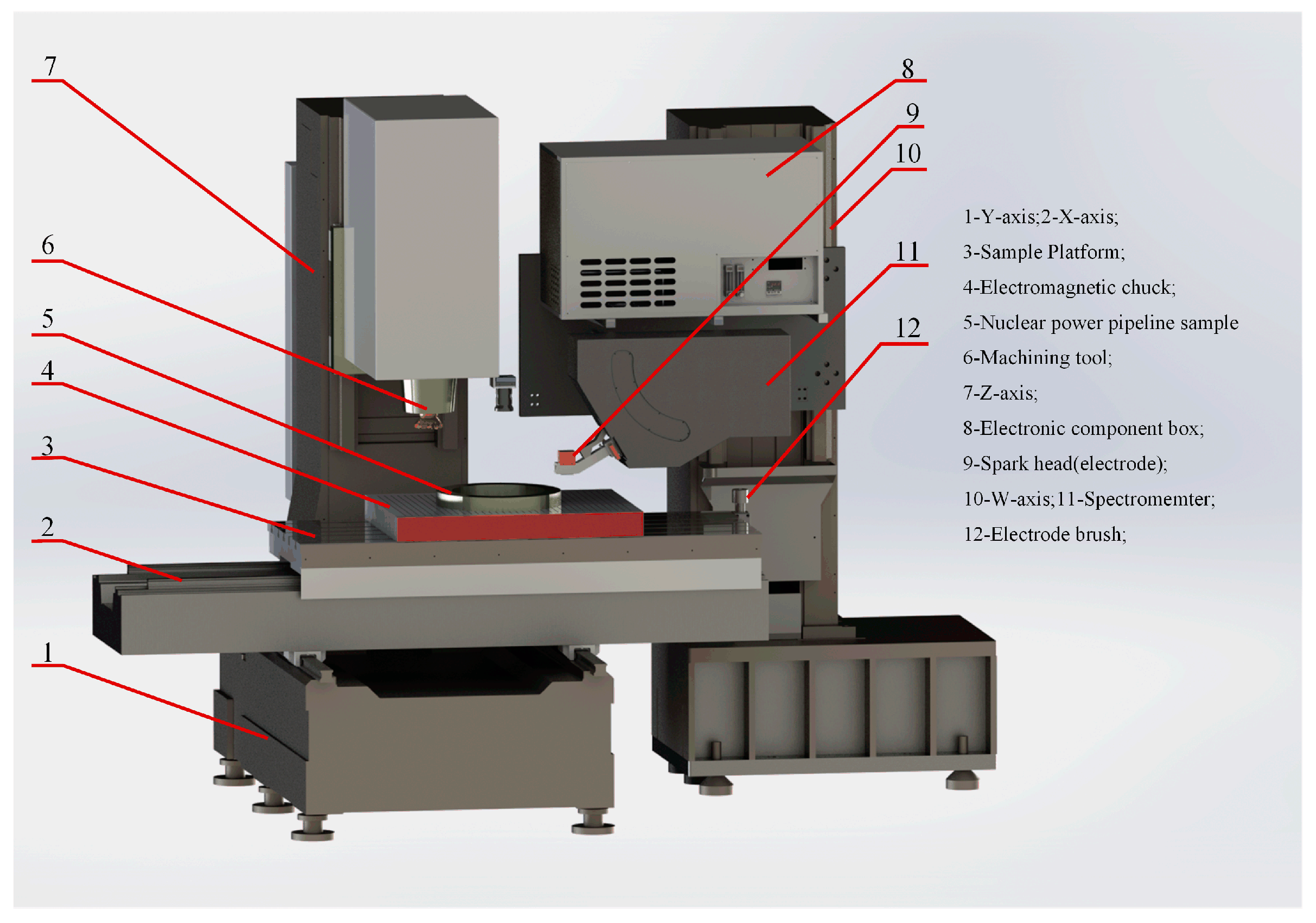
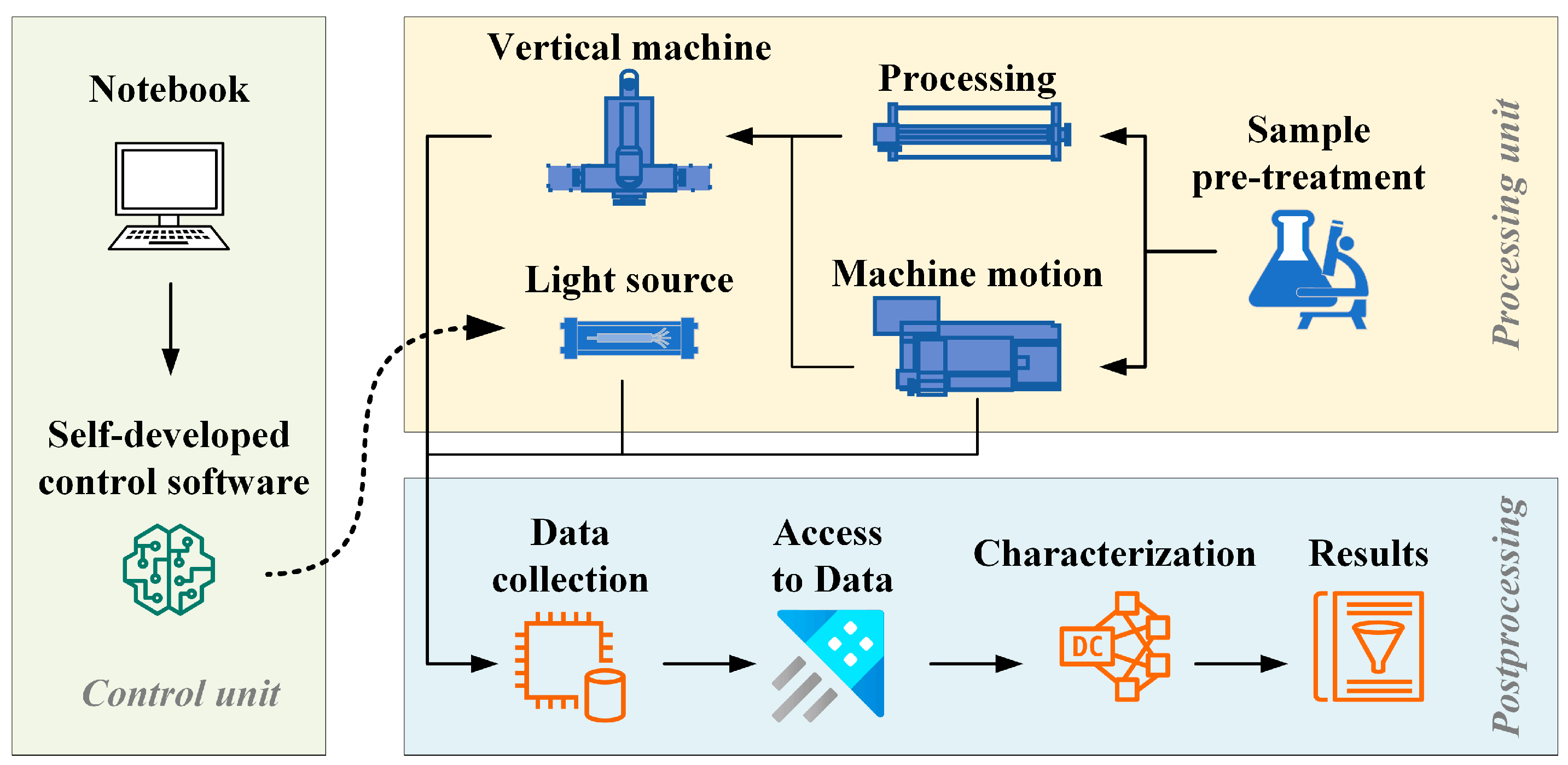
2.1. Equipment
2.2. Samples
2.3. Calibration Curve
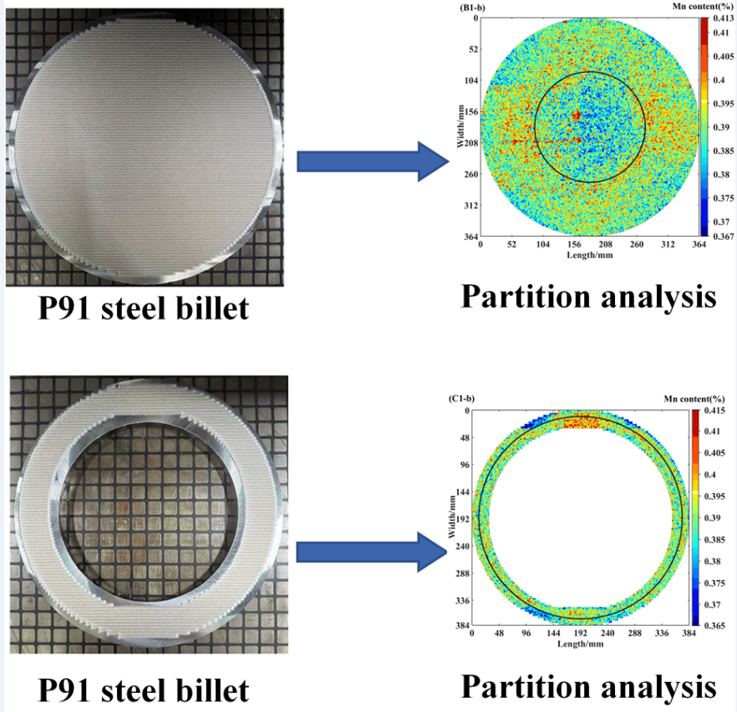
2.4. Partition Analysis Method
3. Results and Discussion
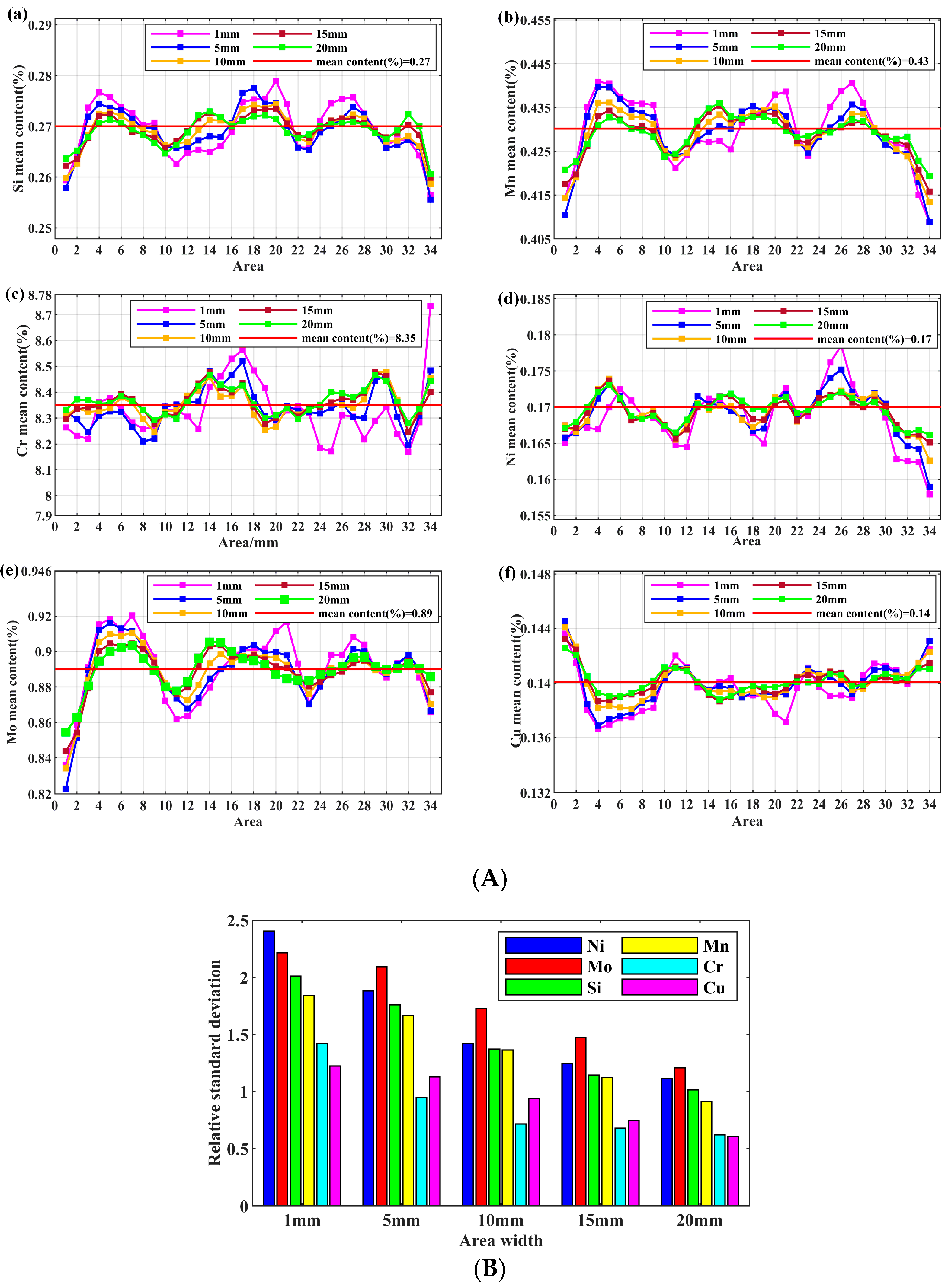
3.1. The Fluctuation Effect on the Different Scanning Areas
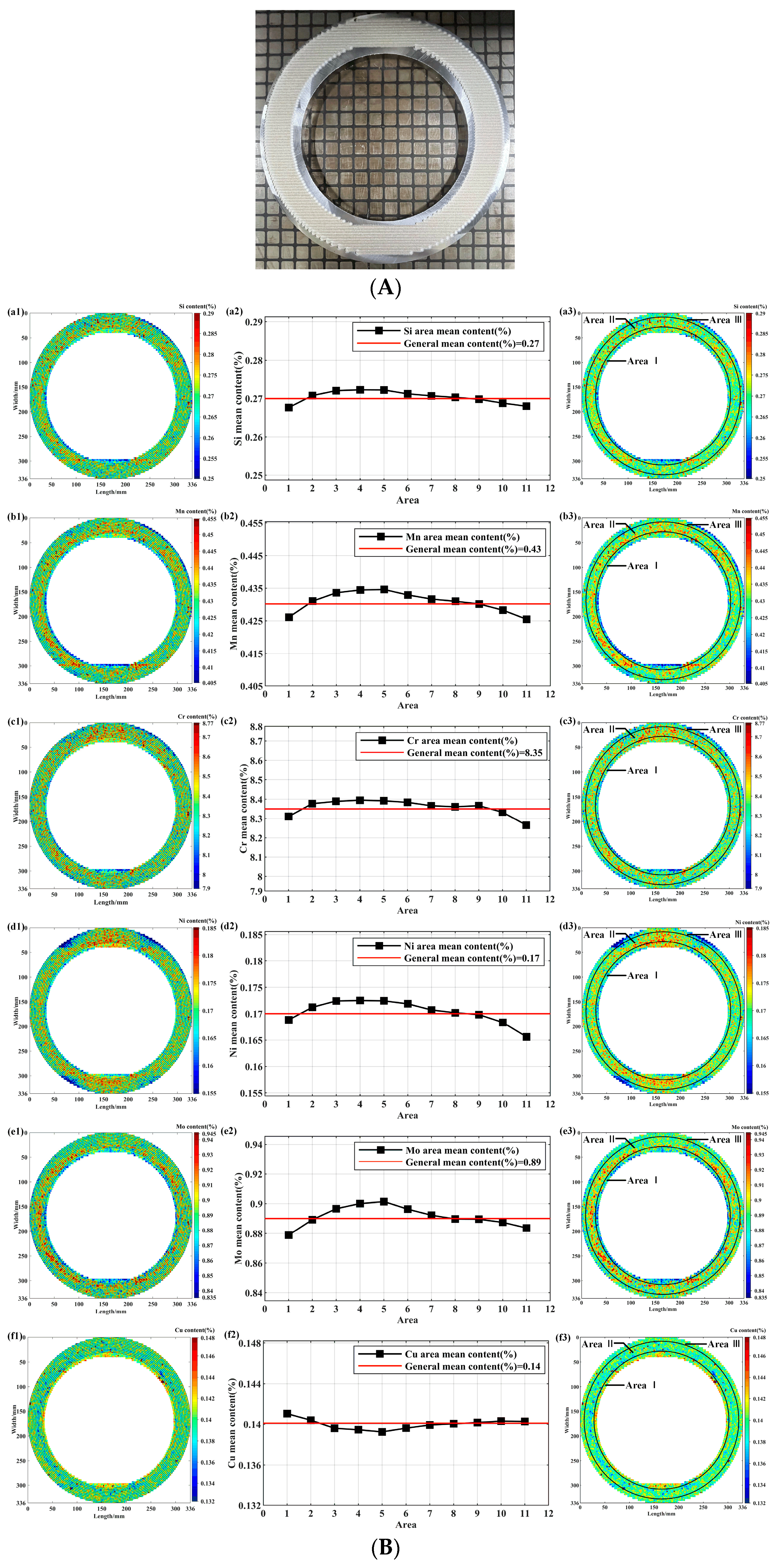
3.2. Data Collection and Analysis of Complete Samples
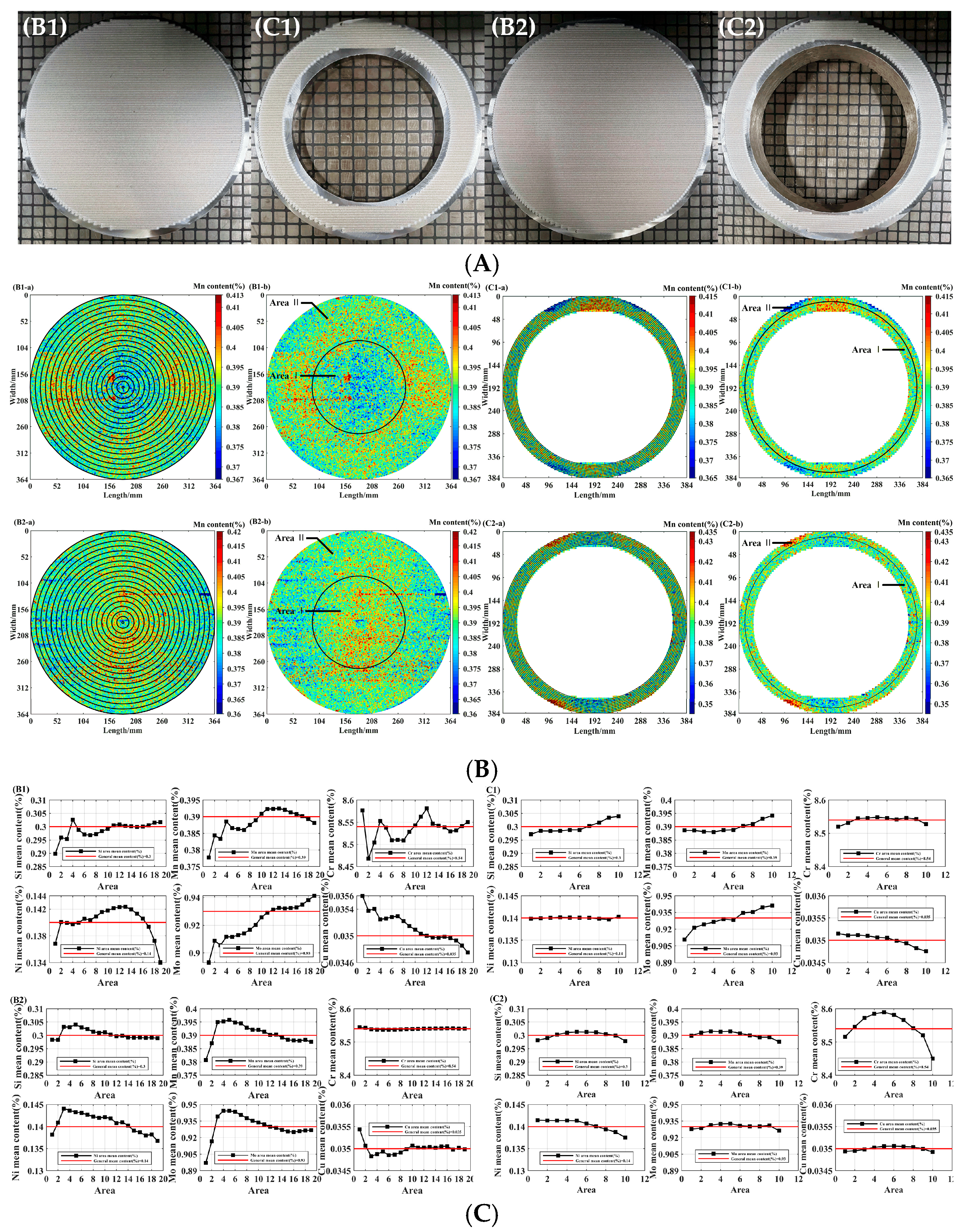
3.3. The Fluctuation Effect on the Different Scanning Areas
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Rerak, M.; Ocłoń, P. Thermal Analysis of Underground Power Cable System. J. Therm. Sci. 2017, 26, 465–471. [CrossRef]
- Pandey, C.; Mahapatra, M.M.; Kumar, P.; Saini, N. Some Studies on P91 Steel and Their Weldments. Journal of Alloys and Compounds 2018, 743, 332–364. [CrossRef]
- Han, K.; Ding, H.; Fan, X.; Li, W.; Lv, Y.; Feng, Y. Study of the Creep Cavitation Behavior of P91 Steel under Different Stress States and Its Effect on High-Temperature Creep Properties. Journal of Materials Research and Technology 2022, 20, 47–59. [CrossRef]
- Maddi, L.; Ballal, A.R.; Peshwe, D.R.; Mathew, M.D. Influence of Normalizing and Tempering Temperatures on the Creep Properties of P92 Steel. High Temperature Materials and Processes 2020, 39, 178–188. [CrossRef]
- Ge, H.; Ren, F.; Li, J.; Hu, Q.; Xia, M.; Li, J. Modelling of Ingot Size Effects on Macrosegregation in Steel Castings. Journal of Materials Processing Technology 2018, 252, 362–369. [CrossRef]
- Zheng, P.; Luo, Y.; Wang, J.; Yang, Y.; Hu, Q.; Mao, X.; Lai, C. Improved Solution Cathode Glow Discharge Micro-Plasma Source with a Geometrically Optimized Stainless Steel Auxiliary Cathode for Optical Emission Spectrometry of Metal Elements. Microchemical Journal 2022, 172, 106883. [CrossRef]
- Adya, V.C.; Sengupta, A.; Thulasidas, S.K.; Natarajan, V. Direct Determination of S and P at Trace Level in Stainless Steel by CCD-Based ICP-AES and EDXRF: A Comparative Study. At.Spectrosc. 2016, 37, 19–24. [CrossRef]
- Liu, R.; Rong, K.; Wang, Z.; Cui, M.; Deguchi, Y.; Tanaka, S.; Yan, J.; Liu, J. Sample Temperature Effect on Steel Measurement Using SP-LIBS and Collinear Long-Short DP-LIBS. ISIJ Int. 2020, 60, 1724–1731. [CrossRef]
- Quackatz, L.; Griesche, A.; Kannengiesser, T. Spatially Resolved EDS, XRF and LIBS Measurements of the Chemical Composition of Duplex Stainless Steel Welds: A Comparison of Methods. Spectrochimica Acta Part B: Atomic Spectroscopy 2022, 193, 106439. [CrossRef]
- Kimura, K.; Kwak, K.; Nambu, S.; Koseki, T. Nondestructive Evaluation of Macro Segregation in Creep Strength Enhanced 9Cr–1Mo–V–Nb Steel. Scripta Materialia 2020, 188, 179–182. [CrossRef]
- Sheng, L.; Yuan, L.; Jia, Y.; Zhao, L.; Zhang, X.; Yu, L.; Zhang, Q.; Wang, H. Full-Scale Spark Mapping of Elements and Inclusions of a High-Speed Train Axle Billet. J. Anal. At. Spectrom. 2022, 37, 1522–1534. [CrossRef]
- Zhang, X.; Jia, Y.; Sheng, L.; Yuan, L.; Li, J. Characterization of Segregation Degree for Large Size Metal Component and Application on High-Speed Train Wheel. Analytica Chimica Acta 2022, 1203, 339719. [CrossRef]
- Wang, H.; Zhao, L.; Jia, Y.; Li, D.; Yang, L.; Lu, Y.; Feng, G.; Wan, W. State-of-the-Art Review of High-Throughput Statistical Spatial-Mapping Characterization Technology and Its Applications. Engineering 2020, 6, 621–636. [CrossRef]
- Li, J.; Xu, X.; Ren, N.; Xia, M.; Li, J. A Review on Prediction of Casting Defects in Steel Ingots: From Macrosegregation to Multi-Defect Model. J. Iron Steel Res. Int. 2022, 29, 1901–1914. [CrossRef]
- Shen, Y.; Yang, S.; Liu, J.; Liu, H.; Zhang, R.; Xu, H.; He, Y. Study on Micro Segregation of High Alloy Fe–Mn–C–Al Steel. steel research int. 2019, 90, 1800546. [CrossRef]
- He, Q.; Wu, H.; Meng, H.; Hu, Z.; Xie, Z. Molten Steel Level Detection by Temperature Gradients With a Neural Network. IEEE Access 2019, 7, 69456–69463. [CrossRef]
- Zhang, Y.; Yi, R.; Wang, P.; Fu, C.; Cai, N.; Ju, J. Self-Piercing Riveting of Hot Stamped Steel and Aluminum Alloy Sheets Base on Local Softening Zone. steel research int. 2021, 92, 2000535. [CrossRef]
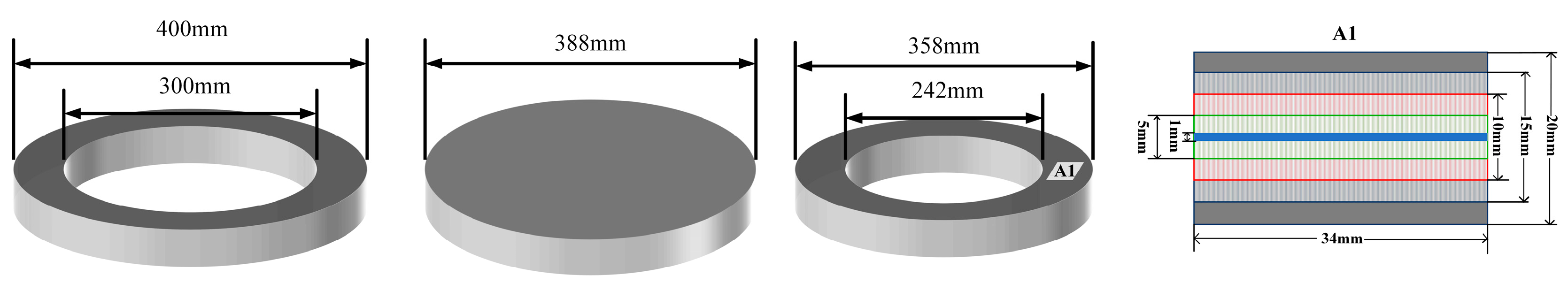
| Sample | Specification | Elemental content | |||||
| Si | Mn | Cr | Ni | Mo | Cu | ||
| A | 358×242×58 | 0.27 | 0.43 | 8.35 | 0.17 | 0.89 | 0.14 |
| B1 | ϕ388 | 0.30 | 0.39 | 8.54 | 0.14 | 0.93 | 0.035 |
| C1 | 400×300×50 | ||||||
| B2 | ϕ388 | ||||||
| C2 | 400×300×50 | ||||||
| Element | Spectral lines(nm) | Content range (%) | Calibration curve | R2 |
| Si | 212.41 | 0.103~1.05 | 0.00035 × I2 + 0.027 × I − 0.053 | 0.994 |
| Mn | 293.30 | 0.126~1.96 | 0.010 × I2 + 0.0066 × I − 0.0019 | 0.993 |
| Cr | 267.71 | 7.4~24.1 | 1.39 × I2 − 0.21 × I − 0.000039 | 0.992 |
| Ni | 218.49 | 0.062~1.432 | 0.015 × I2 + 0.010 × I − 0.019 | 0.997 |
| Mo | 281.61 | 0.089~1.01 | −0.015 × I2 + 0.043 × I − 0.011 | 0.991 |
| Cu | 233.01 | 0.061~0.69 | 0.0018 × I2 + 0.0012 × I − 0.00087 | 0.999 |
| Elements | General mean content(%) | Area | Area mean content(%) | DS(-)(%) | DS(+)(%) | DS(max)(%) | Statistical fitting degree(%) | Specification range (%) | Standard deviation(%) |
| Si | 0.27 | Ⅰ | 0.268 | -4.45 | 5.79 | 16.61 | 100.00 | 0.20~0.50 | 0.0080 |
| Ⅱ | 0.271 | -3.55 | 4.62 | 100.00 | 0.0060 | ||||
| Ⅲ | 0.269 | -4.06 | 5.10 | 100.00 | 0.0070 | ||||
| Mn | 0.43 | Ⅰ | 0.427 | -3.76 | 4.24 | 13.47 | 100.00 | 0.30~0.60 | 0.0090 |
| Ⅱ | 0.433 | -2.92 | 3.65 | 100.00 | 0.0080 | ||||
| Ⅲ | 0.427 | -3.61 | 3.65 | 100.00 | 0.0080 | ||||
| Cr | 8.35 | Ⅰ | 8.325 | -3.31 | 3.68 | 3.53 | 98.98 | 8.00~9.50 | 0.15 |
| Ⅱ | 8.381 | -2.93 | 3.18 | 99.89 | 0.13 | ||||
| Ⅲ | 8.309 | -3.45 | 3.39 | 98.26 | 0.15 | ||||
| Ni | 0.17 | Ⅰ | 0.169 | -5.27 | 6.22 | 20.47 | 100.00 | ≤0.40 | 0.0049 |
| Ⅱ | 0.172 | -4.89 | 5.00 | 100.00 | 0.0043 | ||||
| Ⅲ | 0.167 | -8.13 | 5.93 | 100.00 | 0.0057 | ||||
| Mo | 0.89 | Ⅰ | 0.881 | -4.16 | 4.34 | 9.7 | 95.38 | 0.85~1.05 | 0.019 |
| Ⅱ | 0.895 | -3.61 | 4.20 | 99.72 | 0.018 | ||||
| Ⅲ | 0.886 | -3.47 | 3.66 | 98.87 | 0.016 | ||||
| Cu | 0.14 | Ⅰ | 0.141 | -2.65 | 2.81 | 22.34 | 99.98 | ≤0.20 | 0.0031 |
| Ⅱ | 0.140 | -2.48 | 2.43 | 100.00 | 0.0023 | ||||
| Ⅲ | 0.140 | -2.34 | 2.29 | 99.98 | 0.0028 |
| Element | General mean content(%) | Sample | Area | Mean content(%) | DS(-)(%) | DS(+)(%) | DS(max) (%) | Statistical fitting degree(%) | Specification range (%) | Standard deviation (%) |
| Si | 0.3 | B1 | Ⅰ | 0.298 | -3.87 | 6.52 | 15.84 | 99.96 | 0.20~0.40 | 0.0087 |
| Ⅱ | 0.301 | -3.41 | 4.57 | 100.00 | 0.0062 | |||||
| C1 | Ⅰ | 0.298 | -8.95 | 9.59 | 100.00 | 0.014 | ||||
| Ⅱ | 0.302 | -9.31 | 10.90 | 100.00 | 0.015 | |||||
| B2 | Ⅰ | 0.302 | -3.72 | 6.32 | 100.00 | 0.0077 | ||||
| Ⅱ | 0.299 | -3.81 | 6.68 | 99.98 | 0.0084 | |||||
| C2 | Ⅰ | 0.301 | -3.62 | 3.73 | 100.00 | 0.0057 | ||||
| Ⅱ | 0.300 | -4.36 | 4.26 | 99.98 | 0.0074 | |||||
| Mn | 0.39 | B1 | Ⅰ | 0.388 | -3.77 | 4.52 | 14.07 | 100.00 | 0.30~0.50 | 0.0083 |
| Ⅱ | 0.391 | -3.47 | 3.89 | 100.00 | 0.0074 | |||||
| C1 | Ⅰ | 0.392 | -6.99 | 8.35 | 99.99 | 0.015 | ||||
| Ⅱ | 0.392 | -6.99 | 8.35 | 99.99 | 0.015 | |||||
| B2 | Ⅰ | 0.393 | -4.36 | 5.03 | 100.00 | 0.0094 | ||||
| Ⅱ | 0.389 | -4.42 | 5.20 | 99.99 | 0.01 | |||||
| C2 | Ⅰ | 0.391 | -3.78 | 4.10 | 100.00 | 0.0078 | ||||
| Ⅱ | 0.389 | -4.55 | 4.17 | 99.99 | 0.0086 | |||||
| Cr | 8.54 | B1 | Ⅰ | 8.524 | -4.65 | 5.8 | 3.50 | 99.47 | 8.00~9.50 | 0.23 |
| Ⅱ | 8.545 | -4.52 | 5.37 | 99.68 | 0.22 | |||||
| C1 | Ⅰ | 8.540 | -2.11 | 2.35 | 100.00 | 0.097 | ||||
| Ⅱ | 8.539 | -2.07 | 2.33 | 100.00 | 0.096 | |||||
| B2 | Ⅰ | 8.538 | -0.11 | 0.10 | 100.00 | 0.0047 | ||||
| Ⅱ | 8.541 | -0.11 | 0.10 | 100.00 | 0.0051 | |||||
| C2 | Ⅰ | 8.571 | -3.96 | 4.50 | 99.89 | 0.18 | ||||
| Ⅱ | 8.508 | -5.27 | 4.64 | 98.36 | 0.21 | |||||
| Ni | 0.14 | B1 | Ⅰ | 0.141 | -4.57 | 4.66 | 22.34 | 100.00 | 0~0.20 | 0.0033 |
| Ⅱ | 0.140 | -10.05 | 5.99 | 100.00 | 0.0053 | |||||
| C1 | Ⅰ | 0.140 | -3.14 | 3.18 | 100.00 | 0.0023 | ||||
| Ⅱ | 0.140 | -2.95 | 2.97 | 100.00 | 0.0021 | |||||
| B2 | Ⅰ | 0.143 | -11.34 | 12.05 | 100.00 | 0.0085 | ||||
| Ⅱ | 0.139 | -12.09 | 12.49 | 99.99 | 0.0088 | |||||
| C2 | Ⅰ | 0.141 | -7.16 | 9.37 | 100.00 | 0.0059 | ||||
| Ⅱ | 0.139 | -10.63 | 8.56 | 99.99 | 0.0065 | |||||
| Mo | 0.93 | B1 | Ⅰ | 0.917 | -3.22 | 3.82 | 9.51 | 100.00 | 0.85~1.05 | 0.017 |
| Ⅱ | 0.934 | -2.96 | 3.38 | 100.00 | 0.015 | |||||
| C1 | Ⅰ | 0.924 | -11.24 | 11.05 | 91.28 | 0.053 | ||||
| Ⅱ | 0.938 | -12.4 | 13.22 | 89.05 | 0.062 | |||||
| B2 | Ⅰ | 0.938 | -3.25 | 3.33 | 100.00 | 0.016 | ||||
| Ⅱ | 0.927 | -3.09 | 3.50 | 99.99 | 0.016 | |||||
| C2 | Ⅰ | 0.931 | -4.28 | 4.58 | 99.98 | 0.021 | ||||
| Ⅱ | 0.929 | -4.25 | 4.67 | 99.94 | 0.021 | |||||
| Cu | 0.035 | B1 | Ⅰ | 0.0352 | -1.87 | 1.72 | 41.74 | 100.00 | 0~0.10 | 0.00032 |
| Ⅱ | 0.0349 | -2.02 | 1.80 | 100.00 | 0.00034 | |||||
| C1 | Ⅰ | 0.0351 | -4.56 | 4.53 | 100.00 | 0.00082 | ||||
| Ⅱ | 0.0349 | -5.57 | 4.85 | 100.00 | 0.00092 | |||||
| B2 | Ⅰ | 0.0349 | -1.93 | 9.51 | 100.00 | 0.00080 | ||||
| Ⅱ | 0.0350 | -1.85 | 7.43 | 100.00 | 0.00078 | |||||
| C2 | Ⅰ | 0.0350 | -1.26 | 1.24 | 100.00 | 0.00022 | ||||
| Ⅱ | 0.0350 | -1.37 | 1.29 | 100.00 | 0.00024 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





