Submitted:
08 May 2025
Posted:
09 May 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Protocol and Registration
2.2. Eligibility Criteria
2.2.1. Population
2.2.2. Concept
2.2.3. Context
2.3. Information Sources
2.4. Search Strategy

2.5. Selection of Sources of Evidence
2.6. Data Charting, Extraction and Selection Process
2.7. Quality Assessment of Articles and Level of Evidence Assessment
2.8. Meta Analysis
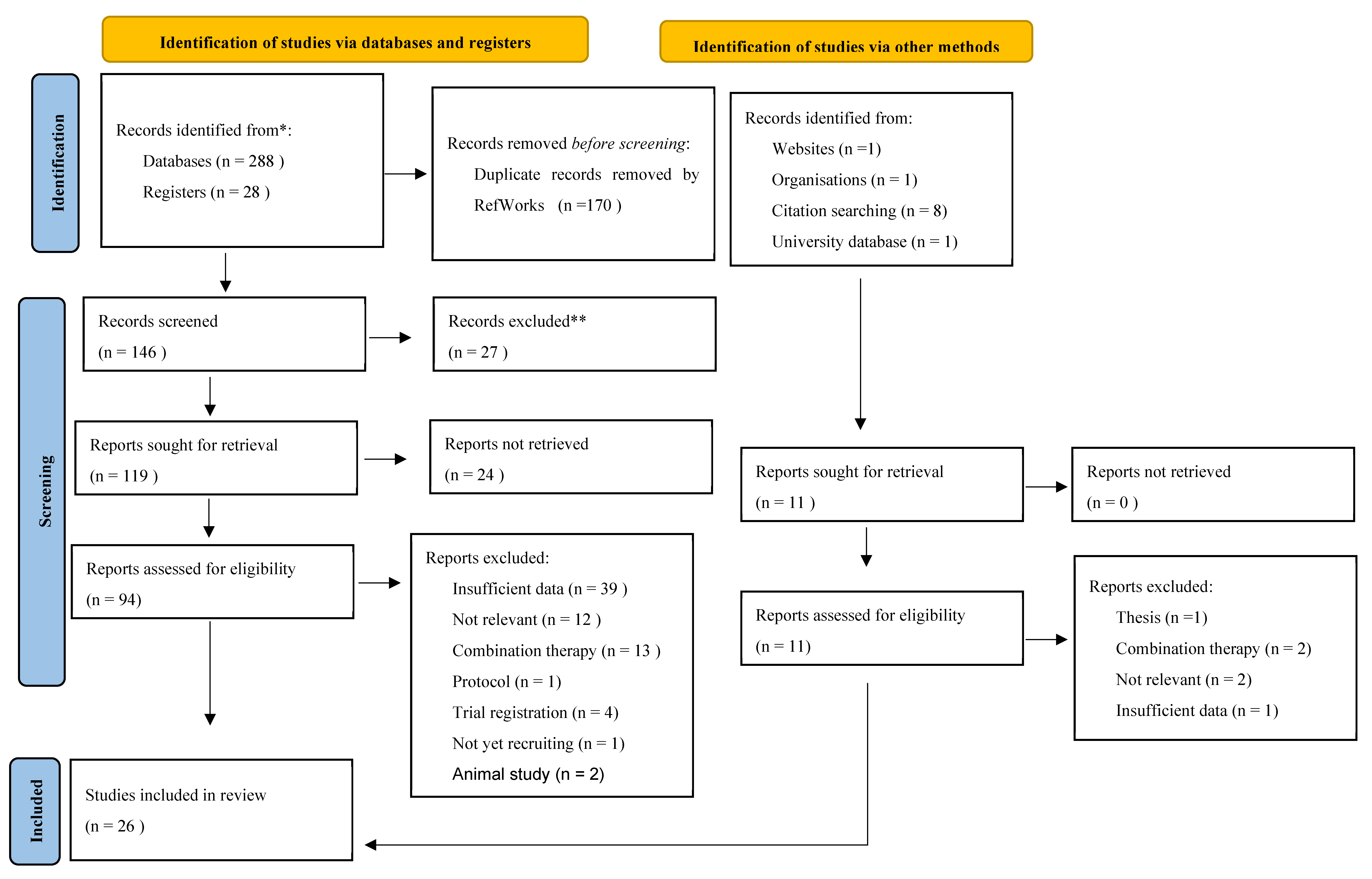
3. Results
3.1. Synthesis of RESULTS
3.2. Selection of Sources of Evidence
3.3. Demographic Summary
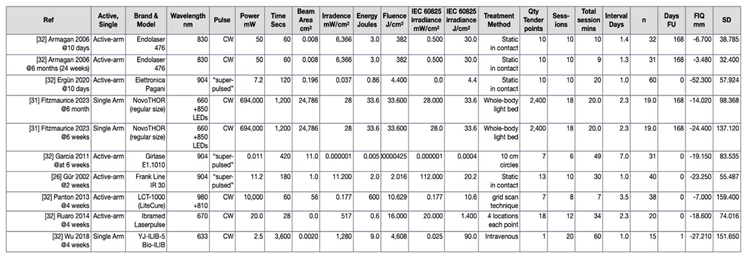
3.4. Descriptive Summary of Interventions (n = 19)
3.5. Summary of Quality Assessment and Level of Evidence Across Interventions (n=19)
3.5. Summary of Outcome Measures Across Interventions (n = 19)
| Paper | Study Type & level of evidence (LoE) | Aims | Demographics | |
|---|---|---|---|---|
| Armagan et al. (2006) [33] | Quantitative RCT LoE:3 |
To investigate efficacy of PBMT in FM |
Gender: 32F PBMT/placebo - Age: 38.94±4.85/37.63±5.90 Pain duration: 5.50±3.03/6.12±3.44 Education: elementary 8/7, high school 5/5, university 3/4 Employment: employed 4/3, not working/retired 4/4, homemaker 8/9 Marital status: married 9/8, single/divorced/widowed 7/8 |
|
| da Silva et al. (2018) [40] | Quantitative Randomized, controlled, blinded LoE:2 |
To evaluate the role of phototherapy and exercise training as well as the combined treatment in general symptoms, pain, and quality of life in women suffering from FM |
Gender: 160F Set 1/Set 2 – Age: 35±3/40±2 Average FM duration: 5±9 BMI: 26±5/27±4 Ethnicity: other or biracial 48/41, white 32/39 Education: elementary 14/9, high school 66/71 Employment: employed or self-employed 52/50, unemployed 28/20 Income (Brazilian Real): <10,000 R$ 9/4, 10,000-30,000 R$ 67/75, 30,000-50,000 R$ 4/2 Drug class (Set 1 and 2): analgesics – paracetamol 109, anti-depressant – amitriptyline 62, fluoxetine 30, citalopram 12, paroxetine 8, duloxetine 58, muscle relaxants – cyclobenzaprine 89, tizanidine 23, carisoprodol 5, hypnotics – benzodiazepines 27 |
|
| de Souza et al. (2018) [42] | Mixed methods: Quantitative, parallel, controlled, randomised. See Table 3 for qualitative LoE:2 |
To compare anaesthetic effect of lidocaine 2% and PBMT by GaAlAs into tender points of patients with orofacial pain and FM |
Gender: 62F 4M Age: 46.14±10.91 FM duration: 76% diagnosed in last 5 years (no differentiation between groups, however, to be no significant difference between groups) |
|
| dos Santos et al. (2020) [32] | Quantitative Randomised, cross-over, clinical-controlled, double-blinded LoE:3 |
To investigate acute effects of PBMT on peripheral muscle strength and resistance in FM patients compared to healthy women |
Gender: 37F FM group/Control group - Age: 44±7/50±8 BMI: 27.69±3.95/29.40±6.37 FM duration: 8 years ±4 TPC: 14±4/not assessed in control group Baseline FIQ: 76±16/not assessed in control group Co-morbidities (n): HTN 7/3, DM 4/0, Osteoporosis 3/0, Heart disease 2/1, Respiratory disease 5/2, Depression 10/1, Other 3/6 |
|
| Ergün et al. (2020) [34] | Quantitative Randomised, placebo-controlled LoE:2 |
To investigate efficiency of PBMT in treatment of clinical symptoms and improvement in QoL in primary FMS |
Gender: 60F PBMT/placebo - Age: 39.4+7.1/40.7+7.3 FM duration: 5.7+5.7/4.5+3.4 |
|
| Fernández García et al. (2011) [35] | Quantitative Randomised, placebo-controlled LoE: 2 |
To assess the effect of the Girlase E1.1010 apparatus on fatigue, sleep difficulties, neck pain, vaginal pain during intercourse and general pain in FM |
Gender: 31F PBMT/placebo - Age: 51.6±6.18/52.4±5.88 FM duration: 4.37±1.41/3.80±1.37 |
|
| Fitzmaurice et al. (2023) [31] | Mixed methods Single-armed, feasibility, embedded qualitative component LoE: 3 |
To investigate the feasibility of whole-body PBMT as a treatment option for reducing pain and pain-related co-morbidities in FM |
Gender: 14F, 5M Age: 47.3±10.9 Ethnicity: Asian/Asian British 5, Black British 1, White British 14 FM duration: 15.6±7.7 BMI: 31.5±5.9 Marital status: married 10, single 6, divorced 1, co-habiting 2, civil partnership 1 Employment: employed full-time 4, employed part-time 1, self-employed 2, unemployed (looking for work) 1, unemployed (not looking for work) 7, sick leave 1, retired 4 Education: some secondary school 1, completed secondary school 2, completed further education (sixth form) 1, higher education 16 Drug class (n): paracetamol 6, anti-inflammatories 4, opioids 17, TCAs 11, SSRIs/SNRIs 11, anticonvulsants 11, anxiolytics 3, sleeping tablet 3, beta blockers 2, migraine prophylaxis and treatment 3, antipsychotic 1 |
|
| Gür et al. (2002) [26] | Quantitative Randomised, placebo-controlled LoE: 3 |
To examine the effectiveness of laser therapy in FM |
Gender: 40F No further demographic breakdown |
|
| Gür et al. (2002) [25] | Quantitative Randomised, placebo-controlled LoE: 2 |
To examine the effectiveness of low power laser and low-dose amitriptyline therapy and to investigate effects of clinical symptoms and QoL in FM |
PBMT/placebo/amitriptyline – Age (years): 30.36±6.91/28.52±6.28/30.14±8.65 FM duration (years): 4.86±4.67/4.63±3.28/4.42±3.14 Gender: 60F 15M Marital status: married 16/15/16, single 5/7/6, divorced 3/2/2, other 1/1/1 Education: elementary 13/14/15, secondary 7/6/6, college/university 5/5/4 Employment: unemployed 2/3/3, employed 3/2/2, retired 1/2/1, homemaker 12/11/11, student 5/5/6, other 2/2/2 |
|
| Molina-Torres et al. (2016) [36] | Quantitative Randomised, single-blinded LoE: 2 |
To investigate therapeutic effects of laser therapy and of an occlusal stabilisation splint for reducing pain and dysfunction and improving quality of sleep in patients with TMD and FM |
Laser/occlusal-splint – Age (years): 51.00±8.32/51.79±7.79 FM duration (%): 1-5 years 3.70/10.71, 6-10 years 29.62/35.71, 11-15 years 44.44/28.57, 16-20 years 14.81/17.85, >20 years 7.40/7.14 TMD duration (%): 1-5 years 22.22/17.85, 6-10 years 37.03/25, 11-15 years 14.81/21.42, 16-20 years 14.81/17.85, >20 years 11.11/17.85 Gender: 55F 3M Profession (%): housewife 70.13/60.71, business 11.11/14.28, administrative staff 18.51/25 Education level (%): primary studies 62.96/67.85, higher education 37.03/32.14 |
|
| Moore & Demchak (2012) [44] | Case report LoE: 4 |
Gender: 1F Age: 19 Ethnicity: Hispanic Employment/Education: college student |
||
| Navarro-Ledesma (2022) [27] | Quantitative Randomised, placebo-controlled, triple blinded LoE: 2 |
To analyse changes in BP values, pain pressure threshold (PPT) and elastic properties of tissue in FM after whole-body PBMT |
Gender: 40F Age: 52.8±7.90 BMI: 29.40±6.36 FM duration: 8.90±2.77 SSS: 8.55±1.29 WPI: 8.13±2.55 Menopausal status: pre = 28, post = 12 |
|
| Navarro-Ledesma et al. (2023) [27] | Quantitative Randomised, placebo-controlled, triple blinded LoE: 2 |
To compare the effects of whole-body PBM on pain, functionality and psychological symptoms in FM |
Gender: 44F Age: 52.83±8.04 BMI: 29.32±6.21 Menopausal status: pre = 29 post = 13 |
|
| Navarro-Ledesma et al. (2024) [29] | Quantitative Prospective, randomised, triple-blinded placebo-controlled LoE: 2 |
To conduct a comparative analysis of effects of whole-body PBM and placebo PBM on pain, functionality and psychological symptoms in FM (6m follow up data) |
Gender: 44F (repeat sample) Age: 52.83±8.04 BMI: 29.32±6.21 Menopausal status: pre = 29 post = 13 (same population as above 2 studies) |
|
| Panton et al. (2013) [37] | Quantitative Randomised, placebo-controlled, double- Blinded LoE: 2 |
To examine the effects of Class IV infrared, therapeutic laser therapy on pain, FM impact and physical function in women with FM |
Gender: 38F, 36 Caucasian, 2 African American Laser + heat/Sham + heat – Age: 52±12/54±11 FM duration: 10±8/11±7 BMI: 31.0±10.0/30.2±6.7 |
|
| Ruaro et al. (2014) [45] | Quantitative Randomised, placebo-controlled LoE: 3 |
To evaluate the effects of PBMT in FM |
PBMT/placebo - Gender: 19F, 1M Age: 43.4/39.4 |
|
| Tramontana et al. (2017) [38] | Pilot, single-blinded, placebo-controlled LoE: 3 |
To evaluate integrative approaches for FM in Physical and Rehabilitation Medicine as an alternative or complementary approach to standard care |
Laser/placebo Gender: 8F, 2M |
|
| White et al. (2018) [41] | Case-based review LoE: 4 |
To evaluated low, intermediate and high level HILT in a patient with longstanding FM |
Gender: 1F Age: 67 FM duration: 7 years Employment: retired veterinarian |
|
| Wu et al. (2018) [39] | Quantitative, single arm (design not stipulated in methods) LoE: 3 |
To investigate clinical effects of intravenous laser irradiation of blood on pain, sleep, mood disorders, QoL in FM |
Gender: 15F Age: 53.77 BMI: 24.30 FM duration: 6.33 years |
|
| Armagan et al. (2006) [33] (Turkey) |
BRIEF NAME: PBMT WHAT: Gal-Al-As diode laser device (Endolaser 476, EnrafNonius,Netherlands) WHO: Assessment by blinded independent physician, all patients treated by same physician WHERE: Physical Therapy and Rehabilitation Dept, Osmangazi University Hospital MODIFICATIONS: None documented HOW WELL: Feasibility/acceptability aspect of this trial/treatment not documented in terms of adherence to a treatment schedule etc “No systemic or local side effects were reported during or after the treatment period.” WHEN: One treatment/day 5d/week, Total = 10 treatments HOW MUCH: Power output = 50 mW; Wavelength = 830 nm; Dose = 2J/tender point; Mode = continuous; 1mm diameter laser beam at each treatment point TAILORING: PBMT - 1 minute at each tender point considered to be one irradiation dose; Placebo - same laser device seemingly working, but with no laser beams transferring to treated area. All painful points irradiated |
| da Silva et al. (2018) [40] (Brazil) |
BRIEF NAME: Photobiomodulation therapy, exercise training WHAT: Multiple light sources (PBMT + LED) Pain Away/PainCure TM 9-diode cluster device (Multi Radiance Medical®, Solon, OH, USA) WHO: Randomisation by independent researcher. Independent research programmed device (on or off). 2nd researcher guided exercise training (blinding to PBM/placebo). Independent assistant controlled PBM for on/off mode. 3rd researcher blinded to all allocations assessed outcomes. 4th research for statistical analysis (blinded) WHERE: 3 Rheumatology centres MODIFICATIONS: None documented HOW WELL: No dropouts following randomisation. No harm or unintended outcomes reported WHEN: Single treatment at baseline; Total = 1 treatment. Treatment time = 300s per tender point HOW MUCH: Aperture of device = 4 cm2; Total energy delivered per tender point = 39.3 J. Device components - 1 super-pulsed infrared laser Laser wavelength = 905 nm; Frequency = 1000 Hz; Average optical output = 0.9 mW; Power density = 2.25 mW/cm2; Peak power = 8.5 W; Dose = 0.3 J; Energy density = 0.75 J/cm2; Laser spot size = 0.4 cm2. 4 red LEDs LED wavelength = 640 nm±10; Frequency = 2 Hz; Average optical output/LED = 15 mW; Power density/LED = 16.66 mW/cm2; Dose/LED = 4.5 J; Energy density/LED = 5 J/cm2; LED spot size = 0.9 cm2. 4 infrared LEDs LED wavelength = 875 nm±10; Frequency = 16 Hz; Average optical output/LED = 17.5 mW; Power density/LED = 19.44 mW/cm2; Dose/LED = 5.25 J; Energy density/LED = 5.83 J/cm2; LED spot size = 0.9 cm2; Magnetic field = 35 mT TAILORING: PBM - 10 tender points which were reported in all patients (occipital, cervical – near C7, trapezius, supraspinatus, 2nd costochondral joint, lateral epicondyle, gluteal/sacrum, greater trochanter, medial knee border) + bilateral TMJ. Exercise - stretching + aerobic training twice/week, 10 weeks. Pain threshold - digital algometer Instrutherm (DD-220). Placed on specific FM tender points and TMJs using rubber tip measuring 1 cm2. Gradual pressure applied until pain felt, displayed values then recorded (once/point). 30s interval between measurements. VAS applied. |
| de Souza et al. (2018) [42] (Brazil) |
BRIEF NAME: PBMT, lidocaine 2% infiltration WHAT: GaALAs diode laser (Twin Flex® MMOptics) WHO: Diagnosis of FM by neurologist with expertise in chronic pain, diagnosis of orofacial pain by an experienced dentist (expert in Oral and Maxillofacial surgery) by manual palpation WHERE: Small capital Northeast of Brazil (recruited from 2 orofacial centres: 1 public, 1 private) MODIFICATIONS: None documented HOW WELL: No dropout. no adverse effects, no complaints of increased pain at study conclusion WHEN: PBMT 2/week, 6 weeks; Total = 12 treatments. Lidocaine 2% 1/week, 4 weeks; Total = 4 treatments HOW MUCH: Power output = 50 mW; Wavelength = 780 nm; Dose = 50 J/cm2; Spot = 0.04 cm2 TAILORING: Prior to treatment, skin disinfected with 70% alcohol, marked with permanent marker. PBMT - applied to selected spots for approx. 40s. Participants exposed to laser application in a spot-skin distance of 1 cm whilst seated in a dental chair, with neck supported. Lidocaine 2% - 30G short needles, 0.5ml infiltrated into each tender point. Stretching after each injection to help distribute solution across muscle. VAS - self-completed 1-day prior to treatment, and 1-day post. Reflecting pain at rest or function in previous 15 days. Tenderness - perpendicular pressure of 2 or 3 fingers on surface of skin, at approx. 4kg/cm2, 5 muscles assessed bilaterally. Participant in supine position. Presence and location of tender points marked on a diagram – used as reference during all treatments. Repeated at end of study. |
| Diniz et al. (2021) [46](Brazil) |
BRIEF NAME: Low intensity red laser therapy and modified/indirect/ transcutaneous ILIB (Intravascular Laser Irradiation of Blood) WHAT: DUO MMP portable laser device (and bracelet for ILIB) WHO: Not documented WHERE: Not documented MODIFICATIONS: None documented HOW WELL: Slept during treatment application, no mention of side effects WHEN: 2 sessions, 5-day interval HOW MUCH: Intensity = 600 mW/cm2; Output area = 3 mm2; Red laser wavelength = 660 nm; Infrared laser wavelength = 808 nm; Dose (fluence) = 200 J/cm2 TAILORING: PBMT - applied at pain trigger points (bilateral TMJ, neck region, between fingers both hands). Modified ILIB - 30 mins each session (right and left wrist) – attached to bracelet over radial artery |
| dos Santos et al. (2020) [32] |
BRIEF NAME: PBMT WHAT: GaAlAs laser device (Laserpulse; Ibramed) WHO: TPC assessment performed by same physiotherapist WHERE: Not documented MODIFICATIONS: None documented HOW WELL: None documented, no mention of side effects WHEN: 1 session (PBMT/placebo) → 7 days → 1 session (PBMT/placebo) HOW MUCH: Laser pen Power output = 30 mW; Wavelength = 840 nm; Dose (intensity) = 4 J; Frequency = 2.5 kHz; Mode = continuous TAILORING: PBMT - applied to 6 different points on the quadriceps. 90s at each point (probe held stationary in skin contact with 90o angle and slight pressure). Placebo - same points with all parameters in zero. All participants were positioned and fixed at the upper body, across the hips and thighs, hip joint aligned 90-100o flexion in seated position, knee positioned 90o flexion |
| Ergün et al. (2020) [34] (Turkey) |
BRIEF NAME: PBMT WHAT: Infrared 27 Gallium-arsenide (GaAs) (Italian made ElettronicaPagani) WHO: Treatment application and assessment by 2 different doctors (assessment doctor blinded) WHERE: Not documented MODIFICATIONS: None documented HOW WELL: None documented, no mention of side effects WHEN: Total = 10 consecutive treatments (days) HOW MUCH: Average power output = 7.2 mW; Wavelength = 904 nm; Dose (energy density/point) = 4.4 J/cm2; Frequency = 3 kHz; Mode = not documented, assumed continuous TAILORING: PBMT - 2 mins per tender point (all tender points). Full contact technique using 90o vertical angle. Placebo - same method, same period and number of sessions with inactive device |
| Fernández García et al. (2011) [35](Spain) |
BRIEF NAME: Laser based program; PBMT WHAT: Portable Laser Girlase E1.1010 WHO: Not documented WHERE: University of Almeria (UAL) MODIFICATIONS: None documented HOW WELL: None documented, no mention of side effects WHEN: Intervention 8 weeks; 1/week on same time and day each week (each treatment approx. 42 mins) HOW MUCH: Wavelength = 905 nm + 10 nm; Mode = pulsed; Peak power of pulse = 1000 mW; Duration of pulses = 70ns; Energy per pulse = 0.70mJ; Frequency of pulses (Hz) = A 292; B 594; C 1168; D 2336; E 4672; F 73; G 146. Individual application of these 6 frequencies (one minute per frequency) TAILORING: PBMT - 7 anatomical areas – (a) anal region; (b) hypogastrium; (c) epigastric region; (d) left chest region; (e) anterior vertical region; (f) crista galli; (g) between bregma and vertex. 2 cycles/second, 1 cm from skin with laser covering 10cm diameter. Placebo - same anatomic sequence as PBMT group. Laser turned off and at a distance of 15 cm from the body All participants in underwear in supine position, head towards ceiling, eyes closed, arms supinated close to body, low limbs spaced at 30 cm |
| Fitzmaurice et al. (2023) [31] (UK) |
BRIEF NAME: Whole-body photobiomodulation therapy WHAT: NovoTHOR® whole-body red and NIR light bed WHO: Treatment provided by all trial investigators, following a short training session WHERE: Clinical Research Facility, Sandwell General Hospital, Sandwell and West Birmingham NHS Trust, West Bromwich, UK MODIFICATIONS: 10 participants received intended treatment schedule of 3x/week over 6 weeks, 10 participants non-adherent (received treatment of 7-9 weeks; total 41 visits re-scheduled): 61% visits due to medical reasons - fibro flare 2, fall 1, poor sleep 2, viral symptoms 5, covid 7, migraine 1, allergic rhinitis 4, elective sinus surgery 3. Practical reasons – lost car keys 1, staffing/investigator availability 3, dissatisfaction with travel expenses 4, DNA 4, unforeseen circumstances 1, work/study 1 HOW WELL: Of 42 who met eligibility criteria, 24 gave consent (18 declined to participate). 21 started treatment (3 no longer met inclusion). 19 completed (1 difficult committing, 1 uncontactable for outcome measure after receiving 17 treatments). Post-treatment physiological parameters did not reveal adverse effects WHEN: 3 treatment/week; 6 weeks; Total = 18 treatments HOW MUCH: Total power output = 694 W; Individual LED power output = 0.289 W (2400 LEDs); Individual LED beam area (LED lens/skin contact area) = 12.0 cm2; Total area of NovoTHOR® emitting surfaces = 26,740 cm2; Red light wavelength = 660 nm; Near-infrared (NIR) wavelength = 850 nm; Ratio red:NIR = 50:50; Mode = continuous wave; Dose (fluence) = 33.6 J/cm2; Irradiance = 0.028 W/cm2 TAILORING: PBM - session 1 = 6 minutes, session 2 = 12 minutes, session 3-18 = 20 minutes. All participants expected to lie horizontally (in underwear or less) in device, with lid as closed as they are comfortable with. Manual Tender Point Survey/Fibromyalgia Intensity Score (MTPS/FIS) - 18 ACR tender points (9 bilateral) assessed with hand-held Wagner FORCE TEN TM FDX digital pressure algometer – incremental increase up to maximum 4kg/cm2. Verbal NRS taken at each point. Anatomical points: low cervical (C5-C7), 2nd costochondral junction, greater trochanter (posterior to trochanteric prominence), knee (medial fat bad proximal to joint line), occiput (suboccipital muscle insertions), trapezius (midpoint upper border), supraspinatus (above scapula spine near medial border), lateral epidocondyle (2 cm distal), gluteal (upper outer buttock quadrants in anterior fold of muscle) |
| Gür et al. (2002) [26] (Turkey) |
BRIEF NAME: Low power laser therapy WHAT: Ga-As infrared laser therapy (class IIIb Laser Product, Frank Line IR 30, Fysiomed, Belgium) WHO: Two physical therapy investigators WHERE: Dept of Physical Therapy and Rehabilitation, University Hospital of Dicle, Diyarbakir, Turkey MODIFICATIONS: None documented HOW WELL: “None of the participants reported any side effects”, no patient reported discomfort related to treatment and no patient complained of an increase in any outcome parameters. WHEN: One treatment/day; 5d/week for 2 weeks; Total = 10 treatments HOW MUCH: Average power output = 11.2 mW; Wavelength = 904 nm; Mode = pulsed; Peak power of pulse = 20 W; Duration of pulse = 200 ns; Frequency of pulse = 2.8 kHz; Dose (energy density/radiant exposure) = 2 J/cm2 TAILORING: PBMT - 3 min at each tender point (1 cm2 surface). Placebo - laser light invisible and emits no heat or other physically detectable indication when active. Same unit used, no laser beam emitted. All participants were treated at same time (in afternoon) and at a temperature of 20oC |
| Gür et al. (2002) [25] (Turkey) |
BRIEF NAME: Low power laser therapy, amitriptyline WHAT: Ga-As infrared laser therapy (class IIIb Laser Product, Frank Line IR 30, Fysiomed, Belgium) WHO: Two physical therapy investigators, depression evaluated by a psychiatrist WHERE: Dept of Physical Therapy and Rehabilitation, University Hospital of Dicle, Diyarbakir, Turkey MODIFICATIONS: None documented HOW WELL: Side effects (or lack of) not directly reported on, but conclusion recommends laser therapy is safe and effective in FM WHEN: One treatment/day; 5d/week for 2 weeks; Total = 10 treatments HOW MUCH: Average power output = 11.2 mW; Wavelength = 904 nm; Mode = pulsed; Peak power of pulse = 20 W; Duration of pulse = 200 ns; Frequency of pulse = 2.8 kHz; Dose (energy density/radiant exposure) = 2 J/cm2 TAILORING: PBMT - 3 min at each tender point. Placebo - same unit used, no laser beam emitted. Amitriptyline - 10mg daily bedtime 8 weeks. All participants were treated at same time (in afternoon) and at a temperature of 20o C |
| Molina-Torres et al. (2016) [36](Spain) |
BRIEF NAME: Laser therapy, occlusal stabilization WHAT: Láser (Enraf-Nonius Ibérica SA, Madrid, Spain) WHO: Assessor blinded to treatment allocation WHERE: Research laboratory, University of Grenada, Grenada, Spain MODIFICATIONS: None documented HOW WELL: Laser group 2 lost to follow up, occlusal-splint group 1 lost to follow-up (unsure why) WHEN: 1 treatment/week; 12 weeks; Total = 12 treatments HOW MUCH: Average power = 50 mW; Peak power = 80 W; Duration of pulse = 1 µs; Frequency of pulse = 1.5 Hz; Dose = 3 J/cm2 TAILORING: Laser - 2 minutes per tender point (selected during 1st examination); Occlusal-splint - fabricated in laboratory of Faculty of Dentistry at University of Grenada. Each participant agreed to wear during sleep every night, average 8 hours per night, for 12 weeks; NTP - palpation of 18 points both sides – (1) 3 points on joint capsules - lateral, posterior, superior; (2) 3 points on masseter – anterior, inferior, deep; (3) 3 points on temporal – anterior, deep middle, origin; (4) 2 points on pterygoid – medial, lateral; (5) 3 points on sternocleidomastoid – upper, middle, lower; (6) 2 points on trapezius – origin and upper; (7) 2 points on splenius capitis muscles; Active mouth opening - asked to open mouth as much as possible for ‘without pain’ and ‘maximal’ measures; Passing mouth opening - measured after application of downward pressure on mandible by participant’s 2nd and 3rd finger; Joint sounds during mouth opening and closing - clicking assessed with examiner’s left index finger on right joint and right finger on preauricular area, fingertip placed anteriorly to tragus. Participant asked to open mouth slowly, as much as possible. After each closing, participant had to place teeth in contact at a maximal intercuspal position (opened + closed 3 times). Total number of sounds recorded for both sides |
| Moore & Demchak (2012) [44] (USA) |
BRIEF NAME: PBMT WHAT: MR4 device (Multi Radiance Medical, Solon, OH), Ga-As laser WHO: Not documented WHERE: Not documented MODIFICATIONS: None documented HOW WELL: None documented, no mention of side effects WHEN: 2d/week for 2 weeks; Total = 4 treatments HOW MUCH: Super-pulsed laser shower transducer. 6 diodes: Power output = 50 W; Wavelength = 905 nm. 4 diodes: Power output = 25 W; Wavelength = 660 nm; Frequency = 5-1000 Hz; Treatment area = 30 cm2 TAILORING: PBMT - 2 mins over each of identified sensitivity points. At beginning of each session, patient identified areas of sensitivity and rated pain level at each point (0-10) before and after PBMT |
| Navarro-Ledesma (2022) [28] (Spain) |
BRIEF NAME: Whole-body photobiomodulation treatment WHAT: NovoTHOR® whole-body red and NIR light bed WHO: Participants, therapists, evaluators, and statistician blinded. Research assistant taught participants BP measurement procedures. Physiotherapist for SEL measurements (10-year experience, expert in MSK imaging) WHERE: Private clinical practice MODIFICATIONS: Further tender point mentioned in methods – Thumbnail. Midfoot – dorsal third metatarsal midpoint HOW WELL: 2 dropouts – omitted due to not completing proposed assessments WHEN: 3 treatment/week; 4 weeks; Total = 12 treatments HOW MUCH: Total power output = 967 W; Individual LED power output = 0.336 W (2880 LEDs); Individual LED beam area (LED lens/skin contact area) = 12.0 cm2; Dimension of emission surface = 35,544 cm2; Red light wavelength = 660 nm; Near-infrared (NIR) wavelength = 850 nm; Ratio red:NIR = 50:50; Mode = continuous wave; Dose (fluence) = 25.2 J/cm2; Irradiance = 0.028 W/cm2 TAILORING: PBM - 20 mins; Placebo - 20 mins. Bed activates heating elements, providing subjects with sensation of active treatment. Goggles worn that emit some red LED light inside. All participants either naked or in underwear lie flat in the device. All treatments between 8am-4pm; BP - daytime BP (0700-0800) and nighttime BP (0000-0100) self-measured over 7 consecutive days; PPT - Up to 4 kg/cm2 to assess 12 ACR tender points (algometer perpendicular, pressure continually increased until pain perceived). Mean of 2 readings at each point; SEL – 15 MHz linear probe, transducer positioned longitudinally to muscle fibres with centre of probe over tender point and control point locations. ~2-5 mm compression applied to tissue. Mean of 3 at each point; PPT and SEL taken at same points - Occiput – suboccipital muscle insertions (1), Low cervical – anterior aspects of C5-C7 intertransverse spaces (2), Trapezius – midpoint of upper border (3), Supraspinatus – origins atop the scapula spine close to the medial border (4), Paraspinous – laterally 3 cm to midline at mid-scapula (5), Lateral pectoral – anterior axillary line at level of 4th rib (6), 2nd rib – just lateral to upper surface of 2nd costochondral junctions (7), Lateral epicondyle – 2 cm distal to epicondyles (8), Medial epicondyle (9) |
| Navarro-Ledesma et al. (2023) [27] (Spain) |
BRIEF NAME: Whole-body photobiomodulation treatment WHAT: NovoTHOR® whole-body red and NIR light bed WHO: Participants, therapists, evaluators, and statistician blinded. Participants screened by physiotherapist to ensure met inclusion criteria. To improve treatment adherence, treating physiotherapist in regular contact with participants to remind them of their time schedule WHERE: Private care practice, Malaga, Spain MODIFICATIONS: None documented HOW WELL: None documented, no mention of side effects WHEN: 3 treatment/week; 4 weeks; Total = 12 treatments HOW MUCH: Total power output = 967 W; Individual LED power output = 0.336 W (2880 LEDs); Individual LED beam area (LED lens/skin contact area) = 12.0 cm2; Dimension of emission surface = 35,544 cm2; Red light wavelength = 660 nm; Near-infrared (NIR) wavelength = 850 nm; Ratio red:NIR = 50:50; Mode = continuous wave; Dose (fluence) = 25.2 J/cm2; Irradiance = 0.028 W/cm2 TAILORING: PBM - 20 mins; Placebo - 20 mins. Bed activates heating elements, providing subjects with sensation of active treatment. Goggles worn that emit some red LED light inside. A switch box selects active or placebo treatment in a way that is undetectable by participant, operator, or observers. All participants lie supine in treatment bed, with no or minimal attire (underwear). LTPAI - 4 components each with 2 levels (light, medium, vigorous). Scores = total hours of activity over preceding 4 weeks |
| Navarro-Ledesma et al. (2024) [29] (Spain) |
BRIEF NAME: Whole-body photobiomodulation treatment WHAT: NovoTHOR® XL whole-body red and NIR light bed WHO: Research assistant evaluated eligibility. To improve treatment adherence, treating physiotherapist in regular contact with participants to remind them of their time schedule WHERE: Private clinic and rehabilitation service, Malaga, Spain MODIFICATIONS: None documented HOW WELL: Two lost to follow up at 6 months WHEN: 3 treatment/week; 4 weeks; Total = 12 treatments HOW MUCH: Total power output = 967 W; Individual LED power output = 0.336 W (2880 LEDs); Individual LED beam area (LED; lens/skin contact area) = 12.0 cm2; Dimension of emission surface = 35,544 cm2; Red light wavelength = 660 nm; Near-infrared (NIR) wavelength = 850 nm; Ratio red:NIR = 50:50; Mode = continuous wave; Dose (fluence) = 25.2 J/cm2;Irradiance = 0.028 W/cm2 TAILORING: PBM - 20 mins; Placebo - 20 mins. Bed activates heating elements, providing subjects with sensation of active treatment. Goggles worn that emit some red LED light inside. A switch box selects active or placebo treatment in a way that is undetectable by participant, operator, or observers. All participants in both groups assumed supine position in treatment bed for 20 min, while adhering to minimal attire requirements. |
| Panton et al. (2013) [37](USA) |
BRIEF NAME: Laser (Class IV) therapy WHAT: LCT-1000 (LiteCure LLC, Newark, DE) solid-state GaAlAs laser WHO: Investigators performing outcome measures and participants blinded to group assignments. Only chiropractor delivering treatment aware of groups. WHERE: Testing at University and Rheumatology office. Tender point assessment by Rheumatologist. Treatment at a chiropractic clinic by a chiropractic physician MODIFICATIONS: None documented HOW WELL: 1 participant did not return after initial assessment. 2 randomised to laser group dropped out due to scheduling conflicts, 1 in laser group could not complete tests due to severe depression. WHEN: 2 treatment/week; 4 weeks; Total = 8 treatments (as per manufacturer’s recommendation) HOW MUCH: Power output = 10 W; Mode = continuous wave; Dual wavelength = 20% 810 nm and 80% 980 nm; Treatment areas = 2.5 inch x 3.5 inch, or ~56.45 cm2; Dose per treatment area = 10.63 J/cm2 (total 600 J); Grid scanning technique utilised to avoid over-heating; Exposure time at each area = 60s TAILORING: Laser - Warm air supply (below) hose bound together with laser’s fibreoptic cable, routed through a hole in the laser handpiece so that warm air could be delivered alone, or in tandem with laser. 7 minutes; application over 7 tender points across neck, shoulders and back; Placebo - Because laser manufacturer mentions “soothing warmth” of laser, sham + heat therapy designed (commercially available air warmer forced through tube, mounted out of view inside vented cart upon which laser mounted to as to appear as single unit); Myalgic score - 0-3 across 18 tender points (total 54). Subjective rating given by the physician to describe sensitivity of tender point when pressure applied; CP-PFP - 10-items to measure functional performance by simulating routine tasks performed at maximal effort within the bounds of safety and comfort. Speed, distance, weight to quantify performance. Weight + speed = (1) pot carrying, (2) carrying groceries. Time = (3) transferring laundry from washer to dryer, dryer to basket, (4) putting jacket on and off, (5) floor sweeping, (6) climbing stairs, (7) getting down and up from floor, (8) picking up 4 scarves from floor. Distance = (9) 6-minute walk, (10) highest reach. Each task scaled 1-100 (higher = higher function). Participants either gowned, or sports bra, to expose skin of cervical, thoracic, lumbar regions. Positioned face down on treatment table or massage chair. Eye protection worn. |
| Ruaro et al. (2014) [45](Brazil) |
BRIEF NAME: PBMT WHAT: Ga-Al-As diode laser (Ibramed, Laserpulse) WHO: Not documented WHERE: Not documented MODIFICATIONS: None documented HOW WELL: None documented, no side effects or complications reported WHEN: 3 treatment/week; 4 weeks; Total = 12 treatments HOW MUCH: Average power output = 20 mW; Wavelength = 670 nm; Dose = 4 J/cm2; Focal spot area = 0.035 cm2; Exposure time at each point = 7s TAILORING: PBMT - 18 tender points, radiation applied at 4 locations around each point, each location separated by a distance of 1 cm (entire area encompassed by PBMT was 1 cm2 per point) = 72 applications/504s, total power density 0.57W/cm2; Placebo - same procedures as PBMT group but received sham laser exposure (0W). Skin cleaned around 18 tender points. Laser pen applied directly to skin at an angle of 90o |
| Tramontana et al. (2017) [38] (Italy and Spain) |
BRIEF NAME: Laser therapy WHAT: Mixed diode (collimated panta-diodic) WHO: Not documented WHERE: Multi-centre private practice and academic institution; Italy (University di Catanzaro and TA SRL outpatients clinic, Reggio) and Spain (Asociación Española Médicos Integrativos, Madrid) MODIFICATIONS: None documented HOW WELL: No side effects or interactions WHEN: 5 sessions/week; 4 weeks; Total = 20 treatments HOW MUCH: Power output = 5 W; Power density = 1.25 W/cm2.; Wavelength = 950 nm; Superpulsed emission mode = 600-1200 Hz; Length of impulses = 125 ns; Energy density = 1125 J/cm2 TAILORING: Laser - 15 minutes per session; Placebo: Laser off, guide-light on |
| White et al. (2018) [41](USA) |
BRIEF NAME: Laser (Class IV) therapy - HILT WHAT: Phoenix Thera-lase device (Phoenix Thera-lase Systems, LLC, Dallas, TX) WHO: Not documented WHERE: McDermott Center for Pain Management, UT Southwestern Medical Center, Dallas, Texas MODIFICATIONS: None documented HOW WELL: None documented, no mention of side effects WHEN: See ‘tailoring’ HOW MUCH: Power output range = 1-75 W; Treatment 1 = 42 W; Treatment 2 = 42 W; Treatment 3 = 1 W; Treatment 4 = 75 W; Wavelength = 1275 nm TAILORING: HILT - Treatment 1 bilateral lower thoracic and lumbar paraspinous region and 10 tender points at shoulder and hip regions. 60s treatments, 4-6inch apart over symptomatic area, laser probe held approx 12 inch from skin surface (total 40 mins); Treatment 2 1 month later, same areas but more abbreviated (total 30 mins); Treatment 3 – 2 weeks later, paraspinous region (total 30 mins); Treatment 4 – 1 week later, same paraspinous region (total 30 mins) |
| Wu et al.(2018) [39] (Taiwan) |
BRIEF NAME: Intravenous (red) Laser Irradiation of Blood (ILIB) WHAT: YJ-ILIB-5, Bio-ILIB (Human Energy Ltd., Taiwan) WHO: Not documented WHERE: Recruited from outpatient clinic in Department of Rehabilitation and Physical Medicine, Taipei Veterans General Hospital, Twaiwan MODIFICATIONS: None documented HOW WELL: No unfavourable events were recorded, no complaints of discomfort WHEN: 10 ILIB sessions; 2 courses; Total = 20 treatments HOW MUCH: Power output = 2.5 mW; Wavelength = 632.8 nm; Mode = continuous wave TAILORING: ILIB - each session was 60 mins; 7-day rest interval between the 2 treatment courses. Participant lying supine on bed, 24G intravenous catheter at antecubital fossa, subsequently replaced with a fibreoptic needle, inserted into inner cannula of IV catheter. Other side of fibreoptic needle connected to laser device. Comparison made with a medication group – details of this not stipulated in methods |
| Paper | Sample size and participants | Outcome measures and findings | |
|---|---|---|---|
| Armagan et al. (2006) [33] |
FM patients = 32 PBMT = 16 Placebo = 16 |
Number of tender points (NTP). Digital palpation across 18 ACR point (+ve = pain reported on palpation) |
Pre/post/6m PBMT 13.68±2.12/11.81±1.80/12.5±1.71 (p<0.01 baseline vs. post, p<0.05 baseline vs. 6m) Pre/post/6m Placebo 13.94±2.11/12.88±2.09/13.95±1.88 (p<0.05 baseline vs. post) |
| Fibromyalgia Impact Questionnaire (FIQ) |
0-100 Pre/post/6m PBMT 65.50±9.01/58.50±10.3/62.02±8.99 (p<0.01 baseline vs. post, p<0.05 baseline vs. 6m) Pre/post/6m Placebo 65.38±9.44/63.63±9.59/66.94±8.44 |
||
| Morning stiffness |
Likert scale 0-4 (none→extreme) Pre/post/6m PBMT 3.00±0.63/2.38±0.62/2.56±0.89 (p<0.01 baseline vs. post, p<0.05 baseline vs. 6m) Pre/post/6m Placebo 3.06±0.77/2.50±0.89/3.25±0.58 (p<0.05 baseline vs. post) |
||
| Global improvement on a verbal scale (VSGI) |
1-5 (great improvement→worsening) Pre/post/6m PBMT 3.44±1.03/2.56±0.63/3.00±0.73 (p<0.01 baseline vs. post, p<0.05 baseline vs. 6m) Pre/post/6m Placebo 3.38±0.96/3.19±0.75/3.69±0.70 |
||
| Total myalgia score |
18 tender points with 4kg digital force 0-3 (no discomfort→pain with grimace/flinch/withdraw). Total 0-54 Pre/post/6m PBMT 25.00±8.66/19.50±6.95/22.44±6.79 Pre/post/6m Placebo 27.56±9.67/26.00±8.95/28.75±9.86 |
||
| da Silva et al. (2018) [40] |
FM patients = 160 Set 1 (acute effect) = 80 Control = 20 PBM = 20 EXT = 20 PBM + EXT = 20 |
Control group/PBM group/exercise group/PBM+exercise group (average of right and left sides). PBM data in bold where there is significant difference to control group. Underlined where there is significant difference to exercise group. Set 1 → Set 2 NB all below scores are with reference to % improvement from baseline |
|
|
Set 2 (long-term effect/10 weeks) = 80 Control = 20 PBM = 20 EXT = 20 PBM + EXT = 20 |
PPT |
TMJ: 7.06/39.87/27.96/38.76 → 8.21/38.51/25.08/46.27 Occipital: 3.29/26.02/19.02/41.66 → 10.45/24.93/25.38/43.14 C7: 2.38/20.81/7.88/28.62 → 13.14/28.36/30.15/42.09 Trapezius: 0.00/20.81/1.79/30.95 → 11.20/28.66/25.52/35.82 Supraspinatus: 1.50/20.74/6.70/12.28 → 8.66/24.63/33.14/36.27 2nd costochondral joint: 0.00/20.82/4.61/10.06 → 5.98/34.03/38.21/45.44 Lateral epicondyle: 1.19/21.14/6.33/9.83 → 5.97/16.72/23.59/42.84 Gluteal/sacrum: 1.78/24.69/5.95/13.6 → 1.50/30.30/10.75/25.33 Greater trochanter: 5.95/28.71/5.06/14.50 → 5.67/27.76/5.67/22.02 Medial knee border: 1.79/32.64/8.45/17.84 à 6.12/33.44/18.06/25.97 |
|
|
VAS TPC FIQ anxiety FIQ depression FIQ stiffness FIQ fatigue FIQ total |
Set 2 13.13/61.41/43.43/66.67 4.04/53.33/24.24/84.85 0.00/8.35/10.26/15.65 8.35/15.65/15.65/20.87 2.78/10.61/5.65/10.09 1.74/10.09/8.00/19.13 0.87/5.74/22.78/24.78 |
||
| Research Diagnostic Criteria (RDC) score Sleep disturbance Night awakenings Trouble sleeping |
0.00/23.65/19.13/33.04 0.00/10.78/23.65/24.34 2.61/6.96/23.48/74.78 |
||
| Quality of life (SF-36) (0-100) Physical functioning Role-emotional Role-physical Social functioning Mental health Vitality General health |
2.65/28.67/14.16/30.97 1.77/15.93/14.34/24.78 5.49/16.81/13.81/28.32 3.10/12.21/14.60/23.01 1.77/7.08/7.08/21.24 5.31/19.12/9.73/40.71 5.66/12.57/16.81/38.94 |
||
| de Souza et al. (2018) [42] |
FM patients = 66 PBMT = 33 Lidocaine = 33 |
Pain intensity (VAS) |
Pre/post PBMT: 7.85±2.22/2.85±1.77 (p=0.0001) Pre/post LA infiltration: 8.08±2.03/3.18±1.87 (p=0.0001) |
| Overall muscle tenderness to palpation |
Pre/post PBMT: 7.85±2.22/2.85±1.77 (p=0.0001) Pre/post LA infiltration: 8.08±2.03/3.18±1.87 (p=0.0001) |
||
|
Posterior masseter Anterior masseter Anterior temporal Medium temporal Posterior temporal |
No. of participants tender to palpation (right pre/post treatment + left pre-post treatment) PBMT: 21/8 (p=0.00) + 17/8 (p=0.01); LA: 19/5 (p=0.00) + 15/4 (p=0.00) PBMT: 14/6 (p=0.00) + 12/6 (p=0.07); LA: 16/2 (p=0.00) + 13/3 (p=0.01) PBMT: 22/11 (p=0.01) + 24/11 (p=0.00); LA: 25/14 (p=0.00) + 25/14 (p=0.00) PBMT: 22/11 (p=0.00) + 25/13 (p=0.00); LA: 23/13 (p=0.01) + 21/13 (p=0.06) PBMT: 28/25 (p=0.45) + 28/24 (p=0.28); LA: 29/24 (p=0.26) + 30/25 (p=0.12) |
||
| dos Santos et al. (2020) [32] |
N = 37 FM group = 20 Healthy (sedentary) with no MSK/CVS/Resp disease = 17 Each group randomised to PBMT/placebo à 7d washout à PBMT/placebo |
Isokinetic dynamometer muscle strength and endurance Dominant quadriceps muscle (3 series of 5 contractions with 60o/s) |
PBMT group (FM/control) Max torque (Nm): 77.75±21.07/101.12±30.43 Torque peak (%): 90.50±53.13/129.06±60.03 Total work (J): 268.00±94.75/383.06±127.58 Power (W): 44.40±16.16/63.59±22.72 Placebo group (FM/control) Max torque (Nm): 84.05±25.64/100.94±33.28 Torque peak (%): 96.80±55.76/127.35±31.28 Total work (J): 282.30±103.80/361.12±144.24 Power (W):49.90±17.58/62.94±26.92 |
| Isokinetic dynamometer muscle resistance Dominant quadriceps muscle (3 series of 5 contractions with 240o/s) |
PBMT group (FM/control) Max torque (Nm): 42.90±14.73/62.47±15.22 Torque peak (%): 48.95±30.71/81.06±34.64 Total work (J): 409.10±166.40/641.82±171.21 Power (W): 57.10±25.22/94.71±26.29 Placebo group (FM/control) Max torque (Nm): 43.90±13.92/60.12±18.47 Torque peak (%): 50.45±31.34/76.41±35.83 Total work (J): 411.80±159.20/621.82±219.70 Power (W): 57.90±24.02/91.82±36.31 |
||
| Ergün et al. (2020) [34] |
FM patients = 60 PBMT = 30 Placebo = 30 |
NTP |
Pre/post PBMT: 13.4+2.4/7.1+4. Pre/post placebo: 13.1+1.9/7.6+3.8 |
| LIKERT TYPE SCALE (0-4; none à intolerable), all p<0.001 | |||
| Pain intensity | Pre/post PBMT: 2.6+0.8/1.4+0.6. Pre/post placebo: 2.7+0.7/1.6+0.7 | ||
| Stiffness | Pre/post PBMT: 2+1.1/1+1. Pre/post placebo: 1.9+0.8/1.1+0.8 | ||
| Sleep disorders | Pre/post PBMT: 1.6+1.1/0.8+1. Pre/post placebo: 1.3+1.3/0.9+0.9 | ||
| Fatigue | Pre/post PBMT: 2.5+0.8/1.4+0.7. Pre/post placebo: 2.6+1/1.3+0.9 | ||
| Muscle spasms | Pre/post PBMT: 1.9+1/0.9+0.9. Pre/post placebo: 2.3+1.1/1.2+1.1 | ||
| Subjective swelling | Pre/post PBMT: 1.3+0.9/0.6+0.7. Pre/post placebo: 1.3+1.1/0.5+0.6 | ||
| Paraesthesia | Pre/post PBMT: 1.7+0.9/0.7+0.5. Pre/post placebo: 1.3+0.9/0.7+0.8 | ||
| Total Likert | Pre/post PBMT: 13.5+3.9/6.8+3.4. Pre/post placebo: 13.3+3.9/6.9+4.2 | ||
| FIQ | Pre/post PBMT: 54.6+11.7/2.3+12.3. Pre/post placebo: 55.6+12.5/33.9+14.8 | ||
| Fitzmaurice et al. (2023) [31] |
FM patients=19 PBMT = 19 |
Pre/post PBM/mean improvement (Cohen’s d)à 6-month follow up: mean improvement (Cohen’s d) | |
| FIQR (0-100) |
79.7±13.26/55.3±19.72 (p≤0.001)/24.44±20.38 (1.49) à 65.68±16.53/Week 6: Week 24 -10.41, p=0.23 (0.57); Baseline: Week 24 14.02, p=0.001 (0.94) | ||
| Patient Global Impression of Change (PGIC) (1-7; 1=no changer or worse, 7 = great deal better) | 6 weeks: 5.47±1.43; 6 months: 3.79±2.1 (0.94) |
||
| Pre/post PBM (Cohen’s d) | |||
| Brief Pain Index-Short Form (BPI-SF): BPI Pain Intensity BPI Pain Interference (0-10) Perceived analgesic efficacy (%) |
7.08±1.28/3.93±1.38 (2.37) 6.59±1.32/4.17±1.99 (1.43) 43.5±17.55/53.89±20.0 |
||
| Fibromyalgia Severity Score (WPI+SSS) (0-31) | 25.1±2.86 (15±2.45 + 10.1±1.45)/16.21±5.78 (9.89±4.21 + 6.32±2.54) (1.95) |
||
| Fatigue severity score (FSS) (1-7) | 6.30±0.86/5.61±1.16 (0.68) |
||
| Jenkins Sleep Questionnaire (JSQ) (0-20) | 17.35±1.90/11.53±6.17 (1.27) |
||
| Hospital Anxiety and Depression Scale (HADS) HADS-A (0-21) HADS-D (0-21) |
14± 3.71/10.53± 4.57 (0.83) 12.5± 3.26/8.21± 3.68 (1.23) |
||
| Stiffness (subsection FIQR) (0-10) | 9.05± 1.02/5.95± 2.56 (1.59) |
||
| Dyscognition (subsection FIQR) (0-10) | 8.35± 1.31/5.58± 2.56 (1.38) |
||
| Fibromyalgia Intensity Score (0-10) Average pressure tolerated (kg/cm2) |
6.35±1.84/5.17± 1.91 (0.52) 1.21± 1.05/1.71± 1.16 (0.49) |
||
| Stroop Test: Total score (in 60s) Accuracy (%) |
27.4±16.0/31.21± 15.11 (0.24) 85.23±24.06/85.45±24.04 (0.01) |
||
| Medications reduced or stopped (n) | Paracetamol 3, anti-inflammatories 2, opioids 9, TCAs 2, SSRIs/SNRIs 2, anticonvulsants 1 | ||
|
Fernández García et al. (2011) ]35] |
FM patients = 31 Ratio not clear |
Impact on FM (FIQ) 0-100 | Pre/post PBMT: 71.45±11.80/52.30±15.22. Pre/post placebo: 60.89±15.28/50.37±24.18 |
| VAS 1-10 (minimal à severe) | |||
| Fatigue |
Pre/post PBMT: 8.25±1.48/3.93±1.76; p<0.049 Pre/post placebo: 7.93±1.79/5.92±3.38 |
||
| Sleeping difficulties (fatigue on waking) |
Pre/post PBMT: 7.53±2.09/5.23±2.56; p<0.044 Pre/post placebo: 5.72±3.13/7.14±2.44 |
||
| General pain |
Pre/post PBMT: 8.43±1.75/6.12±2.91 Pre/post placebo: 7.46±2.44/6.73±2.25 |
||
| Neck pain |
Pre/post PBMT: 8.29±1.64/6.33±2.82 Pre/post placebo: 7.36±2.29/6.81±3.02 |
||
| Vaginal pain during intercourse |
Pre/post PBMT: 6.87±4.34/4.75±2.35 Pre/post placebo: 5.20±2.65/5.73±3.21 |
||
| All other than fatigue and sleep non-significant | |||
| Gür et al. (2002) [26] |
FM patients = 40 PBMT = 20 Placebo = 20 |
NTP |
Pre/post PBMT: 13.18±2.3/6.63±3.86. Pre/post placebo: 12.7±0.71/8.55±4.11 |
| Likert scale 0-4 (none à extreme) | |||
| Pain | Pre/post PBMT: 3.09±0.52/1.270.76. Pre/post placebo: 3.48±0.8/2.44±0.98 | ||
| Skinfold tenderness | Pre/post PBMT: 2.18±0.95/0.90±0.5. Pre/post placebo: 2.10±0.71/1.33±1.37 | ||
| Morning stiffness | Pre/post PBMT: 2.54±0.8/1.09±0.92. Pre/post placebo: 2.7±0.86/2.01±0.8 | ||
| Sleep disturbance | Pre/post PBMT: 2.36±1.25/1.27±1.07. Pre/post placebo: 1.7±1.12/1.66±1.60 | ||
| Fatigue | Pre/post PBMT: 3.09±0.81/1.36±1.17. Pre/post placebo: 2.10±0.71/2.04±1.09 | ||
| Muscle spasm | Pre/post PBMT: 2.27±0.45/0.81±0.73. Pre/post placebo: 2.3±0.47/1.33±0.68 | ||
| P<0.05 improvement in pain, muscle spasm, morning stiffness, and NTP in PBMT group compared with placebo | |||
| Gür et al. (2002) [25] |
FM patients = 75 PBMT = 25 Placebo PBMT = 25 Amitriptyline = 25 |
NTP |
Pre/post PBMT: 13.92±2.30/6.40±3.90. Pre/post placebo: 11.90±2.30/8.00±3.84 Pre/post amitriptyline: 12.72±1.16/7.27±3.20 |
| Likert scale 0-4 (none à extreme) | |||
| Pain intensity |
Pre/post PBMT: 3.04±0.53/1.24±0.72. Pre/post placebo: 3.19±0.87/2.19±0.74 Pre/post amitriptyline: 2.90±0.68/2.09±0.92 |
||
| Skin fold tenderness |
Pre/post PBMT: 2.12±0.92/0.80±0.57. Pre/post placebo: 2.08±0.60/1.64±1.20 Pre/post amitriptyline: 2.27±0.76/1.45±1.18 |
||
| Morning stiffness |
Pre/post PBMT: 2.56±1.01/0.96±0.93. Pre/post placebo: 2.66±0.91/1.90±0.83 Pre/post amitriptyline: 2.45±0.80/1.15±0.67 |
||
| Sleep disturbance |
Pre/post PBMT: 2.40±1.22/1.12±1.09. Pre/post placebo: 2.11±0.80/1.79±1.36 Pre/post amitriptyline: 2.09±1.26/0.81±0.73 |
||
| Muscle spasm |
Pre/post PBMT: 2.28±0.54/0.84±0.68. Pre/post placebo: 2.19±0.40/1.13±0.62 Pre/post amitriptyline: 1.81±0.73/1.00±0.61 |
||
| Fatigue |
Pre/post PBMT: 3.12±0.83/1.32±1.10. Pre/post placebo: 3.04±0.74/2.28±0.90 Pre/post amitriptyline: 2.86±0.90/2.49±1.26 |
||
| Depression | Hamilton Depression Rating Scale 0 - ≥23 (normal → very severe) Pre/post PBMT: 19.24±5.88/11.48±3.96/Pre/post placebo: 18.08±4.13/15.79±4.07 Pre/post amitriptyline: 17.57±4.19/7.16±3.24 |
||
| QoL (FIQ) |
Pre/post PBMT: 56.27±7.57/33.02±11.96Pre/post placebo: 59.94±8.18/50.30±8.87 Pre/post amitriptyline: 57.73±9.11/39.78±8.62 |
||
| Significant improvements in all parameters of PBMT group (p=0.001), and all in amitriptyline group, except fatigue. Significant difference in pain intensity and fatigue in favour of laser group over other groups. |
|||
| Molina-Torres et al. (2016) [36] |
FM + TMD patients = 58 Laser = 29 Occlusal-splint = 29 |
Widespread pain (WPI) (0-19) + Severity of symptoms (SSS) (0-12) |
Between group differences in score changes: -4.138 Pre/post laser/within group score change (Cohen’s d): 15.59±3.50 + 9.72±2.99/14.62±3.75 + 8.69±3.04/0.966 + 1.034 (0.267 + 0.341) Pre/post occlusal-splints/within group score change (Cohen’s d): 15.62±2.89 + 9.72±1.93/13.45±4.16 + 8.07±2.82/2.172 + 1.655 (0.614 + 0.696) Between group differences in score changes: -1.172 + -0.621 |
| Pain intensity (VAS) (0-100) |
Pre/post laser/within group score change (Cohen’s d): 78.62±20.13/70.69±19.07/7.931 (0.404) Pre/post occlusal-splints/within group score change (Cohen’s d): 76.55±14.71/66.55±21.92/10.00 (0.546) |
||
| NTP (0-36) |
Pre/post laser/within group score change (Cohen’s d): 11.69±2.24/7.24±1.81/4.448 (0.200) Pre/post occlusal-splints/within group score change (Cohen’s d): 11.86±2.31/6.76±1.53/5.103 (0.659) Between group differences in score changes: -0.483 |
||
| Quality of sleep (Pittsburgh Quality of Sleep Questionnaire Index – PSQI) (0-21) |
Pre/post laser/within group score change (Cohen’s d): 14.07±4.38/13.45±4.68/0.620 (0.137) Pre/post occlusal-splints/within group score change (Cohen’s d): 16.00±3.17/13.69±4.05/2.310 (0.639) Between group differences in score changes: 0.240 |
||
| Active mouth opening without pain |
Pre/post laser/within group score change (Cohen’s d): 26.10±5.22/27.45±5.27/-1.344 (0.256) Pre/post occlusal-splints/within group score change (Cohen’s d): 27.34±5.15/30.03±5.08/-2.689 (0.525) Between group differences in score changes: 2.586 |
||
| Maximal active + passive mouth opening |
Pre/post laser/within group score change (Cohen’s d): 34.72±5.04 + 38.34±5.32/35.34±5.29 + 39.24±5.74/-0.620 + -0.896 (0.119 + 0.162) Pre/post occlusal-splints/within group score change (Cohen’s d): 37.17±6.23 + 40.79±6.13/38.41±6.29 + 42.47±6.16 (-1.241 + -1.655) Between group differences in score changes: 3.068 + 3.206 |
||
| Clicking sound during palpation when opening (right + left) |
Pre/post laser/within group score change (Cohen’s d): 0.31±0.47/0.17±0.38 + 0.45±0.51/0.24±0.44/0.138 + 0.207 (0.327 + 0.445) Pre/post occlusal-splints/within group score change (Cohen’s d): 0.28±0.46/0.10±0.31 + 0.21±0.41/0.17±0.38/0.172 + 0.034 (0.471 + 0.100) Between group differences in score changes: -0.069 + -0.069 |
||
| Click sound during palpation when closing (right + left) |
Pre/post laser/within group score change (Cohen’s d): 0.34±0.48/0.17±0.38 + 0.41±0.50/0.21±0.41/0.172 + 0.207 (0.391 + 0.438) Pre/post occlusal-splints/within group score change (Cohen’s d): 0.24±0.44/0.03±0.19 + 0.21±0.41/0.03±0.19/0.207 + 0.172 (0.677 + 0.602) Between group differences in score changes: -0.138 + -0.172 |
||
| PGIC (5-point version; much improved à much worse) |
Pre/post laser/within group score change (Cohen’s d): 4.45±0.78/3.83±0.54/0.621 (0.937) Pre/post occlusal-splints/within group score change (Cohen’s d): 4.41±0.87/3.48±1.18/0.931 (0.907) Between group differences in score changes: -0.345 All pre and post intervention values statistically significant, excepting left clicking sound when opening in occlusal-splint group |
||
| Moore & Demchak (2012) [44] | FM patient = 1 |
NTP VAS FIQ Subjective Activity of Daily Living (SADL) – subsection of FIQ |
Pre/post PBMT/2 weeks post PBMT 14/6/14 6/2 82/23/34 20/5/0 |
| Navarro-Ledesma (2022) [28] |
FM patients = 40 |
Circadian BP Index |
Between group difference after intervention: -3.01, p=0.036, SE -0.06 |
| PPT |
PPT in tender points with significant differences (between group differences after intervention) Occiput: -0.273, p=0.039, SE 0.127 Low cervical: -0.254, p=0.035, SE 0.134 Trapezius: -0.235, p=0.037, SE 0.109 2nd rib: -0.632, p=<0.0001, SE 0.109 Medial epicondyle: -0.505, p=0.006, SE 0.173 |
||
| Strain elastography (SEL) (objective alternative for PPT) | SEL in tender points with significant differences (between group differences after intervention), and/or non-significant difference but medium effect size of ~0.5 Trapezius: 0.0522, p=0.028, SE 0.53 Forearm: 0.730, p=<0.001, SE 0.14 Low cervical: dominant -0.004, p=0.808, SE 0.74. non-dominant 0.174, p=0.469, SE 0.62 Supraspinatus: -0.146, p=0.480, SE 0.49 Lateral epicondyle: 0.072, p=0.697, SE 0.60 Anterior tibial: -0.291, p=0.342, SE 0.62 |
||
| Navarro-Ledesma et al. (2023) [27] |
FM patients = 44 |
Baseline = T0, after session 6 (2 weeks) = T1, after session 12 (4 weeks) = T2, 2 weeks after treatment = T3 Significant between group at T1/T2/T3 or non-significant difference but medium effect size of ~0.5 |
|
| Pain intensity (NPRS) – average over preceding 7 days (0-10) | T2; 3.00, p=<0.001, Cohen’s d = 2.06 T3; p=<0.001, Cohen’s d = 2.87 T0 → T1 → T2 → T3: PBMT 8.22 à 7.18 à 5.22 à 4.32; Placebo 8.0 à 7.69 à 7.74 à 8.11 |
||
| Health-related quality of life (HRQL) – average over preceding 7 days (0-10) |
T1; -2.00, p=<0.001, Cohen’s d = -0.129 T2; -3.00, p=<0.001, Cohen’s d = -2.49 T3; -4.00, p=<0.001, Cohen’s d = -3.26 T0 → T1 → T2 → T3: PBMT 3.33 à 4.9 à 6.16 à 7.0; Placebo 2.83 à 3.2 à 2.91 à 3.05 |
||
| Leisure Time Physical Activity Instrument (LTPAI) |
T2; -28.00, p=<0.001, Cohen’s d = -1.90 T3; -43.00, p=<0.001, Cohen’s d = -2.70 T0 → T1 → T2 → T3: PBMT 28 à 25.6 à 47.56 à 72.5; Placebo 30 à 28.4 à 24.4 à 29.17 |
||
| Tampa Scale of Kinesiophobia (11-44; higher score = greater fear movement/injury) |
T1: 6.00, p=<0.008, Cohen’s d = 0.87 T2; 10.00, p=<0.001, Cohen’s d = 1.25 T3; 12.00, p=<0.001, Cohen’s d = 1.49 T0 → T1 → T2 → T3: PBMT 25 à 23.11 à 19.2 à 17.63; Placebo 30.67 à 29.19 à 28.8 à 28.5 |
||
| Self-efficacy questionnaire (0-44; higher score = greater perception confidence to handle situation) | T2; -7.00, p=0.034, Cohen’s d = -0.73 T3; -8.00, p=<0.001, Cohen’s d = -1.33 T0 → T1 → T2 → T3: PBMT 27.93 à 26.93 à 31.03 à 33.80; Placebo 26.55 à 26.13 à 25.71 à 25.86 |
||
| Pain Catastrophising Scale (0-52; higher score = higher catastrophism) |
T0 → T1 → T2 → T3: PBMT 28.21 → 26.21 → 23.18 → 21.43; Placebo 27.14 → 27.38 → 27.14 → 28.5 None statistically significant, all small effect sizes |
||
| Navarro-Ledesma et al. (2024) [29] |
FM patients = 42 |
3 months = T4, 6 months = T5 (see above study for T0, T1, T2, T3 data) Significant between group at T4/T5 and/or non-significant difference but medium effect size of ~0.5 |
|
| Pain intensity (NPRS) – average over preceding 7 days (0-10) |
T4; -1.00, p=0.17, Cohen’s d = -0.53 T5; 2.00, p=0.001, Cohen’s d = 1.16 T4 → T5 PBMT 6.36 à 4.91; Placebo 5.23 à 6.73 |
||
| Health-related quality of life (HRQL) – average over preceding 7 days (0-10) |
T4; -3.03, p=<0.001, Cohen’s d = -3.2 T5; -2.00, p=<0.001, Cohen’s d = -2.35 T4 → T5 PBMT 6.24 à 5.94; Placebo 3.23 à 3.56 |
||
| Leisure Time Physical Activity Instrument (LTPAI) (AKA Godin test) |
T4; -41.23, p=<0.001, Cohen’s d = -2.55 T5; -43.00, p=<0.001, Cohen’s d = -2.86 T4 → T5 PBMT 72.84 à 74.63; Placebo 31.34 à 32.84 |
||
| Tampa Scale of Kinesiophobia (11-44; higher score = greater fear movement/injury) |
T4; 9.52, p=<0.001, Cohen’s d = 1.24 T5; 13.00, p=<0.001, Cohen’s d = 2.16 T4 → T5 PBMT 16.47 à 14.35; Placebo 28.24 à 25.88 |
||
| Self-efficacy questionnaire (0-44; higher score = greater perception confidence to handle situation) | T4; -11.19, p=<0.001, Cohen’s d = -2.31 T5; -12.00, p=<0.001, Cohen’s d = -2.04 T4 → T5 PBMT 36.98 à 38.46; Placebo 18.34 à 17.75 |
||
| Pain Catastrophising Scale (0-52; higher score = higher catastrophism) | T4; 7.00, p=0.05, Cohen’s d = 0.64 T5; 10.00, p=0.006, Cohen’s d = 0.83 T4 → T5 PBMT 19.03 à 19.70; Placebo 25.75 à 29.48 |
||
| Panton et al. (2013) [37] |
FM patients = 38 Laser + heat therapy = 20 Sham + heat therapy = 18 |
Myalgic score 8 tender points (back) Myalgic score (back) |
Pre/post laser + heat: 15±5/12±6 (p≤0.05). Pre/post placebo + heat: 14±4/11±5 (p≤0.05) Pre/post laser: 6±2/5±2. Pre/post placebo: 6±1/5±2 (p≤0.05) Pre/post laser: 16±6/13±7 (p≤0.05). Pre/post placebo: 14±5/11±5 (p≤0.05) |
| NTP |
Pre/post laser + heat: 14±3/11±5 (p≤0.05). Pre/post placebo + heat: 13±3/10±4 (p≤0.05) | ||
| FIQ FIQ Pain subsection |
Pre/post laser: 62±21/55±16 (p≤0.05). Pre/post placebo: 57±11/55±12 Pre/post laser: 7.1±2.3/6.2±2.1 (p≤0.05; ES 12). Pre/post placebo: 5.8±1.3/6.1±1.4 |
||
| Continuous scale physical functional performance (CS-PFP): Upper body strength Upper body flexibility Lower body strength Balance and coordination Endurance CS-PFP total Overall rating of perceived exertion (RPE) post CS-PFP |
Pre/post laser: 33±17/39±16 (p≤0.05). Pre/post placebo: 38±14/42±15 Pre/post laser: 71±17/78±12 (p≤0.05; ES 21). Pre/post placebo: 77±12/77±11 Pre/post laser: 33±15/39±15 (p≤0.05). Pre/post placebo: 39±16/44±16 (p≤0.05) Pre/post laser: 43±16/52±14 (p≤0.05). Pre/post placebo: 51±16/56±16 (p≤0.05) Pre/post laser: 44±15/52±13 (p≤0.05). Pre/post placebo: 52±15/57±16 (p≤0.05) Pre/post laser: 42±15/49±13 (p≤0.05). Pre/post placebo: 49±14/53±15 (p≤0.05) Pre/post laser: 13±2/13±2. Pre/post placebo: 13±1/13±2 FIQ Pain and Upper body flexibility improvements were statistically significant compared with placebo therapy |
||
| Ruaro et al. (2014) [45] |
FM patients = 20 PBMT = 10 Placebo = 10 |
NTP |
Pre/post PBMT: 11.6±2.4/7.3±2. Pre/post placebo: 11.8±1.5/10.4±1.5, p<0.0001 |
| FIQ physical impairment FIQ feel good FIQ work missed FIQ difficult to work FIQ pain FIQ fatigue FIQ rested FIQ stiffness FIQ anxiety FIQ depression FIQ total |
Pre/post PBMT: 4.9±1.8/3.4±1.4. Pre/post placebo: 3.6±2.4/3.0±2.1 1.5 PBMT improvement versus 0.5 placebo, p=0.04 Pre/post PBMT: 7.7±1.8/5.6±1.6. Pre/post placebo: 5.9±4.3/3.6±3.5 2.1 PBMT improvement versus 2.3 placebo, p=0.9 Pre/post PBMT: 2.2±1.9/0.6±1.0. Pre/post placebo: 1.4±1.4/1.3±1.3 1.6 PBMT improvement versus 0.1 placebo, p=0.015 Pre/post PBMT: 7.0±1.9/5.8±1.0. Pre/post placebo: 7.7±2.0/7.3±2.0 1.2 PBMT improvement versus 0.4 placebo, p=0.16 Pre/post PBMT: 8.1±1.6/5.4±1.1. Pre/post placebo: 8.8±1.6/7.7±1.3 2.7 PBMT improvement versus 1.1 placebo, p=0.0075 Pre/post PBMT: 7.6±2.1/5.5±1.2. Pre/post placebo: 8.3±1.6/7.5±1.7 2.1 PBMT improvement versus 0.8 placebo, p=0.0043 Pre/post PBMT: 8.1±1.4/6.4±0.8. Pre/post placebo: 7.9±1.1/7.9±1.4 1.7 PBMT improvement versus 0.0 placebo, p=0.06 Pre/post PBMT: 7.7±1.8/6.0±0.9. Pre/post placebo: 7.8±1.5/8.0±1.6 1.7 PBMT improvement versus 0.2 placebo, p=0.0034 Pre/post PBMT: 7.6±1.6/5.4±1.4. Pre/post placebo: 7.9±1.3/7.4±1.4 2.2 PBMT improvement versus 0.5 placebo, p=0.0012 Pre/post PBMT: 6.7±1.6/4.9±1.3. Pre/post placebo: 7.8±1.6/7.8±1.3 1.8 PBMT improvement versus 0.0 placebo, p<0.0001 Pre/post PBMT: 67.5±13.2/48.9±7.2. Pre/post placebo: 66.7±11.9/61.5±10.0 18.6 PBMT improvement versus 5.2 placebo, p=0.0003 All significant reductions in PBMT group, versus only physical impairment in placebo group |
||
| McGill Pain Questionnaire |
0-78 (no pain → severe pain) Pre/post PBMT: 45/32.1. Pre/post placebo: 47.5/42.6 p=0.0078 Pre/post PBMT: 6.58/4.06. Pre/post placebo: 5.81/5.34 p=0.002 |
||
| VAS |
Pre/post PBMT: 6.58/4.06. Pre/post placebo: 5.81/5.34 p=0.002 |
||
| Tramontana et al. (2017) [38] |
FM patients = 10 Laser = 5 Placebo = 5 |
Reduction of conventional treatment doses (50% average) cortisone, duloxetine, pregabalin |
Laser/placebo First evaluation: 34.85%/42.43% After 15 days: 9.7%/31.06% (p=0.001) |
| White et al. (2018) [41] | FM patient = 1 | VAS |
6-7/1-2 after treatment 1 for 1 week/returned to 3-3.5 after 1 week 0-1 after treatment 4, lasting >10d |
| Baseline scores |
WPI = 10, SSS = 7, FIQ/SIQ = 38.3, SF-36: Physical functioning = 45, role-emotional = 0, role-physical = 0, social functioning = 25, general health = 25, pain = 10, emotional well-being = 56, energy/fatigue = 30 | ||
| NRS pain relief (0 = none, 10 = complete pain relief) |
Post treatment 1 = 7 Post treatment 2 = 6, lasted 4 days Post treatment 3 = 2-3, lasting 2-3 hours. Pain returned to baseline after 1 week Post treatment 4 = 8-9, lasting >10d. After 2w pain symptoms returned to >50% baseline |
||
| Wu et al. (2018) [39] |
FM patients = 15 |
VAS NTP FIQR Beck Depression Inventory (BDI) (0-63, >30 = severe) Pittsburgh Sleep Quality Index (PSQI) |
Pre IV laser irradiation/24 hours after last treatment 7.86/5 (p=0.001) 15/13.27 (p=0.002) 74.08/51.43 (p=0.001) 18.29/8.34 (p=0.003) 15.99/10.72 (p=0.01) |
3.6. Meta-Analysis
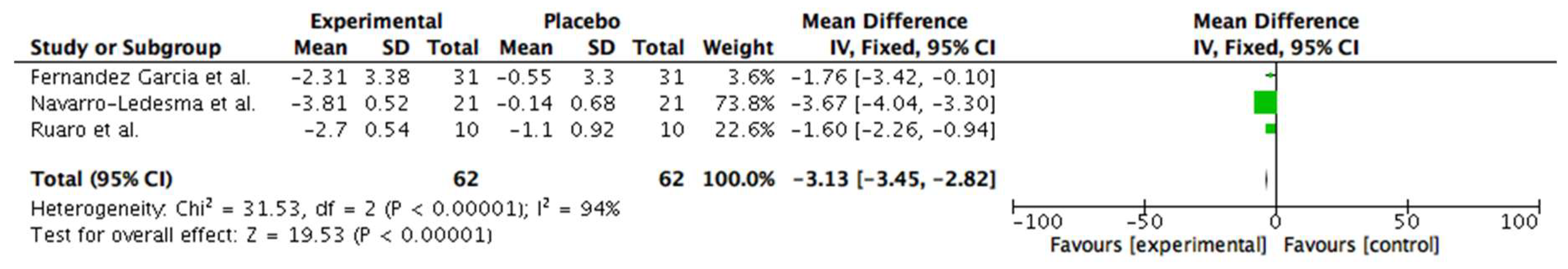
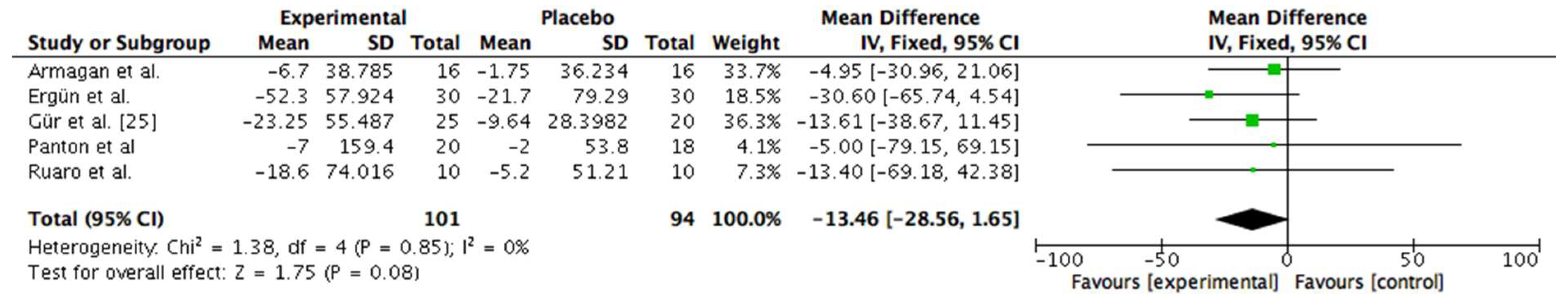
6.3.1. Clinical Heterogenity Considering Application of the Device
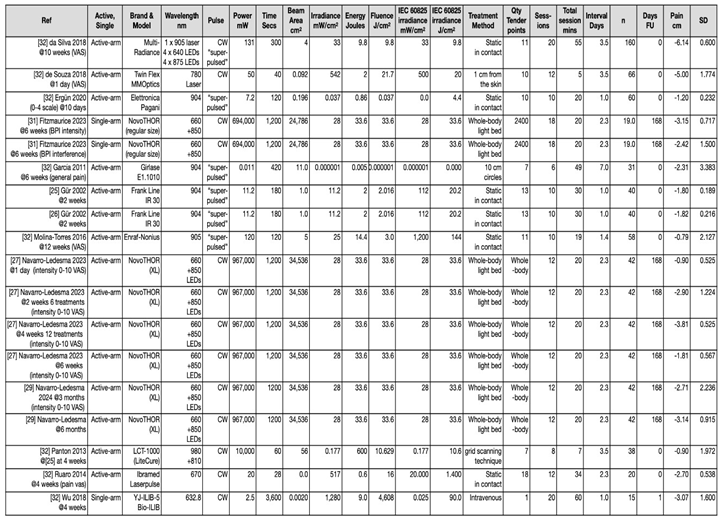
3.6. Systematic Review-Based Evidence and Summary of Meta-Analyses (n=3)
3.7. Qualitative Data (n = 6)
| Paper | Feasibility outcomes pertaining to treatment and schedule | Qualitative outcomes |
|---|---|---|
| de Souza et al. (2018) [42] Mixed methods (n = 66) |
Qualitative component on perceptions of efficacy and well-being: Effectiveness: PBMT Yes: n = 33, No: n = 0; LA Yes: 32 No: 1 Improvement of well-being: PBMT Yes: 32 No: 1; LA Yes: 27 No: 6 |
|
| Diniz et al. (2021) [46] Case report (n = 1) |
During 1st ILIB application for 30 minutes, patient slept sitting in the dental chair. Got up from the chair much better than when she arrived. “She felt a sense of improvement as soon as we completed the laser therapy applications at the trigger points and ILIB” |
Baseline: all teeth aching, pain and burning in face, neck, hips, knees and hands – presented to ED with tooth ache (X rays normal). Poor sleep, waking up with burning hands, patient reported already attempted suicide with husband’s gun to try and end the pain she felt every day. No longer working due to FM: due to severe pain for consecutive days felt too insecure to dedicate herself to activity outside the home. Previously consulted with physiotherapy and psychologist. Medications: escitalopram, pregabalin, topiramate, cyclobenzaprine, bupropion Post treatment: patient reported her pains are completely gone for 2 months, felt much better, feels she seems to have changed, more patient with life events and the people around her, had the courage to travel with her husband. Did not take analgesics, anti-inflammatories, or muscle relaxants during this period. After 2 months, pains were coming back |
| Fitzmaurice et al. (2023) [31] Mixed methods (n = 19) |
Barriers to uptake: 7 could not commit time to treatment schedule, 3 felt would be too fatigued by travel, 1 could not afford petrol (lived >20miles away), 1 worried about personal unreliability due to flare unpredictability, 2 uncontactable, 1 move area, 1 trying for pregnancy, 1 claustrophobic Acceptability of treatment schedule: 12 satisfied with number and frequency of sessions, 5 would have preferred higher frequency (daily), 1 would prefer less due to travel money Acceptability of trial device: 13 felt device easy to access. Suggestions for support rail to make entry and exit easier. 2 were required to remove fentanyl patch for each treatment. Claustrophobic? 79.3% strongly disagree. Comfortable in underwear? 84.1% strongly agree Easy to operate? 100% strongly agree Comfortable to use? 79.3% agree or strongly agree Willingness towards future trial: all participants willing to be involved in future research relating to this device |
Treatment satisfaction Positive experiences: helpful (n = 4), pleasant (n = 3), positive (n = 3), enjoyable (n = 2), comfortable (n = 1), efficient (n = 1), great (n = 1), useful (n = 1), interesting (n = 1), painless (n = 1), quick (n = 1), beneficial (n = 1), easy (n =1), worthwhile (n = 1), necessary (n = 1) Low-energy positive emotions: relaxing (n = 11), calming (n = 3), soothing (n = 2) High-energy positive emotions: pain relief (n = 4), warm (n = 3), better memory (n = 2), good mood (n = 2), better sleep (n = 1), more energy (n = 1), less confused (n = 1), reduced headaches (n = 1), clearer minded (n = 1), addictive (n = 1), fun (n = 1) Future-related: hope (n = 1) Negative experience: pain made it ‘difficult’ to adhere to treatment schedule (n =1) |
| Fitzmaurice et al. (2024) [30] Qualitative (n = 16) |
Subthemes (n = 5) highlighting intervention experience Positive PBMT experience Attributable changes to PBMT Recommendation to others Fear of treatment ending Unanticipated effects |
Themes (n = 3) and subthemes (n = 18) highlighting treatment response Body structure and function Improvement in pain; Reduced lethargy and fatigue; Improved sleep; Mood lifted; Reduction in analgesics and interventional therapy; Reduction in stiffness and improved mobility; Anxiety and agitation decreased; Improved memory and concentration; Reduction in time needed to mobilise; Brain fog cleared; Enjoyment of body warmth; Reduced number and intensity of flares Activities and participation Starting/re-commencing hobbies/enjoyable activities; More able to cope with everyday chores/tasks/work; More willing to engage in activities with others Environment Noticeable physical and emotional improvements; Improved relationships; Insight and reduced reliance on poor habits 4 subsequent processes identified: increased motivation, feeling proud, improved confidence, feeling like ‘old self’ Ultimately identifying a ‘recomposition phase’ in the FM condition, further described by 5 final sub-themes: Ability to cope with and push aside other symptoms; Self-awareness and insight into symptom interlinkage; Improved pain and sleep having substantial effect on mood, energy, confidence, motivation and ability to cope; Improved sleep and more importantly improved sleep hygiene; New-found enjoyment in hobbies secondary to improved memory and concentration |
| Moore et al. (2012) [44] Case report (n = 1) |
Despite an increase in symptoms 2 weeks post PBMT patient felt ADLs were not adversely affected | |
| White et al. (2018) [41] Case report (n = 1) |
Baseline: Difficulty primarily expressed for household chores, lifting and carrying groceries, climbing stairs, prolonged sitting – taken from FIQ. Medical problems stopped her from accomplishing weekly goals. Reported symptoms of depression, difficulty sleeping, memory and balance problems, sensitivity to: touch, loud noises, bright lights, odours, cold. Previously tried a number of treatments including limited series of PBMT (810-980 nm). Hydrocodone 5mg 4-5 times per month for acute FM flares. Post treatment 1: Improved range of motion, mood, level of physical activity, quality of sleep lasting for 1 week. Required no opioid analgesics during this time. Stated she would be willing to pay $90USD out-of-pocket per treatment session to continue receiving 42W and 75W HILT 2-3 times per month Post treatment 4: No longer using opioid analgesics |
3.8. Use of Validated Outcome Measures
3.9. Results to Address NICE Concerns
3.3.1. NICE Critical Outcomes
3.3.1.1. Pain Reduction
3.3.1.2. Health-Related Quality of Life
3.3.1.3. Physical Function
3.3.1.4. Psychological Distress
3.3.1.5. Pain Interference
3.3.1.6. Pain Self-Efficacy
3.3.2. NICE Important Outcomes
3.3.2.1. Use of Healthcare Services
3.3.2.2. Sleep
3.3.2.3. Discontinuation
4. Discussion
4.1. NICE Guidance
4.2. Limitations
4.3. Recommendations for Future Research and Clinical Implications
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgements
Conflicts of Interest
References
- Yeh, S.W., et al., Low-level laser therapy for fibromyalgia: A systematic review and meta-analysis. Pain Physician, 2019. 22(3): p. 241-254.
- National Institute for Health and Care Excellence: Guidelines, in Chronic pain (primary and secondary) in over 16s: assessment of all chronic pain and management of chronic primary pain. 2021, National Institute for Health and Care Excellence (NICE). Copyright © NICE 2021.: London.
- Boomershine, C.S., A comprehensive evaluation of standardized assessment tools in the diagnosis of fibromyalgia and in the assessment of fibromyalgia severity. Pain Res Treat, 2012. 2012: p. 653714.
- Döhmen, A., et al., Are OMERACT recommendations followed in clinical trials on fibromyalgia? A systematic review of patient-reported outcomes and their measures. Qual Life Res, 2023. 32(6): p. 1521-1536.
- Organization, W.H. ICD-11 for Mortality and Morbidity Statistics. 2023 [cited 2023 11th September]; Available from: https://icd.who.int/browse11/l-m/en#!
- Excellence, N.I.f.H.a.C. Chronic pain (primary and secondary) in over 16s: assessment of all chronic pain and management of chronic primary pain. 2021 [cited 2024 3rd March]; Available from: https://www.nice.org.uk/guidance/ng193/chapter/Recommendations.
- Excellence, N.I.f.H.a.C. Chronic pain (primary and secondary) in over 16s: assessment of all chronic pain and management of chronic primary pain [H] Evidence review for electrical physical modalities for chronic primary pain. 2021 [cited 2024 8th October ]; Available from: https://www.nice.org.uk/guidance/ng193/evidence/h-electrical-physical-modalities-for-chronic-primary-pain-pdf-9071987013.
- Health, N.N.I.f. and E. Care, Chronic pain (primary and secondary) in over 16s: assessment of all chronic pain and management of chronic primary pain 2021, NICE - National Institute for Health and Care Excellence.
- Kate, F., et al., Qualitative evidence synthesis for complex interventions and guideline development: clarification of the purpose, designs and relevant methods. BMJ Global Health, 2019. 4(Suppl 1): p. e000882.
- England, P.P.T.N. Patient and Public Voice Partners Policy. 2017 [cited 2023 7th October]; Available from: https://www.england.nhs.uk/wp-content/uploads/2017/08/patient-and-public-voice-partners-policy-july-2017.pdf.
- Fitzmaurice, B.C., et al., The Fibromyalgia Decomposition Phenomenon: A Reflexive Thematic Analysis. Behav Sci (Basel), 2024. 14(1).
- Unni, G. and J.H. Steven, Institutionalising an evidence-informed approach to guideline development: progress and challenges at the World Health Organization. BMJ Global Health, 2018. 3(5): p. e000716.
- Peters, M.D.J., et al., Best practice guidance and reporting items for the development of scoping review protocols. JBI Evid Synth, 2022. 20(4): p. 953-968.
- Tricco, A.C., et al., PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann Intern Med, 2018. 169(7): p. 467-473.
- Excellence, N.I.f.H.a.C. Low-level laser therapy for preventing or treating oral mucositis caused by radiotherapy or chemotherapy (Interventional procedures guidance [IPG615]). 2018 [cited 2020 18th July]; Available from: https://www.nice.org.uk/guidance/ipg615.
- Ltd., T.P. NovoTHOR. 2019-2023 [cited 2021 8th December]; Available from: https://www.novothor.com/?utm_source=google&utm_medium=cpc&keyword=novothor&utm_campaign=602860951&gad=1&gclid=CjwKCAjww7KmBhAyEiwA5-PUSgUI0Ry2inGx2zfW936PictCTP41gfbKRfJXWx3lQ7m5qovrNDs8VBoCnlUQAvD_BwE.
- Magee, K., et al., Implementation of a light therapy team to administer photobiomodulation therapy: A standardized protocol to prevent and treat oral mucositis in the pediatric hematopoietic stem cell transplant population. Pediatr Blood Cancer, 2024. 71(6): p. e30966.
- Pritchard, M., et al., Utilization of Photobiomodulation for the Prevention and Treatment of Oral Mucositis. J Pediatr Hematol Oncol Nurs, 2024. 41(2): p. 107-113.
- Hoffmann, T.C., et al., Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. Bmj, 2014. 348: p. g1687.
- Hong QN, Pluye P, Fàbregues S, Bartlett G, Boardman F, Cargo M, Dagenais P, Gagnon M-P, Griffiths F, Nicolau B, O’Cathain A, Rousseau M-C, Vedel I. Mixed Methods Appraisal Tool (MMAT), version 2018. Registration of Copyright (#1148552), Canadian Intellectual Property Office, Industry Canada.
- Howick, J., Chalmers, I., Glasziou, P., Greenhalgh, T., Heneghan, C., Liberati, A. Moschetti, I., Phillips, B., Thornton, H. (2011). Explanation of the 2011 Oxford Centre for Evidence-Based Medicine (OCEBM) Levels of Evidence (Background Document). Oxford Centre for Evidence-Based Medicine. https://www.cebm.ox.ac.uk/resources/levels-of-evidence/ocebm-levels-of-evidence.
- Shea, B. J., Reeves, B. C., Wells, G., Thuku, M., Hamel, C., Moran, J., Moher, D., Tugwell, P., Welch, V., Kristjannsson, E., Henry, D. A. (2017). AMSTAR 2: A critical appraisal tool for systematic reviews that include rnadomised or non-randomised studies of healthcare interventions, or both. British Medical Journal, 358. [CrossRef]
- PRISMA. PRISMA Flow Diagram 2020; Available from: https://www.prisma-statement.org/prisma-2020-flow-diagram.
- Page, M.J., et al., The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ, 2021. 372: p. n71.
- Gür, A., et al., Effects of low power laser and low dose amitriptyline therapy on clinical symptoms and quality of life in fibromyalgia: a single-blind, placebo-controlled trial. Rheumatol Int, 2002. 22(5): p. 188-93.
- Gür, A., et al., Efficacy of low power laser therapy in fibromyalgia: a single-blind, placebo-controlled trial. Lasers in medical science, 2002. 17(1): p. 57-61.
- Navarro-Ledesma, S., et al., Short-Term Effects of Whole-Body Photobiomodulation on Pain, Quality of Life and Psychological Factors in a Population Suffering from Fibromyalgia: A Triple-Blinded Randomised Clinical Trial. Pain Ther, 2023. 12(1): p. 225-239.
- Navarro-Ledesma S, C.J.G.-M.A.P.L.B.P., Changes in Circadian Variations in Blood Pressure, Pain Pressure Threshold and the Elasticity of Tissue after a Whole-Body Photobiomodulation Treatment in Patients with Fibromyalgia: A Tripled-Blinded Randomized Clinical Trial. Biomedicines, 2022.
- Navarro-Ledesma, S., et al., Outcomes of whole-body photobiomodulation on pain, quality of life, leisure physical activity, pain catastrophizing, kinesiophobia, and self-efficacy: a prospective randomized triple-blinded clinical trial with 6 months of follow-up. Frontiers in Neuroscience, 2024. 18(pagination): p. Article Number: 1264821. Date of Publication: 2024.
- Fitzmaurice, B.C., et al., Whole-Body Photobiomodulation Therapy Propels the Fibromyalgia Patient into the Recomposition Phase: A Reflexive Thematic Analysis. Biomedicines, 2024. 12(5): p. 1116.
- Fitzmaurice, B.C., et al., Whole-Body Photobiomodulation Therapy for Fibromyalgia: A Feasibility Trial. Behavioral sciences (Basel, Switzerland), 2023. 13(9): p. 717. [CrossRef]
- Dos Santos, R.C., et al., Acute low-level laser therapy effects on peripheral muscle strength and resistance in patients with fibromyalgia. Lasers in medical science, 2020. 35(2): p. 505-510.
- Armagan, O., et al., Long-term efficacy of low level laser therapy in women with fibromyalgia: A placebo-controlled study. Journal of Back and Musculoskeletal Rehabilitation, 2006. 19(4): p. 135-140.
- Ergün, G., E. Atalar, and G. Aydın, Assessment of the efficiency of low level laser therapy in women with primary fibromyalgia syndrome: a randomized, placebo-controlled, double-blind study. Acta Oncologica Turcica. 53(2): p. 288-293.
- Fernández García, R., et al., Using a laser based program in patients diagnosed with fibromyalgia. Reumatologia clinica, 2011. 7(2): p. 94-97.
- MolinaTorres, G., et al., Laser Therapy and Occlusal Stabilization Splint for Temporomandibular Disorders in Patients With Fibromyalgia Syndrome: A Randomized, Clinical Trial. Alternative Therapies in Health and Medicine, 2016. 22(5): p. 23-31.
- Panton, L., et al., Effects of class IV laser therapy on fibromyalgia impact and function in women with fibromyalgia. Journal of Alternative and Complementary Medicine, 2013. 19(5): p. 445-452.
- Tramontana, A., et al., Evaluating integrative approaches for fibromyalgia in physical and rehabilitation medicine (PM&R). Archives of Physical Medicine and Rehabilitation, 2017. 98(10): p. e65-e66.
- Yi Wu, P., et al., Effects of Intravenous Laser Irradiation of Blood on Pain, Function and Depression of Fibromyalgia Patients. Genetics in Medicine, 2018. 6: p. 1-8.
- da Silva, M.M., et al., Randomized, blinded, controlled trial on effectiveness of photobiomodulation therapy and exercise training in the fibromyalgia treatment. Lasers Med Sci, 2018. 33(2): p. 343-351.
- White, P.F., et al., Treatment of drug-resistant fibromyalgia symptoms using high-intensity laser therapy: a case-based review. Rheumatology international, 2018. 38(3): p. 517-523.
- de Souza, R.C.V., et al., Low-level laser therapy and anesthetic infiltration for orofacial pain in patients with fibromyalgia: a randomized clinical trial. Medicina oral, patologia oral y cirugia bucal, 2018. 23(1): p. e65-e71.
- Ruaro, J.A., et al., Low-level laser therapy to treat fibromyalgia. Lasers in medical science, 2014. 29(6): p. 1815-1819.
- Moore, J. and T. Demchak, Treatment of fibromyalgia syndrome with low-level laser therapy. International Journal of Athletic Therapy and Training, 2012. 17(4): p. 28-31.
- Ruaro, J.A., et al., Low-level laser therapy to treat fibromyalgia. Lasers Med Sci, 2014. 29(6): p. 1815-9.
- Diniz, V.H.P., A.D. Vial, and R.T.D. Alves. Effectiveness of blood irradiation by modified intravenous laser (ILIB) on the clinical parameters of fibromyalgia. 2021.
- Gikaro, J.M., et al., Efficacy of electrophysical agents in fibromyalgia: A systematic review and network meta-analysis. Clinical rehabilitation, 2023. 37(10): p. 1295-1310.
- Vayvay, E.S., et al., The effect of Laser and taping on pain, functional status and quality of life in patients with fibromyalgia syndrome: A placebo- randomized controlled clinical trial. Journal of back and musculoskeletal rehabilitation, 2016. 29(1): p. 77-83.
- Honda, Y., et al., Effects of Physical-Agent Pain Relief Modalities for Fibromyalgia Patients: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Pain Res Manag, 2018. 2018: p. 2930632.
- Germano Maciel, D., et al., Low-level laser therapy combined to functional exercise on treatment of fibromyalgia: a double-blind randomized clinical trial. Lasers in medical science, 2018. 33(9): p. 1949-1959.
- Matsutani, L.A., et al., Effectiveness of muscle stretching exercises with and without laser therapy at tender points for patients with fibromyalgia. Clinical & Experimental Rheumatology, 2007. 25(3): p. 410-415.
- Bennett, R.M., et al., The Revised Fibromyalgia Impact Questionnaire (FIQR): validation and psychometric properties. Arthritis Res Ther, 2009. 11(4): p. R120.
- Mannerkorpi, K. and C. Hernelid, Leisure Time Physical Activity Instrument and Physical Activity at Home and Work Instrument. Development, face validity, construct validity and test-retest reliability for subjects with fibromyalgia. Disabil Rehabil, 2005. 27(12): p. 695-701.
- Petrini, L. and L. Arendt-Nielsen, Understanding Pain Catastrophizing: Putting Pieces Together. Frontiers in Psychology, 2020. 11.
- Sadovsky, R. (2002). Clinically importance changes in pain severity on VAS. American Family Physician, 65; 1915-1921.
- Williams, D. A., Arnold, L. M. (2011). Measures applied to the Assessment of Fibromyalgia. Arthritis Care Research, 63 (0 11): S86-S97. [CrossRef]
- Liebert, A., et al., Photophysical Mechanisms of Photobiomodulation Therapy as Precision Medicine. Biomedicines, 2023. 11(2): p. 237.
- González-Muñoz, A., et al., Efficacy of Photobiomodulation Therapy in the Treatment of Pain and Inflammation: A Literature Review. Healthcare, 2023. 11(7): p. 938.
- Mease, P., et al., Fibromyalgia syndrome module at OMERACT 9: domain construct. J Rheumatol, 2009. 36(10): p. 2318-29.
- Bell, T., et al., Meta-analysis of cognitive performance in fibromyalgia. J Clin Exp Neuropsychol, 2018. 40(7): p. 698-714.
- Vincent, A., et al., Beyond pain in fibromyalgia: insights into the symptom of fatigue. Arthritis Research & Therapy, 2013. 15(6): p. 221.
- Mease, P.J., et al. OMERACT 7 Workshop Fibromyalgia Syndrome. 2005.
- Fayaz, A., et al., Prevalence of chronic pain in the UK: a systematic review and meta-analysis of population studies. BMJ open, 2016. 6(6): p. e010364.
- Excellence, N.I.f.H.a.C. Low back pain and sciatica in over 16s: assessment and management. NG59 2014 [cited 2024 19th December]; Available from: https://www.nice.org.uk/guidance/ng59.
- NICE. Methods for the development of NICE public health guidance (third edition) [PMG4]. 2012 [cited 2025 8th January ]; Available from: https://www.nice.org.uk/process/pmg4/chapter/reviewing-the-scientific-evidence.
- Hadis, M. A., Zainal, S. A., Holder, M. J., Carroll, J. D., Cooper, P. R., Milward, M. R. Palin, W. M. (2016). The dark art of light measurement: accurate radiometry for low-level light therapy. Lasers Medical Science, 10; 789-809.
- Van Breugel, H.H. and P.R. Bär, Power density and exposure time of He-Ne laser irradiation are more important than total energy dose in photo-biomodulation of human fibroblasts in vitro. Lasers Surg Med, 1992. 12(5): p. 528-37.
- 2014 NAALT/WALT Meeting Nomenclature Breakout. 2015 [cited 2024 19th December]; Available from: https://www.naalt.org/whitepapers/2014-naalt-walt-meeting-nomenclature-breakout/.
- Hadis, M.A., et al., The dark art of light measurement: accurate radiometry for low-level light therapy. Lasers Med Sci, 2016. 31(4): p. 789-809.
- Bjordal, J.M., et al., A systematic review with meta-analysis of the effect of low-level laser therapy (LLLT) in cancer therapy-induced oral mucositis. Support Care Cancer, 2011. 19(8): p. 1069-77.
- Wilson, N., et al., UK healthcare services for people with fibromyalgia: results from two web-based national surveys (the PACFiND study). BMC Health Serv Res, 2022. 22(1): p. 989.
- Hudson, Jemma, Imamura, Mari, Robertson, Clare, Whibley, Daniel, Aucott, Lorna, Gillies, Katie, Manson, Paul, Dulake, Debra, Abhishek, Abhishek, Tang, Nicole K. Y., Macfarlane, Gary and Brazzelli, Miriam (2025) Effects of pharmacological and non-pharmacological interventions for the management of sleep problems in people with fibromyalgia: systematic review and network meta-analysis of randomised controlled trials. Arthritis Care & Research. (In Press) ISSN 2151-464X. [CrossRef]
- Hong-Baik, I., Úbeda-D’Ocasar, E., Cimadevilla-Fernández-Pola, E., Jiménez-Díaz-Benito, V., & Hervás-Pérez, J. P. (2023). The Effects of Non-Pharmacological Interventions in Fibromyalgia: A Systematic Review and Metanalysis of Predominants Outcomes. Biomedicines, 11(9), 2367. [CrossRef]
- Hernando-Garijo I, Jiménez-del-Barrio S, Mingo-Gómez T, Medrano-de-la-Fuente R, Ceballos-Laita L. Effectiveness of non-pharmacological conservative therapies in adults with fibromyalgia: A systematic review of high-quality clinical trials. Journal of Back and Musculoskeletal Rehabilitation. 2022;35(1):3-20. [CrossRef]
- Kundakci, Buraka,b,c,*; Kaur, Jaspreeta,b,d; Goh, Siew Lie; Hall, Michellea,b,f,g; Doherty, Michaela,b,f; Zhang, Weiyaa,b,f; Abhishek, Abhisheka,b,f. Efficacy of nonpharmacological interventions for individual features of fibromyalgia: a systematic review and meta-analysis of randomised controlled trials. PAIN 163(8):p 1432-1445, August 2022. |. [CrossRef]
- Surendran, S., Mithun, C. B. (2018). FRIO647 estimattion of minimum clinically important difference in fibromyalgia for fiqr using bpi as anchor measure. Annals of the Rheumatic Diseases, 77, 845. [CrossRef]
- Lim, M., et al., Increased Low- and High-Frequency Oscillatory Activity in the Prefrontal Cortex of Fibromyalgia Patients. Front Hum Neurosci, 2016. 10: p. 111.
- McCabe, C., K. Claxton, and A.J. Culyer, The NICE Cost-Effectiveness Threshold. PharmacoEconomics, 2008. 26(9): p. 733-744.
- Al-Janabi, H., I. Williams, and M. Powell, Is the NHS underfunded? Three approaches to answering the question. Journal of the Royal Society of Medicine, 2023. 116(12): p. 409-412.
| Paper | Aims, design, outcomes and AMSTAR2 rating | Results synthesis pertaining to FM and PBMT | Author comments/conclusions |
|---|---|---|---|
| Gikaro et al. (2023) [47] Systematic review and network meta-analysis (n = 3045) |
To examine effectiveness of electrophysical agents in FM (looked at 25 treatments) 54 studies, RCTs comparing therapy to control/placebo/no treatment Primary outcomes: pain, functional status, mood Pain assessment: VAS, NPRS, BPI, Likert scale Functional assessment: Original or revised FIQ Mood assessment: Beck’s Depression Inventory, Depression Anxiety Stress Scale-21, Hamilton Depression Rate Scale, Hamilton Depression and Anxiety Scale, HADS – depression domain, Montgomery Asberg Rating Scale AMSTAR2 rating: High |
Node-splitting method showed significant local inconsistency for comparisons between placebo and PBMT (p=0.001), PBMT and no treatment (p=0.000) PBMT comparison to placebo Pain (47 studies): pooled SMD (95% CI) = -1.18 (-4.20 to 1.85), p=0.446, likelihood of being best agent 0.1%, worst agent 0%, surface under the cumulative ranking (SUCRA) 80% Function (31 studies) and mood (26 studies): pooled SMD (95% CI) -0.57 (-1.66 to 0.52), p=0.304, likelihood of being best agent 0.3%, worst agent 0.1%, SUCRA 60%. Consistency model did not show significant improvement after intervention for PBMT alone, but combination of PBMT and exercise therapy had highest SUCRA (80%) Mood: SUCRA revealed PBMT amongst agents most likely to be suitable for managing mood – 70% Side-split analysis from network meta-analysis examining effects expressed as beta coefficients (standard error) PAIN Placebo c/w PBMT: direct -0.86 (0.98), indirect -7.37 (1.72), difference 6.50 (1.98), p=0.001 Amitriptyline c/w PBMT: direct -1.05 (1.94), indirect -4.51 (4.05), difference 3.47 (4.49), p=0.442 Exercise c/w PBMT: direct -3.71 (1.91), indirect -0.14 (2.07), difference -3.57 (2.82), p=0.206 Lidocaine c/w PBMT: direct -0.18 (1.92), indirect -4.93 (632.37), difference 4.75 (632.39), p=0.994 PBMT c/w no treatment: direct 9.54 (1.60), indirect 0.96 (1.05), difference 8.57 (1.92), p=0.000 PBMT c/w occlusal splint: direct -0.20 (1.92), indirect 4.93 (633.02), difference -5.13 (633.02), p=0.994 PBMT c/w taping: direct -0.17 (1.91), indirect 6.13 (3.97), difference -6.30 (4.40), p=0.153 FUNCTION Placebo c/w PBMT: direct -0.89 (0.61), indirect 0.88 (1.29), difference -1.77 (1.42), p=0.213 Amitriptyline c/w PBMT: direct -0.67 (1.20), indirect 2.24 (2.43), difference -2.92 (2.71), p=0.282 Exercise c/w PBMT: direct 1.83 (1.10), indirect -1.27 (1.10), difference 3.09 (1.55), p=0.047 PBMT c/w no treatment: direct 0.57 (1.23), indirect 1.01 (0.99), difference 1.47 (2.54), p=0.562 |
PBMT (and microcurrent) had the highest probability of being among the best modalities for pain relief PBMT (and microcurrent, and transcranial magnetic stimulation) had highest probability of being the best electrophysical agents for managing mood disorders Low-moderate quality evidence that PBMT (and microcurrent, and transcranial magnetic stimulation) are the most effective electrophysical agents for improving at least one outcome in FM |
| Honda et al. (2018) [49] Systematic review and meta-analysis of RCTs (n = 470) |
To investigate the effects of physical-agent modalities (PBMT, thermal therapy, electromagnetic field therapy, TENS) for pain relief in FM. 5 PBMT RCTs out of 11 RCTs analysed Primary outcome: pain relief (VAS) Secondary outcomes: NTP (subjective), FIQ, QoL scores (SF-36) AMSTAR2 rating: Low |
PBMT comparison to control group PAIN No reduction (mean difference: -4.00; 95% CI -23.4 to 15.4, p=0.69) NTP Significant reduction (-2.21; 95% CI -3.51 to -0.92, I2=42%, p=0.0008) FIQ Significant reduction (-4.35; 95% CI -6.69 to -2.01, I2=62%, p=0.03) QoL SF-36 No significant difference (5.80; 95% CI -4.72 to 16.32, p=0.28) |
PBMT had a partial effect on pain relief in FM patients, and this beneficial effect may have a positive influence on FM patients’ health status |
| Yeh et al. (2019) [1] Systematic review and quantitative meta-analysis (n = 325) |
To determine the efficacy of PBMT on patients with FM Analysed all published RCTs (n = 9), evaluating effect of PBMT on FM (with or without exercise programme) Primary outcomes: FIQ, pain severity, NTP Secondary outcomes: fatigue, stiffness, anxiety, depression Pain assessment methods: i. extraction of “pain” subitem from FIQ (0-10), ii. 5-point Likert scale (0 = none → 4 = extreme), iii. VAS NTP: i. points that were reported by patients as being painful, ii. more rigorous definition = patients felt pain at a pressure ≤2.6kgf/cm2 Fatigue: i. extraction of “fatigue” subitem from FIQ (0-10), ii. Likert score Stiffness: i. extraction of “stiffness” subitem from FIQ (0-10), ii. Likert score for ‘morning stiffness’ Anxiety: extraction of “anxiety” subitem from FIQ (0-10) Depression: extraction of “depression” subitem from FIQ (0-10) AMSTAR2 rating: Low |
Note: of the 9 RCTs, only included here the relevant ones to this scoping review. Table 1 and Table 2 describe individual study results. Included here also as extra results and/or presented differently, derived from pooled meta-analysis PBMT comparison to placebo FIQ (p<0.00001) Monowavelength PBMT: overall significant improvement compared with placebo (pooled SMD: 1.16; 95% CI 0.64-1.69, I2 = 47%) Amargan 2006: mean change 7±7.59; placebo 1.75±7.37 (SMD 0.68; 95% CI -0.03-1.40) Garcia 2011: mean change 10.56±10.97; placebo 1.93±8.96 (SMD 0.84; 95% CI 0.10-1.58) Gur 2002: mean change 23.25±8.58; placebo 9.64±6.63 (SMD 1.75; 95% CI 1.09-2.41) Ruaro 2014: mean change 18.6±9.64; placebo 5.2±8.66 (SMD 1.40; 95% CI 0.40-2.40) Combined PBMT/LED phototherapy: 1 RCT, significant improvement da Silva 2018: mean change 0.06±0.06; placebo 0.01±0.02 (SMD 1.10; 95% CI 0.43-1.77) PAIN SEVERITY (p=0.0009) Monowavelength PBMT: overall significant improvement compared with placebo (pooled SMD: 1.18; 95% CI 0.82-1.54, I2 = 0%) Garcia 2011: mean change 2.31±2.1; placebo 0.73±1.82 (SMD 0.78; 95% CI 0.05-1.52) Gur 2002: mean change 1.8±0.51; placebo 1±0.63 (SMD 1.37; 95% CI 0.75-2.00) Gur 2002: mean change 1.82±0.54; placebo 1.04±0.71 (SMD 1.21; 95% CI 0.53-1.89) Ruaro 2014: mean change 2.7±1.14; placebo 1.1±1.16 (SMD 1.33; 95% CI 0.34-2.32) Combined PBMT/LED phototherapy: 1 RCT, significant improvement (larger effect than monowavelength) da Silva 2018: mean change 0.62±0.08; placebo 0.14±0.03 (SMD 7.79; 95% CI 5.89-9.69) NTP (p=0.002) Monowavelength PBMT: overall significant improvement compared with placebo (pooled SMD: 1.01; 95% CI, 0.49-1.52, I2 = 49%) Amargan 2006: mean change 1.87±1.55; placebo 1.06±1.63 (SMD 0.50; 95% CI -0.21-1.20) Gur 2002: mean change 7.52±2.82; placebo 3.9±2.77 (SMD 1.27; 95% CI 0.66-1.99) Gur 2002: mean change 6.55±2.79; placebo 4.15±3.65 (SMD 0.72; 95% CI 0.08-1.37) Ruaro 2014: mean change 4.3±1.74; placebo 1.4±1.16 (SMD 1.88; 95% CI 0.79-2.97) Combined PBMT/LED phototherapy: 1 RCT, significant improvement (larger effect than monowavelength) da Silva 2018: mean change 0.54±0.08; placebo 0.05±0.09 (SMD 5.64; 95% CI 4.20-7.08) FATIGUE (p<0.00001) Monowavelength PBMT: overall significant improvement compared with placebo (pooled SMD: 1.4; 95% CI, 0.96-1.8) Garcia 2011: mean change 4.32±1.28; placebo 2.01±2.48 (SMD 1.15; 95% CI 0.38-1.92) Gur 2002: mean change 1.8±0.79; placebo 0.76±0.65 (SMD 1.42; 95% CI 0.79-2.04) Gur 2002: mean change 1.73±0.84; placebo 0.06±0.78 (SMD 2.02; 95% CI 1.24-2.79) Ruaro 2014: mean change 2.1±1.52; placebo 0.8±1.28 (SMD 0.89; 95% CI -0.04-1.81) Combined PBMT/LED phototherapy: 1 RCT, significant improvement da Silva 2018: mean change 0.08±0.1; placebo 0.02±0.04 (SMD 0.77; 95% CI 0.13-1.42) STIFFNESS (p<0.0001) Monowavelength PBMT: overall significant improvement compared with placebo (pooled SMD: 0.92; 95% CI, 0.36-1.48) Amargan 2006: mean change 0.62±0.48; placebo 0.56±0.65 (SMD 0.10; 95% CI -0.59-0.80) Gur 2002: mean change 1.6±0.75; placebo 0.76±0.68 (SMD 1.15; 95% CI 0.55-1.76) Gur 2002: mean change 1.45±0.68; placebo 0.69±0.65 (SMD 1.12; 95% CI 0.45-1.79) Ruaro 2014: mean change 1.7±1.33; placebo -0.2±1.2 (SMD 1.44; 95% CI 0.43-2.44) Combined PBMT/LED phototherapy: 1 RCT, significant improvement da Silva 2018: mean change 0.11±0.07; placebo 0.03±0.02 (SMD 1.52; 95% CI 0.81-2.44) ANXIETY (p<0.00001) Monowavelength PBMT: overall significant improvement compared with placebo (pooled SMD: 1.46; 95% CI, 0.45-2.47) Ruaro 2014: mean change 2.2±1.18; placebo 0.5±1.05 (SMD 1.46; 95% CI 0.45-2.47) Combined PBMT/LED phototherapy: 1 RCT, significant improvement da Silva 2018: mean change 0.08±0.06; placebo 0.0±0.02 (SMD 1.75; 95% CI 1.01-2.49) DEPRESSION (p=0.001) Monowavelength PBMT: overall significant improvement compared with placebo (pooled SMD: 1.46; 95% CI, 0.93-2.00) Gur 2002: mean change 7.76±4.2; placebo 2.27±3.18 (SMD 1.45; 95% CI 0.82-2.08) Ruaro 2014: mean change 1.8±1.16; placebo 0±1.16 (SMD 1.49; 95% CI 0.47-2.50) Combined PBMT/LED phototherapy: 1 RCT, no significant improvement da Silva 2018: mean change 0.16±0.12; placebo 0.08±0.17 (SMD 0.53; 95% CI -0.10-1.16) |
PBMT is an effective, safe, and well-tolerated treatment for FM. Low-to-middle methodological quality of studies, heterogeneity between groups and PBMT delivery. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).