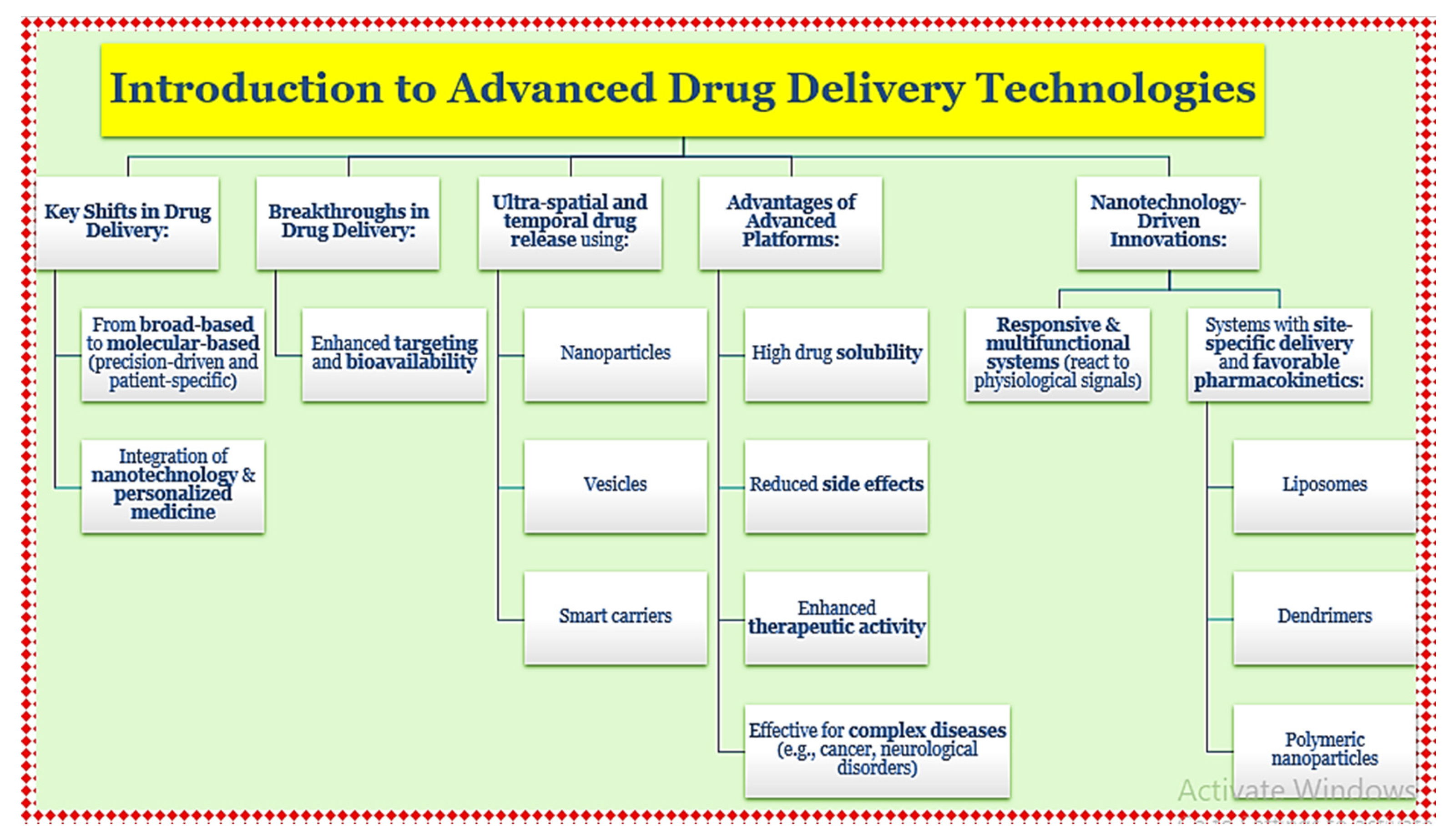
1. Introduction to Advanced Drug Delivery Technologies
The history of drug delivery has reached a revolutionary point, which is facilitated by the convergence of nanotechnology and personalized medicine. Contemporary therapeutics are no longer dealing with dosage and systemic delivery in the traditional sense but are precision-driven, safe, and patient-profiled [
1]. All these are a shift in approach from broad-based to molecular-based therapy.
Figure 1.
Introduction to Advanced Drug Delivery Technologies.
Figure 1.
Introduction to Advanced Drug Delivery Technologies.
One of the most important breakthroughs is the improvement of therapeutic targeting and bioavailability. Complex delivery platforms like nanoparticles, vesicles, and smart carriers are designed with ultra-spacial and temporal drug release [
2]. These advanced platforms offer high drug solubility, reduce side effects, and enhance therapeutic activity, especially for complex diseases like cancer and neurological disorders.
Apart from that, nanotechnology has facilitated the development of responsive and multi-functional drug delivery systems possessing the ability to respond towards physiological signals for site-specific delivery of drugs. Liposomes, dendrimers, and polymeric nanoparticles are only a few of the systems with favorable pharmacokinetics and targeted action against conventional routes [
3]. The new concept is transforming the norm of drug delivery and formulation approach.
Table 1.
Introduction to Advanced Drug Delivery Technologies.
Table 1.
Introduction to Advanced Drug Delivery Technologies.
| Topic |
Details |
| Revolution in Drug Delivery |
The field has shifted from traditional dosage forms to precision-based, patient-profiled therapeutics, enabled by advances in nanotechnology and personalized medicine. |
| Targeting & Bioavailability |
New platforms like nanoparticles and smart carriers allow for ultra-specific spatial and temporal drug release, enhancing solubility and reducing side effects. |
| Complex Diseases Addressed |
These systems are especially beneficial in treating complex conditions such as cancer and neurological disorders. |
| Responsive Systems |
Nanotechnology has led to systems that respond to physiological signals (e.g., pH, temperature) for targeted drug release. |
| Types of Carriers |
Includes liposomes, dendrimers, and polymeric nanoparticles with favorable pharmacokinetics and improved drug targeting compared to conventional routes. |
| Shift in Formulation Approach |
Represents a paradigm shift from broad-based drug delivery to molecular-level, responsive systems tailored to individual patient needs. |
2. Smart Nanocarriers for Precision Medicine
Smart nanocarriers are one of the foundations of precision medicine for the ability to deliver drugs at the desired sites with minimum off-target toxicities. Drug delivery systems such as liposomal, vesicular, and nanoparticles have been made available to achieve greatest targeting specificity and pharmacokinetics [
4]. Such nano-scale nanocarriers are engineered biotherapetically to achieve controlled release as well as high biocompatibility, particularly well-suited to diseases with local therapeutic intervention.
Ligand-targeted liposomes employ surface engineering to specifically target and bind to cellular receptors, also facilitating drug targeting to ailing tissue. With or without stability and immune evasion issues, these systems are a highly attractive path to selective drug delivery [
5]. Likewise, responsive drug release and functional targeting are made possible by four-domain modular architecture of programmable lipid nanoparticles that have shown efficacy in systemic and organ-directed delivery [
6].
Table 2.
Smart Nanocarriers in Precision Medicine.
Table 2.
Smart Nanocarriers in Precision Medicine.
| Topic |
Details |
| Foundation of Precision Medicine |
Smart nanocarriers enable site-specific drug delivery with minimal off-target toxicity, forming a core part of precision medicine. |
| Types of Delivery Systems |
Includes liposomal, vesicular, and nanoparticle-based systems designed for superior pharmacokinetics and targeting specificity. |
| Therapeutic Engineering |
These nano-scale carriers are bioengineered for controlled release, high biocompatibility, and are ideal for localized therapy. |
| Ligand-Targeted Liposomes |
Surface-engineered to bind cellular receptors, allowing precise targeting of diseased tissue and enhancing therapeutic selectivity. |
| Challenges and Benefits |
While immune evasion and stability remain concerns, such systems offer a promising route for selective and effective drug delivery. |
| Programmable Lipid Nanoparticles |
Modular, four-domain structures enable functional targeting and responsive release, proving effective for both systemic and organ-specific drug delivery. |
Nanotechnology application in oncology has been particularly revolutionary, enabling platforms for the controlled, targeted, and effective delivery of chemotherapeutics with reduced systemic toxicity [
7]. Smart drug delivery systems are designed to take advantage of tumor microenvironments, employing stimuli-responsive routes to enhance real-time versatility and optimal drug action [
8].
Lipid vesicles are still valid carriers in dermal and transdermal delivery suitable for entrapment of a wide range of actives with structural integrity in biological systems [
9]. Invasomes, being another of the semipermeable vesicular carrier groups, offer pathways of increasing penetration of therapeutic drugs across the skin [
10]. Alcohol and terpenes based formulation is more prone to flexibility and penetration through stratum corneum, which enhances bioavailability in transdermal [
11].
Progress of current times also consists of multifunctional nanoparticles with the potential to achieve double drug release, photothermal, and photodynamic treatment. These hybrid platforms enable synergistic modalities of therapy with extremely high effectiveness in vascular tumors and widen the scope of nanomedicine towards challenging pathologies [
12].
3. Transdermal and Mucosal Delivery Innovations
Microneedling technology has revolutionized mucosal and transdermal delivery with pain-free, minimally invasive delivery of vaccines and drugs. Pain-free stratum corneum penetration of the skin by the microscopic tips brings the agents into the dermis, increasing bioavailability and patient compliance [
13]. The method is especially effective for large molecules and biologics hitherto bedeviled by passive skin permeability.
Second-generation microneedle systems currently comprise materials and structural designs capable of controlled release, enhanced mechanical stability, and greater therapeutic potency. Their ability to deliver a wide range of drugs — from small molecules to DNA — makes them a versatile nanomedicine platform [
14].
Table 3.
Microneedling Technology in Drug Delivery.
Table 3.
Microneedling Technology in Drug Delivery.
| Aspect |
Details |
| Overview |
Microneedling offers a minimally invasive, pain-free method for mucosal and transdermal drug and vaccine delivery. |
| Mechanism of Action |
Microscopic needle tips penetrate the stratum corneum to deliver drugs into the dermis, increasing bioavailability and improving patient compliance. |
| Advantages for Large Molecules |
Particularly effective for biologics and large molecules that cannot passively permeate the skin. |
| Second-Generation Innovations |
Comprise advanced materials and structural designs allowing controlled release, improved mechanical strength, and higher therapeutic effectiveness. |
| Versatility |
Capable of delivering a broad spectrum of therapeutic agents, including small molecules, peptides, proteins, and nucleic acids (e.g., DNA). |
Invasomal systems, another transdermal technology, produce long-duration therapeutic effect by skin permeation and drug retention. Permeation enhancers like ethanol and terpenes, these vesicles are designed to enable improved penetration into deeper dermis and prolonged release of drugs [
15]. Mucoadhesive invasomes go a step further by targeting mucosal surfaces, providing extended residence time and local action for mucosal drug delivery processes [
16].
Ultradeformable liposomes and elastic liposomes also demonstrate remarkable enhancement of the delivery of active pharmaceutical ingredients (APIs) via the skin. Due to their potential to deform and penetrate small pores without bursting, their percutaneous absorption and drug stability are enhanced [
17]. Even topical formulations like skin creams have been re-optimized with nanotechnology advancements with a view to enhance their drug-loading capacity, homogeneity, and controlled release of the drug [
18].
4. Biodegradable and Sustainable Systems in Drug Delivery
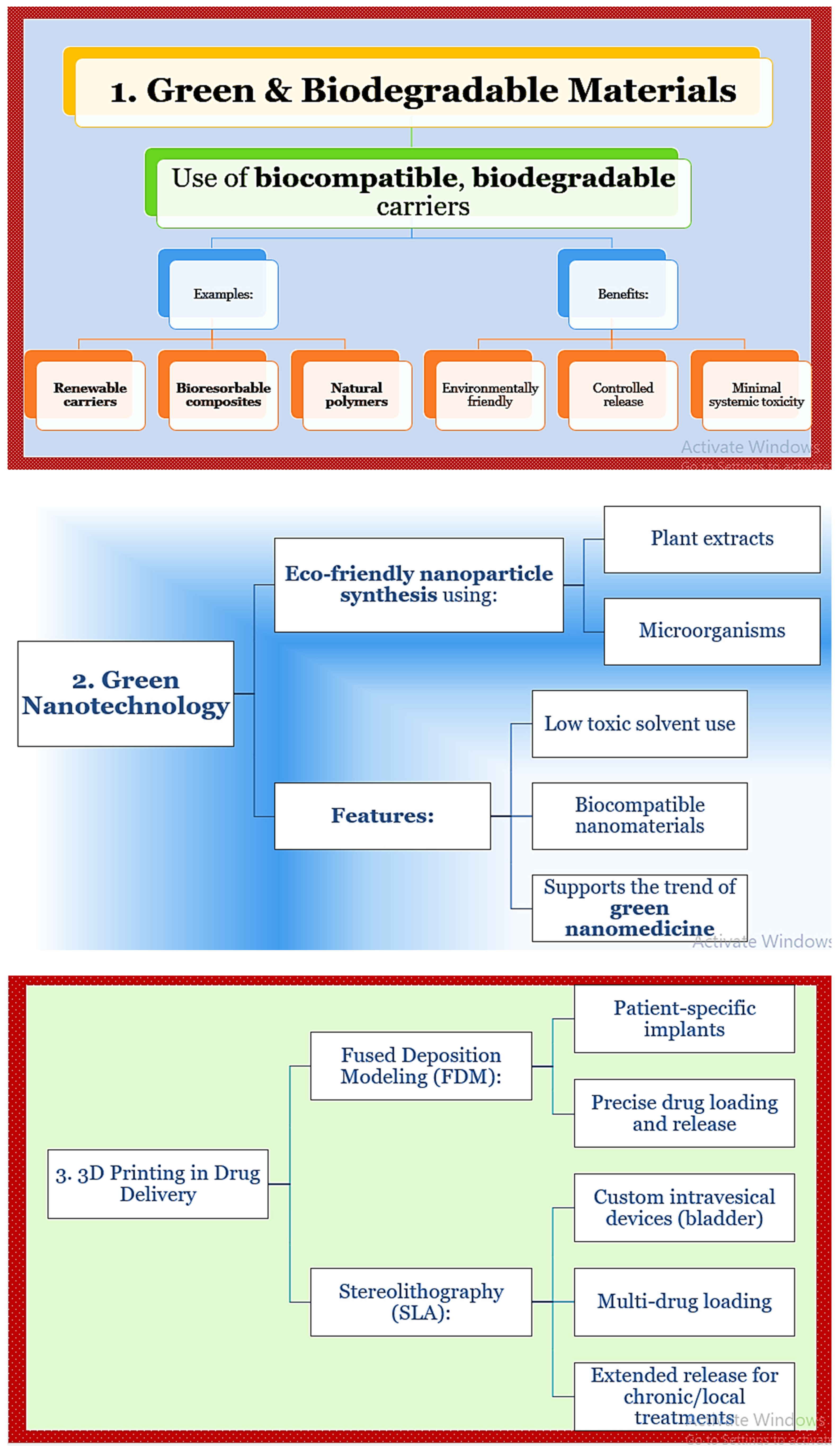
With greater focus on sustainability, it has developed drug delivery systems based on biodegradable and environmentally friendly materials. Besides the addition of minimizing environmental effects, they also provide biocompatible to native environments controlled release and degradative profiles [
19]. Such technologies comprise renewable carriers, bioresorbable composites, and natural polymers that all fall under the philosophy of green healthcare technologies.
Green nanotechnology is also changing this sector by making the synthesis of nanoparticles using plant extracts, microorganisms, and other cells easier. Green processes involving minimal use of toxic solvents and harsh chemical conditions render the end product biocompatible and active nanomaterials as drug delivery systems [
20]. These kinds of methods are also founded on the trend towards green nanomedicine.
Three-dimensional (3D) printing via the technique of fused deposition modeling (FDM) has been explored as a method of printing patient-specific drug-loaded biodegradable polymer-based implantations. FDM provides precise drug loading, release profile, and geometry, and hence patient-specific targeted therapies become achievable [
21].
Likewise, stereolithography (SLA) 3D printing has shown to be viable in producing intravesical drug delivery devices within a bladder. The geometries can be fashioned to be filled with more than one drug and release over an extended period. Such geometries are therefore best suited for local and chronic treatment [
22]. Overall, sustainability combined with frontier manufacturing is the new generation of green precision drug delivery.
5. Role of AI and Smart Systems in Drug Targeting
Artificial intelligence (AI) is increasingly being applied to drug delivery to facilitate data-driven decision-making for individual therapy, pharmacokinetic prediction, and nanoformulation optimization. AI algorithms can be employed to aid in the design of nanocarriers with tailored physicochemical properties and targeting ability, enhancing therapeutic specificity with reduced systemic side effects [
23]. Such astute systems represent a significant leap towards highly responsive and dynamic platforms for therapy.
Concurrently, SORT nanoparticles are transforming tissue-targeted delivery. Organ-level biodistribution profiles are utilized by the engineered carriers to deliver the drug to a targeted site, providing maximum efficacy and reduced toxicity. The technique has proven promising in the delivery of mRNA to organs like the liver and lungs, providing maximum gene expression specificity and translational potential [
24].
Along with chemical targeting, physical targeting methods like percutaneous ablation are being integrated into multimodal regimens. Image-guided such therapies enable drug-targeted alone or drug-delivery nanocarriers delivery to the tumors and lesions with superior outcomes by targeted intervention [
25].
Table 4.
Role of AI and Smart Systems in Drug Targeting.
Table 4.
Role of AI and Smart Systems in Drug Targeting.
| Category |
Description |
| AI in Drug Delivery |
AI facilitates data-driven therapeutic decisions, predicts pharmacokinetics, and optimizes nanocarrier design for enhanced specificity and reduced side effects. |
| Smart Nanocarriers |
AI-designed nanocarriers are tailored for precise physicochemical and targeting properties, representing dynamic and responsive delivery platforms. |
| SORT Nanoparticles |
Engineered to exploit organ-level biodistribution for tissue-specific delivery (e.g., mRNA to liver/lungs), enhancing efficacy and minimizing systemic toxicity. |
| Physical Targeting Techniques |
Image-guided approaches like percutaneous ablation integrate with nanomedicine for localized drug delivery to tumors and lesions. |
6.3. D Bioprinting and Implantable Delivery Devices
3D bioprinting has proven to be an extremely effective method of creating implantable drug delivery devices that are precise and specific. With the help of biocompatible material like hydrogels and alginate, the devices are made to release the drug for specific durations to specific sites. Hydrogels in this regard possess special characteristics as drug delivery systems, including the capability to retain very high percentages of water, and therefore are best suited to be used in tissue engineering and wound healing.
Alginate-based biopolymers have been found to be very useful because they are biodegradable and highly biocompatible. Alginate-based biopolymers have been employed to develop 3D printed implants that are custom designed as per the specific anatomical requirement of the patient which allows improved drug delivery rates and thus, for higher therapeutic efficiency in the treatment of cancer and wound healing [
21].
As explained in
Section 4, the combination of biodegradable polymers and 3D bioprinting greatly enhances the functionality of personalized drug delivery systems. Druggable molecules soluble in the bioenvironment enable such implants to be non-surgically retrievable but therapeutic effects could extend over a long time without influencing safety or causing side effects [
22]. This technology also paves the way for the creation of implantable devices that can deliver the drugs to the target site with highly minimal off-target effects and optimal global treatment effect [
19].
Conclusions
In general, the development of sophisticated drug delivery technology has redefined the landscape of modern-day therapeutics in a total manner. Personalized therapy using new nanotechnologies is transforming drug delivery and increasing specificity and hence minimizing drug side effects. Nanocarriers like liposomes, invasomes, and lipid nanoparticles have advanced far in drug delivery with targeted and efficient delivery, especially anticancer therapy and transdermal delivery. Smart systems with the aid of artificial intelligence also facilitate more efficient drug targeting by tissue-selective drug delivery and real-time optimization of therapy.
It introduces a new generation of green nanotechnology materials that are employed in the production of sustainable and biodegradable materials to be used in 3D bioprinting, whose deployment marks the advent of environmentally friendly drug delivery systems that not only function but are also biocompatible. The carbon footprint is hugely minimized, another benefit that aligns with the future trend towards the sustainability of healthcare services.
Implantable devices and 3D bioprinting also provide tissue-specific, site-directed drug delivery with vast potential for use in cancer and wound healing applications, where precision and individualized methods are of the highest importance. As technologies advance, it is only a matter of time before drug delivery not only becomes more efficient and targeted but also tailored to each patient.
References
- Sengar, A. (2025). Personalized medicine and nanotechnology: Transforming modern therapeutics. Preprints. [CrossRef]
- Sengar, A. (2025). Next-gen drug delivery: Redefining precision, bioavailability, and therapeutic outcomes. Preprints. [CrossRef]
- Sengar, A. (2025). Advancements in drug delivery systems: Targeting strategies, nanotechnology, and vesicular innovations. Preprints. [CrossRef]
- Sengar, A. (2025). Advancements in targeted drug delivery: Innovations in liposomal, nanoparticle, and vesicular systems. Preprints. [CrossRef]
- Noble, G. T., et al. (2014). Ligand-targeted liposome design: Challenges and fundamental considerations. Trends in Biotechnology, 32(1), 32–45. [CrossRef]
- Liu, Z., et al. (2024). Advancements in programmable lipid nanoparticles: Exploring the four-domain model for targeted drug delivery. arXiv. https://arxiv.org/abs/2408.05695.
- Sengar, A. (2025). The role of nanotechnology in revolutionizing cancer treatment. Preprints. [CrossRef]
- Sanchez-Moreno, P., et al. (2024). Smart drug-delivery systems for cancer nanotherapy. arXiv. https://arxiv.org/abs/2401.11192.
- Chacko, I. A., et al. (2020). Lipid vesicles: A versatile drug delivery platform for dermal and transdermal applications. Colloids and Surfaces B: Biointerfaces, 190, 110948. [CrossRef]
- Babaie, S., et al. (2020). Invasome: A novel nanocarrier for transdermal drug delivery. Nanomaterials, 10(5), 1–19.
- Jain, S., et al. (2021). Invasomes: Potential vesicular systems for transdermal delivery of drug molecules. JDDST, 61, 102265. [CrossRef]
- Paris, J. L., et al. (2021). Nanoparticles for multimodal antivascular therapeutics. arXiv. https://arxiv.org/abs/2103.09236.
- Kim, Y.-C., et al. (2012). Microneedles for drug and vaccine delivery. Advanced Drug Delivery Reviews, 64(14), 1547–1568. [CrossRef]
- Larrañeta, E., et al. (2016). Microneedles: A new frontier in nanomedicine delivery. Pharmaceutical Research, 33(5), 1055–1073. [CrossRef]
- El-Tokhy, F. S., et al. (2021). Design of long-acting invasomal nanovesicles. International Journal of Pharmaceutics, 603, 120679. [CrossRef]
- Teaima, M. H., et al. (2022). Propranolol mucoadhesive invasomes. Drug Delivery, 29(1), 1–14. [CrossRef]
- Souto, E. B., et al. (2021). Elastic and ultradeformable liposomes. International Journal of Molecular Sciences, 22(2), 974. [CrossRef]
- Sahu, T., et al. (2016). Skin cream as topical drug delivery system: A review. Journal of Pharmaceutical and Biological Sciences, 4(1), 1–9.
- Ashutosh, S. (2025). Sustainable drug delivery systems with biodegradable innovations. Preprints. [CrossRef]
- Noah, N., & Ndangili, P. (2021). Green synthesis of nanomaterials. arXiv. https://arxiv.org/abs/2112.04740.
- Kempin, W., et al. (2017). Polymers and drug loads for FDM 3D-printed implants. EJPB, 115, 84–93. [CrossRef]
- Goyanes, A., et al. (2015). SLA 3D printing of a bladder device. Materials Science and Engineering: C, 61, 931–938. [CrossRef]
- Sengar, A. (2025). Smart drug delivery: AI, nanotech & future innovations. Preprints. [CrossRef]
- Dilliard, S. A., et al. (2021). Tissue-specific mRNA delivery. PNAS, 118(52), e2109256118. [CrossRef]
- Ahmed, M., et al. (2011). Principles and advances in percutaneous ablation. Radiology, 258(2), 351–369. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).