Submitted:
07 May 2025
Posted:
07 May 2025
You are already at the latest version
Abstract
Keywords:
Introduction
Methods
| Study | ERAS Elements |
| Wang et al. (2022) | Pain control, early mobilization, reduced LOS |
| Lei Wang et al. (2022) | Pain control, reduced PONV, reduced LOS |
| Han et al. (2019) | Pain control, reduced LOS, fewer complications |
| Zaed et al. (2023) | Reduced opioids, early mobilization |
| Elayat et al. (2021) | Pain control, reduced ICU stay |
| McLaughlin et al. (2014) | Reduced LOS and ICU stay |
| Lobatto et al. (2020) | Reduced LOS, fewer complications |
| Study | Country | Design | Sample Size | Population |
| Wang et al. (2022) | China | RCT | 151 | Glioma |
| Lei Wang et al. (2022) | China | RCT | 151 | Elective craniotomy |
| Han et al. (2019) | China | RCT | 84 | Aneurysm clipping |
| Zaed et al. (2023) | Switzerland | Retrospective | 19 | Elderly with glioblastoma |
| Elayat et al. (2021) | India | Non-randomized | 70 | Supratentorial tumors |
| McLaughlin et al. (2014) | USA | Observational | 121 | Pituitary/skull base tumors |
| Lobatto et al. (2020) | Netherlands | Observational | 150 | Meningioma |
Results
| Study ID | Country | Design | Sample Size | Population | Intervention | Comparator | Outcomes and Results |
| Wang et al. (2022) | China | RCT | 151 | Adults with gliomas | ERAS | Conventional care | Reduced LOS (5 vs 7 days), better pain and mobilization |
| Lei Wang et al. (2022) | China | RCT | 151 | Elective craniotomy | ERAS | Standard care | LOS 3 vs 4 days, reduced cost and PONV |
| Han et al. (2019) | China | RCT | 84 | Aneurysm clipping | ERAS | Standard care | Reduced LOS, fewer complications, higher satisfaction |
| Study ID | Country | Design | Sample Size | Population | Intervention | Comparator | Outcomes and Results |
| Zaed et al. (2023) | Switzerland | Retrospective | 19 | Elderly with glioblastoma | ERAS | Historical cohort | Reduced opioid use, increased mobilization |
| Elayat et al. (2021) | India | Non-randomized | 70 | Supratentorial tumor patients | ERAS | Routine care | Reduced ICU stay, better pain control |
| McLaughlin et al. (2014) | USA | Observational | 121 | Pituitary/skull base tumors | ERAS | Historical controls | Reduced LOS and ICU stay |
| Lobatto et al. (2020) | Netherlands | Observational | 150 | Meningioma patients | ERAS | Standard care | LOS reduced from 7.6 to 3 days, fewer complications |
Risk of Bias Assessment
| Study | Randomization | Blinding | Overall Risk |
| Wang et al. (2022) | Low | Low | Low |
| Lei Wang et al. (2022) | Low | Some concerns | Moderate |
| Han et al. (2019) | Unclear | High | High |
| Study | Confounding | Selection Bias | Overall Risk |
| Zaed et al. (2023) | Moderate | Moderate | Moderate |
| Elayat et al. (2021) | Moderate | Moderate | Moderate |
| McLaughlin et al. (2014) | High | Moderate | High |
| Lobatto et al. (2020) | Moderate | Low | Moderate |
Conclusions
Abbreviations and Acronyms
| ERAS | Enhanced Recovery After Surgery |
| LOS | Length of stay |
| PONV | Postoperative nausea and vomiting |
| PRO | Patient-reported outcome |
| RCT | Randomized controlled trial |
Appendix – PRISMA Flowchart
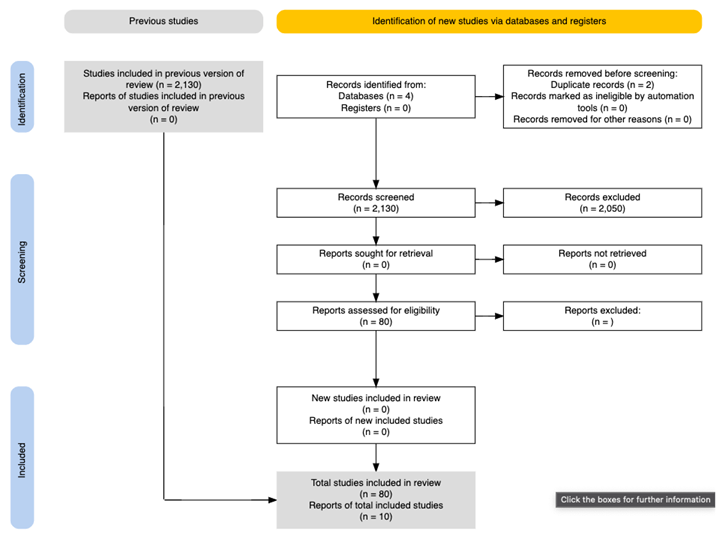
References
- Wang L, Cai H, Wang Y, et al. Enhanced recovery after elective craniotomy: A randomized controlled trial. J Clin Anesth. 2022;76:110575. [CrossRef]
- Lei Wang et al. Enhanced Recovery After Surgery in Neurosurgical Patients Undergoing Elective Craniotomy: A Randomized Study. J Clin Anesth. 2022.
- Kim SH, Choi SH, Moon J, et al. Enhanced Recovery After Surgery for Craniotomies: A Systematic Review and Meta-analysis. J Neurosurg Anesthesiol. 2025;37(1):11–19. [CrossRef]
- Li M, Liu M, Cui Q, et al. Effect of Dexmedetomidine on Postoperative Delirium in Patients Undergoing Awake Craniotomies: Study Protocol of a Randomized Controlled Trial. Trials. 2023;24:607. [CrossRef]
- Elayat A, Jena SS, Nayak S, et al. Enhanced recovery after surgery – ERAS in elective craniotomies: a non-randomized controlled trial. BMC Neurol. 2021;21(1):127. [CrossRef]
- Zaed I, Marchi F, Milani D, et al. Role of Enhanced Recovery after Surgery (ERAS) Protocol in the Management of Elderly Patients with Glioblastoma. J Clin Med. 2023;12(18):6032. [CrossRef]
- McLaughlin N, et al. The Enhanced Recovery After Surgery Pathway for Pituitary Surgery: Early Results. World Neurosurg. 2014;82(5):e661–e669. [CrossRef]
- Lobatto DJ, et al. Enhanced Recovery After Surgery in Meningioma Patients: Results From a Pilot Study. World Neurosurg. 2020;134:e756–e764.
- Han X, et al. Enhanced Recovery After Surgery Protocol for Aneurysmal Clipping: A Randomized Controlled Trial. Chin J Prac Nerv Dis. 2019.
- Wang C, et al. Effect of an Enhanced Recovery After Surgery (ERAS) Program on Perioperative Outcomes in Neurosurgery. J Neurosurg Sci. 2019.
- X-Peters E, Robinson M, Serletis D. Registration for “Enhanced recovery after surgery for patients un dergoing cranial surgery: a systematic review.” PROSPERO international prospective register of systematic reviews. Available at: https://www.crd. york.ac.uk/prospero/display_record.php?RecordID¼ 197187. Accessed February 25, 2021.
- Kim et al. (2025): Uma meta-análise de cinco ensaios clínicos randomizados demonstrou que protocolos ERAS reduziram significativamente o tempo de internação, a dor pós-operatória e a incidência de náuseas e vômitos em pacientes submetidos a craniotomia, sem aumento nas complicações. ResearchGate+1ResearchGate+1.
- Choudhary et al. (2025): Esta revisão sistemática e meta-análise abrangendo 15 estudos concluiu que protocolos ERAS em craniotomias eletivas resultaram em menor tempo de internação, redução de custos hospitalares e melhora no desempenho funcional dos pacientes. ScienceDirect+3Brieflands+3PubMed+3.
- ERAS® Society Guidelines: Embora ainda não existam diretrizes específicas para neurocirurgia, a ERAS® Society fornece recomendações para diversas especialidades, enfatizando a importância de abordagens multimodais e centradas no paciente. SpringerLink+2erassociety.org+2PMC+2Supbumrung et al. (2023): Este estudo destacou que a adesão aos protocolos ERAS em craniotomias neuro-oncológicas está associada a melhores desfechos pós-operatórios, incluindo menor tempo de internação e redução de complicações. thejns.org.
- Jolly et al. (2024): Uma revisão narrativa discutiu os desafios e estratégias para a implementação de protocolos ERAS em neurocirurgia, enfatizando a necessidade de colaboração multidisciplinar e abordagens centradas no paciente. PubMed.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





