1. Introduction
Black stain (BS) is one of the most common types of extrinsic dental pigmentation. It often results in aesthetic concerns and psychological discomfort for patients. BS is more frequently observed in primary dentition but can also occur in permanent teeth. These stains usually appear as small dots or lines, complete or incomplete, following the cervical margin—most commonly on the lingual surface [
1]. In Eurasia, the prevalence of BS among children aged 3 to 15 years is 10.3% (ranging from 2.4% to 19.9%), while in South America, it is 7.4% (3.5% to 14.8%) among children aged 5 to 12 years. No gender predilection has been observed [
2].
The cause of these stains is associated with chromogenic bacteria, microorganisms capable of pigment production [
3]. Studies suggest the pigment results from a black ferric compound formed by the interaction between hydrogen sulfide—produced by anaerobic bacteria in periodontal tissues—and iron from saliva or gingival exudates [
4]. Other contributing factors include the consumption of water with high iron content, high water pH, and elevated salivary pH [
5].
Certain bacterial species—such as
Actinomyces,
Cardiobacterium,
Haemophilus,
Corynebacterium,
Tannerella, and
Treponema—are found more frequently in plaque samples from children with BS, suggesting a microbial correlation.
Actinomyces species, for instance, produce hydrogen sulfide, potentially resulting in ferric sulfide, while
Prevotella species generate pigments ranging from dark brown to black. Notably, patients with BS tend to have fewer
S. mutans, lower salivary pH, and higher concentrations of salivary lactoferrin, calcium, and phosphorus [
4].
The classification system by Koch and later modified by Gasparetto et al. (2003) [
6] defines BS in three levels: pigmented dots or thin lines with incomplete coalescence near the gingival margin; continuous pigmented lines limited to the cervical third; and in more advanced stages, pigmented stains extending beyond the cervical third [
2].
Conventional treatments include dental prophylaxis and ultrasonic scaling, which may result in enamel damage and hypersensitivity. Chlorhexidine mouthwash has also been recommended for its ability to eliminate pigment and inhibit chromogenic bacterial colonization; however, it may cause tooth discoloration, taste alterations, and allergic reactions [
7]. Tooth whitening is another option, using carbamide or hydrogen peroxide for their antibacterial and stain-removal properties. Still, these agents may induce hypersensitivity and alter enamel surface morphology [
1].
More recently, lactoferrin and phototherapy have been studied as promising treatments. Further research using various phototherapy protocols and patient groups is necessary to establish standardized approaches [
4].
Antibacterial photodynamic therapy (aPDT) is an emerging technique for infection control, particularly in dentistry and wound care. It targets microorganisms without promoting resistance. The process involves activating a photosensitizer—typically methylene blue (MB)—with light at specific wavelengths, often red light, to generate reactive oxygen species (ROS) that damage bacterial cell membranes, proteins, and DNA [
8,
9]. aPDT has shown effectiveness in disrupting biofilms and treating antibiotic-resistant infections [
9].
Although aPDT appears to be a promising non-invasive treatment for BS, evidence in the literature remains scarce. To date, only two studies are known: a clinical case report using high-power laser (970 nm, 1.5 W, 10 s/tooth) [
4], and an in vitro study [
6]. The latter evaluated the use of MB and indocyanine green (ICG) with diode lasers (660 nm and 808 nm), demonstrating reduced biofilm density and bacterial colony counts after aPDT application.
Therefore, the aim of this study is to present three clinical case reports in which aPDT was employed as an adjunctive treatment for extrinsic black stains.
2. Case Reports
This report presents the clinical management of three patients with extrinsic black stains, two pediatric cases and one elderly patient—treated using antimicrobial photodynamic therapy (aPDT) as an adjunctive approach.
2.1. Case Report 1 and 2
Two siblings, a 2.5-year-old girl and an 8-year-old boy, were referred for dental consultation due to the presence of black extrinsic stains on all teeth. According to their caregivers, previous attempts to remove the stains through professional prophylaxis performed by other clinicians resulted in temporary stain-free periods of approximately one to two weeks, after which the stains recurred. Interestingly, both parents reported having had similar black stains in their own dentition, which had been resolved after two prophylactic sessions.
Both children were systemically healthy, with no relevant medical history, including no history of iron supplementation or systemic medication. Oral hygiene practices were deemed adequate, with tooth brushing supervised by their parents.
At the initial appointment, manual scaling using Gracey curettes was performed, followed by prophylaxis with extra-fine pumice paste (SS White®) and a Robinson brush. This approach allowed for the removal of only approximately 20% of the stains.
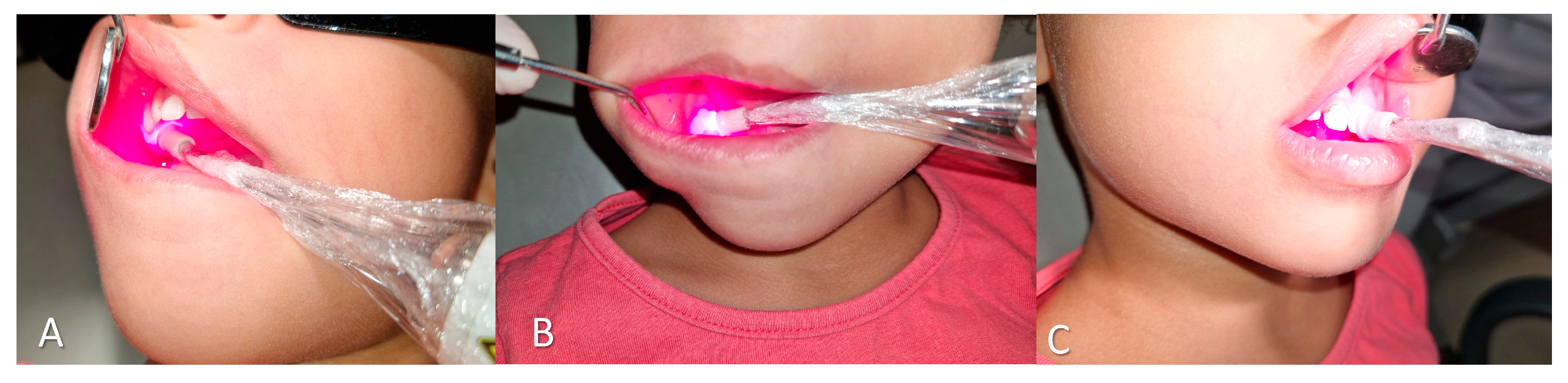
At the one-week follow-up, dietary recall revealed a healthy and balanced diet, rich in fruits and vegetables. Given the persistence of the stains, aPDT was employed using 0.01% methylene blue as the photosensitizing agent. The DMC Therapy EC® laser device was used (one emitter, continuous wave, contact mode, 100 mW, spot size 0.0984 cm², irradiance 1.02 W/cm², wavelength 660 nm, 4 J per point, 40 seconds per point), with application at nine points in the 2.5-year-old and six points in the 8-year-old (
Figure 1).
Following laser application, prophylaxis with extra-fine pumice and a Robinson brush was repeated.
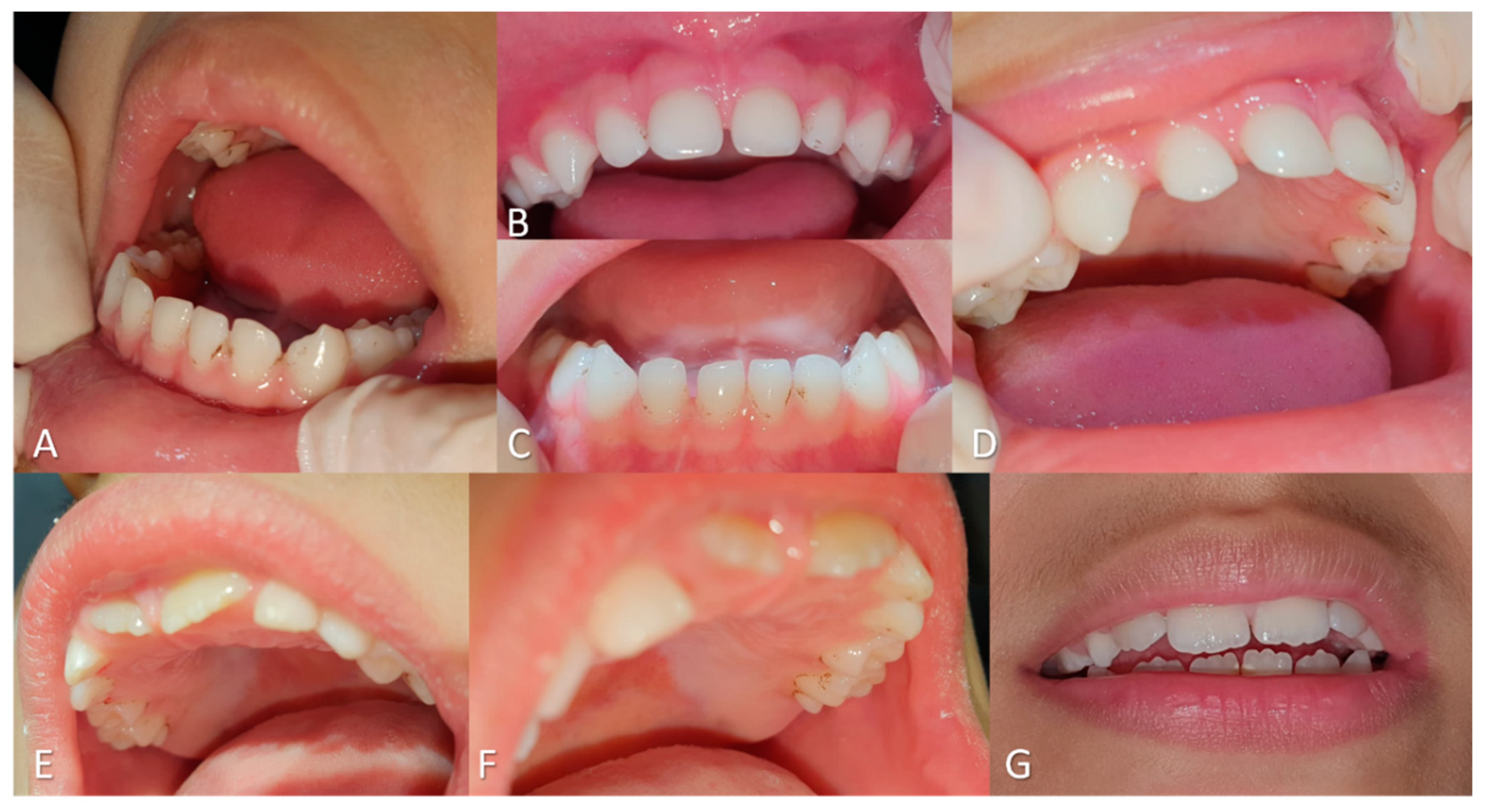
Complete removal of the stains was achieved with ease. At the one-month follow-up, no extrinsic staining was observed, while a slight recurrence was noted at four months in both patients (
Figure 2).
However, at the six-month follow-up, recurrence of the stains was observed in both cases (
Figure 3). The children’s mother reported that, in the absence of laser treatment, stains typically reappeared within 15 days following prophylactic procedures alone.
The aPDT protocol followed by prophylaxis was repeated, and the patients remain under ongoing monitoring. Caregivers and the patients were advised regarding the possibility of stain recurrence and the importance of attending follow-up appointments.
2.1. Case Report 3
The third case involved a 67-year-old retired Caucasian female. During anamnesis, the patient reported allergies to amoxicillin and a medical history of migraine and systemic arterial hypertension, both well-controlled with continuous medication. Her chief complaint was “black stains on the teeth for the past two years.” Additionally, she reported generalized oral mucosal sensitivity. The patient stated that she maintained a balanced diet with frequent intake of vegetables and denied any use of ferrous sulfate.
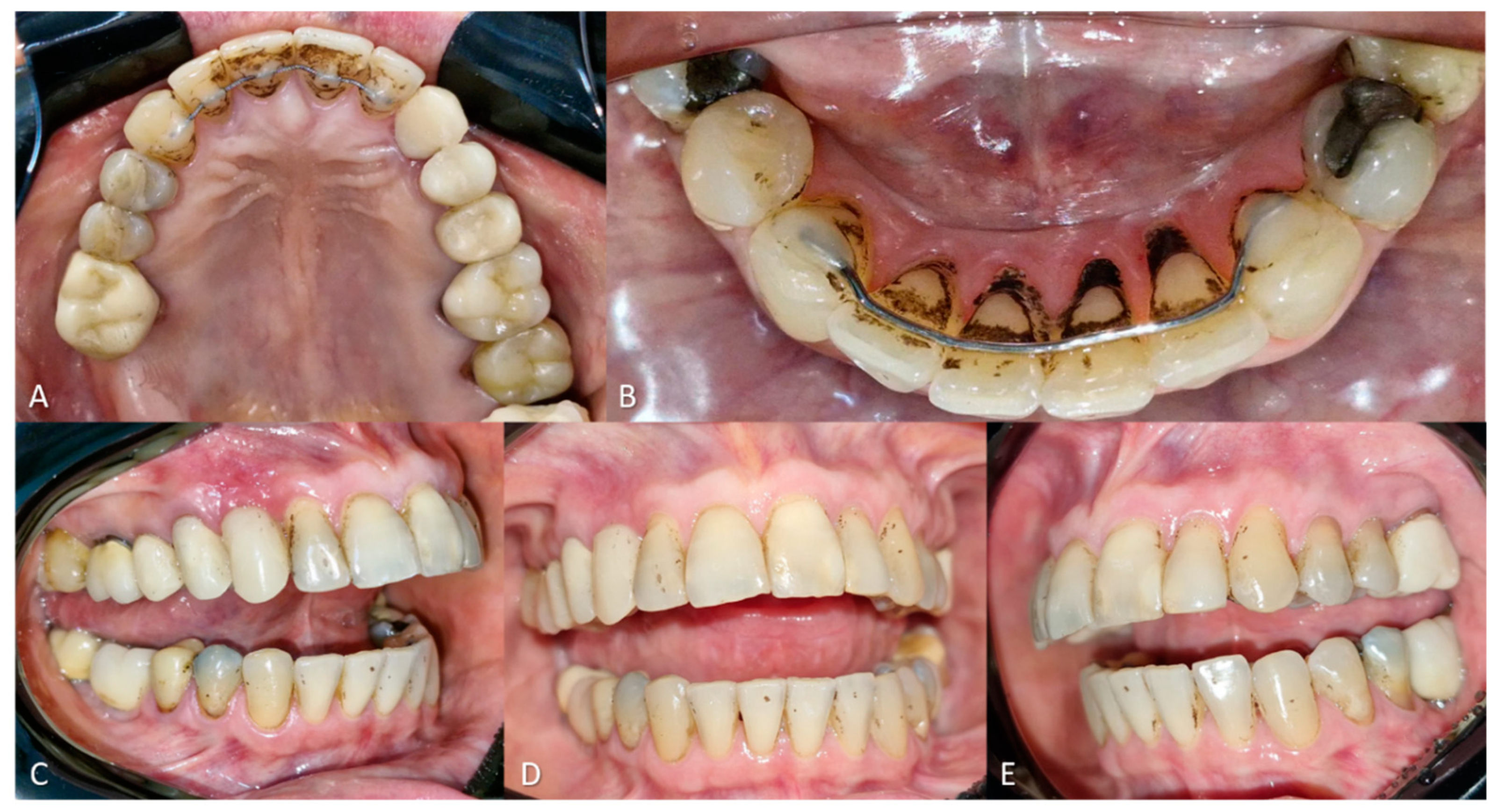
According to the patient, the black stains had appeared two years prior, initially affecting the buccal surfaces of the teeth, compromising esthetics and self-esteem. She mentioned undergoing ultrasonic scaling one month before the consultation, which partially removed the buccal stains but caused considerable dentin hypersensitivity. Intraoral examination revealed a Black Stain score of 3, according to the Gasparetto classification, with stain presence across the palatal surfaces of the maxillary and mandibular incisors, in addition to multiple stains distributed across the buccal surfaces of most teeth (
Figure 4).
The oral mucosa appeared dry during the appointment. Sialometry revealed hyposalivation (unstimulated salivary flow: 0.08 mL/min; stimulated: 0.52 mL/min). The patient also reported a burning sensation during tooth brushing and had switched to a pediatric toothpaste. As a result, the use of a sodium lauryl sulfate-free toothpaste (Cariax® Daily) and artificial saliva (Bioextra® Spray) was prescribed.
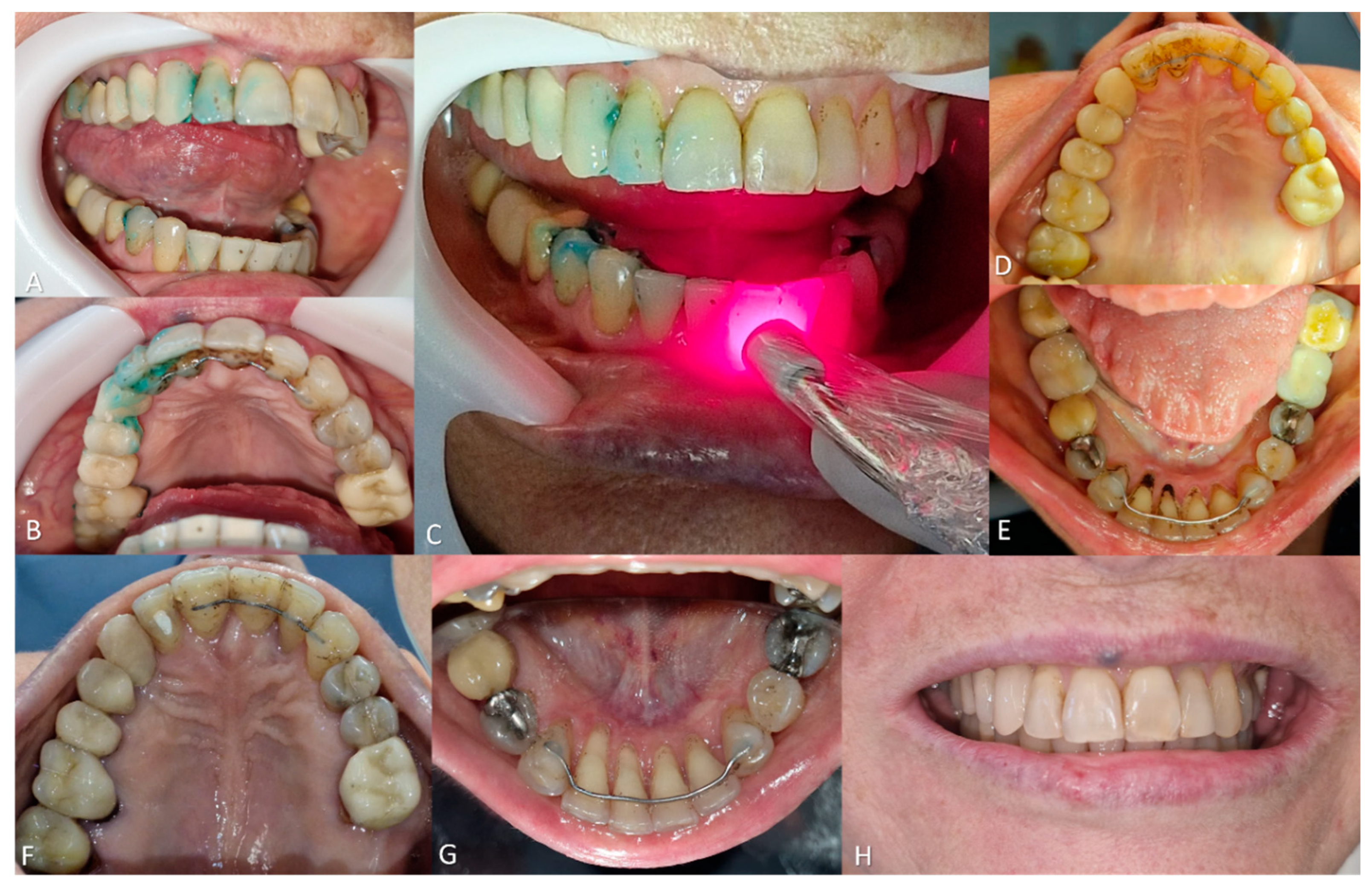
To remove the extrinsic stains, a split-mouth design was employed. Antimicrobial photodynamic therapy (aPDT) was applied using 0.01% methylene blue (MB) gel, compounded for clinical dental use in a certified pharmacy, on the right side. The left side received only red light irradiation without the photosensitizer. A red light laser (660 nm, 60.98 J/cm² fluency, 60 seconds/point, 36 points on buccal and palatal surfaces) was applied, with a total session duration of 20 minutes (
Figure 5).
Additionally, photobiomodulation therapy (PBMT) was performed on the dorsal surface of the tongue due to reported pain caused by habitual pressure against a fixed orthodontic retainer (660 nm, 2 J, 20 seconds/point, 5 points, 20.3 J/cm²) (
Table 1).
Subsequently, prophylaxis was performed using extra-fine pumice (SS White®) and a Robinson brush, resulting in approximately 80% removal of the black stains. Notably, the left side (treated only with red light) exhibited near-complete stain removal compared to the right side, which received both MB and red light. Furthermore, the patient reported significant discomfort during previous prophylactic procedures; however, she experienced no pain during this session, even with the use of water and air jets.
A second session of aPDT was carried out, this time using only red light, with an increased number of irradiation points on the right side. This was followed by another prophylaxis, which led to complete removal of the extrinsic stains and a satisfactory esthetic result. At the 1-month follow-up, no stains were observed on the dental surfaces. At 3 months, some recurrence was noted, and the previously established protocol was repeated. The interval between treatment and recurrence was longer than in previous interventions. At the 6-month follow-up, no stain recurrence was observed.
Furthermore, during the second session, PBMT was initiated on the major and minor salivary glands to improve salivary flow. The patient reported a reduction in oral mucosal sensitivity and increased saliva production. By the third session, visible saliva accumulation in the floor of the mouth was noted, including a visible salivary jet from the sublingual caruncles. In total, ten sessions of PBMT were conducted (one session per week) targeting both minor and major salivary glands (Minor salivary glands: 660 nm, 1 J/point, 82 points, 10.16 J/cm². Major salivary glands: 808 nm, 3 J/point, 25 points, 30.49 J/cm²) (
Table 1). Sialometry assessments conducted in the fifth and eighth sessions continued to show low flow rates (unstimulated: 0.08 mL/min; stimulated: 0.52 mL/min), but at the tenth session, the values were within normal parameters (
Figure 6). The patient remains asymptomatic and under ongoing follow-up.
3. Discussion
Black stains (BS) affect both primary and permanent dentition, having an aesthetic and psychological impact on patients. These stains exhibit distinctive characteristics, such as distribution along the cervical margin of teeth and predominance on the lingual surfaces [
1]. Their prevalence varies by geographical region, ranging from 7.4% to 10.3% in children, underscoring the need for preventive measures and effective treatments [
2].
Patient age is a determining factor in the manifestation and recurrence of BS [
5]. QIAO et al. (2024) [
9] examined 672 preschoolers aged 3–5 years from 12 kindergartens in Qingdao, northern China. BS were observed in 103 children (15.33%), including 3 severe cases (0.45%), 28 moderate (4.17%), and 72 mild (10.71%). It was also noted that children with BS were less likely to develop carious lesions compared to those without BS. In Tunisia, a study involving 393 children aged 3–5 years revealed that 6.1% had BS [
10].
In a study by ORTIZ-LÓPEZ et al. (2018) [
5], involving 94 volunteers aged 18–40 years, it was found that age was inversely associated with the percentage of stained surfaces. As age increased, the proportion of stained surfaces decreased. Contrasting with this, one of the present case reports described a 67-year-old patient with BS on both vestibular and lingual surfaces of all teeth, highlighting the need for personalized treatment approaches.
Studies show that the most commonly affected areas are the lingual surfaces of mandibular teeth, likely due to their proximity to the submandibular salivary glands and the role of saliva in the pathogenesis of BS [
2]. In this particular case, the presence of BS in the reported geriatric patient may be associated with her underlying condition of hyposalivation. It is known that the composition and flow rate of saliva play a critical role in development of BS. In cases of hyposalivation, the reduced mechanical clearance of the oral cavity can lead to greater accumulation of chromogenic bacteria and iron-containing compounds on the dental surface. Moreover, the alteration in salivary composition—frequently observed in patients using antidepressants, as in this case—may result in higher salivary pH and increased availability of free iron. These factors create a favorable environment for the formation of black ferric sulfide precipitates, which adhere to tooth surfaces and manifest as extrinsic pigmentation [
11]. Therefore, the patient’s compromised salivary function likely contributed to the extensive and recurrent pattern of BS observed, supporting the importance of addressing salivary gland dysfunction in the clinical management of this condition [
12].
The accumulation of iron in tissues and secretions, along with chromogenic bacteria, is considered the primary etiology of this condition. Among the metabolic products synthesized by oral bacteria, hydrogen sulfide reacts with available iron in the saliva—under pathological conditions such as iron metabolism disorders—forming black precipitates of ferric sulfide. These deposits adhere to tooth surfaces, typically forming lines that follow the gingival contour, with variable and unpleasant pigmentation intensity [
13]. These findings align with previous studies [
3,
5], which associate BS with specific bacterial activity, elevated salivary pH, and high iron content.
The classification of BS, proposed by KOCH et al. (2001) [
14] and refined by Gasparetto et al. (2003) [
6], guides clinical management based on severity—from fine pigmented lines to extensive, hard-to-remove stains. In the present case series, levels 1 and 3 were observed. Conventional prophylaxis only partially removed more severe stains, demonstrating the need for complementary interventions such as aPDT, particularly in refractory cases.
The standard treatment for BS includes prophylaxis with abrasive pastes, air polishing with sodium bicarbonate, and ultrasonic scaling. However, frequent removal may damage enamel and cause dentin hypersensitivity [
5]. Microabrasion techniques with hydrochloric acid and pumice are indicated in more resistant cases but pose a risk of enamel loss [
15]. In one case, a 67-year-old female patient taking antidepressants developed BS and suffered from hyposalivation and xerostomia. Initial treatment with ultrasonic scaling led to only partial stain removal and increased dentinal sensitivity.
Another treatment alternative is tooth whitening using hydrogen peroxide or carbamide peroxide, which has both antimicrobial action and esthetic benefits. However, it can lead to hypersensitivity and enamel irregularities and is not recommended for pediatric patients—the population most frequently affected by BS—thereby limiting its clinical applicability. Oral administration of bovine lactoferrin (bLf) has also demonstrated a significant reduction in BS due to its antibacterial activity against anaerobic microorganisms and iron-chelating properties that prevent the formation of ferric sulfide. Specific protocols involve orodispersible bLf tablets, which, when used in conjunction with proper oral hygiene, have shown promising results in both children and adults [
13]. Despite therapeutic advancements, no method provides a definitive solution, and treatments must be periodically repeated due to recurrence [
3,
16].
Given this, non-invasive treatment alternatives such as antimicrobial photodynamic therapy (aPDT) are important. The literature on this topic is still limited, with only two published studies on the use of aPDT in BS management [
4,
7]. These studies highlight both the effectiveness and challenges of aPDT, positioning it as a promising approach for managing extrinsic staining.
Results obtained from the split-mouth approach used in one of the present cases revealed that red light irradiation (660 nm) without a photosensitizer was more effective than conventional aPDT using methylene blue (0.01%) for BS removal. This suggests that in the case of BS—where pigmentation is already dark—the use of an additional dye may be unnecessary, as the microorganisms may already be pigmented by the nature of the stain itself. This finding represents a significant advantage for clinical pediatric dentistry, as it simplifies the therapeutic protocol by reducing both chair time and procedural steps—crucial factors when managing young patients who typically exhibit lower compliance during dental visits.
These case reports reinforce the efficacy of aPDT in BS treatment, demonstrating successful stain removal in both pediatric and geriatric patients. The findings corroborate those of previous studies [
4,
7], in which aPDT effectively reduced biofilm and extrinsic stains across various populations and experimental settings.
Although the literature on aPDT for BS remains scarce, the outcomes of this study, in alignment with existing reports, indicate promising potential, without the adverse effects of other treatment modalities. Controlled clinical trials and long-term follow-ups are essential to establish standardized aPDT protocols for various clinical scenarios and age groups. The present study contributes to this growing body of knowledge, emphasizing the importance of integrated and complementary therapeutic strategies—especially in complex and recurrent cases.
4. Conclusions
Antimicrobial photodynamic therapy (aPDT) proved to be effective in the treatment of black dental stains, yielding satisfactory esthetic outcomes in both pediatric and geriatric patients. The application of methylene blue in combination with red light led to effective stain removal, without the adverse effects commonly associated with conventional methods, such as dentin hypersensitivity and enamel damage. Furthermore, the observed difference in stain removal when red light was applied without prior use of methylene blue highlights a promising area for further investigations.
Author Contributions
Conceptualization, M.P.M. and A.T.P.; methodology, M.P.M. formal analysis, A.T.P.; investigation, C.A.; resources, A.T.P.; data curation, R.Y.F.Y., and A.A.O.D.; writing—original draft preparation, L. N.G.C., M.P.M., and A.T.P.; writing—review and editing, R.Y.F.Y., C.A., and A.A.O.D..; supervision, M.P.M.; project administration, A.T.P.; All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Informed Consent Statement
Informed consent was obtained from the patients for publishing the clinical details and images in this article. Informed consent was obtained from all subjects involved in this study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to privacy.
Acknowledgments
This study was supported by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – Brazil (CAPES). CAPES/Brazil scholarship holder – Grant nº 88887.967267/2024-00. This study was financed, in part, by the São Paulo Research Foundation (FAPESP), Brasil. Process Number 2024/06726-8.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- De Lima, I.M.; De Araújo, M.C.L.; De Oliveira, N.C.S.; Da Fonseca, T.S. Pigmentação extrínseca em dente decíduo: revisão de literatura. Rev. Bras. Revisão Saúde 2023, 6, 30346–30357. [CrossRef]
- Yakubova, I.I.; Ostrianko, V.; Skrypnyk, Y.; Volovodovskiy, R. Extrinsic black staining of teeth: a review. Wiad. Lek. 2025, 78, 210–215. [CrossRef]
- Kumar, M.; Madi, M.; Vineetha, R.; Gopinath, D. Chromogenic bacterial staining of teeth: a scoping review. BMC Oral Health 2025, 25, 55. [CrossRef]
- Nosotti, M.G.; Casu, C.; Baruffaldi, A.; Viganò, L. Black stain: A case report. Int. J. Multidiscip. Res. Dev. 2019, April.
- Ortiz-López, C.S.; Veses, V.; Garcia-Bautista, J.A.; Jovani-Sancho, M.D.M. Risk factors for the presence of dental black plaque. Sci. Rep. 2018, 8, 16752. [CrossRef]
- Gasparetto, A.; Conrado, C.A.; Maciel, S.M.; Miyamoto, E.Y.; Chicarelli, M.; Zanata, R.L. Prevalência de manchas pretas nos dentes e cárie dentária em escolares brasileiros. Rev. Bras. Odontol. 2003, 14, 157–161. [CrossRef]
- Nokhbatolfoghahaei, H., Niroomand, A., Chiniforush, N., Najary, S., & Shekarchi, F. (2023). The effect of antibacterial photodynamic therapy with diode laser on chromogenic bacteria associated with dental black staining: An in-vitro study. Photodiagnosis and photodynamic therapy, 44, 103761. [CrossRef]
- Songca, S.P.; Adjei, Y. Applications of antimicrobial photodynamic therapy against bacterial biofilms. Int. J. Mol. Sci. 2022, 23, 3209. [CrossRef]
- Qiao, C.; Han, R.; Yang, J.; Huang, H.; Ma, L. Black stain and dental caries in primary dentition of preschool children in Qingdao, China. J. Clin. Pediatr. Dent. 2024, 48, 200–205. [CrossRef]
- Elelmi, Y.; Mabrouk, R.; Masmoudi, F.; Baaziz, A.; Maatouk, F.; Ghedira, H. Black stain and dental caries in primary teeth of Tunisian preschool children. Eur. Arch. Paediatr. Dent. 2021, 22, 235–240. [CrossRef]
- Garan, A.; Akyüz, S.; Oztürk, L.K.; Yarat, A. Salivary parameters and caries indices in children with black tooth stains. J. Clin. Pediatr. Dent. 2012, 36, 285–288. [CrossRef]
- Tschoppe, P., Wolgin, M., Pischon, N., & Kielbassa, A. M. (2010). Etiologic factors of hyposalivation and consequences for oral health. Quintessence international (Berlin, Germany: 1985), 41(4), 321–333.
- Sangermano, R.; Pernarella, S.; Straker, M.; Lepanto, M.S.; Rosa, L.; Cutone, A.; Valenti, P.; Ottolenghi, L. The treatment of black stain associated with iron metabolism disorders with lactoferrin: a literature search and two case studies. Clin. Ter. 2019, 170, e373–e381. [CrossRef]
- Koch, M.J.; Bove, M.; Schroff, J.; Perlea, P.; García-Godoy, F.; Staehle, H.-J. Mancha preta e cárie dentária em crianças em idade escolar em Potenza, Itália. J. Dent. Child. 2001, 68, 353–355.
- Carvalho, T.A.; de Oliveira, G.K.S.; de Medeiros, K.H.O.; Domingos, P.R.C. Manchas extrínsecas negras em dentes decíduos e permanentes: revisão da literatura. Rev. Eletrônica Acervo Odontol. 2020, 2, e5915. [CrossRef]
- Rosa, L.; Lepanto, M.S.; Cutone, A.; Ianiro, G.; Pernarella, S.; Sangermano, R.; Musci, G.; Ottolenghi, L.; Valenti, P. Lactoferrin and oral pathologies: a therapeutic treatment. Biochem. Cell Biol. 2021, 99, 81–90. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).