Submitted:
29 April 2025
Posted:
30 April 2025
You are already at the latest version
Abstract

Keywords:
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Data Sources and Search Strategies
2.3. Inclusion and Exclusion Criteria
2.4. Screening and Selection Process of Articles
2.6. Methodological Quality Assessment and Data Analysis
3. Results
3.1. Research Effort and Objectives
3.2. Methodologies and Habitats Analysis
3.3. Biodiversity Analysis
3.4. Postprocessing Data Analyses
4. Discussion
4.1. Methodologies and Technological Advances
4.1.1. Direct and Remote Observation Techniques
4.1.2. Acoustic Methodologies
4.1.3. Aerial and Satellite Methodologies
4.1.4. Data Postprocessing
4.2. Habitat Mapping
4.2.1. Methodologies
4.2.2. Biotic-Abiotic Data
4.2.3. Habitat-Related Factors
4.2.4. Final Habitat Map in the Central-Eastern Atlantic Archipelagos
4.3. Challenges and Future Implications
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A
| Habitats | ||||||||||||
| Paper | Reference | Year | Zone | Main objective | Macrophytes | Rhodolith beds | Coral | Habitat map | Geological map | Depth (m) | Substrate | Area size (ha) |
| 1 | [175] | 2021 | Madeira | exploratory | - | x | - | x | - | 35 | mixed | 2,368 |
| 2 | [198] | 2022 | Madeira | monitoring | x | - | - | x | - | 20 | soft | - |
| 3 | [131] | 2000 | Azores | monitoring | x | - | - | - | - | 40 | hard | - |
| 4 | [186] | 2000 | Azores | exploratory | - | - | x | - | - | 3800 | hard | - |
| 5 | [203] | 2008 | Azores | monitoring | x | - | - | x | - | 30 | hard | 400 |
| 6 | [136] | 2015 | Canary Islands | exploratory | - | - | x | - | - | 600 | hard | - |
| 7 | [140] | 2022 | Madeira | exploratory | x | x | x | - | - | 990 | mixed | 1,55 |
| 8 | [165] | 2017 | Canary Islands | mapping | x | - | - | x | - | 20 | mixed | - |
| 9 | [158] | 2022 | Azores | exploratory | - | - | x | - | - | 210 | mixed | 0,019 |
| 10 | [166] | 2015 | Azores | mapping | x | x | x | x | - | n/a | mixed | 167692,2 |
| 11 | [146] | 2023 | Canary Islands | mapping | - | - | x | x | - | 110 | mixed | 48,075 |
| 12 | [116] | 2012 | Azores | exploratory | - | - | x | - | - | 3,3 | mixed | - |
| 13 | [129] | 2017 | Azores, Canary Islands | exploratory | x | - | x | - | - | 734 | mixed | - |
| 14 | [204] | 1992 | Azores | exploratory | x | - | - | x | - | 5,5 | hard | - |
| 15 | [185] | 2013 | Madeira | mapping | - | - | x | x | x | 2660 | soft | 56000 |
| 16 | [143] | 2020 | Azores | exploratory | - | - | x | - | x | 1700 | hard | 0,045 |
| 17 | [196] | 2014 | Canary Islands | monitoring | x | - | - | - | - | 15 | soft | 393,9 |
| 18 | [195] | 2014 | Azores | monitoring | - | - | x | - | x | 1092 | hard | - |
| 19 | [205] | 2021 | Azores | exploratory | - | - | x | - | x | 595 | hard | - |
| 20 | [206] | 2013 | Azores | exploratory | - | - | x | - | x | 1097 | mixed | 2,378 |
| 21 | [176] | 2013 | Azores | exploratory | - | - | x | x | - | 1500 | hard | - |
| 22 | [15] | 2019 | Canary Islands | monitoring | - | x | - | x | - | 50 | mixed | 0,72 |
| 23 | [164] | 2021 | Canary Islands | monitoring | x | - | - | - | - | 75 | soft | - |
| 24 | [14] | 2020 | Canary Islands | monitoring | - | x | - | - | - | 40 | soft | - |
| 25 | [163] | 2024 | Canary Islands | monitoring | x | x | - | x | - | 25 | mixed | 2150 |
| 26 | [182] | 2024 | Cabo Verde | mapping | - | - | x | x | x | 2100 | hard | 118800 |
| 27 | [130] | 2022 | Canary Islands | monitoring | x | - | - | - | - | n/a | hard | - |
| 28 | [114] | 2020 | Madeira | mapping | x | x | x | x | - | 50 | mixed | 240 |
| 29 | [56] | 2024 | Azores | mapping | x | - | x | x | - | 60 | mixed | 394,08 |
| 30 | [207] | 2018 | Canary Islands | mapping | x | - | - | x | - | 50 | mixed | 9138 |
| 31 | [202] | 2013 | Canary Islands | exploratory | x | - | - | - | - | 19 | mixed | - |
| 32 | [208] | 2019 | Madeira | exploratory | x | - | - | - | - | 2,6 | mixed | - |
| 33 | [209] | 2006 | Azores | exploratory | x | - | - | x | - | 20 | hard | - |
| 34 | [200] | 2019 | Canary Islands | exploratory | - | - | x | x | - | 35 | hard | 0,018 |
| 35 | [148] | 2024 | Canary Islands | monitoring | x | - | - | x | - | 50 | hard | - |
| 36 | [173] | 2021 | Madeira | monitoring | x | - | - | - | - | 12 | soft | 0,009 |
| 37 | [4] | 2021 | Azores | mapping | x | - | - | x | - | 15 | mixed | - |
| 38 | [210] | 2012 | Canary Islands | exploratory | x | - | - | - | - | 46 | mixed | - |
| 39 | [211] | 2002 | Canary Islands | mapping | x | - | - | x | - | 10 | mixed | - |
| 40 | [212] | 2023 | Canary Islands | mapping | x | - | - | x | - | n/a | soft | - |
| 41 | [213] | 2012 | Azores | mapping | - | - | x | - | x | 1350 | hard | - |
| 42 | [214] | 2017 | Canary Islands | monitoring | x | - | - | x | - | 20 | hard | 7,4 |
| 43 | [215] | 2020 | Canary Islands | mapping | x | - | - | x | - | 40 | mixed | 746 |
| 44 | [162] | 2020 | Canary Islands | mapping | x | - | - | x | - | 40 | hard | 746 |
| 45 | [194] | 2016 | Canary Islands | mapping | - | - | x | - | x | 2500 | mixed | 367388 |
| 46 | [144] | 2020 | Azores | exploratory | - | - | x | x | x | 4000 | mixed | - |
| 47 | [216] | 2008 | Azores | exploratory | - | - | x | - | x | 2977 | mixed | - |
| 48 | [161] | 2015 | Canary Islands | mapping | x | - | - | x | - | 25 | mixed | - |
| 49 | [197] | 2023 | Azores | mapping | - | - | x | x | - | 2387 | mixed | 154 |
| 50 | [142] | 2012 | Azores | mapping | x | - | - | x | x | 80 | mixed | 53750 |
| 51 | [217] | 2023 | Azores | mapping | - | - | x | x | x | 2700 | hard | - |
| 52 | [199] | 2021 | Azores | exploratory | x | x | - | x | - | 85 | mixed | - |
| 53 | [218] | 2023 | Cabo Verde | exploratory | x | - | - | x | - | 5 | soft | 0,62 |
| 54 | [219] | 2023 | Azores | exploratory | - | - | x | x | - | 2000 | mixed | 2200000 |
| 55 | [220] | 2022 | Canary Islands | mapping | x | - | - | x | - | n/a | mixed | - |
| 56 | [221] | 2008 | Azores | monitoring | x | x | x | - | - | 30 | mixed | - |
| 57 | [46] | 2020 | Canary Islands | mapping | - | - | x | x | - | 173 | hard | 55 |
| 58 | [201] | 2021 | Canary Islands | mapping | - | - | x | x | x | 3000 | mixed | - |
| 59 | [141] | 2013 | Azores | mapping | - | - | x | - | x | 160 | hard | - |
| 60 | [183] | 2020 | Canary Islands | exploratory | x | - | - | x | - | 40 | mixed | - |
| 61 | [184] | 2013 | Canary Islands | mapping | x | - | x | x | - | 50 | mixed | 1574 |
| 62 | [150] | 2019 | Azores | monitoring | x | - | - | x | - | 10 | mixed | - |
| 63 | [167] | 2022 | Azores | exploratory | - | - | x | x | x | 1600 | hard | - |
| 64 | [115] | 2024 | Cabo Verde | exploratory | - | - | x | - | x | 3218 | mixed | 2,807 |
| 65 | [222] | 2013 | Canary Islands | exploratory | x | - | - | x | x | 45 | mixed | - |
| 66 | [160] | 2023 | Canary Islands | mapping | x | - | - | x | - | 20 | mixed | - |
| 67 | [151] | 2024 | Canary Islands | mapping | x | x | - | x | - | 10 | mixed | 58,5 |
| 68 | [149] | 2021 | Madeira | mapping | x | x | - | x | - | 14 | mixed | 10 |
| 69 | [120] | 2008 | Azores | mapping | x | - | - | - | - | 30 | mixed | - |
| Methologies | Data classification | ||||||||||||||
| Paper | Reference | Year | Direct observation | Remote observation | Aerial observation | Satellite observation | Acoustic observation | Sampling | Bibliographic | Modeling | GIS tools | Acoustic / Bathymetry | Image analysis | STATS | Taxonomic / Morphology |
| 1 | [175] | 2021 | x | - | - | - | x | x | x | - | x | - | x | - | - |
| 2 | [198] | 2022 | x | - | - | - | x | x | x | x | x | - | - | - | - |
| 3 | [131] | 2000 | - | - | - | - | - | - | x | - | - | - | - | - | - |
| 4 | [186] | 2000 | - | x | - | - | - | - | - | - | - | - | - | - | - |
| 5 | [203] | 2008 | x | - | - | - | - | x | - | - | - | - | - | - | x |
| 6 | [136] | 2015 | x | x | - | - | - | - | x | - | x | - | - | - | - |
| 7 | [140] | 2022 | - | x | - | - | x | - | - | - | - | - | x | - | - |
| 8 | [165] | 2017 | - | - | - | x | - | - | - | x | - | - | - | - | - |
| 9 | [158] | 2022 | - | x | - | - | - | - | - | - | - | - | x | - | - |
| 10 | [166] | 2015 | - | - | - | - | - | - | x | x | x | x | - | - | - |
| 11 | [146] | 2023 | x | x | - | - | x | - | - | x | x | x | - | - | - |
| 12 | [116] | 2012 | - | x | - | - | - | - | x | - | - | - | - | - | - |
| 13 | [129] | 2017 | x | x | - | - | - | - | - | - | - | - | x | - | - |
| 14 | [204] | 1992 | x | - | - | - | - | x | - | - | - | - | - | - | - |
| 15 | [185] | 2013 | - | x | - | - | x | x | - | x | x | x | - | - | - |
| 16 | [143] | 2020 | - | x | - | - | x | - | - | x | - | - | x | - | - |
| 17 | [196] | 2014 | x | - | - | - | - | x | - | x | - | - | - | - | - |
| 18 | [195] | 2014 | x | x | - | - | - | - | - | x | x | - | - | - | - |
| 19 | [205] | 2021 | - | x | - | - | - | - | - | - | - | - | - | - | - |
| 20 | [206] | 2013 | - | x | - | - | - | - | - | - | - | - | x | - | - |
| 21 | [176] | 2013 | x | x | - | - | - | x | x | - | - | - | - | - | - |
| 22 | [15] | 2019 | x | - | - | - | x | x | - | - | x | - | - | - | - |
| 23 | [164] | 2021 | - | - | - | x | - | - | x | x | x | - | - | - | - |
| 24 | [14] | 2020 | x | - | - | - | x | x | - | - | x | - | - | - | - |
| 25 | [163] | 2024 | - | - | - | x | - | - | - | x | - | - | - | x | - |
| 26 | [182] | 2024 | - | x | - | - | x | - | x | x | - | - | x | x | - |
| 27 | [130] | 2022 | x | - | - | - | - | - | x | x | x | - | - | - | - |
| 28 | [114] | 2020 | x | - | - | - | x | - | x | - | x | - | - | - | - |
| 29 | [56] | 2024 | - | x | - | - | x | - | - | - | x | x | - | - | - |
| 30 | [207] | 2018 | x | x | - | - | - | x | - | x | - | - | - | x | - |
| 31 | [202] | 2013 | x | - | - | - | - | x | - | - | - | - | - | - | x |
| 32 | [208] | 2019 | x | - | - | - | - | x | - | - | - | - | x | - | - |
| 33 | [209] | 2006 | x | - | - | - | - | x | - | - | - | - | - | x | - |
| 34 | [200] | 2019 | x | - | - | - | - | x | - | - | - | - | - | - | - |
| 35 | [148] | 2024 | - | - | x | - | - | - | x | x | x | - | x | - | - |
| 36 | [173] | 2021 | x | - | - | - | - | x | - | - | x | - | x | - | - |
| 37 | [4] | 2021 | x | - | x | - | - | x | - | x | x | - | x | x | - |
| 38 | [210] | 2012 | x | - | - | - | - | x | - | - | - | - | - | - | - |
| 39 | [211] | 2002 | - | x | - | - | - | - | - | x | - | - | x | - | - |
| 40 | [212] | 2023 | x | - | - | - | x | - | x | x | x | - | - | - | - |
| 41 | [213] | 2012 | - | - | - | - | - | x | - | - | - | - | - | - | - |
| 42 | [214] | 2017 | x | - | - | - | - | x | - | - | x | - | - | - | - |
| 43 | [215] | 2020 | - | - | - | x | - | - | x | x | - | - | - | - | - |
| 44 | [162] | 2020 | x | - | - | - | - | x | x | x | - | - | - | - | - |
| 45 | [194] | 2016 | - | x | - | - | x | x | - | x | x | x | - | - | - |
| 46 | [144] | 2020 | - | x | - | - | x | - | - | x | - | x | - | - | - |
| 47 | [216] | 2008 | - | x | - | - | - | x | - | - | - | - | - | - | x |
| 48 | [161] | 2015 | - | - | - | x | - | - | - | - | - | - | - | x | - |
| 49 | [197] | 2023 | - | x | - | - | - | - | - | x | - | x | - | - | - |
| 50 | [142] | 2012 | x | x | - | - | x | x | x | x | x | x | - | - | - |
| 51 | [217] | 2023 | - | x | - | - | x | - | - | x | - | x | - | - | - |
| 52 | [199] | 2021 | x | x | - | - | - | x | - | - | - | - | - | - | - |
| 53 | [218] | 2023 | x | - | - | - | - | - | x | - | x | - | - | - | - |
| 54 | [219] | 2023 | - | - | - | - | - | x | - | x | x | - | - | - | - |
| 55 | [220] | 2022 | - | - | - | - | - | - | x | x | - | - | - | x | - |
| 56 | [221] | 2008 | x | - | - | - | - | - | - | - | - | - | - | - | x |
| 57 | [46] | 2020 | x | - | - | - | x | x | - | - | x | x | - | - | - |
| 58 | [201] | 2021 | - | x | - | - | x | x | - | x | x | x | - | - | - |
| 59 | [141] | 2013 | - | x | - | - | x | - | - | - | x | - | - | - | - |
| 60 | [183] | 2020 | x | - | - | - | - | - | - | - | x | - | - | - | - |
| 61 | [184] | 2013 | - | x | - | - | x | - | - | x | x | x | - | x | - |
| 62 | [150] | 2019 | x | - | x | - | - | - | - | x | x | - | x | - | - |
| 63 | [167] | 2022 | - | x | - | - | - | - | - | - | - | x | x | - | - |
| 64 | [115] | 2024 | - | x | - | - | - | - | x | - | x | x | - | - | - |
| 65 | [222] | 2013 | - | - | - | - | x | - | - | x | x | x | - | - | - |
| 66 | [160] | 2023 | - | - | - | x | - | - | - | x | x | - | x | - | - |
| 67 | [151] | 2024 | x | - | x | - | - | x | - | x | x | - | x | x | - |
| 68 | [149] | 2021 | - | x | x | - | - | - | - | x | x | - | x | x | - |
| 69 | [120] | 2008 | x | - | - | - | - | x | - | x | - | - | x | x | - |
| Species | Order | Family | Studies | Azores | Madeira | Canary Islands | Cabo Verde | Atlantic Mid-Ridge |
| Phyllum Cnidaria | ||||||||
| Subphyllum Anthozoa | ||||||||
| Anthozoa | 1 | x | - | - | - | - | ||
| Class Hexacorallia | ||||||||
| Elatopathes aff. abietina (Pourtalès, 1874) | Antipatharia | Aphanipathidae | 1 | x | - | - | - | - |
| Actiniaria | Actiniaria | 4 | x | - | - | x | x | |
| Anemonia viridis (Forsskål, 1775) | Actiniaria | Actiniidae | 1 | - | x | - | - | - |
| Actinoscyphia aurelia (Stephenson, 1918) | Actiniaria | Actinoscyphiidae | 1 | - | - | x | - | - |
| Parasicyonis ingolfi Carlgren, 1942 | Actiniaria | Actinostolidae | 1 | x | - | - | - | x |
| Parasicyonis Carlgren, 1921 | Actiniaria | Actinostolidae | 1 | x | - | - | - | x |
| Telmatactis cricoides (Duchassaing, 1850) | Actiniaria | Andvakiidae | 1 | - | x | - | - | - |
| Antipatharia | Antipatharia | 3 | x | - | - | - | x | |
| Antipathes furcata Gray, 1857 | Antipatharia | Antipathidae | 1 | - | - | x | - | - |
| Antipathes grayi (Roule, 1902) | Antipatharia | Antipathidae | 1 | x | - | - | - | - |
| Antipathes virgata Esper, 1798 | Antipatharia | Antipathidae | 1 | x | - | - | - | - |
| Antipathes Pallas, 1766 | Antipatharia | Antipathidae | 2 | x | x | - | - | - |
| Stichopathes flagellum Roule, 1902 | Antipatharia | Antipathidae | 1 | x | - | - | - | - |
| Stichopathes gracilis (Gray, 1857) | Antipatharia | Antipathidae | 1 | x | - | x | - | x |
| Stichopathes gravieri Molodtsova, 2006 | Antipatharia | Antipathidae | 2 | x | - | - | - | x |
| Stichopathes richardi Roule, 1902 | Antipatharia | Antipathidae | 1 | x | - | - | - | - |
| Stichopathes Brook, 1889 | Antipatharia | Antipathidae | 4 | x | x | x | - | x |
| Elatopathes Opresko, 2004 | Antipatharia | Aphanipathidae | 1 | x | - | - | - | x |
| Aphanostichopathes dissimilis (Roule, 1902) | Antipatharia | Aphanipathidae | 1 | x | - | - | - | - |
| Distichopathes Opresko, 2004 | Antipatharia | Aphanipathidae | 2 | x | x | - | - | - |
| Phanopathes erinaceus (Roule, 1905) | Antipatharia | Aphanipathidae | 2 | x | - | - | - | - |
| Heteropathes Opresko, 2011 | Antipatharia | Cladopathidae | 1 | x | - | - | - | x |
| Leiopathes expansa Johnson, 1899 | Antipatharia | Leiopathidae | 2 | x | - | - | - | x |
| Leiopathes glaberrima (Esper, 1792) | Antipatharia | Leiopathidae | 3 | x | - | x | - | - |
| Leiopathes grimaldii Roule, 1902 | Antipatharia | Leiopathidae | 1 | x | - | - | - | - |
| Leiopathes Haime, 1849 | Antipatharia | Leiopathidae | 1 | x | - | x | - | x |
| Leiopathes Haime, 1849 | Antipatharia | Leiopathidae | 4 | x | - | x | - | x |
| Antipathella subpinnata (Ellis & Solander, 1786) | Antipatharia | Myriopathidae | 4 | x | - | x | - | x |
| Antipathella wollastoni (Gray, 1857) | Antipatharia | Myriopathidae | 8 | x | - | x | - | x |
| Antipathella Brook, 1889 | Antipatharia | Myriopathidae | 3 | x | - | x | - | x |
| Tanacetipathes squamosa (Koch, 1886) | Antipatharia | Myriopathidae | 1 | x | - | - | - | - |
| Tanacetipathes Opresko, 2001 | Antipatharia | Myriopathidae | 4 | x | x | - | - | x |
| Bathypathes patula Brook, 1889 | Antipatharia | Schizopathidae | 1 | x | - | - | - | - |
| Bathypathes Brook, 1889 | Antipatharia | Schizopathidae | 3 | x | - | x | - | x |
| Stauropathes punctata (Roule, 1905) | Antipatharia | Schizopathidae | 1 | x | - | - | - | - |
| Stauropathes arctica (Lütken, 1871) | Antipatharia | Schizopathidae | 1 | x | - | - | - | x |
| Parantipathes hirondelle Molodtsova, 2006 | Antipatharia | Schizopathidae | 2 | x | - | - | - | - |
| Parantipathes Brook, 1889 | Antipatharia | Schizopathidae | 2 | x | x | - | - | x |
| Ceriantharia | Ceriantharia | 1 | x | - | - | - | - | |
| Pachycerianthus Roule, 1904 | Ceriantharia | Cerianthidae | 1 | - | x | - | - | - |
| Scleractinia | Scleractinia | 2 | x | - | - | - | x | |
| Coenocyathus cylindricus Milne Edwards y Haime, 1848 | Scleractinia | Caryophylliidae | 1 | x | - | - | - | - |
| Concentrotheca laevigata (Pourtalès, 1871) | Scleractinia | Caryophylliidae | 1 | x | - | - | - | - |
| Dasmosmilia lymani (Pourtalès, 1871) | Scleractinia | Caryophylliidae | 1 | x | - | - | - | - |
| Dasmosmilia variegata (Pourtalès, 1871) | Scleractinia | Caryophylliidae | 1 | x | - | - | - | - |
| Desmophyllum dianthus (Esper, 1794) | Scleractinia | Caryophylliidae | 5 | x | - | - | - | x |
| Desmophyllum pertusum (Linnaeus, 1758) | Scleractinia | Caryophylliidae | 8 | x | - | x | - | x |
| Desmophyllum Ehrenberg, 1834 | Scleractinia | Caryophylliidae | 2 | x | - | x | - | x |
| Pourtalosmilia anthophyllites (Ellis & Solander, 1786) | Scleractinia | Caryophylliidae | 1 | x | - | - | - | - |
| Premocyathus cornuformis (Pourtalès, 1868) | Scleractinia | Caryophylliidae | 1 | x | - | - | - | - |
| Anomocora fecunda (Pourtalès, 1871) | Scleractinia | Caryophylliidae | 1 | x | - | - | - | - |
| Paracyathus pulchellus (Philippi, 1842) | Scleractinia | Caryophylliidae | 1 | x | - | - | - | - |
| Phyllangia americana Milne Edwards & Haime, 1849 | Scleractinia | Caryophylliidae | 1 | - | - | x | - | - |
| Polycyathus muellerae (Abel, 1959) | Scleractinia | Caryophylliidae | 1 | - | - | x | - | - |
| Polycyathus senegalensis Chevalier, 1966 | Scleractinia | Caryophylliidae | 1 | - | - | x | - | - |
| Aulocyathus atlanticus Zibrowius, 1980 | Scleractinia | Caryophylliidae | 2 | x | x | - | - | x |
| Caryophyllia (Caryophyllia) abyssorum Duncan, 1873 | Scleractinia | Caryophylliidae | 1 | x | - | - | - | - |
| Caryophyllia (Caryophyllia) alberti Zibrowius, 1980 | Scleractinia | Caryophylliidae | 3 | x | - | - | - | x |
| Caryophyllia (Caryophyllia) atlantica (Duncan, 1873) | Scleractinia | Caryophylliidae | 1 | x | - | - | - | - |
| Caryophyllia (Caryophyllia) calveri Duncan, 1873 | Scleractinia | Caryophylliidae | 1 | x | - | - | - | - |
| Caryophyllia (Caryophyllia) cyathus (Ellis & Solander, 1786) | Scleractinia | Caryophylliidae | 2 | x | - | - | - | x |
| Caryophyllia (Caryophyllia) foresti Zibrowius, 1980 | Scleractinia | Caryophylliidae | 1 | x | - | - | - | - |
| Caryophyllia (Caryophyllia) inornata (Duncan, 1878) | Scleractinia | Caryophylliidae | 2 | x | - | x | - | - |
| Caryophyllia (Caryophyllia) sarsiae Zibrowius, 1974 | Scleractinia | Caryophylliidae | 2 | x | - | - | - | x |
| Caryophyllia (Caryophyllia) smithii Stokes & Broderip, 1828 | Scleractinia | Caryophylliidae | 1 | x | - | - | - | - |
| Caryophyllia (Caryophyllia) Lamarck, 1801 | Scleractinia | Caryophylliidae | 5 | x | - | x | - | x |
| Caryophylliidae Dana, 1846 | Scleractinia | Caryophylliidae | 2 | x | - | - | - | x |
| Solenosmilia variabilis Duncan, 1873 | Scleractinia | Caryophylliidae | 5 | x | - | x | - | x |
| Solenosmilia Duncan, 1873 | Scleractinia | Caryophylliidae | 1 | x | - | - | - | x |
| Tethocyathus variabilis Cairns, 1979 | Scleractinia | Caryophylliidae | 1 | x | - | - | - | - |
| Trochocyathus (Trochocyathus) spinosocostatus Zibrowius, 1980 | Scleractinia | Caryophylliidae | 1 | x | - | - | - | - |
| Deltocyathus eccentricus Cairns, 1979 | Scleractinia | Deltocyathidae | 2 | x | x | - | - | x |
| Deltocyathus italicus (Michelotti, 1838) | Scleractinia | Deltocyathidae | 1 | x | - | - | - | - |
| Deltocyathus moseleyi Cairns, 1979 | Scleractinia | Deltocyathidae | 2 | x | x | - | - | x |
| Dendrophyllia alternata Pourtalès, 1880 | Scleractinia | Dendrophylliidae | 2 | x | - | x | - | x |
| Dendrophyllia cornigera (Lamarck, 1816) | Scleractinia | Dendrophylliidae | 4 | x | x | x | - | x |
| Dendrophyllia ramea (Linnaeus, 1758) | Scleractinia | Dendrophylliidae | 4 | x | x | x | - | x |
| Dendrophyllia de Blainville, 1830 | Scleractinia | Dendrophylliidae | 2 | x | - | - | - | x |
| Enallopsammia pusilla (Alcock, 1902) | Scleractinia | Dendrophylliidae | 1 | x | - | - | - | - |
| Enallopsammia rostrata (Pourtalès, 1878) | Scleractinia | Dendrophylliidae | 4 | x | - | - | x | x |
| Enallopsammia Michelloti, 1871 | Scleractinia | Dendrophylliidae | 1 | x | - | - | - | x |
| Leptopsammia formosa (Gravier, 1915) | Scleractinia | Dendrophylliidae | 2 | x | - | - | - | - |
| Balanophyllia (Balanophyllia) cellulosa Duncan, 1873 | Scleractinia | Dendrophylliidae | 2 | x | - | - | - | x |
| Tubastraea coccinea Lesson, 1830 | Scleractinia | Dendrophylliidae | 1 | - | - | x | - | - |
| Tubastraea tagusensis Wells, 1982 | Scleractinia | Dendrophylliidae | 1 | - | - | x | - | - |
| Tubastraea Lesson, 1830 | Scleractinia | Dendrophylliidae | 1 | - | - | x | - | - |
| Javania cailleti (Duchassaing & Michelotti, 1864) | Scleractinia | Flabellidae | 1 | x | - | - | - | - |
| Javania pseudoalabastra Zibrowius, 1974 | Scleractinia | Flabellidae | 1 | x | - | - | - | - |
| Flabellum (Flabellum) chunii Marenzeller, 1904 | Scleractinia | Flabellidae | 1 | x | - | - | - | - |
| Flabellum (Ulocyathus) alabastrum Moseley, 1876 | Scleractinia | Flabellidae | 2 | x | - | - | - | x |
| Flabellum (Ulocyathus) angulare Moseley, 1876 | Scleractinia | Flabellidae | 2 | x | - | - | - | x |
| Flabellum (Ulocyathus) macandrewi Gray, 1849 | Scleractinia | Flabellidae | 2 | x | - | - | - | x |
| Flabellum Lesson, 1831 | Scleractinia | Flabellidae | 3 | x | - | - | - | x |
| Fungiacyathus (Bathyactis) crispus (Pourtalès, 1871) | Scleractinia | Fungiacyathidae | 2 | x | - | - | - | x |
| Fungiacyathus (Bathyactis) marenzelleri (Vaughan, 1906) | Scleractinia | Fungiacyathidae | 1 | x | - | - | - | - |
| Fungiacyathus (Bathyactis) symmetricus (Pourtalès, 1871) | Scleractinia | Fungiacyathidae | 1 | x | - | - | - | - |
| Fungiacyathus (Fungiacyathus) fragilis Sars, 1872 | Scleractinia | Fungiacyathidae | 3 | x | - | - | - | x |
| Guynia annulata Duncan, 1872 | Scleractinia | Guyniidae | 1 | x | - | - | - | - |
| Madrepora oculata Linnaeus, 1758 | Scleractinia | Madreporidae | 8 | x | x | x | - | x |
| Oculina patagonica de Angelis D’Ossat, 1908 | Scleractinia | Oculinidae | 1 | - | - | x | - | - |
| Oculina Lamarck, 1816 | Scleractinia | Oculinidae | 1 | - | - | x | - | - |
| Madracis pharensis (Heller, 1868) | Scleractinia | Pocilloporidae | 1 | x | - | - | - | - |
| Madracis profunda Zibrowius, 1980 | Scleractinia | Pocilloporidae | 1 | x | - | - | - | - |
| Madracis Milne Edwards & Haime, 1849 | Scleractinia | Pocilloporidae | 1 | - | x | - | - | - |
| Culicia tenella Dana, 1846 | Scleractinia | Rhizangiidae | 1 | - | - | x | - | - |
| Culicia Dana, 1846 | Scleractinia | Rhizangiidae | 1 | - | - | x | - | - |
| Schizocyathidae Stolarski, 2000 | Scleractinia | Schizocyathidae | 1 | x | - | - | - | x |
| Schizocyathus fissilis Pourtalès, 1874 | Scleractinia | Schizocyathidae | 1 | x | - | - | - | - |
| Stenocyathus vermiformis (Pourtalès, 1868) | Scleractinia | Stenocyathidae | 3 | x | x | - | - | x |
| Stephanocyathus (Odontocyathus) nobilis (Moseley, 1876) | Scleractinia | Stephanocyathidae | 1 | x | - | - | - | - |
| Stephanocyathus (Stephanocyathus) crassus (Jourdan, 1895) | Scleractinia | Stephanocyathidae | 1 | x | - | - | - | - |
| Stephanocyathus (Stephanocyathus) diadema (Moseley, 1876) | Scleractinia | Stephanocyathidae | 1 | x | - | - | - | - |
| Stephanocyathus (Stephanocyathus) moseleyanus (Sclater, 1886) | Scleractinia | Stephanocyathidae | 2 | x | - | - | - | x |
| Vaughanella concinna Gravier, 1915 | Scleractinia | Stephanocyathidae | 1 | x | - | - | - | - |
| Peponocyathus stimpsonii (Pourtalès, 1871) | Scleractinia | Turbinoliidae | 1 | x | - | - | - | - |
| Sphenotrochus (Sphenotrochus) andrewianus Milne Edwards & Haime, 1848 | Scleractinia | Turbinoliidae | 1 | x | - | - | - | - |
| Thrypticotrochus Cairns, 1989 | Scleractinia | Turbinoliidae | 1 | - | - | - | - | x |
| Peponocyathus folliculus (Pourtalès, 1868) | Scleractinia | Turbinoliidae | 2 | x | x | - | - | x |
| Zoantharia | Zoantharia | 1 | x | - | - | - | x | |
| Epizoanthus martinsae Carreiro-Silva, Ocaña, Stanković, Sampaio, Porteiro, Fabri & Stefanni, 2017 | Zoantharia | Epizoanthidae | 1 | x | - | - | - | - |
| Parazoanthus Haddon & Shackleton, 1891 | Zoantharia | Parazoanthidae | 1 | x | - | x | - | x |
| Savalia savaglia (Bertoloni, 1819) | Zoantharia | Parazoanthidae | 1 | - | - | x | - | - |
| Palythoa canariensis Haddon & Duerden, 1896 | Zoantharia | Sphenopidae | 1 | - | - | x | - | - |
| Zoanthidae Rafinesque, 1815 | Zoantharia | Zoanthidae | 2 | x | - | - | - | x |
| Class Octocorallia | ||||||||
| Octocorallia | 5 | x | - | x | x | x | ||
| Rolandia coralloides de Lacaze Duthiers, 1900 | Alcyonacea | Clavulariidae | 1 | x | - | - | - | - |
| Malacalcyonacea | Malacalcyonacea | 1 | x | - | - | - | - | |
| Acanthogorgia armata Verrill, 1878 | Malacalcyonacea | Acanthogorgiidae | 3 | x | - | - | - | x |
| Acanthogorgia aspera Pourtalès, 1867 | Malacalcyonacea | Acanthogorgiidae | 1 | x | - | - | - | - |
| Acanthogorgia hirsuta Gray, 1857 | Malacalcyonacea | Acanthogorgiidae | 3 | x | - | x | - | x |
| Acanthogorgia muricata Verrill, 1883 | Malacalcyonacea | Acanthogorgiidae | 1 | x | - | - | - | - |
| Acanthogorgia pico Grasshoff, 1973 | Malacalcyonacea | Acanthogorgiidae | 1 | x | - | - | - | - |
| Acanthogorgia sp. Gray, 1857 | Malacalcyonacea | Acanthogorgiidae | 4 | x | - | x | - | x |
| Bebryce mollis Philippi, 1842 | Malacalcyonacea | Acanthogorgiidae | 3 | x | x | - | - | x |
| Dentomuricea meteor Grasshoff, 1977 | Malacalcyonacea | Acanthogorgiidae | 7 | x | x | x | - | x |
| Dentomuricea Grasshoff, 1977 | Malacalcyonacea | Acanthogorgiidae | 1 | x | - | - | - | x |
| Muriceides lepida Carpine & Grasshoff, 1975 | Malacalcyonacea | Acanthogorgiidae | 1 | x | - | - | - | - |
| Muriceides sceptrum (Studer, 1891) | Malacalcyonacea | Acanthogorgiidae | 2 | x | - | - | - | x |
| Placogorgia becena Grasshoff, 1977 | Malacalcyonacea | Acanthogorgiidae | 1 | x | - | - | - | - |
| Placogorgia coronata Carpine & Grasshoff, 1975 | Malacalcyonacea | Acanthogorgiidae | 1 | x | - | - | - | - |
| Placogorgia intermedia (Thomson, 1927) | Malacalcyonacea | Acanthogorgiidae | 1 | x | - | - | - | - |
| Villogorgia bebrycoides (von Koch, 1887) | Malacalcyonacea | Acanthogorgiidae | 2 | x | - | - | - | x |
| Gersemia clavata (Danielssen, 1887) | Malacalcyonacea | Alcyoniidae | 1 | x | - | - | - | - |
| Azoriella bayeri (López-González & Gili, 2001) | Malacalcyonacea | Cerveridae | 1 | x | - | - | - | - |
| Clavularia arctica (Sars, 1860) | Malacalcyonacea | Clavulariidae | 1 | x | - | - | - | - |
| Clavularia armata Thomson, 1927 | Malacalcyonacea | Clavulariidae | 1 | x | - | - | - | - |
| Clavularia charcoti (Tixier-Durivault & d’Hondt, 1974) | Malacalcyonacea | Clavulariidae | 1 | x | - | - | - | - |
| Clavularia elongata Wright & Studer, 1889 | Malacalcyonacea | Clavulariidae | 1 | x | - | - | - | - |
| Clavularia marioni von Koch, 1890 | Malacalcyonacea | Clavulariidae | 1 | x | - | - | - | - |
| Clavularia tenuis Tixier-Durivault & d’Hondt, 1974 | Malacalcyonacea | Clavulariidae | 1 | x | - | - | - | - |
| Clavularia Blainville, 1830 | Malacalcyonacea | Clavulariidae | 1 | x | - | - | - | x |
| Schizophytum echinatum Studer, 1891 | Malacalcyonacea | Clavulariidae | 2 | x | - | - | - | x |
| Eunicella verrucosa (Pallas, 1766) | Malacalcyonacea | Eunicellidae | 1 | - | x | - | - | - |
| Eunicella Verrill, 1869 | Malacalcyonacea | Eunicellidae | 1 | - | x | - | - | - |
| Pseudotelestula humilis (Thomson, 1927) | Malacalcyonacea | Incrustatidae | 1 | x | - | - | - | - |
| Thesea rigida (Thomson, 1927) | Malacalcyonacea | Malacalcyonacea incertae sedis | 1 | x | - | - | - | - |
| Nephtheidae Gray, 1862 | Malacalcyonacea | Nephtheidae | 1 | - | x | - | - | - |
| Scleronephthya macrospina Thomson, 1927 | Malacalcyonacea | Nephtheidae | 1 | x | - | - | - | - |
| Plexauridae Gray, 1859 | Malacalcyonacea | Plexauridae | 3 | x | - | - | - | x |
| Dacrygorgia modesta (Verrill, 1883) | Malacalcyonacea | Pterogorgiidae | 1 | x | - | - | - | - |
| Sarcophyton Lesson, 1834 | Malacalcyonacea | Sarcophytidae | 1 | - | x | - | - | - |
| Bathytelesto rigida (Wright & Studer, 1889) | Malacalcyonacea | Tubiporidae | 1 | x | - | - | - | - |
| Scyphopodium ingolfi (Madsen, 1944) | Malacalcyonacea | Tubiporidae | 1 | x | - | - | - | - |
| Scleranthelia rugosa (Pourtalès, 1867) | Octocorallia incertae sedis | Octocorallia incertae sedis | 1 | x | - | - | - | - |
| Scleralcyonacea | Scleralcyonacea | 2 | x | - | - | x | x | |
| Chelidonisis aurantiaca Studer, 1890 | Scleralcyonacea | Chelidonisididae | 2 | x | - | - | - | - |
| Chrysogorgia agassizii (Verrill, 1883) | Scleralcyonacea | Chrysogorgiidae | 3 | x | - | - | - | x |
| Chrysogorgia fewkesii Verrill, 1883 | Scleralcyonacea | Chrysogorgiidae | 1 | x | - | - | - | - |
| Chrysogorgia quadruplex Thomson, 1927 | Scleralcyonacea | Chrysogorgiidae | 1 | x | - | - | - | - |
| Chrysogorgia Duchassaing & Michelotti, 1864 | Scleralcyonacea | Chrysogorgiidae | 3 | x | - | x | - | x |
| Parachrysogorgia squamata (Verrill, 1883) | Scleralcyonacea | Chrysogorgiidae | 1 | x | - | - | - | - |
| Radicipes gracilis (Verrill, 1884) | Scleralcyonacea | Chrysogorgiidae | 2 | x | - | - | - | - |
| Chrysogorgia elegans (Verrill, 1883) | Scleralcyonacea | Chrysogorgiidae | 1 | x | - | - | - | - |
| Pleurocorallium johnsoni (Gray, 1860) | Scleralcyonacea | Coralliidae | 3 | x | - | - | - | x |
| Coralliidae Lamouroux, 1812 | Scleralcyonacea | Coralliidae | 4 | x | - | - | - | x |
| Cornularia cornucopiae (Pallas, 1766) | Scleralcyonacea | Cornulariidae | 1 | x | - | - | - | - |
| Viminella flagellum (Johnson, 1863) | Scleralcyonacea | Ellisellidae | 8 | x | x | x | - | x |
| Acanella arbuscula (Johnson, 1862) | Scleralcyonacea | Keratoisididae | 5 | x | - | x | x | x |
| Calyptrophora trilepis (Pourtalès, 1868) | Scleralcyonacea | Primnoidae | 1 | x | - | x | - | x |
| Candidella imbricata (Johnson, 1862) | Scleralcyonacea | Primnoidae | 5 | x | - | - | - | x |
| Narella bellissima (Kükenthal, 1915) | Scleralcyonacea | Primnoidae | 4 | x | - | - | - | x |
| Narella versluysi (Hickson, 1909) | Scleralcyonacea | Primnoidae | 3 | x | - | - | - | x |
| Paracalyptrophora josephinae (Lindström, 1877) | Scleralcyonacea | Primnoidae | 4 | x | - | - | - | x |
| Primnoidae Milne Edwards, 1857 | Scleralcyonacea | Primnoidae | 3 | x | - | - | - | x |
| Thouarella (Euthouarella) grasshoffi Cairns, 2006 | Scleralcyonacea | Primnoidae | 1 | x | - | - | - | - |
| Thouarella (Euthouarella) hilgendorfi (Studer, 1879) | Scleralcyonacea | Primnoidae | 1 | x | - | - | - | x |
| Thouarella (Thouarella) variabilis Wright & Studer, 1889 | Scleralcyonacea | Primnoidae | 1 | x | - | - | - | - |
| Thouarella Gray, 1870 | Scleralcyonacea | Primnoidae | 2 | x | - | - | - | x |
| Sarcodictyon catenatum Forbes in Johnston, 1847 | Scleralcyonacea | Sarcodictyonidae | 2 | x | - | - | - | x |
| Telestula batoni Weinberg, 1990 | Scleralcyonacea | Sarcodictyonidae | 1 | x | - | - | - | - |
| Telestula kuekenthali Weinberg, 1990 | Scleralcyonacea | Sarcodictyonidae | 1 | x | - | - | - | - |
| Telestula tubaria Wright & Studer, 1889 | Scleralcyonacea | Sarcodictyonidae | 1 | x | - | - | - | - |
| Scleroptilum grandiflorum Kölliker, 1880 | Scleralcyonacea | Scleroptilidae | 1 | x | - | - | - | x |
| Titanideum obscurum Thomson, 1927 | Scleralcyonacea | Spongiodermidae | 1 | x | - | - | - | - |
| Paramuricea candida Grasshoff, 1977 | Malacalcyonacea | Acanthogorgiidae | 1 | x | - | - | - | - |
| Paramuricea grayi (Johnson, 1861) | Malacalcyonacea | Acanthogorgiidae | 1 | - | x | - | - | - |
| Paramuricea Kölliker, 1865 | Malacalcyonacea | Acanthogorgiidae | 5 | x | - | x | x | x |
| Alcyoniidae Lamouroux, 1812 | Malacalcyonacea | Alcyoniidae | 3 | x | - | - | - | x |
| Alcyonium bocagei (Saville Kent, 1870) | Malacalcyonacea | Alcyoniidae | 2 | x | - | - | - | x |
| Alcyonium burmedju Sampaio, Stokvis & van Ofwegen, 2016 | Malacalcyonacea | Alcyoniidae | 2 | x | - | - | - | x |
| Alcyonium maristenebrosi (Stiasny, 1937) | Malacalcyonacea | Alcyoniidae | 1 | x | - | - | - | - |
| Alcyonium palmatum Pallas, 1766 | Malacalcyonacea | Alcyoniidae | 1 | x | - | - | - | - |
| Alcyonium profundum Stokvis & van Ofwegen, 2006 | Malacalcyonacea | Alcyoniidae | 1 | x | - | - | - | - |
| Alcyonium Linnaeus, 1758 | Malacalcyonacea | Alcyoniidae | 1 | x | - | - | - | x |
| Anthothela grandiflora (Sars, 1856) | Malacalcyonacea | Alcyoniidae | 1 | x | - | - | - | - |
| Bellonella tenuis Tixier-Durivault & d’Hondt, 1974 | Malacalcyonacea | Alcyoniidae | 1 | x | - | - | - | - |
| Bellonella variabilis (Studer, 1891) | Malacalcyonacea | Alcyoniidae | 1 | x | - | - | - | - |
| Lateothela grandiflora (Tixier-Durivault & d’Hondt, 1974) | Malacalcyonacea | Alcyoniidae | 2 | x | - | - | - | - |
| Isididae Lamouroux, 1812 | Malacalcyonacea | Isididae | 2 | x | - | - | - | x |
| Paralcyonium spinulosum (Delle Chiaje, 1822) | Malacalcyonacea | Paralcyoniidae | 1 | x | - | - | - | - |
| Swiftia dubia (Thomson, 1929) | Malacalcyonacea | Plexauridae | 2 | x | - | - | - | x |
| Swiftia Duchassaing & Michelotti, 1864 | Malacalcyonacea | Plexauridae | 2 | x | - | x | - | - |
| Pennatuloidea Ehrenberg, 1834 | Scleralcyonacea | 2 | x | - | - | - | x | |
| Anthoptilum Kölliker, 1880 | Scleralcyonacea | Anthoptilidae | 1 | x | - | - | - | x |
| Iridogorgia fontinalis Watling, 2007 | Scleralcyonacea | Chrysogorgiidae | 1 | x | - | - | - | - |
| Iridogorgia pourtalesii Verrill, 1883 | Scleralcyonacea | Chrysogorgiidae | 1 | x | - | - | - | - |
| Iridogorgia Verrill, 1883 | Scleralcyonacea | Chrysogorgiidae | 2 | x | - | - | x | x |
| Metallogorgia melanotrichos (Wright & Studer, 1889) | Scleralcyonacea | Chrysogorgiidae | 2 | x | - | x | - | - |
| Metallogorgia Versluys, 1902 | Scleralcyonacea | Chrysogorgiidae | 1 | - | - | - | x | - |
| Corallium Cuvier, 1798 | Scleralcyonacea | Coralliidae | 2 | x | - | x | - | x |
| Hemicorallium niobe (Bayer, 1964) | Scleralcyonacea | Coralliidae | 3 | x | - | x | - | - |
| Hemicorallium tricolor (Johnson, 1899) | Scleralcyonacea | Coralliidae | 2 | x | - | x | - | - |
| Paragorgia arborea (Linnaeus, 1758) | Scleralcyonacea | Coralliidae | 1 | x | - | - | - | x |
| Paragorgia johnsoni Gray, 1862 | Scleralcyonacea | Coralliidae | 4 | x | - | - | - | x |
| Anthomastus canariensis Wright & Studer, 1889 | Scleralcyonacea | Coralliidae | 1 | x | - | - | - | - |
| Anthomastus grandiflorus Verrill, 1878 | Scleralcyonacea | Coralliidae | 1 | x | - | - | - | - |
| Anthomastus Verrill, 1878 | Scleralcyonacea | Coralliidae | 4 | x | - | x | - | x |
| Pseudoanthomastus agaricus (Studer, 1890) | Scleralcyonacea | Coralliidae | 2 | x | - | - | - | x |
| Pseudoanthomastus Tixier-Durivault & d’Hondt, 1974 | Scleralcyonacea | Coralliidae | 1 | x | - | - | - | - |
| Nicella granifera (Kölliker, 1865) | Scleralcyonacea | Ellisellidae | 1 | x | - | - | - | - |
| Gyrophyllum hirondellei Studer, 1891 | Scleralcyonacea | Gyrophyllidae | 1 | x | - | - | - | - |
| Isidella longiflora (Verrill, 1883) | Scleralcyonacea | Keratoisididae | 1 | x | - | - | - | - |
| Lepidisis cyanae Grasshoff, 1986 | Scleralcyonacea | Keratoisididae | 1 | x | - | - | - | - |
| Lepidisis Verrill, 1883 | Scleralcyonacea | Keratoisididae | 1 | x | - | - | - | - |
| Keratoisididae Gray, 1870 | Scleralcyonacea | Keratoisididae | 1 | x | - | - | - | x |
| Keratoisis grayi Wright, 1869 | Scleralcyonacea | Keratoisididae | 1 | x | - | - | - | - |
| Keratoisis Wright, 1869 | Scleralcyonacea | Keratoisididae | 3 | x | - | x | - | x |
| Pennatula Linnaeus, 1758 | Scleralcyonacea | Pennatulidae | 1 | - | - | x | - | - |
| Callogorgia verticillata (Pallas, 1766) | Scleralcyonacea | Primnoidae | 4 | x | - | x | - | x |
| Paracalyptrophora Kinoshita, 1908 | Scleralcyonacea | Primnoidae | 1 | x | - | - | - | x |
| Umbellula Cuvier, [1797] | Scleralcyonacea | Umbellulidae | 1 | x | - | - | - | x |
| Veretillum cynomorium (Pallas, 1766) | Scleralcyonacea | Veretillidae | 1 | - | x | - | - | - |
| Subphyllum Medusozoa | ||||||||
| Class Hydrozoa | ||||||||
| Hydrozoa | 1 | x | - | - | - | x | ||
| Candelabrum phrygium (Fabricius, 1780) | Anthoathecata | Candelabridae | 1 | x | - | - | - | x |
| Candelabrum serpentarii Segonzac & Vervoort, 1995 | Anthoathecata | Candelabridae | 1 | x | - | - | - | x |
| Eudendrium Ehrenberg, 1834 | Anthoathecata | Eudendriidae | 1 | x | - | - | - | x |
| Pennaria disticha Goldfuss, 1820 | Anthoathecata | Pennariidae | 1 | - | x | - | - | - |
| Errina atlantica Hickson, 1912 | Anthoathecata | Stylasteridae | 2 | x | - | - | - | x |
| Errina dabneyi (Pourtalès, 1871) | Anthoathecata | Stylasteridae | 5 | x | - | x | - | x |
| Errina Gray, 1835 | Anthoathecata | Stylasteridae | 2 | x | x | - | - | x |
| Stenohelia Kent, 1870 | Anthoathecata | Stylasteridae | 2 | x | x | - | - | x |
| Stylaster Gray, 1831 | Anthoathecata | Stylasteridae | 1 | - | x | - | - | x |
| Stylasteridae Gray, 1847 | Anthoathecata | Stylasteridae | 5 | x | - | - | - | x |
| Pliobothrus symmetricus Pourtalès, 1868 | Anthoathecata | Stylasteridae | 2 | x | - | - | - | x |
| Crypthelia affinis Moseley, 1879 | Anthoathecata | Stylasteridae | 1 | x | - | - | - | - |
| Crypthelia medioatlantica Zibrowius & Cairns, 1992 | Anthoathecata | Stylasteridae | 1 | x | - | - | - | - |
| Crypthelia tenuiseptata Cairns, 1986 | Anthoathecata | Stylasteridae | 1 | x | - | - | - | - |
| Crypthelia vascomarquesi Zibrowius & Cairns, 1992 | Anthoathecata | Stylasteridae | 1 | x | - | - | - | - |
| Crypthelia Milne Edwards & Haime, 1849 | Anthoathecata | Stylasteridae | 2 | x | - | - | - | x |
| Lepidopora eburnea (Calvet, 1903) | Anthoathecata | Stylasteridae | 1 | x | - | - | - | - |
| Lepidopora Pourtalès, 1871 | Anthoathecata | Stylasteridae | 1 | - | x | - | - | x |
| Ectopleura crocea (Agassiz, 1862) | Anthoathecata | Tubulariidae | 1 | - | x | - | - | - |
| Macrorhynchia philippina Kirchenpauer, 1872 | Leptothecata | Aglaopheniidae | 2 | - | x | - | - | - |
| Macrorhynchia Kirchenpauer, 1872 | Leptothecata | Aglaopheniidae | 1 | - | x | - | - | - |
| Aglaophenia lophocarpa Allman, 1877 | Leptothecata | Aglaopheniidae | 1 | x | - | - | - | x |
| Aglaophenia pluma (Linnaeus, 1758) | Leptothecata | Aglaopheniidae | 1 | - | x | - | - | - |
| Aglaophenia Lamouroux, 1812 | Leptothecata | Aglaopheniidae | 3 | x | x | x | - | x |
| Lytocarpia myriophyllum (Linnaeus, 1758) | Leptothecata | Aglaopheniidae | 5 | x | - | x | - | x |
| Obelia geniculata (Linnaeus, 1758) | Leptothecata | Campanulariidae | 1 | x | - | - | - | - |
| Halecium Oken, 1815 | Leptothecata | Haleciidae | 1 | x | - | - | - | x |
| Polyplumaria flabellata Sars, 1874 | Leptothecata | Halopterididae | 5 | x | - | x | - | x |
| Polyplumaria Sars, 1874 | Leptothecata | Halopterididae | 1 | x | - | - | - | x |
| Antennella secundaria (Gmelin, 1791) | Leptothecata | Halopterididae | 1 | x | - | - | - | x |
| Antennella Allman, 1877 | Leptothecata | Halopterididae | 1 | - | x | - | - | - |
| Kirchenpaueria halecioides (Alder, 1859) | Leptothecata | Kirchenpaueriidae | 1 | - | x | - | - | - |
| Filellum serratum (Clarke, 1879) | Leptothecata | Lafoeidae | 1 | x | - | - | - | x |
| Grammaria abietina (Sars, 1851) | Leptothecata | Lafoeidae | 1 | x | - | - | - | x |
| Acryptolaria conferta (Allman, 1877) | Leptothecata | Lafoeidae | 1 | x | - | - | - | x |
| Acryptolaria crassicaulis (Allman, 1888) | Leptothecata | Lafoeidae | 1 | x | - | - | - | x |
| Acryptolaria Norman, 1875 | Leptothecata | Lafoeidae | 1 | x | - | x | - | x |
| Nemertesia antennina (Linnaeus, 1758) | Leptothecata | Plumulariidae | 3 | x | - | x | - | x |
| Nemertesia ramosa (Lamarck, 1816) | Leptothecata | Plumulariidae | 1 | - | x | - | - | - |
| Nemertesia Lamouroux, 1812 | Leptothecata | Plumulariidae | 1 | x | - | - | - | x |
| Plumulariidae McCrady, 1859 | Leptothecata | Plumulariidae | 1 | x | - | x | - | x |
| Sertularella gayi (Lamouroux, 1821) | Leptothecata | Sertularellidae | 1 | x | - | - | - | x |
| Sertularella Gray, 1848 | Leptothecata | Sertularellidae | 1 | - | x | - | - | x |
| Diphasia alata (Hincks, 1855) | Leptothecata | Sertulariidae | 1 | x | - | x | - | x |
| Diphasia margareta (Hassall, 1841) | Leptothecata | Sertulariidae | 1 | x | - | - | - | x |
| Diphasia Agassiz, 1862 | Leptothecata | Sertulariidae | 2 | x | - | - | - | x |
| Dynamena Lamouroux, 1812 | Leptothecata | Sertulariidae | 1 | - | x | - | - | - |
| Cryptolaria pectinata (Allman, 1888) | Leptothecata | Zygophylacidae | 1 | x | - | - | - | x |
| Cryptolaria Busk, 1857 | Leptothecata | Zygophylacidae | 1 | x | - | x | - | x |
| Zygophylax biarmata Billard, 1905 | Leptothecata | Zygophylacidae | 1 | x | - | - | - | x |
| Phyllum Chlorophyta | ||||||||
| Subphyllum Chlorophytina | ||||||||
| Class Ulvophyceae | ||||||||
| Bryopsis J.V.Lamouroux, 1809 | Bryopsidales | Bryopsidaceae | 1 | - | x | - | - | - |
| Caulerpa cylindracea Sonder, 1845 | Bryopsidales | Caulerpaceae | 1 | - | - | x | - | - |
| Caulerpa mexicana Sonder ex Kützing, 1849 | Bryopsidales | Caulerpaceae | 1 | - | - | x | - | - |
| Caulerpa prolifera (Forsskål) J.V.Lamouroux, 1809 | Bryopsidales | Caulerpaceae | 7 | - | x | x | - | - |
| Caulerpa racemosa (Forsskål) J.Agardh, 1873 | Bryopsidales | Caulerpaceae | 1 | - | - | x | - | - |
| Caulerpa webbiana f. disticha Vickers, 1896 | Bryopsidales | Caulerpaceae | 1 | - | - | x | - | - |
| Caulerpa webbiana Montagne, 1837 | Bryopsidales | Caulerpaceae | 1 | - | x | - | - | - |
| Caulerpa J.V.Lamouroux, 1809 | Bryopsidales | Caulerpaceae | 4 | - | - | x | - | - |
| Codium adhaerens C.Agardh, 1822 | Bryopsidales | Codiaceae | 4 | x | - | - | - | - |
| Codium elisabethiae O.C.Schmidt, 1929 | Bryopsidales | Codiaceae | 2 | x | - | - | - | - |
| Codium fragile subsp. fragile (Suringar) Hariot, 1889 | Bryopsidales | Codiaceae | 1 | x | - | - | - | - |
| Codium fragile (Suringar) Hariot, 1889 | Bryopsidales | Codiaceae | 1 | x | - | - | - | - |
| Avrainvillea canariensis A.Gepp & E.S.Gepp, 1911 | Bryopsidales | Dichotomosiphonaceae | 1 | - | x | - | - | - |
| Halimeda incrassata (J.Ellis) J.V.Lamouroux, 1816 | Bryopsidales | Halimedaceae | 1 | - | - | x | - | - |
| Anadyomene stellata (Wulfen) C.Agardh, 1823 | Cladophorales | Anadyomenaceae | 1 | - | - | x | - | - |
| Microdictyon calodictyon (Montagne) Kützing, 1849 | Cladophorales | Anadyomenaceae | 1 | - | - | x | - | - |
| Microdictyon Decaisne, 1841 | Cladophorales | Anadyomenaceae | 1 | - | - | x | - | - |
| Cladophoropsis membranacea Bang ex C.Agardh) Børgesen, 1905 | Cladophorales | Boodleaceae | 1 | x | - | - | - | - |
| Chaetomorpha aerea (Dillwyn) Kützing, 1849 | Cladophorales | Cladophoraceae | 2 | x | - | - | - | - |
| Chaetomorpha pachynema (Montagne) Kützing, 1847 | Cladophorales | Cladophoraceae | 2 | x | - | - | - | - |
| Chaetomorpha Kützing, 1845 | Cladophorales | Cladophoraceae | 2 | x | - | x | - | - |
| Pseudorhizoclonium africanum (Kützing) Boedeker, 2016 | Cladophorales | Cladophoraceae | 1 | x | - | - | - | - |
| Cladophora albida (Nees) Kutzing, 1843 | Cladophorales | Cladophoraceae | 1 | x | - | - | - | - |
| Cladophora coelothrix Kützing, 1843 | Cladophorales | Cladophoraceae | 1 | x | - | - | - | - |
| Cladophora prolifera (Roth) Kützing, 1843 | Cladophorales | Cladophoraceae | 3 | x | - | - | - | - |
| Cladophora Kützing, 1843 | Cladophorales | Cladophoraceae | 3 | x | - | x | - | - |
| Valonia utricularis (Roth) C.Agardh, 1823 | Cladophorales | Valoniaceae | 1 | x | - | - | - | - |
| Valonia C.Agardh, 1823 | Cladophorales | Valoniaceae | 1 | x | - | - | - | - |
| Cymopolia barbata (Linnaeus) J.V.Lamouroux, 1816 | Dasycladales | Dasycladaceae | 1 | - | - | x | - | - |
| Dasycladus vermicularis (Scopoli) Krasser, 1898 | Dasycladales | Dasycladaceae | 1 | - | x | - | - | - |
| Dasycladus C.Agardh, 1828 | Dasycladales | Dasycladaceae | 1 | - | - | x | - | - |
| Parvocaulis parvulus (Solms-Laubach) S.Berger, Fettweiss, Gleissberg, Liddle, U.Richter, Sawitzky & Zuccarello, 2003 | Dasycladales | Polyphysaceae | 2 | - | - | x | - | - |
| Blidingia minima (Nägeli ex Kützing) Kylin, 1947 | Ulvales | Kornmanniaceae | 1 | x | - | - | - | - |
| Blidingia Kylin, 1947 | Ulvales | Kornmanniaceae | 2 | x | - | - | - | - |
| Ulva clathrata (Roth) C.Agardh, 1811 | Ulvales | Ulvaceae | 1 | x | - | - | - | - |
| Ulva compressa Linnaeus, 1753 | Ulvales | Ulvaceae | 2 | x | - | - | - | - |
| Ulva intestinalis Linnaeus, 1753 | Ulvales | Ulvaceae | 1 | x | - | - | - | - |
| Ulva linza Linnaeus, 1753 | Ulvales | Ulvaceae | 1 | x | - | - | - | - |
| Ulva rigida C.Agardh, 1823 | Ulvales | Ulvaceae | 3 | x | - | - | - | - |
| Ulva torta (Mertens) Trevisan, 1842 | Ulvales | Ulvaceae | 1 | x | - | - | - | - |
| Ulva Linnaeus, 1753 | Ulvales | Ulvaceae | 3 | x | - | - | - | - |
| Ulvaceae J.V. Lamouroux ex Dumortier, 1822 | Ulvales | Ulvaceae | 1 | x | - | - | - | - |
| Class Chlorophyceae | ||||||||
| Pseudotetraspora marina Wille, 1906 | Chlamydomonadales | Palmellopsidaceae | 1 | - | - | x | - | - |
| Phyllum Rhodophyta | ||||||||
| Subphyllum Eurhodophytina | ||||||||
| Class Bangiophyceae | ||||||||
| Porphyra umbilicalis Kützing, 1843 | Bangiales | Bangiaceae | 1 | x | - | - | - | - |
| Porphyra C.Agardh, 1824 | Bangiales | Bangiaceae | 1 | x | - | - | - | - |
| Bangia atropurpurea (Mertens ex Roth) C.Agardh, 1824 | Bangiales | Bangiaceae | 1 | x | - | - | - | - |
| Class Florideophyceae | ||||||||
| Asparagopsis armata f. rufolanosa Harvey, 1856 | Bonnemaisoniales | Bonnemaisoniaceae | 1 | x | - | - | - | - |
| Asparagopsis armata Harvey, 1855 | Bonnemaisoniales | Bonnemaisoniaceae | 4 | x | - | - | - | - |
| Asparagopsis taxiformis (Delile) Trevisan de Saint-Léon, 1845 | Bonnemaisoniales | Bonnemaisoniaceae | 7 | x | x | x | - | - |
| Asparagopsis Montagne, 1840 | Bonnemaisoniales | Bonnemaisoniaceae | 4 | x | - | - | - | - |
| Ceramieales | Ceramiales | 1 | - | - | x | - | - | |
| Chondracanthus teedei (Mertens ex Roth) Kützing, 1843 | Ceramiales | Rhodomelaceae | 1 | x | - | - | - | - |
| Aglaothamnion Feldmann-Mazoyer, 1941 | Ceramiales | Callithamniaceae | 1 | x | - | - | - | - |
| Callithamnion corymbosum (Smith) Lyngbye, 1819 | Ceramiales | Callithamniaceae | 1 | x | - | - | - | - |
| Gaillona hookeri (Dillwyn) Athanasiadis, 2016 | Ceramiales | Callithamniaceae | 1 | x | - | - | - | - |
| Spyridia filamentosa (Wulfen) Harvey, 1833 | Ceramiales | Callithamniaceae | 2 | - | - | x | - | - |
| Spyridia hypnoides (Bory de Saint-Vincent) Papenfuss, 1968 | Ceramiales | Callithamniaceae | 1 | - | - | x | - | - |
| Antithamnion Nägeli, 1847 | Ceramiales | Ceramiaceae | 1 | x | - | - | - | - |
| Centroceras clavulatum (C.Agardh) Montagne, 1846 | Ceramiales | Ceramiaceae | 2 | x | - | x | - | - |
| Ceramieae (Dumortier) Schmitz, 1889 | Ceramiales | Ceramiaceae | 1 | x | - | - | - | - |
| Ceramium ciliatum (J.Ellis) Ducluzeau, 1806 | Ceramiales | Ceramiaceae | 2 | x | - | - | - | - |
| Ceramium diaphanum (Lightfoot) Roth, 1806 | Ceramiales | Ceramiaceae | 2 | x | - | x | - | - |
| Ceramium echionotum J.Agardh, 1844 | Ceramiales | Ceramiaceae | 2 | x | - | - | - | - |
| Ceramium gaditanum (Clemente) Cremades, 1990 | Ceramiales | Ceramiaceae | 1 | x | - | - | - | - |
| Ceramium virgatum Roth, 1797 | Ceramiales | Ceramiaceae | 2 | x | - | - | - | - |
| Ceramium Roth, 1797 | Ceramiales | Ceramiaceae | 2 | - | x | - | - | - |
| Gayliella mazoyerae T.O.Cho, Fredericq & Hommersand, 2008 | Ceramiales | Ceramiaceae | 1 | - | - | x | - | - |
| Stirkia codii (H.Richards) Barros-Barreto & Maggs, 2023 | Ceramiales | Ceramiaceae | 1 | - | - | x | - | - |
| Acrosorium venulosum (Zanardini) Kylin, 1924 | Ceramiales | Delesseriaceae | 1 | x | - | - | - | - |
| Acrosorium Zanardini ex Kützing, 1869 | Ceramiales | Delesseriaceae | 1 | x | - | - | - | - |
| Cottoniella filamentosa (M.A.Howe) Børgesen, 1920 | Ceramiales | Delesseriaceae | 3 | - | - | x | - | - |
| Dasya pedicellata (C.Agardh) C.Agardh, 1824 | Ceramiales | Delesseriaceae | 1 | - | - | x | - | - |
| Dasya C.Agardh, 1824 | Ceramiales | Delesseriaceae | 1 | x | - | - | - | - |
| Delesseriaceae Bory, 1828 | Ceramiales | Delesseriaceae | 1 | x | - | - | - | - |
| Hypoglossum hypoglossoides (Stackhouse) Collins & Hervey, 1917 | Ceramiales | Delesseriaceae | 1 | x | - | - | - | - |
| Chondria capillaris (Hudson) M.J.Wynne, 1991 | Ceramiales | Rhodomelaceae | 1 | x | - | - | - | - |
| Chondria coerulescens (J.Agardh) Sauvageau, 1897 | Ceramiales | Rhodomelaceae | 1 | x | - | - | - | - |
| Chondria dasyphylla (Woodward) C.Agardh, 1817 | Ceramiales | Rhodomelaceae | 2 | x | - | x | - | - |
| Chondria C.Agardh, 1817 | Ceramiales | Rhodomelaceae | 1 | - | x | - | - | - |
| Laurencia viridis Gil-Rodríguez & Haroun, 1992 | Ceramiales | Rhodomelaceae | 1 | x | - | - | - | - |
| Laurencia J.V.Lamouroux, 1813 | Ceramiales | Rhodomelaceae | 5 | x | - | x | - | - |
| Lophocladia trichoclados (C.Agardh) F.Schmitz, 1893 | Ceramiales | Rhodomelaceae | 2 | - | - | x | - | - |
| Lophosiphonia Falkenberg, 1897 | Ceramiales | Rhodomelaceae | 1 | x | - | - | - | - |
| Polysiphonia atlantica Kapraun & J.N.Norris, 1982 | Ceramiales | Rhodomelaceae | 2 | x | - | x | - | - |
| Polysiphonia flexella (C.Agardh) J.Agardh, 1842 | Ceramiales | Rhodomelaceae | 1 | - | - | x | - | - |
| Polysiphonia havanensis Montagne, 1837 | Ceramiales | Rhodomelaceae | 1 | x | - | - | - | - |
| Polysiphonia opaca (C.Agardh) Moris & De Notaris, 1839 | Ceramiales | Rhodomelaceae | 1 | x | - | - | - | - |
| Polysiphonia stricta (Mertens ex Dillwyn) Greville, 1824 | Ceramiales | Rhodomelaceae | 1 | x | - | - | - | - |
| Polysiphonia Greville, 1823 | Ceramiales | Rhodomelaceae | 4 | x | - | x | - | - |
| Symphyocladia marchantioides (Harvey) Falkenberg, 1897 | Ceramiales | Rhodomelaceae | 1 | x | - | - | - | - |
| Carradoriella denudata (Dillwyn) Savoie & G.W.Saunders, 2019 | Ceramiales | Rhodomelaceae | 1 | x | - | - | - | - |
| Halopithys incurva (Hudson) Batters, 1902 | Ceramiales | Rhodomelaceae | 1 | - | - | x | - | - |
| Herposiphonia secunda (C.Agardh) Ambronn, 1880 | Ceramiales | Rhodomelaceae | 1 | - | - | x | - | - |
| Herposiphonia Nägeli, 1846 | Ceramiales | Rhodomelaceae | 1 | x | - | - | - | - |
| Osmundea pinnatifida (Hudson) Stackhouse, 1809 | Ceramiales | Rhodomelaceae | 3 | x | - | - | - | - |
| Osmundea Stackhouse, 1809 | Ceramiales | Rhodomelaceae | 1 | x | - | - | - | - |
| Pterosiphonia Falkenberg, 1897 | Ceramiales | Rhodomelaceae | 1 | x | - | - | - | - |
| Vertebrata fucoides (Hudson) Kuntze, 1891 | Ceramiales | Rhodomelaceae | 1 | x | - | - | - | - |
| Vertebrata subulifera (C.Agardh) Kuntze, 1891 | Ceramiales | Rhodomelaceae | 1 | - | - | x | - | - |
| Vertebrata tripinnata (Harvey) Kuntze, 1891 | Ceramiales | Rhodomelaceae | 1 | x | - | - | - | - |
| Plumaria F.Schmitz, 1896 | Ceramiales | Wrangeliaceae | 1 | x | - | - | - | x |
| Wrangelia penicillata (C.Agardh) C.Agardh, 1828 | Ceramiales | Wrangeliaceae | 1 | - | - | x | - | - |
| Bornetia Thuret, 1855 | Ceramiales | Wrangeliaceae | 1 | - | x | - | - | - |
| Articulated Corallinaceae | Corallinales | 3 | x | - | x | - | - | |
| Rhodolith | Corallinales | 4 | x | - | x | - | - | |
| Corallina officinalis Linnaeus, 1758 | Corallinales | Corallinaceae | 1 | x | - | - | - | - |
| Corallina Linnaeus, 1758 | Corallinales | Corallinaceae | 3 | x | - | - | - | - |
| Ellisolandia elongata (J.Ellis & Solander) K.R.Hind & G.W.Saunders, 2013 | Corallinales | Corallinaceae | 2 | x | - | - | - | - |
| Jania adhaerens J.V.Lamouroux, 1816 | Corallinales | Corallinaceae | 2 | x | - | x | - | - |
| Jania capillacea Harvey, 1853 | Corallinales | Corallinaceae | 1 | x | - | - | - | - |
| Jania longifurca Zanardini, 1844 | Corallinales | Corallinaceae | 2 | x | - | - | - | - |
| Jania rubens (Linnaeus) J.V.Lamouroux, 1816 | Corallinales | Corallinaceae | 3 | x | - | x | - | - |
| Jania virgata (Zanardini) Montagne, 1846 | Corallinales | Corallinaceae | 1 | x | - | - | - | - |
| Jania J.V.Lamouroux, 1812 | Corallinales | Corallinaceae | 5 | x | - | - | - | - |
| Lithophyllum crouaniorum Foslie, 1899 | Corallinales | Lithophyllaceae | 1 | - | - | - | - | - |
| Lithophyllum hibernicum Foslie, 1906 | Corallinales | Lithophyllaceae | 1 | - | - | - | - | - |
| Lithophyllum incrustans Philippi, 1837 | Corallinales | Lithophyllaceae | 4 | - | x | x | - | - |
| Lithophyllum Philippi, 1837 | Corallinales | Lithophyllaceae | 1 | - | x | - | - | - |
| Tenarea tortuosa (Esper) Me.Lemoine, 1910 | Corallinales | Lithophyllaceae | 2 | x | - | - | - | - |
| Amphiroa J.V.Lamouroux, 1812 | Corallinales | Lithophyllaceae | 1 | - | x | - | - | - |
| Neogoniolithon brassica-florida (Harvey) Setchell & L.R.Mason, 1943 | Corallinales | Spongitidaceae | 1 | - | - | - | - | - |
| Gelidiella Feldmann & G.Hamel, 1934 | Gelidiales | Gelidiellaceae | 1 | x | - | - | - | - |
| Gelidium microdon Kützing, 1849 | Gelidiales | Gelidiellaceae | 3 | x | - | - | - | - |
| Gelidium pusillum (Stackhouse) Le Jolis, 1863 | Gelidiales | Gelidiellaceae | 2 | x | - | - | - | - |
| Gelidium spinosum (S.G.Gmelin) P.C.Silva, 1996 | Gelidiales | Gelidiellaceae | 3 | x | - | - | - | - |
| Millerella pannosa (Feldmann) G.H.Boo & L.Le Gall, 2016 | Gelidiales | Gelidiellaceae | 1 | - | - | x | - | - |
| Pterocladiella capillacea (S.G.Gmelin) Santelices & Hommersand, 1997 | Gelidiales | Pterocladiaceae | 5 | x | - | - | - | - |
| Catenella caespitosa (Withering) L.M.Irvine, 1976 | Gigartinales | Caulacanthaceae | 2 | x | - | - | - | - |
| Caulacanthus ustulatus (Turner) Kützing, 1843 | Gigartinales | Caulacanthaceae | 1 | x | - | - | - | - |
| Calliblepharis Kützing, 1843 | Gigartinales | Cystocloniaceae | 1 | - | x | - | - | - |
| Hypnea musciformis (Wulfen) J.V.Lamouroux, 1813 | Gigartinales | Cystocloniaceae | 1 | x | - | - | - | - |
| Hypnea spinella (C.Agardh) Kützing, 1847 | Gigartinales | Cystocloniaceae | 1 | - | - | x | - | - |
| Hypnea J.V.Lamouroux, 1813 | Gigartinales | Cystocloniaceae | 1 | - | - | x | - | - |
| Dudresnaya P.L.Crouan & H.M.Crouan, 1835 | Gigartinales | Dumontiaceae | 1 | - | x | - | - | - |
| Halarachnion ligulatum (Woodward) Kützing, 1843 | Gigartinales | Furcellariaceae | 1 | - | - | x | - | - |
| Chondracanthus acicularis (Roth) Fredericq, 1993 | Gigartinales | Gigartinaceae | 3 | x | - | - | - | - |
| Gigartina pistillata (S.G.Gmelin) Stackhouse, 1809 | Gigartinales | Gigartinaceae | 1 | x | - | - | - | - |
| Callophyllis Kützing, 1843 | Gigartinales | Kallymeniaceae | 1 | - | x | - | - | - |
| Kallymenia reniformis (Turner) J.Agardh, 1842 | Gigartinales | Kallymeniaceae | 1 | - | x | - | - | - |
| Erythrodermis traillii (Holmes ex Batters) Guiry & Garbary, 1990 | Gigartinales | Phyllophoraceae | 1 | x | - | - | - | - |
| Gymnogongrus griffithsiae (Turner) C.Martius, 1833 | Gigartinales | Phyllophoraceae | 1 | x | - | - | - | - |
| Phyllophora gelidioides P.L.Crouan & H.M.Crouan ex Karsakoff, 1896 | Gigartinales | Phyllophoraceae | 1 | x | - | - | - | - |
| Sphaerococcus coronopifolius Stackhouse, 1797 | Gigartinales | Sphaerococcaceae | 2 | x | - | - | - | - |
| Gracilaria Greville, 1830 | Gracilariales | Gracilariaceae | 2 | - | x | x | - | - |
| Dermocorynus dichotomus (J.Agardh) Gargiulo, M.Morabito & Manghisi, 2013 | Halymeniales | Grateloupiaceae | 1 | x | - | - | - | - |
| Lithothamnion corallioides (P.Crouan & H.Crouan) P.Crouan & H.Crouan, 1867 | Hapalidiales | Hapalidiaceae | 3 | - | - | x | - | - |
| Lithothamnion Heydrich, 1897 | Hapalidiales | Hapalidiaceae | 1 | - | x | - | - | - |
| Phymatolithon calcareum (Pallas) W.H.Adey & D.L.McKibbin ex Woelkering & L.M.Irvine, 1986 | Hapalidiales | Hapalidiaceae | 3 | - | - | x | - | - |
| Phymatolithon lusitanicum V.Peña, 2015 | Hapalidiales | Hapalidiaceae | 1 | - | - | - | - | - |
| Phymatolithon Foslie, 1898 | Hapalidiales | Hapalidiaceae | 1 | - | x | - | - | - |
| Mesophyllum lichenoides (J.Ellis) Me.Lemoine, 1928 | Hapalidiales | Mesophyllumaceae | 1 | x | - | - | - | - |
| Mesophyllum sphaericum V.Pena, Bárbara, W.H.Adey, Riosmena-Rodrigues & H.G.Choi, 2011 | Hapalidiales | Mesophyllumaceae | 1 | - | - | - | - | - |
| Mesophyllum Me.Lemoine, 1928 | Hapalidiales | Mesophyllumaceae | 1 | - | x | - | - | - |
| Hildenbrandia rubra (Sommerfelt) Meneghini, 1841 | Hildenbrandiales | Hildenbrandiaceae | 1 | x | - | - | - | - |
| Hildenbrandia Nardo, 1834 | Hildenbrandiales | Hildenbrandiaceae | 4 | x | - | - | - | - |
| Liagora J.V.Lamouroux, 1812 | Nemaliales | Liagoraceae | 1 | - | x | - | - | - |
| Nemalion elminthoides (Velley) Batters, 1902 | Nemaliales | Nemaliaceae | 1 | x | - | - | - | - |
| Scinaia Bivona-Bernardi, 1822 | Nemaliales | Scinaiaceae | 1 | - | - | x | - | - |
| Platoma Schousboe ex F.Schmitz, 1894 | Nemastomatales | Schizymeniaceae | 1 | x | - | - | - | - |
| Schizymenia dubyi (Chauvin ex Duby) J.Agardh, 1851 | Nemastomatales | Schizymeniaceae | 1 | x | - | - | - | - |
| Palmaria palmata (Linnaeus) F.Weber & D.Mohr, 1805 | Palmariales | Palmariaceae | 1 | x | - | - | - | - |
| Peyssonnelia rubra (Greville) J.Agardh, 1851 | Peyssonneliales | Peyssonneliaceae | 1 | x | - | - | - | - |
| Peyssonnelia Decaisne, 1841 | Peyssonneliales | Peyssonneliaceae | 4 | x | - | x | - | - |
| Plocamium cartilagineum (Linnaeus) P.S.Dixon, 1967 | Plocamiales | Plocamiaceae | 2 | x | - | - | - | - |
| Champia parvula (C.Agardh) Harvey, 1853 | Rhodymeniales | Champiaceae | 1 | - | - | x | - | - |
| Champia Desvaux, 1809 | Rhodymeniales | Champiaceae | 1 | - | x | - | - | - |
| Gastroclonium ovatum (Hudson) Papenfuss, 1944 | Rhodymeniales | Champiaceae | 1 | x | - | - | - | - |
| Gastroclonium reflexum (Chauvin) Kützing, 1849 | Rhodymeniales | Champiaceae | 1 | x | - | - | - | - |
| Lomentaria articulata (Hudson) Lyngbye, 1819 | Rhodymeniales | Lomentariaceae | 2 | x | - | - | - | - |
| Rhodymenia holmesii Ardissone, 1893 | Rhodymeniales | Rhodymeniaceae | 2 | x | - | - | - | - |
| Rhodymenia pseudopalmata (J.V.Lamouroux) P.C.Silva, 1952 | Rhodymeniales | Rhodymeniaceae | 1 | x | - | - | - | - |
| Phyllum Ochrophyta | ||||||||
| Class Dictyochophyceae | ||||||||
| Padina pavonica (Linnaeus) Thivy, 1960 | Dictyochales | Dictyochaceae | 10 | x | - | x | - | - |
| Padina Adanson, 1763 | Dictyochales | Dictyochaceae | 1 | - | - | x | - | - |
| Class Phaeophyceae | ||||||||
| Lobophora variegata (J.V.Lamouroux) Womersley ex E.C.Oliveira, 1977 | Dictyotales | Dictyotaceae | 4 | - | - | x | - | - |
| Lobophora J.Agardh, 1894 | Dictyotales | Dictyotaceae | 4 | - | x | x | - | - |
| Stypopodium zonale (J.V.Lamouroux) Papenfuss, 1940 | Dictyotales | Dictyotaceae | 1 | - | x | - | - | - |
| Canistrocarpus cervicornis (Kützing) De Paula & De Clerck, 2006 | Dictyotales | Dictyotaceae | 1 | - | - | x | - | - |
| Dictyopteris polypodioides (A.P.De Candolle) J.V.Lamouroux, 1809 | Dictyotales | Dictyotaceae | 1 | x | - | - | - | - |
| Dictyota bartayresiana J.V.Lamouroux, 1809 | Dictyotales | Dictyotaceae | 1 | x | - | - | - | - |
| Dictyota dichotoma (Hudson) J.V.Lamouroux, 1809 | Dictyotales | Dictyotaceae | 5 | x | - | x | - | - |
| Dictyota fasciola (Roth) J.V.Lamouroux, 1809 | Dictyotales | Dictyotaceae | 1 | - | - | x | - | - |
| Dictyota J.V.Lamouroux, 1809 | Dictyotales | Dictyotaceae | 12 | x | - | x | - | - |
| Zonaria tournefortii (J.V.Lamouroux) Montagne, 1846 | Dictyotales | Dictyotaceae | 6 | x | - | - | - | - |
| Leathesia marina (Lyngbye) Decaisne, 1842 | Ectocarpales | Chordariaceae | 1 | x | - | - | - | - |
| Hydroclathrus clathratus (C.Agardh) M.Howe, 1920 | Ectocarpales | Scytosiphonaceae | 3 | x | - | x | - | - |
| Colpomenia sinuosa (Mertens ex Roth) Derbès & Solier, 1851 | Ectocarpales | Scytosiphonaceae | 5 | x | - | x | - | - |
| Petalonia binghamiae (J.Agardh) K.L.Vinogradova, 1973 | Ectocarpales | Scytosiphonaceae | 1 | x | - | - | - | - |
| Fucus spiralis Linnaeus, 1753 | Fucales | Fucaceae | 3 | x | - | - | - | - |
| Gongolaria abies-marina (S.G.Gmelin) Kuntze 1891 | Fucales | Sargassaceae | 2 | x | - | x | - | - |
| Sargassum furcatum Kützing, 1843 | Fucales | Sargassaceae | 1 | - | - | x | - | - |
| Sargassum C.Agardh, 1820 | Fucales | Sargassaceae | 4 | x | - | x | - | - |
| Cystoseira C.Agardh, 1820 | Fucales | Sargassaceae | 2 | x | - | - | - | - |
| Ericaria selaginoides (Linnaeus) Molinari & Guiry, 2020 | Fucales | Sargassaceae | 1 | x | - | - | - | - |
| Treptacantha abies-marina (S.G.Gmelin) Kützing, 1843 | Fucales | Sargassaceae | 4 | x | - | x | - | - |
| Laminaria ochroleuca Bachelot de la Pylaie, 1824 | Laminariales | Laminariaceae | 4 | x | - | x | - | x |
| Nemoderma tingitanum Schousboe ex Bornet, 1892 | Nemodermatales | Nemodermataceae | 2 | x | - | - | - | - |
| Nemoderma Schousboe ex Bornet, 1892 | Nemodermatales | Nemodermataceae | 1 | - | x | - | - | - |
| Ralfsia Berkeley, 1843 | Ralfsiales | Ralfsiaceae | 1 | x | - | - | - | - |
| Cladostephus spongiosus (Hudson) C.Agardh, 1817 | Sphacelariales | Cladostephaceae | 1 | x | - | - | - | - |
| Sphacelaria Lyngbye, 1818 | Sphacelariales | Sphacelariaceae | 1 | x | - | - | - | - |
| Halopteris filicina (Grateloup) Kützing, 1843 | Sphacelariales | Stypocaulaceae | 9 | x | - | x | - | - |
| Halopteris scoparia (Linnaeus) Sauvageau, 1904 | Sphacelariales | Stypocaulaceae | 5 | x | - | x | - | - |
| Halopteris Kützing, 1843 | Sphacelariales | Stypocaulaceae | 3 | x | x | - | - | - |
| Sporochnus pedunculatus (Hudson) C.Agardh, 1817 | Sporochnales | Sporochnaceae | 2 | - | x | - | - | - |
| Carpomitra costata (Stackhouse) Batters, 1902 | Sporochnales | Sporochnaceae | 1 | x | - | - | - | - |
| Cutleria multifida (Turner) Greville, 1830 | Tilopteridales | Cutleriaceae | 1 | x | - | - | - | - |
| Phyllariopsis brevipes (C.Agardh) E.C.Henry & G.R.South, 1987 | Tilopteridales | Phyllariaceae | 1 | x | - | - | - | - |
| Phyllum Tracheophyta | ||||||||
| Subphyllum Spermatophytina | ||||||||
| Class Magnoliopsida | ||||||||
| Alismatales | Alismatales | 1 | - | - | x | - | - | |
| Cymodocea nodosa (Ucria) Ascherson, 1870 | Alismatales | Cymodoceaceae | 14 | - | x | x | - | - |
| Halodule wrightii Ascherson, 1868 | Alismatales | Cymodoceaceae | 1 | - | - | - | x | - |
| Halophila decipiens Ostenfeld, 1902 | Alismatales | Hydrocharitaceae | 1 | - | - | x | - | - |
| Ruppia maritima Linnaeus, 1753 | Alismatales | Ruppiaceae | 1 | - | - | - | x | - |
References
- Otero-Ferrer, F.; Tuya, F.; Bosch Guerra, N.E.; Herrero-Barrencua, A.; Abreu, A.D.; Haroun, R. Composition, Structure and Diversity of Fish Assemblages across Seascape Types at Príncipe, an Understudied Tropical Island in the Gulf of Guinea (Eastern Atlantic Ocean). Afr J Mar Sci 2020, 42, 381–391. [Google Scholar] [CrossRef]
- Tuya, F.; Vanderklift, M.A.; Hyndes, G.A.; Wernberg, T.; Thomsen, M.S.; Hanson, C. Proximity to Rocky Reefs Alters the Balance between Positive and Negative Effects on Seagrass Fauna. Mar Ecol Prog Ser 2010, 405, 175–186. [Google Scholar] [CrossRef]
- Cosme De Esteban, M.; Haroun, R.; Tuya, F.; Abreu, A.D.; Otero-Ferrer, F. Mapping Marine Habitats in the Gulf of Guinea: A Contribution to the Future Establishment of Marine Protected Areas in Principe Island. Reg Stud Mar Sci 2023, 57, 102742. [Google Scholar] [CrossRef]
- Casas, E.; Fernandez, M.; Gil, A.; Yesson, C.; Prestes, A.; Moreu-Badia, I.; Neto, A.; Arbelo, M. Macroalgae Niche Modelling: A Two-Step Approach Using Remote Sensing and in Situ Observations of a Native and an Invasive Asparagopsis. Biol Invasions 2021, 23, 3215–3230. [Google Scholar] [CrossRef]
- Filbee-Dexter, K.; Wernberg, T.; Barreiro, R.; Coleman, M.A.; Bettignies, T.; Feehan, C.J.; Franco, J.N.; Hasler, B.; Louro, I.; Norderhaug, K.M.; et al. Leveraging the Blue Economy to Transform Marine Forest Restoration. J Phycol 2022, 58, 198–207. [Google Scholar] [CrossRef]
- Schubert, N.; Peña, V.; Salazar, V.W.; Horta, P.A.; Neves, P.; Ribeiro, C.; Otero-Ferrer, F.; Tuya, F.; Espino, F.; Schoenrock, K.; et al. Rhodolith Physiology Across the Atlantic: Towards a Better Mechanistic Understanding of Intra- and Interspecific Differences. Front Mar Sci 2022, 9. [Google Scholar] [CrossRef]
- Buhl-Mortensen, P.; Buhl-Mortensen, L.; Purser, A. Trophic Ecology and Habitat Provision in Cold-Water Coral Ecosystems. In Marine Animal Forests; Springer International Publishing: Cham, 2016; pp. 1–26. [Google Scholar]
- Govenar, B.; Fisher, C.R. Experimental Evidence of Habitat Provision by Aggregations of Riftia Pachyptila at Hydrothermal Vents on the East Pacific Rise. The Authors. Journal compilation a 2007, 28, 3–14. [Google Scholar] [CrossRef]
- Graham, L.; Gaulton, R.; Gerard, F.; Staley, J.T. The Influence of Hedgerow Structural Condition on Wildlife Habitat Provision in Farmed Landscapes. Biol Conserv 2018, 220, 122–131. [Google Scholar] [CrossRef]
- Nielsen, P.; Nielsen, M.M.; McLaverty, C.; Kristensen, K.; Geitner, K.; Olsen, J.; Saurel, C.; Petersen, J.K. Management of Bivalve Fisheries in Marine Protected Areas. Mar Policy 2021, 124. [Google Scholar] [CrossRef]
- Andrews, N.; Bennett, N.J.; Le Billon, P.; Green, S.J.; Cisneros-Montemayor, A.M.; Amongin, S.; Gray, N.J.; Sumaila, U.R. Oil, Fisheries and Coastal Communities: A Review of Impacts on the Environment, Livelihoods, Space and Governance. Energy Res Soc Sci 2021, 75. [Google Scholar] [CrossRef]
- Breen, C.; El Safadi, C.; Huigens, H.; Tews, S.; Westley, K.; Anderou, G.; Vazquez, R.O.; Nikolaus, J.; Blue, L. Integrating Cultural and Natural Heritage Approaches to Marine Protected Areas in the MENA Region. Mar Policy 2021, 132. [Google Scholar] [CrossRef]
- Hattam, C.; Broszeit, S.; Langmead, O.; Praptiwi, R.A.; Lim, V.C.; Creencia, L.A.; Tran, D.H.; Maharja, C.; Mitra Setia, T.; Wulandari, P.; et al. A Matrix Approach to Tropical Marine Ecosystem Service Assessments in South East Asia. Ecosyst Serv 2021, 51. [Google Scholar] [CrossRef]
- Otero-Ferrer, F.; Cosme, M.; Tuya, F.; Espino, F.; Haroun, R. Effect of Depth and Seasonality on the Functioning of Rhodolith Seabeds. Estuar Coast Shelf Sci 2020, 235, 106579. [Google Scholar] [CrossRef]
- Otero-Ferrer, F.; Mannarà, E.; Cosme De Esteban, M.; Falace, A.; Montiel-Nelson, J.A.; Espino, F.; Haroun, R.; Tuya, F. Early-Faunal Colonization Patterns of Discrete Habitat Units: A Case Study with Rhodolith-Associated Vagile Macrofauna. Estuar Coast Shelf Sci 2019, 218, 9–22. [Google Scholar] [CrossRef]
- Perez-Peris, I.; Navarro-Mayoral, S.; de Esteban, M.C.; Tuya, F.; Pena, V.; Barbara, I.; Neves, P.; Ribeiro, C.; Abreu, A.; Grall, J.; et al. Effect of Depth across a Latitudinal Gradient in the Structure of Rhodolith Seabeds and Associated Biota across the Eastern Atlantic Ocean. DIVERSITY-BASEL 2023, 15. [Google Scholar] [CrossRef]
- Jones, C.G.; Lawton, J.H.; Shachak, M. Organisms as Ecosystem Engineers. Oikos 1994, 69, 373. [Google Scholar] [CrossRef]
- Ferreiro, N.; Feijoó, C.; Giorgi, A.; Rosso, J. Macroinvertebrates Select Complex Macrophytes Independently of Their Body Size and Fish Predation Risk in a Pampean Stream. Hydrobiologia 2014, 740, 191–205. [Google Scholar] [CrossRef]
- Cúrdia, J.; Carvalho, S.; Pereira, F.; Guerra-García, J.M.; Santos, M.N.; Cunha, M.R. Diversity and Abundance of Invertebrate Epifaunal Assemblages Associated with Gorgonians Are Driven by Colony Attributes. Coral Reefs 2015, 34, 611–624. [Google Scholar] [CrossRef]
- Cacabelos, E.; Martins, G.M.; Thompson, R.; Prestes, A.C.L.; Azevedo, J.M.N.; Neto, A.I. Material Type and Roughness Influence Structure of Inter-tidal Communities on Coastal Defenses. Marine Ecology 2016, 37, 801–812. [Google Scholar] [CrossRef]
- Carvalho, L.R.S.; Loiola, M.; Barros, F. Manipulating Habitat Complexity to Understand Its Influence on Benthic Macrofauna. J Exp Mar Biol Ecol 2017, 489, 48–57. [Google Scholar] [CrossRef]
- Matias, M.G.; Underwood, A.J.; Hochuli, D.F.; Coleman, R.A. Independent Effects of Patch Size and Structural Complexity on Diversity of Benthic Macroinvertebrates. Ecology 2010, 91, 1908–1915. [Google Scholar] [CrossRef] [PubMed]
- Pianka, E.R. Evolutionary Ecology, 6th ed.; Benjamin Cummings: San Francisco, California, USA, 2000. [Google Scholar]
- Yanovski, R.; Nelson, P.A.; Abelson, A. Structural Complexity in Coral Reefs: Examination of a Novel Evaluation Tool on Different Spatial Scales. Front Ecol Evol 2017, 5, 27. [Google Scholar] [CrossRef]
- Trégarot, E.; D’Olivo, J.P.; Botelho, A.Z.; Cabrito, A.; Cardoso, G.O.; Casal, G.; Cornet, C.C.; Cragg, S.M.; Degia, A.K.; Fredriksen, S.; et al. Effects of Climate Change on Marine Coastal Ecosystems – A Review to Guide Research and Management. Biol Conserv 2024, 289, 110394. [Google Scholar] [CrossRef]
- Gundersen, H. (NIVA); Bryan, T. (GRID-A.; Chen, W. (NIVA); Moy, F.E. (IMR); Sandman, A.N. (AquaBiota); Sundblad, G. (AquaBiota); Schneider, S. (NIVA); Andersen, J.H. (NIVA); Langaas, S. (NIVA); Walday, M.G. (NIVA) Ecosystem Services; Nordic Council of Ministers, 2017; ISBN 978-9-28934-7-488.
- Hemminga, M.A.; Duarte, C.M. Seagrass Ecology; Cambridge University Press, 2000; ISBN 9780521661843.
- Boström, C.; Baden, S.; Bockelmann, A.; Dromph, K.; Fredriksen, S.; Gustafsson, C.; Krause-Jensen, D.; Möller, T.; Nielsen, S.L.; Olesen, B.; et al. Distribution, Structure and Function of Nordic Eelgrass (Zostera Marina) Ecosystems: Implications for Coastal Management and Conservation. Aquat Conserv 2014, 24, 410–434. [Google Scholar] [CrossRef]
- Jahnke, M.; Christensen, A.; Micu, D.; Milchakova, N.; Sezgin, M.; Todorova, V.; Strungaru, S.; Procaccini, G. Patterns and Mechanisms of Dispersal in a Keystone Seagrass Species. Mar Environ Res 2016, 117, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Bekkby, T.; Papadopoulou, N.; Fiorentino, D.; McOwen, C.J.; Rinde, E.; Boström, C.; Carreiro-Silva, M.; Linares, C.; Andersen, G.S.; Bengil, E.G.T.; et al. Habitat Features and Their Influence on the Restoration Potential of Marine Habitats in Europe. Front Mar Sci 2020, 7, 184. [Google Scholar] [CrossRef]
- Smale, D.A.; Burrows, M.T.; Moore, P.; O’Connor, N.; Hawkins, S.J. Threats and Knowledge Gaps for Ecosystem Services Provided by Kelp Forests: A Northeast Atlantic Perspective. Ecol Evol 2013, 3, 4016–4038. [Google Scholar] [CrossRef]
- Filbee-Dexter, K.; Wernberg, T. Rise of Turfs: A New Battlefront for Globally Declining Kelp Forests. Bioscience 2018, 68, 64–76. [Google Scholar] [CrossRef]
- Steller, D.L.; Riosmena-Rodríguez, R.; Foster, M.S.; Roberts, C.A. Rhodolith Bed Diversity in the Gulf of California: The Importance of Rhodolith Structure and Consequences of Disturbance. Aquat Conserv 2003, 13, 5–20. [Google Scholar] [CrossRef]
- Thomsen, M.S.; Wernberg, T.; Altieri, A.; Tuya, F.; Gulbransen, D.; Mcglathery, K.J.; Holmer, M.; Silliman, B.R. Habitat Cascades: The Conceptual Context and Global Relevance of Facilitation Cascades via Habitat Formation and Modification. Integr Comp Biol 2010, 50, 158–175. [Google Scholar] [CrossRef]
- de Figueiredo, M.A.O.; Santos de Menezes, K.; Costa-Paiva, E.M.; Paiva, P.C.; Ventura, C.R.R. Evaluacion Experimental de Rodolitos Como Sustratos Vivos Para La Infauna En El Banco de Abrolhos, Brasil. Cienc Mar 2007, 33, 427–440. [Google Scholar]
- Nordlund, L.M.; Unsworth, R.K.F.; Wallner-Hahn, S.; Ratnarajah, L.; Beca-Carretero, P.; Boikova, E.; Bull, J.C.; Chefaoui, R.M.; de los Santos, C.B.; Gagnon, K.; et al. One Hundred Priority Questions for Advancing Seagrass Conservation in Europe. PLANTS, PEOPLE, PLANET 2024, 6, 587–603. [Google Scholar] [CrossRef]
- Mtwana Nordlund, L.; Koch, E.W.; Barbier, E.B.; Creed, J.C. Seagrass Ecosystem Services and Their Variability across Genera and Geographical Regions. PLoS One 2016, 11, e0163091. [Google Scholar] [CrossRef]
- Campagne, C.S.; Salles, J.-M.; Boissery, P.; Deter, J. The Seagrass Posidonia Oceanica : Ecosystem Services Identification and Economic Evaluation of Goods and Benefits. Mar Pollut Bull 2015, 97, 391–400. [Google Scholar] [CrossRef] [PubMed]
- Roberts, C.; Ormond, R. Habitat Complexity and Coral Reef Fish Diversity and Abundance on Red Sea Fringing Reefs. Mar Ecol Prog Ser 1987, 41, 1–8. [Google Scholar] [CrossRef]
- Roberts, J.M.; Wheeler, A.; Freiwald, A.; Cairns, S. Cold-Water Corals: The Biology and Geology of Deep-Sea Coral Habitats; Cambridge University Press, 2009; ISBN 9780521884853.
- Berke, S.K. Functional Groups of Ecosystem Engineers: A Proposed Classification with Comments on Current Issues. Integr Comp Biol 2010, 50, 147–157. [Google Scholar] [CrossRef]
- Tissot, B.N.; Yoklavich, M.M.; Love, M.S.; York, K.; Amend, M. Benthic Invertebrates That Form Habitat on Deep Banks off Southern California, with Special Reference to Deep Sea Coral. Fishery Bulletin 2006, 104, 167–181. [Google Scholar]
- Herler, J. Microhabitats and Ecomorphology of Coral- and Coral Rock-Associated Gobiid Fish (Teleostei: Gobiidae) in the Northern Red Sea. Marine Ecology 2007, 28, 82–94. [Google Scholar] [CrossRef]
- Cosme De Esteban, M.; Otero Ferrer, F.; Haroun, R. Los Fondos de Rodolitos: El Valor Oculto de Los Ecosistemas Marinos. Okeanos 2020, 10, 26–35. [Google Scholar]
- Buhl-Mortensen, L.; Buhl-Mortensen, P.; Dolan, M.J.F.; Gonzalez-Mirelis, G. Habitat Mapping as a Tool for Conservation and Sustainable Use of Marine Resources: Some Perspectives from the MAREANO Programme, Norway. J Sea Res 2015, 100, 46–61. [Google Scholar] [CrossRef]
- Czechowska, K.; Feldens, P.; Tuya, F.; Cosme de Esteban, M.; Espino, F.; Haroun, R.; Schönke, M.; Otero-Ferrer, F. Testing Side-Scan Sonar and Multibeam Echosounder to Study Black Coral Gardens: A Case Study from Macaronesia. Remote Sens (Basel) 2020, 12, 3244. [Google Scholar] [CrossRef]
- Kaiser, M.J.; Hilborn, R.; Jennings, S.; Amaroso, R.; Andersen, M.; Balliet, K.; Barratt, E.; Bergstad, O.A.; Bishop, S.; Bostrom, J.L.; et al. Prioritization of Knowledge-Needs to Achieve Best Practices for Bottom Trawling in Relation to Seabed Habitats. Fish and Fisheries 2016, 17, 637–663. [Google Scholar] [CrossRef]
- van der Reijden, K.J.; Govers, L.L.; Koop, L.; Damveld, J.H.; Herman, P.M.J.; Mestdagh, S.; Piet, G.; Rijnsdorp, A.D.; Dinesen, G.E.; Snellen, M.; et al. Beyond Connecting the Dots: A Multi-Scale, Multi-Resolution Approach to Marine Habitat Mapping. Ecol Indic 2021, 128, 107849. [Google Scholar] [CrossRef]
- Leslie, H.M.; McLeod, K.L. Confronting the Challenges of Implementing Marine Ecosystem-Based Management. Front Ecol Environ 2007, 5, 540–548. [Google Scholar] [CrossRef]
- Baldwin, K.; Oxenford, H.A. A Participatory Approach to Marine Habitat Mapping in the Grenadine Islands. Coastal Management 2014, 42, 36–58. [Google Scholar] [CrossRef]
- Cogan, C.B.; Todd, B.J.; Lawton, P.; Noji, T.T. The Role of Marine Habitat Mapping in Ecosystem-Based Management. ICES Journal of Marine Science 2009, 66, 2033–2042. [Google Scholar] [CrossRef]
- de Young, C.; Charles, A.; Hjort, A. Human Dimensions of the Ecosystem Approach to Fisheries: An Overview of Context, Concepts, Tools and Methods. FAO Fisheries Technical Paper 2008, 489, 152. [Google Scholar]
- Tallis, H.; Levin, P.S.; Ruckelshaus, M.; Lester, S.E.; McLeod, K.L.; Fluharty, D.L.; Halpern, B.S. The Many Faces of Ecosystem-Based Management: Making the Process Work Today in Real Places. Mar Policy 2010, 34, 340–348. [Google Scholar] [CrossRef]
- Christie, P.; White, A.T. Best Practices for Improved Governance of Coral Reef Marine Protected Areas. Coral Reefs 2007, 26, 1047–1056. [Google Scholar] [CrossRef]
- Katsanevakis, S.; Stelzenmüller, V.; South, A.; Sørensen, T.K.; Jones, P.J.S.; Kerr, S.; Badalamenti, F.; Anagnostou, C.; Breen, P.; Chust, G.; et al. Ecosystem-Based Marine Spatial Management: Review of Concepts, Policies, Tools, and Critical Issues. Ocean Coast Manag 2011, 54, 807–820. [Google Scholar] [CrossRef]
- Cosme De Esteban, M.; Feldens, P.; Haroun, R.; Tuya, F.; Gil, A.; Otero Ferrer, F. Habitat Mapping of the Vila Franca Do Campo Marine Reserve (Azores) and Recommendations for Its Improvement. Estuar Coast Shelf Sci 2024, 303, 108809. [Google Scholar] [CrossRef]
- Floor, J.R.; van Koppen, C.S.A. (Kris); van Tatenhove, J.P.M. Science, Uncertainty and Changing Storylines in Nature Restoration: The Case of Seagrass Restoration in the Dutch Wadden Sea. Ocean Coast Manag 2018, 157, 227–236. [Google Scholar] [CrossRef]
- Balado, J.; Olabarria, C.; Martínez-Sánchez, J.; Rodríguez-Pérez, J.R.; Pedro, A. Semantic Segmentation of Major Macroalgae in Coastal Environments Using High-Resolution Ground Imagery and Deep Learning. Int J Remote Sens 2021, 42, 1785–1800. [Google Scholar] [CrossRef]
- Bell, J.J.; Micaroni, V.; Harris, B.; Strano, F.; Broadribb, M.; Rogers, A. Global Status, Impacts, and Management of Rocky Temperate Mesophotic Ecosystems. Conservation Biology 2024, 38. [Google Scholar] [CrossRef] [PubMed]
- de la Mare, W.K. Marine Ecosystem-Based Management as a Hierarchical Control System. Mar Policy 2005, 29, 57–68. [Google Scholar] [CrossRef]
- Sini, M.; Katsanevakis, S.; Koukourouvli, N.; Gerovasileiou, V.; Dailianis, T.; Buhl-Mortensen, L.; Damalas, D.; Dendrinos, P.; Dimas, X.; Frantzis, A.; et al. Assembling Ecological Pieces to Reconstruct the Conservation Puzzle of the Aegean Sea. Front Mar Sci 2017, 4. [Google Scholar] [CrossRef]
- Mumby, P.J.; Green, E.P.; Edwards, A.J.; Clark, C.D. The Cost-Effectiveness of Remote Sensing for Tropical Coastal Resources Assessment and Management. J Environ Manage 1999, 55, 157–166. [Google Scholar] [CrossRef]
- Harris, P.; Baker, E. (eds) Seafloor Geomorphology as Benthic Habitat; Elsevier: Amsterdam, The Netherlands, 2020; ISBN 9780128149607. [Google Scholar]
- Jordan, A.; Lawler, M.; Halley, V.; Barrett, N. Seabed Habitat Mapping in the Kent Group of Islands and Its Role in Marine Protected Prea Planning. Aquat Conserv 2005, 15, 51–70. [Google Scholar] [CrossRef]
- Che Hasan, R.; Ierodiaconou, D.; Laurenson, L. Combining Angular Response Classification and Backscatter Imagery Segmentation for Benthic Biological Habitat Mapping. Estuar Coast Shelf Sci 2012, 97, 1–9. [Google Scholar] [CrossRef]
- Li, D.; Tang, C.; Xia, C.; Zhang, H. Acoustic Mapping and Classification of Benthic Habitat Using Unsupervised Learning in Artificial Reef Water. Estuar Coast Shelf Sci 2017, 185, 11–21. [Google Scholar] [CrossRef]
- Roff, J.C.; Taylor, M.E.; Laughren, J. Geophysical Approaches to the Classification, Delineation and Monitoring of Marine Habitats and Their Communities. Aquat Conserv 2003, 13, 77–90. [Google Scholar] [CrossRef]
- Stelzenmüller, V.; Breen, P.; Stamford, T.; Thomsen, F.; Badalamenti, F.; Borja, Á.; Buhl-Mortensen, L.; Carlstöm, J.; D’Anna, G.; Dankers, N.; et al. Monitoring and Evaluation of Spatially Managed Areas: A Generic Framework for Implementation of Ecosystem Based Marine Management and Its Application. Mar Policy 2013, 37, 149–164. [Google Scholar] [CrossRef]
- Moura, R.L.; Secchin, N.A.; Amado-Filho, G.M.; Francini-Filho, R.B.; Freitas, M.O.; Minte-Vera, C.V.; Teixeira, J.B.; Thompson, F.L.; Dutra, G.F.; Sumida, P.Y.G.; et al. Spatial Patterns of Benthic Megahabitats and Conservation Planning in the Abrolhos Bank. Cont Shelf Res 2013, 70, 109–117. [Google Scholar] [CrossRef]
- Brown, C.J.; Collier, J.S. Mapping Benthic Habitat in Regions of Gradational Substrata: An Automated Approach Utilising Geophysical, Geological, and Biological Relationships. Estuar Coast Shelf Sci 2008, 78, 203–214. [Google Scholar] [CrossRef]
- Li, R.; Liu, J.-K.; Sukcharoenpong, A.; Yuan, J.; Zhu, H.; Zhang, S. A Systematic Approach toward Detection of Seagrass Patches from Hyperspectral Imagery. Marine Geodesy 2012, 35, 271–286. [Google Scholar] [CrossRef]
- Micallef, A.; Le Bas, T.P.; Huvenne, V.A.I.; Blondel, P.; Hühnerbach, V.; Deidun, A. A Multi-Method Approach for Benthic Habitat Mapping of Shallow Coastal Areas with High-Resolution Multibeam Data. Cont Shelf Res 2012, 39–40, 14–26. [Google Scholar] [CrossRef]
- Lurton, X. An Introduction to Underwater Acoustics: Principles and Applications; 2nd ed.; Springer/Praxis Publishing: Berlin, Germany, 2010. [Google Scholar]
- Brock, J.C.; Purkis, S.J. The Emerging Role of Lidar Remote Sensing in Coastal Research and Resource Management. J Coast Res 2009, 53, 1–5. [Google Scholar] [CrossRef]
- Lafon, V.; Froidefond, J.M.; Lahet, F.; Castaing, P. SPOT Shallow Water Bathymetry of a Moderately Turbid Tidal Inlet Based on Field Measurements. Remote Sens Environ 2002, 81, 136–148. [Google Scholar] [CrossRef]
- Lecours, V.; Dolan, M.F.J.; Micallef, A.; Lucieer, V.L. Characterising the Ocean Frontier: A Review of Marine and Coastal Geomorphometry. Hydrol Earth Syst Sci 2016, 1–66. [Google Scholar] [CrossRef]
- Su, H.; Liu, H.; Wang, L.; Filippi, A.M.; Heyman, W.D.; Beck, R.A. Geographically Adaptive Inversion Model for Improving Bathymetric Retrieval From Satellite Multispectral Imagery. IEEE Transactions on Geoscience and Remote Sensing 2014, 52, 465–476. [Google Scholar] [CrossRef]
- Wang, C.-K.; Philpot, W.D. Using Airborne Bathymetric Lidar to Detect Bottom Type Variation in Shallow Waters. Remote Sens Environ 2007, 106, 123–135. [Google Scholar] [CrossRef]
- Agardy, T.; Bridgewater, P.; Crosby, M.P.; Day, J.; Dayton, P.K.; Kenchington, R.; Laffoley, D.; McConney, P.; Murray, P.A.; Parks, J.E.; et al. Dangerous Targets? Unresolved Issues and Ideological Clashes around Marine Protected Areas. Aquat Conserv 2003, 13, 353–367. [Google Scholar] [CrossRef]
- Spalding, M.D.; Fox, H.E.; Allen, G.R.; Davison, N.; Ferdaña, Z.A.; Finlayson, M.; Halpern, B.S.; Jorge, M.A.; Lombana, A.; Lourie, S.A.; et al. Marine Ecoregions of the World: A Bioregionalization of Coastal and Shelf Areas. Bioscience 2007, 57, 573–583. [Google Scholar] [CrossRef]
- Hughes, T.P.; Barnes, M.L.; Bellwood, D.R.; Cinner, J.E.; Cumming, G.S.; Jackson, J.B.C.; Kleypas, J.; van de Leemput, I.A.; Lough, J.M.; Morrison, T.H.; et al. Coral Reefs in the Anthropocene. Nature 2017, 546, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Pham, C.K.; Ramirez-Llodra, E.; Alt, C.H.S.; Amaro, T.; Bergmann, M.; Canals, M.; Company, J.B.; Davies, J.; Duineveld, G.; Galgani, F.; et al. Marine Litter Distribution and Density in European Seas, from the Shelves to Deep Basins. PLoS One 2014, 9, e95839. [Google Scholar] [CrossRef]
- Larrea, A.; Torres, P.; Seijo, C.; Ventura, M.A.; Costa, A.C.; Parente, M.I.; Lopes, E.; Castaño, D.; Botelho, A.Z. Environmental Coastal Research: A Systematic Review for Azores and Cabo Verde, Two Peripherical Macaronesian Archipelagos. Front Mar Sci 2023, 10. [Google Scholar] [CrossRef]
- Cicin-Sain, B.; Belfiore, S. Linking Marine Protected Areas to Integrated Coastal and Ocean Management: A Review of Theory and Practice. Ocean Coast Manag 2005, 48, 847–868. [Google Scholar] [CrossRef]
- Wagner, D.; van der Meer, L.; Gorny, M.; Sellanes, J.; Gaymer, C.F.; Soto, E.H.; Easton, E.E.; Friedlander, A.M.; Lindsay, D.J.; Molodtsova, T.N.; et al. The Salas y Gómez and Nazca Ridges: A Review of the Importance, Opportunities and Challenges for Protecting a Global Diversity Hotspot on the High Seas. Mar Policy 2021, 126, 104377. [Google Scholar] [CrossRef]
- Bax, N.; Novaglio, C.; Maxwell, K.H.; Meyers, K.; McCann, J.; Jennings, S.; Frusher, S.; Fulton, E.A.; Nursey-Bray, M.; Fischer, M.; et al. Ocean Resource Use: Building the Coastal Blue Economy. Rev Fish Biol Fish 2022, 32, 189–207. [Google Scholar] [CrossRef]
- Gari, S.R.; Newton, A.; Icely, J.D. A Review of the Application and Evolution of the DPSIR Framework with an Emphasis on Coastal Social-Ecological Systems. Ocean Coast Manag 2015, 103, 63–77. [Google Scholar] [CrossRef]
- Calado, H.; Borges, P.; Phillips, M.; Ng, K.; Alves, F. The Azores Archipelago, Portugal: Improved Understanding of Small Island Coastal Hazards and Mitigation Measures. Natural Hazards 2011, 58, 427–444. [Google Scholar] [CrossRef]
- Castro, N.; Félix, P.M.; Gestoso, I.; Costa, J.L.; Canning-Clode, J. Management of Non-Indigenous Species in Macaronesia: Misconceptions and Alerts to Decision-Makers. Mar Pollut Bull 2024, 204, 116506. [Google Scholar] [CrossRef] [PubMed]
- McIvor, A.J.; Williams, C.T.; Alves, F.; Dinis, A.; Pais, M.P.; Canning-Clode, J. The Status of Marine Megafauna Research in Macaronesia: A Systematic Review. Front Mar Sci 2022, 9, 819581. [Google Scholar] [CrossRef]
- Hawkins, S.J.; Corte-Real, H.B.S.M.; Pannacciulli, F.G.; Weber, L.C.; Bishop, J.D.D. Thoughts on the Ecology and Evolution of the Intertidal Biota of the Azores and Other Atlantic Islands. Hydrobiologia 2000, 440, 3–17. [Google Scholar] [CrossRef]
- MacArthur, R.H.; Wilson, E.O. An Equilibrium Theory of Insular Zoogeography. Evolution (N Y) 1963, 17, 373–387. [Google Scholar] [CrossRef]
- Kueffer, C.; Drake, D.R.; Fernández-Palacios, J.M. Island Biology: Looking towards the Future. Biol Lett 2014, 10, 20140719. [Google Scholar] [CrossRef]
- Graham, N.R.; Gruner, D.S.; Lim, J.Y.; Gillespie, R.G. Island Ecology and Evolution: Challenges in the Anthropocene. Environ Conserv 2017, 44, 323–335. [Google Scholar] [CrossRef]
- Nogué, S.; Santos, A.M.C.; Birks, H.J.B.; Björck, S.; Castilla-Beltrán, A.; Connor, S.; de Boer, E.J.; de Nascimento, L.; Felde, V.A.; Fernández-Palacios, J.M.; et al. The Human Dimension of Biodiversity Changes on Islands. Science (1979) 2021, 372, 488–491. [Google Scholar] [CrossRef]
- Misiuk, B.; Brown, C.J. Benthic Habitat Mapping: A Review of Three Decades of Mapping Biological Patterns on the Seafloor. EarthArXiv 2023, 71. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. Ann Intern Med 2009, 151, 264. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. Declaración PRISMA 2020: Una Guía Actualizada Para La Publicación de Revisiones Sistemáticas. Rev Esp Cardiol 2021, 74, 790–799. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Palacios, J.M.; Whittaker, R.J. The Canaries: An Important Biogeographical Meeting Place. J Biogeogr 2008, 35, 379–387. [Google Scholar] [CrossRef]
- EEA. Europe’s Biodiversity - Biogeographical Regions and Seas; 2002. [Google Scholar]
- Haroun, R. El Mar Canario. In Naturaleza de las Islas Canarias. Ecología y Conservación; Fernández-Palacios, J.M., Martín-Esquivel, J.L., Eds.; Editorial Turquesa, 2001; pp. 103–107.
- Morton, B.; Britton, J.C.; De Frias, A.M.; Martins, H. Coastal Ecology of the Azores; 1998. [Google Scholar]
- Oceanographic and Biological Features in the Canary Current Large Marine Ecosystem; Valdés, L., Déniz-González, I. (eds), Eds.; 2015. [Google Scholar]
- Ávila, S.P.; Melo, C.; Berning, B.; Cordeiro, R.; Landau, B.; da Silva, C.M. Persististrombus Coronatus (Mollusca: Strombidae) in the Lower Pliocene of Santa Maria Island (Azores, NE Atlantic): Paleoecology, Paleoclimatology and Paleobiogeographic Implications. Palaeogeogr Palaeoclimatol Palaeoecol 2016, 441, 912–923. [Google Scholar] [CrossRef]
- Tuya, F.; Haroun, R.J. Phytogeography of Lusitanian Macaronesia: Biogeographic Affinities in Species Richness and Assemblage Composition. Eur J Phycol 2009, 44, 405–413. [Google Scholar] [CrossRef]
- Freitas, R.; Romeiras, M.; Silva, L.; Cordeiro, R.; Madeira, P.; González, J.A.; Wirtz, P.; Falcón, J.M.; Brito, A.; Floeter, S.R.; et al. Restructuring of the ‘Macaronesia’ Biogeographic Unit: A Marine Multi-Taxon Biogeographical Approach. Sci Rep 2019, 9, 15792. [Google Scholar] [CrossRef] [PubMed]
- Carine, M.A.; Schaefer, H. The Azores Diversity Enigma: Why Are There so Few Azorean Endemic Flowering Plants and Why Are They so Widespread? J Biogeogr 2010, 37, 77–89. [Google Scholar] [CrossRef]
- Emerson, B.C. Evolution on Oceanic Islands: Molecular Phylogenetic Approaches to Understanding Pattern and Process. Mol Ecol 2002, 11, 951–966. [Google Scholar] [CrossRef]
- Juan, C.; Emerson, B.C.; Oromı́, P.; Hewitt, G.M. Colonization and Diversification: Towards a Phylogeographic Synthesis for the Canary Islands. Trends Ecol Evol 2000, 15, 104–109. [Google Scholar] [CrossRef]
- Whittaker, R.J.; Fernández-Palacios, J.M. Island Biogeography: Ecology, Evolution, and Conservation, Second ed; Oxford University Press, 2007. [Google Scholar]
- Haddaway, N.R.; Page, M.J.; Pritchard, C.C.; McGuinness, L.A. PRISMA2020: An R Package and Shiny App for Producing PRISMA 2020-compliant Flow Diagrams, with Interactivity for Optimised Digital Transparency and Open Synthesis. Campbell Systematic Reviews 2022, 18. [Google Scholar] [CrossRef]
- Mayorga-Ponce, R.B.; Monroy-Hernández, A.; Hernández-Rubio, J.; Roldan-Carpio, A.; Reyes-Torres, S.B. Programa SPSS. Educación y Salud Boletín Científico Instituto de Ciencias de la Salud Universidad Autónoma del Estado de Hidalgo 2021, 10, 282–284. [Google Scholar] [CrossRef]
- Hammer, Ø.; Harper, D.A. Past: Paleontological Statistics Software Package for Educaton and Data Anlysis. Palaeontologia electronica 2001, 4, 9. [Google Scholar]
- Ribeiro, C.; Neves, P. Habitat Mapping of Cabo Girão Marine Park (Madeira Island): A Tool for Conservation and Management. J Coast Conserv 2020, 24, 22. [Google Scholar] [CrossRef]
- Scepanski, D.; Augustin, N.; Dünn, M.; Scherwaß, A.; Xavier, J.R.; Werner, J.; Waldvogel, A.M.; Arndt, H. Vertical Distribution of Epibenthic Megafauna of a Large Seamount West of Cape Verde Islands (Tropical North Atlantic). Marine Biodiversity 2024, 54, 6. [Google Scholar] [CrossRef]
- Tempera, F.; Pereira, J.N.; Henriques, A.B.; Porteiro, F.M.; Morato, T.; de Matos, V.; Souto, M.; Guillaumont, B.; Serrão Santos, R. Cataloguing Deep-Sea Biological Facies of the Azores. Revista de Investigación Marina 2012, 19, 36–38. [Google Scholar]
- Doxa, A.; Holon, F.; Deter, J.; Villéger, S.; Boissery, P.; Mouquet, N. Mapping Biodiversity in Three-Dimensions Challenges Marine Conservation Strategies: The Example of Coralligenous Assemblages in North-Western Mediterranean Sea. Ecol Indic 2016, 61, 1042–1054. [Google Scholar] [CrossRef]
- Coggan, R.; Populus, J.; White, J.; Sheehan, K.; Fitzpatrick, F.; Piel, S. Review of Standards and Protocols for Seabed Habitat Mapping. In Mapping European Seabed Habitats (MESH); Peterborough, UK., 2007; pp. 106–203.
- Van Rein, H.; Brown, C.J.; Quinn, R.; Breen, J. A Review of Sublittoral Monitoring Methods in Temperate Waters: A Focus on Scale. Underwater Technology 2009, 28, 1–15. [Google Scholar] [CrossRef]
- Álvaro, N. V.; Wallenstein, F.F.M.M.; Neto, A.I.; Nogueira, E.M.; Ferreira, J.; Santos, C.I.; Amaral, A.F. The Use of Digital Photography for the Definition of Coastal Biotopes in Azores. Hydrobiologia 2008, 596, 143–152. [Google Scholar] [CrossRef]
- Ferretti, R.; Bibuli, M.; Caccia, M.; Chiarella, D.; Odetti, A.; Ranieri, A.; Zereik, E.; Bruzzone, G. Machine Learning Methods for Acoustic-Based Automatic Posidonia Meadows Detection by Means of Unmanned Marine Vehicles. In Proceedings of the OCEANS 2017 - Aberdeen; IEEE; 2017; pp. 1–6. [Google Scholar]
- Ierodiaconou, D.; Schimel, A.C.G.; Kennedy, D.; Monk, J.; Gaylard, G.; Young, M.; Diesing, M.; Rattray, A. Combining Pixel and Object Based Image Analysis of Ultra-High Resolution Multibeam Bathymetry and Backscatter for Habitat Mapping in Shallow Marine Waters. Marine Geophysical Research 2018, 39, 271–288. [Google Scholar] [CrossRef]
- Moore, C.H.; Harvey, E.S.; Van Niel, K.P. Spatial Prediction of Demersal Fish Distributions: Enhancing Our Understanding of Species–Environment Relationships. ICES Journal of Marine Science 2009, 66, 2068–2075. [Google Scholar] [CrossRef]
- Kocak, D.M.; Dalgleish, F.R.; Caimi, F.M.; Schechner, Y.Y. A Focus on Recent Developments and Trends in Underwater Imaging. Mar Technol Soc J 2008, 42, 52–67. [Google Scholar] [CrossRef]
- Robert, K.; Jones, D.O.B.; Tyler, P.A.; Van Rooij, D.; Huvenne, V.A.I. Finding the Hotspots within a Biodiversity Hotspot: Fine-scale Biological Predictions within a Submarine Canyon Using High-resolution Acoustic Mapping Techniques. Marine Ecology 2015, 36, 1256–1276. [Google Scholar] [CrossRef]
- Pearman, T.R.R.; Robert, K.; Callaway, A.; Hall, R.; Lo Iacono, C.; Huvenne, V.A.I. Improving the Predictive Capability of Benthic Species Distribution Models by Incorporating Oceanographic Data – Towards Holistic Ecological Modelling of a Submarine Canyon. Prog Oceanogr 2020, 184, 102338. [Google Scholar] [CrossRef]
- Williams, S.B.; Pizarro, O.; Jakuba, M.; Barrett, N. AUV Benthic Habitat Mapping in South Eastern Tasmania. In; 2010; pp. 275–284.
- Williams, S.B.; Pizarro, O.R.; Jakuba, M. V.; Johnson, C.R.; Barrett, N.S.; Babcock, R.C.; Kendrick, G.A.; Steinberg, P.D.; Heyward, A.J.; Doherty, P.J.; et al. Monitoring of Benthic Reference Sites: Using an Autonomous Underwater Vehicle. IEEE Robot Autom Mag 2012, 19, 73–84. [Google Scholar] [CrossRef]
- Gomes-Pereira, J.N.; Carmo, V.; Catarino, D.; Jakobsen, J.; Alvarez, H.; Aguilar, R.; Hart, J.; Giacomello, E.; Menezes, G.; Stefanni, S.; et al. Cold-Water Corals and Large Hydrozoans Provide Essential Fish Habitat for Lappanella Fasciata and Benthocometes Robustus. Deep Sea Res 2 Top Stud Oceanogr 2017, 145, 33–48. [Google Scholar] [CrossRef]
- Martín García, L.; Rancel-Rodríguez, N.M.; Sangil, C.; Reyes, J.; Benito, B.; Orellana, S.; Sansón, M. Environmental and Human Factors Drive the Subtropical Marine Forests of Gongolaria Abies-Marina to Extinction. Mar Environ Res 2022, 181, 105759. [Google Scholar] [CrossRef]
- Tittley, I.; Neto, A.I. A Provisional Classification of Algal-Characterised Rocky Shore Biotopes in the Azores. Hydrobiologia 2000, 440, 19–25. [Google Scholar] [CrossRef]
- Van Rein, H.; Brown, C.J.; Quinn, R.; Breen, J.; Schoeman, D. An Evaluation of Acoustic Seabed Classification Techniques for Marine Biotope Monitoring over Broad-Scales (>1 Km2) and Meso-Scales (10 M2-1 Km2). Estuar Coast Shelf Sci 2011, 93, 336–349. [Google Scholar] [CrossRef]
- Sabol, B.; Graves, M.; Preston, J. Evaluation of Multibeam Hydrographic System Performance in Dense Seagrass. Proceedings Hydro2007 2007, 14–17. [Google Scholar]
- Fish, J.P.; Carr, A.H. Sound Underwater Images. A Guide to the Generation and Interpretation of Side-Scan Sonar Data; American Underwater Search and Surveys Ltd., Lower Cape Publishing Orleans, MA. 1990. [Google Scholar]
- Kenny, A.J.; Cato, I.; Desprez, M.; Fader, G.; Schüttenhelm, R.T.E.; Side, J. An Overview of Seabed-Mapping Technologies in the Context of Marine Habitat Classification. ICES Journal of Marine Science 2003, 60, 411–418. [Google Scholar] [CrossRef]
- Giusti, M.; Cerrano, C.; Angiolillo, M.; Tunesi, L.; Canese, S. An Updated Overview of the Geographic and Bathymetric Distribution of Savalia Savaglia. Mediterr Mar Sci 2015, 16, 128–135. [Google Scholar] [CrossRef]
- Green, M.; Cunningham, D. Seabed Imaging Techniques. Hydro International 1998, 2, 4. [Google Scholar]
- Foster-Smith, R.; Gilliland, P. Mapping the Seabed. Coast-NET 1997, 2, 1. [Google Scholar]
- Hughes Clarke, J.E. Detecting Small Seabed Targets Using High-Frequency Multibeam Sonar. Sea Technology 1998, 6, 87–90. [Google Scholar]
- Braga-Henriques, A.; Buhl-Mortensen, P.; Tokat, E.; Martins, A.; Silva, T.; Jakobsen, J.; Canning-Clode, J.; Jakobsen, K.; Delgado, J.; Voirand, T.; et al. Benthic Community Zonation from Mesophotic to Deep Sea: Description of First Deep-Water Kelp Forest and Coral Gardens in the Madeira Archipelago (Central NE Atlantic). Front Mar Sci 2022, 9, 973364. [Google Scholar] [CrossRef]
- Kalwa, J.; Carreiro-Silva, M.; Tempera, F.; Fontes, J.; Santos, R.S.; Fabri, M.-C.; Brignone, L.; Ridao, P.; Birk, A.; Glotzbach, T.; et al. The MORPH Concept and Its Application in Marine Research. In Proceedings of the 2013 MTS/IEEE OCEANS - Bergen; IEEE; 2013; pp. 1–8. [Google Scholar]
- Tempera, F.; MacKenzie, M.; Bashmachnikov, I.; Puotinen, M.; Santos, R.S.; Bates, R. Predictive Modeling of Dominant Macroalgae Abundance on Temperate Island Shelves (Azores, Northeast Atlantic). In Seafloor Geomorphology as Benthic Habitat; Elsevier Inc., 2012; pp. 169–184 ISBN 9780123851406.
- Girard, F.; Sarrazin, J.; Arnaubec, A.; Cannat, M.; Sarradin, P.M.; Wheeler, B.; Matabos, M. Currents and Topography Drive Assemblage Distribution on an Active Hydrothermal Edifice. Prog Oceanogr 2020, 187, 102397. [Google Scholar] [CrossRef]
- Somoza, L.; Medialdea, T.; González, F.J.; Calado, A.; Afonso, A.; Albuquerque, M.; Asensio-Ramos, M.; Bettencourt, R.; Blasco, I.; Candón, J.A.; et al. Multidisciplinary Scientific Cruise to the Northern Mid-Atlantic Ridge and Azores Archipelago. Front Mar Sci 2020, 7, 568035. [Google Scholar] [CrossRef]
- De Falco, G.; Tonielli, R.; Di Martino, G.; Innangi, S.; Simeone, S.; Michael Parnum, I. Relationships between Multibeam Backscatter, Sediment Grain Size and Posidonia Oceanica Seagrass Distribution. Cont Shelf Res 2010, 30, 1941–1950. [Google Scholar] [CrossRef]
- Feldens, P.; Held, P.; Otero-Ferrer, F.; Bramanti, L.; Espino, F.; Schneider von Deimling, J. Can Black Coral Forests Be Detected Using Multibeam Echosounder “Multi-Detect” Data? Frontiers in Remote Sensing 2023, 4, 988366. [Google Scholar] [CrossRef]
- Purkis, S.J.; Gleason, A.C.R.; Purkis, C.R.; Dempsey, A.C.; Renaud, P.G.; Faisal, M.; Saul, S.; Kerr, J.M. High-Resolution Habitat and Bathymetry Maps for 65,000 Sq. Km of Earth’s Remotest Coral Reefs. Coral Reefs 2019, 38, 467–488. [Google Scholar] [CrossRef]
- Valdazo, J.; Coca, J.; Haroun, R.; Bergasa, O.; Viera-Rodríguez, M.A.; Tuya, F. Local and Global Stressors as Major Drivers of the Drastic Regression of Brown Macroalgae Forests in an Oceanic Island. Reg Environ Change 2024, 24, 65. [Google Scholar] [CrossRef]
- Monteiro, J.G.; Jiménez, J.L.; Gizzi, F.; Přikryl, P.; Lefcheck, J.S.; Santos, R.S.; Canning-Clode, J. Novel Approach to Enhance Coastal Habitat and Biotope Mapping with Drone Aerial Imagery Analysis. Sci Rep 2021, 11, 574. [Google Scholar] [CrossRef] [PubMed]
- Kellaris, A.; Gil, A.; Faria, J.; Amaral, R.; Moreu-Badia, I.; Neto, A.; Yesson, C. Using Low-cost Drones to Monitor Heterogeneous Submerged Seaweed Habitats: A Case Study in the Azores. Aquat Conserv 2019, 29, 1909–1922. [Google Scholar] [CrossRef]
- Valdazo, J.; Ferrer, N.; Vega, C.; Martín, J.; Luque, Á.; Bergasa, O. Mapping Marine Habitats in a Shallow Beach-Reef Environment Combining Direct Methods and Hyperspectral Remote Sensing. Ocean Coast Manag 2024, 255, 107231. [Google Scholar] [CrossRef]
- Cerdeira-Estrada, S.; Heege, T.; Kolb, M.; Ohlendorf, S.; Uribe, A.; Muller, A.; Garza, R.; Ressl, R.; Aguirre, R.; Marino, I.; et al. Benthic Habitat and Bathymetry Mapping of Shallow Waters in Puerto Morelos Reefs Using Remote Sensing with a Physics Based Data Processing. In Proceedings of the IEEE International Geoscience and Remote Sensing Symposium; IEEE; 2012; pp. 4383–4386. [Google Scholar]
- Poursanidis, D.; Traganos, D.; Teixeira, L.; Shapiro, A.; Muaves, L. Cloud-native Seascape Mapping of Mozambique’s Quirimbas National Park with Sentinel-2. Remote Sens Ecol Conserv 2021, 7, 275–291. [Google Scholar] [CrossRef]
- Borfecchia, F.; Consalvi, N.; Micheli, C.; Carli, F.M.; Cognetti De Martiis, S.; Gnisci, V.; Piermattei, V.; Belmonte, A.; De Cecco, L.; Bonamano, S.; et al. Landsat 8 OLI Satellite Data for Mapping of the Posidonia Oceanica and Benthic Habitats of Coastal Ecosystems. Int J Remote Sens 2019, 40, 1548–1575. [Google Scholar] [CrossRef]
- Doukari, M.; Topouzelis, K. UAS Data Acquisition Protocol For Marine Habitat Mapping: An Accuracy Assessment Study. The International Archives of the Photogrammetry, Remote Sensing and Spatial Information Sciences 2020, XLIII-B3-2020, 1321–1326. [Google Scholar] [CrossRef]
- Ventura, D.; Bonifazi, A.; Gravina, M.F.; Belluscio, A.; Ardizzone, G. Mapping and Classification of Ecologically Sensitive Marine Habitats Using Unmanned Aerial Vehicle (UAV) Imagery and Object-Based Image Analysis (OBIA). Remote Sens (Basel) 2018, 10, 1331. [Google Scholar] [CrossRef]
- Hedley, J.; Roelfsema, C.; Chollett, I.; Harborne, A.; Heron, S.; Weeks, S.; Skirving, W.; Strong, A.; Eakin, C.; Christensen, T.; et al. Remote Sensing of Coral Reefs for Monitoring and Management: A Review. Remote Sens (Basel) 2016, 8, 118. [Google Scholar] [CrossRef]
- Rovelli, L.; Carreiro-Silva, M.; Attard, K.M.; Rakka, M.; Dominguez-Carrió, C.; Bilan, M.; Blackbird, S.; Morato, T.; Wolff, G.A.; Glud, R.N. Benthic O2 Uptake by Coral Gardens at the Condor Seamount (Azores). Mar Ecol Prog Ser 2022, 688, 19–31. [Google Scholar] [CrossRef]
- Zweng, M.M.; Reagan, J.R.; Antonov, J.I.; Locarnini, R.A.; Mishonov, A.V.; Boyer, T.P.; Garcia, H.E.; Baranova, O.K.; Johnson, D.R.; Seidov, D.; et al. World Ocean Atlas 2013, Volume 2: Salinity. In NOAA Atlas NESDIS; Levitus, S., Mishonov, A., Eds.; 2013; Vol. 74, p. 39.
- Eugenio, F.; Mederos-Barrera, A.; Marcello, J. High-Resolution Satellite Monitoring of Vulnerable Coastal Ecosystems Using Advanced Artificial Intelligence Techniques. In Proceedings of the International Geoscience and Remote Sensing Symposium (IGARSS); IEEE, 2023; Vol. 2023-July; pp. 4104–4107. [Google Scholar]
- Marcello, J.; Eugenio, F.; Marques, F.; Martin, J. Precise Classification of Coastal Benthic Habitats Using High Resolution Worldview-2 Imagery. In Proceedings of the International Geoscience and Remote Sensing Symposium (IGARSS); Institute of Electrical and Electronics Engineers Inc., 2015; Vol. 2015-November; pp. 2307–2310. [Google Scholar]
- Mendoza, J.C.; Clemente, S.; Hernández, J.C. Modeling the Role of Marine Protected Areas on the Recovery of Shallow Rocky Reef Ecosystem after a Catastrophic Submarine Volcanic Eruption. Mar Environ Res 2020, 155, 104877. [Google Scholar] [CrossRef]
- Mederos-Barrera, A.; Sevilla, J.; Marcello, J.; Espinosa, J.M.; Eugenio, F. Effects of the Construction of Granadilla Industrial Port in Seagrass and Seaweed Habitats Using Very-High-Resolution Multispectral Satellite Imagery. Remote Sens (Basel) 2024, 16, 945. [Google Scholar] [CrossRef]
- Casas, E.; Martín-García, L.; Otero-Ferrer, F.; Tuya, F.; Haroun, R.; Arbelo, M. Economic Mapping and Assessment of Cymodocea Nodosa Meadows as Nursery Grounds for Commercially Important Fish Species. A Case Study in the Canary Islands. One Ecosystem 2021, 6. [Google Scholar] [CrossRef]
- Eugenio, F.; Marcello, J.; Martin, J.; Rodríguez-Esparragón, D. Benthic Habitat Mapping Using Multispectral High-Resolution Imagery: Evaluation of Shallow Water Atmospheric Correction Techniques. Sensors (Switzerland) 2017, 17, 2639. [Google Scholar] [CrossRef]
- Vasquez, M.; Mata Chacón, D.; Tempera, F.; O’Keeffe, E.; Galparsoro, I.; Sanz Alonso, J.L.; Gonçalves, J.M.S.; Bentes, L.; Amorim, P.; Henriques, V.; et al. Broad-Scale Mapping of Seafloor Habitats in the North-East Atlantic Using Existing Environmental Data. J Sea Res 2015, 100, 120–132. [Google Scholar] [CrossRef]
- Puerta, P.; Mosquera-Giménez, Á.; Reñones, O.; Domínguez-Carrió, C.; Rueda, J.L.; Urra, J.; Carreiro-Silva, M.; Blasco-Ferre, J.; Santana, Y.; Gutiérrez-Zárate, C.; et al. Variability of Deep-Sea Megabenthic Assemblages along the Western Pathway of the Mediterranean Outflow Water. Deep Sea Res 1 Oceanogr Res Pap 2022, 185, 103791. [Google Scholar] [CrossRef]
- Marcos, C.; Díaz, D.; Fietz, K.; Forcada, A.; Ford, A.; García-Charton, J.A.; Goñi, R.; Lenfant, P.; Mallol, S.; Mouillot, D.; et al. Reviewing the Ecosystem Services, Societal Goods, and Benefits of Marine Protected Areas. Front Mar Sci 2021, 8, 613819. [Google Scholar] [CrossRef]
- Brown, C.J.; Smith, S.J.; Lawton, P.; Anderson, J.T. Benthic Habitat Mapping: A Review of Progress towards Improved Understanding of the Spatial Ecology of the Seafloor Using Acoustic Techniques. Estuar Coast Shelf Sci 2011, 92, 502–520. [Google Scholar] [CrossRef]
- Mayer, L.A. Frontiers in Seafloor Mapping and Visualization. Mar Geophys Res (Dordr) 2006, 27, 7–17. [Google Scholar] [CrossRef]
- Lecours, V.; Dolan, M.F.J.; Micallef, A.; Lucieer, V.L. A Review of Marine Geomorphometry, the Quantitative Study of the Seafloor. Hydrol Earth Syst Sci 2016, 20, 3207–3244. [Google Scholar] [CrossRef]
- Grohmann, C.H. Morphometric Analysis in Geographic Information Systems: Applications of Free Software GRASS and R. Comput Geosci 2004, 30, 1055–1067. [Google Scholar] [CrossRef]
- Schäfer, S.; Monteiro, J.; Castro, N.; Gizzi, F.; Henriques, F.; Ramalhosa, P.; Parente, M.I.; Rilov, G.; Gestoso, I.; Canning-Clode, J. Lost and Found: A New Hope for the Seagrass Cymodocea Nodosa in the Marine Ecosystem of a Subtropical Atlantic Island. Reg Stud Mar Sci 2021, 41, 101575. [Google Scholar] [CrossRef]
- Gumusay, M.U.; Bakirman, T.; Tuney Kizilkaya, I.; Aykut, N.O. A Review of Seagrass Detection, Mapping and Monitoring Applications Using Acoustic Systems. Eur J Remote Sens 2019, 52, 1–29. [Google Scholar] [CrossRef]
- Neves, P.; Silva, J.; Peña, V.; Ribeiro, C. “Pink Round Stones”—Rhodolith Beds: An Overlooked Habitat in Madeira Archipelago. Biodivers Conserv 2021, 30, 3359–3383. [Google Scholar] [CrossRef]
- Braga-Henriques, A.; Porteiro, F.M.; Ribeiro, P.A.; De Matos, V.; Sampaio, Í.; Ocaña, O.; Santos, R.S. Diversity, Distribution and Spatial Structure of the Cold-Water Coral Fauna of the Azores (NE Atlantic). Biogeosciences 2013, 10, 4009–4036. [Google Scholar] [CrossRef]
- Warren, D.L.; Glor, R.E.; Turelli, M. ENVIRONMENTAL NICHE EQUIVALENCY VERSUS CONSERVATISM: QUANTITATIVE APPROACHES TO NICHE EVOLUTION. Evolution (N Y) 2008, 62, 2868–2883. [Google Scholar] [CrossRef]
- Melo-Merino, S.M.; Reyes-Bonilla, H.; Lira-Noriega, A. Ecological Niche Models and Species Distribution Models in Marine Environments: A Literature Review and Spatial Analysis of Evidence. Ecol Modell 2020, 415, 108837. [Google Scholar] [CrossRef]
- Pearson, R.G.; Dawson, T.P.; Liu, C. Modelling Species Distributions in Britain: A Hierarchical Integration of Climate and Land-cover Data. Ecography 2004, 27, 285–298. [Google Scholar] [CrossRef]
- Hu, Z.; Hu, J.; Hu, H.; Zhou, Y. Predictive Habitat Suitability Modeling of Deep-Sea Framework-Forming Scleractinian Corals in the Gulf of Mexico. Science of The Total Environment 2020, 742, 140562. [Google Scholar] [CrossRef]
- Cordero-Penín, V.; Abramic, A.; García-Mendoza, A.; Otero-Ferrer, F.; Haroun, R. Mapping Marine Ecosystem Services Potential across an Oceanic Archipelago: Applicability and Limitations for Decision-Making. Ecosyst Serv 2023, 60, 101517. [Google Scholar] [CrossRef]
- Vinha, B.; Murillo, F.J.; Schumacher, M.; Hansteen, T.H.; Schwarzkopf, F.U.; Biastoch, A.; Kenchington, E.; Piraino, S.; Orejas, C.; Huvenne, V.A.I. Ensemble Modelling to Predict the Distribution of Vulnerable Marine Ecosystems Indicator Taxa on Data-Limited Seamounts of Cabo Verde (NW Africa). Divers Distrib 2024, 30, e13896. [Google Scholar] [CrossRef]
- Gonzalez-Lorenzo, G.; Toledo-Guedes, K.; Rodríguez-Riesco, E.; González-Méndez, E.; González-Rodríguez, Y.; Piñeiro-Corbeira, C. The Underreported Presence of the Subtidal Seagrass Cymodocea Nodosa in Rocky Intertidal Pools of the Canary Islands. Marine Biology Research 2020, 16, 550–555. [Google Scholar] [CrossRef]
- Martín-García, L.; González-Lorenzo, G.; Brito-Izquierdo, I.T.; Barquín-Diez, J. Use of Topographic Predictors for Macrobenthic Community Mapping in the Marine Reserve of La Palma (Canary Islands, Spain). Ecol Modell 2013, 263, 19–31. [Google Scholar] [CrossRef]
- Wienberg, C.; Wintersteller, P.; Beuck, L.; Hebbeln, D. Coral Patch Seamount (NE Atlantic) – a Sedimentological and Macrofaunal Reconnaissance Based on Video and Hydroacoustic Surveys. Biogeosciences Discussions 2012, 9, 18707–18753. [Google Scholar] [CrossRef]
- Desbruyères, D.; Almeida, A.; Biscoito, M.; Comtet, T.; Khripounoff, A.; Le Bris, N.; Sarradin, P.M.; Segonzac, M. A Review of the Distribution of Hydrothermal Vent Communities along the Northern Mid-Atlantic Ridge: Dispersal vs. Environmental Controls. Hydrobiologia 2000, 440, 201–216. [Google Scholar] [CrossRef]
- ICES. Report of the Working Group on Marine Habitat Mapping (WGMHM); Galway, Ireland, 2006. [Google Scholar]
- Shumchenia, E.J.; King, J.W. Comparison of Methods for Integrating Biological and Physical Data for Marine Habitat Mapping and Classification. Cont Shelf Res 2010, 30, 1717–1729. [Google Scholar] [CrossRef]
- Galparsoro, I.; Connor, D.W.; Borja, Á.; Aish, A.; Amorim, P.; Bajjouk, T.; Chambers, C.; Coggan, R.; Dirberg, G.; Ellwood, H.; et al. Using EUNIS Habitat Classification for Benthic Mapping in European Seas: Present Concerns and Future Needs. Mar Pollut Bull 2012, 64, 2630–2638. [Google Scholar] [CrossRef] [PubMed]
- Strong, J.A.; Clements, A.; Lillis, H.; Galparsoro, I.; Bildstein, T.; Pesch, R. A Review of the Influence of Marine Habitat Classification Schemes on Mapping Studies: Inherent Assumptions, Influence on End Products, and Suggestions for Future Developments. ICES Journal of Marine Science 2019, 76, 10–22. [Google Scholar] [CrossRef]
- Ierodianconou, D.; Monk, J.; Rattray, A.; Laurenson, L.; Versace, V.L. Comparison of Automated Classification Techniques for Predicting Biological Communities Using Hydroacoutics and Video Observations. Cont Shelf Res 2011, 31, S28–S38. [Google Scholar] [CrossRef]
- Diaz, R.J.; Solan, M.; Valente, R.M. A Review of Approaches for Classifying Benthic Habitats and Evaluating Habitat Quality. J Environ Manage 2004, 73, 165–181. [Google Scholar] [CrossRef]
- Laban, C. Seabed Mapping. Hydro International 1998, 2, 4. [Google Scholar]
- Rivera, J.; Canals, M.; Lastras, G.; Hermida, N.; Amblas, D.; Arrese, B.; Martín-Sosa, P.; Acosta, J. Morphometry of Concepcion Bank: Evidence of Geological and Biological Processes on a Large Volcanic Seamount of the Canary Islands Seamount Province. PLoS One 2016, 11, e0156337. [Google Scholar] [CrossRef] [PubMed]
- Pham, C.K.; Diogo, H.; Menezes, G.; Porteiro, F.; Braga-Henriques, A.; Vandeperre, F.; Morato, T. Deep-Water Longline Fishing Has Reduced Impact on Vulnerable Marine Ecosystems. Sci Rep 2014, 4, 4837. [Google Scholar] [CrossRef] [PubMed]
- Tuya, F.; Ribeiro-Leite, L.; Arto-Cuesta, N.; Coca, J.; Haroun, R.; Espino, F. Decadal Changes in the Structure of Cymodocea Nodosa Seagrass Meadows: Natural vs. Human Influences. Estuar Coast Shelf Sci 2014, 137, 41–49. [Google Scholar] [CrossRef]
- Duncan, E.M.; Vieira, N.; González-Irusta, J.M.; Dominguez-Carrió, C.; Morato, T.; Carreiro-Silva, M.; Jakobsen, J.; Jakobsen, K.; Porteiro, F.; Schläpfer, N.; et al. Predicting the Distribution and Abundance of Abandoned, Lost or Discarded Fishing Gear (ALDFG) in the Deep Sea of the Azores (North Atlantic). Science of the Total Environment 2023, 900, 166579. [Google Scholar] [CrossRef]
- Ribeiro, C.; Neves, P.; Kaufmann, M.; Araújo, R.; Riera, R. A Baseline for Prioritizing the Conservation of the Threatened Seagrass Cymodocea Nodosa in the Oceanic Archipelago of Madeira. J Nat Conserv 2022, 68, 126224. [Google Scholar] [CrossRef]
- Tempera, F.; Milla-Figueras, D.; Sinde-Mano, A.L.; Atchoi, E.; Afonso, P. Range Extension of Mesophotic Kelps (Ochrophyta: Laminariales and Tilopteridales) in the Central North Atlantic: Opportunities for Marine Forest Research and Conservation. J Phycol 2021, 57, 1140–1150. [Google Scholar] [CrossRef]
- López, C.; Clemente, S.; Moreno, S.; Ocaña, O.; Herrera, R.; Moro, L.; Monterroso, O.; Rodríguez, A.; Brito, A. Invasive Tubastraea Spp. and Oculina Patagonica and Other Introduced Scleractinians Corals in the Santa Cruz de Tenerife (Canary Islands) Harbor: Ecology and Potential Risks. Reg Stud Mar Sci 2019, 29, 100713. [Google Scholar] [CrossRef]
- Somoza, L.; Rueda, J.L.; Sánchez-Guillamón, O.; Medialdea, T.; Rincón-Tomás, B.; González, F.J.; Palomino, D.; Madureira, P.; López-Pamo, E.; Fernández-Salas, L.M.; et al. The Interactive Role of Hydrocarbon Seeps, Hydrothermal Vents and Intermediate Antarctic/Mediterranean Water Masses on the Distribution of Some Vulnerable Deep-Sea Habitats in Mid Latitude NE Atlantic Ocean. Oceans 2021, 2, 351–385. [Google Scholar] [CrossRef]
- Riera, R.; Rodríguez, M.; Ramos, E.; Monterroso, Ó.; Delgado, J.D. Hard and Soft-Bottom Macrozoobenthos in Subtidal Communities around an Inactive Harbour Area (Gran Canaria, Canary Islands). Life and Environment 2013, 63, 23–34. [Google Scholar]
- Wallenstein, F.M.; Neto, A.I.; Álvaro, N. V.; Santos, C.I. Algae-Based Biotopes of the Azores (Portugal): Spatial and Seasonal Variation. Aquat Ecol 2008, 42, 547–559. [Google Scholar] [CrossRef]
- Neto, A.I. Contribution to the Taxonomy and Ecology of the Azorean Benthic Marine Algae. Biological Journal of the Linnean Society 1992, 46, 163–176. [Google Scholar] [CrossRef]
- Morato, T.; Dominguez-Carrió, C.; Mohn, C.; Ocaña Vicente, O.; Ramos, M.; Rodrigues, L.; Sampaio, Í.; Taranto, G.H.; Fauconnet, L.; Tojeira, I.; et al. Dense Cold-Water Coral Garden of Paragorgia Johnsoni Suggests the Importance of the Mid-Atlantic Ridge for Deep-Sea Biodiversity. Ecol Evol 2021, 11, 16426–16433. [Google Scholar] [CrossRef]
- Porteiro, F.M.; Gomes-Pereira, J.N.; Pham, C.K.; Tempera, F.; Santos, R.S. Distribution and Habitat Association of Benthic Fish on the Condor Seamount (NE Atlantic, Azores) from in Situ Observations. Deep Sea Res 2 Top Stud Oceanogr 2013, 98, 114–128. [Google Scholar] [CrossRef]
- Sangil, C.; Martín-Garciá, L.; Afonso-Carrillo, J.; Barquín, J.; Sansón, M. Halimeda Incrassata (Bryopsidales, Chlorophyta) Reaches the Canary Islands: Mid- A Nd Deep-Water Meadows in the Eastern Subtropical Atlantic Ocean. Botanica Marina 2018, 61, 103–110. [Google Scholar] [CrossRef]
- Cacabelos, E.; Gestoso, I.; Ramalhosa, P.; Riera, L.; Neto, A.I.; Canning-Clode, J. Intertidal Assemblages across Boulders and Rocky Platforms: A Multi-Scaled Approach in a Subtropical Island. Marine Biodiversity 2019, 49, 2709–2723. [Google Scholar] [CrossRef]
- Wallenstein, F.F.M.M.; Neto, A.I. Intertidal Rocky Shore Biotopes of the Azores: A Quantitative Approach. Helgol Mar Res 2006, 60, 196–206. [Google Scholar] [CrossRef]
- Riera, R.; Delgado, J.D.; Rodríguez, M.; Monterroso, Ó.; Ramos, E. Macrofaunal Communities of Threatened Subtidal Maerl Seabeds on Tenerife (Canary Islands, North-East Atlantic Ocean) in Summer. Acta Oceanologica Sinica 2012, 31, 98–105. [Google Scholar] [CrossRef]
- Keller, N.B.; Pasternak, F.A. Madreporic and Gorgonian Corals (Anthozoa: Scleractinia, Gorgonacea) in the Benthos of the African Shelf and Slope in the Canary Upwelling Area. Oceanology (Wash D C) 2002, 42, 521–526. [Google Scholar]
- Montero-Hidalgo, M.; Tuya, F.; Otero-Ferrer, F.; Haroun, R.; Santos-Martín, F. Mapping and Assessing Seagrass Meadows Changes and Blue Carbon under Past, Current, and Future Scenarios. Science of the Total Environment 2023, 872, 162244. [Google Scholar] [CrossRef]
- Tempera, F.; Giacomello, E.; Mitchell, N.C.; Campos, A.S.; Braga Henriques, A.; Bashmachnikov, I.; Martins, A.; Mendonça, A.; Morato, T.; Colaço, A.; et al. Mapping Condor Seamount Seafloor Environment and Associated Biological Assemblages (Azores, Ne Atlantic). In Seafloor Geomorphology as Benthic Habitat; Elsevier Inc., 2012; pp. 807–818 ISBN 9780123851406.
- Valdazo, J.; Viera-Rodríguez, M.A.; Espino, F.; Haroun, R.; Tuya, F. Massive Decline of Cystoseira Abies-Marina Forests in Gran Canaria Island (Canary Islands, Eastern Atlantic). Sci Mar 2017, 81, 499. [Google Scholar] [CrossRef]
- Mendoza, J.C.; Clemente, S.; Hernández, J.C. Modeling the Role of Marine Protected Areas after a Catastrophic Submarine Volcanic Eruption. Mar Environ Res 2020, 155, 104877. [Google Scholar] [CrossRef] [PubMed]
- Mortensen, P.B.; Buhl-Mortensen, L.; Gebruk, A. V.; Krylova, E.M. Occurrence of Deep-Water Corals on the Mid-Atlantic Ridge Based on MAR-ECO Data. Deep Sea Res 2 Top Stud Oceanogr 2008, 55, 142–152. [Google Scholar] [CrossRef]
- Tojeira, I.; Pinto-Ribeiro, L.; Rafael, T.; Albuquerque, M.; Simões, M.; Calado, A.; Afonso, A.; Ramos, B.; Souto, M.; Bettencourt, R.; et al. Preliminary Deep-Sea Data Analysis Collected at Gloria Seamount, Azores-Biscay Rise. Cont Shelf Res 2023, 267, 105100. [Google Scholar] [CrossRef]
- Sidi Cheikh, M.A.; Bandeira, S.; Soumah, S.; Diouf, G.; Diouf, E.M.; Sanneh, O.; Cardoso, N.; Kujabie, A.; Ndure, M.; John, L.; et al. Seagrasses of West Africa: New Discoveries, Distribution Limits and Prospects for Management. Diversity (Basel) 2023, 15, 5. [Google Scholar] [CrossRef]
- Taranto, G.H.; González-Irusta, J.M.; Dominguez-Carrió, C.; Pham, C.K.; Tempera, F.; Ramos, M.; Gonçalves, G.; Carreiro-Silva, M.; Morato, T. Spatial Distributions, Environmental Drivers and Co-Existence Patterns of Key Cold-Water Corals in the Deep Sea of the Azores (NE Atlantic). Deep Sea Res 1 Oceanogr Res Pap 2023, 197, 104028. [Google Scholar] [CrossRef]
- Casas, E.; Martín-García, L.; Hernández-Leal, P.; Arbelo, M. Species Distribution Models at Regional Scale: Cymodocea Nodosa Seagrasses. Remote Sens (Basel) 2022, 14, 4334. [Google Scholar] [CrossRef]
- Wallenstein, F.F.M.M.; Neto, A.I.; Álvaro, N. V.; Tittley, I. Subtidal Rocky Shore Communities of the Azores: Developing a Biotope Survey Method. J Coast Res 2008, 1, 244–249. [Google Scholar] [CrossRef]
- Fontán Bouzas, A.; Alcántara-Carrió, J.; Montoya Montes, I.; Barranco Ojeda, A.; Albarracín, S.; Rey Díaz de Rada, J.; Rey Salgado, J. Distribution and Thickness of Sedimentary Facies in the Coastal Dune, Beach and Nearshore Sedimentary System at Maspalomas, Canary Islands. Geo-Marine Letters 2013, 33, 117–127. [Google Scholar] [CrossRef]

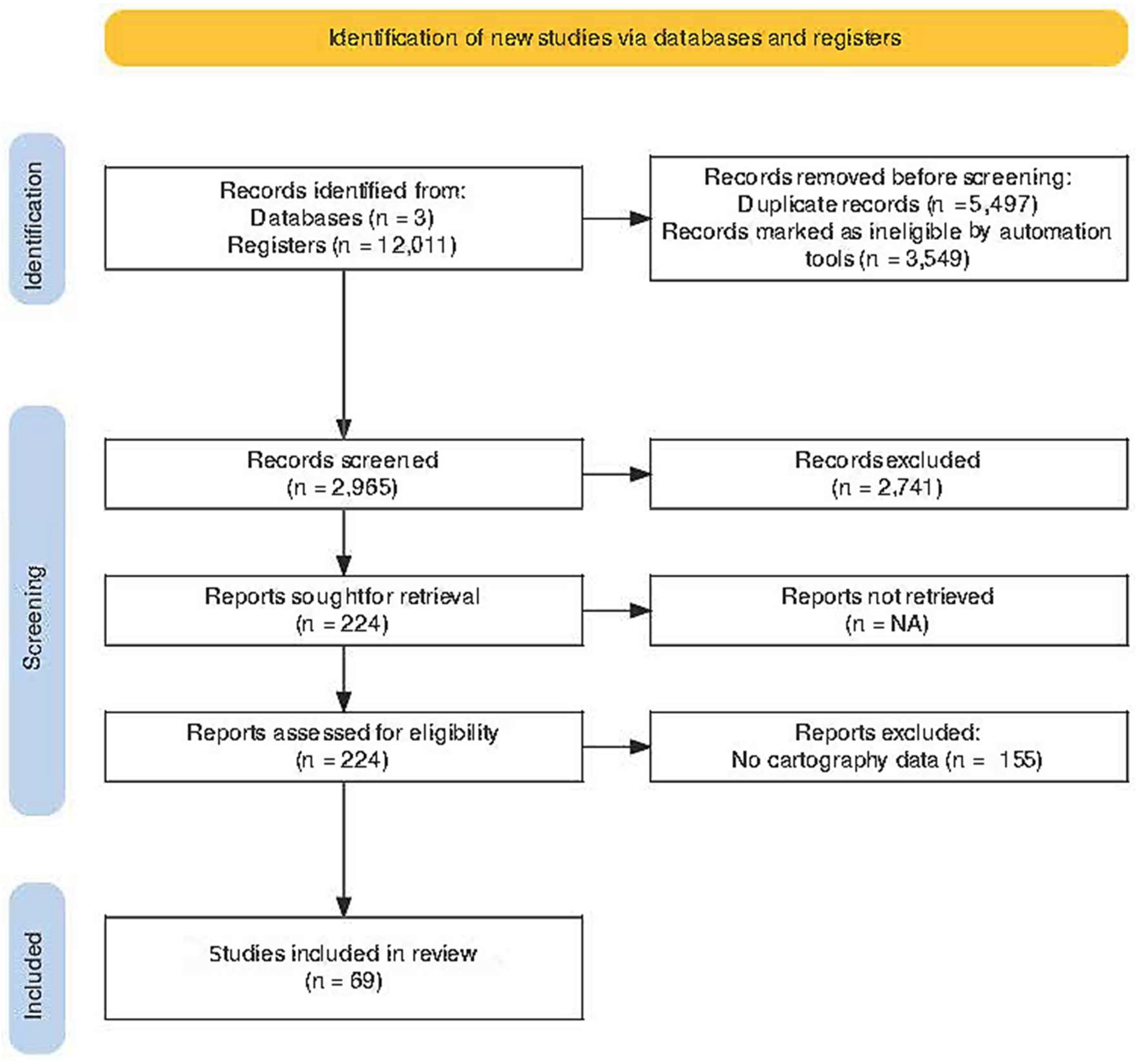

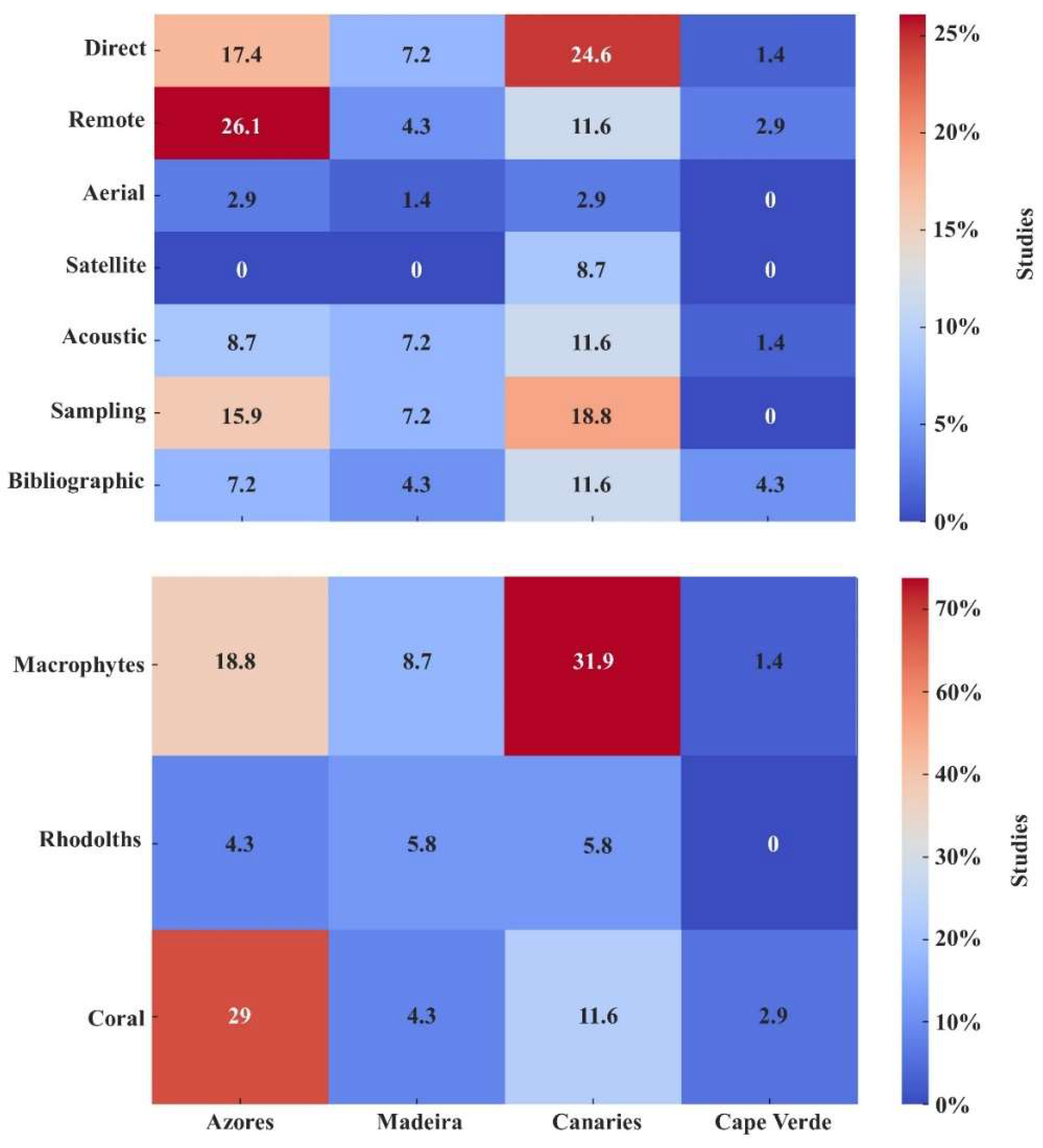
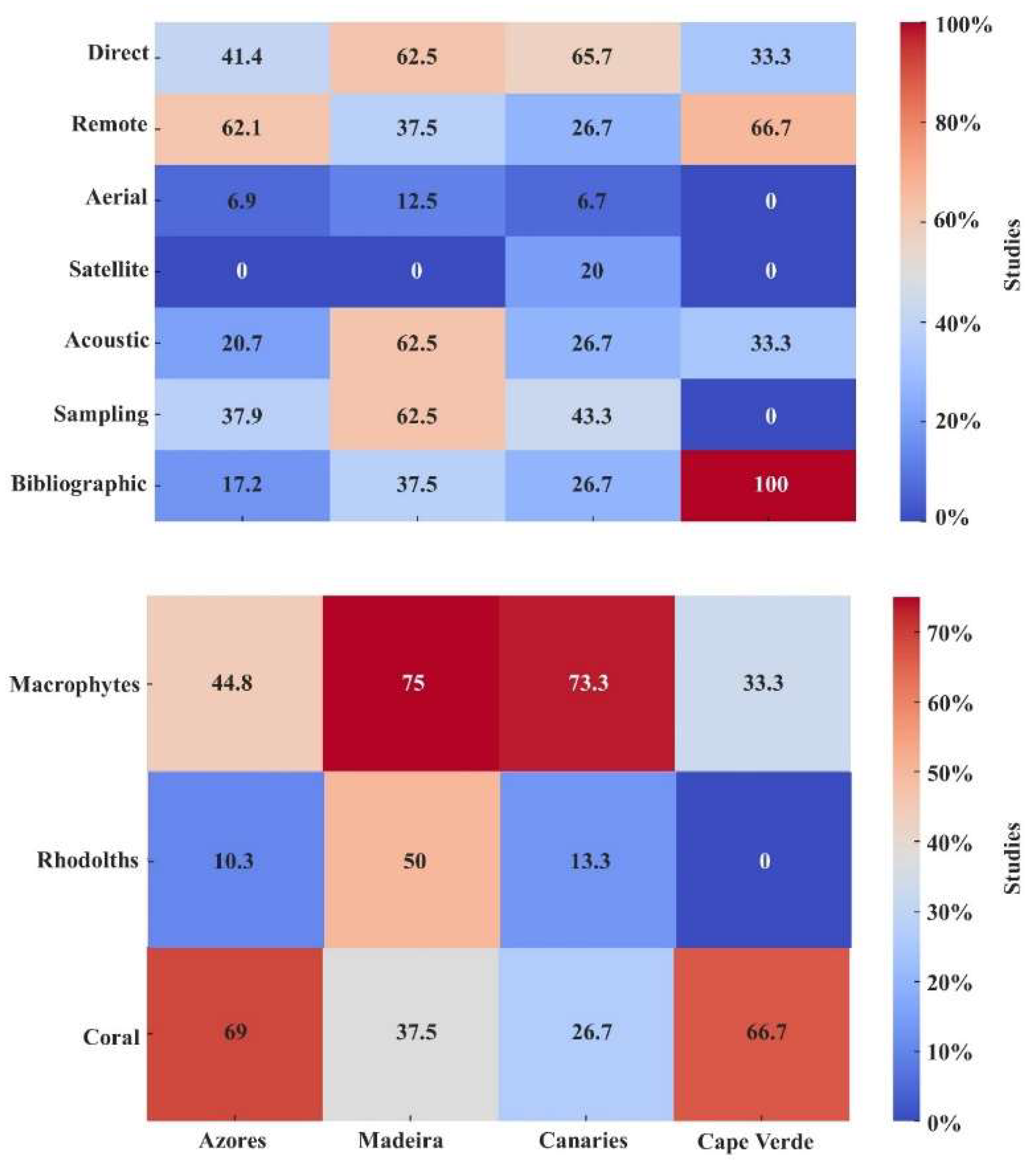
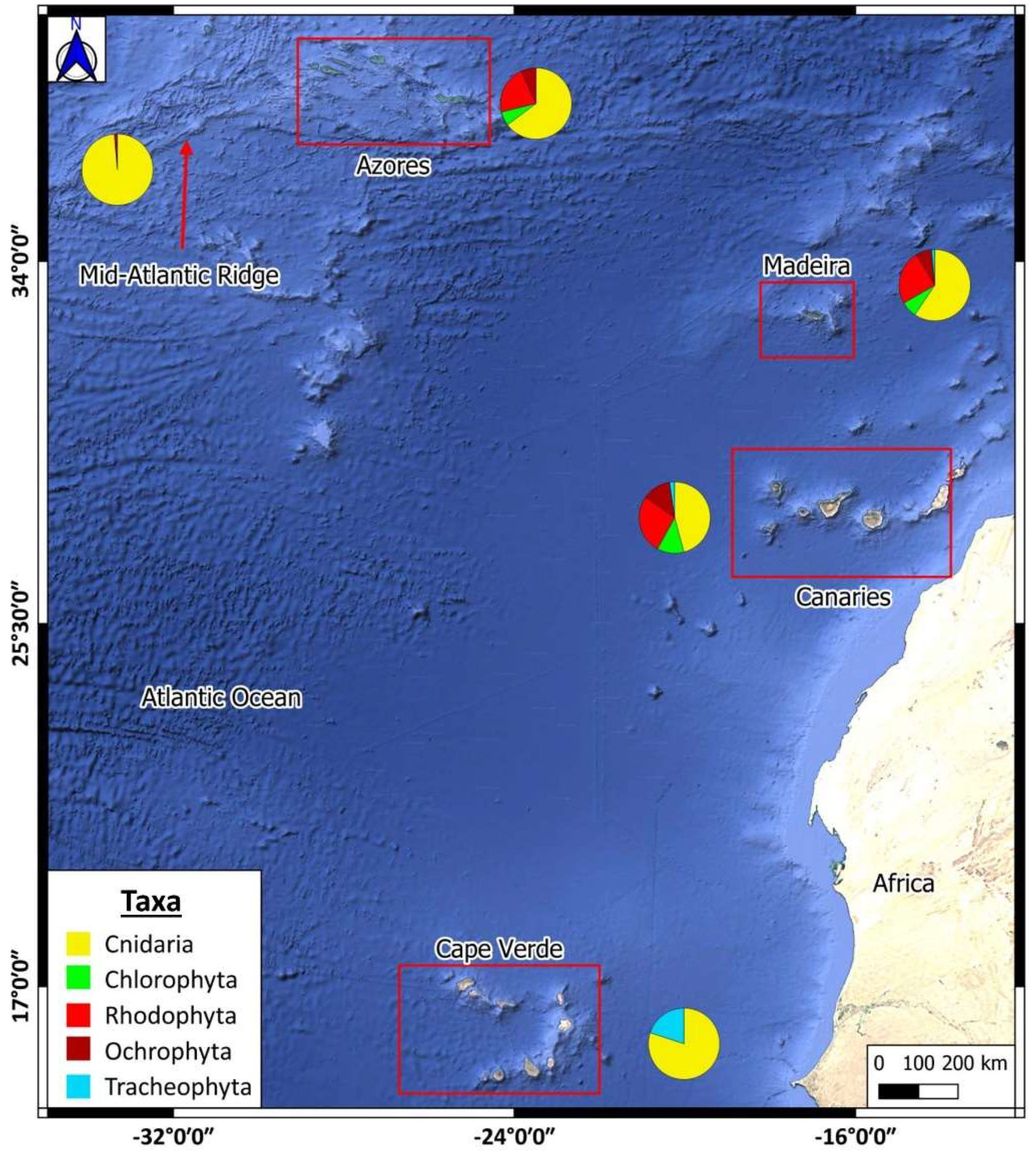
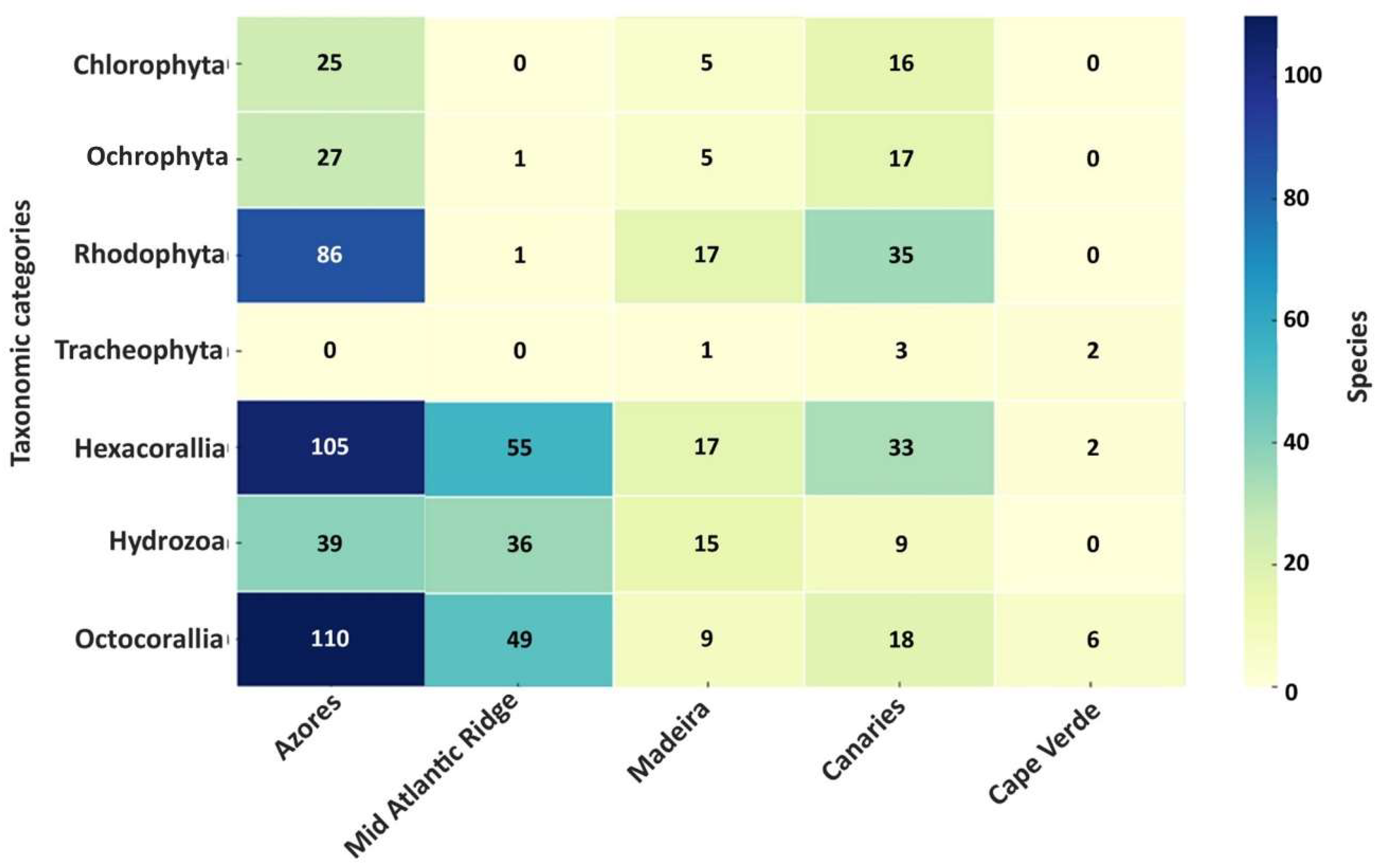
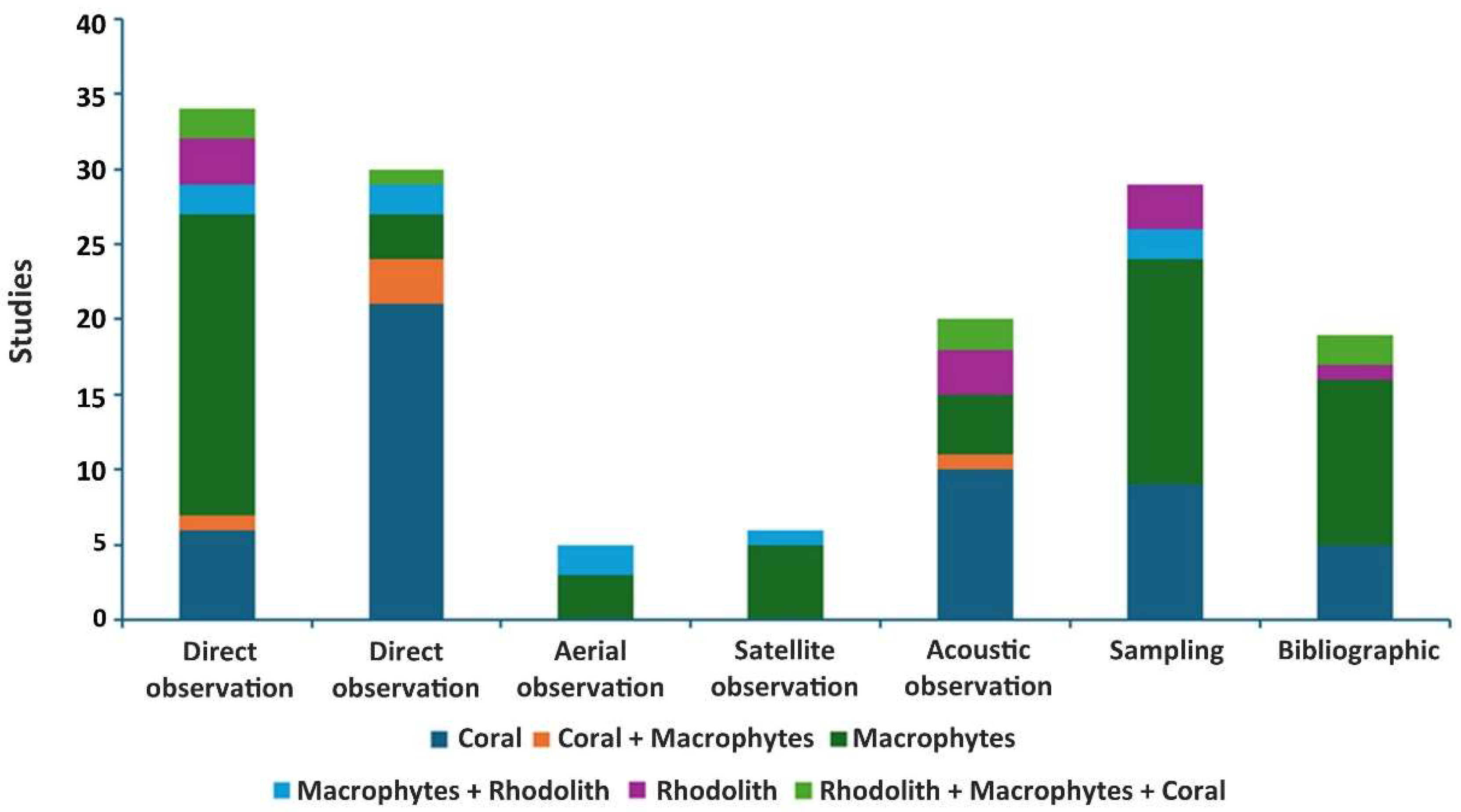

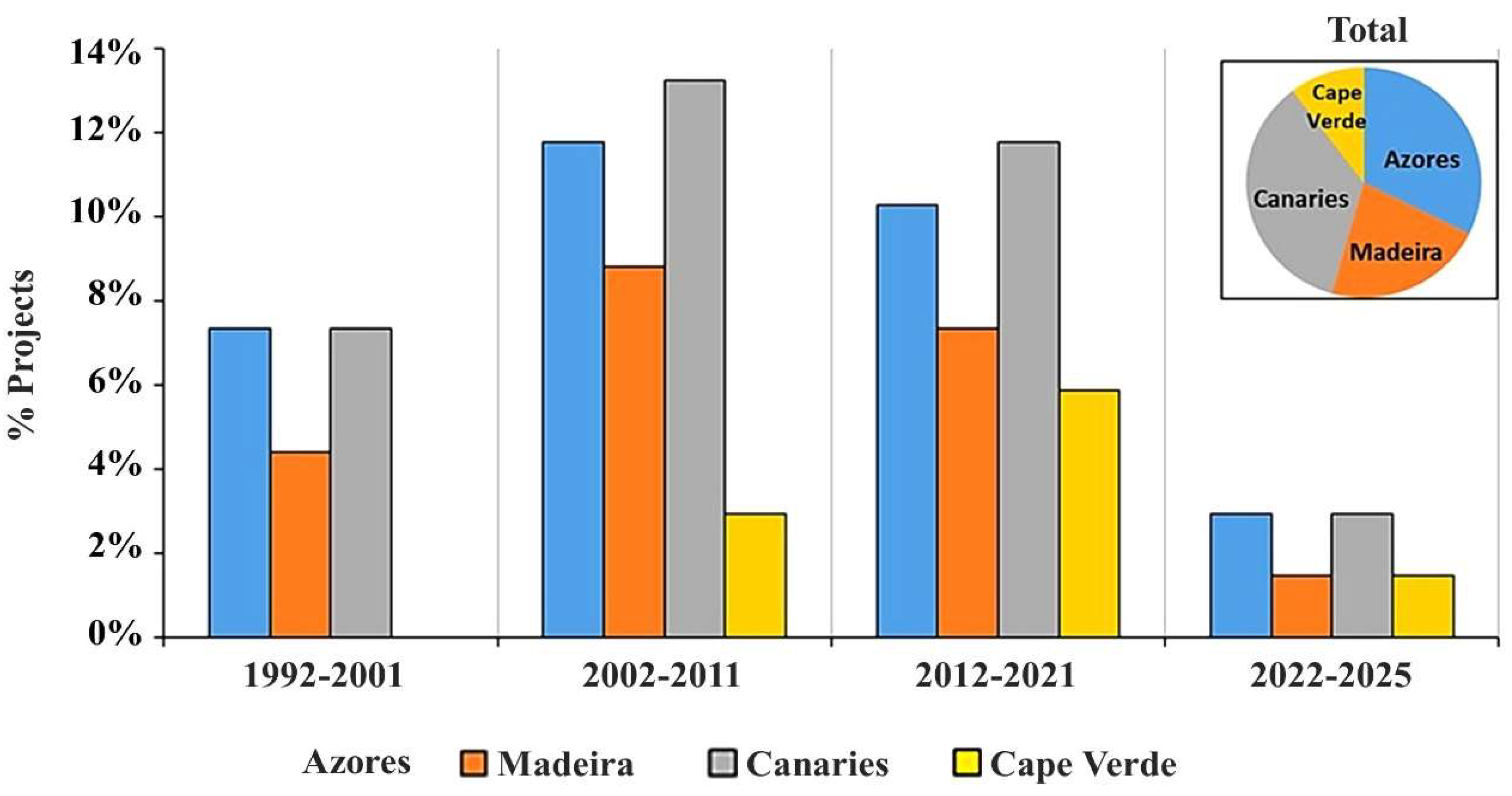
| Area | Target | Habitat / Species | |
| Macaronesia | Acoustic | Cartography | Zostera |
| Webbensia | Aerial | Coastal Ecosystem Services | Aquatic Vegetation |
| Azores | Backscatter | Conservation | Black Coral |
| Açores | Bathymetric | Distribution | Coral |
| Madeira | Drone | Geospatial Data | Eelgrass |
| Wild Island | GIS(1) | Habitat | Forest |
| Islas Salvajes | Hydroacoustic | Habitat Connectivity | Garden |
| Ilhas Selvagens | Hydroacoustic Survey | Habitat Mapping | Halodule |
| Desertas Islands | LIDAR(2) | Imaging | Halophila |
| Islas Desertas | Multispectral Imaging | Map | Maerl |
| Canary | Radar | Mapping | MAF(5) |
| Canarias | Remote Sensing | Marine Environmental Assessment | Nodule |
| Canaries | Satellite | Marine Flora Conservation | Rhodolith |
| Canary Islands | Scanning | Marine Flora Mapping | Seagrass |
| Islas Canarias | Side Scan Sonar | Marine Habitat Protection | Seaweed |
| Cabo Verde | Sonar | Marine Protected Areas | |
| Cape Verde | Sonic | MBES | |
| Sound | Monitoring | ||
| Spatial Mapping | MPA(4) | ||
| SSS(3) | Seabed Mapping | ||
| Telemetry | Spatial Analysis | ||
| Thermal Imaging | Multibeam | ||
| |
| Parameter | Option / Detail |
| Mapping techniques | Direct (SCUBA, in-situ observations, etc.) |
| Remote (ROVs, submersibles, etc.) | |
| Aerial (drones, airborne sensors, etc.) | |
| Satellite (MODIS, etc.) | |
| Acoustic (side scan sonar, multi-frequency sonar, etc.) | |
| Sampling (dredges, corers, nets, CTD Rosette, traps etc.) | |
| Bibliographic studies | |
| Study area | Archipelago id (Azores, Madeira, Canary Islands & Cabo Verde) |
| Year of the study | Year in which the research was conducted |
| Main objective | Exploratory (generation of original data) |
| Monitoring (tracking variables over time) | |
| Mapping (mapping of habitats and distributions) | |
| Bibliographic review (analysis of existing literature) | |
| Postprocessed maps | Type (Habitat and/or geological map) |
| Habitat type | Macrophyte beds |
| Rhodolith beds | |
| Coral reefs or gardens | |
| Depth | Maximum depth range recorded |
| Substrate type | Hard / Soft / Mixed |
| Data modelling | Use of advanced statistical algorithms |
| Cartographic data analysis | Geographic Information Systems (GIS) |
| Bathymetric or acoustic analysis | |
| Image analysis and computer vision | |
| Statistics and modeling (including ML) | |
| Taxonomic or morphological approaches | |
| Mapped zones area | Reported area (when available) |
| |
| Parameter | Option / Detail |
| Taxonomy | Revised taxonomic classification in WORMS and AlgaeBase |
| Number of studies | Number of studies in which the species appears |
| Number of archipelagos in which its presence is documented | |
| Zone | Archipelago id (Azores, Madeira, Canary Islands & Cabo Verde) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





