1. Introduction
The significant diffusion of thermally processed canned food (especially sterilized) is justified by the high food safety of this preservation technology, combined with good nutritional and organoleptic quality and low costs [
1,
2].
The first and second Bigelow laws [
3] describing the influence of temperature and time on the death of the microbial population and on the alteration of constituents such as vitamins, enzymes and proteins, have given rise to the so-called general method for calculating the thermal process. It consists in experimentally measuring the temperature-time curve (also called heat penetration curve) of the critical point, i.e. the coldest point and in proceeding graphically or numerically to the calculation of the process lethality
F by repeating the experiment until this process lethality reaches the desired value that ensures the commercial thermal death of the microbial population. The result will be the heating time of the canned food (steam-on in the retort). Over time the general method has been subject to improvements [
4,
5,
6,
7], and an optimization [
8].
However, the general method, certainly accurate, is long, repetitive and expensive precisely because it has no predictive capacity. Only by adding the non-steady state heat transfer equation that describes heat penetration into the mass of canned food, a faster and less expensive predictive method can be made.
The first author to propose this approach was Ball [
9] and his method took the name of original formula method. This method has also been refined over time with the contribution of Ball and Olson [
10] and expanded by Stumbo [
11]. The contributions of Hemdon et Al. [
12], Griffin et Al. [
13,
14], Hayakawa [
15], Larkin [
16], Steele and Board [
17] and Larkin and Berry [
18] should also be noted. However, Smith and Tung [
19] have carried out a comparative evaluation, through which Ball's method continues to provide the most accurate estimate in all the different conditions of food thermal processes.
Numerical methods for the calculation of thermal processes have also been proposed in the literature [
20,
21,
22,
23,
24] as well as reviews dedicated to the development of mathematical procedures for thermal process calculations are available [
25,
26,
27,
28].
More recently, works have appeared in literature in which the calculation of thermal processes is based on computational thermo-fluid dynamics (CFD) modelling. The use of CFD methods has proven to be an interesting tool [
29,
30,
31,
32]. However, their use requires high computing power and, in any case, long computing times [
33]. Furthermore, their use requires a lot of experience (right choice of mesh, etc.), that food technologists cannot have, and an accurate knowledge of multiple input data such as the thermal diffusivity of the food, the convective heat transfer coefficient of the heating and cooling fluid and other processing conditions.
Ultimately, the formula methods are still current, and Ball's remains the most accurate, allowing the calculation of the heating time with the guarantee of the desired microbial lethality and of organoleptic and nutritional quality. However, the original Ball formula method [
10] (pp. 313-358) requires the consultation of tables and linear interpolations of his data.
First, the tabulated data are due to the presence of the exponential integral function Ei, as the solution of the differential equation obtained by combining the two Bigelow laws of thermal death with the equation of heat penetration in canned food. The exponential integral is not an elementary function, so its values must be obtained through an infinite series and are available in [
34]. The series can be truncated, thus introducing an approximation which will be acceptable if there are at least 40 terms in the equation resulting from the truncation of the series. Therefore, the use of such a long equation as an alternative to the tabulated discrete values used by Ball is not easy.
Secondly, Ball [
9,
10] described with a hyperbola the temperature-time relationship in the initial cooling period, corresponding to the lag with respect to the asymptotic behavior. The hyperbola equation combined with Bigelow's laws [
3], had forced Ball to perform a numerical and graphical integration and consequently, also in this case, Ball produced a tabulation of the results.
The data in the Ball’s tables [
10] correlate the process lethality
F (or
U) with the difference
g, between the retort temperature (steam) and the temperature in the critical point of the canned food arises at the steam-off, and other quantities such as the thermo-bacteriological quantity
z and the heating rate index
f that depends on the canned food size.
To computerize the Ball’s tables, the data contained were used by Stoforos [
35] to produce an interesting equation using nonlinear regression. With similar methods [
36,
37,
38], mathematical modelling of the Stumbo’s tables [
11] were also obtained, which are used as an alternative to the Ball’s tables.
The present work also pursues the mathematical modelling of the Ball’s tables [
10] but with a different approach than that used so far by the other authors as Stoforos which consisted in obtaining equations with the regression of the tables data.
It was chosen to retrace the procedure for integrating the differential equation for the process lethality
F, which is the combination of the two laws of Bigelow [
3] of thermal death and the equation of heat penetration. This latter presents three phases: heating with asymptotic increase in temperatures, initial cooling and cooling with asymptotic decrease in temperatures.
During this critical analysis of the Ball construction of the formula method, two changes were developed. The first step was to replace the hyperbola used by Ball to describe the initial cooling. An exponential function was chosen such that after the integration of the process lethality equation, the result was an exponential integral function, like asymptotic heating and cooling. The second step was to develop an analytical approximation of the exponential integral function Ei by obtaining two equations, one for positive domain and one for negative domain of the Ei function, which contain only elementary functions (logarithm and exponential).
As a result of these two developments, the process lethality of all three phases (heating, initial cooling and final cooling) was represented by the exponential integral function Ei and that this function had a very accurate approximate analytical representation.
Such mathematical modelling, which replaces the Ball’s tables [
10], is useful for speeding up the calculation of the thermal process and reducing the risk of error but can also be useful for automation of controlling the process by implementing the PLC systems. Furthermore, unlike other mathematical modelling obtained instead by directly approximating the data of the Ball’s tables, the approach adopted in this work lends itself to be used also to approximate tables of other methods of the formula such as that of Stumbo.
2. Materials and Methods
2.1. Breaf Review of Ball’s Formula Method
2.1.1. Kinetics of Microbial Destruction at Constant Temperature
When a food with a spoilage microbial population at the same stage of development is subjected to a lethal temperature
T, a decrease in the population occurs over time proportional to the number of microorganisms
N. The rate constant
kT (s
-1) depends on the type of microorganism, the temperature and the chemical-physical characteristics of the food. This is a microbial destruction that follows first-order kinetics:
where
N is the number of microorganisms at time
t. Equation (1) can be integrated between initial population
N0 at time 0 and a population
N at time
t as follows:
For easier graphical representation it is useful to rewrite (2) with the decimal logarithm log instead of the natural logarithm ln:
where
is defined as decimal reduction time, i.e. the time required to destroy 90% of the initial population
N0.
2.1.2. Reduction Exponent and Thermal Death Time
If an absolute final sterility is imposed, i.e.
N = 0, equation (3) shows that the total time
t at the lethal temperature
T becomes infinite. Therefore, a final number of microorganisms,
N > 0, must be accepted. This introduces commercial sterility, which in turn defines the reduction exponent
n, also called the number of decimal reductions, as follows:
Therefore, for a given food with a given spoiler microbial population, performing a thermal sterilization process is equivalent to defining the reduction exponent
n with reference to the most resistant microorganism. Consequently, the thermal death time
tT (to reach commercial sterility at that given constant lethal temperature
T) is immediately defined as:
Equation (5) is known as Bigelow's 1st law.
2.1.3. Influence of Temperature on the Kinetics of Microbial Destruction
An increase in the process temperature from
T1 to
T2 determines a decrease in the decimal reduction time
. The rate constant
follows the Arrhenius’ law regarding its dependence on temperature. Recalling the definition
and applying the Arrhenius’ law, the following equation is obtained, known as Bigelow's 2
nd law:
The quantity z is the temperature increase to be implemented to decrease the decimal reduction time by tenfold.
The combination of the two Bigelow laws (5) and (6), provides:
Since laboratory experiments have been conducted to determine the thermal death time of various microorganisms at a reference temperature of 121.1°C (250°F),
, then equation (7) gives the new thermal death time
tT for temperatures
T other than the reference temperature:
Clearly (min). If T is in °C, z-value must also be in °C.
The thermal death time at a constant reference temperature of 121.1°C (250°F), is also defined required lethality
and, based on equation (5), it results:
As mentioned above, both the values of the decimal reduction time D121.1 at the reference temperature of 121.1°C (250°F) and the reduction exponent n are known experimentally for the various microorganisms present in the food to be thermally processed. Therefore, based on equation (9), the required lethality F at a constant reference temperature of 121.1°C is easy to determine.
2.1.4. Thermal Death at Variable Temperature
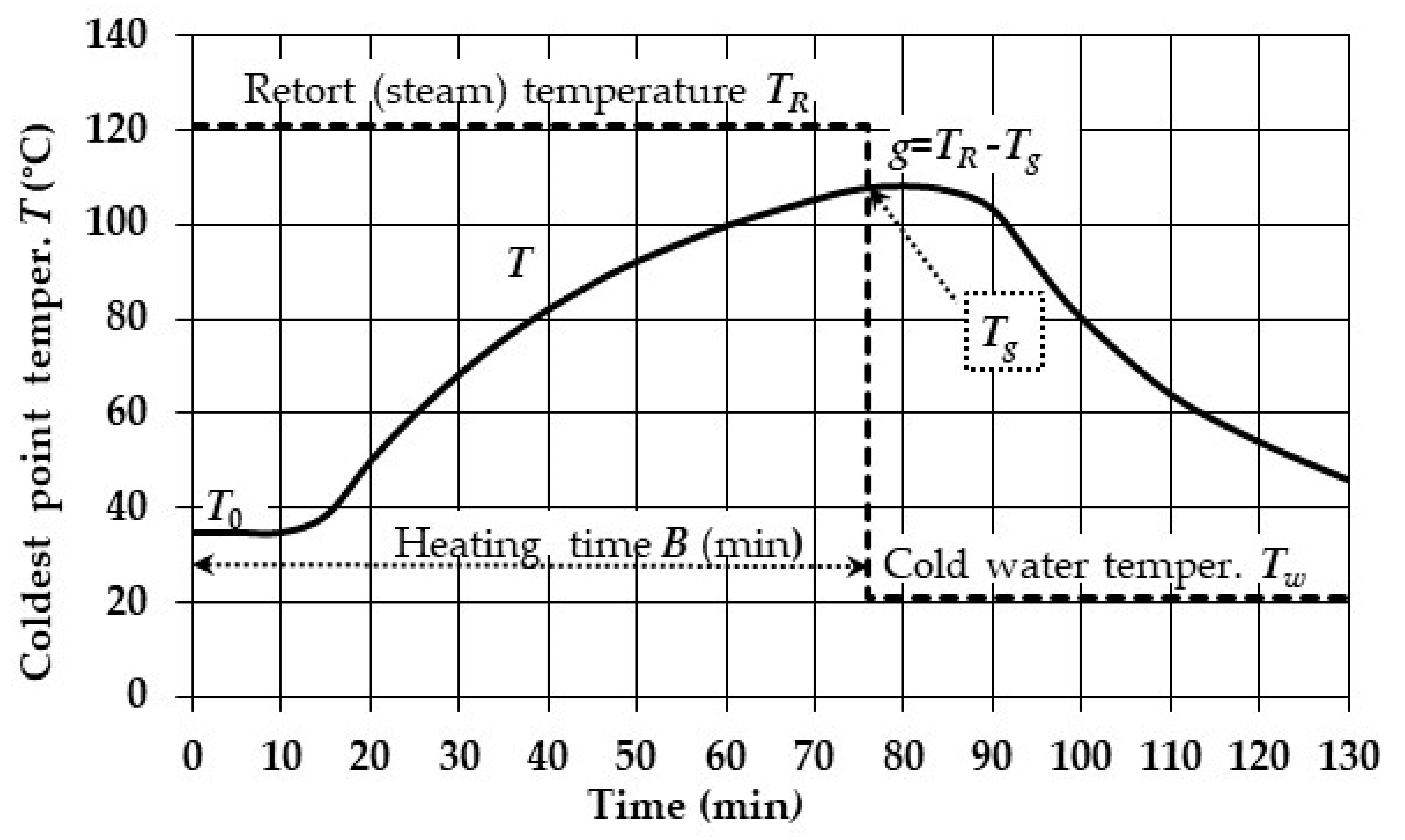
The thermal processes (sterilization, cooking, pasteurization, etc.) of canned food occur in two phases, one of heating and one of cooling during which the temperature inside the canned food varies with respect to time and volume (
Figure 1).
To overcome the problem of variable temperature
T in the food volume, Ball [
9,
10] considered only the critical point, that is, the coldest point of the canned food, often the geometric center of the volume of the package.
The variation of the temperature
T(t) with respect to the time
t at the critical point produces a variation of the decimal reduction time now called
DT, according to equation (6):
. Considering an elemental time interval
dt, during which the temperature is considered equal to
T and the decimal reduction time equal to
DT, equation (5) provides the following relationship for an infinitesimal increase in the reduction exponent:
. Integrating and using equation (6), the following relationship is obtained:
Since
D121,1 is constant, equation (10) becomes:
The left side of equation (11), according to equation (9), is the required lethality F to ensure the desired reduction in microbial population i.e. commercial thermal death. For this commercial thermal death to occur, the equality of the required lethality F with the right-hand integral must be satisfied. This integral is defined as the process lethality at variable temperature.
The solution of integral (11), which must be done for both the heating and cooling periods, requires the relationship between the critical point temperature
T of the canned food and the time
t. This relationship is also called heat penetration curve (
Figure 1).
2.1.5. Exponential Decay Heating Curve
To obtain the temperature-time relationship during the heating period (heating curve), Ball [
9,
10] considered that, after a possible initial lag which had no influence on lethality, the temperature difference between the retort and the critical point
(
Figure 1) had an exponential decay with respect to time
t:
where:
TR (°C) is the retort temperature,
T0 (°C) is the initial food temperature,
Jch is the lag factor and
f (min) is the heating rate index, i.e. the time to reduce tenfold the temperature difference between the retort steam and the critical point of the canned food.
Highlighting the time
t from equation (12) and differentiating with respect to the temperature
T,
dt is:
Combining equations (13) and (11), Ball [
10] obtained:
where:
is the difference between the retort temperature
TR and the canned food critical point temperature
Tg at the end of the heating period (figure 1);
is the lower integration limit, i.e. it is the initial temperature difference of the heating period that Ball [
10] imposed equal to 44.4°C (80°F), a value that ensures the counting of all contributions to lethality;
Fh is the process lethality during the heating period.
Adding and subtracting the retort temperature
TR to the exponent, equation (14) becomes:
The integral of which is:
where Ei is the exponential integral function. The expression
appearing in equation (16) is also indicated with the symbol
[
35]. Ball defined
Uh as the sterilizing value. When the retort temperature
TR is equal to the reference temperature 121.1°C (250°F), the sterilizing value,
Uh, coincides with the lethality
Fh. If the retort temperature
TR is higher than 121.1°C (250°F) then
and the time
tTR, i.e. the sterilizing value,
is lower than the lethality
Fh, meaning that the same result in terms of thermal death is now obtained by a time
Uh shorter than
Fh.
When
z-value is less than 15°C (26°F), then
. Therefore, equation (16) simplifies:
The Exponential Integral function Ei is not an elementary function, its values must be obtained through an infinite series and are available in tables [
34]. Ball [
9,
10] then used the tabulated values of Ei to produce his own tables with the data of some process parameters of his formula method.
2.1.6. Cooling Curve
The cooling curve starts at the steam-off point which coincides with the cold-water-on point (
Figure 1). Unlike the heating curve which has a single temperature-time relationship, namely equation (11), the cooling curve requires two equations. The first one describes the temperature during the initial period, i.e. before the temperature difference between the critical point and the cold water
undergoes an exponential decay vs time
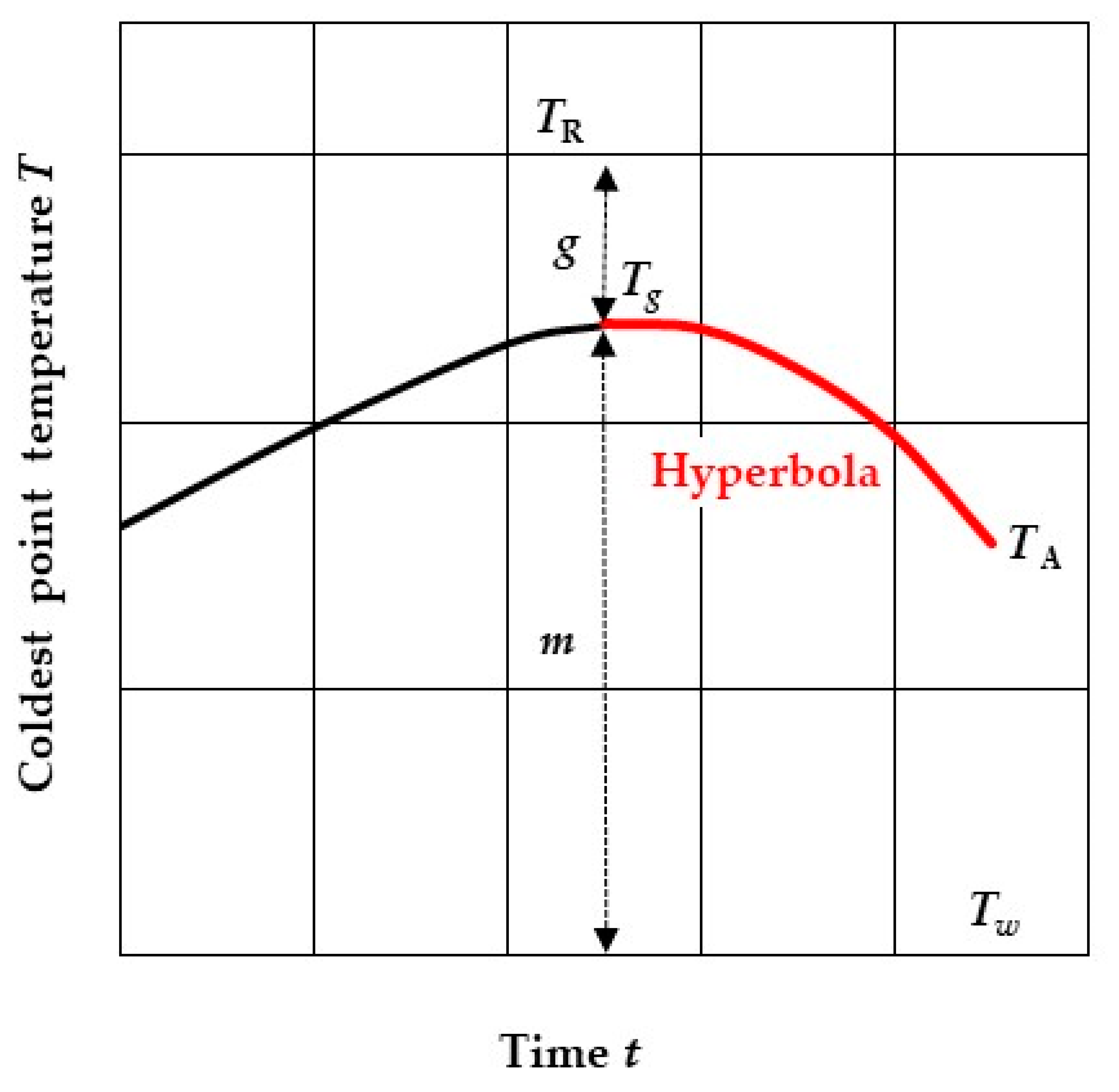
tc, and the second equation describes precisely this exponential decay. To describe the initial cooling curve, Ball, based on careful empirical evaluations, chose a hyperbola (
Figure 2) which has the following equation of temperature
T vs time
tc, where
tc has its origin at the steam-off point:
where:
Tg is the critical point temperature of the canned food at the time of steam-off; the coefficient
; the coefficient
. Ball established these two relationships under the assumption that the lag factor
Jcc during cooling is constant and equal to 1.41 and that the cooling rate index
fc (min) is equal to the heating rate index:
. The index
fc is the time to increase tenfold the temperature difference between the critical point of the canned food and the cold water.
Ball [
9,
10] established that the temperature value at the end of the initial cooling represented by equation (18) is empirically:
(
Figure 2). Ball then integrated to obtain the sterilizing value
Uic of this initial cooling:
The quantity E hides an integral that Ball had to calculate numerically and graphically [
10]. This numerical/graphical calculation of E was an additional reason to the one already reported with equation (17), which forced Ball to prepare the tables that accompany the formula method.
When the temperature
TA is reached (figure 2), cooling begins with the exponential decay of the temperature difference between the critical point and the cold water
. Like the exponential decay heating curve (2.1.5), Ball derived the equation that gives the sterilizing value of the exponential decay cooling curve
Uc:
where (
Figure 2):
is equal to the difference between the critical point temperature
Tg and the cold water temperature
Tw;
is equal to the difference between the retort temperature
TR and the critical point temperature
Tg;
is equal to the difference between the retort temperature
TR and cold water temperature
Tw. The values of the exponential integral function of equation (20) are also available in tabular form [
34], so this is the third reason why Ball was forced to prepare his tables which make the original formula method non-computerizable.
2.1.7. Tables and Formula of Ball
The sum of the sterilizing values of equations (17), (19) and (20) combined with some algebraic steps, gives:
Ball's tables have a first row with the z-values ranging from a minimum of 3.33°C (6°F) to a maximum of 15°C (26°F) and a first column on the left with the values of the f/U ratio. The range of values of this ratio are the result of the combination of the f and U that the canning industry adopts. The rest of the boxes in the tables are filled with the values of g that Ball calculated by solving equation (21) in the implicit unknown g that appears within the three sterilizing values Uh, Uic and Uc present in equations (17), (19) and (20).
Ultimately, the formula method using Ball's tables consists of: determining the value of the required lethality
F with equation (9) starting from
D121,1 and
n, known from thermo-bacteriology; calculating the sterilizing value
U with the following:
; experimentally conducting a test to detect the values of index
f (Ball assumes that the cooling rate index
fc (min) is equal to the heating rate index:
and the cooling lag factor
Jch = 1.41); calculating the ratio
f/U; entering this value into the Ball’s tables, together with the
z-value, known from thermo-bacteriology, and obtaining
; finally, calculating the heating time
B until Steam-off, applying the following Formula derived from equation (12):
where log is the decimal logarithm.
2.2. Development of an Analytical Approximation of the Exponential Integral Function Ei
Ball's tables make computerization of the formula method impossible. As mentioned above, Ball's tables are needed primarily because of the presence of the non-elementary exponential integral function Ei as a solution to the differential equation for lethality during the heating and cooling with temperature difference exponentially decaying. In fact, the Ei function requires to be represented by an infinite series. Using the nomenclature of Ball and Olson [
10], the Ei function for heating period can be described by the following series:
The series can be truncated, thus introducing an approximation, which to be acceptable requires the presence of at least 40 terms in the equation resulting from the truncated series. Therefore, the use of such a long equation as an alternative to the tabulated values that Ball used, is not at all easy. However, for values of x < 0.1, the series (23) truncated to the first three terms: , can represent the Ei function with an excellent approximation.
For the cooling period, the Ei function can instead be described by the following series:
Also in this case, what was said to be valid for the series (23) and that for x < 0.4 the series (24) can be truncated at the third term ensuring an excellent approximation of the Ei function:
By imposing approximations of the two functions Ei, described by the infinite series (23) and (24), such as to ensure an average error of about 0.1%, the first possibility is the truncation to at least 40 terms of the same series. As already said above, these are final equations that are not at all easy. Therefore, equations with few terms of elementary functions that can represent Ei functions, must be developed.
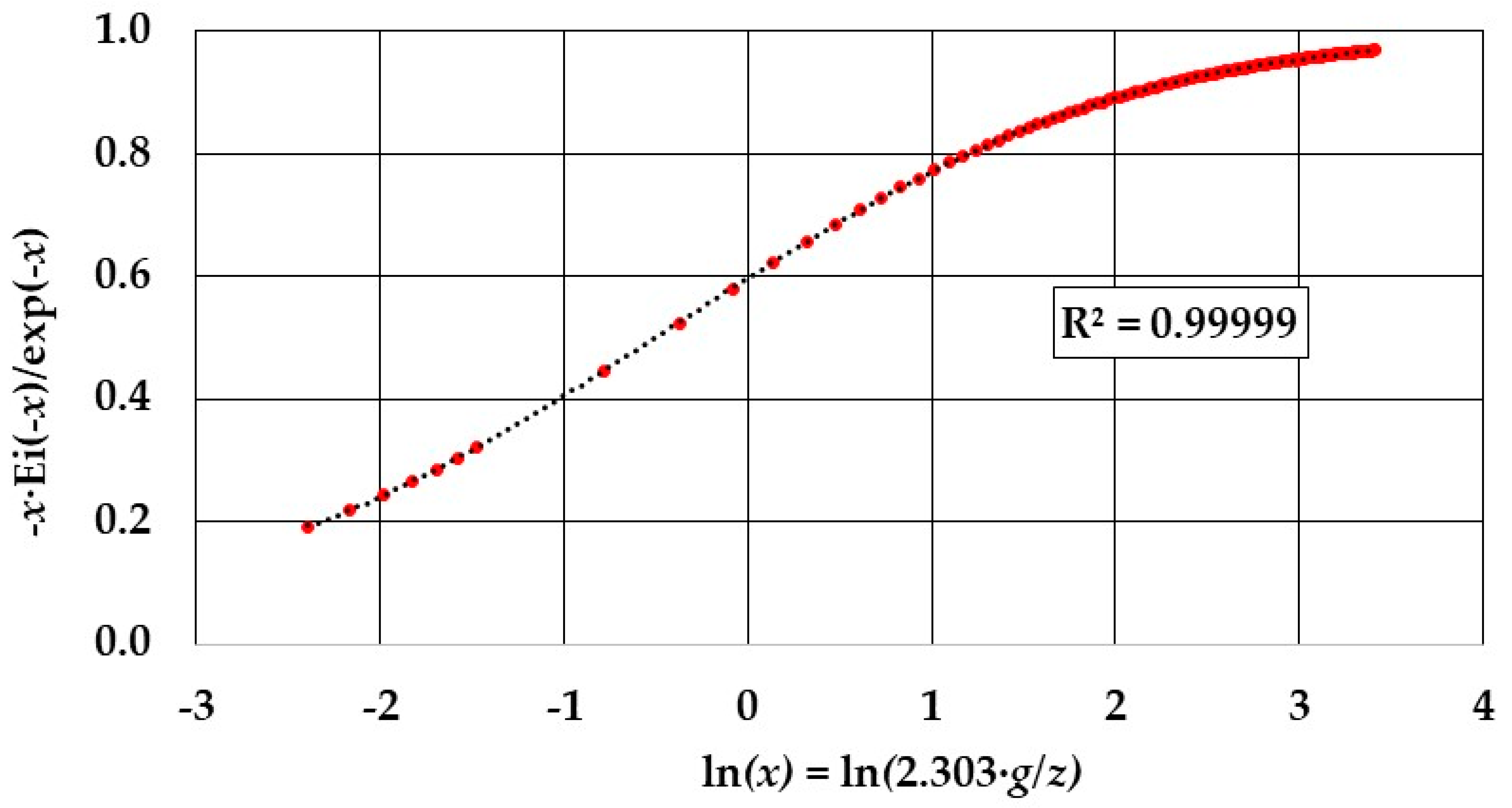
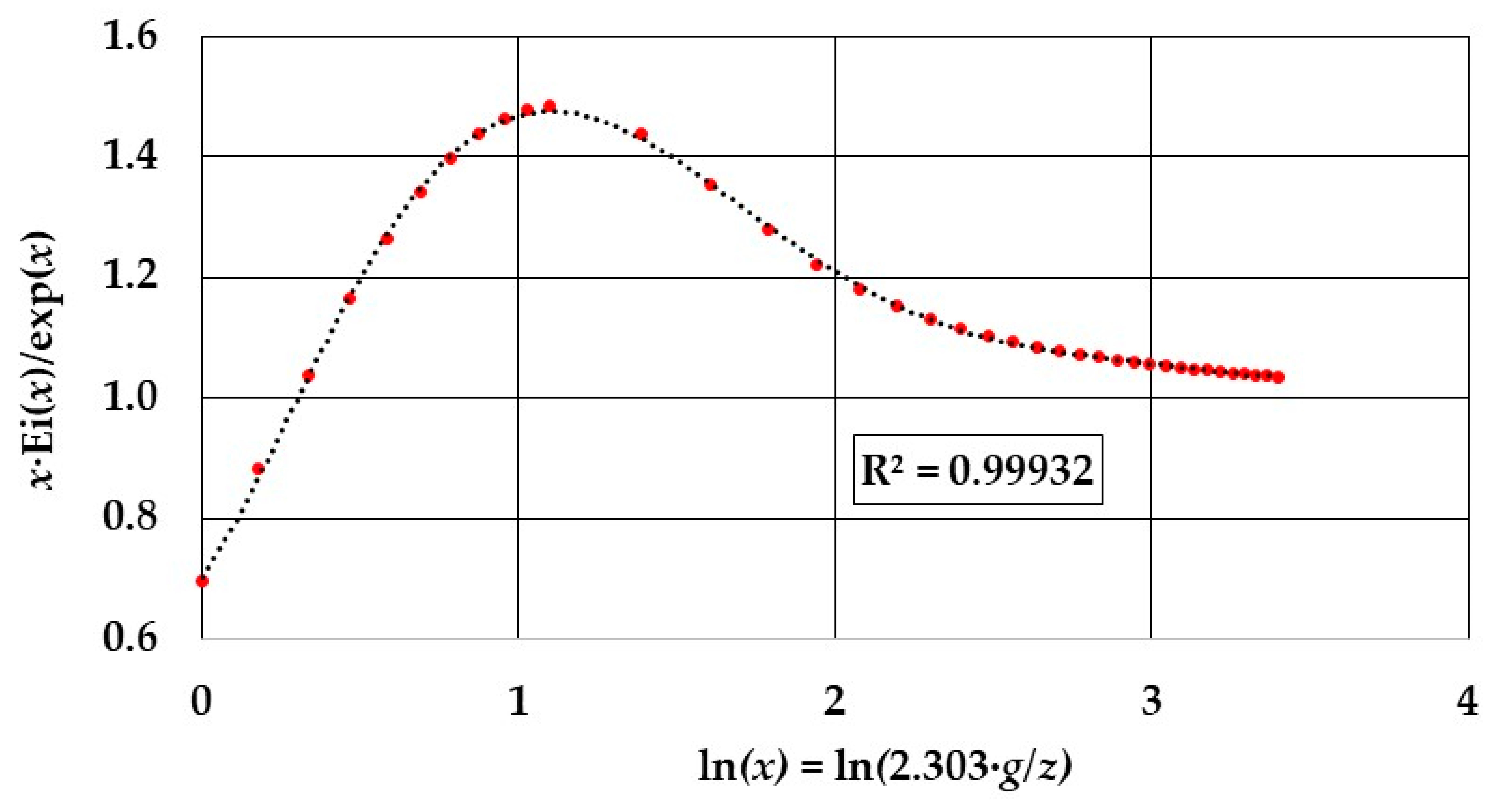
In many cases it is easier to approximate, through regression, the ratio between a function and its derivative than the function itself. For example, the ratio of a polynomial to its derivative is a linear function. Another example is provided by the ratio of the exponential function to its derivative which is the trivial function y = 1. Furthermore, the derivative of the exponential integral function Ei(x) has a very easy representation since it is the multiplication of two elementary functions, the exponential one and that of the equilateral hyperbola: .
Therefore, with the exact values of the two exponential integral functions, Ei(-
x) and Ei(
x) tabulated [
34] or downloaded from [
39] and with the values of their respective derivatives
, and
the two respective diagrams of the ratio
and
vs
were plotted (
Figure 3 and
Figure 4).
The regression (R
2=0.99999) of the curve in
Figure 3 produced the following 5° polynomial of elementary functions:
valid for
. Equation (25) provides negative values because the polynomial in square brackets representing the curve in figure 3 provides positive values, the
e-x function is positive, and the negative sign remains in front of
x in the denominator. The relative mean error is MRE = 0.05% and the standard deviation SD = 0.03%. For
, the series (23) truncated at the third term can be used:
The regression (R
2=0.99932) of the curve in
Figure 4 gave the following 6° polynomial of elementary functions:
valid for
. The mean relative error is MRE = 0.79% and the standard deviation SD = 0.54%. For
, the series (24) truncated at the fourth term can be used:
2.3. The Ball’s Formula Method in Combination with the Analytical Approximation of the Exponential Integral Function
2.3.1. Heating Curve
It is sufficient to insert into equation (17) the polynomial (25) or (26) depending on the value of .
2.3.2. Initial Cooling Curve
This is the cooling from the point at temperature
Tg (steam-off) (
Figure 2) to the point at temperature
TA. This initial cooling corresponds to the lag portion of the cooling curve that Ball approximated with hyperbola. Difficulties in solving the integral (19) analytically led Ball to perform numerical and graphical calculations and then to tabulate the results. To overcome this impossibility of a closed solution of equation (19), in this work Ball's hyperbola has been replaced by a portion of an exponential function such as the following:
where:
tc (min) is the cooling time with the origin at the cold-water-on (and steam-off);
Tg is the temperature of the critical point (coldest point of the canned food) at the steam-off;
k is determined by the condition (
Figure 2) that when
then
. Therefore, from equation (29), the quantity
k is:
During the subsequent cooling, starting from the point at temperature
TA (
Figure 2) where the temperature difference
begins to follow the asymptotic decay law, the critical point temperature follows an equation like (12) which at point A appears as follows:
where the lag factor of the cooling curve,
Jcc, was established by Ball to be 1.41. Combining the two equations (30) and (31), considering that:
the quantity
k becomes equal to coefficient
a of Ball’s Hyperbola (18):
Isolating the time
tc from equation (29), with
k = a, and differentiating, the differential
dtc is:
Combining equations (11) and (33), the process lethality during initial cooling
Fic is:
By adding and subtracting the sum
in the exponent and performing some algebraic transformations, equation (34) becomes:
The integral of which is:
The sterilizing value
Uic obtained with equation (35) is lower than that obtained by Ball with (19) due to the slightly lower exponential curve of the Ball’s hyperbola. Therefore, the coefficient
a must be reduced, dividing it by a coefficient
α that depends on
z, g and
m. The relationship,
, was found with a nonlinear multiple regression:
where
is equal to 180°F if all other quantities present (
g, m and
z) are in °F. Otherwise if
g, m and
z are in °C, then
.
Ultimately, also remembering that:
and
equation (35) becomes:
Now, it is sufficient to insert into equation (35) the polynomial (25) or (26) depending on the values of and respectively .
2.3.3. Exponential Decay Cooling Curve
It is sufficient to insert the polynomial (27) twice into equation (20), first with and then with .
The temperature of 44.4°C corresponds to 80°F, therefore the number 44.4 is fine if all the temperatures, m, g and z, in the equation are in °C, but if 80 is used then all the temperatures, m, g and z, must be in °F.
2.4. The Proposed Mathematical Modelling and Stoforo’s Modelling for a Comparison
In place of reading the Ball’s tables to obtain f/U with respect to g and z and the need to interpolate the data contained therein, the following mathematical modelling, just developed, can be used. It consists of the equation (21), into which the results of the three equations, (17), (20) and (37) are inserted. In equation (17) the result of equation (25) or (26) must be inserted, in equation (20) the result of equation (27) must be inserted, in equation (37) the results of equations (25) and (36) must be inserted.
To evaluate the results obtained by applying the modelling just proposed, the Stoforos’ modelling [
35] was taken as a benchmark, which is the most accurate and recent available in literature. It consists of an algebraic equation resulting from a non-linear regression made directly on the pairs of values (
g, f/U) as the
z value varies:
where the eight numerical constants
a1 to
a7 and
zc have values that depend on
m+g. Stoforos provided two sets of values for the eight constants, one for
m+g of 100°C (180°F) and the other for
m+g of 72.2°C (130°F), noting that for intermediate values of
m+g, interpolation is necessary.
Ball's tables present
f/U data down to a minimum
g of 0.055°C (0.1°F). Ball also suggested that for
g<0.055°C (
g<0.1°F), the corresponding
f/U value should be calculated with the following relationship, also used by Stoforos in his mathematical modelling, where
z and
g are expressed in °F:
Instead, the mathematical modelling proposed in this work does not require different coefficients for m+g of 130°F compared to 180°F and does not require interpolations for values of m+g different from 180 or 130°F because equations (17), (20), (21), (25), (27), (36) and (37) are valid for any value of m+g.
Furthermore, for values of g < 0.1°F the same equations maintain their ability to calculate the value of f/U up to g = 10-8 °F with a maximum relative error vs Ball's relationship (39) equal to 1.5%. It is only necessary not to use equation (36) that provides the coefficient α, because, with g < 0.1°F, α is simply equal to 1.
Finally, equations (17), (20), (21), (25), (27), (36) and (37) of the mathematical modelling can be used indifferently, with all the temperature differences present in the equations, i.e. , , and z-value, in °C, or with all the temperature differences and z-value in °F.
3. Results and Discussion
Equations (17), (20), (21), (25), (27), (36) and (37) of the mathematical modelling were used to calculate the values of
f/U by inserting the values of
g read on the two Ball’s tables, the first for
m+g=100°C (180°F) and the second for
m+g=72.2°C (130°F). From both tables all the
z-values were considered, excluding the
z-value equal to 3.3°C (6°F) because this value was excluded by Stumbo [
11] as unlikely in practice. For each
z-value (from 26°F up to 8°F) the rows of the tables involved in the comparison were 38 out of a total of 53 on average. The choice included all the rows from 20 up to 500 of
f/U-values, while for
f/U between 0.6 and 17.5, fifteen rows were alternately excluded because they were considered non-essential. The total number of data points was therefore 375 for each of the two tables and therefore for each of the two values of
m+g.
The values of f/U obtained from the equations of this work were compared with those of the Ball’s tables to obtain the mean relative error and the standard deviation SD (%) for each z-value.
Stoforos’ mathematical modelling was also used to calculate the f/U values and to calculate vs Ball’s tables, the MRE and SD values, with the same m+g values, the same z values and the same data points.
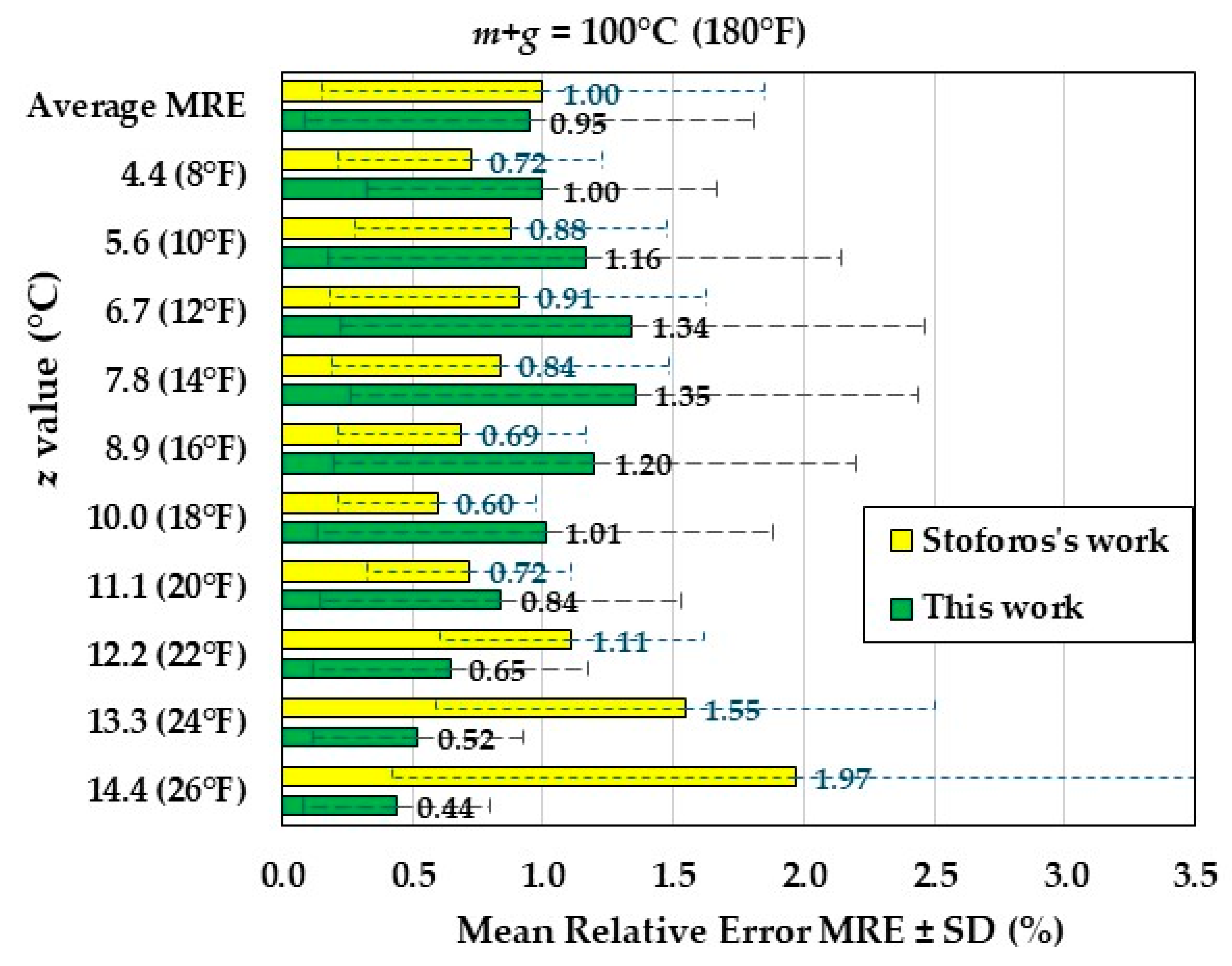
The MRE values for
m+g = 100°C (180°F) of both this work and the Stoforos' work are shown in
Figure 5. The maximum MRE occurs for
z = 7.8°C (14°F) with a value of 1.35% ± 1.09%, while with the Stoforos' equations the maximum MRE occurs for
z = 14.4°C (26°F) with a value of 1.97% ± 1.55%. The average MRE, calculated for all
z-values , was 0.95% ± 0.86%, while with the Stoforos' equations the average MRE was 1.00% ± 0.85%. Therefore, the average MRE of the two modellings have almost equal values.
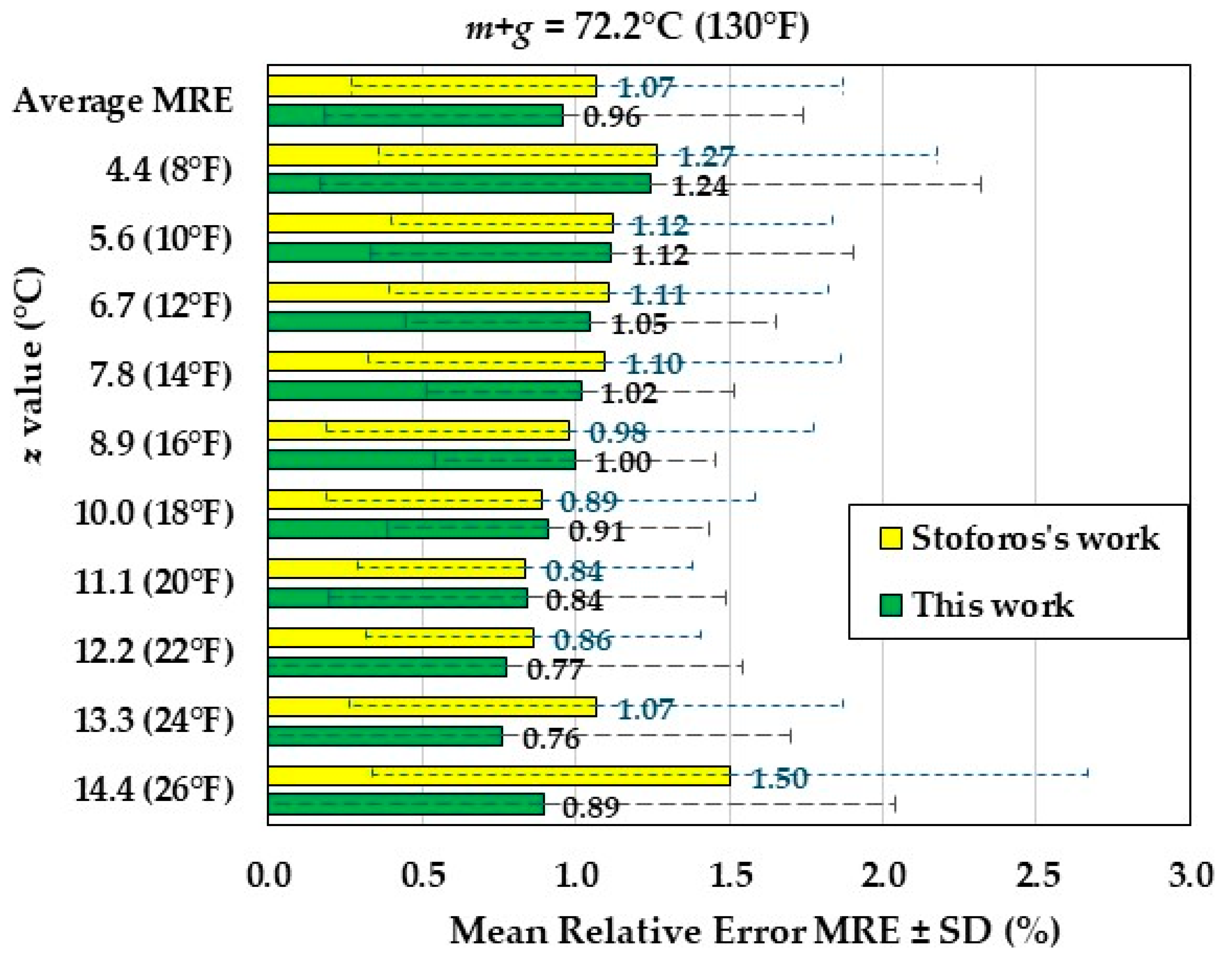
The MRE values for
m+g = 72.2°C (130°F) of both this work and the Stoforos’ work are shown in
Figure 6. The maximum MRE occurs for
z = 4.4°C (8°F) with a value of 1.24% ± 1.08%, while with the Stoforos’ equations the maximum MRE occurs for
z = 14.4°C (26°F) with a value of 1.50% ± 1.16%. The average MRE, calculated for all
z-values, was 0.96% ± 0.78%, while with the Stoforos’ equations the average MRE was 1.07% ± 0.80%. Therefore, also for
m+g = 72.2°C (130°F) the average MRE of the two modellings have almost equal values.
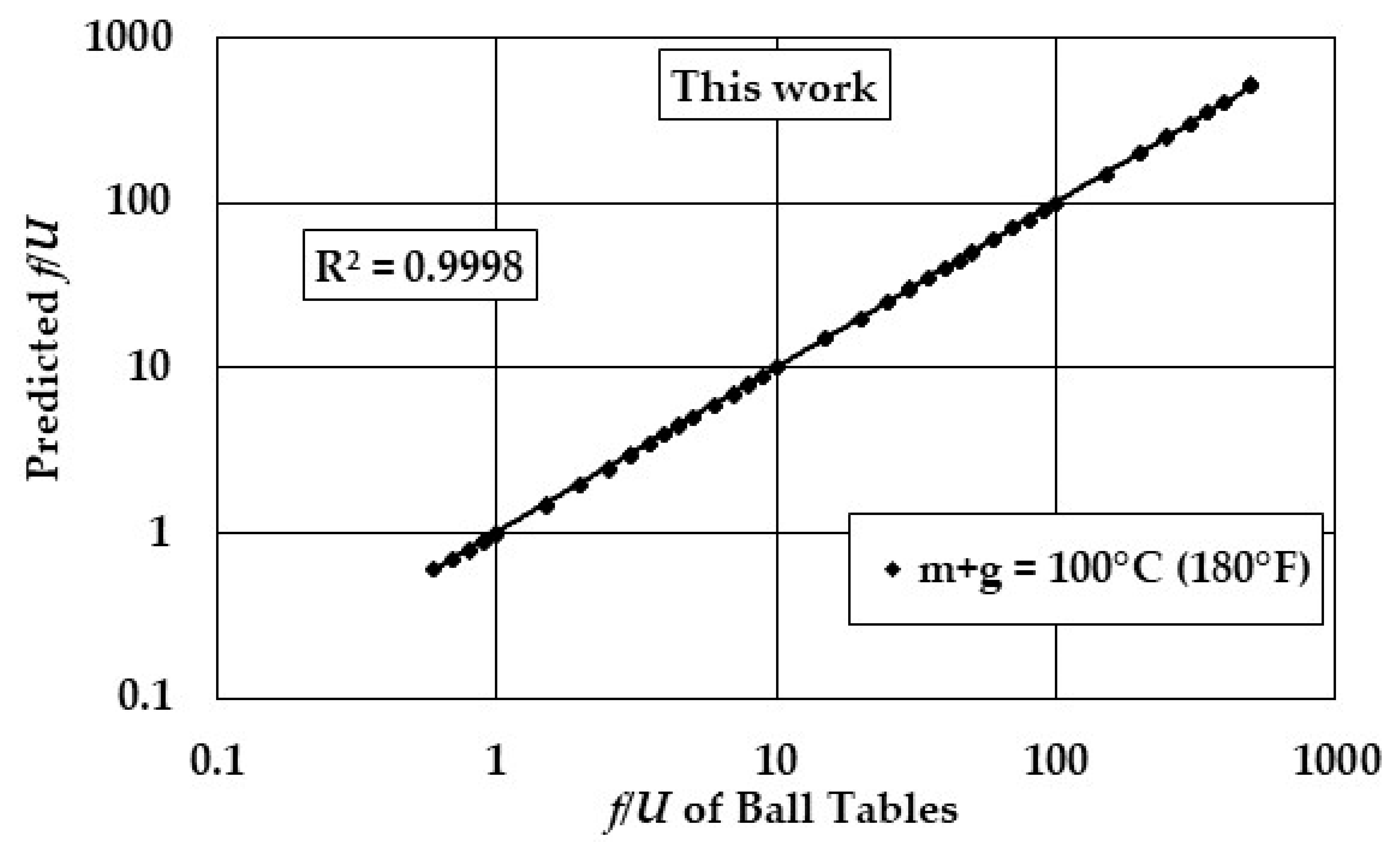
For further information, the same
f/U values, for all
z-values predicted by the mathematical modelling of this work were compared with those of Ball’ tables in
Figure 7 and
Figure 8 for
m+g of 100°C (180°F) and 72.2°C (130°F) respectively. The two diagrams present, in addition to the data, also the regression line with R
2 of 0.9998 and 0.9996 respectively, values that confirm the high accuracy of this mathematical modelling.
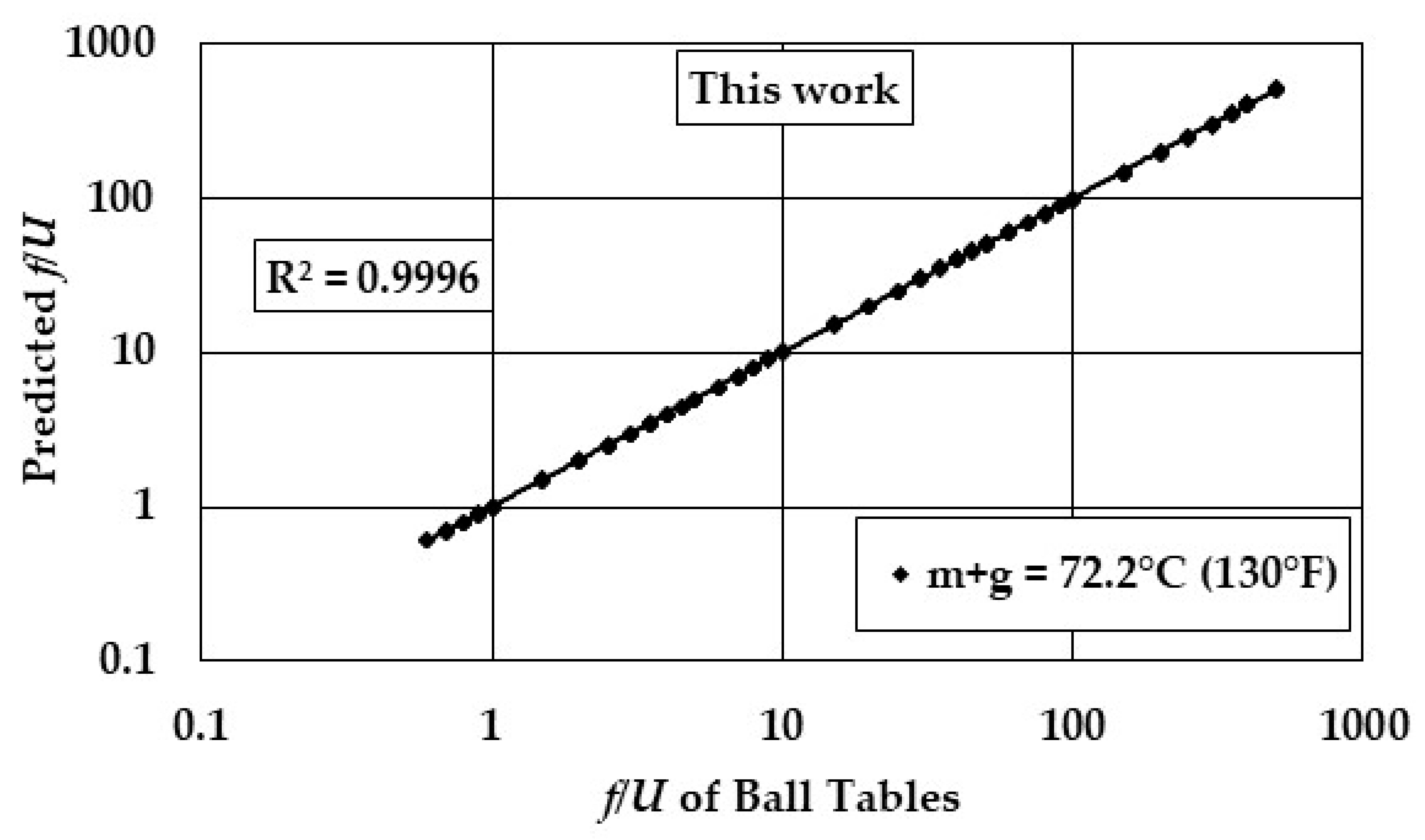
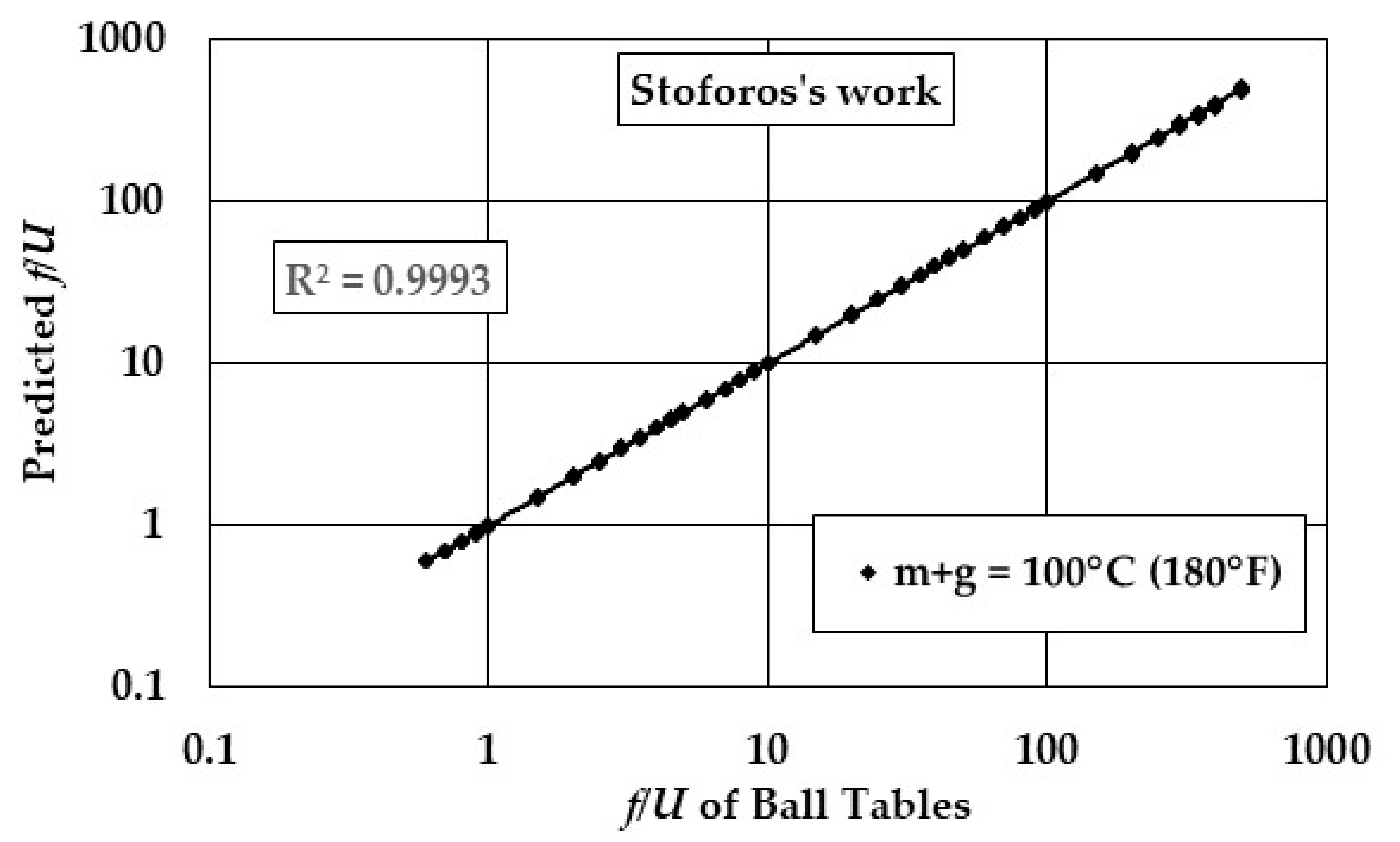
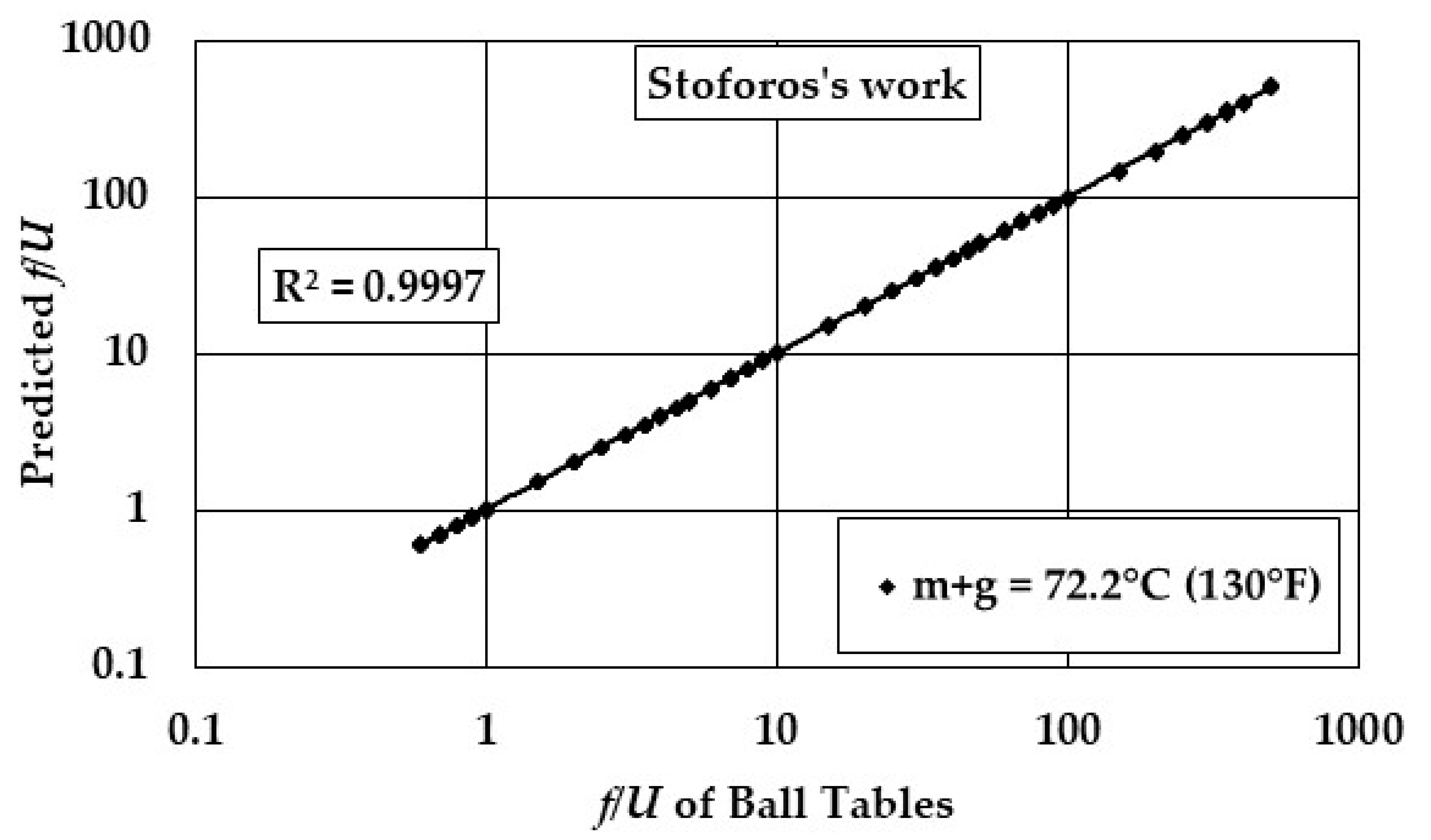
The
f/U values predicted by the Stoforos' mathematical modelling were also compared with those of the Ball’s tables in
Figure 9 and
Figure 10, respectively for
m+g of 100°C (180°F) and 72.2°C (130°F). In this case the regression line produced R
2 of 0.9993 and 0.9997 respectively, values very similar to those in
Figure 7 and
Figure 8, which also confirm the high accuracy of the Stoforos' modelling.
In summary, the differences in the results of the
f/U calculation between this mathematical modelling and the Stoforos’ one, are minimal, with a slight increase in accuracy in favor of the one proposed here. Stoforos' equations (38) and (39) are only two, so his method of calculating
f/U is easier. Furthermore, equation (38) can be used in a mirror manner to obtain
g with respect to
f/U, although eight new values for the constants
a1-a7 and
zc must be used: values that Stoforos proposed [
35].
The equations of the method proposed here are more numerous and therefore the algorithm is more laborious, but they are of general nature. They do not require new values of the constants when the value of
g+m is changed and when
g < 10
-1 °F. In fact, they are valid for
g up to 10
-8 °F. It was the nonlinear regression of the exponential integral function and not the regression of the data from the Ball tables that made the equations in this work general in nature. For this same reason, the equations could also be used to simulate Stumbo’s tables [
11] where
z extends up to the value of 100°C (180°F), simply by modifying only equation (36).
4. Conclusions
To overcome the long and tedious procedure of calculating food thermal processes provided by the general method, the so-called formula methods have been established since the second half of the last century.
Furthermore, in the last 20 years studies have been carried out to use computational thermo-fluid dynamics (CFD). This approach seemed interesting, but it requires high computing power, long calculation times and considerable experience in the use of CFD that food technologists working in the canning industry do not have. The use of CFD also requires accurate knowledge of multiple data such as the food thermal diffusivity, the convective heat transfer coefficient during the heating and cooling, ecc.
Therefore, the formula methods are still very current. Among these, Ball's original remains the most used for its accuracy and safety. The dark side of the original Ball’s formula method is in the need to consult tables that Ball prepared and, in the need to make linear interpolations of the table data. This is due first to the impossibility of an analytical solution of the differential equation resulting from the combination of the bacteriological laws on thermal death and the hyperbola of temperature as a function of time adopted by Ball to describe the first cooling phase of canned food. Secondly, and regarding heating and cooling where both follow the exponential decay law, the solution presents the exponential integral function which, being non-elementary, is available in an exact way only with the relative tables.
A mathematical modelling that replaces Ball tables would be of great use to speed up the thermal process calculations and to automate the process control through implementation in PLC systems. Several proposals have been made in this direction, among which the Stoforos’ one stands out for its accuracy and simplicity. It is based on an algebraic equation obtained directly from the data of the tables with regression/optimization methods. The algebraic equation must be completed by knowing the values of the eight numerical coefficients it contains. Unfortunately, these coefficient values are not constant but depend on the difference in temperature of the steam for heating TR and that of the water for cooling Tw: .
Therefore, Stoforos provided two sets of values of the eight constants, one for m+g of 100°C (180°F) and the other for m+g of 72.2°C (130°F), pointing out that for intermediate values of m+g it is necessary to proceed with an interpolation or accept approximations that, if they favor microbiological safety on the other hand produce over sterilizations.
To overcome these limitations, trying to maintain the high accuracy of the results of the Stofors’ modelling, in this work mathematical modelling of the Ball’s tables was found with a different approach. Instead of relying on the data of the Ball’s tables to obtain equations through regression/optimization, the integration procedure of the three differential equations for the process lethality F was retraced, one for each of the three period: heating with asymptotic temperature increase, initial part of cooling and cooling with asymptotic temperature decrease.
The first step was to develop an analytical approximation of the exponential integral function Ei by obtaining two polynomial equations, one for positive values and one for negative values of the Ei function domain, which presented only elementary functions (logarithmic function and exponential function). The second step was to replace the hyperbola of the initial cooling adopted by Ball, with an appropriate exponential function. In this way, the result of the integration of the process lethality equation contained the exponential integral function similarly to what happens in heating and cooling with asymptotic decay.
The resulting set of equations allowed the calculation of the process lethality F, then the sterilizing value U and finally the dimensionless ratio f/U. Then the values of this ratio were compared with those of the two Ball tables, obtaining, for the first table relating to m+g = 100°C (180°F), the mean relative error and the standard deviation MRE ± SD of 0.95% ± 0.86%, and for the second table relating to m+g = 72.2°C (130°F) a MRE ± SD of 0.96% ± 0.78%. Similarly, it was done using the equations proposed by Stoforos, obtaining respectively MRE ± SD = 1.00% ± 0.85% for m+g = 100°C and MRE ± SD = 1.07% ± 0.80% for m+g = 72.2°C. Therefore, the mean relative errors were almost equal with a slight improvement by the equations of this work.
In terms of ease of calculation, the Stoforos’ method is better because it has fewer equations and furthermore, they can be used formally to obtain the temperature difference g necessary to calculate the heating time B. However, the equations to calculate this g require to use a new series of eight values of the coefficients present in them.
Ultimately, the equations of the method proposed here are more numerous and therefore the algorithm is more laborious, and to obtain the value of the quantity g that is implicit, an iterative method of a spreadsheet must be used. However, the equations have a much more general character because they are the result of non-linear regressions of the exponential integral function and not of the data tabulated by Ball. That is, they do not need new values of the coefficients when the conditions are changed such as the value of m+g, or when the quantity g should be less than 10-1 °F.
Finally, the method of the formula with the Stumbo tables is also widely used in the canning industry. These Stumbo's tables were obtained in a similar way to those of Ball but present values of z that extend up to 100 °C (180 °F). Therefore, as the equations of this work are usable for any value of m+g, they will also be usable for any value of z, unlike the mathematical models based on the data of Ball's tables. Such extensions of the equations will be carried out in future work.
Funding
This research received no external funding.
Data Availability Statement
The data presented in this study are contained within the article.
Conflicts of Interest
The author declares no conflicts of interest.
References
- Featherstone, S. A review of developments in and challenges in thermal processing over the past 200 years – A tribute to Nicolas Appert. Food Res Int 2012, 47(2), 156-160. [CrossRef]
- Mafart, P. Food engineering and predictive microbiology: on the necessity to combine biological and physical kinetics. Int J Food Microbiol 2005, 100, 239-251. [CrossRef]
- Bigelow, W.D.; Bohart, G.S.; Richardson, A.C; Ball, C.O. Heat Penetration in Processing Canned Foods, Bulletin 16-L Res, 1st ed.; Laboratory National Canners Associated: Washington, USA, 1920; pp. 1-128.
- Ball, C.O. Mathematical solution of problems on thermal processing of canned food, Berkley: Publication Health, 1st ed.; University of California Publications in Public Health: Berkeley, 1928; pp. 15-245.
- Schultz, O.T.; Olson, F. C. Thermal processing of canned foods in tin containers. III. Recent improvements in the General Method of thermal process calculation. A special coordinate paper and methods of converting initial retort temperature. J Food Sci 1940, 5, 399-407. [CrossRef]
- Patashnik, M. A simplified procedure for thermal process evaluation. Food Technol 1953, 7(1), 1-6.
- Hayakawa, K.I. A procedure for calculating the sterilizing value of a thermal process. Food Technol 1968, 22(7), 93-95.
- Simpson, R.; Almonacid, S.; Teixeira, A.A. Bigelow’s General Method revisited: development of a new calculation technique. JFood Sci 2003, 68(4), 1324-1333. [CrossRef]
- Ball, C.O. Thermal Process Times for Canned Foods, Bulletin 37, 1st ed.; National research Council: Washington, 1923; pp. 1-76.
- Ball, C.O.; Olson, F.C. Sterilization in Food Technology, 1st ed.; McGraw-Hill: New York, 1957; pp. 1-507.
- Stumbo, C.R. Thermobacteriology in Food Processing, 2nd ed.; Academic Press: New York, 1973; pp. 1-329.
- Hemdon, D.H.; Griffin, R.C. Jr; Ball C.O. Use of computer derived tables to calculate sterilizing processes for packaged foods. Food Technol 1968, 22(4), 473–484.
- Griffin, R.C., Jr; Hemdon, D.H.; Ball. C.O. Use of computer derived tables to calculate sterilizing processes for packaged foods. 2. Application to broken line heating curves. Food Technol 1969, 23(4), 519–524.
- Griffin, R.C. Jr; Hemdon, D.H.; Ball, C.O. Use of computer derived tables to calculate sterilizing processes for packaged foods 3. Application to cooling curves. Food Technol 1971, 25(2), 134–143.
- Hayakawa, K. Experimental formulas for accurate estimation of transient temperature of food and their application to thermal process evaluation. Food Technol 1970, 24(12), 1407–1418.
- Larkin, J.W. Use of a modified Ball’s formula method to evaluate aseptic processing of foods containing particulates. Food Technol 1989, 43(3), 124–131.
- Steele, R.J.; Board, P.W. Thermal process calculations using sterilizing ratios. J Food Technol 1979, 14, 227-235. [CrossRef]
- Larkin, J.W.; Berry R.B. Estimating cooling process lethality for different cooling j values. J Food Sci 1991, 56(4), 1063–1067.
- [14] Smith, T.; Tung, M.A. Comparison of formula methods for calculating thermal process lethality. J Food Sci 1982, 47, 626-630. [CrossRef]
- Kim, K.H.; Teixeira, A.A.; Bichier, J.; Tavares, J. STERILIMATE: software for designing and evaluating thermal sterilization processes. ASAE paper No 93–4051. 1993, St. Joseph, MI.
- Noronha, J.; Hendrickx, M.; Van Loey, A.; Tobback, P. New semi-empirical approach to handle time-variable boundary conditions during sterilization of non-conductive heating foods. J Food Eng 1995, 24, 249–268. [CrossRef]
- Teixeira, A.A.; Balaban, M.O.; Germer, S.P.M.; Sadahira, M.S.; Teixeira-Neto Vitali, R.O. Heat Transfer Model Performance in Simulation of Process Deviations. J Food Sci 1999, 64, 488-493. [CrossRef]
- Holdsworth, D.; Simpson, R. Thermal Processing of Packaged Foods, 2nd ed.; Springer: Berlin, 2016; pp. 1-516.
- Miranda Zamora, W.R.; Sanchez Chero, M.J.; Sanchez Chero, J.A. (2020). Software for the Determination of the Time and the F Value in the Thermal Processing of Packaged Foods Using the Modified Ball Method. In Intelligent Human Systems Integration (IHSI 2020): Integrating People and Intelligent Systems; Ahram, T., Karwowski, W., Vergnano, A., Leali, F., Taiar, R., Eds.; Springer: Modena, Italy, 2020; Volume 1131, pp. 498-502. [CrossRef]
- Cleland A.C.; Robertson G.L. Determination of thermal processes to ensure commercial sterility of foods in cans. In: Developments in food preservation, 1st ed.; Thorne S. Ed.; Elsevier Applied Science: London, 1985; volume 3, pp. 1-315.
- Hayakawa, K.I. A critical review of mathematical procedures for determining proper heat sterilization processes. Food Technol 1978, 32(3), 59-65.
- Stoforos, N.G.; Noronha, J.; Hendrickx, M.; Tobback, P.A. Critical analysis of mathematical procedures for the evaluation and design of in-container thermal processes for foods. Crit Rev Food Sci Nutr 1997, 37(5), 411–441. [CrossRef]
- Sojecka, A.A.; Drozd-Rzoska, A.; Rzoska, S.J. Food Preservation in the Industrial Revolution Epoch: Innovative High Pressure Processing (HPP, HPT) for the 21st-Century Sustainable Society. Foods 2024, 13, 3028. [CrossRef]
- Ghani, A.G.A.; Farid, M. M. Using the computational fluid dynamics to analyze the thermal sterilization of solid-liquid food mixture in cans. Innov Food Sci Emerg 2006, 7, 55-61. [CrossRef]
- Augusto, P. E. D.; Cristianini, M. Numerical simulation of packed liquid food thermal process using computational fluid dynamics (CFD). Int J Food Eng 2011, 7(4), Article 16. [CrossRef]
- Erdogdu, F. Optimization in Food Engineering, 1st ed.; CRC Press: Boca Raton, USA, 2008; pp. 585-594. [CrossRef]
- Dimou, A.; Panagou, E.; Stoforos, N.G.; Yanniotis, S. Analysis of Thermal Processing of Table Olives Using Computational Fluid Dynamics. J Food Sci 2013, 78(11), 1695-1703. [CrossRef]
- Calomino, G.E.; Minim, L.A.; Coimbra, J.S.D.; Rodrigues Minim, V.P. Thermal process calculation using artificial neural networks and other traditional methods. J Food Process Eng 2006, 29, 162-173. [CrossRef]
- Gautschi, W.; Cahill, W.F. Exponential integral and related functions. In: Handbook of mathematical functions, 1st ed.; Abramowitz, M.; Stegun, I.A., Eds.; National Bureau of Standards: Washington, USA, 1964; Appl Math Ser 55, pp. 227-237.
- Stoforos, N.G. Thermal process calculations through Ball’s original formula method: a critical presentation of the method and simplification of its use through regression equations. Food Eng Rev 2010, 2, 1-16. [CrossRef]
- Friso, D. A new mathematical model for food thermal process prediction, Model Simul Eng 2013, 2013, 1-8. [CrossRef]
- Friso, D. A Mathematical Solution for Food Thermal Process Design, Appl Math Scie 2015, 9(6), 255-270. [CrossRef]
- Friso, D. The Check Problem of Food Thermal Processes: a Mathematical Solution, Appl Math Scie 2015, 9(113), 6529-6546. [CrossRef]
- 39. Wolframalpha. Available online: https://www.wolframalpha.com.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).