1. Introduction
The burgeoning fields of artificial intelligence (AI) and machine learning (ML) have begun to permeate various aspects of scientific and technological advancement, with their potential to revolutionize industries being increasingly recognized. One such promising area is the development and optimization of hydrogel microneedle technology, which holds significant promise in the field of transdermal drug delivery and other biomedical applications (Omidian and Dey Chowdhury, 2025). These microneedles, composed of hydrogel materials, represent a paradigm shift in drug delivery systems, offering innovative pathways for both diagnostics and therapeutics.
Hydrogel microneedles offer a non-invasive alternative to conventional hypodermic needles, thereby presenting an avenue to improve patient compliance and accessibility to medical interventions (Zhou et al., 2023). Their unique ability to painlessly penetrate the skin and deliver therapeutic agents into the dermal layer has made them a focal point in biomedical engineering. The intersection of AI and ML with hydrogel microneedle technology offers unprecedented opportunities to enhance the design, functionality, and application of these devices (Biswas et al., 2024). A review of 27 seminal research and review papers delves into the application of AI and ML strategies in this field, providing insights and proposing a comprehensive framework and roadmap for future endeavors.
The exploration of AI and ML in hydrogel microneedle technology is rooted in the need for precision, efficiency, and personalization in biomedical applications. AI and ML algorithms can process vast amounts of data, identify patterns, and make predictions that are beyond the reach of traditional analytical techniques (Boppana, 2022). This capability becomes particularly valuable in the development of hydrogel microneedles, where multiple variables such as hydrogel composition, microneedle geometry, insertion mechanics, and drug diffusion must be optimized concurrently for safety, efficacy, and biocompatibility (Abdullah et al., 2024).
The reviewed literature highlights how AI techniques such as neural networks, genetic algorithms, and deep learning models have been applied to model and predict the behavior of hydrogel materials, assess their mechanical properties, and optimize the microneedle design for specific biomedical applications. This AI-driven approach to design and prototyping facilitates rapid iteration cycles, thereby accelerating innovation and reducing developmental costs.
One of the key insights from the review is the role of AI in the material selection process for hydrogel microneedles. AI algorithms can analyze extensive databases of material properties to identify suitable candidates for hydrogel formation (Negut and Bita, 2023). This reduces the time and resources traditionally spent on experimental trial-and-error testing and accelerates the material discovery process. Machine learning models can be trained on datasets containing hydrogel properties such as swelling capacity, tensile strength, degradation rate, and drug encapsulation efficiency to predict the performance of new compositions, enabling researchers to focus only on the most promising candidates (Negut and Bita, 2023). This predictive capability is critical in developing microneedles that meet the stringent demands of biomedical use, including skin penetration ability, biocompatibility, and controlled release kinetics.
The reviewed papers also emphasize the importance of AI in optimizing the geometric design of hydrogel microneedles. The shape, height, base diameter, and tip sharpness of the microneedles play a crucial role in their ability to penetrate the stratum corneum and efficiently deliver drugs into underlying tissue layers (Qallaf and Das, 2008). AI and ML algorithms can be used to simulate the mechanical and biological behavior of various microneedle designs under physiological conditions. These simulations help identify the geometries that optimize skin insertion and drug permeation while minimizing tissue damage and patient discomfort. This design approach allows the microneedle arrays to be customized for specific therapeutic applications, such as insulin delivery, vaccine administration, or interstitial fluid monitoring.
In addition to design optimization, AI and ML strategies have been employed to enhance the manufacturing processes of hydrogel microneedles (Biswas et al., 2024). AI-based process control tools, such as reinforcement learning and evolutionary algorithms, can significantly improve the precision, scalability, and consistency of microneedle production. These algorithms help fine-tune processing parameters—such as curing temperature, molding conditions, and polymer concentrations—to ensure reproducible product quality (Biswas et al., 2024). Moreover, these techniques can reduce production costs by optimizing resource allocation, minimizing material waste, and shortening cycle times (Luciano et al., 2020). The integration of AI into manufacturing pipelines ensures that hydrogel microneedles are fabricated with the required dimensions, mechanical integrity, and drug-loading efficiency, thereby improving their reliability for large-scale deployment (Biswas et al., 2024).
The roadmap for harnessing AI and ML in hydrogel microneedle technology involves several key steps. First, the development of comprehensive datasets encompassing the physicochemical properties and performance metrics of hydrogel materials and microneedle designs is critical. These datasets should be standardized, interoperable, and made available to the research community to facilitate collaborative model training and benchmarking. Collaborative efforts between academic institutions, medical centers, and industry stakeholders are necessary to build these shared databases.
Next, the development of specialized AI algorithms tailored to microneedle applications is essential. These algorithms must be capable of integrating diverse datasets—from material science, mechanical engineering, and pharmacology—and capturing the nonlinear interactions between them (Abdullah et al., 2024). Interdisciplinary collaboration between data scientists, biomedical engineers, and clinicians is fundamental to ensure that these AI models are scientifically sound, clinically relevant, and computationally efficient (Akhtar, 2024).
Another essential pillar of the roadmap is the implementation of AI-driven platforms for real-time monitoring and quality assurance in microneedle manufacturing. These platforms can use computer vision, predictive maintenance, and anomaly detection algorithms to identify defects or deviations in the production process, ensuring that each batch of microneedles meets stringent quality standards. Such systems also facilitate continuous process improvement by integrating real-world data into feedback loops that refine manufacturing protocols over time.
However, the integration of AI and ML into hydrogel microneedle systems must also address broader ethical and regulatory challenges (Biswas et al., 2024). As AI becomes more entrenched in biomedical product development, issues surrounding algorithm transparency, data privacy, bias mitigation, and explainability become increasingly important. Regulatory frameworks must evolve to support AI-enabled healthcare technologies, ensuring that they are validated for clinical safety and efficacy (Williamson and Prybutok, 2024). Active involvement of regulatory agencies is required to develop new standards and certification pathways tailored to AI-driven microneedle technologies (Biswas et al., 2024).
Artificial Intelligence (AI) and Machine Learning (ML) have also become pivotal in broader biomedical device innovation, demonstrating remarkable capabilities in data analysis, optimization, real-time control, and personalization (Ahmed et al., 2020). These technologies are transforming the conceptualization, fabrication, and clinical application of not only microneedles but also hydrogels and biosensors (Wang et al., 2024). Microneedles, characterized by their minimally invasive approach, enable transdermal drug delivery, biosensing, and fluid extraction (Ahmad et al., 2021). Hydrogels, renowned for their tunability and compatibility with human tissue, are ideal for drug encapsulation, wound healing, and regenerative medicine applications (Xue et al., 2021). Meanwhile, biosensors integrated into wearable devices enable continuous, non-invasive monitoring of physiological markers, contributing to preventive and personalized healthcare (Vila and Wang, 2019). The role of AI in this ecosystem extends from signal interpretation and feature extraction to automated diagnosis and adaptive therapeutic response (Da Silva, 2024).
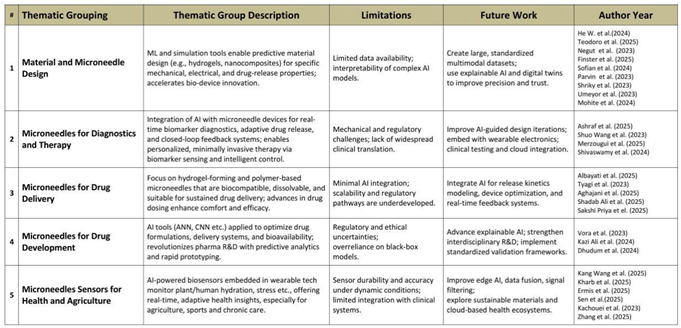
Despite the growing volume of publications in these areas, most reviews tend to focus on narrow domains or individual material classes. Such compartmentalized perspectives, while useful, often overlook the larger picture of AI integration across biomedical platforms. This umbrella review addresses that gap by synthesizing findings from 27 papers published between 2023 and 2025. It organizes insights into five thematic groupings: (1) material and microneedle design, (2) microneedles for diagnostics and therapy, (3) microneedles for drug delivery, (4) microneedles for drug development, and (5) microneedle sensors for health and agriculture.
These thematic classifications reveal research trends, identify underexplored areas, and provide a structured foundation for cross-disciplinary learning. For example, the integration of CNNs, GANs, support vector machines, reinforcement learning, and hybrid AI models such as ANN-GA and neuro-fuzzy systems have consistently demonstrated value across these domains. These tools improve accuracy in material property prediction, design validation, biosignal interpretation, and clinical decision support.
To translate these insights into action, the review introduces a novel five-pillar developmental framework called AIM-DO: (1) material discovery and optimization, (2) structural and mechanical design, (3) manufacturing process optimization, (4) biomedical performance enhancement, and (5) advanced AI integration. These pillars guide the deployment of AI technologies in microneedle development from bench to bedside. They enable predictive design, intelligent quality control, and real-time data integration, thereby supporting the broader shift toward precision medicine and adaptive healthcare.
In conclusion, this umbrella review not only consolidates current knowledge from 27 significant publications but also outlines a strategic roadmap to guide future innovation in AI-enabled hydrogel microneedle systems. It emphasizes the value of collaboration, data transparency, and regulatory foresight, while highlighting the transformative potential of AI and ML in shaping the future of transdermal drug delivery and personalized biomedical solutions.
3. Umbrella Review of Five Thematic Groupings
3.1. Thematic Group 1: Material and Microneedle Design
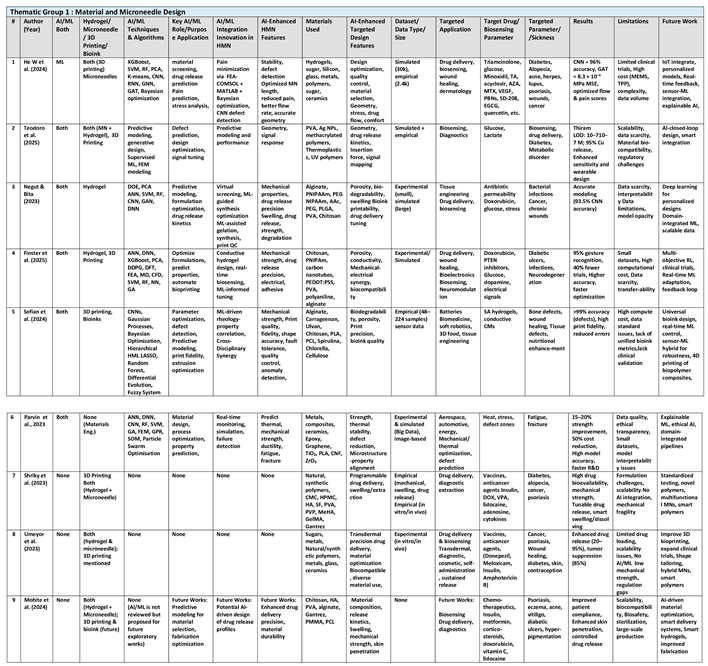
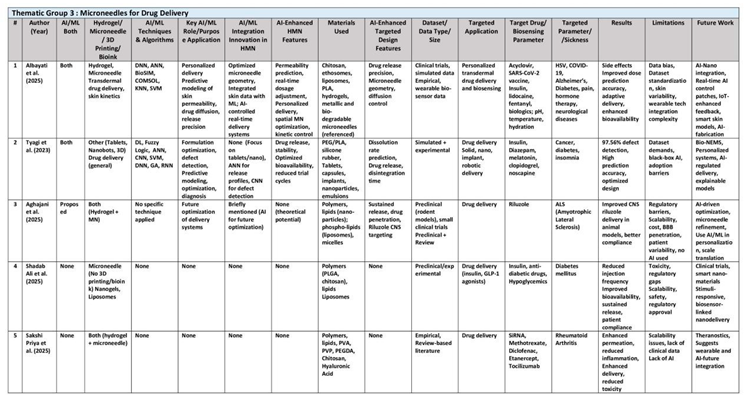
AI/ML and simulation tools enable predictive material design (e.g., new hydrogels, nanocomposites) for specific mechanical, electrical, and drug-release properties; accelerates material-based microneedle innovation. Together, these authors harness the power of computational models to transform traditional material development from an empirical process to one driven by data and simulation. This thematic group illustrates that machine learning can accurately forecast material properties like swelling behavior, mechanical strength, and drug release kinetics, enabling microneedle design with highly specific performance criteria. They emphasize the integration of digital tools and simulation platforms to accelerate the material discovery pipeline. Their thematic research offers compelling evidence that AI-guided material design can lead to the creation of customized nanocomposite systems for a range of biomedical applications. (See
Table 2
For instance, this review by Wenqing He et al. (2024) introduces basic ML concepts (neural networks, decision trees), uses ML for optimizing fabrication such as predicting best etching parameters or molding conditions and cover ML algorithms for analyzing skin images or insertion force data with ML to refine microneedle designs. ML algorithms like Bayesian optimization now enable precise geometry tuning and balancing pain reduction with MN structural integrity. For instance, a COMSOL-MATLAB LiveLink framework minimized pain levels by 37% through iterative parameter adjustments. Graph Attention Networks (GAT) further accelerated material screening by predicting von Mises stress distributions with high accuracy MSE of 8.3×10⁻⁵ MPa, enabling rapid screening of polymers and metals for microneedle fabrication reducing laboratory validation time. ML's impact extends to 3D printing, where convolutional neural networks (CNNs) achieved 96% accuracy in detecting manufacturing defects. ML-driven drug release models, such as XGBoost predicting skin penetration rates, outperforming traditional Fick’s law. In biosensing, ML-enhanced MN patches enabled real-time pH monitoring in diabetic wounds using smartphone-based image analysis. Furthermore, RF and gradient boosting algorithms optimized interstitial fluid extraction rates, while transformers improved biomarker detection in graph-structured data. Future directions aim to merge IoT and multimodal ML models with microneedle technology.
Similarly, Teodoro et al. (2025) study focus mainly on microneedle MN microfabrication technology, but does not delve in-depth into AI techniques, ensuring efficient tissue penetration and biomarker detection. AI models assist in monitoring and predicting imperfections during the 3D printing process, ensuring high-resolution and defect-free MN fabrication, maintaining consistency in mass production. AI models aid in identifying and selecting suitable materials for specific 3D printing technologies, while making parametric design adjustments to balance geometric and mechanical properties like mechanical strength, biocompatibility, biodegradability, conductivity, and cost. AI-driven analytics process data from MN-based sensors for real-time biomarker detection (e.g., glucose, urea, pH), where machine learning models classify and interpret weak signals, improving accuracy in noisy environments. The authors highlight IoT integration where AI algorithms wirelessly transmit biomarker data to cloud platforms for remote patient monitoring. Future research prioritize biodegradable materials and expand ML datasets to include diverse environmental stressors.
Notably, Negut et al. (2023) used neural nets, support vector machines, and regression models to map composition–property relationships, and mentions numerical simulations (finite element modeling) coupled with ML, to comprehensively review how AI/ML techniques enhance the design, characterization, and optimization of hydrogels for biomedical applications. This review is limited to hydrogels, not microneedles. For example, Random Forest (RF) and Artificial Neural Networks (ANNs) analyze vast datasets to predict hydrogels’ complex structure-property relationships like swelling ratios and mechanical strength, to enhance hydrogel’s biocompatibility and tunable properties, which are pivotal in drug delivery, tissue engineering, and wound healing. RF models have been used to predict antibiotic skin permeability in hydrogels, achieving 75% accuracy in classifying cardiomyocyte yields. Convolutional Neural Networks (CNNs) further enhance 3D bioprinting precision, with studies reporting 93.5% accuracy in differentiating optimal hydrogel prints. AI techniques like reinforcement learning and generative adversarial networks (GANs) accelerate the discovery of optimal hydrogel formulations by virtually screening vast chemical spaces and iteratively refining synthesis parameters. Key AI/ML approaches include ML-guided synthesis optimization, where algorithms iteratively adjust parameters like crosslinker concentration and temperature to achieve desired outcomes. Supervised learning techniques map input variables (e.g., polymer composition) to outputs (e.g., drug release kinetics), enabling tailored hydrogel formulations. For example, ANNs have modeled pH- and temperature-responsive hydrogels for controlled doxorubicin delivery, minimizing side effects in cancer therapy. Unsupervised learning methods, such as clustering, identify hidden patterns in hydrogel datasets, facilitating the discovery of novel material classes. Future directions indicates that Reinforcement Learning and generative adversarial networks (GANs) hold promise for designing hydrogels with unprecedented functionalities, such as self-healing or stimuli-responsive behaviors.
Likewise, the review by Finster et al. (2025) underscores how AI/ML techniques (e.g. Supervised Learning:ANN, DNN, CNN, XGBoost, SVR; Unsupervised Learning: PCA; Reinforcement Learning: DDPG) and computational methods (such as DFT, FEA, MD, CFD, Multiphysics Modeling) synergize to overcome traditional trial-and-error limitations, enabling precise customization of hydrogels for applications ranging from wound healing to biosensing. This review is focused mainly on hydrogel, but not microneedles. For example, AI techniques like artificial neural networks (ANN) and extreme gradient boosting (XGBoost) accelerate the discovery of optimal polymer compositions, as demonstrated in chitosan-gelatin bioinks where Bayesian optimization reduced experimental iterations by 40%. Supervised learning models, including artificial and deep neural networks (ANN, DNN), predict mechanical properties such as tensile strength and swelling behavior, critical for tissue engineering scaffolds. ANN models are trained on swelling behavior and drug release kinetics enabled doxorubicin drug release from PNIPAm, while DNNs are trained on alginate-calcium chloride cross-linking ratios data improving 3D bioprinting extrusion accuracy, ensuring consistent pore structures for cell proliferation. Reinforcement learning (RL), particularly deep deterministic policy gradient (DDPG), optimizes multi-parameter systems, balancing hydrogel porosity and conductivity for adaptive bioelectronics. Unsupervised learning methods like principal component analysis (PCA) streamline high-dimensional data, identifying key variables in gelatin stiffness modulation. A real-time-biosensing hydrogels integrated with CNN models interpret colorimetric pH changes in wound dressings, achieving 95% accuracy in infection detection. ML-guided polyaniline hydrogels coupled with piezoelectric nanogenerators accelerated diabetic ulcer healing from 12 to 3 days by synchronizing drug release with electrical stimulation. CNN achieved >95% gesture recognition in conductive hydrogels. Bayesian optimization reduced experimental trials by 40%. Future directions emphasize multi-objective RL for holistic hydrogel design and in vivo clinical validation of AI-designed hydrogels.
Also, the review by Sofian et al. (2024) does not refer specifically to hydrogel microneedles but surveys a range of ML techniques applied to additive manufacturing of bioinks and biopolymers such as alginate, carrageenan, spirulina. Sofian explores the synergistic potential of ML algorithms and biopolymer printing, addressing mechanical instability and printability issues which necessitate advanced computational interventions. For example, ML techniques, including Random Forest, Gaussian processes, SciPy differential evolution, convolutional neural networks (CNNs) are employed to optimize printing parameters such as nozzle temperature, flow rate, and layer height, improving bioink print fidelity by correlating biopolymer rheology with printing outcomes. Printing dimensional deviation was reduced to <10% using hierarchical machine learning (HML) frameworks. Bayesian optimization reduced experimental iterations by 50% for GelMA bio-inks, achieving precise control over scaffold porosity. Hierarchical ML improved alginate-based scaffold stiffness by 3000% via fiber reinforcement. Similarly, LASSO regression simplified high-dimensional parameter spaces, enhancing accuracy for alginate-based hydrogels. Deep learning CNNs trained on real-time camera images achieved 84–99.7% accuracy in detecting printing defects like layer misalignment and infill inconsistencies for better quality control. Furthermore, ML models predicted mechanical properties of spirulina composites with 91% correlation to experimental data, enabling tailored designs for bone tissue engineering. ML mitigates these issues between mechanical strength and biodegradability.by optimizing composite formulations; for example, adding hydroxyapatite (HAP) to PLA increased stiffness by 393 GPa while maintaining biocompatibility. Future directions emphasize hybrid ML models for multi-material systems and expansion into 4D/5D printing.
In another review, Parvin et al. (2023) highlights use of AI algorithms like genetic algorithms, particle swarm optimization, and ANN models to predict and optimize material properties. Also covers scientometric AI (text mining) to discover material trends. While the study does not explore hydrogels or microneedles, its methodologies offer a blueprint for AI-driven innovation in structural and industrial materials to enhance mechanical properties such as strength, ductility, and thermal stability by systematically reviewing AI’s role in material design, process optimization, and predictive analytics, For example, genetic algorithms optimized magnesium alloy compositions for biomedical implants, achieving yield strength improvements of 20–25%. By coupling finite element method (FEM) simulations with ML, the study achieved real-time monitoring of parameters like temperature and humidity during additive 3D printing. This integration of AI’s application into additive manufacturing reduced material waste by 30% and enhanced the tensile strength of polylactic acid (PLA) components by 15–20% through optimized infill patterns.
Similarly, the review article by Shriky et al. (2023) review does not include AI, but it is focused on materials and engineering for HMNs. This study comprehensively examines hydrogel-based microneedles (HMN), focused on materials and engineering, especially on their materials, fabrication, characterization, and challenges. All review analysis is experimental: material formulation and physical testing. The paper underscores HMNs' applications in transdermal drug delivery, notably for insulin, vaccines, and anticancer agents, with insulin-loaded HMNs achieving up to 96.6% bioavailability. Diagnostic uses, such as interstitial fluid extraction for biomarker detection in psoriasis, are also reviewed. While the study does not incorporate AI/ML, it identifies opportunities for future integration, such as optimizing formulation parameters or predicting drug release kinetics via machine learning.
Likewise, the review paper by Umeyor et al. (2023) does not incorporate artificial intelligence (AI) or machine learning (ML) methodologies in its exploration of biomimetic microneedles but comprehensively examines advancements in biomimetic microneedle (MN) systems for transdermal delivery of therapeutics. While the paper does not incorporate AI or ML, it emphasizes material innovation, fabrication techniques, and biomedical applications. The authors highlight the potential of advanced manufacturing methods like stereolithography, but unfortunately there is no discussion of computational tools such as neural networks or predictive modeling to optimize microneedle design or drug release kinetics. The absence of AI/ML in this review underscores a gap in the current research landscape of data-driven optimization of microneedle design.
Lastly, the review by Mohite et al. (2024) consolidates advancements in hydrogel-forming microneedles (HMNs) biomedical applications and materials for treating dermal disorders, but study does not include any AI/ML component. A notable gap is the absence of AI/ML in current HMN research. The authors propose future integration of machine learning for predictive modeling of hydrogel behavior and drug release kinetics. AI could optimize material selection (e.g., balancing chitosan’s antimicrobial properties with mechanical stability) and enhance 3D-printed microneedle designs for deeper skin penetration. For instance, AI might refine bioink formulations to improve printability and drug-loading efficiency.
By merging theoretical modeling with experimental validation, these 9 studies pave the way for more efficient and targeted material synthesis. This thematic group highlights the potential of interdisciplinary collaboration between materials scientists, engineers, and data scientists to unlock new therapeutic possibilities. Their findings are crucial for the development of next-generation biosensors and implantable devices that require precise material characteristics. They also address the challenges of data scarcity and model interpretability while proposing robust solutions. The authors’ collective efforts signify a paradigm shift in how advanced materials and nanocomposites are designed and optimized for clinical applications. Their research not only advances scientific understanding but also provides practical strategies for the rapid prototyping of biomedical devices. Ultimately, the group’s work underscores the transformative potential of machine learning in driving innovation in the field of material science and nanocomposite design.
3.2. Thematic Group 2: Microneedles for Diagnostics and Therapy
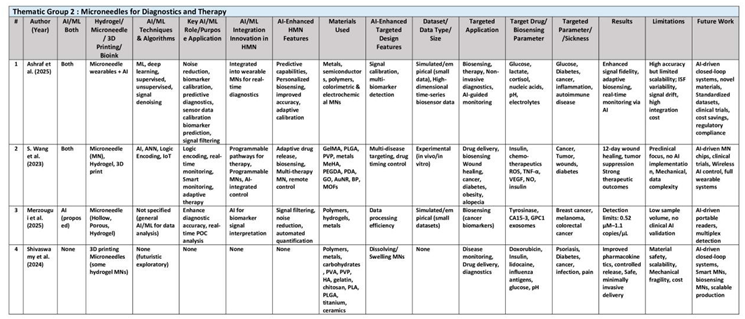
This thematic group describes Integration of AI with microneedle devices for real-time diagnostics, adaptive drug release, and closed-loop feedback systems; enables personalized, minimally invasive therapy through biosensing and intelligent control. Their research collectively illustrates a convergence of biomedical engineering, materials science, and computational intelligence that is reshaping transdermal drug delivery. These studies underline how AI-powered signal recognition and logic encoding can transform traditional devices into adaptive, closed-loop therapeutic systems. The authors demonstrate that integrating real-time data feedback into microneedle design can enable responsive drug dosing tailored to individual patient needs. Their work not only enhances the accuracy and precision of treatments but also reduces systemic side effects by targeting therapy at the specific site of need. The interdisciplinary nature of their contributions is pivotal for addressing complex challenges in chronic disease management and cancer therapy. By merging sensor technology with drug delivery, they present a paradigm shift toward minimally invasive, patient-friendly healthcare solutions. (See
Table 3)
For instance, Ashraf et al. (2025) covers how machine learning, possibly deep learning techniques are used to calibrate, process and interpret the biosensor signal data from microneedles. AI algorithms for pattern recognition in signal processing, sensor readouts and predictive analytics for personalized health trends. Machine learning models, including deep learning architectures such CNNs and RNNs, process high-dimensional data from optical/electrochemical microneedle sensors to de-noise raw data, calibrate signals, and do pattern recognition. AI enhances non-invasive ISF microneedle systems rich in biomarkers like lactate and proteins by optimizing microneedle wearable sensor performance through AI adaptive algorithms that account for signal instability and physiological variations. Unsupervised learning such as clustering algorithms correlate protein/DNA biomarker patterns essential for disease subtyping, early disease detection and personalized therapy. AI-driven platforms trained on longitudinal datasets can perform real-time stress monitoring using cortisol-level predictions to identify early warning signs. Reinforcement learning algorithms detect subtle fluctuations in glucose levels in a closed-loop with 98.4% accuracy in clinical trials, offering diabetic patients continuous monitoring without invasive discomfort of traditional lancets. However, clinical translation remains hindered by ISF composition variability and small-scale datasets, while larger diverse datasets are needed to train robust AI models capable of generalizing across diverse patient populations.
Similarly, the 2023 review by Shuo Wang et al. briefly mentions of AI/ML techniques which include programmable intelligent MNs integrating AI, neural networks, logic encoding, and big data analytics for optimizing therapy efficacy. Specific algorithmic or technical details are not elaborated extensively, indicating a broad conceptual but superficial coverage of AI techniques. While AI/ML is not yet experimentally implemented, Wang envisions its future role in optimizing therapeutic outcomes. Wang proposes that AI-driven microneedle systems capable of real-time health monitoring and adaptive therapy, which could enable programmable MNs for logic-encoded treatment pathways to autonomously execute complex treatment protocols. By integrating photothermal therapy and adjusting drug release in response to real-time biomarker data thresholds such as blood glucose levels detected via biosensors, AI-powered MNs optimizes efficacy while minimizing off-target effects. Machine learning algorithms might analyze large-scale datasets from wearable MN sensors to predict disease progression or personalize dosages. The review highlights intelligent MN patches that integrate with neural networks for adaptive photodynamic therapy, potentially reducing side effects through precision targeting.
Likewaise, the 2025 review by Merzougui et al. focuses mainly on microneedle (MN) array-based dermal interstitial fluid (ISF) biopsy as a tool for cancer diagnosis, but the AI/ML framework remains undeveloped with no specific algorithms or datasets disclosed. While the paper primarily focuses on material and sensor innovations, it briefly suggests the conceptual use of artificial intelligence (AI) to address challenges in data interpretation, such as filtering noise from low-abundance biomarkers and enabling real-time analysis. Although electrochemical and fluorescent microneedles sensors demonstrate promising results in detecting tyrosinase at 0.52 μM for melanoma screening, AI/ML technqiues are necessary to automate biomarker quantification and improve diagnostic accuracy, particularly for multiplex detection.
Lastly, Shivaswamy et al. (2024) does not incorporate AI or ML in his review of microneedle technology, but mentions AI/ML role in transdermal drug delivery and disease monitoring. it highlights future opportunities for AI/ML to advance MN systems. This paper cites that AI-driven closed-loop systems could revolutionize personalized medicine by enabling real-time biomarker monitoring and dynamic drug dosage adjustments. Machine learning algorithms might optimize microneedle design and fabrication processes, reducing costs and improving scalability. Furthermore, AI could predicting patient-specific pharmacokinetic responses and automating stimuli-triggered drug release mechanisms.
For this thematic group, these collective findings suggest that future microneedle systems will seamlessly integrate with wearable electronics and cloud-based data analysis platforms. They also highlight the importance of addressing mechanical and regulatory challenges through iterative design improvements powered by AI. This body of research supports the idea that intelligent microneedle systems can deliver both diagnostics and therapeutics in a single, cohesive platform. The group’s work paves the way for next-generation devices that are not only smart but also capable of predictive and adaptive responses. Their comprehensive approaches illustrate the feasibility of transitioning from laboratory prototypes to clinically viable, commercial products. In sum, the contributions of S. Wang, Merzougui, Teodoro, Ashraf, W. He, and Shivaswamy collectively establish the foundation for smart microneedle systems that promise to revolutionize digital health through AI integration. Their research offers a roadmap for designing devices that are both technologically advanced and clinically transformative, ultimately setting new standards for personalized, minimally invasive therapy.
3.3. Thematic Group 3: Microneedles for Drug Delivery
Focus on hydrogel-forming and polymer-based microneedles that are biocompatible, dissolvable, and suitable for sustained drug delivery. Collectively, these authors create a narrative focused on the synthesis and application of hydrogel and polymer-based microneedles as robust platforms for transdermal drug delivery. Their research illustrates the importance of selecting the right polymer blends and fabrication techniques to achieve both drug delivery efficacy and patient comfort. They show that controlling the swelling behavior of hydrogels is essential for creating reservoirs that release drugs in a sustained manner. Their work underscores the transformative potential of combining material science with advanced engineering to overcome clinical challenges. The group highlights how integrating smart materials into microneedle designs can significantly enhance therapeutic outcomes for diseases such as diabetes, cancer, and inflammatory skin disorders. Their interdisciplinary approaches bridge the gap between chemical engineering and biomedical applications, providing a pathway to personalized medicine. They emphasize the need for scalability, regulatory compliance, and reproducibility in transitioning these systems from bench to bedside. (See
Table 4)
For instance, Albayati et al. (2025) systematically explore how AI-driven innovations enhance hydrogel and microneedle-based TDDS, focusing on predictive modeling, formulation optimization, and personalized medicine. Albayati describes ML models predicting skin permeability of drug molecules, and AI algorithms to optimize formulation (amount of enhancer, microneedle length) for different skin types. Using Deep Neural Networks (DNN) and Support Vector Machines (SVM), the study predicted skin permeability (LogKp) for drugs such as acyclovir, enabling rapid identification of candidates suitable for transdermal delivery. AI models trained on molecular datasets identified optimal combinations of penetration enhancers, improving the permeation flux of sodium salt acyclovir by 50% compared to traditional methods. The authors highlight the use of COMSOL and BioSIM software to model temperature-dependent drug diffusion, revealing that a 10°C increase in skin temperature doubles nicotine absorption. Hydrogels, such as fluorocarbon-modified chitosan, are tailored using ANN to achieve sustained release profiles, critical for chronic conditions like Alzheimer’s. Sensor-integrated patches exemplify AI’s real-time adaptability, dynamically adjusting drug doses based on physiological feedback, thereby reducing systemic side effects. For example, AI reduced clinical trial durations for a transdermal rivastigmine patch by 30% through predictive enrollment. Future directions propose hybrid AI-nanotechnology platforms to enhance targeting precision, particularly for oncological applications.
Similarly, the study by Tyagi et al. (2023) explores the transformative role of AI and machine learning (ML) in pharmaceutical formulation design, emphasizing enhanced drug delivery and bioavailability. The authors highlight how AI techniques are employed to optimize drug formulations, predict release profiles, and detect manufacturing defects. For instance, ANN models demonstrated high accuracy (85%) in predicting tablet disintegration times, while CNNs achieved 97.56% precision in identifying capsule defects, significantly reducing manual inspection efforts. A key innovation lies in integrating AI with nanotechnology, where ANNs optimized nanoparticle size and polymer-drug ratios, improving solubility for drugs like carbamazepine. The study also details AI-driven 3D printing of tablets, where infill patterns and density were optimized using ANN models to control diazepam dissolution rates. Furthermore, AI-powered DNA nanobots and biohybrid systems represent a paradigm shift in targeted drug delivery, utilizing biosensors and pharmacokinetic simulations to navigate physiological environments and deliver therapeutics with minimal off-target effects. Despite these advancements, challenges include the "black box" nature of deep learning algorithms, and dependency on high-quality datasets and strong regulatory barriers. The authors advocate for explainable AI (XAI) to enhance model transparency and multi-omics data integration for personalized medicine.
In addition, the review by Aghajani et al. (2025) does not involve artificial intelligence or machine learning AI/ML, but it is focused on pharmacological and delivery mechanisms using hydrogel microneedle HMN for ALS drug riluzole delivery. Aghajani briefly mentioned AI for future optimization but explores advanced drug delivery systems (DDS) for riluzole in ALS treatment, emphasizing overcoming pharmacokinetic barriers such as low bioavailability and blood-brain barrier (BBB) penetration. The authors envisioned AI and machine learning as future pivotal tools for optimizing nanoparticle and hydrogel formulations, enabling predictive modeling of drug-release kinetics and material interactions to enhance scalability and reproducibility. AI approach is suggested to facilitate the design of patient-centric microneedle systems, tailoring geometry and composition to individual patient needs while overcoming riluzole’s physicochemical limitations.
Similarly, the 2025 review by Shadab Ali et al. does not incorporate artificial intelligence (AI) or machine learning (ML) methodologies in its analysis of nanotechnology for diabetes management. While the study extensively discusses innovations such as glucose-responsive nanogels and microneedle arrays, these advancements rely solely on material science and biochemical engineering principles without leveraging AI/ML for optimization or predictive modeling. The absence of AI/ML integration highlights a gap in utilizing computational tools to enhance drug release (GLP-1 receptor agonists, Insulin) precision, predict patient-specific responses, or optimize nanocarrier designs.
Likewise, the review article by Sakshi Priya and colleagues (2025) focused on empirical and experimental approaches to optimize drug delivery systems for rheumatoid arthritis (RA) but does not incorporate artificial intelligence (AI) or machine learning (ML) techniques. The paper highlights hydrogels and microneedles (MN) as promising platforms for transdermal delivery of RA inflammatory drugs Methotrexate, Diclofenac, etc emphasizing nanotechnology and novel formulations. While this review excels in detailing nanotechnological innovations, it overlooks computational approaches that could accelerate material selection and reduce experimental iterations. AI could streamline clinical trial design by optimizing formulation parameters or predicting therapeutic outcomes and identifying high-response patient subgroups.
This thematic body of work demonstrates that the fusion of hydrogels with intelligent design principles can lead to devices that are both effective and minimally invasive. The authors also propose that future innovations will benefit from further integration of AI biosensors to monitor therapeutic efficacy in real time. In essence, their research collectively propels the field of transdermal drug delivery forward by leveraging smart polymers and advanced fabrication methods. Their contributions offer significant insights into creating next-generation microneedle systems that are dosage-effective, patient-centric, and clinically impactful. Their work not only advances the science of drug delivery but also paves the way for more sustainable, cost-effective therapeutic solutions.
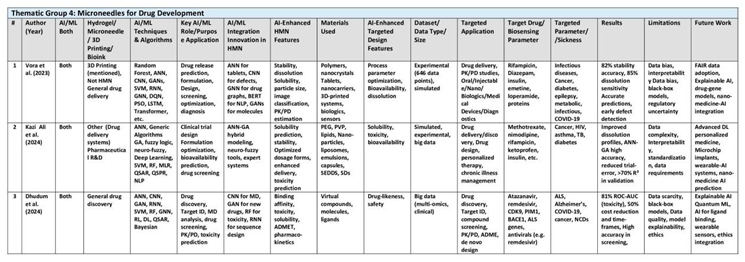
3.4. Thematic Group 4: Microneedles for Drug Development
AI tools (ANN, CNN, GA, etc.) applied to optimize drug formulations, drug discovery, and bioavailability; revolutionizes pharmaceutical R&D with predictive analytics and rapid prototyping. Collectively, these authors illustrate that the application of computational intelligence is transforming traditional pharmaceutical R&D into a more precise, data-driven process. Their research emphasizes the significant advantages of reducing the trial-and-error approach in drug formulation development through predictive analytics. They demonstrate that AI-driven methodologies not only improve drug efficacy but also enhance patient selectivity/safety by better predicting adverse effects. Their interdisciplinary approaches merge data science, chemistry, and clinical insights, offering a holistic view of modern drug delivery challenges. They advocate for the development of standardized datasets and robust validation techniques to further integrate AI into the drug development lifecycle. (See
Table 5)
For instance, the 2023 review by Vora et al. comprehensively explores the integration of artificial intelligence (AI) and machine learning (ML) in pharmaceutical technology and drug delivery design. The authors emphasize AI’s transformative role in accelerating drug discovery, optimizing formulations, and personalizing therapies. Supervised learning techniques, such as random forest RF, support vector machines (SVMs) and artificial neural networks (ANNs), are extensively utilized for predicting drug-target interactions and pharmacokinetic parameters. For instance, ANN models have demonstrated 85% accuracy in classifying dissolution profiles of sustained-release tablets, significantly reducing experimental iterations. Unsupervised learning methods, including principal component analysis (PCA) and clustering, aid in identifying patient subgroups for precision medicine. A pivotal application discussed is AI’s role in de novo drug design using generative adversarial networks (GANs), which generate novel molecular structures with desired properties. The review highlights tools like DeepChem and AutoDock Vina, which streamline virtual screening and molecular docking for novel drug candidates. In dosage form development, AI optimizes 3D-printed tablets by analyzing infill density and temperature parameters, enhancing drug release kinetics. For example, Vora cited Obeid et al. [
43] employed ANNs to predict diazepam release from 3D-printed tablets, achieving higher dissolution rates through parameter optimization. The paper underscores AI’s impact on pharmacokinetic modeling, particularly physiologically based pharmacokinetic (PBPK) simulations. Machine learning algorithms, such as XGBoost, reduce the need for animal testing by predicting human PK parameters from in vitro data. The Vora also highlighted Lin et al.’s work [
44] on tumor prediction where ML predicts nanoparticle toxicity and tumor targeting efficiency. Future works include reinforcement learning for adaptive clinical trials.
Similarly, the review by Kazi Ali et al. (2024) highlights how AI technologies such as fuzzy logic, neuro-fuzzy systems, artificial neural networks (ANN) and genetic algorithms (GA) are used for multi-parameter optimization in drug development, but does not mention hydrogel microneedles. Deep learning is highlighted for its ability to handle the massive datasets in drug discovery and ADMET (absorption, distribution, metabolism, excretion, toxicity) prediction; for example, deep neural networks (DNN) have outperformed traditional ML in predicting ADMET properties of compounds. Specific AI tools like XenoSite, FAME, SMARTCyp (for predicting metabolic sites on molecules) underscores the variety of algorithmic techniques from expert systems to modern deep learning. Fuzzy logic handles uncertainty in formulation parameters, and neuro-fuzzy systems combine pattern recognition with decision-making. ANN-GA hybrid optimize drug design, formulation, and clinical trials. ANN mimics human brain processing to predict drug solubility and bioavailability, while GA refines solid-nanoparticle formulations through evolutionary principles. Hybrid ANN-GA systems reduced trial-and-error in hydrochlorothiazide tablet formulations. ANN application is highlighted in controlled-release tablets, where ANN models predict dissolution profiles using inputs like polymer ratios and compression forces. For example, ANN models improved the solubility of BCS Class II drugs in hard gelatin capsules. For drug discovery, machine learning models like QSAR modeling (Quantitative Structure–Activity Relationship models) and random forests accelerate drug discovery through virtual screening, target validation, and drug repurposing of millions of chemical compounds to screen, identify and select suitable bioactive candidates. In clinical trials, AI can efficiently identifies patient cohorts likely to respond to treatments and real-time monitors adverse effects, reducing costs and timelines. Solid dispersions, nanoemulsions, and self-emulsifying systems (SEDDS) benefit from AI’s ability to predict stability and optimize surfactant-oil ratios. AI also enhances manufacturing via Process Analytical Technology (PAT), enabling continuous production with minimal lot-to-lot variation. Despite successes, challenges such as data complexity and heterogeneity limit AI’s accuracy in predicting complex biological outcomes like drug efficacy and toxicity, while small datasets for niche formulations constrain model generalizability.
In another review, Dhudum et al. (2024) comprehensively review AI applications across all stages of drug development, using neural networks and QSAR models to predict drug-target interactions and ADMET properties, emphasizing how ML algorithms like Random Forest (RF) and Convolutional Neural Networks (CNNs) enhance target identification, compound screening, and toxicity assessment. For instance, AI-driven platforms such as PandaOmics leverage multi-omics data and deep learning models to prioritize therapeutic targets for complex diseases like amyotrophic lateral sclerosis (ALS), identifying 18 novel targets with experimental validation in Drosophila models. In molecular dynamics (MD) simulations, deep learning models like recurrent neural networks RNNs and Graph Neural Networks (GNNS) analyze protein-ligand binding affinities with atomic precision, enabling rapid virtual screening of billion-compound libraries while reducing computational costs. The authors highlight ML-based scoring functions, such as RF-Score and DeepTox, which outperform traditional methods in virtual screening by minimizing false positives. For toxicity prediction, ensemble models achieve 81% ROC-AUC in detecting drug-induced liver injury (DILI), demonstrating AI’s potential to mitigate clinical trial risks. Generative adversarial networks (GANs) and reinforcement learning (RL) enable *de novo* drug design by synthesizing novel molecular structures with optimized pharmacokinetic profiles, exemplified by the discovery of novel CDK9 and PIM1 kinase inhibitors. These AI-generated compounds undergo iterative optimization cycles, reducing synthesis timelines. In pharmacokinetics, multitask learning models predict oral bioavailability and plasma protein binding with mean absolute errors as low as 0.17, streamlining lead optimization. Case-studies illustrate AI’s real-world impact, such as Benevolent AI’s identification of repurposed drugs (e.g., baricitinib) for COVID-19 and IBM Watson’s oncology applications. Despite these advancements, challenges persist, including data heterogeneity, model interpretability, and the need for explainable AI (XAI) for transparent decision-making.
This group’s work highlights the importance of collaboration between AI experts and pharmaceutical scientists to create adaptive, personalized drug options. Their collective contributions underscore the potential for AI to revolutionize every stage of pharmaceutical development—from molecule design to clinical application. They provide a drug development roadmap for achieving faster, more reliable drug formulations that meet the high standards of modern medicine. Their research is poised to set new benchmarks for efficiency and precision in the pharmaceutical industry. In essence, this thematic group represents a transformative shift toward intelligent drug discovery procedures that are both innovative and clinically impactful.
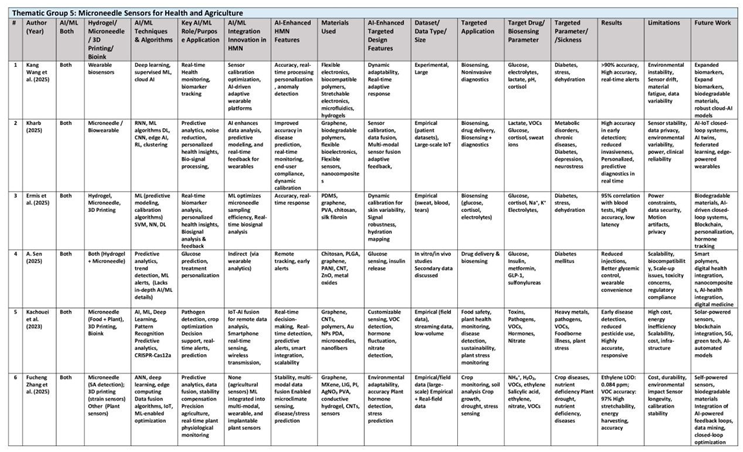
3.5. Thematic Group 5: Microneedles Sensors for Health and Agriculture
AI-powered sensors embedded in wearable technology monitor human and horticulture such as plant hydration, plant stress, human glucose etc offering real-time, adaptive health insights, especially for agriculture, sports and chronic care. Together, these researchers build a compelling case for the role of smart wearables in revolutionizing personalized plant and human health monitoring for athletic performance optimization and sustainable plant growth. Their studies illustrate how the integration of advanced biosensing with AI-driven data analytics can enable early detection of physiological anomalies and optimize training regimens. They show that such systems have the potential to offer continuous, personalized monitoring that is critical for proactive agri-health management. Their interdisciplinary efforts merge material science, electronics, and computational analytics, creating a seamless interface between the user and real-time health data. They emphasize that the development of robust, adaptive wearables can lead to breakthroughs in both preventive interventions for human and horticulture enhancement. (See
Table 6)
For instance, the 2025 review by Kang Wang and colleagues emphasizes coupling Deep learning, Supervised ML, Cloud AI with wearable sensors in general (including microneedle-based sensors) to enhance non-invasive health monitoring. Machine learning algorithms that calibrate wearable sensors in real-time and personalize the readings to automatically account for individual baseline differences. Study cites big data approaches to correlate sensor readings with clinical outcomes. These AI-driven sensors integrates with flexible electronics and microfluidics to analyze biomarkers in sweat, saliva, and interstitial fluid, offering a painless alternative to traditional blood tests. AI/ML offers continuous monitoring, real-time data processing and environmental adaptability. AI algorithms are employed to calibrate sensors dynamically, compensating for variables like temperature and humidity that traditionally degrade performance. Machine learning models, trained on large datasets from clinical trials, predict biomarker trends such as glucose levels with high accuracy, reducing reliance on invasive methods. Future directions emphasize sustainable material synthesis and expanding biomarker detection to include proteins and genetic markers, facilitated by AI-driven multi-omics integration.
In addition, the review by Kharb (2025) examines the integration of AI and machine learning (ML) with non-invasive health monitoring microneedle patches, smart tattoos, and implantable biosensors to analyze biomarkers in interstitial fluid, sweat, and breath. AI techniques are used in filtering noise from continuous sensor data, enabling precise detection of biomarkers such as glucose, lactate, and cortisol. For instance, deep learning models like recurrent neural networks (RNNs) and convolutional neural networks (CNNs) are employed to diagnose infections such as COVID-19 using data from wearable devices like the Oura smart ring. AI models correlates biomarker trends with disease progression, enabling early interventions for diabetes and cardiovascular conditions. MN-based wearable sensors calibration remains a significant hurdle, as environmental factors and individual physiological variations can skew data accuracy. Training AI models on diverse datasets are necessary to mitigate data discrepancies. Despite these advancements, useful AI/ML wearables require addressing technical limitations, ethical considerations, and the need for cross-disciplinary collaboration. Additionally, privacy concerns arise from continuous generation of sensitive health data, necessitating robust encryption and ethical data usage frameworks.
Similarly, the 2025 review by Ermis et al. does not mention AI/ML as a core focus, does not discuss AI/ML algorithms, but briefly mentions AI/ML applications conceptually. Ermis covers mainly about wearable sensor devices and materials in sports and healthcare, briefly mentioning the conceptual integration of AI and machine learning (ML) with advanced materials like hydrogels and microneedles. AI-driven predictive analytics offering personalized hydration and stress management strategies for athletes. Machine learning models calibrate microneedle-based glucose sensors using light polarization data to achieve 95% correlation with invasive blood tests.
Likewise, this review by Sen (2025) synthesizes recent advancements in polymer nanocomposite PNC devices, emphasizing their dual role in enhancing glucose biosensors and enabling controlled insulin release, but did not discuss AI/ML in-depth. While the paper does not focus on AI/ML and AI/ML is not directly integrated into the discussed polymer nanocomposite (PNC) sensor devices, it contextualizes AI/ML technologies as an aspirational frontier within broader diabetes care trends, such as AI-driven predictive analytics for real-time glucose monitoring. .
Also, the 2023 review by Kachouei et al. explores the transformative role of IoT-enabled sensors augmented with artificial intelligence (AI) and machine learning (ML). While specific AI/ML algorithms are not listed in detail, it implies the conceptual use of machine learning models for advancing sustainable agriculture and food safety. Study reported CRISPR-Cas12a detection systems coupled with AI-assisted colorimetric assays achieve ultrasensitive detection of food-borne pathogens, demonstrating LoDs as low as 3.4 × 10² CFU/mL, useful for early detection of contaminants. Machine learning models are leveraged for high-throughput machine learning-driven plant phenotyping, analyzing multi-spectral imaging data from drones to assess crop health, transforming IoT-enabled sensors into predictive tools, enabling real-time analysis of contaminants and stressors in food and plants. AI/ML techniques can interpret volatile organic compounds (VOCs) emitted by stressed plants, enabling early intervention against drought or fungal infections. However, limitations include the high energy consumption of IoT devices, data standardization and the complexity of calibrating AI models for diverse agricultural environments.
Lastly, Zhang et al. (2025) explored the transformative role of AI and ML in analyzing plant sensor data in agriculture (for yield prediction, disease outbreak forecasting from sensor data), machine learning for sensor calibration and combining multimodal data (soil moisture + nutrient levels + weather) to provide actionable farming outcomes. ML technqiues such as artificial neural networks (ANN), are deployed to interpret electrochemical data from hormone sensors, enabling real-time quantification of indole-3-acetic acid (IAA) with a detection limit as low as 10.8 pg/mL. For instance, wireless ethylene sensors leverage ANN models to predict crop stress responses, achieving a linear detection range of 0.5–20 ppm. Deep learning frameworks are integrated with hyperspectral sensing to identify disease-specific volatile organic compounds (VOCs), achieving over 97% classification accuracy for early blight detection in tomatoes. Additionally, 3D-printed strain sensors with PEG/AgNO₃ composites utilize ML to monitor micron-level plant growth deformations, demonstrating a fatigue resistance threshold of 40.87 J/m². Despite these advancements, signal drift under field conditions and the high cost of nano-material-based sensors remains a problem. Future directions propose AI-powered self-calibration mechanisms and biodegradable sensor designs to align with sustainable farming goals.
Their research supports the notion that the AI/ML-powered future of healthcare and agriculture lies in personalized, non-invasive wearable monitoring sensors that are both comfortable and reliable. By addressing challenges such as sensor accuracy, data integration, and user adaptability, their work paves the way for next-generation AI wearables. The collective contributions of these authors underscore the transformative impact of combining cutting-edge biosensor technology with smart data processing. Their innovative AI/ML approaches offer a vision of agri-healthcare where continuous monitoring and timely interventions become standard practice. Ultimately, their work not only benefits agriculture, individual health and athletic performance but also has broader implications for public health and preventive care. Their research provides a strong foundation for the development of intelligent, wearable AI sensors that transform the landscape of personalized interventions for humans and horticulture.
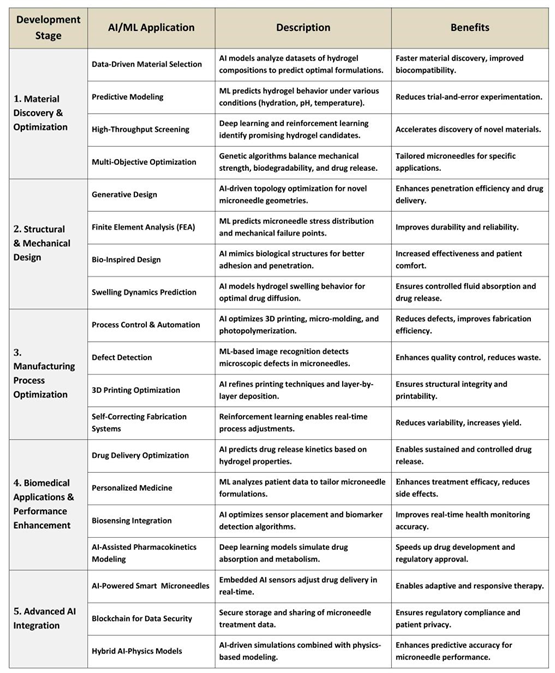
4. Development Framework for AI/ML Applications in Hydrogel Microneedles 873 words
Microneedles have emerged as a promising technology for transdermal drug delivery due to their biocompatibility, tunable properties, and minimally invasive nature. However, the optimization of their materials, structural design, manufacturing processes, and biomedical applications requires a data-driven approach to enhance efficiency and performance. The integration of artificial intelligence (AI) and machine learning (ML) into the development of hydrogel microneedles represents a transformative paradigm in drug delivery systems. This paper presents a comprehensive framework that integrates artificial intelligence (AI) and machine learning (ML) for accelerated development of hydrogel microneedles. (See
Table 7)
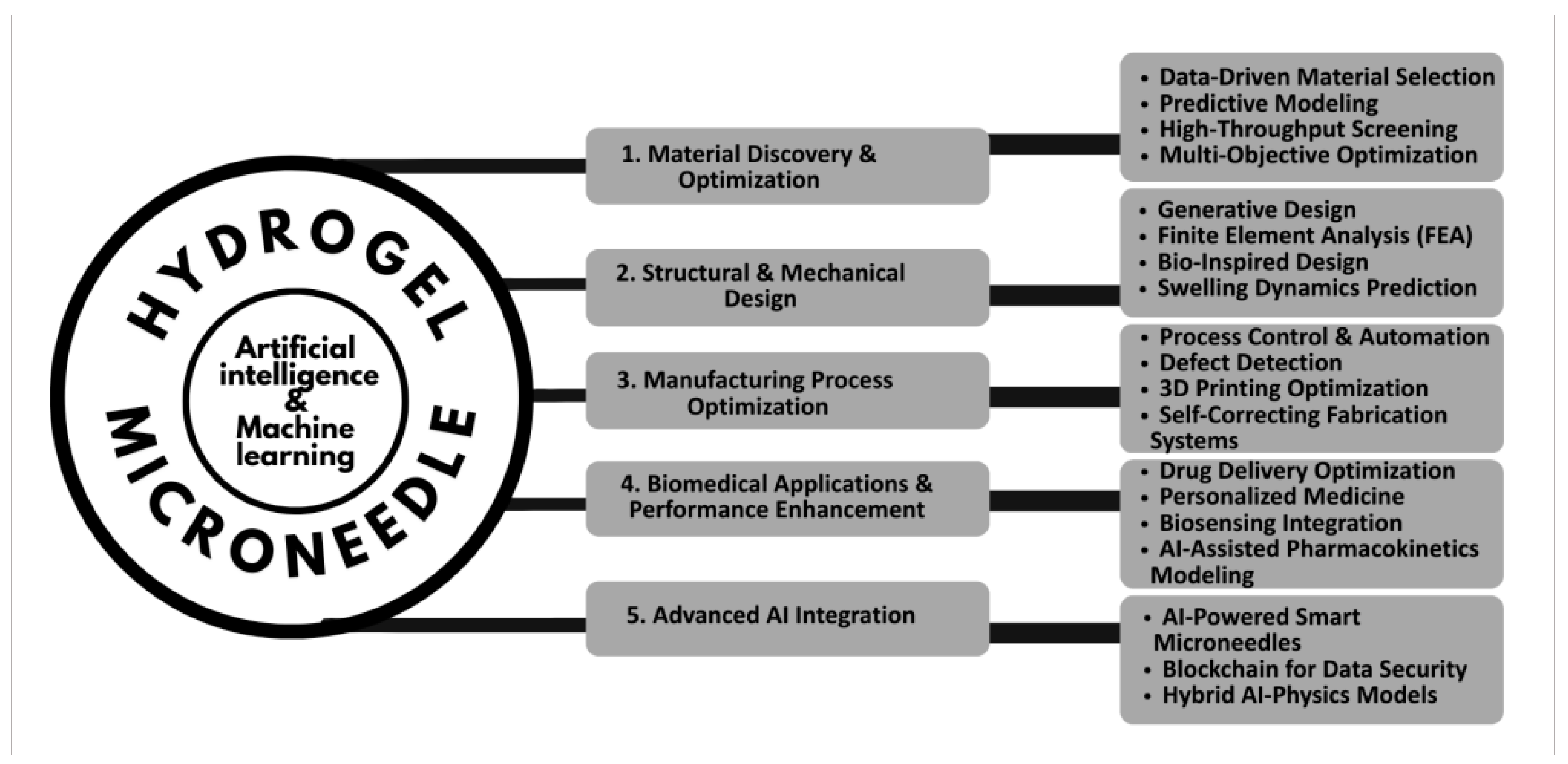
This novel framework, termed AIM-DO (AI-driven Microneedle Design Optimization), establishes a multi-disciplinary approach to address critical challenges in material science, structural engineering, manufacturing, and biomedical application. The framework is structured into five strategic domains: (1) material discovery and optimization, (2) structural and mechanical design, (3) manufacturing process optimization, (4) biomedical applications and performance enhancement, and (5) advanced AI integration. By leveraging AI/ML, the framework accelerates innovation across five interconnected pillars, each contributing uniquely to the optimization of hydrogel microneedle performance. (See
Figure 1)
Together, these five pillars provide a conceptual roadmap for deploying and promoting AI-driven innovation and fostering the next generation of hydrogel microneedles through cross-domain synergy and structured translational pathways.
4.1. Pillar 1. Material Discovery & Optimization
The foundation of the framework lies in data-driven material selection, where ML algorithms analyze vast datasets of polymer properties, crosslinking mechanisms, and biocompatibility profiles to identify optimal hydrogel compositions. Predictive modeling enables the simulation of swelling kinetics, degradation rates, and drug-loading capacities, reducing reliance on trial-and-error experimentation. High-throughput screening, powered by automated robotic systems, rapidly validates computational predictions, ensuring alignment between theoretical and empirical results. Multi-objective optimization algorithms balance competing parameters—such as mechanical strength versus biodegradability—to tailor hydrogels for specific therapeutic needs. For instance, models may prioritize rapid swelling for burst drug release or sustained degradation for prolonged delivery.
4.2. Pillar 2. Structural & Mechanical Design
Generative design algorithms explore vast design spaces to propose microneedle architectures that maximize skin penetration while minimizing pain response. Finite element analysis (FEA) simulates mechanical stress distribution under varying loads, ensuring structural integrity during insertion. Bio-inspired designs, informed by natural systems such as insect proboscises or plant microstructures, enhance insertion efficiency and biocompatibility. Swelling dynamics prediction models, trained on experimental data, forecast hydrogel expansion post-insertion, enabling precise control over drug release kinetics. These computational tools enable the creation of microneedles with tunable geometries (e.g., conical, pyramidal) and pore architectures, optimized for specific drug molecular weights and patient demographics.
4.3. Pillar 3. Manufacturing Process Optimization
AI-driven process control systems dynamically adjust parameters (e.g., temperature, humidity) during hydrogel synthesis to mitigate batch-to-batch variability. Computer vision algorithms integrated with microscopy enable real-time defect detection, such as microcracks or uneven crosslinking, improving yield rates. In 3D printing optimization, reinforcement learning agents iteratively refine nozzle speed, layer thickness, and UV curing duration to fabricate high-resolution microneedle arrays. Self-correcting fabrication systems autonomously recalibrate equipment in response to sensor feedback, ensuring reproducibility. These advancements address scalability challenges, bridging the gap between lab-scale prototypes and industrial production.
4.4. Pillar 4. Biomedical Applications & Performance Enhancement
Drug delivery optimization models correlate microneedle properties (e.g., swelling ratio, pore size) with pharmacokinetic profiles to predict drug bioavailability. Personalized medicine frameworks leverage patient-specific data (e.g., skin thickness, metabolic rates) to customize microneedle designs via digital twins. Reasoning integration—a hybrid of symbolic AI and neural networks—enables adaptive dosing strategies, where microneedles respond to biomarkers (e.g., glucose levels) for closed-loop therapy. AI-assisted pharmacokinetics modeling accelerates preclinical testing by simulating drug diffusion through dermal layers, reducing animal trials. Case studies in transdermal insulin delivery demonstrate how these tools enhance temporal precision and minimize off-target effects.
4.5. Pillar 5. Advanced AI Integration
AI-powered smart microneedles embed microsensors to monitor drug release in real time, transmitting data to cloud platforms for clinician review. Blockchain technology ensures secure, immutable records of formulation data and clinical outcomes, fostering collaboration while protecting intellectual property. Hybrid AI-physics models merge deep learning with continuum mechanics equations, enhancing predictive accuracy in complex biological environments. Federated learning frameworks enable decentralized model training across institutions, addressing data privacy concerns. These innovations position hydrogel microneedles as IoT-enabled medical devices, capable of integrating with broader healthcare ecosystems.
4.6. Synergistic Impact and Future Directions
The AIMDO framework for hydrogel microneedle development offers a transformative approach to optimizing material selection, design, manufacturing, and biomedical applications. By leveraging predictive modeling, automation, and real-time feedback mechanisms, AI accelerates the innovation cycle and enhances the clinical efficacy of microneedle-based drug delivery systems.
The AIM-DO framework’s interdisciplinary approach unites materials science, mechanical engineering, and biomedical research under a unified AI/ML infrastructure. By automating iterative design processes, it reduces development timelines from years to months, a critical advantage in responding to emerging health crises.
Future work will focus on clinical translation, including human trials to validate AI predictions, and expansion into vaccines, biologics, and gene therapy. Challenges such as model interpretability and regulatory compliance remain, yet the framework’s modular design allows continuous integration of emerging technologies (e.g., quantum computing for molecular simulations). Ultimately, this paradigm shift promises to democratize access to personalized drug delivery, advancing global health equity through precision medicine.
5. Results and Discussion 1517
The comprehensive review of 27 peer-reviewed articles between 2023 and 2025 reveals how artificial intelligence (AI) and machine learning (ML) are being increasingly integrated across hydrogel microneedle (HMN) research. These technologies now extend beyond supplementary analysis to serve as central agents for intelligent design, predictive modeling, therapeutic customization, and performance optimization. Organized under five thematic categories—material and microneedle design, diagnostics and therapy, drug delivery, drug development, and microneedle sensors—this umbrella review synthesizes cross-domain AI applications while highlighting methodological gaps, ethical concerns, and translational potential. These insights are structured within the AIM-DO (AI-driven Microneedle Design Optimization) framework proposed in this study.
5.1. Thematic Group 1: Material and Microneedle Design
This thematic group includes similar reviews that employ AI or ML predictive modeling of hydrogel behavior, strength, and drug release kinetics to enhance microneedle and hydrogel design, with particular emphasis on mechanical properties, drug-loading efficiency, and simulation models.
For instance, He et al. (2024) investigated machine learning frameworks, such as decision trees and neural network, for predicting hydrogel behavior under varying stimuli to refine fabrication parameters, enabling accelerated prototyping and testing. Teodoro et al. (2025) highlighted the use of AI-based simulation tools in 3D-printed microneedle systems, particularly for for defect prediction and sensor calibration, materials optimization and shape prediction in 3D-printed microneedle arrays. Other contributions in this group include Negut et al. (2023), who focused on AI-enabled predictions of hydrogel conductivity and gelation time, utilized regression models and random forests to map hydrogel composition-property relationships, and Finster et al. (2024), who integrated supervised and reinforcement learning for extrusion optimization and explored high-throughput synthesis guided by ML for photodegradable hydrogels. Sofian et al. (2024) utilized CNNs to reduce bioprinting defects, achieving up to 99.7% detection accuracy demonstrating how AI-enhanced 3D printing using algae-based biopolymers could lead to more sustainable design. Parvin et al. (2023) and Shriky et al. (2023) reviewed mechanical property enhancement strategies informed by AI modeling, particularly for swelling hydrogels. Finally, Umeyor et al. (2023) discussed the use of biomimetic microstructures and predictive ML in optimizing microneedle penetration and release behavior, while Mohite et al. (2024) reviewed how AI-assisted polymer analysis could refine hydrogel microneedle formulation strategies.
In summary, these efforts align with Pillar 1 and Pillar 2 of the AIM-DO framework, supporting material discovery and microneedle design. However, several studies (e.g., Mohite et al., 2024; Shriky et al., 2023) focus on HMNs from an engineering perspective without incorporating AI, suggesting room for deeper integration of computational tools in material formulation. Common limitations included the need for explainable AI, model generalization challenges, and limited open-access datasets. Future work emphasized the development of standardized materials databases and simulation-integrated AI platforms for real-time feedback.
5.2. Thematic Group 2: Microneedles for Diagnostics and Therapy
This thematic group covers reviews centered on AI-enhanced diagnostic microneedles and therapeutic feedback systems, which demonstrates the potential for real-time biosensing and closed-loop systems.
Ashraf et al. (2025) examined the integration of microneedle wearables with deep learning for personalized biosensing, personalize stress-related interventions and adaptive drug administration. Shuo Wang et al. (2023) envisioned programmable MNs capable of logic-encoded drug release using machine learning for real-time health parameter monitoring, particularly emphasizing versatility and integration in closed-loop systems. Merzougui et al. (2025) reviewed microneedle-based interstitial fluid biopsies for cancer diagnostics, enabled by AI-assisted signal processing and predictive modeling, but AI was only conceptually proposed for signal processing. Meanwhile, Shivaswamy et al. (2024) focused on minimally invasive microneedles capable of detecting biochemical fluctuations and adjusting therapy dosage accordingly via cloud-linked AI analytics, but lacked algorithmic implementation.
In summary, these findings support AIM-DO Pillar 4—biomedical application and performance enhancement—showing how AI can tailor therapeutic delivery. However, technological gaps persist in real-time sensor calibration, cloud data security, and in-situ miniaturization of AI processors. Challenges in this group revolved around device miniaturization, real-time data processing, and validation in clinical environments. Proposed future directions included embedding edge AI processors directly in microneedle patches, federated learning models for data privacy, and adaptive therapy control using closed feedback loops.
5.3. Thematic Group 3: Microneedles for Drug Delivery
This thematic group emphasizes AI-assisted modeling is applied to sustained-release microneedle platforms empowered by AI to optimize transdermal drug delivery.
Albayati et al. (2025) discussed AI/ML strategies for overcoming transdermal barriers using predictive modeling and skin kinetic simulations, deep neural networks and SVMs to simulate skin permeability for drugs like acyclovir, achieving a 50% improvement in permeation flux. Tyagi et al. (2023) elaborated on AI-assisted drug delivery systems that adjust dose-release patterns based on patient physiology and behavior. Aghajani et al. (2025) examined riluzole microneedle applications for neurodegenerative conditions, suggesting ML to guide optimal formulation and delivery protocols. Sahada Ali et al. (2025) proposed nanotechnology-enhanced drug systems regulated via AI-based pharmacokinetic feedback. Similarly, Sakshi Priya et al. (2025) analyzed emerging cutaneous delivery platforms for rheumatoid arthritis, advocating for AI-driven formulation adjustment and precision delivery.
In summary, Aghajani et al. (2025), Shadab Ali et al. (2025), and Sakshi Priya et al. (2025) highlight empirical advances in drug-loaded HMNs without integrating AI. These limitations underscore the underutilization of AI for tuning release kinetics, reducing trial iterations, and predicting patient-specific pharmacokinetics, which are critical functions central to AIM-DO Pillars 3 and 4. Limitations reported included the complexity of modeling drug-polymer interactions and the lack of regulatory pathways for AI-guided devices. Future research suggested digital twin technologies, ML-regulated release kinetics, and integrated sensors to monitor therapeutic outcomes.
5.4. Thematic Group 4: Microneedles for Drug Development
This thematic group investigates the application of AI to the pharmaceutical formulation and drug development process.
Both Vora et al. (2023) and Dhudum et al. (2024) described AI-driven formulation design using GANs, reinforcement learning, and deep QSAR models for predicting ADMET properties and streamlining clinical trials. Vora et al. (2023) described how AI in drug delivery design enhances bioavailability and molecular targeting, using models such as deep neural networks and support vector machines. Dhudum et al. (2024) synthesized insights on how AI accelerates drug discovery and formulation optimization. Kazi Ali et al. (2024) evaluated the influence of AI techniques (such as hybrid ANN-GA systems for drug solubility, solid dispersion design, and nanoparticle formulation) on modern pharmaceutical formulation, particularly for hydrogel-embedded drugs.
In summary, these thematic findings reinforce the relevance of AIM-DO’s Pillar 5: Advanced AI Integration, and illustrate how AI accelerates decision-making in pharmaceutical R&D while reducing experimental burden. These papers highlighted how ML algorithms reduce development time by identifying promising drug-polymer combinations and optimizing pharmacodynamic profiles. Despite progress, challenges remain in AI interpretability, data heterogeneity, and regulatory compliance. Methodological limitations in this group included lack of AI/ML model transparency and challenges in tailoring formulations to diverse populations. Authors recommended advancing toward interpretable AI, establishing patient-specific formulation models, and developing ethically aligned, regulatory-compliant AI systems.
5.5. Thematic Group 5: Microneedle Sensors for Health and Agriculture
This final group focuses on microneedle-integrated biosensors used in wearable or environmental applications.
Kang Wang et al. (2025) examined non-invasive fluid analysis powered by smart wearable sensors and edge AI for real-time sweat analysis and health monitoring, highlighting applications in sports and personal health. Kharb et al. (2025) proposed biochemical wearables for real-time physiological tracking, using AI models such as CNNs and RNNs for COVID-19 early disease detection. Zhang et al. (2025) expanded this to smart sensors in plants, discussing adaptive learning systems for water stress monitoring while achieving 97% accuracy in identifying early blight in tomatoes using VOC sensors and neural networks. Kachouei et al. (2023) presented IoT-enabled food and plant sensors for agricultural sustainability, with AI used to classify environmental stressors. Ermis et al. (2025) explored body fluid monitoring sensors embedded in athletic wear, capable of continuous data collection and feedback. Sen et al. (2025) assessed biosensors for diabetes management using polymer nanocomposites and AI-powered analyte detection. Although Ermis et al. (2025) and Sen (2025) discussed sensor systems, they lacked concrete AI methodologies.
In summary, these studies showcase the expanding application of microneedles beyond clinical healthcare, into precision agriculture and personalized sports management. However, AIM-DO aims to resolve challenges such as energy constraints, signal drift, and secure data transmission remain. Challenges in this group included durability of sensors under variable conditions and energy constraints for continuous data processing. Future innovations included solar-powered edge devices, generative AI for signal calibration, and cloud-based analytics platforms for multi-sensor environments.
5.6. Challenges & Future Work
In conclusion, this review identifies AI/ML as key catalysts in transforming hydrogel microneedle systems into intelligent, personalized, and multifunctional platforms. However, widespread integration is contingent upon overcoming data, ethical, and regulatory barriers through interdisciplinary collaboration and strategic foresight.AI implementation often lacks standardization, leading to reproducibility concerns and hindered scalability. across all themes, a shared limitation is the fragmentation of datasets and inconsistent performance metrics. Additionally, ethical concerns—algorithmic bias, patient consent, and data ownership—remain unresolved and require proactive regulatory alignment (Williamson & Prybutok, 2024). The AIM-DO framework directly addresses these challenges through its modular roadmap, supporting harmonized data curation, AI explainability, and scalable deployment pathways, offering a unifying structure for accelerating innovation from bench to bedside and beyond.
6. Conclusions
This umbrella review has systematically analyzed 27 peer-reviewed review articles published between 2023 and 2025, highlighting the expanding role of artificial intelligence (AI) and machine learning (ML) in advancing hydrogel microneedle (HMN) systems across biomedical and biosensing applications. Through a thematic grouping strategy, we organized the literature into five domains—material and microneedle design, diagnostics and therapy, drug delivery, drug development, and health/agriculture sensing. These categories not only capture the diversity of AI applications but also expose the fragmented state of research and uneven implementation of computational tools across domains.
A key contribution of this review is the introduction of the AIM-DO (AI-driven Microneedle Design Optimization) framework, which provides a five-pillar roadmap for deploying AI/ML across HMN development pipelines: (1) material discovery and optimization, (2) structural and mechanical design, (3) manufacturing process optimization, (4) biomedical application and performance enhancement, and (5) advanced AI integration. This framework emphasizes the need for harmonized datasets, predictive modeling, quality control, and real-time feedback loops, all of which are critical for transitioning HMNs from research labs to clinical and commercial applications.
Across the literature, AI has demonstrated value in simulating hydrogel behaviors, predicting drug release profiles, automating fabrication, and enabling biosensing through edge-AI and cloud-based analytics. However, challenges remain in model interpretability, ethical governance, data standardization, and regulatory compliance. These barriers must be addressed through cross-disciplinary collaboration involving data scientists, material engineers, clinicians, and policy makers.
What sets this review apart is its cross-sector synthesis, bridging insights from diverse AI-enabled domains while offering a unified development strategy. As AI/ML capabilities continue to evolve, their responsible integration into microneedle systems promises to accelerate innovation in precision drug delivery, minimally invasive diagnostics, and real-time health monitoring. By aligning technological progress with ethical foresight and translational strategies, the future of AI-enhanced HMNs can move toward personalized, intelligent, and scalable biomedical solutions.