1. Introduction
Neuromuscular diseases (NMD) encompass a spectrum of conditions with varying degrees of muscle weakness, fatigue, and impairment in movement. NMD has a significant impact on an individual’s ability to perform physical activities due to progressive muscle weakness, contributing to a sedentary lifestyle that further exacerbates their symptoms [
1]. In addition, the reduced physical activity level increases the risk of cardiovascular and pulmonary diseases, as well as muscle atrophy [
1,
2]. Exercise recommendations in NMD have been varied, reflecting the diverse evidence base and incomplete understanding of the mechanisms underlying exercise recovery and response in this population [
3,
4].
Recent evidence suggests that aerobic exercise is a safe and effective treatment option for most individuals with NMD. Still, there remains a lack of clarity regarding exercise prescription and recovery time, and it remains unclear what metrics are most important in determining positive responses to exercise interventions [
5,
6]. In a recent survey of neuromuscular clinicians, 81% of respondents believed that exercise should be integrated into care protocols. Nevertheless, a primary barrier identified was insufficient knowledge regarding accurately prescribing and monitoring exercise responses in this population [
7].
Several physiological processes are involved in exercise recovery, including removing metabolic waste products, repairing tissues, and replenishing energy [
8]. The effectiveness and duration of recovery depend on factors such as exercise intensity, individual fitness level, and underlying health conditions [
9]. Addressing factors that may impair recovery, such as chronic inflammation or metabolic disorders, may improve exercise performance and overall physical health [
8].
Cardiorespiratory fitness (CRF) is a critical metric determining an individual’s ability to use oxygen efficiently during physical exertion. Cardiopulmonary exercise testing (CPET) is the gold-standard method for evaluating CRF, which measures variables such as peak oxygen consumption (VO
2), ventilation (VE), and carbon dioxide output (VCO
2) during incremental exercise [
10,
11]. Recent work has focused on analyzing the recovery patterns following CPET, as it may provide valuable insights into the underlying mechanisms of various diseases; as they provide valuable insights into the underlying mechanisms of various diseases, and recovery patterns can provide important insights into understanding cardiopulmonary training effects in different chronic conditions [
12,
13,
14,
15,
16,
17].
While heart rate (HR) recovery is an established diagnostic and prognostic indicator for cardiovascular disease, understanding gas exchange recovery kinetics remains limited. Yet, gas exchange recovery kinetics is hypothesized to provide complementary information and may improve prognosis and diagnosis related to cardiorespiratory disease [
14,
18,
19]. Recent studies have demonstrated that individuals with diabetes and chronic heart failure (CHF) exhibit slower VO
2 recovery after exercise. Specifically, CHF individuals require more time to return to baseline VO
2 values, and the severity of the disease negatively impacts their VO
2 recovery [
15,
20,
21,
22,
23].
For individuals with NMD, exercise recovery can be compromised by both cardiorespiratory difficulties and muscle impairment, resulting in mitochondrial abnormalities, metabolic dysfunction, and inflammatory responses to exercise [
24]. These factors can lead to delayed-onset muscle soreness, prolonged fatigue, and impaired muscle regeneration, thus highlighting the need to consider exercise-specific muscle responses in treatment strategies.
In this study, we aimed to evaluate the differences in physiological recovery variables between individuals with NMD and healthy controls.
2. Materials and Methods
Patients with NMD were prospectively recruited from the Neuroscience Health Center at Stanford University between 2023 and 2024. All had limited mobility, which was defined as the inability to perform cycle ergometry or treadmill ambulation safely. Individuals were excluded if they could not provide informed consent/assent, had engaged in regular treadmill or upright cycle ergometry exercise within the last six months, or were deemed unable to exercise vigorously by the study physicians. Participants in the control group were recruited from the local community, did not have medical diagnoses that would prohibit maximal exercise testing performance, and efforts were made to match for age and sex. The study was approved by the Institutional Review Board at Stanford University. All research staff were trained in good clinical practice, and all participants provided written informed consent or assent before any study procedures.
Thirty-six participants with NMD were initially included in the study; four patients were excluded due to technical errors that resulted in incomplete and/or unreliable CPET data (two with myotonic dystrophy, one with Duchenne muscular dystrophy, and one with limb-girdle muscular dystrophy. Thirty-two patients with NMD and fifteen controls were included.
2.1. Cardiopulmonary Exercise Testing (CPET)
CPET was performed using a CosMed K5 wearable metabolic system (COSMED USA Inc, Concord, CA, USA) and a Keiser wheelchair-accessible total body trainer (Keiser Corporation, Fresno, CA, USA) that allowed individuals to use lower limb strength in an elliptical pattern and upper limb strength in a push-pull pattern.
CPET was performed by clinical exercise physiologists and physical therapists and monitored by a physician familiar with the individuals and the study protocols. Respiratory gas exchange data were collected and analyzed after applying a 30-second rolling average filter (sampled every 10 seconds) and were calculated as the highest 30-second average during the last phase of the CPET at the point of peak ventilation. The percentage of age-predicted peak VO
2 reached by participants was calculated using the Fitness Registry and Importance of Exercise National Database (FRIEND) Registry protocol [
25]. Peak HR (bpm), rating of perceived exertion (RPE 6–20; Borg Perception AB, Akersberga, Sweden), and workload (W) were calculated as the maximal number recorded during exercise. The VE/VCO
2 slope was calculated for the entire exercise period [
25].
Recovery was defined as the period from the end of exercise until the cessation of the measurement (a five-minute period). Respiratory gasses were analyzed as a percentage change from the time of exercise cessation to the time to reach 50% (T1/2) of peak VO2 during recovery. The overshoot percent change from peak values of the metrics displaying an overshoot during recovery −respiratory exchange ratio (RER or VCO2/VO2 ratio), ventilatory equivalents for oxygen (VE/VO2) and carbon dioxide (VE/VCO2), and partial pressures of oxygen (PETO2) were calculated as the magnitude of the overshoot (i.e., the maximum percentage increase from peak). The half-time of recovery for respiratory metrics − volume of exhaled oxygen (VO2), volume of exhaled carbon dioxide (VCO2), and ventilation (VE) − were defined as the time required to reach 50% of peak.
2.2. Statistical Analysis
CPET variables of interest were extracted from the metabolic cart filtered as described above. Data were analyzed using the R statistical programming language in Rstudio IDE (R 4.3.2). Before conducting inferential statistics, data normality was assessed using the Shapiro-Wilk test, which indicated a non-normal distribution of all data. Consequently, we adopted a non-parametric test approach. We applied a Wilcoxon rank-sum test for differences between groups (NMD vs. Control), setting the statistical significance at an alpha level of 0.05. The descriptive statistics, including mean and standard deviation and median and interquartile range (IQR), were calculated and presented in the results section in the text and accompanying tables.
3. Results
Table 1 presents the demographic statistics of the 32 individuals by disease group. There were no significant differences in age, height, body weight, and BMI between the control and NMD groups.
Table 2 shows significant differences between the control and NMD groups during CPET. Peak workload was significantly higher in the control group compared to the NMD group (199 ± 58.9 W vs 66.8 ± 42.7 W; p<0.001). Absolute and relative peak VO
2 were significantly higher in the control group (2.51 ± 0.81 vs. 1.44 ± 0.62 L/min, 35.9 ± 10.3 kg/ml/min vs. 20.6 ± 6.87 ml/kg/min; both p<0.001). Similar results were observed regarding the percentage of age-predicted peak VO
2 (106 ± 27.6% vs. 59.0 ± 27.3%; p<0.001). Peak RER and peak HR were also significantly higher in the control group (1.16 ± 0.11 vs. 1.07 ± 0.14; p <0.02 and 172 ± 9.95 bpm vs. 152 ± 22.8 bpm; p<0.001). The surrogate marker for stroke volume, O
2 pulse, calculated as the ratio of VO
2 to HR and expressed as the volume of oxygen consumed with each cardiac contraction, was significantly higher in the control group, with a mean value of 16.1 ± 4.71 ml/bpm, compared to the NMD group, which had a mean value of 9.55 ± 4.32 ml/bpm (p<0.001).
As shown in
Table 3, physiological recovery parameters after CPET differed significantly between the control group and the NMD group. For T1/2 VO
2, the control group had a faster O
2 recovery (76.0±36.4 s) versus the NMD group (105 ± 43.4 s; p=0.02). The recovery time for the O
2 pulse (T1/2 O
2 pulse) was also significantly longer in the NMD group compared to the control group (172±60.3 s vs. 114±62.2 s; p<0.001).
Significant differences were observed between the groups for the overshoot parameters. The control group exhibited a mean overshoot of RER of 28.8 ± 9.03%, significantly higher than the NMD group’s mean of 17.1 ± 13.0% (p<0.001). The mean overshoot of VE/VO2 was also higher in the control group; 52.2 ± 23.5% versus 31.9 ± 28.3%, respectively (p=0.01). A difference was also noted in the overshoot of VE/VCO2, with the control group having a mean of 27.9 ± 15.7% versus the NMD group mean of 20.3 ± 20.8% (p=0.04).
4. Discussion
This study investigated recovery metrics following peak exercise among individuals with NMD. Established metrics for CPET testing, including peak VO
2 and ventilatory efficiency (VE/VCO
2 slope), have been used as indicators of CRF. However, physiological responses during recovery provide additional insights into fatigability, exercise intolerance, and muscle recovery mechanisms. Although HR has been a well-established method to assess recovery, understanding gas exchange kinetics may provide more insight into central versus peripheral muscular determinants for aerobic capacity [
18]. A study by Cohen-Solal et al. found that delayed VO
2 recovery may explain the delayed replenishment of muscle energy stores [
26]. This deficiency in energy stores has been associated with poor aerobic efficiency.
Recent research underscores the significance of overshoot metrics during recovery as metabolic and cardiovascular function markers across various patient populations [
7,
14,
15,
16,
21]. Vecchiato et al. demonstrated that elevated RER overshoot in young CHD patients reflected impaired oxygen delivery and exercise intolerance [
15,
16]. In contrast, low RER overshoot during recovery in patients with heart failure with reduced ejection fraction (HFrEF) is associated with poorer clinical outcomes [
15,
16]. Similarly, studies by Takayanagi and Patti et al. showed that reduced overshoot metrics, particularly RER, correlate with the severity of heart failure [
14,
21]. We observed that patients with NMD and low mobility showed distinct impairments in recovery, particularly in overshoot parameters, especially RER overshoot, compared with controls. However, unlike CHD and HFrEF, where recovery limitations are primarily driven by cardiovascular dysfunction, recovery impairments in NMD likely stem from skeletal muscle dysfunction. These findings highlight the value of RER overshoot as a key metric for understanding recovery physiology and tailoring interventions for diverse clinical populations, including those with neuromuscular diseases. Future studies should explore its prognostic relevance and potential role in guiding individualized rehabilitation strategies for NMD patients.
Our study findings reveal substantial differences in recovery exercise variables between individuals with NMD and controls. The peak exercise variables highlight significant distinctions in exercise capacity between the two groups. Notably, the control group demonstrated higher peak workload, peak VO
2, percentage of age-predicted peak VO
2, peak HR, and O
2 pulse versus the NMD group. These findings coincide with symptoms in individuals with NMD due to impaired muscle function and other factors associated with functional limitations [
5,
10,
27,
28].
Regarding recovery metrics, the NMD exhibited longer VO
2 recovery time (T1/2 VO2) compared to controls. They also showed lower overshoot values for RER and VE/VO2 ratio, likely driven by CO
2 output and decreased plasma pH. These delayed recovery patterns are similar to those observed in patients with CVD [
14,
15,
26], likely reflecting impaired muscle energy replenishment and cardiovascular limitations restricting oxygen delivery and utilization [
26].
While delayed VE and VCO
2 recovery reflect impaired cardiac function for individuals with CHF [
21,
26,
29], the absence of differences in the T1/2 of VE and VCO
2 between the control and NMD groups suggest that recovery limitations in NMD may stem from distinct peripheral mechanisms rather than cardiovascular limitations to oxygen delivery; the prolonged oxygen uptake kinetics likely stem primarily from skeletal muscle impairments that restrict cellular energy replenishment after peak exertion. However, this difference from cardiac patients may depend on the specific NMD subtype, as some neuromuscular disorders have associated cardiac involvement [
24,
30]. Thus, while some recovery metrics such as T1/2 VO
2 mirror patterns seen in CHF, preserved VE and VCO
2 recovery indicate the participation of different pathological processes centered in the muscle itself. However, cardiovascular comorbidities in certain NMD subpopulations could display hybrid cardiac-muscular recovery phenotypes [
14,
26].
Future studies should explore the reasons behind limitations in oxygen utilization and identify whether they stem from muscle-level factors or are associated with factors related to ventilatory insufficiency. Including CPET recovery parameters among individuals with NMD will enhance our ability to stratify NMD cohorts with known cardiovascular deficits compared to those without cardiorespiratory complications, providing valuable insights into potential impairments in muscle metabolomics or cardiorespiratory responses. Additionally, integrating dual-energy X-ray absorptiometry (DEXA) will enhance our understanding of the interplay between muscle and cardiopulmonary impairments, enabling a more individualized approach to exercise prescriptions. These insights into recovery have the potential to enhance the measurement of function and fatigue following physical exertion and inform recommendations for improving recovery responses in NMD. With additional CPET data in NMD, we may be better equipped to differentiate between individuals based on factors such as ventilatory insufficiency or muscle composition.
The current study, as in many studies in NMD, was limited by a relatively small and heterogeneous sample of convenience. Nonetheless, the sample represents a real-world example of individuals with NMD, reflecting those who would be seen in a typical NMD clinic. Another limitation of our analysis was the inability to investigate recovery metrics over time. In addition, we lacked DEXA scans, which would have provided valuable insights into body composition, particularly muscle mass. Integrating DEXA scans with our CPET data could have elucidated how variations in muscle mass impact exercise performance, allowing for a more comprehensive understanding of exercise recovery among individuals with differing body composition.
5. Conclusions
Many individuals with NMD experience exertional impairments characterized by fatigue, exercise intolerance, breathlessness, limited ability for sustained activity, and reduced engagement in an active lifestyle [
5,
6,
31]. Analyzing cardiorespiratory recovery responses from maximal exercise offers insights into peripheral and central mechanisms that contribute to reduced oxygen utilization. A thorough grasp of recovery metrics enhances precision in tailoring home exercise programs and understanding rest, recovery, and fatigability. Improved comprehension of these metrics may offer a novel approach to assessing improvements in CRF, serving as a valuable endpoint for measuring exercise capacity and function for individuals with NMD.
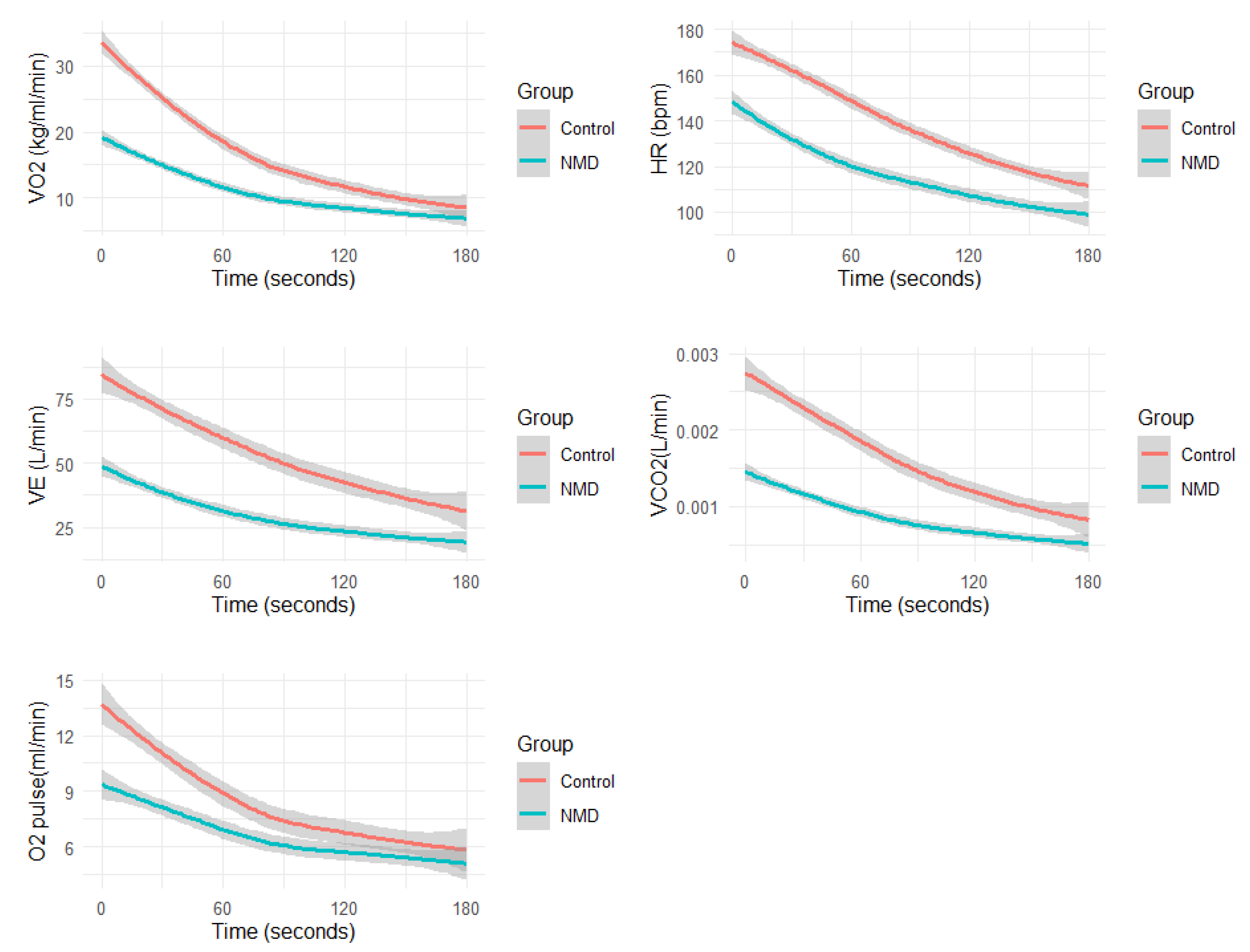
Figure 1.
Average physiological responses during recovery: Oxygen Consumption (VO2), Carbon Dioxide Production (VCO2), Minute Ventilation (VE), Oxygen Consumption per Heart Rate (VO2/HR), and Heart Rate (HR).
Figure 1.
Average physiological responses during recovery: Oxygen Consumption (VO2), Carbon Dioxide Production (VCO2), Minute Ventilation (VE), Oxygen Consumption per Heart Rate (VO2/HR), and Heart Rate (HR).
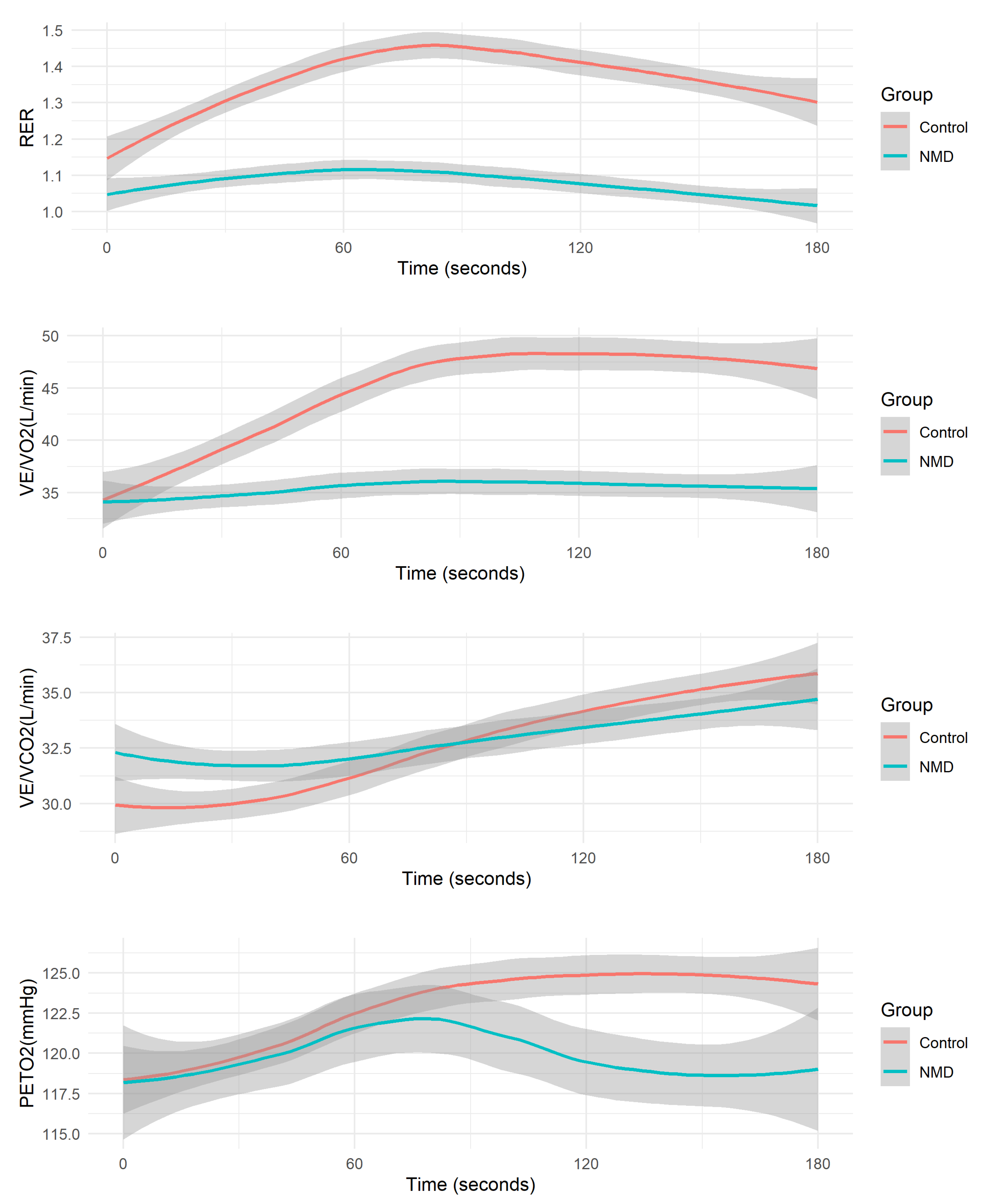
Figure 2.
Average Overshoot Metrics during Recovery: Respiratory Exchange Ratio (RER), Ventilation to Oxygen Consumption Ratio (VE/VO2), Ventilation to Carbon Dioxide Production Ratio (VE/VCO2), and Partial Pressure of Oxygen (PETO2).
Figure 2.
Average Overshoot Metrics during Recovery: Respiratory Exchange Ratio (RER), Ventilation to Oxygen Consumption Ratio (VE/VO2), Ventilation to Carbon Dioxide Production Ratio (VE/VCO2), and Partial Pressure of Oxygen (PETO2).
Author Contributions
Conceptualization and Methodology: YB, JWC, TD, AP; Data Collection, Analysis, and Interpretation: CDM, PEA, WJT, SM, AP, CDM, DA; Data Collection and Manuscript Review: SDY, NNG, DMP, JM; Writing—Original Draft: YB, JWC, SM, CDM; Supervision and Review: JWC, TD, YB, JWD, MW, EAA, FH; Methodology, Software, Validation: SM, DA; Editing and Proofreading: Word tune AI-assisted technology.
Funding
This research received no external funding.
References
- Schillings, M.L.; Kalkman, J.S.; Janssen, H.M.; van Engelen, B.G.; Bleijenberg, G.; Zwarts, M.J. Experienced and physiological fatigue in neuromuscular disorders. Clin Neurophysiol 2007, 118, 292–300. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Torres, R.S.; Uher, D.; Gay, E.L.; Coratti, G.; Dunaway Young, S.; Rohwer, A.; Muni Lofra, R.; De Vivo, D.C.; Hirano, M.; Glynn, N.W.; et al. Measuring Fatigue and Fatigability in Spinal Muscular Atrophy (SMA): Challenges and Opportunities. J Clin Med 2023, 12. [Google Scholar] [CrossRef] [PubMed]
- Lanfranconi, F.; Marzorati, M.; Tremolizzo, L. Editorial: Strategies to Fight Exercise Intolerance in Neuromuscular Disorders. Front Physiol 2020, 11, 968. [Google Scholar] [CrossRef]
- Siciliano, G.; Chico, L.; Lo Gerfo, A.; Simoncini, C.; Schirinzi, E.; Ricci, G. Exercise-Related Oxidative Stress as Mechanism to Fight Physical Dysfunction in Neuromuscular Disorders. Front Physiol 2020, 11, 451. [Google Scholar] [CrossRef] [PubMed]
- Markert, C.D.; Case, L.E.; Carter, G.T.; Furlong, P.A.; Grange, R.W. Exercise and Duchenne muscular dystrophy: where we have been and where we need to go. Muscle Nerve 2012, 45, 746–751. [Google Scholar] [CrossRef]
- Anziska, Y.; Sternberg, A. Exercise in neuromuscular disease. Muscle Nerve 2013, 48, 3–20. [Google Scholar] [CrossRef]
- Voorn, E.L.; Koopman, F.; Nollet, F.; Brehm, M.A. Aerobic exercise in adult neuromuscular rehabilitation: A survey of healthcare professionals. J Rehabil Med 2019, 51, 518–524. [Google Scholar] [CrossRef]
- Peake, J.M. Recovery after exercise: what is the current state of play? Current Opinion in Physiology 2019, 10, 17–26. [Google Scholar] [CrossRef]
- Romero, S.A.; Minson, C.T.; Halliwill, J.R. The cardiovascular system after exercise. J Appl Physiol (1985) 2017, 122, 925–932. [Google Scholar] [CrossRef]
- Christle, J.W.; Duong, T.; Parker, D.; Stevens, V.; Dunaway Young, S.; Kaufman, B.D.; Tang, W.; Sampson, J.; Myers, J.; Ashley, E.A.; et al. Cardiopulmonary Exercise Testing for Patients With Neuromuscular Disease and Limited Mobility. Journal of Clinical Exercise Physiology 2023, 12, 12–17. [Google Scholar] [CrossRef]
- Arena, R.; Myers, J.; Williams, M.A.; Gulati, M.; Kligfield, P.; Balady, G.J.; Collins, E.; Fletcher, G.; American Heart Association Committee on Exercise, R.; Prevention of the Council on Clinical, C.; et al. Assessment of functional capacity in clinical and research settings: a scientific statement from the American Heart Association Committee on Exercise, Rehabilitation, and Prevention of the Council on Clinical Cardiology and the Council on Cardiovascular Nursing. Circulation 2007, 116, 329–343. [Google Scholar] [CrossRef] [PubMed]
- Lim, Z.X.; Gyanwali, B.; Soh, J.; Koh, A.S.; Goh, J. The potential benefits of assessing post-cardiopulmonary exercise testing (CPET) in aging: a narrative review. BMC Sports Sci Med Rehabil 2023, 15, 68. [Google Scholar] [CrossRef]
- Luttrell, M.J.; Halliwill, J.R. Recovery from exercise: vulnerable state, window of opportunity, or crystal ball? Front Physiol 2015, 6, 204. [Google Scholar] [CrossRef]
- Patti, A.; Blumberg, Y.; Hedman, K.; Neunhauserer, D.; Haddad, F.; Wheeler, M.; Ashley, E.; Moneghetti, K.J.; Myers, J.; Christle, J.W. Respiratory gas kinetics in patients with congestive heart failure during recovery from peak exercise. Clinics (Sao Paulo) 2023, 78, 100225. [Google Scholar] [CrossRef]
- Vecchiato, M.; Neunhaeuserer, D.; Zanardo, E.; Quinto, G.; Battista, F.; Aghi, A.; Palermi, S.; Babuin, L.; Tessari, C.; Guazzi, M.; et al. Respiratory exchange ratio overshoot during exercise recovery: a promising prognostic marker in HFrEF. Clin Res Cardiol 2024. [Google Scholar] [CrossRef]
- Vecchiato, M.; Ermolao, A.; Zanardo, E.; Battista, F.; Ruvoletto, G.; Palermi, S.; Quinto, G.; Degano, G.; Gasperetti, A.; Padalino, M.A.; et al. Overshoot of the Respiratory Exchange Ratio during Recovery from Maximal Exercise Testing in Young Patients with Congenital Heart Disease. Children (Basel) 2023, 10. [Google Scholar] [CrossRef]
- Myers, J.N.; Gujja, P.; Neelagaru, S.; Hsu, L.; Burkhoff, D. Noninvasive measurement of cardiac performance in recovery from exercise in heart failure patients. Clinics (Sao Paulo) 2011, 66, 649–656. [Google Scholar] [CrossRef]
- Cole, C.R.; Blackstone, E.H.; Pashkow, F.J.; Snader, C.E.; Lauer, M.S. Heart-rate recovery immediately after exercise as a predictor of mortality. N Engl J Med 1999, 341, 1351–1357. [Google Scholar] [CrossRef]
- Sydo, N.; Sydo, T.; Gonzalez Carta, K.A.; Hussain, N.; Farooq, S.; Murphy, J.G.; Merkely, B.; Lopez-Jimenez, F.; Allison, T.G. Prognostic Performance of Heart Rate Recovery on an Exercise Test in a Primary Prevention Population. J Am Heart Assoc 2018, 7. [Google Scholar] [CrossRef]
- Takahashi, T.; Niizeki, K.; Miyamoto, Y. Respiratory responses to passive and active recovery from exercise. Jpn J Physiol 1997, 47, 59–65. [Google Scholar] [CrossRef]
- Takayanagi, Y.; Koike, A.; Nagayama, O.; Nagamine, A.; Qin, R.; Kato, J.; Nishi, I.; Himi, T.; Kato, Y.; Sato, A.; et al. Clinical significance of the overshoot phenomena of respiratory gas indices during recovery from maximal exercise testing. J Cardiol 2017, 70, 598–606. [Google Scholar] [CrossRef]
- Bailey, C.S.; Wooster, L.T.; Buswell, M.; Patel, S.; Pappagianopoulos, P.P.; Bakken, K.; White, C.; Tanguay, M.; Blodgett, J.B.; Baggish, A.L.; et al. Post-Exercise Oxygen Uptake Recovery Delay: A Novel Index of Impaired Cardiac Reserve Capacity in Heart Failure. JACC Heart Fail 2018, 6, 329–339. [Google Scholar] [CrossRef]
- Tanabe, Y.; Takahashi, M.; Hosaka, Y.; Ito, M.; Ito, E.; Suzuki, K. Prolonged recovery of cardiac output after maximal exercise in patients with chronic heart failure. J Am Coll Cardiol 2000, 35, 1228–1236. [Google Scholar] [CrossRef] [PubMed]
- Feingold, B.; Mahle, W.T.; Auerbach, S.; Clemens, P.; Domenighetti, A.A.; Jefferies, J.L.; Judge, D.P.; Lal, A.K.; Markham, L.W.; Parks, W.J.; et al. Management of Cardiac Involvement Associated With Neuromuscular Diseases: A Scientific Statement From the American Heart Association. Circulation 2017, 136, e200–e231. [Google Scholar] [CrossRef]
- Kaminsky, L.A.; Arena, R.; Myers, J.; Peterman, J.E.; Bonikowske, A.R.; Harber, M.P.; Medina Inojosa, J.R.; Lavie, C.J.; Squires, R.W. Updated Reference Standards for Cardiorespiratory Fitness Measured with Cardiopulmonary Exercise Testing: Data from the Fitness Registry and the Importance of Exercise National Database (FRIEND). Mayo Clin Proc 2022, 97, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Cohen-Solal, A.; Laperche, T.; Morvan, D.; Geneves, M.; Caviezel, B.; Gourgon, R. Prolonged kinetics of recovery of oxygen consumption after maximal graded exercise in patients with chronic heart failure. Analysis with gas exchange measurements and NMR spectroscopy. Circulation 1995, 91, 2924–2932. [Google Scholar] [CrossRef] [PubMed]
- Barroso de Queiroz Davoli, G.; Bartels, B.; Mattiello-Sverzut, A.C.; Takken, T. Cardiopulmonary exercise testing in neuromuscular disease: a systematic review. Expert Rev Cardiovasc Ther 2021, 19, 975–991. [Google Scholar] [CrossRef]
- Torri, F.; Lopriore, P.; Montano, V.; Siciliano, G.; Mancuso, M.; Ricci, G. Pathophysiology and Management of Fatigue in Neuromuscular Diseases. Int J Mol Sci 2023, 24. [Google Scholar] [CrossRef]
- Patti, A.; Neunhaeuserer, D.; Gasperetti, A.; Baioccato, V.; Vecchiato, M.; Battista, F.; Marchini, F.; Bergamin, M.; Furian, L.; Ermolao, A. Overshoot of the Respiratory Exchange Ratio during Recovery from Maximal Exercise Testing in Kidney Transplant Recipients. Int J Environ Res Public Health 2021, 18. [Google Scholar] [CrossRef]
- Finsterer, J.; Stollberger, C. Heart Disease in Disorders of Muscle, Neuromuscular Transmission, and the Nerves. Korean Circ J 2016, 46, 117–134. [Google Scholar] [CrossRef]
- Voet, N.B.M. Exercise in neuromuscular disorders: a promising intervention. Acta Myol 2019, 38, 207–214. [Google Scholar] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).