1. Introduction
Hyperbranched Polyglycerols (HPG) are highly functional, hydrophilic, aliphatic polyol polyethers that have been explored and developed as one of the most promising products for applications in industry, drug delivery, and other biomedical purposes [
1]. Repeated branching within a molecule is known as a dendritic molecule. Dendritic structures are a class of macromolecules with monodisperse macrostructures featuring numerous branches. Dendritic molecules are divided into cascades, dendrimers, and hyperbranched molecules. Cascades consist of molecules of the same type and weight, resulting in a more perfect structure. In contrast, hyperbranched molecules exhibit repeated branching that can differ in type and molecular weight [
2]. Dendrimers and dendritic molecules are recognised as new polymers with structural forms and applications distinct from conventional polymers.
Glycerol polymerization into polyglycerol occurs through ring-opening polymerization with a base catalyst, forming a dendrimer structure. The reaction occurs due to a nucleophilic attack on the hydroxyl group. Dendritic polyglycerol has a branched structure with many hydroxyl groups at the ends of its structure. Wilms et al. (2009) have developed several strategies to create hyperbranched materials that more closely resemble dendrimers. Sunder and Mülhaupt (2000) described the multibranching polymerization of glycidol ring-opening (ROMBP). The ring-opening reaction of glycidol with secondary hydroxyl groups of glycerol or oligomers forms branched polyglycerol. The primary reaction is the attack of a primary or secondary alkoxide anion on the carbon atom of the glycidol ring, leading to the formation of hydroxyalkyl ether with an epoxide ring opening [
3].
Glycerol polymerization is generally performed by indirect polymerization by forming epichlorohydrin and glycidol [
4]. The drawback of this method is the very low product formation and the high formation of by-products in the form of chloride salts, requiring further separation processes [
5,
6,
7]. Additionally, epichlorohydrin is a highly toxic and carcinogenic compound [
4]. Another method is direct polymerization, where glycerol polymerization uses strong homogeneous base catalysts such as KOH or NaOH [
8,
9]. The disadvantage of this process is that it occurs at a temperature of 200–275°C with a process duration of 8–24 hours under a CO2/N2 gas flow. In other studies, using homogeneous and heterogeneous catalysts in the glycerol polymerization process can influence the degree of polymerization. The use of heterogeneous calcium oxide (CaO) catalysts can promote the polymerization of glycerol into low molecular weight polyglycerol at low temperatures (200–230°C) and induce higher degrees of polymerization at higher temperatures >230°C, which is better compared to other catalysts. The drawback of this catalyst is the solubility of CaO due to water formation during the process, leading to decreased activation power in the reaction process [
4]. Based on calculating the diglycerol formation reaction mechanism following the SN2 route using HyperChem software, the base catalyst (NaOH) interaction energy with glycerol is 529.38 kJ/mol [
10].
The use of ultrasonic technology (USG) in chemistry began in the 1930s, and since then, USG has been successfully applied to various chemical reactions and processes. Ultrasonication was proven to be a beneficial method for depolymerising macromolecules, as it can reduce molecular weight by breaking the weakest chemical bonds without causing changes in the polymer's chemical properties [
11,
12]. Gandhi’s use of ultrasonic methods in biodiesel synthesis showed higher yields at lower temperatures and shorter reaction times than conventional methods. At a temperature of 45°C and a reaction time of 45 minutes with a molar ratio of oil to alcohol of 1:9 and 0.5% KOH catalyst weight, a conversion of 93% was achieved using Ultrasonic Horn, 55% with Ultrasonic Batch. In contrast, conventional methods under the same conditions only resulted in 39% conversion [
13].
Not many publications explain the optimal conditions for glycerol polymerization synthesis to obtain hyperbranched/dendritic polyglycerol. On the other hand, although USG technology has been widely applied in various processes, its role in the synthesis of HPG is not well understood. The USG process is a versatile method that can be applied to various monomers, solvents, and polymerization techniques, including free radical polymerization and ring-opening polymerization [
14,
15]. Therefore, this research aims to determine the effect of USG technology in the synthesis of HPG and its characterisation based on the amount of glycerol monomer formed according to molecular weight using the Ostwald viscometer method and the chemical structure of the formed polyglycerol.
2. Materials and Methods
2.1. Material
The materials used in the synthesis of HPG using sonification are technical grade glycerol 96% (PT. Brataco), Analytic grade potassium hydroxide ≥ 85% (Merck) and Analytic grade sodium hydroxide ≥ 99% (Merck).
2.2. Synthesis Process
The HPG synthesis process using USG technology employed an Ultrasonic Horn unit, model VCX750/VCX500, from Sonic & Material, Inc. The method used in this activity is direct polymerization, where glycerol polymerization is carried out using strong homogeneous base catalysts such as KOH or NaOH. In this activity, several process variables that affect the formation of glycerol monomers were investigated, considering the molecular weight and chemical structure of the resulting HPG. The process variables include using 4% KOH and NaOH catalysts and variations in USG power at 100, 150, and 200 watts. The process begins by reacting 98% glycerol with 4% KOH/NaOH catalyst by weight of 98% glycerol for 150 minutes. After the process, purification is performed to separate the KOH/NaOH catalyst from the product using absorption techniques with cation exchange resin. After achieving a neutral pH of 6-7, the product is characterised using FTIR, NMR, and molecular weight analysis via the Ostwald viscometer method.
Figure 1.
Ultrasonic Horn.
Figure 1.
Ultrasonic Horn.
2.3. Identification and Characterisation
The HPG product's identification and characterisation included determining functional groups. Identification was performed using a Fourier Transform Infrared Spectrophotometer (FTIR Shimadzu/IR Prestige-21), chemical structure analysis was conducted using Nuclear Magnetic Resonance Spectroscopy (H-NMR and C-NMR BRUKER ASCEND 700MHz), and molecular weight was determined using the Ostwald viscometry method (Mark-Houwink).
2.4. Determination of Viscosity of Glycerol and HPG
2.4.1. Determination of Glycerol Viscosity
The viscosity of glycerol solutions with concentrations of 10%, 20%, 30%, 50%, 70%, and 100% was measured using an Ostwald viscometer. The time required for the sample to flow from the upper limit to the lower limit of the viscometer was recorded. Measurements were taken three times.
2.4.2. Determination of HPG Viscosity
The viscosity of HPG solutions with concentrations of 10%, 20%, 30%, 50%, 70%, and 100% was measured using an Ostwald viscometer. The time required for the sample to flow from the upper limit to the lower limit of the viscometer was recorded. Measurements were taken three times.
2.5. Determination of Density/Mass Density of Glycerol and HPG
Density measures the amount of substance contained in a unit volume. The density of the solution was measured using a 25 ml pycnometer. The solution was made by mixing glycerol with water and HPG with water at various concentrations. The formula for determining density is:
where:
| (Rho) |
= |
Density (g/ml) |
| m |
= |
Mass of sample (g) |
| v |
= |
Volume of sample (ml) |
2.5.1. Determination of Glycerol Density
Weigh the empty pycnometer and record its mass. Insert the glycerol solution sample with concentrations of 10%, 20%, 30%, 50%, 70%, and 100% up to the neck of the pycnometer, weigh the filled pycnometer, and then record and calculate the density.
2.5.2. Determination of HPG Density
Weigh the empty pycnometer and record its mass. Insert the HPG solution sample with concentrations of 10%, 20%, 30%, 50%, 70%, and 100% up to the neck of the pycnometer, weigh the filled pycnometer, and then record and calculate the density.
2.6. Determination of Intrinsic Viscosity Using the Least Square Method
The intrinsic viscosity [η] was determined using the Least Square method by calculating the relative viscosity [ŋ
rel], specific viscosity [ŋ
sp], and reduced viscosity [ŋ
red]. Relative viscosity was determined by the ratio between the sample solution flow time (t) and the pure solvent flow time (t
0). Specific viscosity (η
sp) and reduced viscosity (η
red) were determined using the following equations [
15]:
2.7. Determination of Molecular Weight Using the Mark-Houwink Equation
Molecular weight was determined by determining the intrinsic viscosity obtained from the curve between reduced viscosity and concentration. Intrinsic viscosity was calculated using the Mark-Houwink equation [
16]. Here, η (intrinsic viscosity), M (molecular weight), and α, constant K for a specific solvent system. K and α are Mark-Houwink constants: K = 2×10
−5 l/g, α = 0,71 (for glycerol and HPG solvent at 25°C).
The Mark-Houwink equation is as follows:
2.8. Determination of Degree of Polymerization (DP)
The degree of polymerization was calculated based on the ratio between the obtained molecular weight and the molecular weight of the structural unit.
3. Results
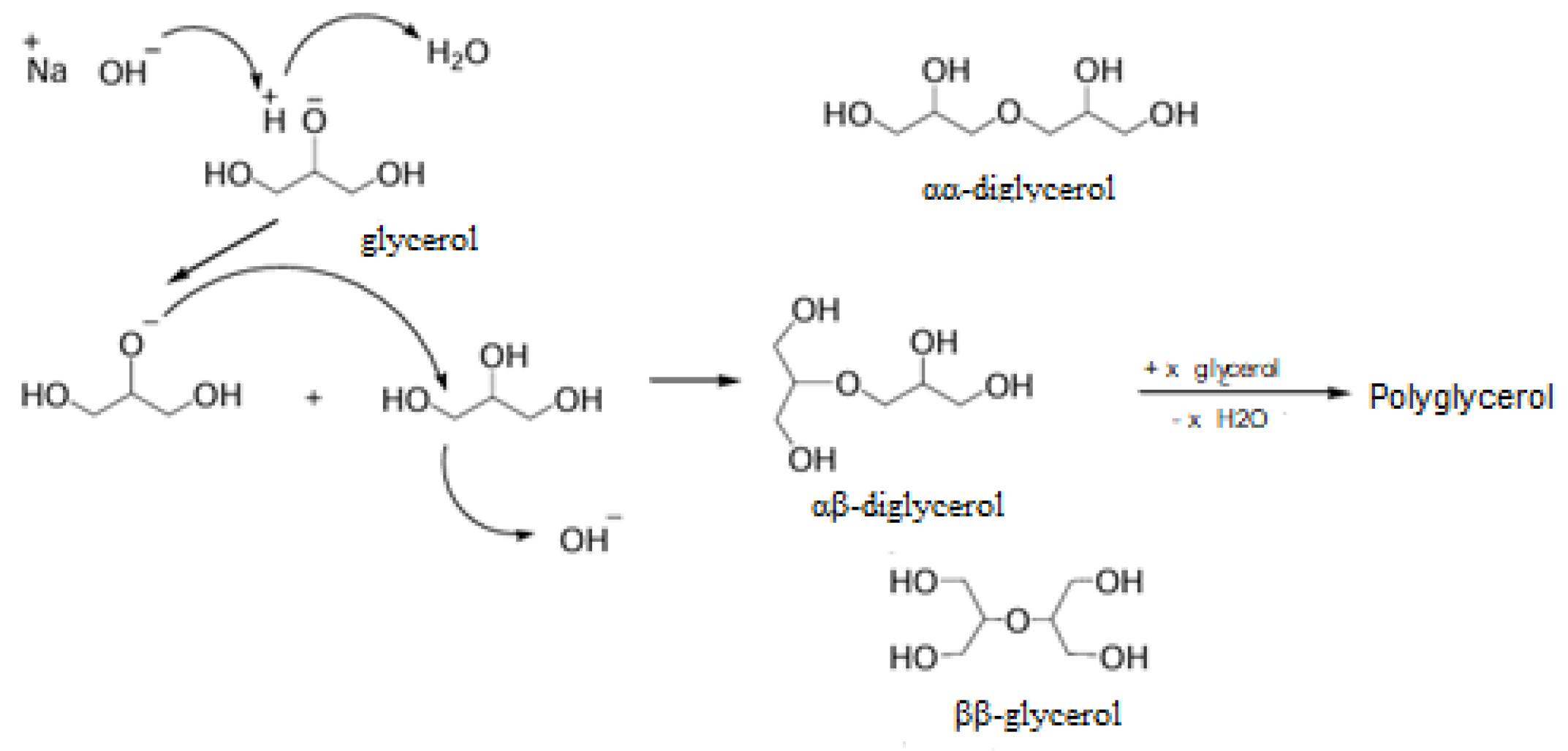
The HPG synthesis process utilises ultrasonic technology (USG) through an Ultrasonic Horn unit. This process involves direct polymerization, where the NaOH/KOH base catalyst interacts with glycerol, weakening one of the OH bonds in glycerol and enhancing the nucleophilic character of the oxygen in the hydroxyl group. Subsequently, glycerol rapidly attacks the carbon of a second glycerol molecule, forming an ether bond between the molecules, producing diglycerol and releasing water.
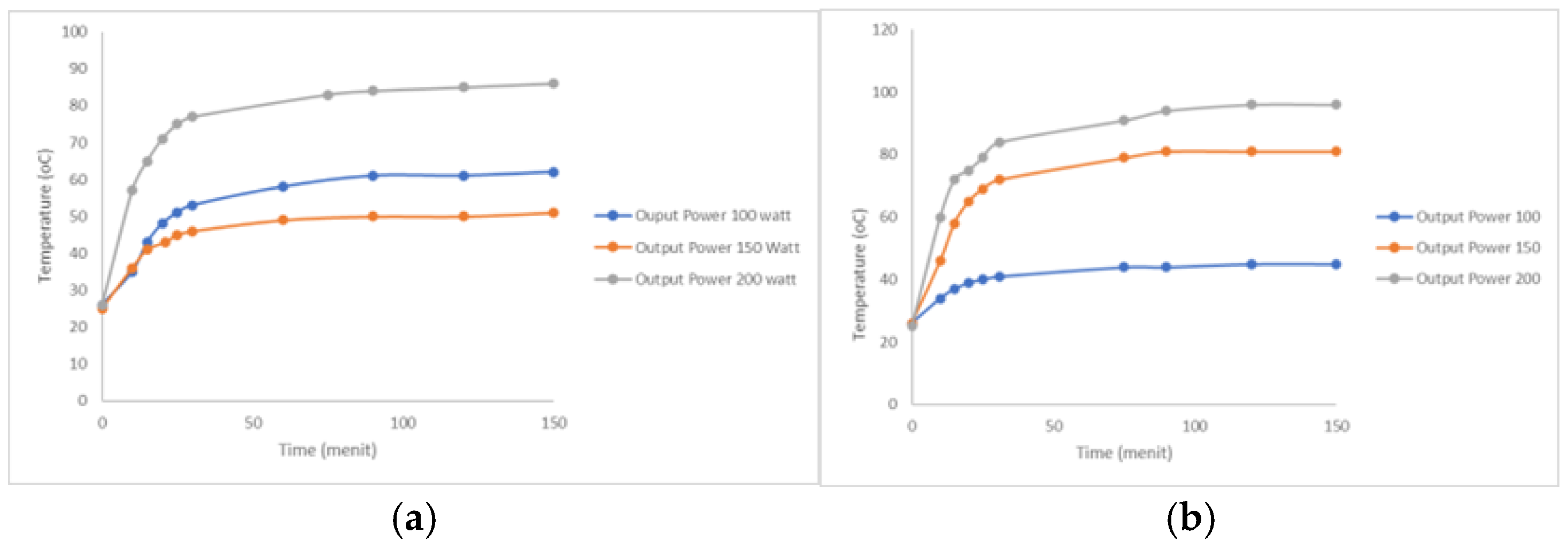
3.1. Effect of USG on Process Temperature Increase
The glycerol polymerization stage was carried out at room temperature for 150 minutes with different ultrasonic power (100, 150, and 200 W) and different catalysts (KOH and NaOH) at 4%.
Figure 2 shows the effect of ultrasonic power on temperature increase.
Increasing the ultrasonic power can lead to a rise in temperature during the process. In
Figure 2 (a), (b), there appears to be a significant increase in temperature up to the first 30 minutes, after which the temperature stabilises up to the 150th minute. This is because the higher the ultrasonic power, the greater the cavitation effect and stirring, which promotes the transfer of active components. In a liquid system at an ultrasonic frequency of 20 kHz, each cavitation bubble rupture acts as a local “hotspot”, producing temperatures of around 5000 oC and pressures of 2000 atmospheres [
17,
18]. The highest temperature for the KOH catalyst reached 86°C at an ultrasonic power of 200 W. Meanwhile, for the NaOH catalyst, the highest temperature reached 96°C at an ultrasonic power of 200 W.
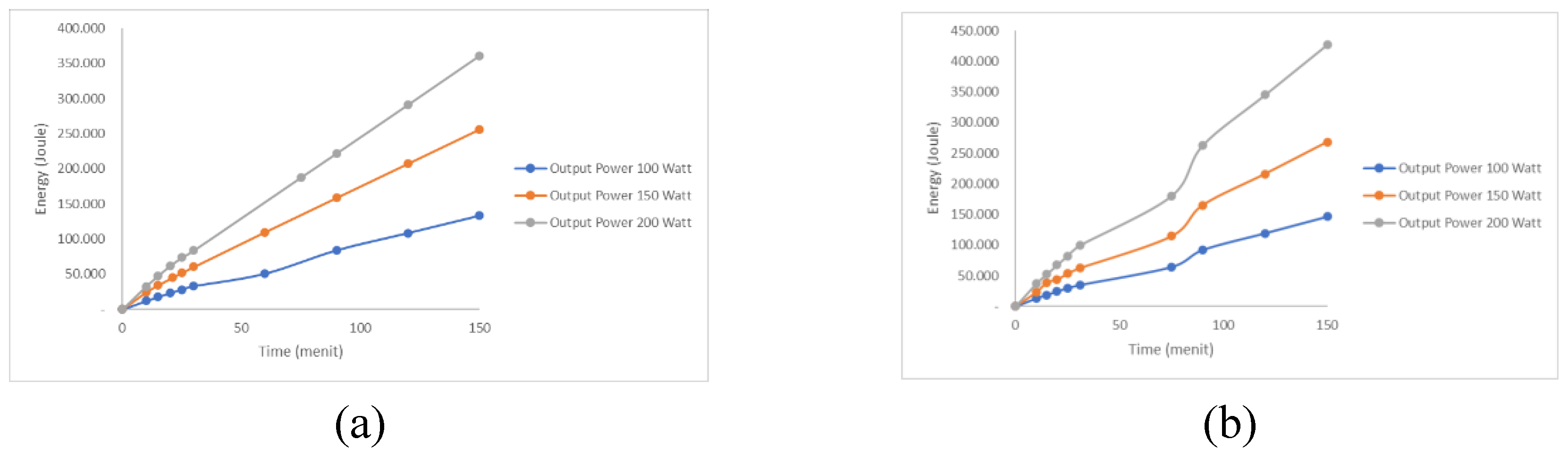
3.2. Effect of USG on Process Energy Increase
The increase in ultrasonic power also increases the energy provided during the polymerization synthesis process for 150 minutes with different ultrasonic power (100, 150, and 200 W) and different catalysts (KOH and NaOH) at 4%. The effect of ultrasonic power on the energy increase is shown in
Figure 3.
Increasing ultrasonic power enhances the amount of energy delivered during the process. This is because the higher the ultrasonic power, the greater the cavitation effect and stirring, which promotes the transfer of active components. The highest energy with the KOH catalyst is 360.593 Joules at 200 W ultrasonic power, whereas the NaOH catalyst is 427.182 Joules at 200 W ultrasonic power.
3.3. Effect of USG on Molecular Weight (MW) and Degree of Polymerization (DP) Increase
The effect of ultrasonic power by increasing temperature and energy provided during the glycerol polymerization process to form HPG was conducted for 150 minutes with different ultrasonic power (100, 150, and 200 W) and different types of catalysts (KOH and NaOH) at 4%, resulting in physical changes in the product from its initial state. Physical changes were correlated with changes in the molecular weight and degree of polymerization of the resulting product (HPG). The effect of ultrasonic power on the polymerization process can be seen in the changes in the product's colour obtained using either KOH or NaOH catalyst. The changes in product colour can be seen in
Figure 4.
Physical changes resulting from the effect of varying ultrasonic power include changes in the viscosity of the produced HPG. Viscosity measurements are used to determine the molecular weight (BM) and degree of polymerization (DP) of HPG.
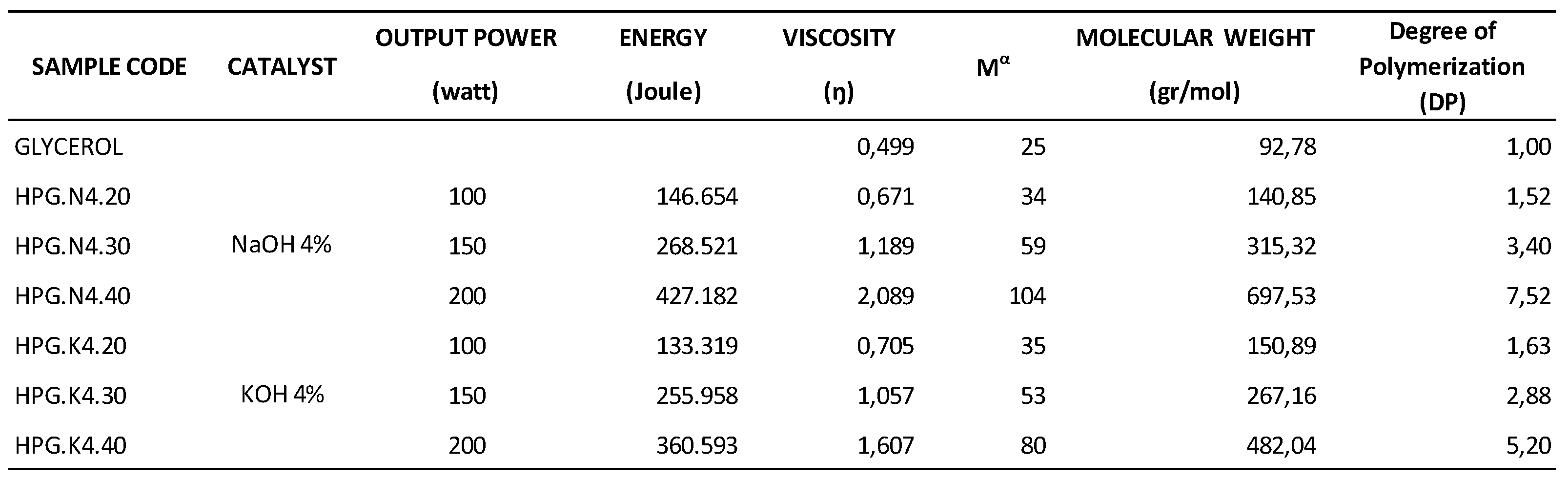
Table 1 shows the effect of varying ultrasonic power on determining BM and DP.
Table 1 shows the relationship between USG power and the resulting MW and DP, indicating that the greater the energy USG provides, the greater the MW and DP of HPG produced compared to the initial raw material (glycerol). The highest MW was observed in the HPG.N4.40 sample code, with a value of 697,53 g/mol and a DP of 7,52. In HPG.N4.40, the polymerization process was conducted using a 4% w/w NaOH catalyst with 200-watt USG power and an energy output of 427,182 Joules for 150 minutes.
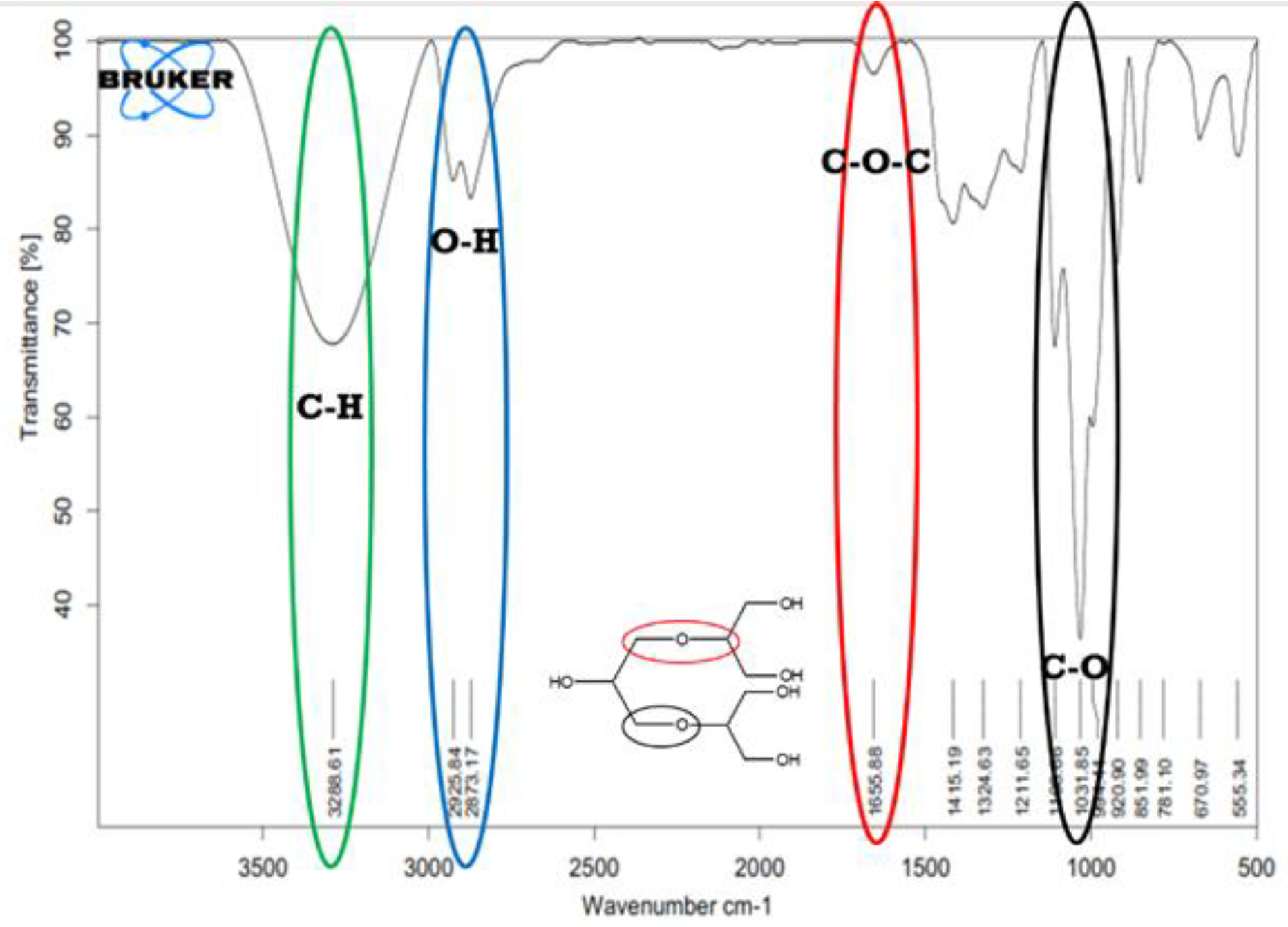
3.4. Characterisation of HPG Using FTIR
The functional group analysis aims to determine the success of the polymerization process by identifying the functional groups and specific absorption present in HPG.
Figure 5 shows the FTIR spectrum of HPG resulting from glycerol polymerization synthesis.
Based on
Figure 5, the functional group analysis using FT-IR on the HPG.N4.40 product shows a broad valley-shaped spectrum at wavelengths between 3000 cm-1 and 3700 cm-1, with a spectrum peak at 3284 cm-1 indicating the presence of hydroxyl groups (O-H). A sharp split spectrum at wavelengths between 2940 cm-1 and 2700 cm-1 with spectrum peaks at 2933 cm-1 and 2879 cm-1 was identified as C-H groups. The spectrum peak at 1650 cm-1 was identified as C=O or C-O-C groups. The identification of C-O-C groups is further supported by the appearance of spectrum peaks between 1033 cm-1 and 1210 cm-1. The characteristic of dendritic polyglycerol structure formation in FTIR analysis is the appearance of O-H groups at the ends of the structure and C-O-C groups in the middle of the polyglycerol structure [
19].
3.5. Characterisation of HPG using C-NMR and H-NMR
Figure 6 shows the formation of the HPG chemical structure at the highest molecular weight using NaOH and KOH catalysts, as revealed by NMR analysis (C-NMR and H-NMR).
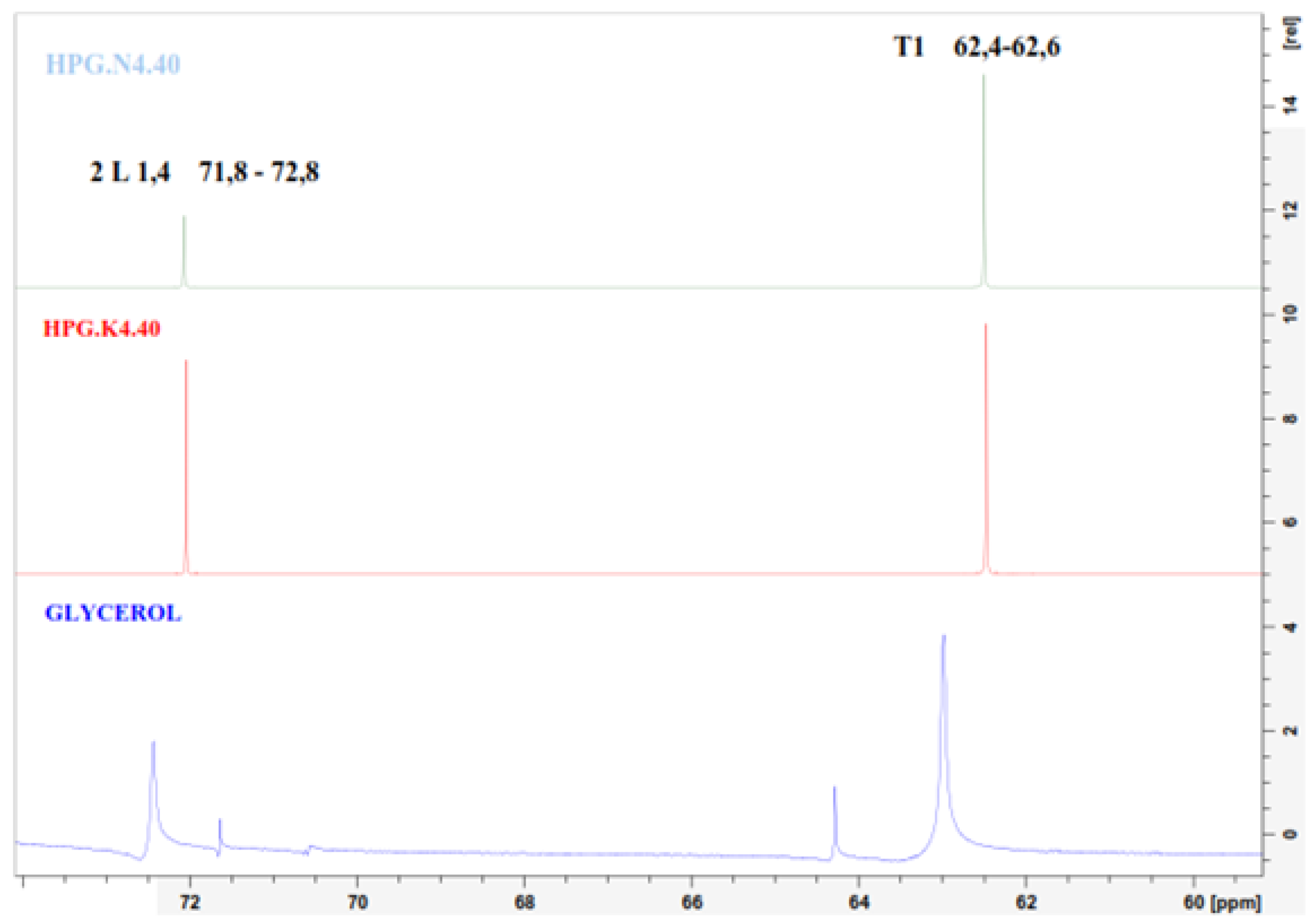
From the
13C NMR spectrum, it is observed that there is a shift in the spectrum between glycerol and the product in both HPG.N4.40 and HPG.K4.40. In both products, only one peak at 71.8 ppm is observed, indicating that the formed polymer is diglycerol. The peak shift occurs at 71.5 to 72.8 ppm, which characterises the presence of an HPG structure. The spectrum peak from 72.0 to 73.5 ppm represents the hyperbranched polymer structure of the dendritic type [
20].
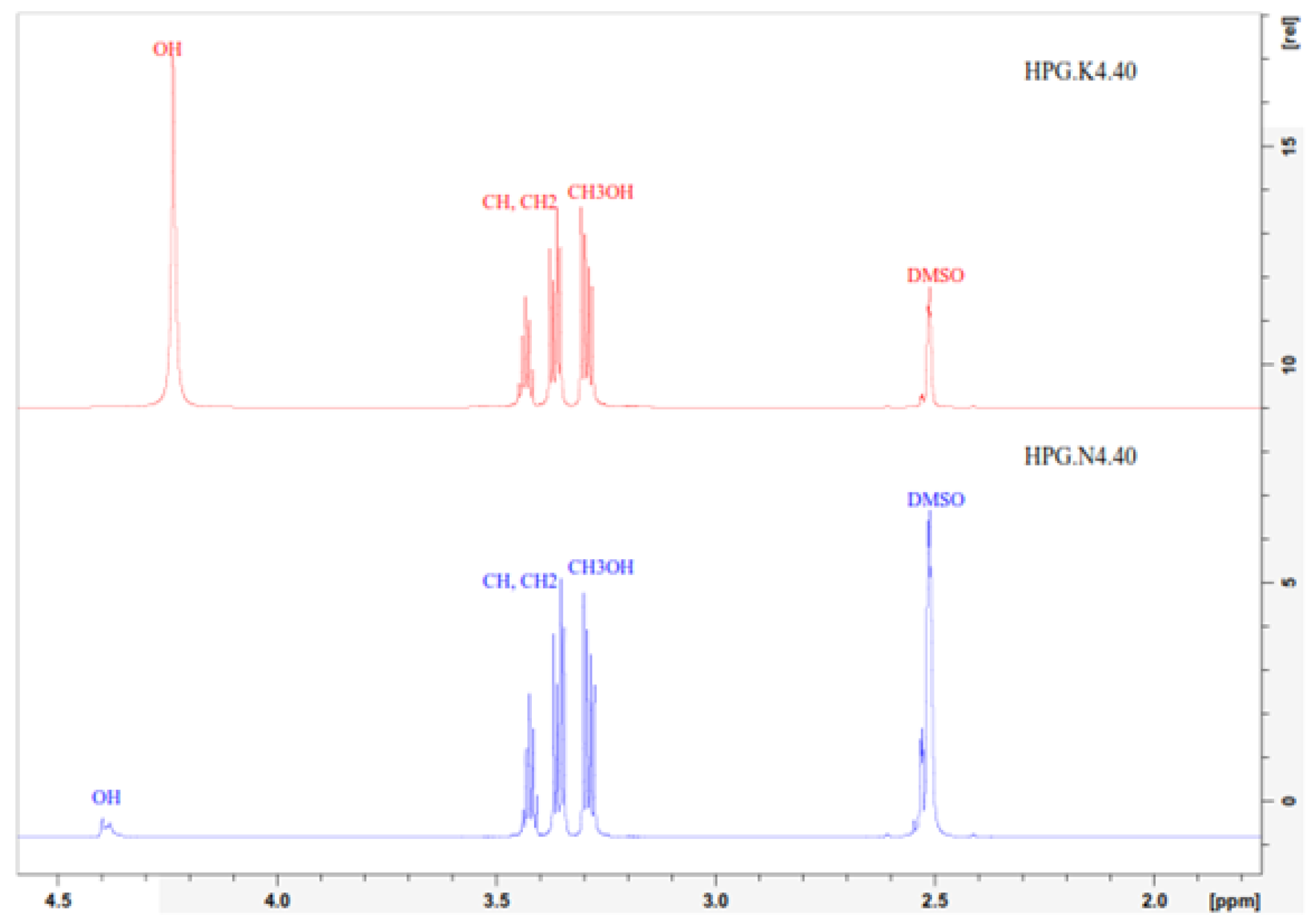
In the
1H NMR spectrum shown in
Figure 7, the methylene and methine protons of the polyglycerol HPG.K4.40 and HPG.N4.40 appear at a chemical shift between 3.2 and 3.6 ppm, with three peaks observed. These peaks at this chemical shift are indicated as CH-O and CH CH2 [
20]. The OH group attached to the end of the HPG.K4.40 structure appears at a shift between 4.1 and 4.3 ppm, while in HPG.N4.40, it appears at a shift between 4.3 and 4.4 ppm. The results of the NMR analysis shown in
Figure 6 and
Figure 7 show that from the results of this activity, the glycerol polymerization process produces HPG products with a Dendritic structure.
4. Discussion
The results of this activity show that ultrasound (USG) technology has proven to be effective in the glycerol polymerization process. With USG technology, polymerization can occur at room temperature and in a shorter time. Based on the NMR analysis results in
Figure 6 and
Figure 7, HPG with a dendritic structure has been formed. The mechanism of the glycerol polymerization reaction is that carried out by Martin and Richter, 2011. The glycerol polymerization formation reaction stages can be seen in
Figure 8.
The highest molecular weight using the NaOH catalyst was 697.53 g/mol, with a degree of polymerization 7.5. In contrast, with the KOH catalyst, the molecular weight was 482.04 g/mol, with a degree of polymerization of 5.2. The USG energy applied for the NaOH catalyst was 427.182 Joules, while for the KOH catalyst, it was 360.593 Joules.
Ultrasound produces high-intensity sound waves that create rapid cycles of compression and dilution in the reaction medium. This causes the formation, growth and collapse of microscopic bubbles (cavitation). When these bubbles collapse, they produce localized “hot spots” with very high temperatures (about 5000°C) and pressures (2000 atmospheres), which facilitate the activation of reactants and enhance the reaction kinetics. The cavitation and agitation produced by ultrasound provide intense mixing and energy transfer in the reaction medium. This improves the interaction between the glycerol molecules and the catalyst (NaOH or KOH), reducing the time required to reach the desired product.
In conventional methods, HPG polymerization reactions generally use high temperatures. In USG, the localized heating effect can reduce the need for high temperatures. Local conditions that occur continuously will accelerate the reaction without requiring high temperatures. Ultrasound promotes nucleophilic attack and bond cleavage more efficiently due to the localized energy input, thereby reducing the energy barrier to the reaction and enabling rapid polymerization at room temperature. Several studies have been conducted on the glycerol polymerization process, generally carried out at high temperatures and long processing times.
Yusuf (2017) conducted the other comparison. Based on reaction mechanism calculations for diglycerol formation following the SN2 route using HyperChem software, the interaction energy between the base catalyst (NaOH) and glycerol was 529.38 kJ/mol.
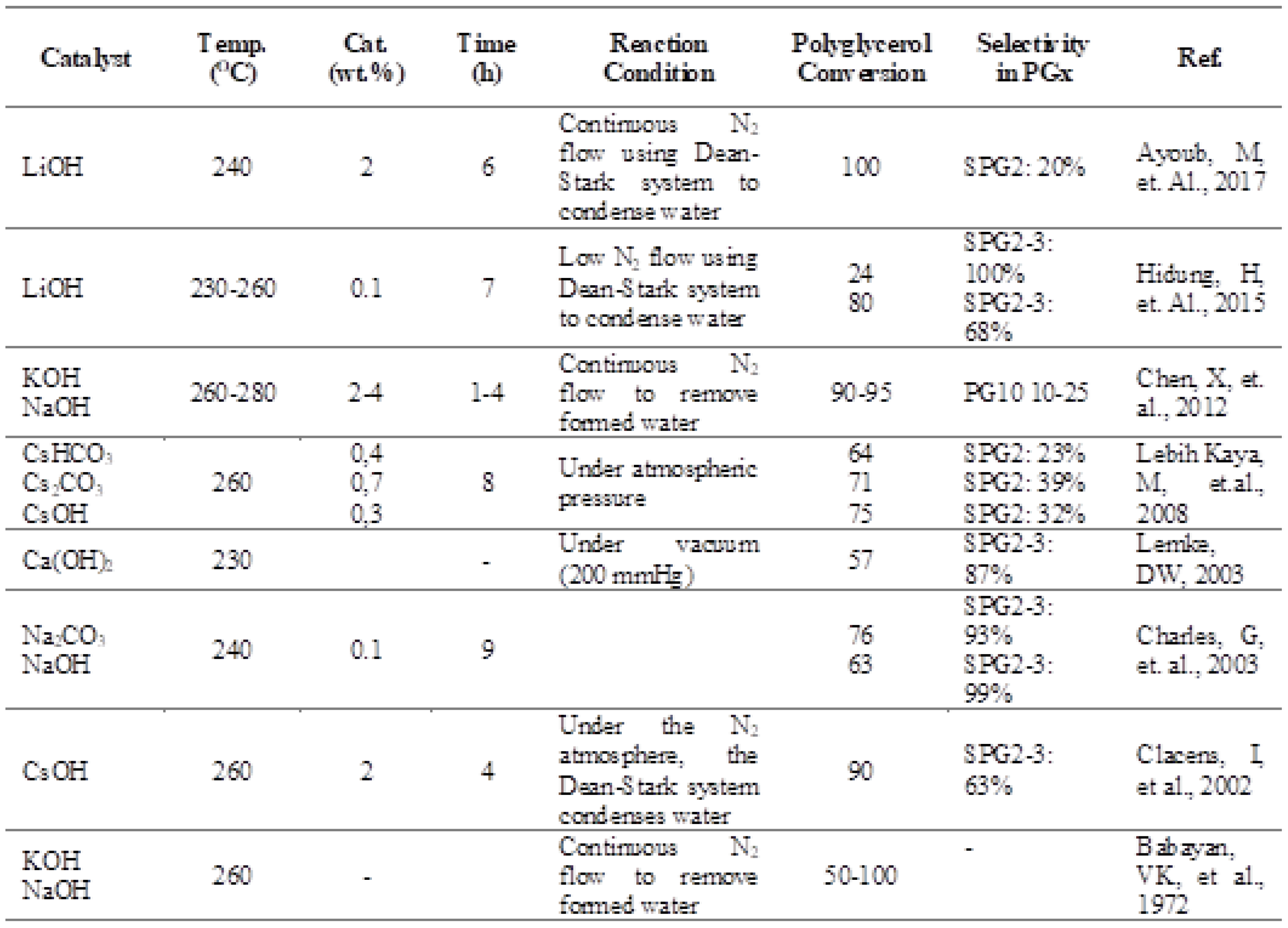
Table 2.
Comparison of Polimerisation Glycerol [
22].
Table 2.
Comparison of Polimerisation Glycerol [
22].
5. Conclusions
The HPG product from glycerol polymerization synthesis using USG under process conditions with 200-watt power and energy of 427,182 Joules for 150 minutes yielded a molecular weight of 1.432 g/mol with a DP of 7,518. Testing with FTIR showed the characteristic formation of dendritic polyglycerol structures, indicated by the formation of ether or C-O-C groups at spectrum peaks between 1033 cm-1 and 1210 cm-1 and a spectrum peak at 3284 cm-1 indicating the presence of hydroxyl (O-H) groups. Identification using 13C NMR revealed peak shifts in the spectrum 71.5 to 72.8 ppm, identifying the HPG product as having a dendrimer structure. The 1H NMR spectrum showed peak shifts in the spectrum 3.2 to 3.6, identifying CH-O, indicating a dendritic structure.
Acknowledgements
The authors gratefully acknowledged the financial support for publication by The Ministry of Research and Higher Education of Indonesia’s financial support through the research grant “Hibah Penelitian Disertasi Doktor” (PDD) 2024 (NKB-947/UN.2RST/HKP.05.00/2024). The authors also acknowledge the financial support from Universitas Indonesia through the research grant “Riset Kolaborasi Indonesia” 2024 (contract number: NKB-790/UN2.RST/HKP.05.00/2024). The authors thank Mr. Giant Pramana for his assistance in interpreting the analysis data using 13C NMR and 1H NMR.
Credit authorship contribution statement
Yan Irawan: Writing – original draft, Conceptualization, Investigation. Yenny Meliana: Writing – review & editing, Supervision, Project administration. Muhammad Adiguna Rabbuka: Writing – review & editing, Investigation. Ghislaine Ndonkeu Mangoumou: Writing – review & editing, Investigation. Kasbawati: Writing – review & editing, Validation. Efri Mardawati: Writing – review & editing, Validation. Biqiong Chen: Writing – review & editing. Yeyen Nurhamiyah: Writing – review & editing, Validation. Misri Gozan: Writing – review & editing, Supervision, Project administration.
References
- Dadkhah, M., Shamlooei, H., Mohammadifar, E., Adeli, M. 2018. Synthesis of hyperbranched polyglycerols using ascorbic acid as an activator. RSC Adv. 8, 217-221. [CrossRef]
- Rahmi, D., Yunilawati, R., Riyanto, A., Nuraeni, C. 2017. Characteristic of local minerals as catalyst on hyperbranched polyglycerol synthesis. Jurnal Kimia dan Kemasan. 39(2), 39-46.
- Ionescu, M., Petrović, Z. S. 2018. On the mechanism of base catalyed glycerol polymerization and copolymerization. European Journal of Lipid Science and Technology. 120(6). [CrossRef]
- Negisa Ebadipour, Sébastien Paul, Benjamin Katryniok and Franck Dumeignil (2020). ” Alkaline-Based Catalysts for Glycerol PolymerizationReaction: A Review”, Catalysts 2020,10,1021.
- Milewski, A., Dydo, P., Jakóbik-Kolon, A., Czechowicz, D., Babilas, D., Burek, M., Wa´skiewicz, S., Byczek-Wyrostek, A., Krawczyk, T., Kasprzycka, A. 2018. Preparation of triglycerol from glycerol and epichlorohydrin at room temperature: synthesis optimization and toxicity studies. ACS Sustainable Chem. Eng. 6, 13208–13216. [CrossRef]
- Cespi, D., Cucciniello, R., Ricciardi, M., Capacchione, C., Vassura, I., Passarini, F., Proto, A. 2016. A simplified early stage assessment of process intensification: glycidol as a value-added product from epichlorohydrin industry wastes. Green Chem. 18, 4559–4570. [CrossRef]
- Mukbaniani, O.V., Abadie, M. J. M., Tatrishvili, T.N. (Eds.). 2017. Chemical Engineering of Polymers: Production of Functional and Flexible Materials, first ed. Apple Academic Press, New York.
- Babayan, V.K.; Lehman, H. Process for Preparation and Purification of Polyglycerols and Esters Thereof. U.S. Patent 3,637,774, 25 January 1972. [Google Scholar]
- Lemke, D.W. Processes for Preparing Linear Polyglycerols and Polyglycerol Esters. U.S. Patent 6,620,904, 16 September 2003. [Google Scholar]
- Yusuf, M. 2017. Mechanism study of the glycerol oligomerization reaction using ab initio method. Jurnal Pendidikan Kimia (JPKim). 9(1), 236-243. [CrossRef]
- Babu AS, Mohan RJ, Parimalavalli R..”Effect of single and dual-modifications on stability and structural characteristics of foxtail millet starch”, J. Food chemistry 271:457-65. [CrossRef]
- Zitron, R., Wajsfeld, T., Klautau, G. B., da Silva, C. B., Nigro, S. 2016. Concentration of sonication fluid through centrifugation is superior to membrane filtration for microbial diagnosis of orthopedic implant-associated infection. Journal of Clinical Microbiology. 54,788-90. [CrossRef]
- Sudhir, S. Gandhi, Parag R. Gogate,” Process intensification of fatty acid ester production using esterification followed by transesterification of high acid value mahua (lluppai ennai) oil: Comparison of the ultrasonic reactors”, Fuel 294 (2021) 120560. [CrossRef]
- Kumar, A. R. S. S., Padmakumar, A., Kalita, U., Samanta, S., Baral, A., Singha, N. K., Ashokkumar, M., Qiao, C. G. 2023. Ultrasonics in polymer science: applications and challenges. Progress in Material Science.136, 101113. [CrossRef]
- Arunjunai, R.S. Santha Kumar, Amrishkumar Padmakumar, Uddhab Kalita, Sarthik Samanta, Anshul Baral, Nikhil K. Singha, Muthupandian Ashokkumar, Greg G. Qiao,” Ultrasonics in polymer science: applications and challenges”, Progress in Material Science 136 (2023) 101113. [CrossRef]
- Muharja, M., Zikrillah, M., Damayanti, F. F., Batuthoh, M. W. I., Khamil, A. I. 2023. metoda baru perhitungan viskositas intrinsic dan berat molekul polihidroksialkanoat untuk produksi plastik biodegradable. Jurnal Penelitian IPTEKS. 8(2), 188-195.
- Mason, T. J. 1999. Sonochemistry. Oxford Science Publications, Oxford.
- Suslick, K.S., Hammerton, D. A., Cline, D.E. 1986. Sonochemical hot spot. Journal of the American Chemical Society. 108(18), 5641–5642. [CrossRef]
- Dwinna Rahmi, Retno Yunilawati, dan Arief Riyanto,” SINTESIS KATALIS LOGAM BERPENYANGGA DENDRIMER POLIGLISEROL BERBASIS TURUNAN KELAPA SAWIT”, Jurnal Kimia dan Kemasan 38(2):61. [CrossRef]
- Istratov, V. V., Vasnev, V. A., Markova, G. D. 2021. Biodegradable and biocompatible silatrane polymers. Molecules. 26(7). [CrossRef]
- Martin, A., Richter, M. 2010. Oligomerization of glycerol - a critical review. European Journal of Lipid Science and Technology. 113(1), 100–117. [CrossRef]
- Ebadipour, N., Paul, S., Katryniok, B., Dumeignil, F. 2020. Alkaline-based catalysts for glycerol polymerization reaction: a review. Catalysts. 10(9), 1021. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).