1. Introduction
Over the past few years Rhythmic Auditory Stimulation (RAS) has become a widely used technique to target motor functions in neurological conditions. RAS is directed to improve gait in individuals with neurological impairment by influencing the physiological effects of auditory rhythm on the motor system, consecutively enhancing the control of movement [
1,
2]. Neurological conditions with deficits in gait that can benefit from the application of RAS include Parkinson’s disease, cerebral palsy, cerebrovascular accidents (CVA)/stroke, traumatic brain injury, multiple sclerosis, and orthopaedic patients [
3]. Numerous meta-analytic studies and systematic reviews has investigated the beneficial effect of RAS on gait and motor performance in patients with neurological conditions. A recent systematic review of 21 studies and meta-analysis of 14 studies to determine the effectiveness of RAS in improving motor and gait disturbances in Parkinson’s disease. The results revealed improvement in gait parameters, walking and balance function as well as daily living activities in Parkinson’s disease patients [
4]. Similarly, another systematic review of 22 studies and meta-analysis of 18 studies was conducted to determine the effectiveness of RAS on the motor performance in patients with stroke [
4]. The results reported enhanced performance in gait parameters, balance ability and walking functions in patients with stroke [
4]. Another meta-analysis of 5 studies involving 188 participants, to study the effectiveness of rhythmic auditory cueing in gait rehabilitation in multiple sclerosis patients shows improvement in spatiotemporal parameters of gait such as cadence (a large effect size, Hedge’s g: 1.00), stride length (a medium effect size, Hedge’s g: 0.71) and velocity (a medium effect size, Hedge’s g: 0.67) [
5]. Similarly, distinct variation of music therapy such as RAS, rhythmic harmonic stimulation and rhythmic exercise program is found to have positive medium effect (Hedge’s g: 0.534) improvements on spatiotemporal aspects of gait in individuals with traumatic brain injury [
6].
What is RAS?
Rhythmic Auditory Stimulation (RAS) is a neurologic music therapy technique in which the individual synchronizes their steps or movement in accordance to a rhythmic beat, ideally provided by a metronome or music with an amplified beat. This technique is primarily used to facilitate the rehabilitation of difficulties with gait or gait-related activities, which are considered biologically rhythmic [
7,
8]. RAS acts by providing an extrinsic reference for the initiation and ongoing timing of movements [
9], and is administered to improve the cadence, by secondarily influencing the stride length and speed of walking [
7].
Therapeutic Principles of RAS
Rhythm is a component of music, and by definition is the element of "time" in music. Activities like walking, writing, typing and talking require temporal patterns for attunement of fine movement [
10]. However, neurological conditions can disrupt the precision of co-ordinated movement patterns. The auditory system being highly sensitive to rhythmic beat can influence the motor system by providing a regularizing temporal input [
10].
A number of research studies have shown that rhythm is primarily responsible for orienting attention to music [
11,
12,
13]. Rhythm can trigger an intrinsically created sense of beat, which once ingrained can perpetuate in the mind of the listener regardless of the continuity of music [
14]. And this process of synchronization of the intrinsic sensations of the beat with an extrinsic rhythm of movement pattern is known as entrainment [
10]. Entrainment is the mechanism of integrating periodic inputs and endogenous brain rhythms (i.e., internal oscillators) [
15]. After the establishment of the synchronization between the internal oscillators and external periodic stimulations, phases of optimal cortical excitability concur with the incoming stimuli and promotes their processing [
16]. Entrainment is basically the mechanism aiding the interconnection between the brain and the incoming sensory stimuli [
17]. As an example, it is our capacity to attend to and comprehend speech. Entrainment is considered as the most multifaceted method for precisely influencing endogenous brain activity [
18].
Rhythmic entrainment is one of the key therapeutic principles of RAS, it is the capacity of the motor function to integrate with the auditory system and initiate movement. The local spinal cord circuits, known as the
Central Pattern Generators (CPG) link the auditory stimulus to appropriate motor neurons to enable movement function [
9]. This movement occurs without learning cognitively and below the levels of conscious perception, as CPG has the ability to reproduce movement of the limbs without any intervention from the brain [
9]. Other principles of RAS include priming, cueing of the movement period, and step-wise limit cycle entrainment (SLICE).
Priming is the stimulation of the motor neurons at the spinal cord level via the external auditory stimulus, resulting in the entrainment of the activation of the limb muscle, while walking [
9].
Cueing of the movement period is due to time stability synchronization throughout the movement trajectory and is not coincidental with the rhythmic beat at the movement endpoints [
8].
Stepwise limit cycle entrainment, is the process of progressive entrainment of step cadence in individuals, with deficit in limit-cycle i.e., optimal step cadence, due to neurological conditions or injury, to premorbid levels [
9].
2. Neural Bases of RAS
Multiple areas of the brain are responsible for the processing of rhythm including the prefrontal cortex, and the cerebellum, leading to stimulation of several neural networks [
19]. The facilitation of movement by creating an anticipatory template of time sequence marked by rhythmic beats is enabled due to the rich neural connectivity between the auditory and motor systems [
10]. Additionally, the auditory and motor system is well-connected to several cortical, sub-cortical, and spinal levels [
20]. Numerous studies on the neural basis of synchronicity to music have indicated that perception of rhythm is linked to brain areas responsible for motor output and production [
21]. It suggests that the motor regions including both cortical and subcortical areas such as the basal ganglia and cerebellum are responsible for beat extraction [
21,
22]. The motor pathway is influenced by auditory sensation via the reticulospinal connections, priming and altering the spinal motor neuron activity [
23]. The excitability of motor neurons can be directly increased with sound, resulting in the reduction of time necessary for a response of the muscle to a given motor command [
10]. It has been demonstrated that musical rhythms result in priming and timing of motor responses via the audiospinal route [
23]. The cerebellum receives auditory projections via the pontine, whereas the striatum of the basal ganglia receives via the inferior colliculus as being part of the ascending auditory pathway [
24]. As an output of the basal ganglia the striatum projects to the globus pallidus reaching the cortical motor areas including the premotor cortex and supplementary motor area. Timing, sequencing, and selection of behavioral responses occur as a result of the reciprocal connection between the basal ganglia and the auditory association areas [
20].
The intricate interconnection of rhythmic cues with areas spread throughout the brain plays a crucial role in the facilitative effect on motor output and engages patients with motor disabilities. This process can circumvent the functional and psychological impairment in patients and result in therapeutic outcomes [
19]. Research in psychophysics demonstrated that auditory rhythm affects motor function as a result of speed and time resolution of temporal processing in the auditory system. As concluded from such research findings, an
oscillatorentrainment model has been proposed, which posits that the "rhythmic processes in neural motor networks become entrained to rhythmic timekeeper networks in the auditory system [
20].” This network is propelled externally by rhythmic cues such as metronome or music.
Neuroimaging studies have shown that even at the pre-attentive level [
25], increased co-ordination of neural activities is observed between auditory and premotor cortex during rhythm processing [
26]. Areas in the brain responsible for rhythm processing overlap with the areas involved in motor functioning, viz., premotor cortex, supplementary motor areas (SMA), cerebellum, and basal ganglia [
27]. Putamen of the basal ganglia is specifically involved in the sequencing of the rhythmic events (McIntosh, Brown, Rice, & Thaut, 1997). Processing the rhythmic patterns and regulating motor behavior to altering tempos by controlling the rhythmic auditory-motor synchronization is primarily implicated by the cerebellum [
28]. Healthy successional movements are regulated by the functional loop formed by the basal ganglia and supplementary motor area. The predictable anticipatory movement is initiated by the supplementary motor area. Once, the movement is initiated, SMA activity is paused and the cycle engages the basal ganglia for subsequent sub-movement [
29]. An internal cue is necessary to regulate the cycle. However, in neurological conditions such as Parkinson's disease, this internal cue is either dysfunctional or irregular, or absent. Other brain areas that are responsible for modulating timing include the anterior and posterior cerebellar hemisphere, the inferior parietal cortex, and the cerebellar vermis [
30]. Kotz and Schawartze (2010), have proposed a resolute temporal processing network known as the subcortico-thalamo-cortical network including the basal ganglia, cerebellum, pre-SMA, and SMA, that implements the synchronized motor activity. Neuroimaging findings revealed that movement that is self-initiated or self-paced is impaired in patients related to decreased putamen or associated cortical and cortico-striatal activity [
31]. However, this is not the case in movement that is directed by external cues, as it reduces the reliance on automatized processes [
32]. Therefore, rhythmic beats can influence sensorimotor network activity by circumventing the basal-ganglia- SMA loop and initiating direct movement [
10].
3. Can RAS affect Neurocognition?
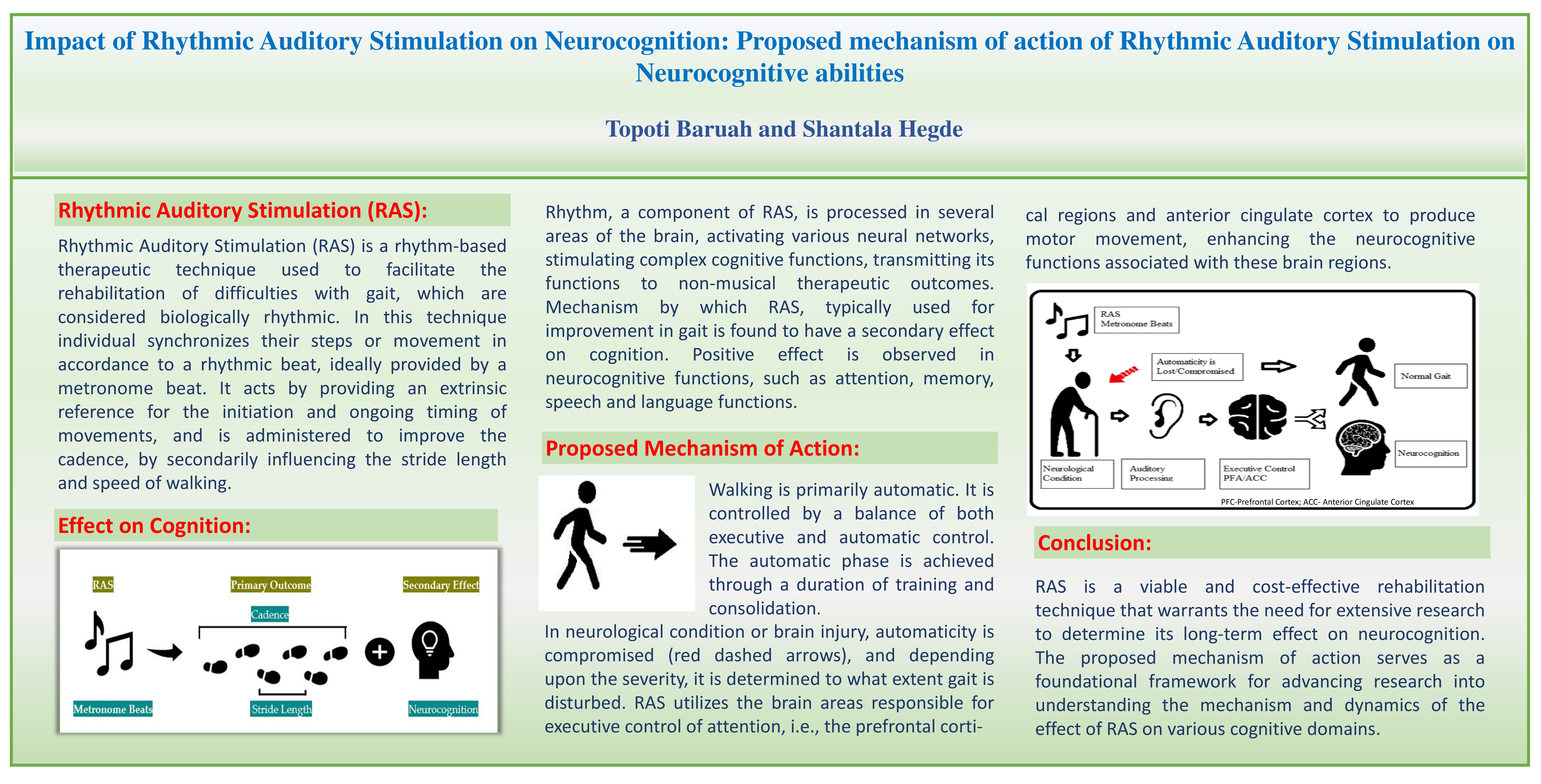
Extensive research studies have demonstrated the efficacy of RAS in rehabilitating motor disabilities resulting from neurological conditions or traumatic brain injury. Neurological conditions can give rise to motor as well as non-motor symptoms and cognitive deficits is one of the most prevalent non-motor symptoms with an insidious onset. Furthermore, injury to the brain can also result in impairment of various cognitive abilities. Therefore, it becomes crucial to explore whether rehabilitation of motor disability via RAS can also affect the cognitive function of patients.
Rhythm as a component of music is processed in several areas of the brain, activating various neural networks, stimulating complex cognitive functions thereby transmitting its functions to non-musical therapeutic outcomes [
33]. The highly complex interaction of music through different areas of the brain can capacitate patients with impaired executive functions, and result in therapeutic effect (Tomaino, 1993). A recent meta-analytic study conducted by Wang et al (2024), to determine the potential impact of RAS in improving cognitive abilities. Findings synthesised from 11 Randomized Controlled Trials (RCTs) revealed that RAS have a significant effect on overall cognition (MD=1.19), attention (MD=-1.86), memory (MD=0.71) and executive function (MD=-.0.23), and no effect was found on verbal memory (MD=-0.51) in individuals with impaired cognition [
34]. The studies reviewed, reported 1 to 3 sessions per week, each lasting 30 to 70 minutes, with intervention durations ranging widely from 3 to 40 weeks.
3.1. Rhythm and Movement Initiation
One of the most important difficulties faced by patients with head injury or neurological conditions is the initiation of movement. They report that, even if they want to move, they have to plan through the movement pattern and that it does not occur spontaneously. The initiation or decision-making process is impaired due to damage or deterioration of brain areas responsible for executive functions in such patients. And it was found that the patients can be driven into automated functioning with the appropriate stimulus [
35].
3.2. Rhythmic Intervention and Speech/Language
Areas of the brain involved in smooth motor movement, overlap brain areas responsible for key neurocognitive functions [
36]. OPERA hypothesis proposed by Patel (2011) suggests that musical training improves the neural encoding of speech via mechanisms of adaptive neuroplasticity when the following five conditions are met, viz., 1. Shared anatomical
overlap in the brain that process both music and speech. 2. Higher level of
precision is required for music to operate in these shared brain networks than speech does. 3. Provocation of strong positive
emotion induced by musical activities that involves these networks. 4. Repeated engagement of the networks during musical experience. 5. Focussed
attention during engagement in music reinforces these brain networks frequently [
37]. Elaborating on this hypothesis, Fujii and Wan (2014), proposed SEP hypothesis, specific to rhythm to elucidate on speech and language rehabilitation. SEP is “sound envelope processing” and “synchronization and entrainment to pulse”. This framework proposes that rhythm-based therapy has the potential to activate brain networks including (i) the auditory-afferent pathway, which involves the brainstem, thalamus, cerebellum, and temporal cortex for accurate processing of sound patterns and timing; (ii) the subcortical-prefrontal pathway, responsible for emotional and reward-related processing; (iii) the basal ganglia-thalamus-cortex pathway, related to beat-based timing; and (iv) the cortical-motor efferent pathway, associated with motor output [
38].
Basal ganglia and cerebellum control the motor as well as perceptual timing, and dysfunction of these brain structures can lead to motor speech difficulty, resulting in unintelligible speech. Speech is considered rhythmic, in the temporal structural range of 2-8Hz across variety of human languages; and the intelligibility of speech is associated with the speech temporal structure [
39]. Rhythmic capacity is crucial for the successful acquisition of language and the ability of temporal prediction in the brain aids in parsing speech streams and understanding syntax and semantics [
40]. Rhythmic cueing following the tempo of the target word or sentences is found to significantly increase the intelligibility of speech and without cues, the speech sounds slurred [
41].
Additionally, several research studies have demonstrated that in idiopathic Parkinson's disease (IPD) syntactic computation and semantic information are altered due to neurodegenerative or structural changes in the basal ganglia [
22,
42]. It has also been observed that the computation deficits related to syntax and semantics are caused due to the temporal processing deficits observed in idiopathic Parkinson's disease, resulting in language deficits [
42,
43]. Kotz and Gunter (2015), have proposed that temporal deficit in IPD results in not only impairment of smooth co-ordination of motor movement but also language and speech. Therefore, external auditory cues (metronome) used for remediation of gait dysfunction that occurs as a result of timing deflection in basal ganglia can also help improve higher neurocognitive abilities such as language and speech [
44].
The process of stabilizing movement by temporally predicting the metronome beats and generating an internal plan of action to take steps is facilitated by the cerebello–thalamo–cortical circuit [
10,
45] that confluences with the dysfunctional striato–cortical circuit [
46]. Kotz and Gunter conducted a research study to explore whether auditory cueing can remediate language processing deficits in IPD. In the study the patients were subjected to thee conditions, viz., metrically aligned speech accent structure, waltz, and no auditory cue, all three conditions were played before listening to either grammatically and semantically correct or incorrect naturally spoken sentences. Results indicated that the sentences cued to march beat only led to improvement of syntactic computation and semantic information. The authors concluded that the involvement of the cerebello–thalamo–cortical circuit to compensate for impaired striato-cortical timing is more potent in the case of marching rhythm. Furthermore, timely processing of linguistic information embedded in the temporally variable speech signal can be enhanced by reinforcing temporal realignment [
44].
In another study by Przybylski and colleagues (2013), the effect of RAS in investigated in children with language developmental disorder, who shows impaired language as well as meter and rhythm perception. In the study, children listened to regular or irregular musical prime sequences followed by blocks of grammatically correct and incorrect sentences. They have to report their grammaticality judgments for the sentences that are presented auditorily. It was found that the children performed better after the regular prime sequences than after the irregular prime sequences. The authors concluded that the rhythmic stimulus has the potential to improve linguistic structure processing [
47].
3.3. Rhythm and Attention
Rhythmic stimulation has been found to influence attentional processing, physical endurance, and interpersonal communication in persons with dementia [
48]. Repetition and duration of engagement in an activity are crucial to increase the chance of building a meaningful response, thereby improving attention and physical endurance that persist in other aspects of functioning, boosting the quality of life [
35]. In a study conducted by Lei and colleagues (2018), the effect of RAS and AMS (Auditory Motor Stimulation) is evaluated in Parkinson’s Disease patients, in which the participants underwent an auditory odd-ball paradigm. The results of the study revealed that RAS enhances attentional processing of temporally predictable external events in Parkinson's' Disease patients as well as healthy participants. However, stimulus processing has no effect on temporal encoding via motor movement, because of dysfunctional basal ganglia in patients [
36].
4. The Secondary Effect of RAS on Neurocognition
Aging and history of falls can result in the reduction of self-confidence and "fear of falling". This fear can lead to unstable and "cautious gait" which can result in stiff, slow movement and adversely affect the pattern of gait [
49]. It can also lead to intrinsic attentional focus [
50], and explicit motor control [
51]. Additionally, the capacity to direct attention can be influenced by clinical symptoms such as depression and fatigue, resulting in interference with performance of complex tasks [
52]. Rhythm is considered a highly complex cue, immune to neurodegeneration, and is reported to have a broad effect on neurocognition in both healthy and diseased individuals [
53]. In addition to the auditory and motor cortex, the deeper brain structure involved in emotion, memory, and reward system such as the amygdala, orbitofrontal, and anterior cingulate cortex, hippocampus, and mesolimbic reward structures are activated by rhythmic stimulus. This activation is found to alleviate depression and anxiety, evoke emotion, and memory, and feel rewarding [
54]. External auditory cues enhance gait by focussing attention on the task of walking [
55]. Slow auditory stimuli can also improve the excessive involuntary movements in conditions such as Parkinson’s disease or Huntington's chorea. It can result in the necessary auditory entrainment and improve overactive heart rate and respiration eventuating relaxation and sleep. Thus, rhythm is found to alleviate the cognitive load caused due to depression, anxiety, and lack of proper sleep and relaxation in both healthy and diseased individuals resulting in improved neurocognitive functions [
35].
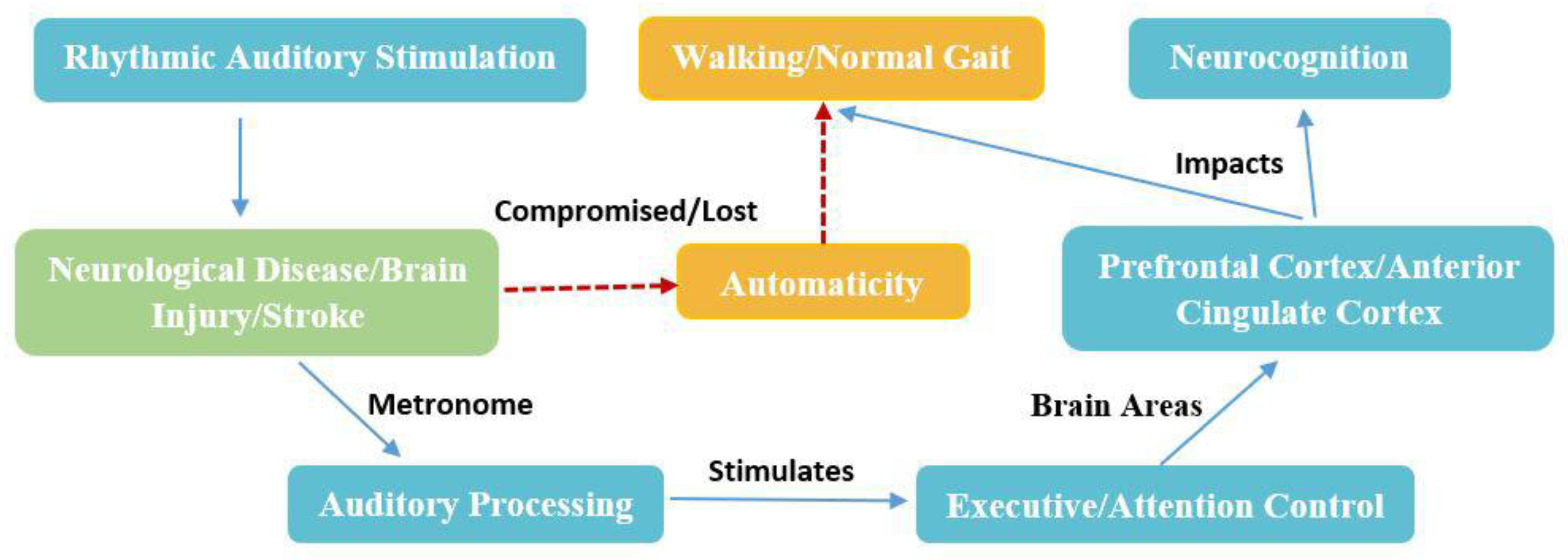
5. Proposed Mechanism of Action: RAS Effect on Neurocognition
Walking is controlled by a balance between automatic control and executive control processes based on the complexity of the task and ability of the individual [
56]. The capacity to execute motor movements requiring lower precisions for example, walking, eating, brushing teeth etc. without allocating attention towards the details of the movements performed, is known as automaticity [
57]. The automatic phase is achieved through a duration of training and consolidation [
58]. In automaticity the sequence of nodes gets automatically activated by input configuration without the requirement of attention by the individual [
59]. Whereas, in controlled cognitive processing, the sequence of nodes gets activated through the necessary function of attention by the individual, specifically applied in situations where the required motor sequence is not previously learned [
59], requiring longer duration for the peripheral stimulus to be dispatched and processed in the cortex prior to consequent incorporation with the pattern of gait [
56].
Investigations on the neural correlates of automaticity have shown increased connectivity between cerebellum, cingulate motor area, supplementary motor area (SMA), and putamen [
60], depicting improved synaptic strength as a result of automatic process [
61]. Contrarily, weakened activity is found in the prefrontal cortex, dorsolateral prefrontal cortex (DLPFC) and anterior cingulate cortex (ACC), the critical attentional areas, due to less reliance on attentional networks [
62,
63].
In neurological condition and in brain damaged individual, the automaticity of walking, either deteriorates or is lost. In RAS, use of external cues in the form of rhythmic cues or metronome beats, to direct attention to the sequence of motor performance utilizes more of attentional regulated behaviour instead of reliance on automatic pattern.
An increased activity in the prefrontal cortex has been observed in individuals with neurological conditions, indicating higher executive demand as a compensatory mechanism for deterioration or loss of automaticity resulting from their condition [
64]. Additionally, research involving healthy participants, found higher activation of the DLPFC, ACC and pre-SMA after re-attending to automated motor task as compared to the automatic stage [
60]. Whereas no considerable activation in prefrontal cortex is found in steady state walking [
65,
66].
6. Discussion
RAS utilizes the executive control of attention to produce movement, the prefrontal cortical regions and anterior cingulate cortex known to be responsible for executive control is also stimulated [
67]. Furthermore, it has been suggested that RAS relies on the residual function of the cortico-striatal circuity or compensates by circumventing the impaired brain regions by depending on substitute pathways such as the cerebello-thalamo-cortical circuitry [
68].
Neuroimaging studies have shown that the cerebello-thalamo-cortical pathway is stretched across a wide region of the brain [
69]. The cerebellum is connected via the thalamus with the prefrontal, frontal and parietal cortices [
69]. Multiple studies have shown that the cerebellum is associated with the prefrontal cortex in a “closed-loop” connection [70-72]. Therefore, it has been suggested that cerebellum impacts both cognitive functions via association with multiple areas of the prefrontal cortex and motor and sensory tasks through associations with frontal and parietal cortices [
73]. Therefore, it can be hypothesized that the neurocognitive functions associated with prefrontal cortex primarily speech, language, executive control, attention etc. is impacted with RAS.
7. Conclusions and Future Direction
Research has shown that in addition to improvement in gait and motor function, RAS can influence attentional processes, memory, language and speech production, and semantics. However, this topic remains highly under-investigated and there is a need for understanding the mechanism and dynamics of the effect of RAS on various cognitive domains. RAS is cost-efficient, viable, and easily comprehensible even by individuals with impaired cognition. This method can also be carried out as a home-based intervention tool even in lower and middle-income countries. The proposed mechanism of action serves as a foundational framework for advancing research into understanding the mechanism and dynamics of the effect of RAS on various cognitive domains. Therefore, strongly warranting the need for extensive research on such cost-effective rehabilitation technique to unravel its impact on neurocognition.
Supplementary Materials
The following supporting information can be downloaded at:
www.mdpi.com/xxx/s1,
Figure 5.1: Schematic representation of the mechanism of action of RAS on Neurocognition.
Acknowledgments
Topoti Baruah is a PhD scholar and has received WT-DBT India Alliance funding (WT-DBT/002/203/2018/001136). Dr. Shantala Hegde is a Clinical and Public Health Intermediate Fellow of the DBT/Wellcome Trust India Alliance (IA/CPHI/17/1/503348).
Conflicts of Interest
There is no conflict of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| RAS |
Rhythmic Auditory Stimulation |
| CVA |
Cerebrovascular Accident |
| CPG |
Central Pattern Generators |
| SLICE |
Step-wise Limit Cycle Entrainment |
| RCT |
Randomized Controlled Trial |
| SEP |
Sound Envelope Processing |
| IPD |
Idiopathic Parkinson’s Disease |
| AMS |
Auditory Motor Stimulation |
| SMA |
Supplementary Motor Area |
| DLPFC |
Dorsolateral Prefrontal Cortex |
| ACC |
Anterior Cingulate Cortex |
References
- Thaut, M. H. Rhythm, Music, and the Brain: Scientific Foundations and Clinical Applications; Routledge: 2005.
- McIntosh, G. C.; Brown, S. H.; Rice, R. R.; Thaut, M. H. Rhythmic Auditory-Motor Facilitation of Gait Patterns in Patients with Parkinson’s Disease. J. Neurol. Neurosurg. Psychiatry 1997, 62(1), 22–26. [CrossRef]
- Zhang, M.; Li, F.; Wang, D.; Ba, X.; Liu, Z. Mapping Research Trends from 20 Years of Publications in Rhythmic Auditory Stimulation. Int. J. Environ. Res. Public Health 2023, 20(1), 215.
- Wang, L.; Peng, J. L.; Xiang, W.; et al. Effects of Rhythmic Auditory Stimulation on Motor Function and Balance Ability in Stroke: A Systematic Review and Meta-Analysis of Clinical Randomized Controlled Studies. Front. Neurosci. 2022, 16, 1043575. [CrossRef]
- Ghai, S.; Ghai, I. Effects of Rhythmic Auditory Cueing in Gait Rehabilitation for Multiple Sclerosis: A Mini Systematic Review and Meta-Analysis. Front. Neurol. 2018, 9, 386. [CrossRef]
- Ghai, S. Does Music Therapy Improve Gait after Traumatic Brain Injury and Spinal Cord Injury? A Mini Systematic Review and Meta-Analysis. Brain Sci. 2023, 13(3), 522. [CrossRef]
- Lim, I.; van Wegen, E.; de Goede, C.; et al. Effects of External Rhythmical Cueing on Gait in Patients with Parkinson’s Disease: A Systematic Review. Clin. Rehabil. 2005, 19(7), 695–713. [CrossRef]
- Thaut, M. H.; McIntosh, G. C.; Rice, R. R. Rhythmic Facilitation of Gait Training in Hemiparetic Stroke Rehabilitation. J. Neurol. Sci. 1997, 151(2), 207–212. [CrossRef]
- Thaut, M.; Hoemberg, V., Eds. Handbook of Neurologic Music Therapy; Oxford University Press: 2014.
- Nombela, C.; Hughes, L. E.; Owen, A. M.; Grahn, J. A. Into the Groove: Can Rhythm Influence Parkinson’s Disease? Neurosci. Biobehav. Rev. 2013, 37(10 Pt 2), 2564–2570. [CrossRef]
- Drake, C.; Jones, M. R.; Baruch, C. The Development of Rhythmic Attending in Auditory Sequences: Attunement, Referent Period, Focal Attending. Cognition 2000, 77(3), 251–288. [CrossRef]
- Jones, M. R.; Ralston, J. T. Some Influences of Accent Structure on Melody Recognition. Mem. Cognit. 1991, 19(1), 8–20. [CrossRef]
- Klein, J. M.; Jones, M. R. Effects of Attentional Set and Rhythmic Complexity on Attending. Percept. Psychophys. 1996, 58(1), 34–46. [CrossRef]
- Palmer, C.; Krumhansl, C. L. Mental Representations for Musical Meter. J. Exp. Psychol. Hum. Percept. Perform. 1990, 16(4), 728–741. [CrossRef]
- Keitel, C.; Ruzzoli, M.; Dugué, L.; Busch, N. A.; Benwell, C. S. Y. Rhythms in Cognition: The Evidence Revisited. Eur. J. Neurosci. 2022, 55(11–12), 2991–3009. [CrossRef]
- Schroeder, C. E.; Lakatos, P. Low-Frequency Neuronal Oscillations as Instruments of Sensory Selection. Trends Neurosci. 2009, 32(1), 9–18. [CrossRef]
- Tavano, A.; Maess, B.; Poeppel, D.; Schröger, E. Neural Entrainment via Perceptual Inferences. Eur. J. Neurosci. 2022, 55(11–12), 3277–3287. [CrossRef]
- Hauswald, A.; Keitel, A.; Chen, Y. P.; Rösch, S.; Weisz, N. Degradation Levels of Continuous Speech Affect Neural Speech Tracking and Alpha Power Differently. Eur. J. Neurosci. 2022, 55(11–12), 3288–3302. [CrossRef]
- Tomaino, C. M. Music Therapy: Evidence-Based Practice to Benefit People with Neurologic Issues. J. Nurse Life Care Plan. 2021, 21(1).
- Thaut, M. H.; Abiru, M. Rhythmic Auditory Stimulation in Rehabilitation of Movement Disorders: A Review of Current Research. Music Percept. 2010, 27(4), 263–269.
- Ravignani, A.; Honing, H.; Kotz, S. A. Editorial: The Evolution of Rhythm Cognition: Timing in Music and Speech. Front. Hum. Neurosci. 2017, 11, 303. [CrossRef]
- Grahn, J. A.; Brett, M. Rhythm and Beat Perception in Motor Areas of the Brain. J. Cogn. Neurosci. 2007, 19(5), 893–906.
- Rossignol, S.; Jones, G. M. Audio-Spinal Influence in Man Studied by the H-Reflex and Its Possible Role on Rhythmic Movements Synchronized to Sound. Electroencephalogr. Clin. Neurophysiol. 1976, 41(1), 83–92. [CrossRef]
- Koziol, L. F.; Budding, D. E. Subcortical Structures and Cognition: Implications for Neuropsychological Assessment; Springer Science & Business Media: 2009.
- Tecchio, F.; Salustri, C.; Thaut, M. H.; Pasqualetti, P.; Rossini, P. M. Conscious and Preconscious Adaptation to Rhythmic Auditory Stimuli: A Magnetoencephalographic Study of Human Brain Responses. Exp. Brain Res. 2000, 135(2), 222–230. [CrossRef]
- Grahn, J. A.; Rowe, J. B. Feeling the Beat: Premotor and Striatal Interactions in Musicians and Non-Musicians During Beat Perception. J. Neurosci. 2009, 29(23), 7540–7548. [CrossRef]
- Ullén, F.; Bengtsson, S. L. Independent Processing of the Temporal and Ordinal Structure of Movement Sequences. J. Neurophysiol. 2003, 90(6), 3725–3735. [CrossRef]
- Bijsterbosch, J. D.; Lee, K. H.; Hunter, M. D.; et al. The Role of the Cerebellum in Sub- and Supraliminal Error Correction During Sensorimotor Synchronization: Evidence from fMRI and TMS. J. Cogn. Neurosci. 2011, 23(5), 1100–1112. [CrossRef]
- Mushiake, H.; Inase, M.; Tanji, J. Selective Coding of Motor Sequence in the Supplementary Motor Area of the Monkey Cerebral Cortex. Exp. Brain Res. 1990, 82(1), 208–210. [CrossRef]
- Thaut, M. H. Neural Basis of Rhythmic Timing Networks in the Human Brain. Ann. N. Y. Acad. Sci. 2003, 999, 364–373. [CrossRef]
- Wu, T.; Chan, P.; Hallett, M. Effective Connectivity of Neural Networks in Automatic Movements in Parkinson’s Disease. Neuroimage 2010, 49(3), 2581–2587. [CrossRef]
- Morris, M. E.; Iansek, R.; Matyas, T. A.; Summers, J. J. Stride Length Regulation in Parkinson’s Disease: Normalization Strategies and Underlying Mechanisms. Brain 1996, 119(Pt 2), 551–568. [CrossRef]
- Cohen, N. S. The Use of Superimposed Rhythm to Decrease the Rate of Speech in a Brain-Damaged Adolescent. J. Music Ther. 1988, 25(2), 85–93.
- Wang, Y. N.; Wen, X. N.; Chen, Y.; et al. Effects of Movement Training Based on Rhythmic Auditory Stimulation in Cognitive Impairment: A Meta-Analysis of Randomized Controlled Clinical Trials. Front. Neurosci. 2024, 18, 1360935.
- Tomaino, C. M. Using Rhythmic Auditory Stimulation for Rehabilitation: Concetta M. Tomaino. In Music, Science, and the Rhythmic Brain; Routledge: 2012; pp 120–130.
- Lei, J.; Conradi, N.; Abel, C.; et al. Cognitive Effects of Rhythmic Auditory Stimulation in Parkinson’s Disease: A P300 Study. Brain Res. 2019, 1716, 70–79. [CrossRef]
- Patel, A. D. Why Would Musical Training Benefit the Neural Encoding of Speech? The OPERA Hypothesis. Front. Psychol. 2011, 2, 142.
- Fujii, S.; Wan, C. Y. The Role of Rhythm in Speech and Language Rehabilitation: The SEP Hypothesis. Front. Hum. Neurosci. 2014, 8, 777.
- Poeppel, D.; Assaneo, M. F. Speech Rhythms and Their Neural Foundations. Nat. Rev. Neurosci. 2020, 21(6), 322–334.
- Fiveash, A.; Bedoin, N.; Gordon, R. L.; Tillmann, B. Processing Rhythm in Speech and Music: Shared Mechanisms and Implications for Developmental Speech and Language Disorders. Neuropsychology 2021, 35(8), 771.
- Tomaino, C.; Wilkens, J. Combined Music Therapy and Speech Therapy to Improve Intelligibility in Patients with Dysarthria. In Press.
- Harrington, D. L.; Castillo, G. N.; Greenberg, P. A.; et al. Neurobehavioral Mechanisms of Temporal Processing Deficits in Parkinson’s Disease. PLoS One 2011, 6(2). [CrossRef]
- Wu, T.; Wang, J.; Wang, C.; et al. Basal Ganglia Circuits Changes in Parkinson’s Disease Patients. Neurosci. Lett. 2012, 524(1), 55–59. [CrossRef]
- Kotz, S. A.; Schwartze, M.; Schmidt-Kassow, M. Non-Motor Basal Ganglia Functions: A Review and Proposal for a Model of Sensory Predictability in Auditory Language Perception. Cortex 2009, 45(8), 982–990. [CrossRef]
- Kotz, S. A.; Schwartze, M. Cortical Speech Processing Unplugged: A Timely Subcortico-Cortical Framework. Trends Cogn. Sci. 2010, 14(9), 392–399. [CrossRef]
- Lewis, M. M.; Slagle, C. G.; Smith, A. B.; et al. Task Specific Influences of Parkinson's Disease on the Striato-Thalamo-Cortical and Cerebello-Thalamo-Cortical Motor Circuitries. Neuroscience 2007, 147(1), 224–235. [CrossRef]
- Przybylski, L.; Bedoin, N.; Krifi-Papoz, S.; et al. Rhythmic Auditory Stimulation Influences Syntactic Processing in Children with Developmental Language Disorders. Neuropsychology 2013, 27(1), 121–131. [CrossRef]
- Clair, A. A.; Memmott, J. Therapeutic Uses of Music with Older Adults; American Music Therapy Association: 2008.
- Young, W. R.; Williams, A. M. How Fear of Falling Can Increase Fall-Risk in Older Adults: Applying Psychological Theory to Practical Observations. Gait Posture 2015, 41(1), 7–12. [CrossRef]
- Young, W. R.; Olonilua, M.; Masters, R. S.; Dimitriadis, S.; Williams, A. M. Examining Links Between Anxiety, Reinvestment and Walking When Talking by Older Adults During Adaptive Gait. Exp. Brain Res. 2016, 234(1), 161–172. [CrossRef]
- de Melker Worms, J. L.; Stins, J. F.; van Wegen, E. E.; Loram, I. D.; Beek, P. J. Influence of Focus of Attention, Reinvestment and Fall History on Elderly Gait Stability. Physiol. Rep. 2017, 5(1). [CrossRef]
- Rochester, L.; Hetherington, V.; Jones, D.; et al. The Effect of External Rhythmic Cues (Auditory and Visual) on Walking During a Functional Task in Homes of People with Parkinson's Disease. Arch. Phys. Med. Rehabil. 2005, 86(5), 999–1006. [CrossRef]
- Ghai, S.; Driller, M.; Ghai, I. Effects of Joint Stabilizers on Proprioception and Stability: A Systematic Review and Meta-Analysis. Phys. Ther. Sport 2017, 25, 65–75. [CrossRef]
- Ghai, S.; Ghai, I.; Effenberg, A. O. Effect of Rhythmic Auditory Cueing on Aging Gait: A Systematic Review and Meta-Analysis. Aging Dis. 2018, 9(5), 901–923. [CrossRef]
- Thaut, M. H.; McIntosh, G. C.; Rice, R. R.; et al. Rhythmic Auditory Stimulation in Gait Training for Parkinson’s Disease Patients. Mov. Disord. 1996, 11(2), 193–200. [CrossRef]
- Clark, D. J. Automaticity of Walking: Functional Significance, Mechanisms, Measurement and Rehabilitation Strategies. Front. Hum. Neurosci. 2015, 9, 246.
- Bernstein, N. The Co-ordination and Regulation of Movements; Pergamon Press Ltd: 1967.
- Fitts, P. M. Perceptual-Motor Skill Learning. In Categories of Human Learning; Academic Press: 1964; pp 243–285.
- Schneider, W.; Shiffrin, R. M. Controlled and Automatic Human Information Processing. I. Detection, Search and Attention. Psychol. Rev. 1977, 84(1), 1–66. [CrossRef]
- Wu, T.; Liu, J.; Zhang, H.; et al. Attention to Automatic Movements in Parkinson's Disease: Modified Automatic Mode in the Striatum. Cereb. Cortex 2015, 25(10), 3330–3342.
- Hebb, D. O. The Organization of Behavior: A Neuropsychological Theory; Science Editions: 1949.
- Wu, T.; Kansaku, K.; Hallett, M. How Self-Initiated Memorized Movements Become Automatic: A Functional MRI Study. J. Neurophysiol. 2004, 91(4), 1690–1698.
- Poldrack, R. A.; Sabb, F. W.; Foerde, K.; et al. The Neural Correlates of Motor Skill Automaticity. J. Neurosci. 2005, 25(22), 5356–5364.
- Seidler, R. D.; Bernard, J. A.; Burutolu, T. B.; et al. Motor Control and Aging: Links to Age-Related Brain Structural, Functional and Biochemical Effects. Neurosci. Biobehav. Rev. 2010, 34, 721–733. [CrossRef]
- Suzuki, M.; Miyai, I.; Ono, T.; et al. Prefrontal and Premotor Cortices Are Involved in Adapting Walking and Running Speed on the Treadmill: An Optical Imaging Study. Neuroimage 2004, 23, 1020–1026. [CrossRef]
- Meester, D.; Al-Yahya, E.; Dawes, H.; Martin-Fagg, P.; Pinon, C. Associations Between Prefrontal Cortex Activation and H-Reflex Modulation During Dual Task Gait. Front. Hum. Neurosci. 2014, 8, 78. [CrossRef]
- Benes, F. M. Emerging Principles of Altered Neural Circuitry in Schizophrenia. Brain Res. Rev. 2000, 31(2–3), 251–269.
- Bella, S. D.; Benoit, C. E.; Farrugia, N.; et al. Gait Improvement Via Rhythmic Stimulation in Parkinson’s Disease Is Linked to Rhythmic Skills. Sci. Rep. 2017, 7, 42005. [CrossRef]
- Palesi, F.; Tournier, J. D.; Calamante, F.; et al. Contralateral Cerebello-Thalamo-Cortical Pathways with Prominent Involvement of Associative Areas in Humans In Vivo. Brain Struct. Funct. 2015, 220(6), 3369–3384. [CrossRef]
- Watson, T. C.; Becker, N.; Apps, R.; Jones, M. W. Back to Front: Cerebellar Connections and Interactions with the Prefrontal Cortex. Front. Syst. Neurosci. 2014, 8, 4. [CrossRef]
- Mittleman, G.; Goldowitz, D.; Heck, D. H.; Blaha, C. D. Cerebellar Modulation of Frontal Cortex Dopamine Efflux in Mice: Relevance to Autism and Schizophrenia. Synapse 2008, 62(7), 544–550. [CrossRef]
- Arguello, P. A.; Enquist, L. W.; Wang, S. S. H. Long-Distance Connectivity Between Prefrontal Cortex and Cerebellum in Mouse. Neurosci. Meet. Planner. New Orleans, LA: Soc Neurosci. 2012, 104, 30.
- D’Angelo, E.; Casali, S. Seeking a Unified Framework for Cerebellar Function and Dysfunction: From Circuit Operations to Cognition. Front. Neural Circuits 2013, 6, 116.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).