1. Introduction
Clinical pharmacy constitutes both a professional discipline and a research field dedicated to optimizing pharmacotherapy, with the goal of enhancing patient-centered outcomes and advancing public health [
1,
2,
3]. From this perspective, medication review and reconciliation are essential tools for tailoring drug therapy, particularly in complex or understudied settings like pediatric patients.
The limited availability of efficacy and safety data in pediatric populations, combined with the frequent off-label use of medications and the need for dosage form adaptations to ensure adherence, renders children a particularly complex group in drug therapy. [
2,
4,
5].
Additionally, children exhibit lower precision in in symptom reporting compared to adults, with verbal reliability influenced by factors such as age, previous experiences with medications and illnesses, self-esteem, and resilience [
6,
7].
Although most research on therapeutic errors focuses on adult populations, children are up to three times more likely to experience adverse drug events and reactions resulting from such errors [
8].
In this context, the clinical pharmacist plays a pivotal role in optimizing pharmacological management, as demonstrated in several studies.
A study conducted at Hacettepe University, Ankara, evaluated the impact of clinical pharmacist-led intervention for drug related problems (DRPs) in neonatal intensive care units. The results revealed a significantly lower incidence of medication errors in the intervention group (35%), in which clinical pharmacists were involved, compared to the control group (53%), along with improved accuracy in drug prescribing and administration. However, the incidence of adverse drug reactions—most commonly electrolyte imbalances and nephrotoxicity—did not differ significantly between groups [
9].
These findings underscore the need for personalized and targeted approaches in pediatric drug use to enhance safety and improve care quality. The clinical pharmacist plays a critical role in advancing the care of hospitalized pediatric patients by contributing to the development and implementation of individualized therapeutic strategies. Effective integration of this role necessitates strong interdisciplinary collaboration, often within the core care team, to facilitate shared decision-making and optimize patient outcomes [
10].
Physicians' acceptance of clinical pharmacist recommendations has been widely explored in the literature, with findings showing considerable variability. Although some studies report high acceptance rates, others highlight fluctuations influenced by clinical settings and the nature of interprofessional relationships. Key factors affecting acceptance include the level of trust in pharmacists, he clarity and clinical relevance of the recommendations, and the robustness of the supporting evidence [
11].
This study aims to comprehensively assess the role of clinical pharmacists in the Pediatric Neurology and Neurophysiology Unit, with a particular focus on physician acceptance of pharmacists’ suggestions and the types of recommendations most frequently implemented over a 13-week period. Given that the majority of patients in this unit are treated for epilepsy, this setting presents a critical area for clinical pharmacy interventions, as antiepileptic therapies require careful monitoring to ensure both efficacy and safety.
2. Materials and Methods
2.1. Study Design
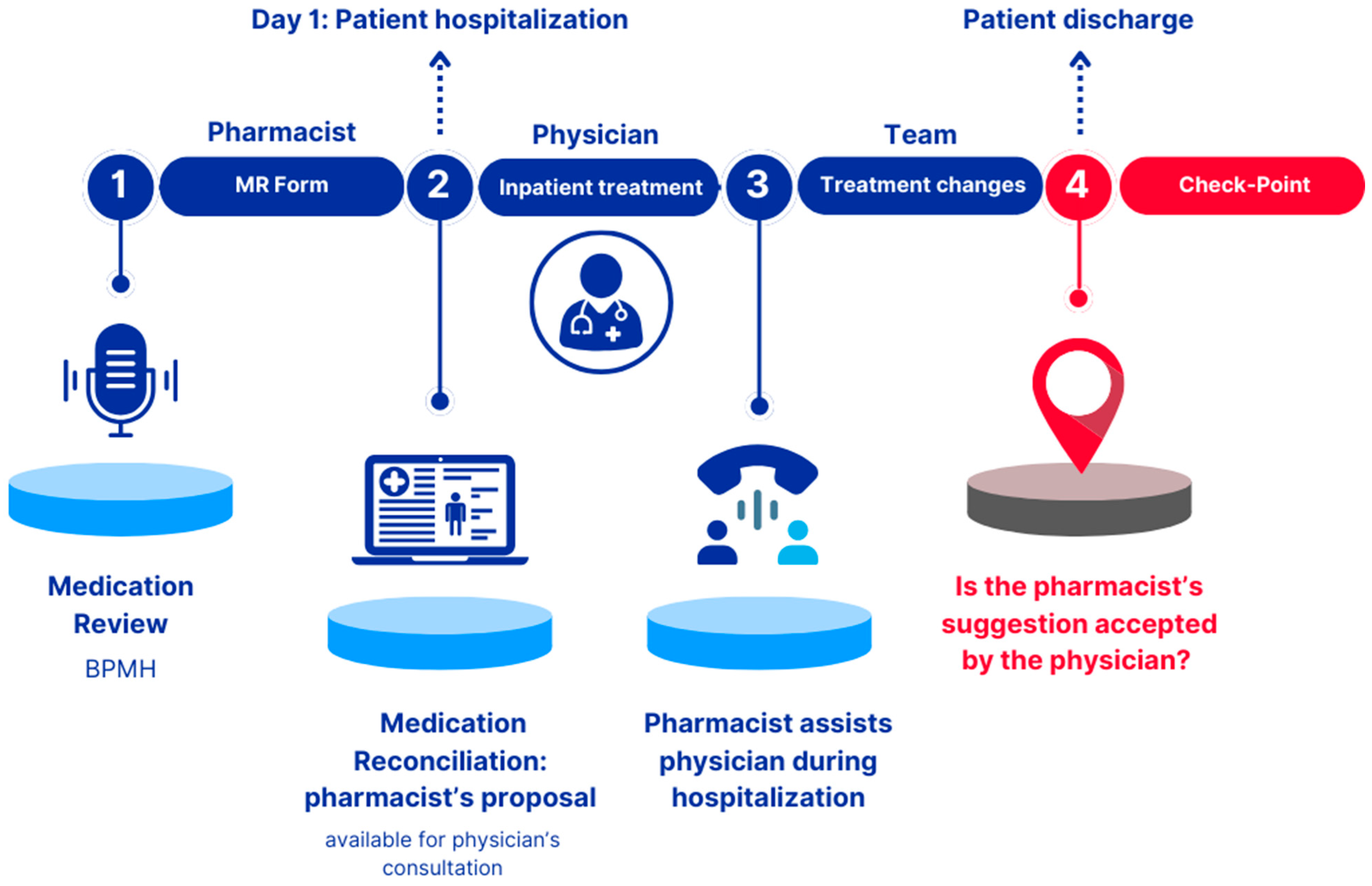
A retrospective observational non-profit study conducted over a 13-week period over 13 weeks, from June to September 2023, in the Pediatric Neurology and Neurophysiology Unit of a university hospital in Northern Italy. The clinical pharmacist was involved across three distinct phases, as illustrated in
Figure 1.
2.1.1. Phase 1 “Pharmacist: Medication Review Form”
Phase 1 was conducted in a pre-hospitalization setting and involved collecting the Best Possible Medication History (BPMH) for each patient, followed by the completion of the Medication Review (MR) Form. The BPMH was gathered using two separate sources of information::
A phone interview with the patient’s parent/caregiver;
A review of clinical documentation, available through the electronic medical record.
An in-depth analysis of each patient’s therapy was subsequently performed. To facilitate this, the following resources were utilized:
Evidence-based point-of-care medical resources, including UpToDate and Merative Micromedex) [
12,
13];
Summary of Product Characteristics (SmPC), Instructions For Use (IFU) or product sheet databases for medicinal products, medical devices or non-medicinal products, respectively. The completed MR Form was signed by the pharmacist and uploaded into the patient's electronic medical record. The MR Form collected the following information: patient’s anonymized name, surname, and date of birth (for data analysis); allergies and/or intolerances; recent adverse drugs reactions; recently discontinued therapies; meal administration times and feeding route; details of in-use medicinal products, including name, dosage, posology, pharmaceutical form, posology and route/method of administration; non-medicinal products (e.g., medical devices, homeopathic remedies, supplements); drug-drug, food-drug and drug-other non-medicinal products contraindications or major interactions; and any other relevant information (e.g. drug handling, supply or administration issues). The key areas of focus for the reconciliation proposal included pediatric dosages, formulation stability data and strategies to improve drug administration, either orally or via Percutaneous Endoscopic Gastrostomy (PEG) (Percutaneous Endoscopic Gastrostomy) or Nasogastric Tube (NGT).
2.1.2. Phase 2 “Physician: Inpatient Treatment”
The MR Form, generated in Phase 1, was available for consultation by physicians and served as a medication reconciliation proposal. In this phase, the pharmacist's recommendations were either confirmed or rejected by the physicians.
2.1.3. Phase 3 “Team: Treatment Changes”
During patient hospitalization, the clinical pharmacist provided support to physicians regarding drug administration, supply and handling. A dedicated telephone line was established between doctors and the clinical pharmacist, who was also actively involved in the Unit's weekly meetings. During this phase, the pharmacist’s recommendations could still be accepted or rejected by the physicians.
2.1.4. Check-Point
After the patient's discharge, a follow-up was conducted to determine whether the pharmacist's recommendations regarding both in-hospital and post-discharge therapy were accepted by the prescribing physician. Additionally, the specific suggestions that were accepted were identified. This checkpoint represents the primary objective of the study.
2.2. Cluster Model
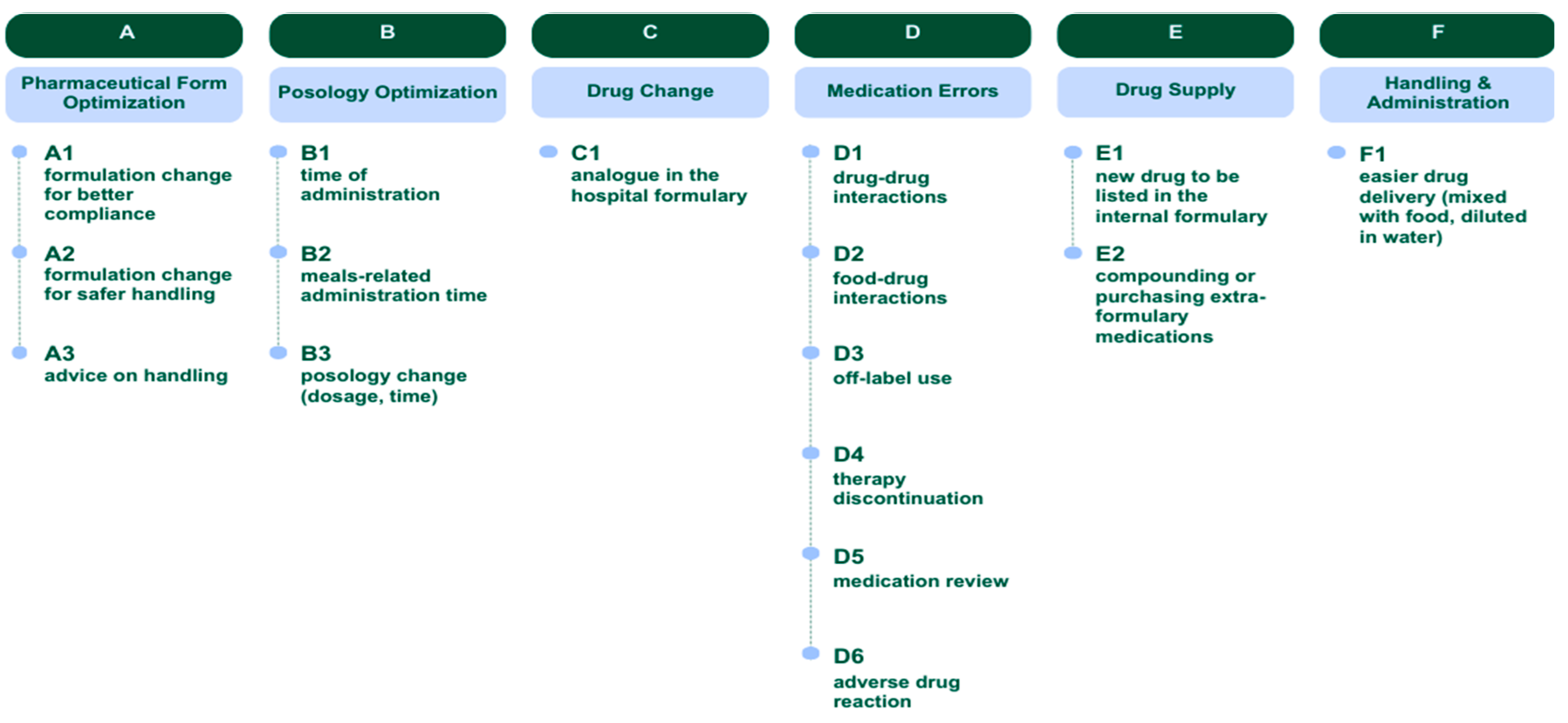
Following a thorough evaluation of the PCNE Classification for Drug-Related Problems V9.1 [
14], a classification system for the potential recommendations provided by clinical pharmacists was developed.
Six clusters of suggestions were identified: Pharmaceutical Form Optimization (A), Posology Optimization (B), Drug Change (C), Medication Errors (D), Drug Supply (E), Handling & Administration (F). Additionally, each recommendation was assigned an alphanumeric code (letter+digit), as illustrated in
Figure 2.
2.3. Inclusion and Exclusion Criteria
Patient selection was based on the following criteria:
Patients with a scheduled hospitalization in the Pediatric Neurology and Neurophysiology Unit;
Patients prescribed at least two concomitant daily medications;
Hospitalized patients with a high care burden (e.g., requiring multiple daily administrations and/or manipulations of the pharmaceutical form);
Patients capable of providing Informed Consent or whose guardians provided consent on their behalf.
Lack of a scheduled hospitalization or absence of pharmacological therapy were not considered exclusion criteria.
2.4. Outcome Measures
The primary outcome was the degree of physician acceptance of the medication review activities performed by the clinical pharmacist.
Secondary outcomes included the types of recommendations most commonly accepted by physicians and the correlation between time to intervention and the number of accepted recommendations. Time to intervention was measured in weeks from baseline (day 0).
The percentages of physician-accepted suggestions out of the total number of pharmacist-supplied recommendations were calculated for each patient, categorized by type of suggestion and over time. These percentages represented the primary and secondary endpoints.
2.5. Statistical Analysis
Continuous normally and non-normally distributed variables were reported as median (interquartile range, IQR), whereas categorical variables were expressed as absolute values and percentages.
For correlation analysis, a positive correlation was considered when R2 > 0.50.
Data analysis was performed using Jamovi software [
15].
2.6. Ethics Approval
The study was conducted in compliance with Good Clinical Practice (GCP) standards as outlined in in the ICH guideline for Good Clinical Practice E6(R2) Step 5 [
16].
Informed Consent for all patients enrolled in the study was obtained from their legal guardians [
17]. Formal approval granted by the Ethics Committee on March 7
th 2024 (code: CET-ACEV: 469n/AO/24).
3. Results
3.1. Baseline Characteristics of the Cohort
A total of 63 patients were initially enrolled in the study with a clinical pharmacist completing an MR Form for each participant. However, 6 patients scheduled for hospitalization were not admitted, leading to their exclusion from the analysis, resulting in a final cohort of 57 patients.
Of the 57 patients, 30 (53%) were male. The median age of the patients was 3 years (IQR: 1.00-10.25), with 19% being younger than one year and a similar proportion being adolescents. Regarding their pharmacological therapy, 16% of the patients were prescribed at least 5 medications per day, resulting in a median daily drug intake of 3.2 medications (IQR: 1.25-4.00).
Baseline characteristics of the patients and the details of their drug therapies are summarized in
Table 1.
3.2. Evaluation of Suggestions Shared by the Clinical Pharmacist with Clinicians
During the observation period the clinical pharmacist provided a total of 138 recommendations for 57 patients, with a median of 2 suggestions for each patient (range 1-3).
The most frequently shared suggestion clusters were “Medication Errors” (D, 45%), “Drug Supply” (E, 26%) and “Pharmaceutical Form Optimization” (A, 18%). When examining individual recommendation types, the most common suggestions provided by the pharmacist were related to “drug-drug interactions” (D1, 23%), followed by “compounding or purchasing medications not included in local formulary” (E2, 17%).
3.3. Evaluation of Suggestions Accepted by Clinicians
The analysis of shared pharmaceutical recommendations revealed a total of 138 suggestions, of which 42% (n=58) were accepted and 58% (n=80) were not. The most frequently shared category was medication errors (D) (45%, n=62), particularly related to drug-drug interactions (D1, 23%), medication review (D5, 10%), and food-drug interactions (D2, 6%). Among these, 32% (n=20) were accepted. Drug supply (E) was the second most common cluster (26%, n=36), mainly driven by compounding or purchasing extra-formulary medications (E2, 17%). Among “E cluster”, 42% (n=15) of shared suggestions were accepted. Pharmaceutical form optimization (A) accounted for 18% (n=25) of the suggestions, particularly formulation changes for safer handling (A2, 8%) and advice on handling (A3, 9%), with an overall acceptance of 64% (n=16). Conversely, posology optimization (B), drug change (C) and handling & administration (F) were less frequently shared. Within the same suggestion categories (A, B, C, D, E, or F), the suggestions most frequently accepted by clinicians involved handling & administration (F), pharmaceutical form optimization (A), drug change (C) and drug supply (E).
These findings highlight the key areas where interventions were most and least likely to be implemented, suggesting a higher feasibility for drug supply adjustments and formulation changes, while modifications related to medication errors required further assessment before acceptance. See
Table 2, for further information.
During the observation period the clinical pharmacist shared 138 suggestions for 57 patients, with a median of 2 suggestions for each patient (range 1-3).
3.4. Analysis of Time to Intervention and Acceptance Rate Correlation
During the 13 weeks of the study, a comprehensive analysis of the degree of acceptance of shared recommendations was conducted.
Throughout this period, the median number of recommendations sent per week was 12.
The weekly trend of shared/accepted recommendations provides an indication of how the acceptance rate correlates with the consolidation of the clinical pharmacist's activity.
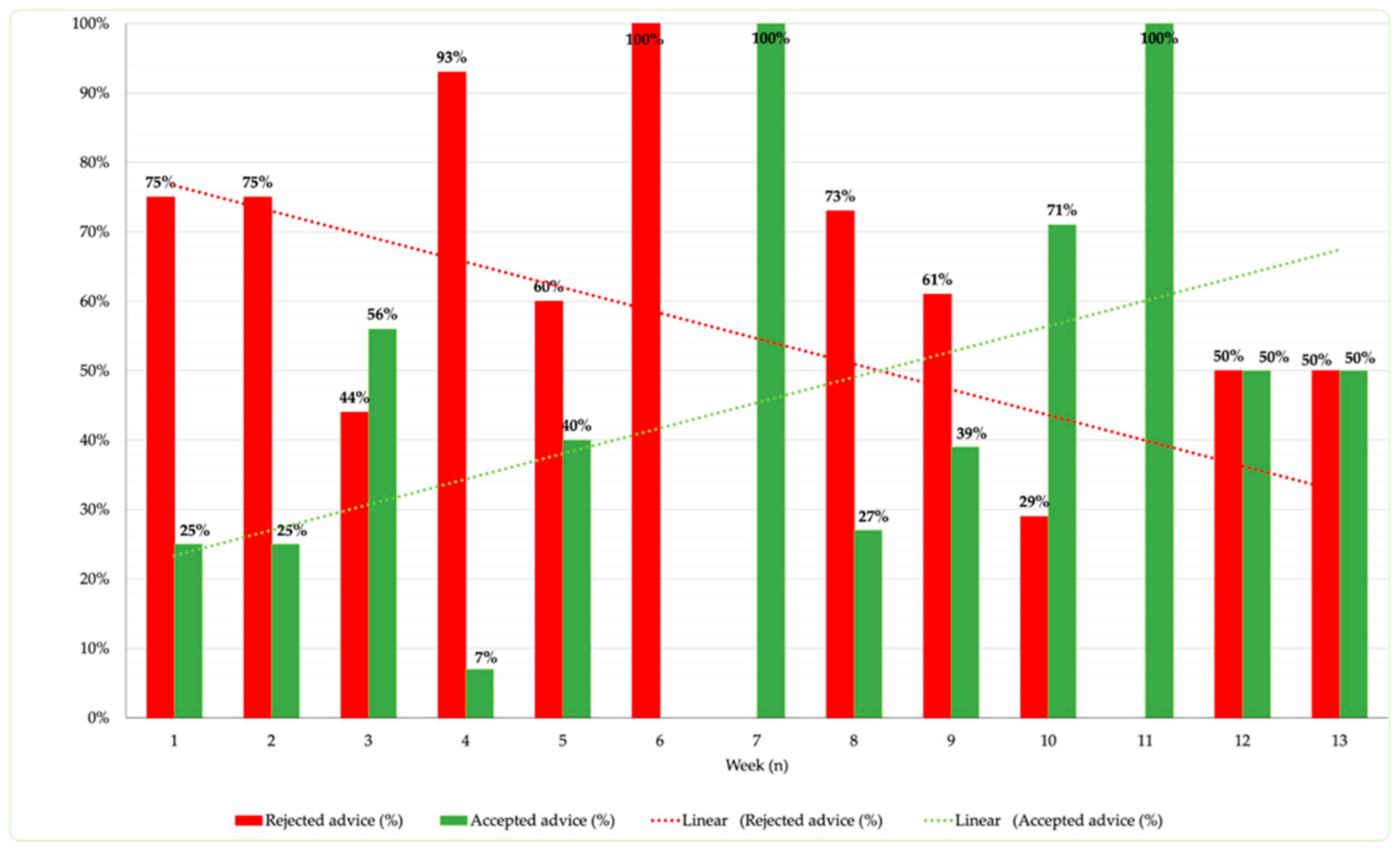
As reported in
Figure 3, indeed, it is noted that, as the weeks of project implementation progress, the proportion of accepted recommendations per week increases. With the exception of week 6, the trend demonstrates an increasingly higher frequency of accepted recommendations, with two peak values (100%) reached in weeks 7 and 11. The introduction of the clinical pharmacist in the weekly departmental meetings has facilitated an increase in trust from the department clinicians, resulting in a percentage increase in accepted recommendations. This observation is supported by the increasing trend, positively correlated with the weeks of observation (R
2 = 0.59).
The structuring of weekly commitment and the implementation of discussion sessions, such as clinical case review meetings, have contributed to improving the acceptance of recommendations at Pediatric Neurology and Neurophysiology Unit.
4. Discussion
The setting selected in this study involves pediatric patients with epilepsy, representing an important area of study and requiring carefully monitored antiepileptic therapy to ensure both treatment efficacy and patient safety.
The concurrent use of multiple antiepileptic drugs poses complex challenges related to pharmacological interactions, potentially affecting treatment response and tolerability. Continuous treatment is crucial, as non-adherence can lead to recurrent seizures, significantly impacting the patient's quality of life.
An essential aspect is the careful evaluation of drugs used in pediatric patients, with particular attention to the use of drugs not specifically approved for this population or not intended for the treatment of epileptic seizures. This carries the risk of unforeseen side effects and requires careful assessment by the clinical pharmacist.
In our study, the impact of the clinical pharmacist's intervention was analyzed by observing the acceptance level of shared suggestions with physicians over a 13-week period. During this time, a total of 138 suggestions were shared with the analyzed patients, of which 42% were accepted by the prescribers. Although this outcome serves more as a methodological indicator than an effectiveness measure, it provided valuable insights into the actual perception of the pharmacist's activities.
The "traditional" support of the pharmacist in medication reviews involves identifying potential DRPs but requires time and the establishment of a "culture" to achieve a satisfactory acceptance level [
18].
For this reason, we clustered the types of recommendations provided by the pharmacist to analyze areas where the professional's opinion was well accepted versus areas requiring sensitization among the healthcare providers receiving the suggestions.
The most shared suggestion was about medication errors (45%), which remains the responsibility of clinical pharmacists, as highlighted by the study Zhang et al. [
19]. However, the acceptance rate of type D suggestions was only 32%. Other areas had proven more suitability to changes, like drug supply adjustments and formulation changes.
The longitudinal analysis of suggestions provided over 13 weeks revealed significant variability in acceptance and rejection rates. The acceptance of suggestions varied throughout the study, driven by increased collaboration between pharmacists and clinicians through weekly meetings and the clinical pharmacist's growing understanding of the patient cohort. These fluctuations could be attributed to several factors, including the hospital staff's increasing familiarity with the clinical pharmacist, the complexity of cases treated, and inter-professional communication [
20].
It is noteworthy that some weeks recorded a 100% acceptance rate of recommendations, suggesting a better understanding and trust in the role of the clinical pharmacist. On the other hand, weeks with higher rejection rates may reflect initial resistance to change or inadequate communication.
4.1. Strengths and Weaknesses (Study Limitations)
The study, while providing important results, presents some limitations.
Firstly, the only parameter used to evaluate the impact of the clinical pharmacist was the number of suggestions accepted or rejected by physicians. However, this endpoint may not fully capture the effectiveness of the pharmacist's role, as it does not account for other clinically relevant variables, such as the effects of therapeutic modifications on patients, drug-related problems, hospitalization length, and other outcome indicators.
A second aspect to consider is the relatively short observation period. This, combined with a ward organization that included the closure of scheduled admissions between weeks 10 and 13, could have influenced the data and the overall representativeness of the results.
Additionally, the lack of specific training for the ward staff may have impacted their perception and collaboration with the clinical pharmacist. The absence of dedicated resources for continuous pharmacist involvement is another obstacle, limiting regular interventions in the ward.
Expanding the activities with more continuous participation in specialist meetings, as well as involvement in ward rounds and all medication review phases, not only for patients with scheduled admissions, will be crucial to verify the definitive impact of the clinical pharmacist in an Italian pediatric setting.
4.2. Further Research
An aspect to examine is the potential implementation of medication reconciliation during care transitions, namely immediately after admission to the ward and upon transfers to other operating units or home. This initiative aims to optimize the management of pharmacological therapy throughout the entire care pathway, contributing to reducing potential errors or inefficiencies.
A necessary step, once all information on the ongoing project has been collected, is to promote the clinical pharmacist figure in all pediatric settings, particularly those where polypharmacotherapy is frequent. This sharing would allow for the analysis of the clinical pharmacist's impact on the pediatric patient from a 360-degree perspective, enriching the evaluation framework.
To promote even closer collaboration with the medical team, it could be considered to involve the clinical pharmacist more continuously in ward activities, through participation in daily ward meetings and patient visits. This would ensure a constant presence and facilitate more effective synergy among the professional figures involved.
Finally, it would be useful to expand consultation activities, extending support not only to patients undergoing pre-admission, but also to those admitted in emergency. This approach aims to improve medication management and the quality of care provided throughout all stages of the care pathway.
These prospects represent potentially useful directions for enriching the project and deepening the evaluation of the clinical pharmacist's effectiveness within the pediatric hospital setting.
5. Conclusions
In the context of the Pediatric Neurology and Neurophysiology Unit, the introduction of the clinical pharmacist has proven to be a valuable element in patient care.
Our research has highlighted how this intervention had a significant impact on increasing adherence to recommendations over the 13-week study period.
The clinical pharmacist has established effective communication within the multidisciplinary team, playing a crucial role in monitoring therapeutic appropriateness and in training healthcare staff and caregivers to optimize and make drug administration safer. This research project has clearly demonstrated how involving a clinical pharmacist in a multidisciplinary clinical setting is a valuable resource for managing complex patients.
While acceptance of shared recommendations still has room for improvement, it is fascinating to observe how this process has sparked constructive debate and promoted increased communication and collaboration between clinical pharmacists and physicians, with the latter becoming more open to collaboration and sharing.
Through research projects like this and by implementing tailored training opportunities on clinical pharmacy from university education onwards, it will be possible to institutionalize this professional role in Italy, enhancing patient care and fostering an important and innovative professionalism for pharmacists.
Author Contributions
Conceptualization, D.M., L.C.; methodology, D.M, M.Z, A.T, L.C.; validation, M.Z., D.M. and L.C.; formal analysis, M.Z. and D.M.; investigation, M.Z.; data curation, M.Z.; writing—original draft preparation, M.Z., A.T. and D.M.; writing—review and editing, M.Z., A.T., D.M., M.C.G., L.C., I.T., S.S., M.F.P., M.N., C.A. and G.P.; visualization, M.Z., A.T. and D.M.; supervision, D.M., L.C., F.V., I.T., S.S. and G.P.; project administration, D.M., F.V. and G.P.; All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Ethics Committee Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethic Committee (code: CET-ACEV: 469n/AO/24).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study. Written informed consent has been obtained from the patients to publish this paper.
Data Availability Statement
Data are available from the authors upon request to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Dreischulte, T. , van den Bemt, B. , Steurbaut, S. et al. European Society of Clinical Pharmacy definition of the term clinical pharmacy and its relationship to pharmaceutical care: a position paper. Int J Clin Pharm 2022, 44, 837–842. [Google Scholar] [CrossRef]
- Sifact. Available online: https://www.sifact.it/cose-la-farmacia-clinica/ (accessed on November 22nd, 2024).
- Bernocchi, B., Casula, C., Tragni, E. Medication review: an effective tool for appropriate prescribing. GIFF 2016, 8, 21-30. http://www.sefap.it/web/upload/GIFF2016-3_21_30.pdf.
- Zanin, A. , Baratiri, F., Roverato, B., et al. Polypharmacy in Children with Medical Complexity: A Cross-Sectional Study in a Pediatric Palliative Care Center. Children 2024, 11, 821. [Google Scholar] [CrossRef] [PubMed]
- Mengato, D. , Zanin, A., Baratiri, F., et al. Polypharmacy in Pediatric Palliative Care: Exploring Discrepancies Between Physicians and Pharmacists. Children 2025, 12, 124. [Google Scholar] [CrossRef] [PubMed]
- Corny, J. , Bailey, B., Lebel, D., Bussières, J.-F. Unlicensed and off-label drug use in paediatrics in a mother-child tertiary care hospital. Paediatr Child Health 2016, 21, 83–87. [Google Scholar] [CrossRef] [PubMed]
- Cite Eriksson, T. , & Melander, A. C. Clinical pharmacists' services, role and acceptance: a national Swedish survey. European journal of hospital pharmacy: science and practice 2021, 28, 203–206. [Google Scholar] [CrossRef]
- Sin, C. M. , Huynh, C., Dahmash, D., Maidment, I. D. Factors influencing the implementation of clinical pharmacy services on paediatric patient care in hospital settings. Eur J Hosp Pharm 2022, 29, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Yalçın, N. , Kaşıkcı, M., Çelik, H. T., et al. Impact of clinical pharmacist-led intervention for drug-related problems in neonatal intensive care unit a randomized controlled trial. Front Pharmacol 2023, 14, 1242779. [Google Scholar] [CrossRef] [PubMed]
- McKay, C. , Vest, M. H., Doligalski, C., et al. Implementation and results of a standardized process for identifying ambulatory pharmacy clinical outcome measures. Am J Health Syst Pharm 2023, 80, 860–867. [Google Scholar] [CrossRef] [PubMed]
- Maffre, I. , Leguelinel-Blache, G., Soulairol, I. A systematic review of clinical pharmacy services in pediatric inpatients. Drugs Ther Perspect 2021, 37, 363–375. [Google Scholar] [CrossRef]
- UptoDate. Available online: https://wkhealthce.my.site.com/customers/s/?language=it (accessed on December 2nd, 2024).
- Meritive Micromedex. Available online: https://www.micromedexsolutions.com/micromedex2/librarian/ (accessed on December 2nd, 2024).
- PCNE Classification for Drug-Related Problems 2020, Volume 9.1 Available online: https://www.pcne.org/upload/files/555_09_PCNE_classification_V9-1_final.pdf.
- The jamovi project (Version 2.5)[Computer Software] https://www.jamovi.org (accessed on May 2nd, 2023).
- European Medicines Agency, Guideline for good clinical practice E6(R2). Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/ich-guideline-good-clinical-practice-e6r2-step-5_en.
- EMA updated version of EnprEMA "Informed Consent for Paediatric Clinical Trials in Europe". Available online: https://www.ema.europa.eu/en/events/annual-workshop-european-network-paediatric-research-ema-enpr-ema (accessed on May 2nd, 2023).
- Clyne, W., Blenkinsopp, A., Seal, R. A Guide to Medication Review 2008. Available online: https://www.cff.org.br/userfiles/52%20-%20CLYNE%20W%20A%20guide%20to%20medication%20review%202008.pdf.
- Zhang, L. , Hu, Y., Pan, P., et al. Estimated Manipulation of Tablets and Capsules to Meet Dose Requirements for Chinese Children: A Cross-Sectional Study. Front Pediatr 2021, 9, 747499. [Google Scholar] [CrossRef] [PubMed]
- Naseef, H. , Amria, A., Asrawi, A., et al. The acceptance and awareness of healthcare providers towards doctor of pharmacy (Phram D) in the Palestinian health care system. Saudi Pharm J 2020, 28, 1068–1074. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).