Submitted:
10 April 2025
Posted:
11 April 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Management Strategies
2.1. Preventive Measures
2.1.1. Crops’ Exploitation
2.1.2. Modifications on Sowing and Transplanting Dates
2.1.3. Solarization
2.1.4. Tillage, Mechanical Cultivation
2.1.5. Fertilization
2.1.6. Use of Synthetic Stimulants, Suicidal Germination
2.1.7. Polyethylene Plastic Mulching
2.1.8. Use of Crop Tolerance Inducers
2.1.9. Use of Resistant/Tolerant cvs
2.2. Curative Measures
2.2.1. Seed Dressing Treatments
2.2.2. Foliar Herbicides
2.2.3. Herbigation
2.2.4. Biological Control
2.2.5. Beneficial Insects
2.2.6. Hand Weeding
3. Recognition of Parasitic Plant Molecular Patterns by Hosts
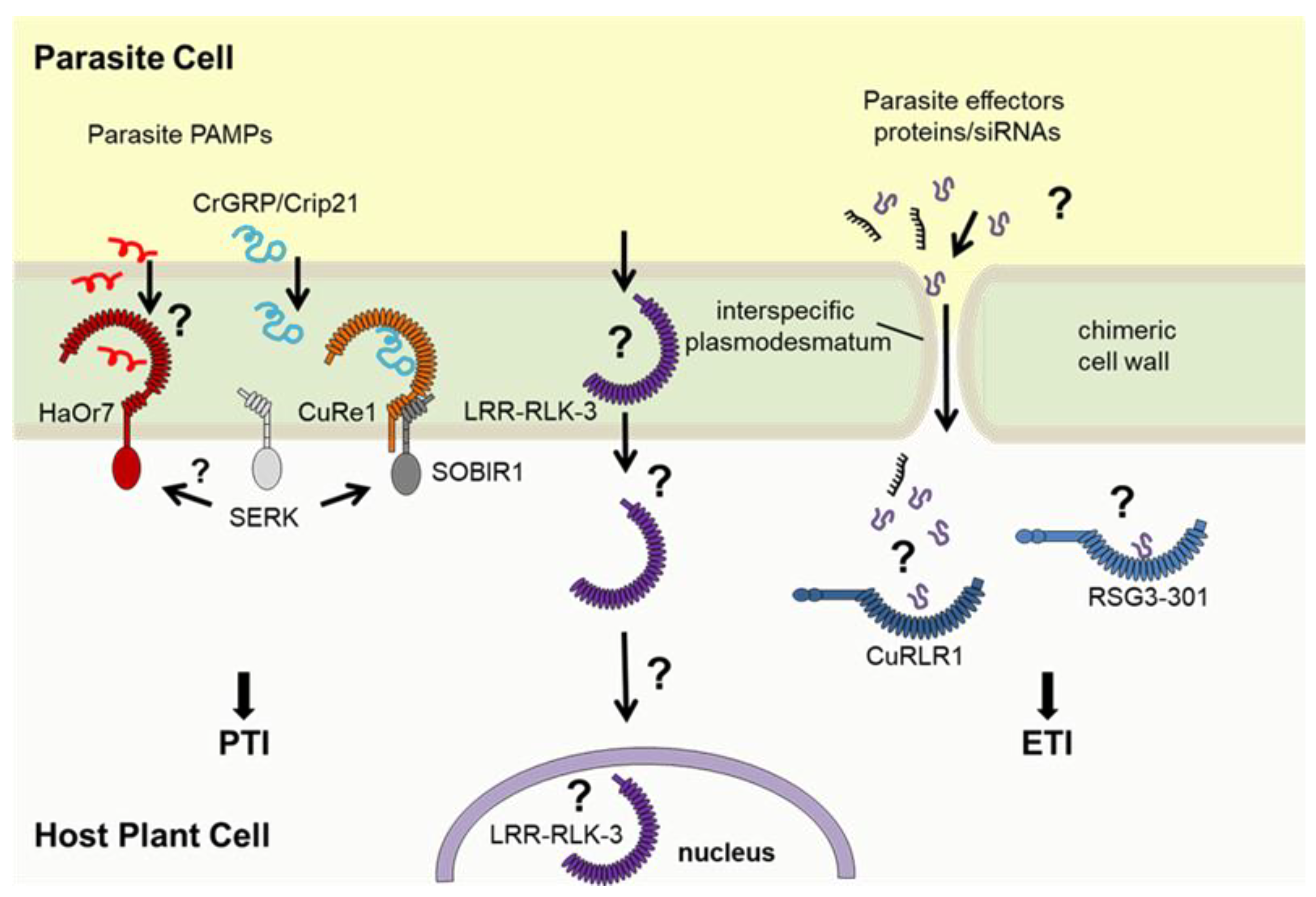
4. Hormonal Crosstalk Between Crop-Parasite
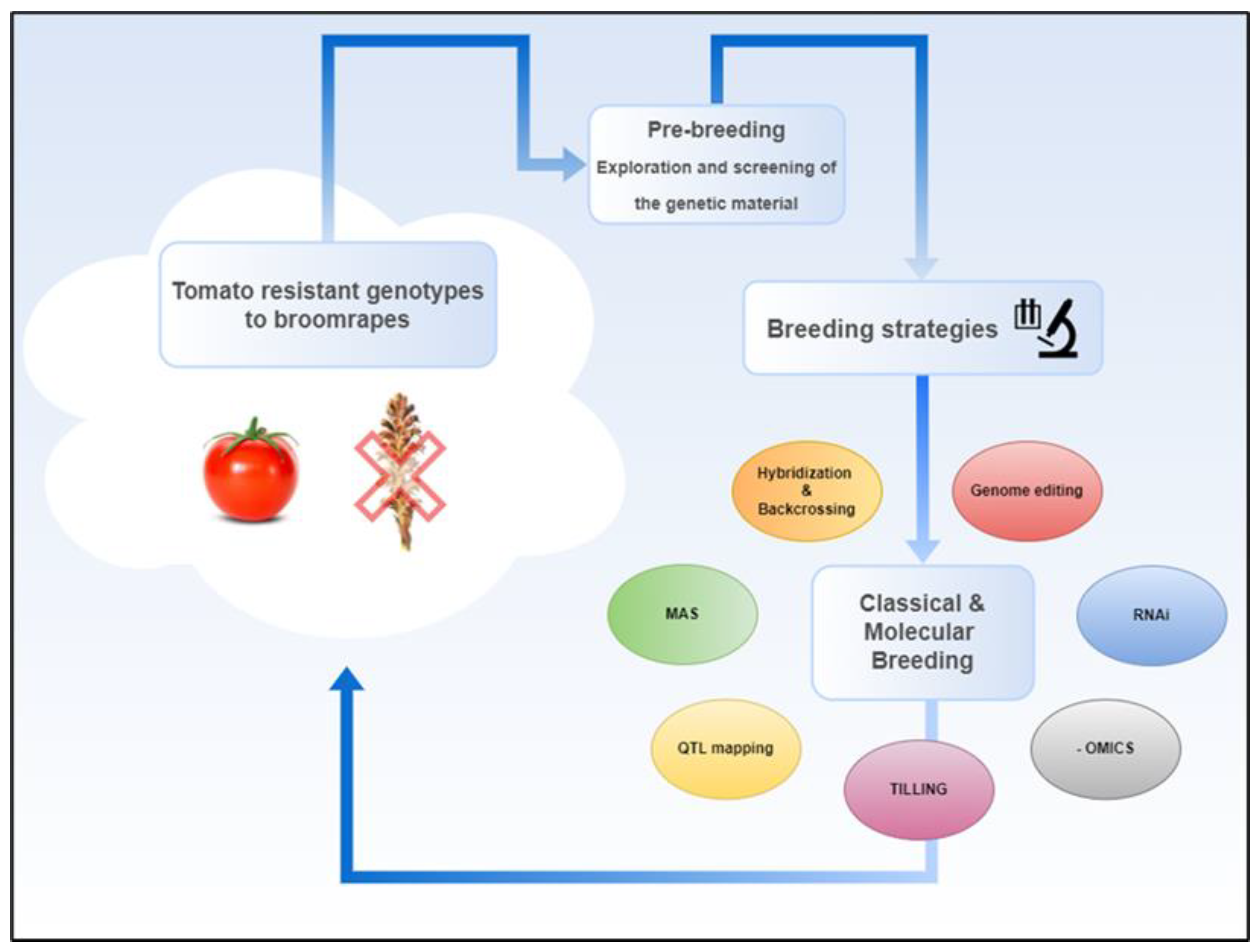
5. Breeding for Resistance
5.1. Industrial Tomato
5.2. Legumes
6. Conclusions
6.1. Management Strategies
6.2. Recognition of Parasitic Plant Molecular Patterns by Hosts
6.3. Hormonal Crosstalk Between Crop-Parasite
6.4. Breeding for Resistance
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Parker, C. Parasitic Weeds: A World Challenge. Weed science 2012, 60, 269–276. [Google Scholar] [CrossRef]
- Fernández-Aparicio, M.; Reboud, X.; Gibot-Leclerc, S. Broomrape Weeds. Underground Mechanisms of Parasitism and Associated Strategies for Their Control: A Review. Frontiers in plant science 2016, 7, 135. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Aparicio, M.; Delavault, P.; Timko, M.P. Management of Infection by Parasitic Weeds: A Review. Plants 2020, 9, 1184. [Google Scholar] [CrossRef] [PubMed]
- Rubiales, D.; Fernández-Aparicio, M. Innovations in Parasitic Weeds Management in Legume Crops. A Review. Agronomy for Sustainable Development 2012, 32, 433–449. [Google Scholar] [CrossRef]
- Rubiales, D. Broomrape Threat to Agriculture. Outlooks on Pest Management 2020, 31, 141–145. [Google Scholar] [CrossRef]
- Eizenberg, H.; Goldwasser, Y. Control of Egyptian Broomrape in Processing Tomato: A Summary of 20 Years of Research and Successful Implementation. Plant Disease 2018, 102, 1477–1488. [Google Scholar] [CrossRef]
- Qasem, J.R. Broomrapes (Orobanche Spp.) the Challenge and Management: A Review. Jordan Journal of Agricultural Sciences 2021, 17, 117–150. [Google Scholar] [CrossRef]
- Dor, E.; Goldwasser, Y. “Parasitic Weeds: Biology and Control” Special Issue Editors Summary. Plants 2022, 11, 1891. [Google Scholar] [CrossRef]
- Negewo, T.; Ahmed, S.; Tessema, T.; Tana, T. Biological Characteristics, Impacts, and Management of Crenate Broomrape (Orobanche Crenata) in Faba Bean (Vicia Faba): A Review. Frontiers in Agronomy 2022, 4, 708187. [Google Scholar] [CrossRef]
- Rubiales, D. Managing Root Parasitic Weeds to Facilitate Legume Reintroduction into Mediterranean Rain-Fed Farming Systems. Soil Systems 2023, 7, 99. [Google Scholar] [CrossRef]
- Fernández-Aparicio, M.; Flores, F.; Rubiales, D. The Effect of Orobanche Crenata Infection Severity in Faba Bean, Field Pea, and Grass Pea Productivity. Frontiers in Plant Science 2016, 7, 1409. [Google Scholar] [CrossRef]
- Lops, F.; Frabboni, L.; Carlucci, A.; Tarantino, A.; Raimondo, M.L.; Disciglio, G. Management of Branched Broomrape in Field Processing Tomato Crop. In Tomato-From Cultivation to Processing Technology; IntechOpen: Rijeka, Croatia, 2022. [Google Scholar]
- Rubiales, D.; Fernández-Aparicio, M.; Wegmann, K.; Joel, D.M. Revisiting Strategies for Reducing the Seedbank of Orobanche and Phelipanche Spp. Weed Research 2009, 49, 23–33. [Google Scholar] [CrossRef]
- Grenz, J.H.; Manschadi, A.M.; Uygur, F.N.; Sauerborn, J. Effects of Environment and Sowing Date on the Competition between Faba Bean (Vicia Faba) and the Parasitic Weed Orobanche Crenata. Field Crops Research 2005, 93, 300–313. [Google Scholar] [CrossRef]
- Krishnamurthy, S.; Rao, N.K. Effect of Submergence on Bearing Capacity. Soils and Foundations 1975, 15, 61–66. [Google Scholar] [CrossRef]
- Zhelev, Z.; Zheleva, A.; Halacheva, K. Preparation of a β-Amanitin-Concanavalin A Conjugate of Low Toxicity. Toxicon 1987, 25, 981–987. [Google Scholar] [CrossRef] [PubMed]
- Musselman, L.J.; Rodenburg, J. Parasitic Weed Management. 2023.
- Abbes, Z.; Kharrat, M.; Chaibi, W. Seed Germination and Tubercle Development of Orobanche Foetida and Orobanche Crenata in Presence of Different Plant Species. Tunisian Journal of Plant Protection 2008, 3, 101. [Google Scholar]
- Pérez-de-Luque, A.; Eizenberg, H.; Grenz, J.H.; Sillero, J.C.; Avila, C.; Sauerborn, J.; Rubiales, D. Broomrape Management in Faba Bean. Field Crops Research 2010, 115, 319–328. [Google Scholar] [CrossRef]
- Habimana, S.; Nduwumuremyi, A.; Chinama, R.J.D. Managementof Orobanche in Field Crops: A Review. Journal of soil science and plant nutrition 2014, 14, 43–62. [Google Scholar] [CrossRef]
- Parker, C.; Riches, C.R. Parasitic Weeds of the World: Biology and Control; 1993.
- Jestin, C.; Molenat, D.; Boulet, C.; Bamme, B.; Leflon, M. Impact of Catch and Non-Host Crops in Cropping Systems to Control Branched Broomrape [Conference Poster]. 2013.
- Restuccia, A.; Marchese, M.; Mauromicale, G.; Restuccia, G. Biological Characteristics and Control of Orobanche Crenata Forsk., a Review. Italian Journal of Agronomy 2009, 4, 53–68. [Google Scholar] [CrossRef]
- Hegazi, E.; Zeid, A.; Attia, M.; Hasaneen, M.; Shall, A.; Eryan, M.; Aly, N.; Showiel, S.; El-Rahman, S.; Taleb, H.; et al. Effect of Intercropping by Flax, Radish and Fenugreek on Faba Bean, Vicia Faba L., Production and Reduction of Orobanche Crenata Forsk Seed Bank. AFF 2024, 13, 52–59. [Google Scholar] [CrossRef]
- Rubiales, D.; Fernández-Aparicio, M.; Pérez-de-Luque, A.; Castillejo, M.A.; Prats, E.; Sillero, J.C.; Rispail, N.; Fondevilla, S. Breeding Approaches for Crenate Broomrape (Orobanche Crenata Forsk.) Management in Pea ( Pisum Sativum L.). Pest Management Science 2009, 65, 553–559. [Google Scholar] [CrossRef]
- Acharya, B.D.; Khattri, G.B.; Chettri, M.K.; Srivastava, S.C. Effect of Brassica Campestris Var. Toria as a Catch Crop on Orobanche Aegyptiaca Seed Bank. Crop protection 2002, 21, 533–537. [Google Scholar] [CrossRef]
- Nadal, S.; Moreno, M.T.; Cubero, J.I.; Rubiales, D. Determinate Faba Bean Young Pod Response to Glyphosate and Crenate Broomrape ( Orobanche Crenata). Journal of Sustainable Agriculture 2005, 25, 19–27. [Google Scholar] [CrossRef]
- Perronne, R.; Makowski, D.; Goffaux, R.; Montalent, P.; Goldringer, I. Temporal Evolution of Varietal, Spatial and Genetic Diversity of Bread Wheat between 1980 and 2006 Strongly Depends upon Agricultural Regions in France. Agriculture, Ecosystems & Environment 2017, 236, 12–20. [Google Scholar]
- Qasem, J.R.S. Parasitic Weeds of Jordan: Species, Hosts, Distribution and Management; Bentham Science Publishers, 2022.
- Fernández-Aparicio, M.; Emeran, A.A.; Rubiales, D. Control of Orobanche Crenata in Legumes Intercropped with Fenugreek (Trigonella Foenum-Graecum). Crop protection 2008, 27, 653–659. [Google Scholar] [CrossRef]
- Evidente, A.; Fernández-Aparicio, M.; Andolfi, A.; Rubiales, D.; Motta, A. Trigoxazonane, a Monosubstituted Trioxazonane from Trigonella Foenum-Graecum Root Exudate, Inhibits Orobanche Crenata Seed Germination. Phytochemistry 2007, 68, 2487–2492. [Google Scholar] [CrossRef]
- Khan, Z.R.; Hassanali, A.; Overholt, W.; Khamis, T.M.; Hooper, A.M.; Pickett, J.A.; Wadhams, L.J.; Woodcock, C.M. Control of Witchweed Striga Hermonthica by Intercropping with Desmodium Spp., and the Mechanism Defined as Allelopathic. Journal of chemical ecology 2002, 28, 1871–1885. [Google Scholar] [CrossRef]
- Chon, S.-U.; Kim, Y.-M. Herbicidal Potential and Quantification of Suspected Allelochemicals from Four Grass Crop Extracts. J Agronomy Crop Science 2004, 190, 145–150. [Google Scholar] [CrossRef]
- Baghestani, A.; Lemieux, C.; Leroux, G.D.; Baziramakenga, R.; Simard, R.R. Determination of Allelochemicals in Spring Cereal Cultivars of Different Competitiveness. Weed Science 1999, 47, 498–504. [Google Scholar] [CrossRef]
- Aksoy, E.; Arslan, Z.F.; Tetik, Ö.; Eymirli, S. Using the Possibilities of Some Trap, Catch and Brassicaceaen Crops for Controlling Crenate Broomrape a Problem in Lentil Fields. International Journal of Plant Production 2016, 10, 53–62. [Google Scholar]
- Kitiş, Y.E.; Grenz, J.H.; Sauerborn, J. Effects of Some Cereal Root Exudates on Germination of Broomrapes (Orobanche Spp. and Phelipanche Spp.). Mediterranean Agricultural Sciences 2019, 32, 145–150. [Google Scholar] [CrossRef]
- Haidar, M.A.; Bibi, W.; Abdel-Khalek, N. Effect of Wheat and Barley Residues on Branched Broomrape (Orobanche Ramosa) Growth and Development in Potatoes. 1995.
- Hosh, M.; Tabbache, S.; Haddad, D.; Habak, H. The Effect of Dried Powdered Leaves of Radish (Rhaphanus Sativus L.) in Decreasing the Parasitism of Orobanche Ramosa L. on Tomato (Solanum Lycopersicum L.) Grown in Greenhouses. 2022.
- Calha, I.M.; Santos, J.; Amaral, A.; Cachado, J.; Nunes, A.P. Cover Cropping Effect on P. Ramosa Parasitation in Tomato under Controlled Conditions. In Proceedings of the Actas XVIII Congreso de la Sociedad Española de Malherbología; Centro de Investigaciones Científicas y Tecnológicas de Extremadura (CICYTEX), 2022; pp. 215–220.
- Kebreab, E.; Murdoch, A.J. Modelling the Effects of Water Stress and Temperature on Germination Rate of Orobanche Aegyptiaca Seeds. Journal of Experimental Botany 1999, 50, 655–664. [Google Scholar] [CrossRef]
- Pérez-de-Luque, A.; Jorrín, J.V.; Rubiales, D. Crenate Broomrape Control in Pea by Foliar Application of Benzothiadiazole (BTH). Phytoparasitica 2004, 32, 21–29. [Google Scholar] [CrossRef]
- Kemal, S.A. Narrowing the Yield Gap of Food Legumes through Integrated Management of Parasitic Weeds in the Highlands of Ethiopia. 2015.
- Rubiales, D.; Alcántara, C.; Gil, J.; Sillero, J.C. Infection of Chickpea (Cicer Arietinum) by Crenate Broomrape (Orobanche Crenata) as Influenced by Sowing Date and Weather Conditions. Agronomie 2003, 23, 359–362. [Google Scholar] [CrossRef]
- Kacan, K.; Tursun, N. Effect of Planting Time and Tomato Varieties on Broomrape (Phelipanche Aegyptiaca) Emergence and Tomato Yield in Western Turkey. Research on Crop 2012, 13, 1070–1077. [Google Scholar]
- Manschadi, A.M.; Sauerborn, J.; Stützel, H. Quantitative Aspects of Orobanche Crenata Infestation in Faba Beans as Affected by Abiotic Factors and Parasite Soil Seedbank. Weed Research 2001, 41, 311–324. [Google Scholar] [CrossRef]
- Jacobsohn, R.; Greenberger, A.; Katan, J.; Levi, M.; Alon, H. Control of Egyptian Broomrape (Orobanche Aegyptiaca) and Other Weeds by Means of Solar Heating of the Soil by Polyethylene Mulching. Weed Science 1980, 28, 312–316. [Google Scholar] [CrossRef]
- Mauromicale, G.; Monaco, A.L.; Longo, A.M.; Restuccia, A. Soil Solarization, a Nonchemical Method to Control Branched Broomrape (Orobanche Ramosa) and Improve the Yield of Greenhouse Tomato. Weed science 2005, 53, 877–883. [Google Scholar] [CrossRef]
- Boz, Ö.; Doğan, M.N.; Öğöt, D. The Effect of Duration of Solarization on Controlling Branched Broomrape (Phelipanche Ramosa L.) and Some Weed Species. 2012.
- Goldwasser, Y.; Rodenburg, J. Integrated Agronomic Management of Parasitic Weed Seed Banks. In Parasitic Orobanchaceae; Joel, D.M., Gressel, J., Musselman, L.J., Eds.; Springer Berlin Heidelberg: Berlin, Heidelberg, 2013; pp. 393–413. ISBN 978-3-642-38145-4. [Google Scholar]
- Eizenberg, H.; Lande, T.; Achdari, G.; Roichman, A.; Hershenhorn, J. Effect of Egyptian Broomrape (Orobanche Aegyptiaca) Seed-Burial Depth on Parasitism Dynamics and Chemical Control in Tomato. Weed science 2007, 55, 152–156. [Google Scholar] [CrossRef]
- Ghersa, C.M.; Martınez-Ghersa, M.A. Ecological Correlates of Weed Seed Size and Persistence in the Soil under Different Tilling Systems: Implications for Weed Management. Field Crops Research 2000, 67, 141–148. [Google Scholar] [CrossRef]
- Van Delft, G.J.; Graves, J.D.; Fitter, A.H.; Van Ast, A. Striga Seed Avoidance by Deep Planting and No-Tillage in Sorghum and Maize. International Journal of Pest Management 2000, 46, 251–256. [Google Scholar] [CrossRef]
- Tarantino, E.; Lops, F.; Disciglio, G.; Carlucci, A.; Gatta, G.; Frabboni, L. Contain Phelipanche Ramosa on Processing Tomatoes. 2015.
- Disciglio, G.; Frabboni, L.; Tarantino, A.; Stasi, A. Association between Dynamic Agrivoltaic System and Cultivation: Viability, Yields and Qualitative Assessment of Medical Plants. Sustainability 2023, 15, 16252. [Google Scholar] [CrossRef]
- Jain, R.; Foy, C.L. Nutrient Effects on Parasitism and Germination of Egyptian Broomrape (Orobanche Aegyptiaca). Weed Technology 1992, 6, 269–275. [Google Scholar] [CrossRef]
- Parker, C. Observations on the Current Status of Orobanche and Striga Problems Worldwide. Pest Management Science 2009, 65, 453–459. [Google Scholar] [CrossRef] [PubMed]
- Westwood, J.H.; Foy, C.L. Influence of Nitrogen on Germination and Early Development of Broomrape (Orobanche Spp.). Weed Science 1999, 47, 2–7. [Google Scholar] [CrossRef]
- Yoneyama, K.; Xie, X.; Kim, H.I.; Kisugi, T.; Nomura, T.; Sekimoto, H.; Yokota, T.; Yoneyama, K. How Do Nitrogen and Phosphorus Deficiencies Affect Strigolactone Production and Exudation? Planta 2012, 235, 1197–1207. [Google Scholar] [CrossRef]
- Johnson, A.W.; Rosebery, G.; Parker, C. A Novel Approach to Striga and Orobanche Control Using Synthetic Germination Stimulants. Weed Research 1976, 16, 223–227. [Google Scholar] [CrossRef]
- Babiker, A.G.T.; Hamdoun, A.M.; Rudwan, A.; Mansi, N.G.; Faki, H.H. Influence of Soil Moisture on Activity and Persistence of the Strigol Analogue GR 24. Weed Research 1987, 27, 173–178. [Google Scholar] [CrossRef]
- Zwanenburg, B.; Mwakaboko, A.S.; Kannan, C. Suicidal Germination for Parasitic Weed Control. Pest Management Science 2016, 72, 2016–2025. [Google Scholar] [CrossRef]
- Lechat, M.-M.; Brun, G.; Montiel, G.; Véronési, C.; Simier, P.; Thoiron, S.; Pouvreau, J.-B.; Delavault, P. Seed Response to Strigolactone Is Controlled by Abscisic Acid-Independent DNA Methylation in the Obligate Root Parasitic Plant, Phelipanche Ramosa L. Pomel. Journal of experimental botany 2015, 66, 3129–3140. [Google Scholar] [CrossRef]
- Hỳlová, A.; Pospíšil, T.; Spíchal, L.; Mateman, J.J.; Blanco-Ania, D.; Zwanenburg, B. New Hybrid Type Strigolactone Mimics Derived from Plant Growth Regulator Auxin. New biotechnology 2019, 48, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Dvorakova, M.; Hylova, A.; Soudek, P.; Petrova, S.; Spichal, L.; Vanek, T. Triazolide Strigolactone Mimics as Potent Selective Germinators of Parasitic Plant Phelipanche Ramosa. Pest Management Science 2019, 75, 2049–2056. [Google Scholar] [CrossRef]
- Cala, A.; Ghooray, K.; Fernández-Aparicio, M.; Molinillo, J.M.; Galindo, J.C.; Rubiales, D.; Macías, F.A. Phthalimide-derived Strigolactone Mimics as Germinating Agents for Seeds of Parasitic Weeds. Pest Management Science 2016, 72, 2069–2081. [Google Scholar] [CrossRef]
- Vouzounis, N.A.; Americanos, P.G. Control of Orobanche (Broomrape) in Tomato and Eggplant. 1998.
- Sokat, Y.; Demirkan, H. Research on the Methods for Controlling Broomrape (Phelipanche Ramosa (L.) Pomel.), Problem in Eggplant Production Areas in Turkey. 2020.
- Sillero, J.C.; Rojas-Molina, M.M.; Ávila, C.M.; Rubiales, D. Induction of Systemic Acquired Resistance against Rust, Ascochyta Blight and Broomrape in Faba Bean by Exogenous Application of Salicylic Acid and Benzothiadiazole. Crop Protection 2012, 34, 65–69. [Google Scholar] [CrossRef]
- Kusumoto, D.; Goldwasser, Y.; Xie, X.; Yoneyama, K.; Takeuchi, Y.; Yoneyama, K. Resistance of Red Clover (Trifolium Pratense) to the Root Parasitic Plant Orobanche Minor Is Activated by Salicylate but Not by Jasmonate. Annals of botany 2007, 100, 537–544. [Google Scholar] [CrossRef] [PubMed]
- Bakewell-Stone, P. Orobanche Ramosa (Branched Broomrape) 2024, 37747.
- Al-Wakeel, S.A.M.; Moubasher, H.; Gabr, M.M.A.; Madany, M.M.Y. Induction of Systemic Resistance in Tomato Plants against Orobanche Ramosa L. Using Hormonal Inducers. 2012.
- Gonsior, G.; Buschmann, H.; Szinicz, G.; Spring, O.; Sauerborn, J. Induced Resistance—An Innovative Approach to Manage Branched Broomrape (Orobanche Ramosa) in Hemp and Tobacco. Weed science 2004, 52, 1050–1053. [Google Scholar] [CrossRef]
- Vurro, M.; Boari, A.; Pilgeram, A.L.; Sands, D.C. Exogenous Amino Acids Inhibit Seed Germination and Tubercle Formation by Orobanche Ramosa (Broomrape): Potential Application for Management of Parasitic Weeds. Biological Control 2006, 36, 258–265. [Google Scholar] [CrossRef]
- Mabrouk, Y.; Simier, P.; Arfaoui, A.; Sifi, B.; Delavault, P.; Zourgui, L.; Belhadj, O. Induction of Phenolic Compounds in Pea ( Pisum Sativum L.) Inoculated by Rhizobium Leguminosarum and Infected with Orobanche Crenata. Journal of Phytopathology 2007, 155, 728–734. [Google Scholar] [CrossRef]
- Gozzo, F. Systemic Acquired Resistance in Crop Protection: From Nature to a Chemical Approach. J. Agric. Food Chem. 2003, 51, 4487–4503. [Google Scholar] [CrossRef]
- Khalil, S.; Malhotra, R.; Saxena, M.C.; Erskine, W.; Kharrat, M. Breeding Faba Bean for Orobanche Resistance. 2004.
- Nassib, A.M.; Ibrahim, A.A.; Saber, H.A. Broomrape (Orobanche Crenata) Resistance in Broad Beans: Breeding Work in Egypt. In Food legume improvement and development: Proceedings...; IDRC: Ottawa, ON, CA, 1978. [Google Scholar]
- Kharrat, M.; Abbes, Z.; Amri, M. A New Faba Bean Small Seeded Variety Najeh Tolerant to Orobanche Registered in the Tunisian Catalogue. Tunisian Journal of Plant Protection 2010, 5. [Google Scholar]
- Amri, M.; Trabelsi, I.; Abbes, Z.; Kharrat, M. Release of a New Faba Bean Variety" Chourouk" Resistant to the Parasitic Plants Orobanche Foetida and O. Crenata in Tunisia. 2019.
- Rubiales, D.; Flores, F.; Emeran, A.A.; Kharrat, M.; Amri, M.; Rojas-Molina, M.M.; Sillero, J.C. Identification and Multi-Environment Validation of Resistance against Broomrapes (Orobanche Crenata and Orobanche Foetida) in Faba Bean (Vicia Faba). Field Crops Research 2014, 166, 58–65. [Google Scholar] [CrossRef]
- El-Mehy, A.A.; El-Gendy, H.M.; Aioub, A.A.; Mahmoud, S.F.; Abdel-Gawad, S.; Elesawy, A.E.; Elnahal, A.S. Response of Faba Bean to Intercropping, Biological and Chemical Control against Broomrape and Root Rot Diseases. Saudi Journal of Biological Sciences 2022, 29, 3482–3493. [Google Scholar] [CrossRef]
- Rubiales, D.; Moral, A.; Flores, F. Agronomic Performance of Broomrape Resistant and Susceptible Faba Bean Accession. Agronomy 2022, 12, 1421. [Google Scholar] [CrossRef]
- Jurado-Expósito, M.; Castejón-Muñoz, M.; García-Torres, L. Broomrape (Orobanche Crenata) Control with Imazethapyr Applied to Pea (Pisum Sativum) Seed. Weed technology 1996, 10, 774–780. [Google Scholar] [CrossRef]
- Punia, S.S. Biology and Control Measures of Orobanche. 2014.
- Misganaw, M. Integrated Weed (Orobanche Crenata) Management on Faba Bean. American Journal of Agricultural Research 2016, 1, 0029–0034. [Google Scholar]
- Garcia-Torres, L.; Lopez-Granados, F. Progress of Herbicide Control of Broomrape (Orobanche Spp.) in Legumes and Sunflower (Helinanthus Annuus L.). 1991.
- Garcia-Torres, L.; Lopez-Granados, F.; Jurado-Exposito, M.; Díaz Sánchez, J. The Present State of Orobanche Spp. Infestations in Andalusia and the Prospects for Its Management. 1998.
- Foy, C.L.; Jacobsohn, R.; Jain, R. Screening of Lycopersicon Spp. for Glyphosate and/or Orobanche Aegyptiaca Pers. Resistance. Weed Research 1988, 28, 383–391. [Google Scholar] [CrossRef]
- Eizenberg, H.; Aly, R.; Cohen, Y. Technologies for Smart Chemical Control of Broomrape (Orobanche Spp. and Phelipanche Spp.). Weed Science 2012, 60, 316–323. [Google Scholar] [CrossRef]
- Hershenhorn, J.; Eizenberg, H.; Dor, E.; Kapulnik, Y.; Goldwasser, Y. Phelipanche Aegyptiaca Management in Tomato. Weed Research 2009, 49, 34–47. [Google Scholar] [CrossRef]
- Gressel, J. Enhancing Microbiocontrol of Weeds. Microbe 2003, 69, 498–502. [Google Scholar]
- Boari, A.; Vurro, M. Evaluation of Fusarium Spp. and Other Fungi as Biological Control Agents of Broomrape (Orobanche Ramosa). Biological Control 2004, 30, 212–219. [Google Scholar] [CrossRef]
- Gibot-Leclerc, S.; Guinchard, L.; Edel-Hermann, V.; Dessaint, F.; Cartry, D.; Reibel, C.; Gautheron, N.; Bernaud, E.; Steinberg, C. Screening for Potential Mycoherbicides within the Endophyte Community of Phelipanche Ramosa Parasitizing Tobacco. FEMS Microbiology Ecology 2022, 98, fiac024. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, A. Biological Control of Orobanche Ramosa by Fusarium Solani. International Journal of Advanced Biological and Biomedical Research 2014, 2, 2751–2755. [Google Scholar]
- Vurro, M. Are Root Parasitic Broomrapes Still a Good Target for Bioherbicide Control? Pest Management Science 2024, 80, 10–18. [Google Scholar] [CrossRef]
- Klein, M.; Braverman, Y.; Chizov-Ginzburg, A.; Gol’berg, A.; Blumberg, D.; Khanbegyan, Y.; Hackett, K.J. Infectivity of Beetle Spiroplasmas for New Host Species. BioControl 2002, 47, 427–433. [Google Scholar] [CrossRef]
- Tóth, B.; Mesterházy, Á.; Horváth, Z.; Bartók, T.; Varga, M.; Varga, J. Genetic Variability of Central European Isolates of the Fusarium Graminearum Species Complex. European Journal of Plant Pathology 2005, 113, 35–45. [Google Scholar] [CrossRef]
- Kapralov, S.I. Phytomyza against Broomrape. 1974.
- Ranf, S. Sensing of Molecular Patterns through Cell Surface Immune Receptors. Current opinion in plant biology 2017, 38, 68–77. [Google Scholar] [CrossRef]
- Shiu, S.-H.; Bleecker, A.B. Plant Receptor-Like Kinase Gene Family: Diversity, Function, and Signaling. Sci. STKE 2001, 2001. [Google Scholar] [CrossRef]
- Shiu, S.-H.; Bleecker, A.B. Expansion of the Receptor-like Kinase/Pelle Gene Family and Receptor-like Proteins in Arabidopsis. Plant physiology 2003, 132, 530–543. [Google Scholar] [CrossRef] [PubMed]
- Shiu, S.-H.; Li, W.-H. Origins, Lineage-Specific Expansions, and Multiple Losses of Tyrosine Kinases in Eukaryotes. Molecular biology and evolution 2004, 21, 828–840. [Google Scholar] [CrossRef] [PubMed]
- Albert, M. Peptides as Triggers of Plant Defence. Journal of experimental botany 2013, 64, 5269–5279. [Google Scholar] [CrossRef] [PubMed]
- Boller, T.; Felix, G. A Renaissance of Elicitors: Perception of Microbe-Associated Molecular Patterns and Danger Signals by Pattern-Recognition Receptors. Annu. Rev. Plant Biol. 2009, 60, 379–406. [Google Scholar] [CrossRef]
- Duriez, P.; Vautrin, S.; Auriac, M.-C.; Bazerque, J.; Boniface, M.-C.; Callot, C.; Carrère, S.; Cauet, S.; Chabaud, M.; Gentou, F. A Receptor-like Kinase Enhances Sunflower Resistance to Orobanche Cumana. Nature Plants 2019, 5, 1211–1215. [Google Scholar] [CrossRef]
- Zhang, L.; Yao, Z.; Cao, X.; Chen, M.; Chen, S.; Zhao, Q.; Zhao, S. A Leucine-Rich Repeat Receptor-like Protein Kinase Enhances Tomato Resistance to Phelipanche Aegyptiaca. Scientia Horticulturae 2024, 337, 113353. [Google Scholar] [CrossRef]
- Kaiser, B.; Vogg, G.; Fürst, U.B.; Albert, M. Parasitic Plants of the Genus Cuscuta and Their Interaction with Susceptible and Resistant Host Plants. Frontiers in plant science 2015, 6, 45. [Google Scholar] [CrossRef]
- Ihl, B.; Tutakhil, N.; Hagen, A.; Jacob, F. Studien an Cuscuta Reflexa Roxb: VII. Zum Abwehrmechanismus von Lycopersicon Esculentum Mill. Flora 1988, 181, 383–393. [Google Scholar] [CrossRef]
- Jiang, L.; Wijeratne, A.J.; Wijeratne, S.; Fraga, M.; Meulia, T.; Doohan, D.; Li, Z.; Qu, F. Profiling mRNAs of Two Cuscuta Species Reveals Possible Candidate Transcripts Shared by Parasitic Plants. PLoS ONE 2013, 8, e81389. [Google Scholar] [CrossRef]
- Ranjan, A.; Ichihashi, Y.; Farhi, M.; Zumstein, K.; Townsley, B.; David-Schwartz, R.; Sinha, N.R. Focus on Weed Control: De Novo Assembly and Characterization of the Transcriptome of the Parasitic Weed Dodder Identifies Genes Associated with Plant Parasitism. Plant Physiology 2014, 166, 1186. [Google Scholar] [CrossRef]
- Runyon, J.B.; Mescher, M.C.; Felton, G.W.; De Moraes, C.M. Parasitism by Cuscuta Pentagona Sequentially Induces JA and SA Defence Pathways in Tomato. Plant Cell & Environment 2010, 33, 290–303. [Google Scholar] [CrossRef]
- Werner, T.; Motyka, V.; Strnad, M.; Schmülling, T. Regulation of Plant Growth by Cytokinin. Proc. Natl. Acad. Sci. U.S.A. 2001, 98, 10487–10492. [Google Scholar] [CrossRef] [PubMed]
- Albert, M.; Werner, M.; Proksch, P.; Fry, S.C.; Kaldenhoff, R. The Cell Wall-Modifying Xyloglucan Endotransglycosylase/Hydrolase LeXTH1 Is Expressed during the Defence Reaction of Tomato against the Plant Parasite Cuscuta Reflexa. Plant Biology 2004, 6, 402–407. [Google Scholar] [CrossRef] [PubMed]
- Hegenauer, V.; Fürst, U.; Kaiser, B.; Smoker, M.; Zipfel, C.; Felix, G.; Stahl, M.; Albert, M. Detection of the Plant Parasite Cuscuta Reflexa by a Tomato Cell Surface Receptor. Science 2016, 353, 478–481. [Google Scholar] [CrossRef] [PubMed]
- Chitwood, D.H.; Kumar, R.; Headland, L.R.; Ranjan, A.; Covington, M.F.; Ichihashi, Y.; Fulop, D.; Jiménez-Gómez, J.M.; Peng, J.; Maloof, J.N. A Quantitative Genetic Basis for Leaf Morphology in a Set of Precisely Defined Tomato Introgression Lines. The Plant Cell 2013, 25, 2465–2481. [Google Scholar] [CrossRef]
- Eshed, Y.; Zamir, D. An Introgression Line Population of Lycopersicon Pennellii in the Cultivated Tomato Enables the Identification and Fine Mapping of Yield-Associated QTL. Genetics 1995, 141, 1147–1162. [Google Scholar] [CrossRef]
- Liebrand, T.W.H.; Van Den Berg, G.C.M.; Zhang, Z.; Smit, P.; Cordewener, J.H.G.; America, A.H.P.; Sklenar, J.; Jones, A.M.E.; Tameling, W.I.L.; Robatzek, S.; et al. Receptor-like Kinase SOBIR1/EVR Interacts with Receptor-like Proteins in Plant Immunity against Fungal Infection. Proc. Natl. Acad. Sci. U.S.A. 2013, 110, 10010–10015. [Google Scholar] [CrossRef]
- Liebrand, T.W.; van den Burg, H.A.; Joosten, M.H. Two for All: Receptor-Associated Kinases SOBIR1 and BAK1. Trends in plant science 2014, 19, 123–132. [Google Scholar] [CrossRef]
- Hegenauer, V.; Slaby, P.; Körner, M.; Bruckmüller, J.-A.; Burggraf, R.; Albert, I.; Kaiser, B.; Löffelhardt, B.; Droste-Borel, I.; Sklenar, J. The Tomato Receptor CuRe1 Senses a Cell Wall Protein to Identify Cuscuta as a Pathogen. Nature communications 2020, 11, 5299. [Google Scholar] [CrossRef]
- Fürst, U.; Hegenauer, V.; Kaiser, B.; Körner, M.; Welz, M.; Albert, M. Parasitic Cuscuta Factor(s) and the Detection by Tomato Initiates Plant Defense. Communicative & Integrative Biology 2016, 9, e1244590. [Google Scholar] [CrossRef]
- Johnsen, H.R.; Striberny, B.; Olsen, S.; Vidal-Melgosa, S.; Fangel, J.U.; Willats, W.G.T.; Rose, J.K.C.; Krause, K. Cell Wall Composition Profiling of Parasitic Giant Dodder ( C Uscuta Reflexa ) and Its Hosts: A Priori Differences and Induced Changes. New Phytologist 2015, 207, 805–816. [Google Scholar] [CrossRef] [PubMed]
- Jhu, M.-Y.; Farhi, M.; Wang, L.; Philbrook, R.N.; Belcher, M.S.; Nakayama, H.; Zumstein, K.S.; Rowland, S.D.; Ron, M.; Shih, P.M. Heinz-Resistant Tomato Cultivars Exhibit a Lignin-Based Resistance to Field Dodder (Cuscuta Campestris) Parasitism. Plant Physiology 2022, 189, 129–151. [Google Scholar] [CrossRef] [PubMed]
- Albert, M.; Axtell, M.J.; Timko, M.P. Mechanisms of Resistance and Virulence in Parasitic Plant–Host Interactions. Plant physiology 2021, 185, 1282–1291. [Google Scholar] [CrossRef]
- Ohlson, E.W.; Timko, M.P. Race Structure of Cowpea Witchweed (Striga Gesnerioides) in West Africa and Its Implications for Striga Resistance Breeding of Cowpea. Weed science 2020, 68, 125–133. [Google Scholar] [CrossRef]
- Li, J.; Lis, K.E.; Timko, M.P. Molecular Genetics of Race-specific Resistance of Cowpea to Striga Gesnerioides (Willd.). Pest Management Science 2009, 65, 520–527. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Timko, M.P. Gene-for-Gene Resistance in Striga -Cowpea Associations. Science 2009, 325, 1094. [Google Scholar] [CrossRef]
- Jubic, L.M.; Saile, S.; Furzer, O.J.; El Kasmi, F.; Dangl, J.L. Help Wanted: Helper NLRs and Plant Immune Responses. Current opinion in plant biology 2019, 50, 82–94. [Google Scholar] [CrossRef]
- Yoshida, S.; Cui, S.; Ichihashi, Y.; Shirasu, K. The Haustorium, a Specialized Invasive Organ in Parasitic Plants. Annual Review of Plant Biology 2016, 67, 643–667. [Google Scholar] [CrossRef]
- Mishev, K.; Dobrev, P.I.; Lacek, J.; Filepová, R.; Yuperlieva-Mateeva, B.; Kostadinova, A.; Hristeva, T. Hormonomic Changes Driving the Negative Impact of Broomrape on Plant Host Interactions with Arbuscular Mycorrhizal Fungi. International Journal of Molecular Sciences 2021, 22, 13677. [Google Scholar] [CrossRef]
- Yoshida, S.; Shirasu, K. Plants That Attack Plants: Molecular Elucidation of Plant Parasitism. Current opinion in plant biology 2012, 15, 708–713. [Google Scholar] [CrossRef]
- Jamil, F.; Mukhtar, H.; Fouillaud, M.; Dufossé, L. Rhizosphere Signaling: Insights into Plant–Rhizomicrobiome Interactions for Sustainable Agronomy. Microorganisms 2022, 10, 899. [Google Scholar] [CrossRef] [PubMed]
- Aloni, R. Ecophysiological Implications of Vascular Differentiation and Plant Evolution. Trees 2015, 29, 1–16. [Google Scholar] [CrossRef]
- Ishida, J.K.; Wakatake, T.; Yoshida, S.; Takebayashi, Y.; Kasahara, H.; Wafula, E.; DePamphilis, C.W.; Namba, S.; Shirasu, K. Local Auxin Biosynthesis Mediated by a YUCCA Flavin Monooxygenase Regulates Haustorium Development in the Parasitic Plant Phtheirospermum Japonicum. The Plant Cell 2016, 28, 1795–1814. [Google Scholar] [CrossRef] [PubMed]
- Bouwmeester, H.; Sinha, N.; Scholes, J. Parasitic Plants: Physiology, Development, Signaling, and Ecosystem Interactions. Plant Physiology 2021, 185, 1267–1269. [Google Scholar] [CrossRef]
- López-Ráez, J.A.; Shirasu, K.; Foo, E. Strigolactones in Plant Interactions with Beneficial and Detrimental Organisms: The Yin and Yang. Trends in plant science 2017, 22, 527–537. [Google Scholar] [CrossRef]
- Zagorchev, L.; Stöggl, W.; Teofanova, D.; Li, J.; Kranner, I. Plant Parasites under Pressure: Effects of Abiotic Stress on the Interactions between Parasitic Plants and Their Hosts. International Journal of Molecular Sciences 2021, 22, 7418. [Google Scholar] [CrossRef]
- Cheng, X.; Floková, K.; Bouwmeester, H.; Ruyter-Spira, C. The Role of Endogenous Strigolactones and Their Interaction with ABA during the Infection Process of the Parasitic Weed Phelipanche Ramosa in Tomato Plants. Frontiers in plant science 2017, 8, 392. [Google Scholar] [CrossRef]
- Inoue, M.; Xie, X.; Yoneyama, K. Barley Is a Potential Trap Crop for Root Parasitic Broomrape Weeds. Journal of Pesticide Science 2024, D24–D34. [Google Scholar] [CrossRef]
- Bao, Y.Z.; Yao, Z.Q.; Cao, X.L.; Peng, J.F.; Xu, Y.; Chen, M.X.; Zhao, S.F. Transcriptome Analysis of Phelipanche Aegyptiaca Seed Germination Mechanisms Stimulated by Fluridone, TIS108, and GR24. PLoS ONE 2017, 12, e0187539. [Google Scholar] [CrossRef]
- Cui, S.; Kubota, T.; Nishiyama, T.; Ishida, J.K.; Shigenobu, S.; Shibata, T.F.; Toyoda, A.; Hasebe, M.; Shirasu, K.; Yoshida, S. Ethylene Signaling Mediates Host Invasion by Parasitic Plants. Sci. Adv. 2020, 6, eabc2385. [Google Scholar] [CrossRef]
- Goyet, V.; Billard, E.; Pouvreau, J.-B.; Lechat, M.-M.; Pelletier, S.; Bahut, M.; Monteau, F.; Spíchal, L.; Delavault, P.; Montiel, G. Haustorium Initiation in the Obligate Parasitic Plant Phelipanche Ramosa Involves a Host-Exudated Cytokinin Signal. Journal of Experimental Botany 2017, 68, 5539–5552. [Google Scholar] [CrossRef] [PubMed]
- Harb, A.M.; Hameed, K.M.; Shibli, R.A. Effect of Triiodobenzoic Acid on Broomrape (Orobanche Ramosa) Infection and Development in Tomato Plants. The Plant Pathology Journal 2004, 20, 81–84. [Google Scholar] [CrossRef]
- Xiao, X.; Zhang, J.; Satheesh, V.; Meng, F.; Gao, W.; Dong, J.; Zheng, Z.; An, G.-Y.; Nussaume, L.; Liu, D. SHORT-ROOT Stabilizes PHOSPHATE1 to Regulate Phosphate Allocation in Arabidopsis. Nature Plants 2022, 8, 1074–1081. [Google Scholar] [CrossRef]
- Wakatake, T.; Ogawa, S.; Yoshida, S.; Shirasu, K. An Auxin Transport Network Underlies Xylem Bridge Formation between the Hemi-Parasitic Plant Phtheirospermum Japonicum and Host Arabidopsis. Development 2020, 147, dev187781. [Google Scholar] [CrossRef]
- Goyet, V.; Wada, S.; Cui, S.; Wakatake, T.; Shirasu, K.; Montiel, G.; Simier, P.; Yoshida, S. Haustorium Inducing Factors for Parasitic Orobanchaceae. Front. Plant Sci. 2019, 10. [Google Scholar] [CrossRef]
- Smith, J.L.; De Moraes, C.M.; Mescher, M.C. Jasmonate- and Salicylate-mediated Plant Defense Responses to Insect Herbivores, Pathogens and Parasitic Plants. Pest Management Science 2009, 65, 497–503. [Google Scholar] [CrossRef]
- Rispail, N.; Dita, M.-A.; González-Verdejo, C.; Pérez-de-Luque, A.; Castillejo, M.-A.; Prats, E.; Román, B.; Jorrín, J.; Rubiales, D. Plant Resistance to Parasitic Plants: Molecular Approaches to an Old Foe. New Phytologist 2007, 173, 703–712. [Google Scholar] [CrossRef]
- Torres-Vera, R.; García, J.M.; Pozo, M.J.; López-Ráez, J.A. Expression of Molecular Markers Associated to Defense Signaling Pathways and Strigolactone Biosynthesis during the Early Interaction Tomato-Phelipanche Ramosa. Physiological and Molecular Plant Pathology 2016, 94, 100–107. [Google Scholar] [CrossRef]
- Frost, A.; López-Gutiérrez, J.C.; Purrington, C.B. Fitness of Cuscuta Salina (Convolvulaceae) Parasitizing Beta Vulgaris (Chenopodiaceae) Grown under Different Salinity Regimes. American Journal of Botany 2003, 90, 1032–1037. [Google Scholar] [CrossRef]
- Wiese, A.; Elzinga, N.; Wobbes, B.; Smeekens, S. A Conserved Upstream Open Reading Frame Mediates Sucrose-Induced Repression of Translation. The Plant Cell 2004, 16, 1717–1729. [Google Scholar] [CrossRef]
- Foyer, C.H.; Rasool, B.; Davey, J.W.; Hancock, R.D. Cross-Tolerance to Biotic and Abiotic Stresses in Plants: A Focus on Resistance to Aphid Infestation. Journal of experimental botany 2016, 67, 2025–2037. [Google Scholar] [CrossRef]
- Martínez-Melgarejo, P.A.; Ormazabal, M.; López-García, J.A.; Guerrero-Franco, J.J.; Martín-Rodríguez, J.A.; Martínez-Andújar, C.; Pérez-Alfocea, F. Solanum Rootstock Biodiversity to Deal with Holoparasitism and Fertilizer Use Efficiency. In Proceedings of the IV Asian Horticultural Congress-AHC2023 1404; 2023; pp. 513–520.
- Baghyalakshmi, K.; Sarala, K.; Prabhakararao, K.; Reddy, D.D. Orobanche Menace in Crop Plants: Host Resistance as a Potential Tool to Control. Journal of Pharmacognosy and Phytochemistry 2019, 8, 93–102. [Google Scholar]
- Gerakari, M.; Kotsira, V.; Kapazoglou, A.; Tastsoglou, S.; Katsileros, A.; Chachalis, D.; Hatzigeorgiou, A.G.; Tani, E. Transcriptomic Approach for Investigation of Solanum Spp. Resistance upon Early-Stage Broomrape Parasitism. Current Issues in Molecular Biology 2024, 46, 9047. [Google Scholar] [CrossRef] [PubMed]
- Tokasi, S.; Bannayan Aval, M.; Mashhadi, H.R.; Ghanbari, A. Screening of Resistance to Egyptian Broomrape Infection in Tomato Varieties. Planta Daninha 2014, 32, 109–116. [Google Scholar] [CrossRef]
- Kapazoglou, A.; Gerakari, M.; Lazaridi, E.; Kleftogianni, K.; Sarri, E.; Tani, E.; Bebeli, P.J. Crop Wild Relatives: A Valuable Source of Tolerance to Various Abiotic Stresses. Plants 2023, 12, 328. [Google Scholar] [CrossRef] [PubMed]
- Sawafta, M.; Shtaya, M. Search for Resistance to Egyptian Broomrape (Orobanche Aegyptiaca) in Tomato Germplasm. 2016.
- Sawafta, M.S. Resistance of Some Tomato Species to Orobanche Aegyptiaca (Comparative Study). PhD Thesis, 2012.
- Ghani, M.A.; Abbas, M.M.; Amjad, M.; Ziaf, K.; Ali, B.; Shaheen, T.; Awan, F.S.; Khan, A.N. Production and Characterisation of Tomato Derived from Interspecific Hybridisation between Cultivated Tomato and Its Wild Relatives. The Journal of Horticultural Science and Biotechnology 2020, 95, 506–520. [Google Scholar] [CrossRef]
- Dalela, G.G.; Mathur, R.L. Resistance of Varieties of Eggplant, Tomato and Tobacco to Broomrape (Orobanche Cernua Loefl.). PANS Pest Articles & News Summaries 1971, 17, 482–483. [Google Scholar] [CrossRef]
- Avdeyev, Y.I.; Scherbinin, B.M.; Ivanova, L.M.; Avdeyev, A.Y. Studying of Tomato Resistance to Broomrape and Breeding Varieties for Processing. In Proceedings of the VIII International Symposium on the Processing Tomato 613; 2002; pp. 283–290.
- Draie, R. Differential Responses of Commercial Tomato Rootstocks to Branched Broomrape. Science and Education 2017, 5, 15–25. [Google Scholar] [CrossRef]
- Rubiales, D.; Araújo, S.S.; Vaz Patto, M.C.; Rispail, N.; Valdés-López, O. Advances in Legume Research. Frontiers in Plant Science 2018, 9, 501. [Google Scholar] [CrossRef]
- Cuccurullo, A.; Nicolia, A.; Cardi, T. Resistance against Broomrapes (Orobanche and Phelipanche Spp.) in Vegetables: A Comprehensive View on Classical and Innovative Breeding Efforts. Euphytica 2022, 218, 82. [Google Scholar] [CrossRef]
- Exploration of Resistance to Phelipanche Aegyptiaca in Tomato - Bai - 2020 - Pest Management Science - Wiley Online Library. Available online: https://scijournals.onlinelibrary.wiley.com/doi/abs/10.1002/ps.5932?casa_token=6C7CoD5d5SkAAAAA%3ACMmxpiLH4ef35n7x8bfzPRp7GiGX7rm2bfpkqs43fHiWYI6l5c6AVEhpzzaiHUIgR1t_f8POcYswGJLP (accessed on 9 January 2025).
- Koltai, H.; LekKala, S.P.; Bhattacharya, C.; Mayzlish-Gati, E.; Resnick, N.; Wininger, S.; Dor, E.; Yoneyama, K.; Yoneyama, K.; Hershenhorn, J. A Tomato Strigolactone-Impaired Mutant Displays Aberrant Shoot Morphology and Plant Interactions. Journal of Experimental Botany 2010, 61, 1739–1749. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, S.; Tsutsumi, T.; Fukushima, S.; Okabe, Y.; Saito, J.; Katayama, M.; Shindo, M.; Yamada, Y.; Shimomura, K.; Yoneyama, K. Low Infection of Phelipanche Aegyptiaca in Micro-Tom Mutants Deficient in CAROTENOID CLEAVAGE DIOXYGENASE 8. International journal of molecular sciences 2018, 19, 2645. [Google Scholar] [CrossRef] [PubMed]
- Dor, E.; Yoneyama, K.; Wininger, S.; Kapulnik, Y.; Yoneyama, K.; Koltai, H.; Xie, X.; Hershenhorn, J. Strigolactone Deficiency Confers Resistance in Tomato Line SL-ORT1 to the Parasitic Weeds Phelipanche and Orobanche Spp. Phytopathology® 2011, 101, 213–222. [Google Scholar] [CrossRef]
- Karniel, U.; Koch, A.; Bar Nun, N.; Zamir, D.; Hirschberg, J. Tomato Mutants Reveal Root and Shoot Strigolactone Involvement in Branching and Broomrape Resistance. Plants 2024, 13, 1554. [Google Scholar] [CrossRef] [PubMed]
- En-Nahli, Y.; Abbes, Z.; Trabelsi, I.; Makaza, W.; Kharrat, M.; Ghanem, M.E.; Es-Safi, N.E.; Amri, M. Host–Parasitic Plant Interaction: The Key Role of Strigolactones. In Strigolactones. In Strigolactones, Alkamides and Karrikins in Plants; CRC Press: Boca Raton, FL, USA, 2023; pp. 87–99. [Google Scholar]
- Vogel, J.T.; Walter, M.H.; Giavalisco, P.; Lytovchenko, A.; Kohlen, W.; Charnikhova, T.; Simkin, A.J.; Goulet, C.; Strack, D.; Bouwmeester, H.J.; et al. SlCCD7 Controls Strigolactone Biosynthesis, Shoot Branching and Mycorrhiza-Induced Apocarotenoid Formation in Tomato: SlCCD7 Controls Tomato Branching. The Plant Journal 2009, 61, 300–311. [Google Scholar] [CrossRef]
- Kohlen, W.; Charnikhova, T.; Lammers, M.; Pollina, T.; Tóth, P.; Haider, I.; Pozo, M.J.; De Maagd, R.A.; Ruyter-Spira, C.; Bouwmeester, H.J.; et al. The Tomato CAROTENOID CLEAVAGE DIOXYGENASE 8 ( S l CCD 8 ) Regulates Rhizosphere Signaling, Plant Architecture and Affects Reproductive Development through Strigolactone Biosynthesis. New Phytologist 2012, 196, 535–547. [Google Scholar] [CrossRef]
- Dubey, N.K.; Eizenberg, H.; Leibman, D.; Wolf, D.; Edelstein, M.; Abu-Nassar, J.; Marzouk, S.; Gal-On, A.; Aly, R. Enhanced Host-Parasite Resistance Based on Down-Regulation of Phelipanche Aegyptiaca Target Genes Is Likely by Mobile Small RNA. Front. Plant Sci. 2017, 8. [Google Scholar] [CrossRef]
- Radi, A.; Dina, P.; Guy, A. Expression of Sarcotoxin IA Gene via a Root-Specific Tob Promoter Enhanced Host Resistance against Parasitic Weeds in Tomato Plants. Plant Cell Rep 2006, 25, 297–303. [Google Scholar] [CrossRef]
- Bari, V.K.; Nassar, J.A.; Kheredin, S.M.; Gal-On, A.; Ron, M.; Britt, A.; Steele, D.; Yoder, J.; Aly, R. CRISPR/Cas9-Mediated Mutagenesis of CAROTENOID CLEAVAGE DIOXYGENASE 8 in Tomato Provides Resistance against the Parasitic Weed Phelipanche Aegyptiaca. Scientific reports 2019, 9, 11438. [Google Scholar] [CrossRef]
- Bari, V.K.; Nassar, J.A.; Aly, R. CRISPR/Cas9 Mediated Mutagenesis of MORE AXILLARY GROWTH 1 in Tomato Confers Resistance to Root Parasitic Weed Phelipanche Aegyptiaca. Scientific reports 2021, 11, 3905. [Google Scholar] [CrossRef]
- Bari, V.K.; Nassar, J.A.; Meir, A.; Aly, R. Targeted Mutagenesis of Two Homologous ATP-Binding Cassette Subfamily G (ABCG) Genes in Tomato Confers Resistance to Parasitic Weed Phelipanche Aegyptiaca. J Plant Res 2021, 134, 585–597. [Google Scholar] [CrossRef] [PubMed]
- Wakabayashi, T.; Moriyama, D.; Miyamoto, A.; Okamura, H.; Shiotani, N.; Shimizu, N.; Mizutani, M.; Takikawa, H.; Sugimoto, Y. Identification of Novel Canonical Strigolactones Produced by Tomato. Frontiers in Plant Science 2022, 13, 1064378. [Google Scholar] [CrossRef] [PubMed]
- Punia, S.; Dhull, S.B.; Sandhu, K.S.; Kaur, M. Faba Bean ( Vicia Faba ) Starch: Structure, Properties, and in Vitro Digestibility—A Review. Legume Science 2019, 1, e18. [Google Scholar] [CrossRef]
- Alharbi, N.H.; Adhikari, K.N. Factors of Yield Determination in Faba Bean (Vicia Faba). Crop Pasture Sci. 2020, 71, 305–321. [Google Scholar] [CrossRef]
- Khamassi, K.; Babay, E.; Rouissi, M.; Dakhlaoui, A.; Ben Ayed, R.; Hanana, M. Genetic Variability of Tunisian Faba Beans (Vicia Faba L.) Based on Seeds’ Morphophysical Properties as Assessed by Statistical Analysis. Journal of Food Quality 2021, 2021, 1–10. [Google Scholar] [CrossRef]
- Babay, E.; Khamassi, K.; Sabetta, W.; Miazzi, M.M.; Montemurro, C.; Pignone, D.; Danzi, D.; Finetti-Sialer, M.M.; Mangini, G. Serendipitous in Situ Conservation of Faba Bean Landraces in Tunisia: A Case Study. Genes 2020, 11, 236. [Google Scholar] [CrossRef]
- Hadou el hadj, D.; Tellah, S.; Goumeida, K.; Aitouakli, S.; Tifest, C.; Ammi, N.; Ratet, P.; Pulvento, C.; Sellami, M.H. Evaluation of Adaptability of Different Faba Bean Landraces under Mediterranean Field Conditions of Central-Northern Algeria. Agronomy 2022, 12, 1660. [Google Scholar] [CrossRef]
- Bouraoui, M.; Abbes, Z.; Rouissi, M.; Abdi, N.; Hemissi, I.; Kouki, S.; Sifi, B. Effect of Rhizobia Inoculation, N and P Supply on Orobanche Foetida Parasitising Faba Bean (Vicia Faba Minor) under Field Conditions. Biocontrol Science and Technology 2016, 26, 776–791. [Google Scholar] [CrossRef]
- Khamassi, K.; Abbes, Z.; Tani, E.; Katsileros, A.; Guenni, K.; Rouissi, M.; Khoufi, S.; Chaabane, R.; Chachalis, D.; Kharrat, M. Genetic Structure and Diversity Analysis of Tunisian Orobanche Spp. and Phelipanche Spp. Using Molecular Markers. Applied Sciences 2023, 13, 11622. [Google Scholar] [CrossRef]
- Pujadas-Salvà, A.J.; Plaza-Arregui, L. Studies on Thapsia (Apiaceae) from North-Western Africa: A Forgotten and a New Species. Botanical Journal of the Linnean Society 2003, 143, 433–442. [Google Scholar] [CrossRef]
- Fernández-Aparicio, M.; Flores, F.; Rubiales, D. Escape and True Resistance to Crenate Broomrape (Orobanche Crenata Forsk.) in Grass Pea (Lathyrus Sativus L.) Germplasm. Field Crops Research 2012, 125, 92–97. [Google Scholar] [CrossRef]
- Fernández-Aparicio, M.; Kisugi, T.; Xie, X.; Rubiales, D.; Yoneyama, K. Low Strigolactone Root Exudation: A Novel Mechanism of Broomrape ( Orobanche and Phelipanche Spp.) Resistance Available for Faba Bean Breeding. J. Agric. Food Chem. 2014, 62, 7063–7071. [Google Scholar] [CrossRef] [PubMed]
- Rubiales, D.; Rojas-Molina, M.M.; Sillero, J.C. Characterization of Resistance Mechanisms in Faba Bean (Vicia Faba) against Broomrape Species (Orobanche and Phelipanche Spp.). Frontiers in Plant Science 2016, 7, 1747. [Google Scholar] [CrossRef] [PubMed]
- Briache, F.Z.; El Amri, M.; Ennami, M.; Amri, M.; Triqui, Z.E.A.; Mentag, R. Induction of Systemic Resistance to Orobanche Crenata in Lentil by Exogenous Application of Salicylic Acid and Indole Acetic Acid. Journal of Plant Protection Research 2023, 83–96. [Google Scholar]
- Thebti, S.; Bouallegue, A.; Rzigui, T.; En-Nahli, Y.; Horchani, F.; Hosni, T.; Kharrat, M.; Amri, M.; Abbes, Z. Potential Physiological Tolerance Mechanisms in Faba Bean to Orobanche Spp. Parasitism. Frontiers in Plant Science 2024, 15, 1497303. [Google Scholar] [CrossRef]
- Abbes, Z.; Kharrat, M.; Delavault, P.; Chaïbi, W.; Simier, P. Nitrogen and Carbon Relationships between the Parasitic Weed Orobanche Foetida and Susceptible and Tolerant Faba Bean Lines. Plant Physiology and Biochemistry 2009, 47, 153–159. [Google Scholar] [CrossRef]
- Abbes, Z.; Bouallegue, A.; Trabelsi, I.; Trabelsi, N.; Taamalli, A.; Amri, M.; Mhadhbi, H.; Kharrat, M. Investigation of Some Biochemical Mechanisms Involved in the Resistance of Faba Bean (Vicia Faba L.) Varieties to Orobanche Spp. Plant Protection Science 2020, 56. [Google Scholar] [CrossRef]
- Trabelsi, I.; Abbes, Z.; Amri, M.; Kharrat, M. Study of Some Resistance Mechanisms to Orobanche Spp. Infestation in Faba Bean ( Vicia Faba L.) Breeding Lines in Tunisia. Plant Production Science 2016, 19, 562–573. [Google Scholar] [CrossRef]
- Trabelsi, I.; Yoneyama, K.; Abbes, Z.; Amri, M.; Xie, X.; Kisugi, T.; Kim, H.I.; Kharrat, M. Characterization of Strigolactones Produced by Orobanche Foetida and Orobanche Crenata Resistant Faba Bean (Vicia Faba L.) Genotypes and Effects of Phosphorous, Nitrogen, and Potassium Deficiencies on Strigolactone Production. South African Journal of Botany 2017, 108, 15–22. [Google Scholar] [CrossRef]
- Mejri, S.; Mabrouk, Y.; Belhadj, O.; Saidi, M. Orobanche Foetida Resistance in Two New Faba Bean Genotypes Produced by Radiation Mutagenesis. International Journal of Radiation Biology 2018, 94, 671–677. [Google Scholar] [CrossRef]
- Pavan, S.; Schiavulli, A.; Marcotrigiano, A.R.; Bardaro, N.; Bracuto, V.; Ricciardi, F.; Charnikhova, T.; Lotti, C.; Bouwmeester, H.; Ricciardi, L. Characterization of Low-Strigolactone Germplasm in Pea (Pisum Sativum L.) Resistant to Crenate Broomrape (Orobanche Crenata Forsk.). MPMI 2016, 29, 743–749. [Google Scholar] [CrossRef] [PubMed]
- Boukteb, A.; Sato, K.; Gan, P.; Kharrat, M.; Sakouhi, H.; Shibata, A.; Shirasu, K.; Ichihashi, Y.; Bouhadida, M. Global Changes in Gene Expression during Compatible and Incompatible Interactions of Faba Bean (Vicia Faba L.) during Orobanche Foetida Parasitism. PLoS ONE 2024, 19, e0301981. [Google Scholar] [CrossRef] [PubMed]
| Technique | Efficacy Levels(a) | Mechanism; primary Effects | |
|---|---|---|---|
| Legumes | Ind. tomato | ||
| I. Preventive | |||
| Rotation | + | + | (M): Disruption of the parasite life cycle by avoiding soil seed bank replenishing (E): soil seed bank; seed germination; pre- and early-attachment of haustorium |
| Winter cover crops | na1 | + | (M): Disruption of the parasite life cycle by creating a hostile soil environment (E): soil seed bank; seed germination; pre-attachment of haustorium |
| Use of trap, catch allelopathic crops; intercropping | + | + | (M): Disruption of the parasite life cycle by reducing final haustorium numbers (multiple tactics) (E): soil seed bank; seed germination; pre-attachment of haustorium |
| Modify sowing, transplanting dates | + | + | (M): Disruption of best conditions for the onset of parasitism (E): soil seed bank; seed germination; pre- and early-attachment of haustorium |
| Solarization | +++ | na2 | (M): Reduction of active soil seed bank by seed decay (E): soil seed bank; seed germination |
| Tillage, mechanical cultivation | ++ | ++ | (M): Reduction of active soil seed bank by physical misplacement of seeds (E): soil seed bank; seed germination |
| Fertilization | + | ++ | (M): Disruption of the parasite life cycle by employing direct (on the parasite seeds) and indirect (on enhancing crop competitive ability) conditions (E): soil seed bank; seed germination; pre-, early-, and late-attachment of haustorium |
| Use of synthetic stimulants | + | + | (M): Lower the onset of parasitism by suicidal germination tactics (E): soil seed bank; seed germination |
| Polyethylene plastic mulching | na3 | +++ | (M): Disruption of the parasite life cycle by physical barriers (E): seed germination; pre-, early-, and late-attachment of haustorium |
| Use of crop tolerance inducers | + | + | (M): Disruption of the parasite life cycle by enhancing crop competitive ability (E): seed germination; pre-, and early-attachment of haustorium |
| Use of resistant/tolerant cvs | ++ | na4 | (M): Low or minimum onset of parasitism by natural crop tolerance/resistance development (E): seed germination; pre-, and early-attachment of haustorium |
| II. Curative | |||
| Seed dressing treatments | ++ | ++ | (M): Lower onset of parasitism by utilizing synthetic substances applied as seed coating, film or pellets (E): seed germination; pre-, and early-attachment of haustorium |
| Foliar herbicides | ++ | + | (M): Lower onset of parasitism by utilizing herbicides to reach target sites via translocation of the haustorium (E): seed germination; pre-, and early-attachment of haustorium |
| Herbigation | na5 | ++ | (M): Lower onset of parasitism by utilizing herbicides to be applied with irrigation (E): seed germination; pre-, early- and late-attachment of haustorium |
| Biological control; beneficial insects | na6 | (M): Disruption of the parasite life cycle by biological agents (multiple tactics) (E): soil seed bank; seed germination; pre-, and early-attachment of haustorium |
|
| Hand weeding | +++ | +++ | (M): Disruption of the parasite life cycle by physical weed removal (E): soil seed bank; late-attachment of haustorium |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





