Submitted:
09 April 2025
Posted:
09 April 2025
You are already at the latest version
Abstract
Keywords:
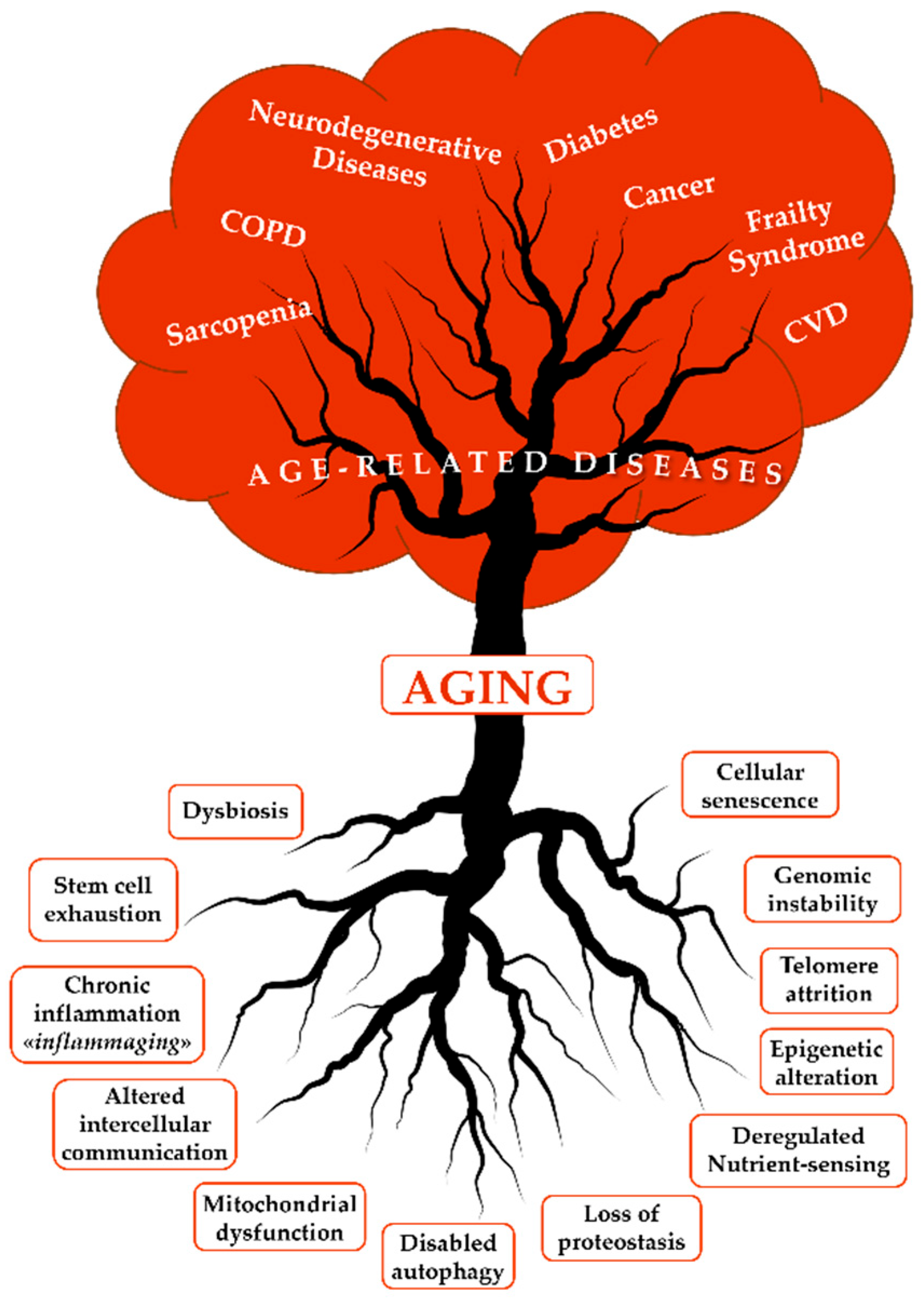
1. Introduction and Background
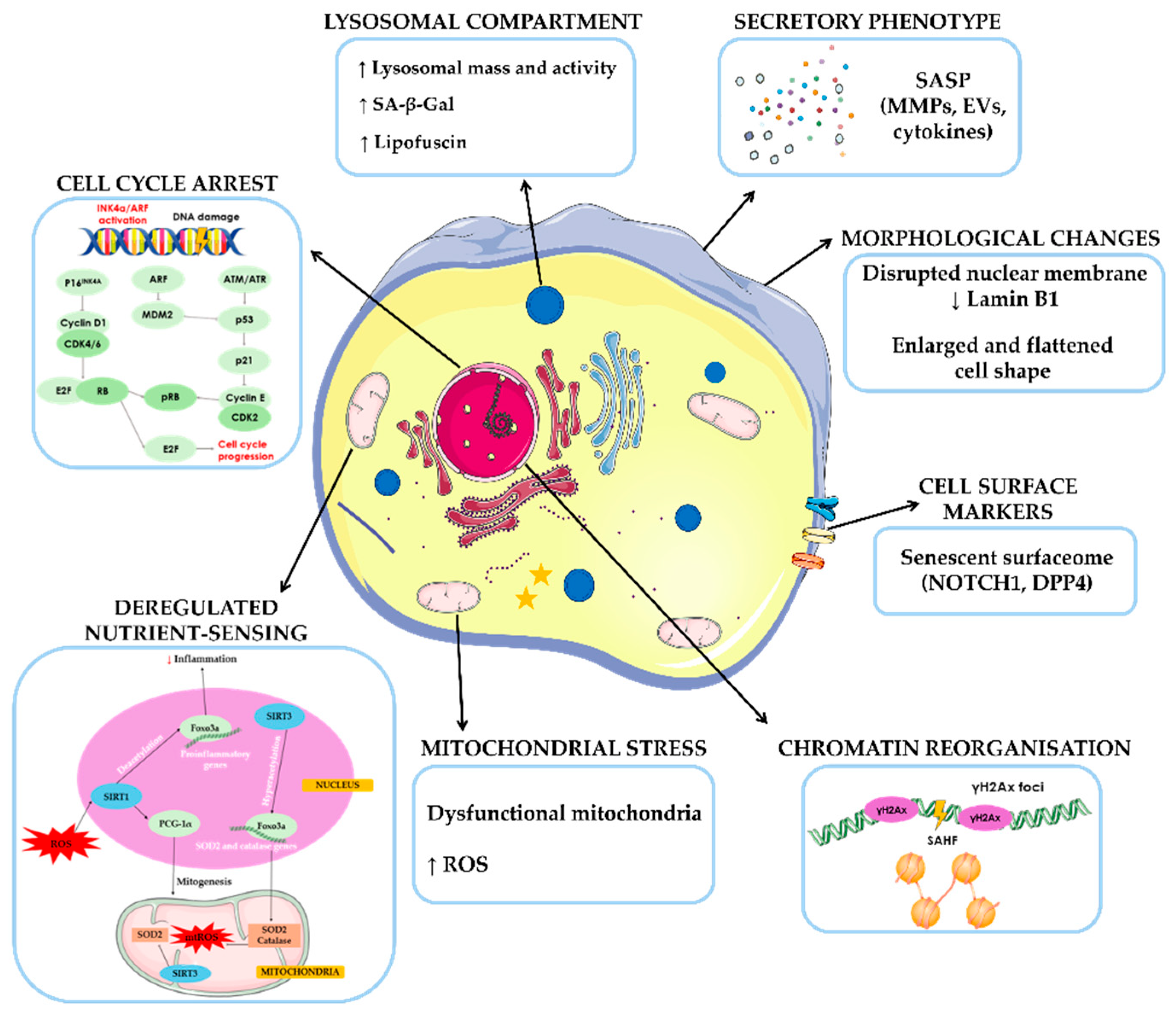
2. The Role of Senescence in Aging and Neurodegenerative Diseases
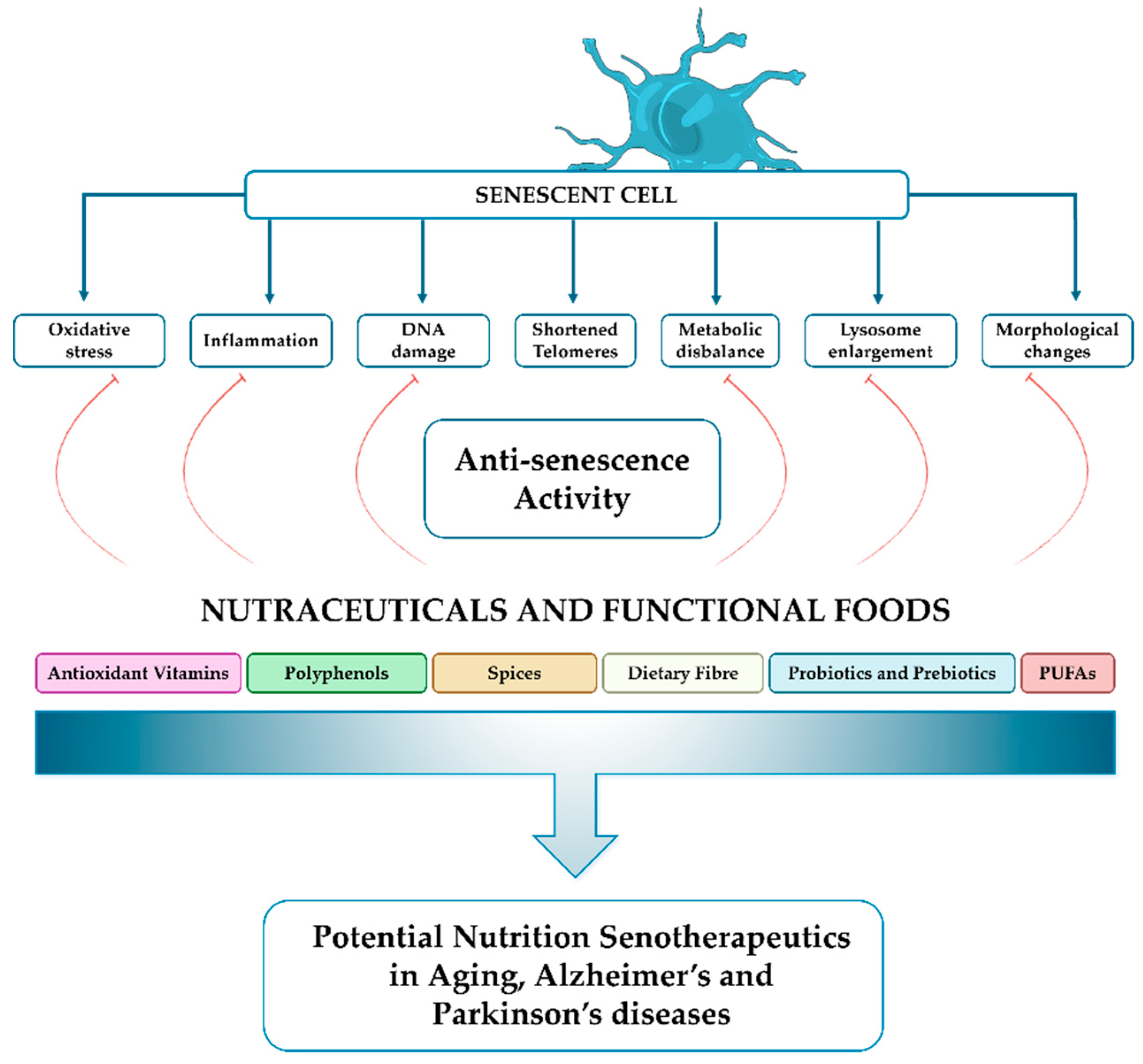
3. Nutritional Interventions to Slow Down Aging
4. Nutraceutical Interventions in Neurodegenerative Disorders: Focus on Parkinson’s and Alzheimer’s Diseases
4.1. Antioxidant Vitamins
4.2. Polyphenols
4.3. Spices
4.4. Dietary Fiber
4.5. Probiotics and Prebiotics
4.6. Polyunsaturated Fatty Acids (PUFAs)
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| αT | α-tocopherol |
| αTP | α-tocopheryl phosphate |
| Aβ | Amyloid-β peptide |
| AA | Arachidonic acid |
| AAC | Ascorbic acid |
| AD | Alzheimer’s Disease |
| ARDs | Age-related diseases |
| BACE1 | β-secretase 1 |
| CEppt | Cinnamon extract |
| CNS | Central Nervous System |
| CR | Caloric Restriction |
| D+Q | Dasatinib plus Quercetin |
| DHA | Docosahexaenoic Acid |
| DOPA | Dihydroxyphenylalanine |
| EPA | Eicosapentaenoic Acid |
| FD | Fiber deficiency |
| GLP-1 | Glucagon-like peptide 1 |
| GSH | Glutathione |
| GSs | Geriatric Syndromes |
| IL | Interleukin |
| LCPUFAs | Long-chain polyunsaturated fatty acids |
| MAO-B | Monoamine Oxidase B |
| MDA | Malondialdehyde |
| MedDiet | Mediterranean Diet |
| MPTP | 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine |
| NFTs | Neurofibrillary tangles |
| PD | Parkinson’s Disease |
| PUFAs | Polyunsaturated Fatty Acids |
| ROS | Reactive Oxygen Species |
| SASP | Senescence-Associated Secretory Phenotype |
| SA-β-gal | Senescence-Associated β-Galactosidase |
| SAHFs | Senescence-associated heterochromatic foci |
| SCFAs | Short-Chain Fatty Acids |
| SIRT | Sirtuin |
References
- WHO World Population Prospects 2022; 2022; ISBN 978-92-1 -148373-4.
- Santana, P.; Grant, M. Global Aging and Health Determinants in a Changing World; INC, 2023; ISBN 9780128237618.
- World Health Organisation World Population Ageing 2019; 2019; ISBN 9789211483260.
- Nemitz, J. Increasing Longevity and Life Satisfaction: Is There a Catch to Living Longer? J Popul Econ 2022, 35, 557–589. [Google Scholar] [CrossRef]
- Olshansky, S.J. From Lifespan to Healthspan. JAMA 2018, 320, 1323. [Google Scholar] [CrossRef] [PubMed]
- Garmany, A.; Yamada, S.; Terzic, A. Longevity Leap: Mind the Healthspan Gap. NPJ Regen Med 2021, 6, 57. [Google Scholar] [CrossRef] [PubMed]
- Ostan, R.; Bucci, L.; Capri, M.; Salvioli, S.; Scurti, M.; Pini, E.; Monti, D.; Franceschi, C. Immunosenescence and Immunogenetics of Human Longevity. Neuroimmunomodulation 2008, 15, 224–240. [Google Scholar] [CrossRef]
- Cevenini, E.; Bellavista, E.; Tieri, P.; Castellani, G.; Lescai, F.; Francesconi, M.; Mishto, M.; Santoro, A.; Valensin, S.; Salvioli, S.; Capri, M.; Zaikin, A.; Monti, D.; de Magalhaes, J.; Franceschi, C. Systems Biology and Longevity: An Emerging Approach to Identify Innovative Anti- Aging Targets and Strategies. Curr Pharm Des 2010, 16, 802–813. [Google Scholar] [CrossRef]
- Li, X.; Li, C.; Zhang, W.; Wang, Y.; Qian, P.; Huang, H. Inflammation and Aging: Signaling Pathways and Intervention Therapies. Signal Transduct Target Ther 2023, 8, 239. [Google Scholar] [CrossRef]
- Franceschi, C.; Bonafè, M.; Valensin, S.; Olivieri, F.; De Luca, M.; Ottaviani, E.; De Benedictis, G. Inflamm-Aging. An Evolutionary Perspective on Immunosenescence. Ann N Y Acad Sci 2000, 908, 244–254. [Google Scholar] [CrossRef]
- Franceschi, C.; Capri, M.; Monti, D.; Giunta, S.; Olivieri, F.; Sevini, F.; Panourgia, M.P.; Invidia, L.; Celani, L.; Scurti, M.; et al. Inflammaging and Anti-Inflammaging: A Systemic Perspective on Aging and Longevity Emerged from Studies in Humans. Mech Ageing Dev 2007, 128, 92–105. [Google Scholar] [CrossRef]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. Hallmarks of Aging: An Expanding Universe. Cell 2023, 186, 243–278. [Google Scholar] [CrossRef]
- Franceschi, C.; Garagnani, P.; Morsiani, C.; Conte, M.; Santoro, A.; Grignolio, A.; Monti, D.; Capri, M.; Salvioli, S. The Continuum of Aging and Age-Related Diseases: Common Mechanisms but Different Rates. Front Med (Lausanne) 2018, 5. [Google Scholar] [CrossRef]
- Sierra, F.; Kohanski, R. Geroscience and the Trans-NIH Geroscience Interest Group, GSIG. 2017, 1–5. [CrossRef]
- Franceschi, C.; Garagnani, P.; Morsiani, C.; Conte, M.; Santoro, A.; Grignolio, A.; Monti, D.; Capri, M.; Salvioli, S. The Continuum of Aging and Age-Related Diseases: Common Mechanisms but Different Rates. Front Med (Lausanne) 2018, 5. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.; Mehdi, M.M. Oxidative Stress, Inflammation and Hormesis: The Role of Dietary and Lifestyle Modifications on Aging. Neurochem Int 2023, 164, 105490. [Google Scholar] [CrossRef] [PubMed]
- Prattichizzo, F.; Frigé, C.; Pellegrini, V.; Scisciola, L.; Santoro, A.; Monti, D.; Rippo, M.R.; Ivanchenko, M.; Olivieri, F.; Franceschi, C. Organ-Specific Biological Clocks: Ageotyping for Personalized Anti-Aging Medicine. Ageing Res Rev 2024, 96, 102253. [Google Scholar] [CrossRef]
- Fjell, A.M.; Walhovd, K.B. Structural Brain Changes in Aging: Courses, Causes and Cognitive Consequences. Rev Neurosci 2010, 21, 187–221. [Google Scholar] [CrossRef] [PubMed]
- Rodrigue, K.M.; Kennedy, K.M. The Cognitive Consequences of Structural Changes to the Aging Brain; Seventh Ed.; Elsevier Inc., 2011; ISBN 9780123808820.
- Nyberg, L.; Wåhlin, A. The Many Facets of Brain Aging. Elife 2020, 9, 18–20. [Google Scholar] [CrossRef]
- Murman, D.L. The Impact of Age on Cognition. Semin Hear 2015, 36, 111–121. [Google Scholar] [CrossRef]
- Fjell, A.M.; Sneve, M.H.; Grydeland, H.; Storsve, A.B.; Amlien, I.K.; Yendiki, A.; Walhovd, K.B. Relationship between Structural and Functional Connectivity Change across the Adult Lifespan: A Longitudinal Investigation. Hum Brain Mapp 2017, 38, 561–573. [Google Scholar] [CrossRef]
- Seidler, R.D.; Bernard, J.A.; Burutolu, T.B.; Fling, B.W.; Gordon, M.T.; Gwin, J.T.; Kwak, Y.; Lipps, D.B. Motor Control and Aging: Links to Age-Related Brain Structural, Functional, and Biochemical Effects. Neurosci Biobehav Rev 2010, 34, 721–733. [Google Scholar] [CrossRef]
- Park, D.C.; Reuter-Lorenz, P. The Adaptive Brain: Aging and Neurocognitive Scaffolding. Annu Rev Psychol 2009, 60, 173–196. [Google Scholar] [CrossRef]
- Zhang, Q.; Yang, G.; Luo, Y.; Jiang, L.; Chi, H.; Tian, G. Neuroinflammation in Alzheimer’s Disease: Insights from Peripheral Immune Cells. Immunity & Ageing 2024, 21, 38. [Google Scholar] [CrossRef]
- Kumari, R.; Jat, P. Mechanisms of Cellular Senescence: Cell Cycle Arrest and Senescence Associated Secretory Phenotype. Front Cell Dev Biol 2021, 9, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Hayflick, L. The Limited in vitro Lifetime of Human Diploid Cell Strains. Exp Cell Res 1965, 37, 614–636. [Google Scholar] [CrossRef] [PubMed]
- Campisi, J.; D’Adda Di Fagagna, F. Cellular Senescence: When Bad Things Happen to Good Cells. Nat Rev Mol Cell Biol 2007, 8, 729–740. [Google Scholar] [CrossRef]
- González-Gualda, E.; Baker, A.G.; Fruk, L.; Muñoz-Espín, D. A Guide to Assessing Cellular Senescence in vitro and in vivo. FEBS Journal 2021, 288, 56–80. [Google Scholar] [CrossRef]
- Campisi, J. Aging, Cellular Senescence, and Cancer. Annu Rev Physiol 2013, 75, 685–705. [Google Scholar] [CrossRef]
- M. Laura Idda; Waverly G. McClusky; Valeria Lodde; Rachel Munk; Kotb Abdelmohsen; Martina Rossi; Myriam Gorospe Survey of Senescent Cell Markers with Age in Human Tissues. Aging 2020, 12, 4052–4066. [Google Scholar]
- Krishnamurthy, J.; Torrice, C.; Ramsey, M.R.; Kovalev, G.I.; Al-Regaiey, K.; Su, L.; Sharpless, N.E. Ink4a/Arf Expression Is a Biomarker of Aging. Journal of Clinical Investigation 2004, 114, 1299–1307. [Google Scholar] [CrossRef]
- Yousefzadeh, M.J.; Wilkinson, J.E.; Hughes, B.; Gadela, N.; Ladiges, W.C.; Vo, N.; Niedernhofer, L.J.; Huffman, D.M.; Robbins, P.D. Heterochronic Parabiosis Regulates the Extent of Cellular Senescence in Multiple Tissues. Geroscience 2020, 42, 951–961. [Google Scholar] [CrossRef]
- Si, Z.; Sun, L.; Wang, X. Evidence and Perspectives of Cell Senescence in Neurodegenerative Diseases. Biomedicine and Pharmacotherapy 2021, 137, 111327. [Google Scholar] [CrossRef]
- Boyko, A.A.; Troyanova, N.I.; Kovalenko, E.I.; Sapozhnikov, A.M. Similarity and Differences in Inflammation-Related Haracteristics of the Peripheral Immune System of Patients with Parkinson’s and Alzheimer’s Diseases. Int J Mol Sci 2017, 18. [Google Scholar] [CrossRef]
- Nakajima, K.; Kohsaka, S. Microglia: Neuroprotective and Neurotrophic Cells in the Central Nervous System. Curr Drug Targets Cardiovasc Haematol Disord 2004, 4, 65–84. [Google Scholar] [CrossRef] [PubMed]
- Diwan, B.; Sharma, R. Nutritional Components as Mitigators of Cellular Senescence in Organismal Aging: A Comprehensive Review. Food Sci Biotechnol 2022, 31, 1089–1109. [Google Scholar] [CrossRef] [PubMed]
- Głowacka, P.; Oszajca, K.; Pudlarz, A.; Szemraj, J.; Witusik-Perkowska, M. Postbiotics as Molecules Targeting Cellular Events of Aging Brain—The Role in Pathogenesis, Prophylaxis and Treatment of Neurodegenerative Diseases. Nutrients 2024, 16. [Google Scholar] [CrossRef] [PubMed]
- Bhat, R.; Crowe, E.P.; Bitto, A.; Moh, M.; Katsetos, C.D.; Garcia, F.U.; Johnson, F.B.; Trojanowski, J.Q.; Sell, C.; Torres, C. Astrocyte Senescence as a Component of Alzheimer’s Disease. PLoS One 2012, 7. [Google Scholar] [CrossRef]
- Walker, L.; Jacobs, E.; McAleese, K.E.; Johnson, M.; Attems, J. Do Senescent Cells Play a Role in Alzheimer’s Disease? Alzheimer’s & Dementia 2020, 16, 43820. [Google Scholar] [CrossRef]
- Simmnacher, K.; Krach, F.; Schneider, Y.; Alecu, J.E.; Mautner, L.; Klein, P.; Roybon, L.; Prots, I.; Xiang, W.; Winner, B. Unique Signatures of Stress-Induced Senescent Human Astrocytes. Exp Neurol 2020, 334, 113466. [Google Scholar] [CrossRef]
- Si, Z.; Sun, L.; Wang, X. Evidence and Perspectives of Cell Senescence in Neurodegenerative Diseases. Biomedicine and Pharmacotherapy 2021, 137, 111327. [Google Scholar] [CrossRef]
- Chinta, S.J.; Woods, G.; Demaria, M.; Rane, A.; Zou, Y.; McQuade, A.; Rajagopalan, S.; Limbad, C.; Madden, D.T.; Campisi, J.; et al. Cellular Senescence Is Induced by the Environmental Neurotoxin Paraquat and Contributes to Neuropathology Linked to Parkinson’s Disease. Cell Rep 2018, 22, 930–940. [Google Scholar] [CrossRef]
- Wang, W.Y.; Tan, M.S.; Yu, J.T.; Tan, L. Role of Pro-Inflammatory Cytokines Released from Microglia in Alzheimer’s Disease. Ann Transl Med 2015, 3, 1–15. [Google Scholar] [CrossRef]
- Bae, E.J.; Choi, M.; Kim, J.T.; Kim, D.K.; Jung, M.K.; Kim, C.; Kim, T.K.; Lee, J.S.; Jung, B.C.; Shin, S.J.; Rhee, K. H.; Lee, S. J. TNF-α Promotes α-Synuclein Propagation through Stimulation of Senescence-Associated Lysosomal Exocytosis. Exp Mol Med 2022, 54, 788–800. [Google Scholar] [CrossRef]
- Hou, Y.; Wei, Y.; Lautrup, S.; Yang, B.; Wang, Y.; Cordonnier, S.; Mattson, M.P.; Croteau, D.L.; Bohr, V.A. NAD+ Supplementation Reduces Neuroinflammation and Cell Senescence in a Transgenic Mouse Model of Alzheimer’s Disease via CGAS-STING. Proc Natl Acad Sci U S A 2021, 118. [Google Scholar] [CrossRef] [PubMed]
- Dehkordi, S.K.; Walker, J.; Sah, E.; Bennett, E.; Atrian, F.; Frost, B.; Woost, B.; Bennett, R.E.; Orr, T.C.; Zhou, Y.; Andhey, P. S.; Colonna, M.; Sudmant, P. H.; Xu, P.; Wang, M.; Zhang, B.; Zare, H.; Orr, M. E. Profiling Senescent Cells in Human Brains Reveals Neurons with CDKN2D/P19 and Tau Neuropathology. Nat Aging 2021, 1, 1107–1116. [Google Scholar] [CrossRef] [PubMed]
- Bussian, T.; Aziz, A.; Meyer, C.; Swenson, B.; Deursen, J.; Baker, D. Clearance of Senescent Glial Cells Prevents Tau-Dependent Pathology and Cognitive Decline. Nature 2018, 562. [Google Scholar] [CrossRef] [PubMed]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The Hallmarks of Aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef]
- Mathers, J.C. Impact of Nutrition on the Ageing Process. British Journal of Nutrition 2015, 113, S18–S22. [Google Scholar] [CrossRef]
- Biesalski, H.K.; Dragsted, L.O.; Elmadfa, I.; Grossklaus, R.; Müller, M.; Schrenk, D.; Walter, P.; Weber, P. Bioactive Compounds: Definition and Assessment of Activity. Nutrition 2009, 25, 1202–1205. [Google Scholar] [CrossRef]
- Morris, M.C. Nutrition and Risk of Dementia: Overview and Methodological Issues. Ann N Y Acad Sci 2016, 1367, 31–37. [Google Scholar] [CrossRef]
- Scuto, M.; Rampulla, F.; Reali, G.M.; Spanò, S.M.; Trovato Salinaro, A.; Calabrese, V. Hormetic Nutrition and Redox Regulation in Gut–Brain Axis Disorders. Antioxidants 2024, 13. [Google Scholar] [CrossRef]
- Aliper, A.; Belikov, A. V.; Garazha, A.; Jellen, L.; Artemov, A.; Suntsova, M.; Ivanova, A.; Venkova, L.; Borisov, N.; Buzdin, A.; Mamoshina, P.; Putin, E.; Swick, A. G.; Moskalev, A.; Zhavoronkov, A. In Search for Geroprotectors: In Silico Screening and in vitro Validation of Signalome-Level Mimetics of Young Healthy State. Aging 2016, 8, 2127–2152. [Google Scholar] [CrossRef]
- Aliper, A.; Jellen, L.; Cortese, F.; Artemov, A.; Semper, D.K.; Moskalev, A.; Swick, A.G.; Zhavoronkov, A. Natural Mimetics of Rapamycin and Minoxidil Obtained via Computational Methods. Aging (Albany NY) 2017, 9, 2245–2268. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, L.; Liang, Y.; Zhang, C.; Xu, Z.; Zhang, L.; Fuji, R.; Mu, W.; Li, L.; Jiang, J.; Ju, Y.; Wang, Z. Cyclic AMP Mimics the Anti-Ageing Effects of Calorie Restriction by Up-Regulating Sirtuin. Sci Rep 2015, 5, 12012. [Google Scholar] [CrossRef] [PubMed]
- Naisam, S.; Mohan, A.; Sreekumar, N. Epigenetic Regulation of Human Sirtuin 1 Insights into Aging Mechanisms 2024.
- Effect of Calorie Restriction on the Expression of Sirtuin1 as an Antiaging Biomarker. Makara J Sci 2023, 27. [CrossRef]
- Dhillon, R.S.; Qin, Y.; van Ginkel, P.R.; Fu, V.X.; Vann, J.M.; Lawton, A.J.; Green, C.L.; Manchado-Gobatto, F.B.; Gobatto, C.A.; Lamming, D.W.; Prolla, T.A.; Denu, J.M. SIRT3 Deficiency Decreases Oxidative Metabolism Capacity but Increases Lifespan in Male Mice under Caloric Restriction. Aging Cell 2022, 21. [Google Scholar] [CrossRef]
- Gurău, F.; Baldoni, S.; Prattichizzo, F.; Espinosa, E.; Amenta, F.; Procopio, A.D.; Albertini, M.C.; Bonafè, M.; Olivieri, F. Anti-Senescence Compounds: A Potential Nutraceutical Approach to Healthy Aging. Ageing Res Rev 2018, 46, 14–31. [Google Scholar] [CrossRef]
- Hadem, I.K.H.; Majaw, T.; Kharbuli, B.; Sharma, R. Beneficial Effects of Dietary Restriction in Aging Brain. J Chem Neuroanat 2019, 95, 123–133. [Google Scholar] [CrossRef]
- Krishnamurthy, J.; Torrice, C.; Ramsey, M.R.; Kovalev, G.I.; Al-Regaiey, K.; Su, L.; Sharpless, N.E. Ink4a/Arf Expression Is a Biomarker of Aging. Journal of Clinical Investigation 2004, 114, 1299–1307. [Google Scholar] [CrossRef]
- Fontana, L.; Mitchell, S.E.; Wang, B.; Tosti, V.; van Vliet, T.; Veronese, N.; Bertozzi, B.; Early, D.S.; Maissan, P.; Speakman, J.R.; Demaria, M. The Effects of Graded Caloric Restriction: XII. Comparison of Mouse to Human Impact on Cellular Senescence in the Colon. Aging Cell 2018, 17, 4–8. [Google Scholar] [CrossRef]
- Wang, C.; Maddick, M.; Miwa, S.; Jurk, D.; Czapiewski, R.; Saretzki, G.; Langie, S.A.S.; Godschalk, R.W.L.; Cameron, K.; von Zglinicki, T. Adult-Onset, Short-Term Dietary Restriction Reduces Cell Senescence in Mice. Aging 2010, 2, 555–566. [Google Scholar] [CrossRef]
- Aversa, Z.; White, T.A.; Heeren, A.A.; Hulshizer, C.A.; Saul, D.; Zhang, X.; Molina, A.J.A.; Redman, L.M.; Martin, C.K.; Racette, S.B.; et al. Calorie Restriction Reduces Biomarkers of Cellular Senescence in Humans. Aging Cell 2024, 23, 1–11. [Google Scholar] [CrossRef]
- Forni, C.; Facchiano, F.; Bartoli, M.; Pieretti, S.; Facchiano, A.; D’Arcangelo, D.; Norelli, S.; Valle, G.; Nisini, R.; Beninati, S.; Tabolacci, C.; Jadeja, R.N. Beneficial Role of Phytochemicals on Oxidative Stress and Age-Related Diseases. Biomed Res Int 2019. [Google Scholar] [CrossRef]
- Ooi, T.C.; Meramat, A.; Rajab, N.F.; Shahar, S.; Ismail, I.S.; Azam, A.A.; Sharif, R. Intermittent Fasting Enhanced the Cognitive Function in Older Adults with Mild Cognitive Impairment by Inducing Biochemical and Metabolic Changes: A 3-Year Progressive Study. Nutrients 2020, 12, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Teng, N.I.M.F.; Shahar, S.; Rajab, N.F.; Manaf, Z.A.; Johari, M.H.; Ngah, W.Z.W. Improvement of Metabolic Parameters in Healthy Older Adult Men Following a Fasting Calorie Restriction Intervention. Aging Male 2013, 16, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Chiavaroli, L.; Nishi, S.K.; Khan, T.A.; Braunstein, C.R.; Glenn, A.J.; Mejia, S.B.; Rahelić, D.; Kahleová, H.; Salas-Salvadó, J.; Jenkins, D.J.A.; Kendall, C.W.C.; Sievenpiper, J.L. Portfolio Dietary Pattern and Cardiovascular Disease: A Systematic Review and Meta-Analysis of Controlled Trials. Prog Cardiovasc Dis 2018, 61, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Ndanuko, R.N.; Tapsell, L.C.; Charlton, K.E.; Neale, E.P.; Batterham, M.J. Dietary Patterns and Blood Pressure in Adults: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Advances in Nutrition 2016, 7, 76–89. [Google Scholar] [CrossRef]
- Fontana, L.; Mitchell, S.E.; Wang, B.; Tosti, V.; van Vliet, T.; Veronese, N.; Bertozzi, B.; Early, D.S.; Maissan, P.; Speakman, J.R.; et al. The Effects of Graded Caloric Restriction: XII. Comparison of Mouse to Human Impact on Cellular Senescence in the Colon. Aging Cell 2018, 17, 4–8. [Google Scholar] [CrossRef]
- Barbaresko, J.; Koch, M.; Schulze, M.B.; Nöthlings, U. Dietary Pattern Analysis and Biomarkers of Low-Grade Inflammation: A Systematic Literature Review. Nutr Rev 2013, 71, 511–527. [Google Scholar] [CrossRef]
- Grande de França, N.A.; Rolland, Y.; Guyonnet, S.; de Souto Barreto, P. The Role of Dietary Strategies in the Modulation of Hallmarks of Aging. Ageing Res Rev 2023, 87, 101908. [Google Scholar] [CrossRef]
- Ravussin, E.; Redman, L.M.; Rochon, J.; Das, S.K.; Fontana, L.; Kraus, W.E.; Romashkan, S.; Williamson, D.A.; Meydani, S.N.; Villareal, D.T.; Smith, Steven R. ; Stein, R.I.; Scott, T.M.; Stewart, T.M.; Saltzman, E.; Klein, S.; Bhapkar, M.; Martin, C.K.; Gilhooly, C.H.; Holloszy, J.O.; Hadley, E.C.; Roberts, S.B. A 2-Year Randomized Controlled Trial of Human Caloric Restriction: Feasibility and Effects on Predictors of Health Span and Longevity. J Gerontol A Biol Sci Med Sci 2015, 70, 1097–1104. [Google Scholar] [CrossRef]
- Panda, S.; Maier, G.; Villareal, D.T. Targeting Energy Intake and Circadian Biology to Engage Mechanisms of Aging in Older Adults With Obesity: Calorie Restriction and Time-Restricted Eating. The Journals of Gerontology: Series A 2023, 78, 79–85. [Google Scholar] [CrossRef]
- Sofi, F.; Cesari, F.; Abbate, R.; Gensini, G.F.; Casini, A. Adherence to Mediterranean Diet and Health Status: Meta-Analysis. BMJ 2008, 337, a1344. [Google Scholar] [CrossRef]
- Xavier Medina, F. Mediterranean Diet, Culture and Heritage: Challenges for a New Conception. Public Health Nutr 2009, 12, 1618–1620. [Google Scholar] [CrossRef] [PubMed]
- Marin, C.; Delgado-Lista, J.; Ramirez, R.; Carracedo, J.; Caballero, J.; Perez-Martinez, P.; Gutierrez-Mariscal, F.M.; Garcia-Rios, A.; Delgado-Casado, N.; Cruz-Teno, C.; Yubero-Serrano, E.M.; Tinahones, F.; Malagon, M.D.M.; Perez-Jimenez, F.; Lopez-Miranda, J. Mediterranean Diet Reduces Senescence-Associated Stress in Endothelial Cells. Age (Omaha) 2012, 34, 1309–1316. [Google Scholar] [CrossRef] [PubMed]
- Salas-Salvadó, J.; Guasch-Ferré, M.; Lee, C.H.; Estruch, R.; Clish, C.B.; Ros, E. Protective Effects of the Mediterranean Diet on Type 2 Diabetes and Metabolic Syndrome. Journal of Nutrition 2016, 146, 920S–927S. [Google Scholar] [CrossRef] [PubMed]
- Wade, A.T.; Davis, C.R.; Dyer, K.A.; Hodgson, J.M.; Woodman, R.J.; Keage, H.A.D.; Murphy, K.J. A Mediterranean Diet to Improve Cardiovascular and Cognitive Health: Protocol for a Randomised Controlled Intervention Study. Nutrients 2017, 9, 1–29. [Google Scholar] [CrossRef]
- Fekete, M.; Varga, P.; Ungvari, Z.; Tibor, J.; Annamaria, F.; Ágnes, B.; Lehoczki, A.; Mózes, N.; Grosso, G.; Godos, J.; Menyhart, O.; Munkácsy, G.; Tarantini, S.; Yabluchanskiy, A.; Ungvari, A.; Győrffy, B. The Role of the Mediterranean Diet in Reducing the Risk of Cognitive Impairement, Dementia, and Alzheimer ‘s Disease: A Meta - Analysis. Geroscience 2025. [Google Scholar] [CrossRef]
- Andreo-López, M.C.; Contreras-Bolívar, V.; Muñoz-Torres, M.; García-Fontana, B.; García-Fontana, C. Influence of the Mediterranean Diet on Healthy Aging. Int J Mol Sci 2023, 24, 4491. [Google Scholar] [CrossRef]
- Marin, C.; Ramirez, R.; Delgado-Lista, J.; Yubero-Serrano, E.M.; Perez-Martinez, P.; Carracedo, J.; Garcia-Rios, A.; Rodriguez, F.; Gutierrez-Mariscal, F.M.; Gomez, P.; Perez-Jimenez, F.; Lopez-Maria, J. Mediterranean Diet Reduces Endothelial Damage and Improves the Regenerative Capacity of Endothelium. American Journal of Clinical Nutrition 2011, 93, 267–274. [Google Scholar] [CrossRef]
- Mantilla-Escalante, D.C.; López de las Hazas, M.C.; Crespo, M.C.; Martín-Hernández, R.; Tomé-Carneiro, J.; del Pozo-Acebo, L.; Salas-Salvadó, J.; Bulló, M.; Dávalos, A. Mediterranean Diet Enriched in Extra-Virgin Olive Oil or Nuts Modulates Circulating Exosomal Non-Coding RNAs. Eur J Nutr 2021, 60, 4279–4293. [Google Scholar] [CrossRef]
- Bacalini, M.G.; Friso, S.; Olivieri, F.; Pirazzini, C.; Giuliani, C.; Capri, M.; Santoro, A.; Franceschi, C.; Garagnani, P. Present and Future of Anti-Ageing Epigenetic Diets. Mech Ageing Dev 2014, 136–137, 101–115. [Google Scholar] [CrossRef]
- Lee, P.S.; Chiou, Y.S.; Ho, C.T.; Pan, M.H. Chemoprevention by Resveratrol and Pterostilbene: Targeting on Epigenetic Regulation. BioFactors 2018, 44, 26–35. [Google Scholar] [CrossRef]
- Bekdash, R.A. Epigenetics, Nutrition, and the Brain: Improving Mental Health through Diet. Int J Mol Sci 2024, 25, 4036. [Google Scholar] [CrossRef] [PubMed]
- Pazoki-Toroudi, H.; Amani, H.; Ajami, M.; Nabavi, S.F.; Braidy, N.; Kasi, P.D.; Nabavi, S.M. Targeting MTOR Signaling by Polyphenols: A New Therapeutic Target for Ageing. Ageing Res Rev 2016, 31, 55–66. [Google Scholar] [CrossRef] [PubMed]
- Gillette-Guyonnet, S.; Secher, M.; Vellas, B. Nutrition and Neurodegeneration: Epidemiological Evidence and Challenges for Future Research. Br J Clin Pharmacol 2013, 75, 738–755. [Google Scholar] [CrossRef]
- Vellas, B.; Carrie, I.; Gillette-Guyonnet, S.; Touchon, J.; Dantoine, T.; Dartigues, J.F.; Cuffi, M.N.; Bordes, S.; Gasnier, Y.; Robert, P.; et al. Alzheimer’s Disease : Design and Baseline Data. J Prev Alzheimers Dis 2014, 1, 13–22. [Google Scholar]
- Ngandu, T.; Lehtisalo, J.; Solomon, A.; Levälahti, E.; Ahtiluoto, S.; Antikainen, R.; Bäckman, L.; Hänninen, T.; Jula, A.; Laatikainen, T.; Lindström, J.; Mangialasche, F.; Paajanen, T.; Pajala, S.; Peltonen, M.; Rauramaa, R.; Stigsdotter-Neely, A.; Strandberg, T.; Tuomilehto, J.; Soininen, H.; Kivipelto, M. A 2 Year Multidomain Intervention of Diet, Exercise, Cognitive Training, and Vascular Risk Monitoring versus Control to Prevent Cognitive Decline in at-Risk Elderly People (FINGER): A Randomised Controlled Trial. The Lancet 2015, 385, 2255–2263. [Google Scholar] [CrossRef]
- Palou, A.; Serra, F.; Pico, C. General Aspects on the Assessment of Functional Foods in the European Union. Eur J Clin Nutr 2003, 57, S12–S17. [Google Scholar] [CrossRef]
- Siró, I.; Kápolna, E.; Kápolna, B.; Lugasi, A. Functional Food. Product Development, Marketing and Consumer Acceptance—A Review. Appetite 2008, 51, 456–467. [Google Scholar] [CrossRef]
- Jeck, W.R.; Siebold, A.P.; Sharpless, N.E. Review: A Meta-analysis of GWAS and Age-associated Diseases. Aging Cell 2012, 11, 727–731. [Google Scholar] [CrossRef]
- Martucci, M.; Conte, M.; Bucci, L.; Giampieri, E.; Fabbri, C.; Palmas, M.G.; Izzi, M.; Salvioli, S.; Zambrini, A.V.; Orsi, C.; Brigidi, P.; Santoro, A.; Capri, M.; Monti, D.; Franceschi, C. Twelve-Week Daily Consumption of Ad Hoc Fortified Milk with ω-3, D, and Group B Vitamins Has a Positive Impact on Inflammaging Parameters: A Randomized Cross-over Trial. Nutrients 2020, 12, 1–19. [Google Scholar] [CrossRef]
- Tillisch, K.; Labus, J.; Kilpatrick, L.; Jiang, Z.; Stains, J.; Ebrat, B.; Guyonnet, D.; Legrain–Raspaud, S.; Trotin, B.; Naliboff, B.; Mayer, E.A. Consumption of Fermented Milk Product With Probiotic Modulates Brain Activity. Gastroenterology 2013, 144, 1394–1401.e4. [Google Scholar] [CrossRef]
- Navarro-Hortal, M.D.; Romero-Márquez, J.M.; Jiménez-Trigo, V.; Xiao, J.; Giampieri, F.; Forbes-Hernández, T.Y.; Grosso, G.; Battino, M.; Sánchez-González, C.; Quiles, J.L. Molecular Bases for the Use of Functional Foods in the Management of Healthy Aging: Berries, Curcumin, Virgin Olive Oil and Honey; Three Realities and a Promise. Crit Rev Food Sci Nutr 2023, 63, 11967–11986. [Google Scholar] [CrossRef] [PubMed]
- Driver, C. Mitochondrial Interventions in Aging and Longevity. In Modulating Aging and Longevity; Springer Netherlands: Dordrecht, 2003; pp. 205–217. [Google Scholar]
- Reiter, R.J.; Tan, D.X.; Manchester, L.C.; El-Sawi, M.R. Melatonin Reduces Oxidant Damage and Promotes Mitochondrial Respiration: Implications for Aging. Ann N Y Acad Sci 2002, 959, 238–250. [Google Scholar] [CrossRef] [PubMed]
- Mahoney, D.J.; Parise, G.; Tarnopolsky, M.A. Nutritional and Exercise-Based Therapies in the Treatment of Mitochondrial Disease. Curr Opin Clin Nutr Metab Care 2002, 5, 619–629. [Google Scholar] [CrossRef] [PubMed]
- Ames, B.N.; Shigenaga, M.K.; Hagen, T.M. Oxidants, Antioxidants, and the Degenerative Diseases of Aging. Proc Natl Acad Sci U S A 1993, 90, 7915–7922. [Google Scholar] [CrossRef]
- Lyras, L.; Cairns, N.J.; Jenner, A.; Jenner, P.; Halliwell, B. An Assessment of Oxidative Damage to Proteins, Lipids, and DNA in Brain from Patients with Alzheimer’s Disease. J Neurochem 1997, 68, 2061–2069. [Google Scholar] [CrossRef]
- Bej, E.; Cesare, P.; Volpe, A.R.; d’Angelo, M.; Castelli, V. Oxidative Stress and Neurodegeneration: Insights and Therapeutic Strategies for Parkinson’s Disease. Neurol Int 2024, 16, 502–517. [Google Scholar] [CrossRef]
- Valko, M.; Rhodes, C.J.; Moncol, J.; Izakovic, M.; Mazur, M. Free Radicals, Metals and Antioxidants in Oxidative Stress-Induced Cancer. Chem Biol Interact 2006, 160, 1–40. [Google Scholar] [CrossRef]
- Stadtman, E.R. Role of Oxidant Species in Aging. Curr Med Chem 2004, 11, 1105–1112. [Google Scholar] [CrossRef]
- von Zglinicki, T. Oxidative Stress and Cell Senescence as Drivers of Ageing: Chicken and Egg. Ageing Res Rev 2024, 102, 102558. [Google Scholar] [CrossRef]
- May, J.M. Vitamin C Transport and Its Role in the Central Nervous System. In Water Soluble Vitamins. Subcellular Biochemistry; O. Stanger; 2012; Volume 56, pp. 85–103.
- Harrison, F.E.; May, J.M. Vitamin C Function in the Brain: Vital Role of the Ascorbate Transporter SVCT2. Free Radic Biol Med 2009, 46, 719–730. [Google Scholar] [CrossRef]
- Harrison, F.E.; Hosseini, A.H.; McDonald, M.P.; May, J.M. Vitamin C Reduces Spatial Learning Deficits in Middle-Aged and Very Old APP/PSEN1 Transgenic and Wild-Type Mice. Pharmacol Biochem Behav 2009, 93, 443–450. [Google Scholar] [CrossRef] [PubMed]
- Dhingra, D.; Parle, M.; Kulkarni, S.K. Comparative Brain Cholinesterase-Inhibiting Activity of Glycyrrhiza Glabra, Myristica Fragrans, Ascorbic Acid, and Metrifonate in Mice. J Med Food 2006, 9, 281–283. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Chang, M.; Park, C.; Kim, H.; Kim, J.; Son, H.; Lee, Y.; Lee, S. Ascorbate-induced Differentiation of Embryonic Cortical Precursors into Neurons and Astrocytes. J Neurosci Res 2003, 73, 156–165. [Google Scholar] [CrossRef]
- Harrison, F.; Bowman, G.; Polidori, M. Ascorbic Acid and the Brain: Rationale for the Use against Cognitive Decline. Nutrients 2014, 6, 1752–1781. [Google Scholar] [CrossRef]
- Nualart, F. Vitamin C Transporters, Recycling and the Bystander Effect in the Nervous System: SVCT2 versus Gluts. J Stem Cell Res Ther 2014, 04. [Google Scholar] [CrossRef]
- Belluzzi, E.; Bisaglia, M.; Lazzarini, E.; Tabares, L.C.; Beltramini, M.; Bubacco, L. Human SOD2 Modification by Dopamine Quinones Affects Enzymatic Activity by Promoting Its Aggregation: Possible Implications for Parkinson’s Disease. PLoS One 2012, 7, e38026. [Google Scholar] [CrossRef]
- Man Anh, H.; Linh, D.M.; My Dung, V.; Thi Phuong Thao, D. Evaluating Dose- and Time-Dependent Effects of Vitamin C Treatment on a Parkinson’s Disease Fly Model. Parkinsons Dis 2019, 2019, 1–14. [Google Scholar] [CrossRef]
- Casani, S.; Gómez-Pastor, R.; Matallana, E.; Paricio, N. Antioxidant Compound Supplementation Prevents Oxidative Damage in a Drosophila Model of Parkinson’s Disease. Free Radic Biol Med 2013, 61, 151–160. [Google Scholar] [CrossRef]
- Etminan, M.; Gill, S.S.; Samii, A. Intake of Vitamin E, Vitamin C, and Carotenoids and the Risk of Parkinson’s Disease: A Meta-Analysis. Lancet Neurol 2005, 4, 362–365. [Google Scholar] [CrossRef]
- Grünewald, R.A. Ascorbic Acid in the Brain. Brain Res Rev 1993, 18, 123–133. [Google Scholar] [CrossRef]
- Mefford, I.N.; Oke, A.F.; Adams, R.N. Regional Distribution of Ascorbate in Human Brain. Brain Res 1981, 212, 223–226. [Google Scholar] [CrossRef] [PubMed]
- Figueroa-Méndez, R.; Rivas-Arancibia, S. Vitamin C in Health and Disease: Its Role in the Metabolism of Cells and Redox State in the Brain. Front Physiol 2015, 6. [Google Scholar] [CrossRef] [PubMed]
- Seitz, G.; Gebhardt, S.; Beck, J.F.; Böhm, W.; Lode, H.N.; Niethammer, D.; Bruchelt, G. Ascorbic Acid Stimulates DOPA Synthesis and Tyrosine Hydroxylase Gene Expression in the Human Neuroblastoma Cell Line SK-N-SH. Neurosci Lett 1998, 244, 33–36. [Google Scholar] [CrossRef] [PubMed]
- Nagayama, H.; Hamamoto, M.; Ueda, M.; Nito, C.; Yamaguchi, H.; Katayama, Y. The Effect of Ascorbic Acid on the Pharmacokinetics of Levodopa in Elderly Patients with Parkinson Disease. Clin Neuropharmacol 2004, 27, 270–273. [Google Scholar] [CrossRef]
- Nikolova, G.; Karamalakova, Y.; Gadjeva, V. Reducing Oxidative Toxicity of L-Dopa in Combination with Two Different Antioxidants: An Essential Oil Isolated from Rosa Damascena Mill., and Vitamin C. Toxicol Rep 2019, 6, 267–271. [Google Scholar] [CrossRef]
- Yan, J.; Studer, L.; McKay, R.D.G. Ascorbic Acid Increases the Yield of Dopaminergic Neurons Derived from Basic Fibroblast Growth Factor Expanded Mesencephalic Precursors. J Neurochem 2001, 76, 307–311. [Google Scholar] [CrossRef]
- Hughes, K.C.; Gao, X.; Kim, I.Y.; Rimm, E.B.; Wang, M.; Weisskopf, M.G.; Schwarzschild, M.A.; Ascherio, A. Intake of Antioxidant Vitamins and Risk of Parkinson’s Disease. Movement Disorders 2016, 31, 1909–1914. [Google Scholar] [CrossRef]
- Yang, F.; Wolk, A.; Håkansson, N.; Pedersen, N.L.; Wirdefeldt, K. Dietary Antioxidants and Risk of Parkinson’s Disease in Two Population-based Cohorts. Movement Disorders 2017, 32, 1631–1636. [Google Scholar] [CrossRef]
- Miyake, Y.; Fukushima, W.; Tanaka, K.; Sasaki, S.; Kiyohara, C.; Tsuboi, Y.; Yamada, T.; Oeda, T.; Miki, T.; Kawamura, N.; Fukuyama, H.; Hirota, Y.; Nagai, M. ; Dietary Intake of Antioxidant Vitamins and Risk of Parkinson’s Disease: A Case–Control Study in Japan. Eur J Neurol 2011, 18, 106–113. [Google Scholar] [CrossRef]
- Schirinzi, T.; Martella, G.; Imbriani, P.; Di Lazzaro, G.; Franco, D.; Colona, V.L.; Alwardat, M.; Sinibaldi Salimei, P.; Mercuri, N.B.; Pierantozzi, M.; Pisani, A. ; Dietary Vitamin E as a Protective Factor for Parkinson’s Disease: Clinical and Experimental Evidence. Front Neurol 2019, 10. [Google Scholar] [CrossRef]
- Casani, S.; Gómez-Pastor, R.; Matallana, E.; Paricio, N. Antioxidant Compound Supplementation Prevents Oxidative Damage in a Drosophila Model of Parkinson’s Disease. Free Radic Biol Med 2013, 61, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Cohen, G.; Kesler, N. Monoamine Oxidase and Mitochondrial Respiration. J Neurochem 1999, 73, 2310–2315. [Google Scholar] [CrossRef] [PubMed]
- Ithayarasi, A.P.; Shyamala Devi, C. Effect of Alpha-Tocopherol on Mitochondrial Electron Transport in Experimental Myocardial Infarction in Rats. Indian J Biochem Biophys 1998, 35, 115–119. [Google Scholar] [PubMed]
- Nakaso, K.; Tajima, N.; Horikoshi, Y.; Nakasone, M.; Hanaki, T.; Kamizaki, K.; Matsura, T. The Estrogen Receptor β-PI3K/Akt Pathway Mediates the Cytoprotective Effects of Tocotrienol in a Cellular Parkinson’s Disease Model. Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease 2014, 1842, 1303–1312. [Google Scholar] [CrossRef]
- Nakaso, K.; Horikoshi, Y.; Takahashi, T.; Hanaki, T.; Nakasone, M.; Kitagawa, Y.; Koike, T.; Matsura, T. Estrogen Receptor-Mediated Effect of δ-Tocotrienol Prevents Neurotoxicity and Motor Deficit in the MPTP Mouse Model of Parkinson’s Disease. Neurosci Lett 2016, 610, 117–122. [Google Scholar] [CrossRef]
- Odunze, I.N.; Klaidman, L.K.; Adams, J.D. MPTP Toxicity in the Mouse Brain and Vitamin E. Neurosci Lett 1990, 108, 346–349. [Google Scholar] [CrossRef]
- Perry, T.L.; Yong, V.W.; Clavier, R.M.; Jones, K.; Wright, J.M.; Foulks, J.G.; Wall, R.A. Partial Protection from the Dopaminergic Neurotoxin N-Methyl-4-Phenyl-1,2,3,6-Tetrahydropyridine by Four Different Antioxidants in the Mouse. Neurosci Lett 1985, 60, 109–114. [Google Scholar] [CrossRef]
- Hao, X.; Li, H.; Li, Q.; Gao, D.; Wang, X.; Wu, C.; Wang, Q.; Zhu, M. Dietary Vitamin E Intake and Risk of Parkinson’s Disease: A Cross-Sectional Study. Front Nutr 2024, 10. [Google Scholar] [CrossRef]
- Feng, Y.; Wang, X. Antioxidant Therapies for Alzheimer’s Disease. Oxid Med Cell Longev 2012, 2012, 1–17. [Google Scholar] [CrossRef]
- Choudhry, F.; Howlett, D.R.; Richardson, J.C.; Francis, P.T.; Williams, R.J. Pro-Oxidant Diet Enhances β/γ Secretase-Mediated APP Processing in APP/PS1 Transgenic Mice. Neurobiol Aging 2012, 33, 960–968. [Google Scholar] [CrossRef]
- Huang, J.; May, J.M. Ascorbic Acid Protects SH-SY5Y Neuroblastoma Cells from Apoptosis and Death Induced by β-Amyloid. Brain Res 2006, 1097, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Rosales-Corral, S.; Tan, D.; Reiter, R.J.; Valdivia-Velázquez, M.; Martínez-Barboza, G.; Pablo Acosta-Martínez, J.; Ortiz, G.G. Orally Administered Melatonin Reduces Oxidative Stress and Proinflammatory Cytokines Induced by Amyloid- β Peptide in Rat Brain: A Comparative, in vivo Study versus Vitamin C and E. J Pineal Res 2003, 35, 80–84. [Google Scholar] [CrossRef] [PubMed]
- Niki, E. Role of Vitamin E as a Lipid-Soluble Peroxyl Radical Scavenger: In vitro and in vivo Evidence. Free Radic Biol Med 2014, 66, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Ulatowski, L.M.; Manor, D. Vitamin E and Neurodegeneration. Neurobiol Dis 2015, 84, 78–83. [Google Scholar] [CrossRef]
- Yatin, S.M.; Varadarajan, S.; Butterfield, D.A. Vitamin E Prevents Alzheimer’s Amyloid ß-Peptide (1-42)-Induced Neuronal Protein Oxidation and Reactive Oxygen Species Production. Journal of Alzheimer’s Disease 2000, 2, 123–131. [Google Scholar] [CrossRef]
- Yatin, S.M.; Varadarajan, S.; Butterfield, D.A. Vitamin E Prevents Alzheimer’s Amyloid ß-Peptide (1-42)-Induced Neuronal Protein Oxidation and Reactive Oxygen Species Production. Journal of Alzheimer’s Disease 2000, 2, 123–131. [Google Scholar] [CrossRef]
- Scimemi, A.; Meabon, J.S.; Woltjer, R.L.; Sullivan, J.M.; Diamond, J.S.; Cook, D.G. Amyloid-β 1–42 Slows Clearance of Synaptically Released Glutamate by Mislocalizing Astrocytic GLT-1. The Journal of Neuroscience 2013, 33, 5312–5318. [Google Scholar] [CrossRef]
- Mattson, M.P. Pathways towards and Away from Alzheimer’s Disease. Nature 2004, 430, 631–639. [Google Scholar] [CrossRef]
- Pérez, M.; Cuadros, R.; Smith, M.A.; Perry, G.; Avila, J. Phosphorylated, but Not Native, Tau Protein Assembles Following Reaction with the Lipid Peroxidation Product, 4-hydroxy-2-nonenal. FEBS Lett 2000, 486, 270–274. [Google Scholar] [CrossRef]
- Gamblin, T.C.; King, M.E.; Kuret, J.; Berry, R.W.; Binder, L.I. Oxidative Regulation of Fatty Acid-Induced Tau Polymerization. Biochemistry 2000, 39, 14203–14210. [Google Scholar] [CrossRef]
- Jiang, Q.; Yin, X.; Lill, M.A.; Danielson, M.L.; Freiser, H.; Huang, J. Long-Chain Carboxychromanols, Metabolites of Vitamin E, Are Potent Inhibitors of Cyclooxygenases. Proceedings of the National Academy of Sciences 2008, 105, 20464–20469. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Elson-Schwab, I.; Courtemanche, C.; Ames, B.N. γ-Tocopherol and Its Major Metabolite, in Contrast to α-Tocopherol, Inhibit Cyclooxygenase Activity in Macrophages and Epithelial Cells. Proceedings of the National Academy of Sciences 2000, 97, 11494–11499. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.-H.; Kim, M.-B.; Kim, C.; Hwang, J.-K. Inhibitory Effect of Vitamin C on Intrinsic Aging in Human Dermal Fibroblasts and Hairless Mice. Food Sci Biotechnol 2017. [Google Scholar] [CrossRef] [PubMed]
- Teawcharoensopa, C.; Srisuwan, T. The Potential Use of Ascorbic Acid to Recover the Cellular Senescence of Lipopolysaccharide-Induced Human Apical Papilla Cells: An in vitro Study. Clin Oral Investig 2023, 28, 49. [Google Scholar] [CrossRef]
- La Fata, G.; Seifert, N.; Weber, P.; Mohajeri, M.H. Vitamin E Supplementation Delays Cellular Senescence In vitro. Biomed Res Int 2015, 2015, 1–11. [Google Scholar] [CrossRef]
- Ricciarelli, R.; Azzi, A.; Zingg, J. Reduction of Senescence-associated Beta-galactosidase Activity by Vitamin E in Human Fibroblasts Depends on Subjects’ Age and Cell Passage Number. BioFactors 2020, 46, 665–674. [Google Scholar] [CrossRef]
- Mandel, S.A.; Amit, T.; Weinreb, O.; Youdim, M.B.H. Understanding the Broad-Spectrum Neuroprotective Action Profile of Green Tea Polyphenols in Aging and Neurodegenerative Diseases. Journal of Alzheimer’s Disease 2011, 25, 187–208. [Google Scholar] [CrossRef]
- Williams, R.J.; Spencer, J.P.E.; Rice-Evans, C. Flavonoids: Antioxidants or Signalling Molecules? Free Radic Biol Med 2004, 36, 838–849. [Google Scholar] [CrossRef]
- Menicacci, B.; Cipriani, C.; Margheri, F.; Mocali, A.; Giovannelli, L. Modulation of the Senescence-Associated Inflammatory Phenotype in Human Fibroblasts by Olive Phenols. Int J Mol Sci 2017, 18, 2275. [Google Scholar] [CrossRef]
- Lim, H.; Park, H.; Kim, H.P. Effects of Flavonoids on Senescence-Associated Secretory Phenotype Formation from Bleomycin-Induced Senescence in BJ Fibroblasts. Biochem Pharmacol 2015, 96, 337–348. [Google Scholar] [CrossRef]
- Bientinesi, E.; Lulli, M.; Becatti, M.; Ristori, S.; Margheri, F.; Monti, D. Doxorubicin-Induced Senescence in Normal Fibroblasts Promotes in vitro Tumour Cell Growth and Invasiveness: The Role of Quercetin in Modulating These Processes. Mech Ageing Dev 2022, 206. [Google Scholar] [CrossRef] [PubMed]
- Bientinesi, E.; Ristori, S.; Lulli, M.; Monti, D. Quercetin Induces Senolysis of Doxorubicin-Induced Senescent Fibroblasts by Reducing Autophagy, Preventing Their pro-Tumour Effect on Osteosarcoma Cells. Mech Ageing Dev 2024, 220. [Google Scholar] [CrossRef] [PubMed]
- Maurya, P.K.; Kumar, P.; Nagotu, S.; Chand, S.; Chandra, P. Multi-Target Detection of Oxidative Stress Biomarkers in Quercetin and Myricetin Treated Human Red Blood Cells. RSC Adv 2016, 6, 53195–53202. [Google Scholar] [CrossRef]
- Jung, U.J.; Kim, S.R. Beneficial Effects of Flavonoids Against Parkinson’s Disease. J Med Food 2018, 21, 421–432. [Google Scholar] [CrossRef]
- Guo, S.; Yan, J.; Yang, T.; Yang, X.; Bezard, E.; Zhao, B. Protective Effects of Green Tea Polyphenols in the 6-OHDA Rat Model of Parkinson’s Disease Through Inhibition of ROS-NO Pathway. Biol Psychiatry 2007, 62, 1353–1362. [Google Scholar] [CrossRef]
- Levites, Y.; Youdim, M.B.H.; Maor, G.; Mandel, S. Attenuation of 6-Hydroxydopamine (6-OHDA)-Induced Nuclear Factor-KappaB (NF-ΚB) Activation and Cell Death by Tea Extracts in Neuronal Cultures. Biochem Pharmacol 2002, 63, 21–29. [Google Scholar] [CrossRef]
- Rojas, P.; Serrano-García, N.; Mares-Sámano, J.J.; Medina-Campos, O.N.; Pedraza-Chaverri, J.; Ögren, S.O. EGb761 Protects against Nigrostriatal Dopaminergic Neurotoxicity in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced Parkinsonism in Mice: Role of Oxidative Stress. European Journal of Neuroscience 2008, 28, 41–50. [Google Scholar] [CrossRef]
- Wu, W.-R.; Zhu, X.-Z. Involvement of Monoamine Oxidase Inhibition in Neuroprotective and Neurorestorative Effects of Ginkgo Biloba Extract against MPTP-Induced Nigrostriatal Dopaminergic Toxicity in C57 Mice. Life Sci 1999, 65, 157–164. [Google Scholar] [CrossRef]
- Khan, Mohd. M.; Ahmad, A.; Ishrat, T.; Khan, M.B.; Hoda, Md.N.; Khuwaja, G.; Raza, S.S.; Khan, A.; Javed, H.; Vaibhav, K.; et al. Resveratrol Attenuates 6-Hydroxydopamine-Induced Oxidative Damage and Dopamine Depletion in Rat Model of Parkinson’s Disease. Brain Res 2010, 1328, 139–151. [Google Scholar] [CrossRef]
- Wang, H.; Dong, X.; Liu, Z.; Zhu, S.; Liu, H.; Fan, W.; Hu, Y.; Hu, T.; Yu, Y.; Li, Y.; et al. Resveratrol Suppresses Rotenone-induced Neurotoxicity Through Activation of SIRT1/Akt1 Signaling Pathway. Anat Rec 2018, 301, 1115–1125. [Google Scholar] [CrossRef]
- Gao, Z.-B.; Chen, X.-Q.; Hu, G.-Y. Inhibition of Excitatory Synaptic Transmission by Trans-Resveratrol in Rat Hippocampus. Brain Res 2006, 1111, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Wang, S.-J. Inhibitory Effect of Glutamate Release from Rat Cerebrocortical Nerve Terminals by Resveratrol. Neurochem Int 2009, 54, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Yáñez, M.; Fraiz, N.; Cano, E.; Orallo, F. Inhibitory Effects of Cis- and Trans-Resveratrol on Noradrenaline and 5-Hydroxytryptamine Uptake and on Monoamine Oxidase Activity. Biochem Biophys Res Commun 2006, 344, 688–695. [Google Scholar] [CrossRef] [PubMed]
- Palazzi, L.; Bruzzone, E.; Bisello, G.; Leri, M.; Stefani, M.; Bucciantini, M.; Polverino de Laureto, P. Oleuropein Aglycone Stabilizes the Monomeric α-Synuclein and Favours the Growth of Non-Toxic Aggregates. Sci Rep 2018, 8, 8337. [Google Scholar] [CrossRef]
- Mohammad-Beigi, H.; Aliakbari, F.; Sahin, C.; Lomax, C.; Tawfike, A.; Schafer, N.P.; Amiri-Nowdijeh, A.; Eskandari, H.; Møller, I.M.; Hosseini-Mazinani, M.; Christiansen, G.; Ward, J.L.; Morshedi, D.; Otzen, D.E. ; Oleuropein Derivatives from Olive Fruit Extracts Reduce α-Synuclein Fibrillation and Oligomer Toxicity. Journal of Biological Chemistry 2019, 294, 4215–4232. [Google Scholar] [CrossRef]
- Sun, W.; Wang, X.; Hou, C.; Yang, L.; Li, H.; Guo, J.; Huo, C.; Wang, M.; Miao, Y.; Liu, J.; Kang, Y. ; Oleuropein Improves Mitochondrial Function to Attenuate Oxidative Stress by Activating the Nrf2 Pathway in the Hypothalamic Paraventricular Nucleus of Spontaneously Hypertensive Rats. Neuropharmacology 2017, 113, 556–566. [Google Scholar] [CrossRef]
- Lange, K.W.; Li, S. Resveratrol, Pterostilbene, and Dementia. BioFactors 2018, 44, 83–90. [Google Scholar] [CrossRef]
- Ader, P. Bioavailability and Metabolism of the Flavonol Quercetin in the Pig. Free Radic Biol Med 2000, 28, 1056–1067. [Google Scholar] [CrossRef]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; Abete, P. ; Oxidative Stress, Aging, and Diseases. Clin Interv Aging 2018, Volume 13, 757–772. [Google Scholar] [CrossRef]
- Vauzour, D.; Rodriguez-Mateos, A.; Corona, G.; Oruna-Concha, M.J.; Spencer, J.P.E. Polyphenols and Human Health: Prevention of Disease and Mechanisms of Action. Nutrients 2010, 2, 1106–1131. [Google Scholar] [CrossRef]
- Zheng, Q.; Kebede, M.T.; Kemeh, M.M.; Islam, S.; Lee, B.; Bleck, S.D.; Wurfl, L.A.; Lazo, N.D. Inhibition of the Self-Assembly of Aβ and of Tau by Polyphenols: Mechanistic Studies. Molecules 2019, 24, 2316. [Google Scholar] [CrossRef] [PubMed]
- Jaeger, B.N.; Parylak, S.L.; Gage, F.H. Mechanisms of Dietary Flavonoid Action in Neuronal Function and Neuroinflammation. Mol Aspects Med 2018, 61, 50–62. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Kishimoto, Y.; Grammatikakis, I.; Gottimukkala, K.; Cutler, R.G.; Zhang, S.; Abdelmohsen, K.; Bohr, V.A.; Misra Sen, J.; Gorospe, M.; et al. Senolytic Therapy Alleviates Aβ-Associated Oligodendrocyte Progenitor Cell Senescence and Cognitive Deficits in an Alzheimer’s Disease Model. Nat Neurosci 2019, 22, 719–728. [Google Scholar] [CrossRef]
- Van houcke, J.; Mariën, V.; Zandecki, C.; Ayana, R.; Pepermans, E.; Boonen, K.; Seuntjens, E.; Baggerman, G.; Arckens, L. A Short Dasatinib and Quercetin Treatment Is Sufficient to Reinstate Potent Adult Neuroregenesis in the Aged Killifish. NPJ Regen Med 2023, 8. [Google Scholar] [CrossRef]
- Currais, A.; Farrokhi, C.; Dargusch, R.; Armando, A.; Quehenberger, O.; Schubert, D.; Maher, P. Fisetin Reduces the Impact of Aging on Behavior and Physiology in the Rapidly Aging SAMP8 Mouse. Journals of Gerontology - Series A Biological Sciences and Medical Sciences 2018, 73, 299–307. [Google Scholar] [CrossRef]
- Wissler Gerdes, E.O.; Misra, A.; Netto, J.M.E.; Tchkonia, T.; Kirkland, J.L. Strategies for Late Phase Preclinical and Early Clinical Trials of Senolytics. Mech Ageing Dev 2021, 200. [Google Scholar] [CrossRef]
- Song, S.; Lam, E.W.F.; Tchkonia, T.; Kirkland, J.L.; Sun, Y. Senescent Cells: Emerging Targets for Human Aging and Age-Related Diseases. Trends Biochem Sci 2020, 45, 578–592. [Google Scholar] [CrossRef]
- Hartman, R.E.; Shah, A.; Fagan, A.M.; Schwetye, K.E.; Parsadanian, M.; Schulman, R.N.; Finn, M.B.; Holtzman, D.M. Pomegranate Juice Decreases Amyloid Load and Improves Behavior in a Mouse Model of Alzheimer’s Disease. Neurobiol Dis 2006, 24, 506–515. [Google Scholar] [CrossRef]
- Ho, L.; Chen, L.H.; Wang, J.; Zhao, W.; Talcott, S.T.; Ono, K.; Teplow, D.; Humala, N.; Cheng, A.; Percival, S.S.; Ferruzzi, M.; Janle, E.; Dickstein, D.L.; Pasinetti, G.M. ; Heterogeneity in Red Wine Polyphenolic Contents Differentially Influences Alzheimer’s Disease-Type Neuropathology and Cognitive Deterioration. Journal of Alzheimer’s Disease 2009, 16, 59–72. [Google Scholar] [CrossRef]
- Mori, T.; Rezai-Zadeh, K.; Koyama, N.; Arendash, G.W.; Yamaguchi, H.; Kakuda, N.; Horikoshi-Sakuraba, Y.; Tan, J.; Town, T. Tannic Acid Is a Natural β-Secretase Inhibitor That Prevents Cognitive Impairment and Mitigates Alzheimer-like Pathology in Transgenic Mice. Journal of Biological Chemistry 2012, 287, 6912–6927. [Google Scholar] [CrossRef]
- Wang, J.; Ho, L.; Zhao, W.; Ono, K.; Rosensweig, C.; Chen, L.; Humala, N.; Teplow, D.B.; Pasinetti, G.M. Grape-Derived Polyphenolics Prevent A Oligomerization and Attenuate Cognitive Deterioration in a Mouse Model of Alzheimer’s Disease. Journal of Neuroscience 2008, 28, 6388–6392. [Google Scholar] [CrossRef] [PubMed]
- Nugraheni, N.; Ahlina, F.N.; Salsabila, I.A.; Haryanti, S.; Meiyanto, E. Anti-Senescence Activity of Indonesian Black Pepper Essential Oil (Piper Nigrum L.) on Ovarian CHO-K1 and Fibroblast NIH-3T3 Cells; Vol. 45.
- Chilelli, N.; Ragazzi, E.; Valentini, R.; Cosma, C.; Ferraresso, S.; Lapolla, A.; Sartore, G. Curcumin and Boswellia Serrata Modulate the Glyco-Oxidative Status and Lipo-Oxidation in Master Athletes. Nutrients 2016, 8, 745. [Google Scholar] [CrossRef] [PubMed]
- Karimian, M.S.; Pirro, M.; Majeed, M.; Sahebkar, A. Curcumin as a Natural Regulator of Monocyte Chemoattractant Protein-1. Cytokine Growth Factor Rev 2017, 33, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Grabowska, W.; Suszek, M.; Wnuk, M.; Lewinska, A.; Wasiak, E.; Sikora, E.; Bielak-Zmijewska, A. Curcumin Elevates Sirtuin Level but Does Not Postpone in vitro Senescence of Human Cells Building the Vasculature. Oncotarget 2016, 7, 19201–19213. [Google Scholar] [CrossRef]
- Taka, T.; Changtam, C.; Thaichana, P.; Kaewtunjai, N.; Suksamrarn, A.; Lee, T.R.; Tuntiwechapikul, W. Curcuminoid Derivatives Enhance Telomerase Activity in an in vitro TRAP Assay. Bioorg Med Chem Lett 2014, 24, 5242–5246. [Google Scholar] [CrossRef]
- Kim, T.; Davis, J.; Zhang, A.J.; He, X.; Mathews, S.T. Curcumin Activates AMPK and Suppresses Gluconeogenic Gene Expression in Hepatoma Cells. Biochem Biophys Res Commun 2009, 388, 377–382. [Google Scholar] [CrossRef]
- Liu, Z.; Cui, C.; Xu, P.; Dang, R.; Cai, H.; Liao, D.; Yang, M.; Feng, Q.; Yan, X.; Jiang, P. Curcumin Activates AMPK Pathway and Regulates Lipid Metabolism in Rats Following Prolonged Clozapine Exposure. Front Neurosci 2017, 11. [Google Scholar] [CrossRef]
- Ray Hamidie, R.D.; Yamada, T.; Ishizawa, R.; Saito, Y.; Masuda, K. Curcumin Treatment Enhances the Effect of Exercise on Mitochondrial Biogenesis in Skeletal Muscle by Increasing CAMP Levels. Metabolism 2015, 64, 1334–1347. [Google Scholar] [CrossRef]
- Juturu, V.; Sahin, K.; Pala, R.; Tuzcu, M.; Ozdemir, O.; Orhan, C.; Sahin, N. Curcumin Prevents Muscle Damage by Regulating NF-KB and Nrf2 Pathways and Improves Performance: An in vivo Model. J Inflamm Res 2016, Volume 9, 147–154. [Google Scholar] [CrossRef]
- Namirah, I.; Wimbanu, K.S.; Rompies, A.M.E.; Prayogo, Y.S.; Arozal, W.; Fadilah, F.; Hanafi, M.; Hardiany, N.S. The Effect of Ethanol-Based Coriander (Coriandrum Sativum L.) Seed Extract on Oxidative Stress, Antioxidant Level and Cellular Senescence in the Heart of Obese Rat. J Pharm Pharmacogn Res 2024, 12, 1111–1120. [Google Scholar] [CrossRef]
- Hardiany, N.S.; Dewi, P.K.K.; Dewi, S.; Tejo, B.A. Exploration of Neuroprotective Effect from Coriandrum Sativum L. Ethanolic Seeds Extracts on Brain of Obese Rats. Sci Rep 2024, 14, 603. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.-Y.; Cheng, Y.; Cui, J.; Zhang, Y.; Yu, L.-C. Potential Protection of Curcumin against Amyloid β-Induced Toxicity on Cultured Rat Prefrontal Cortical Neurons. Neurosci Lett 2009, 463, 158–161. [Google Scholar] [CrossRef] [PubMed]
- Muthian, G.; Mackey, V.; Prasad, K.; Charlton, C. Curcumin and an Antioxidant Formulation Protect C57BL/6J Mice from MPTP-Induced Parkinson’s Disease like Changes: Potential Neuroprotection for Neurodegeneration. Journal of Parkinsonism and Restless Legs Syndrome 2018, Volume 8, 49–59. [Google Scholar] [CrossRef]
- Canistro, D.; Chiavaroli, A.; Cicia, D.; Cimino, F.; Curro, D.; Dell Agli, M.; Ferrante, C.; Giovannelli, L.; Leone, S.; Martinelli, G.; Milella, L.; Pagano, E.; Piazza, S.; Ponticelli, M.; Recinella, L.; Ristori, S.; Sangiovanni, E.; Smeriglio, A.; Speciale, A.; Trombetta, D.; Vivarelli, F. ; The Pharmacological Basis of the Curcumin Nutraceutical Uses: An Update. Pharmadvances 2021, 03, 421. [Google Scholar] [CrossRef]
- Tønnesen, H.H.; Karlsen, J.; Henegouwen, G.B. Studies on Curcumin and Curcuminoids VIII. Photochemical Stability of Curcumin. Zeitschrift für Lebensmittel-Untersuchung und -Forschung 1986, 183, 116–122. [Google Scholar] [CrossRef]
- Zbarsky, V.; Datla, K.P.; Parkar, S.; Rai, D.K.; Aruoma, O.I.; Dexter, D.T. Neuroprotective Properties of the Natural Phenolic Antioxidants Curcumin and Naringenin but Not Quercetin and Fisetin in a 6-OHDA Model of Parkinson’s Disease. Free Radic Res 2005, 39, 1119–1125. [Google Scholar] [CrossRef]
- Wang, J.; Du, X.-X.; Jiang, H.; Xie, J.-X. Curcumin Attenuates 6-Hydroxydopamine-Induced Cytotoxicity by Anti-Oxidation and Nuclear Factor-KappaB Modulation in MES23.5 Cells. Biochem Pharmacol 2009, 78, 178–183. [Google Scholar] [CrossRef]
- Huang, W.-T.; Niu, K.-C.; Chang, C.-K.; Lin, M.-T.; Chang, C.-P. Curcumin Inhibits the Increase of Glutamate, Hydroxyl Radicals and PGE2 in the Hypothalamus and Reduces Fever during LPS-Induced Systemic Inflammation in Rabbits. Eur J Pharmacol 2008, 593, 105–111. [Google Scholar] [CrossRef]
- Yang, W.; Chen, Y.-H.; Liu, H.; Qu, H.-D. Neuroprotective Effects of Piperine on the 1-Methyl-4-Phenyl-1,2,3,6-Tetrahydropyridine-Induced Parkinson’s Disease Mouse Model. Int J Mol Med 2015, 36, 1369–1376. [Google Scholar] [CrossRef]
- Chaudhri, S.K.; Jain, S. A Systematic Review of Piperine as a Bioavailability Enhancer. Journal of Drug Delivery and Therapeutics 2023, 13, 133–136. [Google Scholar] [CrossRef]
- Singh, S.; Jamwal, S.; Kumar, P. Neuroprotective Potential of Quercetin in Combination with Piperine against 1-Methyl-4-Phenyl-1,2,3,6-Tetrahydropyridine-Induced Neurotoxicity. Neural Regen Res 2017, 12, 1137. [Google Scholar] [CrossRef] [PubMed]
- Bae, W.-Y.; Choi, J.-S.; Jeong, J.-W. The Neuroprotective Effects of Cinnamic Aldehyde in an MPTP Mouse Model of Parkinson’s Disease. Int J Mol Sci 2018, 19, 551. [Google Scholar] [CrossRef] [PubMed]
- Ramazani, E.; YazdFazeli, M.; Emami, S.A.; Mohtashami, L.; Javadi, B.; Asili, J.; Tayarani-Najaran, Z. Protective Effects of Cinnamomum Verum, Cinnamomum Cassia and Cinnamaldehyde against 6-OHDA-Induced Apoptosis in PC12 Cells. Mol Biol Rep 2020, 47, 2437–2445. [Google Scholar] [CrossRef]
- Shaltiel-Karyo, R.; Davidi, D.; Frenkel-Pinter, M.; Ovadia, M.; Segal, D.; Gazit, E. Differential Inhibition of α-Synuclein Oligomeric and Fibrillar Assembly in Parkinson’s Disease Model by Cinnamon Extract. Biochimica et Biophysica Acta (BBA) - General Subjects 2012, 1820, 1628–1635. [Google Scholar] [CrossRef]
- El-Demerdash, F.M.; Yousef, M.I.; Radwan, F.M.E. Ameliorating Effect of Curcumin on Sodium Arsenite-Induced Oxidative Damage and Lipid Peroxidation in Different Rat Organs. Food and Chemical Toxicology 2009, 47, 249–254. [Google Scholar] [CrossRef]
- Venkatesan, N.; Punithavathi, D.; Arumugam, V. Curcumin Prevents Adriamycin Nephrotoxicity in Rats. Br J Pharmacol 2000, 129, 231–234. [Google Scholar] [CrossRef]
- Strimpakos, A.S.; Sharma, R.A. Curcumin: Preventive and Therapeutic Properties in Laboratory Studies and Clinical Trials. Antioxid Redox Signal 2008, 10, 511–546. [Google Scholar] [CrossRef]
- Ganguli, M.; Chandra, V.; Kamboh, M.I.; Johnston, J.M.; Dodge, H.H.; Thelma, B.K.; Juyal, R.C.; Pandav, R.; Belle, S.H.; DeKosky, S.T. Apolipoprotein E Polymorphism and Alzheimer Disease. Arch Neurol 2000, 57, 824. [Google Scholar] [CrossRef]
- Ng, T.-P.; Chiam, P.-C.; Lee, T.; Chua, H.-C.; Lim, L.; Kua, E.-H. Curry Consumption and Cognitive Function in the Elderly. Am J Epidemiol 2006, 164, 898–906. [Google Scholar] [CrossRef]
- Zhang, L.; Fiala, M.; Cashman, J.; Sayre, J.; Espinosa, A.; Mahanian, M.; Zaghi, J.; Badmaev, V.; Graves, M.C.; Bernard, G.; et al. Curcuminoids Enhance Amyloid-β Uptake by Macrophages of Alzheimer’s Disease Patients. Journal of Alzheimer’s Disease 2006, 10, 1–7. [Google Scholar] [CrossRef]
- Ambegaokar, S.S.; Wu, L.; Alamshahi, K.; Lau, J.; Jazayeri, L.; Chan, S.; Khanna, P.; Hsieh, E.; Timiras, P.S. Curcumin Inhibits Dose-Dependently and Time-Dependently Neuroglial Cell Proliferation and Growth. Neuro Endocrinol Lett 2003, 24, 469–473. [Google Scholar] [PubMed]
- Kim, G.-Y.; Kim, K.-H.; Lee, S.-H.; Yoon, M.-S.; Lee, H.-J.; Moon, D.-O.; Lee, C.-M.; Ahn, S.-C.; Park, Y.C.; Park, Y.-M. Curcumin Inhibits Immunostimulatory Function of Dendritic Cells: MAPKs and Translocation of NF-ΚB as Potential Targets. The Journal of Immunology 2005, 174, 8116–8124. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-Y.; Kim, D.S.H.L. Discovery of Natural Products from Curcuma l Onga That Protect Cells from Beta-Amyloid Insult: A Drug Discovery Effort against Alzheimer’s Disease. J Nat Prod 2002, 65, 1227–1231. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.S.H.L.; Park, S.-Y.; Kim, J.-Y. Curcuminoids from Curcuma Longa L. (Zingiberaceae) That Protect PC12 Rat Pheochromocytoma and Normal Human Umbilical Vein Endothelial Cells from ΒA(1–42) Insult. Neurosci Lett 2001, 303, 57–61. [Google Scholar] [CrossRef]
- Jiang, J.; Wang, W.; Sun, Y.J.; Hu, M.; Li, F.; Zhu, D.Y. Neuroprotective Effect of Curcumin on Focal Cerebral Ischemic Rats by Preventing Blood–Brain Barrier Damage. Eur J Pharmacol 2007, 561, 54–62. [Google Scholar] [CrossRef]
- Liu, H.; Li, Z.; Qiu, D.; Gu, Q.; Lei, Q.; Mao, L. The Inhibitory Effects of Different Curcuminoids on β-Amyloid Protein, β-Amyloid Precursor Protein and β-Site Amyloid Precursor Protein Cleaving Enzyme 1 in SwAPP HEK293 Cells. Neurosci Lett 2010, 485, 83–88. [Google Scholar] [CrossRef]
- Ono, K.; Hasegawa, K.; Naiki, H.; Yamada, M. Curcumin Has Potent Anti-amyloidogenic Effects for Alzheimer’s Β-amyloid Fibrils in vitro. J Neurosci Res 2004, 75, 742–750. [Google Scholar] [CrossRef]
- Pagliari, S.; Forcella, M.; Lonati, E.; Sacco, G.; Romaniello, F.; Rovellini, P.; Fusi, P.; Palestini, P.; Campone, L.; Labra, M.; Bulbarelli, A.; Bruni, I.; Antioxidant and Anti-Inflammatory Effect of Cinnamon (Cinnamomum Verum J. Presl) Bark Extract after In vitro Digestion Simulation. Foods 2023, 12, 452. [CrossRef]
- Moselhy, S.S.; Ali, H.K.H. Hepatoprotective Effect of Cinnamon Extracts against Carbon Tetrachloride Induced Oxidative Stress and Liver Injury in Rats. Biol Res 2009, 42, 93–98. [Google Scholar] [CrossRef]
- Gunawardena, D.; Karunaweera, N.; Lee, S.; van Der Kooy, F.; Harman, D.G.; Raju, R.; Bennett, L.; Gyengesi, E.; Sucher, N.J.; Münch, G. Anti-Inflammatory Activity of Cinnamon (C. Zeylanicum and C. Cassia) Extracts – Identification of E-Cinnamaldehyde and o-Methoxy Cinnamaldehyde as the Most Potent Bioactive Compounds. Food Funct 2015, 6, 910–919. [Google Scholar] [CrossRef]
- Schink, A.; Naumoska, K.; Kitanovski, Z.; Kampf, C.J.; Fröhlich-Nowoisky, J.; Thines, E.; Pöschl, U.; Schuppan, D.; Lucas, K. Anti-Inflammatory Effects of Cinnamon Extract and Identification of Active Compounds Influencing the TLR2 and TLR4 Signaling Pathways. Food Funct 2018, 9, 5950–5964. [Google Scholar] [CrossRef] [PubMed]
- Frydman-Marom, A.; Levin, A.; Farfara, D.; Benromano, T.; Scherzer-Attali, R.; Peled, S.; Vassar, R.; Segal, D.; Gazit, E.; Frenkel, D.; Ovadia, M. ; Orally Administrated Cinnamon Extract Reduces β-Amyloid Oligomerization and Corrects Cognitive Impairment in Alzheimer’s Disease Animal Models. PLoS One 2011, 6, e16564. [Google Scholar] [CrossRef] [PubMed]
- Chonpathompikunlert, P.; Wattanathorn, J.; Muchimapura, S. Piperine, the Main Alkaloid of Thai Black Pepper, Protects against Neurodegeneration and Cognitive Impairment in Animal Model of Cognitive Deficit like Condition of Alzheimer’s Disease. Food and Chemical Toxicology 2010, 48, 798–802. [Google Scholar] [CrossRef] [PubMed]
- Subedee, L. Preventive Role of Indian Black Pepper in Animal Models of Alzheimer’s Disease. Journal of Clinical And Diagnostic Research 2015. [Google Scholar] [CrossRef]
- Auti, S.T.; Kulkarni, Y.A. Neuroprotective Effect of Cardamom Oil Against Aluminum Induced Neurotoxicity in Rats. Front Neurol 2019, 10. [Google Scholar] [CrossRef]
- Shi, H.; Ge, X.; Ma, X.; Zheng, M.; Cui, X.; Pan, W.; Zheng, P.; Yang, X.; Zhang, P.; Hu, M.; Tang, R.; Zheng, K.; Huang, X.; Yu, Y. ; A Fiber-Deprived Diet Causes Cognitive Impairment and Hippocampal Microglia-Mediated Synaptic Loss through the Gut Microbiota and Metabolites. Microbiome 2021, 9, 1–20. [Google Scholar] [CrossRef]
- Gareau, M.G.; Gareau, M.G.; Lyte, M.; Cryan, J.F. Microbiota-Gut-Brain Axis and Cognitive Function Abbreviations 5-HT Serotonin ANS Autonomic Nervous System BDNF Brain Derived Neurotropic Factor CD Crohn’s Disease CREB CAMP Response Element Binding Protein CRF Corticotrophin-Releasing Factor DA Dopamine. Adv Exp Med Biol 2014, 817, 357–371. [Google Scholar] [CrossRef]
- Unger, M.M.; Spiegel, J.; Dillmann, K.U.; Grundmann, D.; Philippeit, H.; Bürmann, J.; Faßbender, K.; Schwiertz, A.; Schäfer, K.H. Short Chain Fatty Acids and Gut Microbiota Differ between Patients with Parkinson’s Disease and Age-Matched Controls. Parkinsonism Relat Disord 2016, 32, 66–72. [Google Scholar] [CrossRef]
- Matt, S.M.; Allen, J.M.; Lawson, M.A.; Mailing, L.J.; Woods, J.A.; Johnson, R.W. Butyrate and Dietary Soluble Fiber Improve Neuroinflammation Associated with Aging in Mice. Front Immunol 2018, 9. [Google Scholar] [CrossRef]
- Martins, I.J.; Fernando, W.M.A.D.B. High Fibre Diets and Alzheimer’s Disease. Food Nutr Sci 2014, 05, 410–424. [Google Scholar] [CrossRef]
- Den Besten, G.; Van Eunen, K.; Groen, A.K.; Venema, K.; Reijngoud, D.J.; Bakker, B.M. The Role of Short-Chain Fatty Acids in the Interplay between Diet, Gut Microbiota, and Host Energy Metabolism. J Lipid Res 2013, 54, 2325–2340. [Google Scholar] [CrossRef] [PubMed]
- Zilberter, Y.; Zilberter, M. The Vicious Circle of Hypometabolism in Neurodegenerative Diseases: Ways and Mechanisms of Metabolic Correction. J Neurosci Res 2017, 95, 2217–2235. [Google Scholar] [CrossRef] [PubMed]
- Erny, D.; Hrabě de Angelis, A.L.; Jaitin, D.; Wieghofer, P.; Staszewski, O.; David, E.; Keren-Shaul, H.; Mahlakoiv, T.; Jakobshagen, K.; Buch, T.; Schwierzeck, V.; Utermöhlen, O.; Chun, E.; Garrett, W.S.; McCoy, K.D.; Diefenbach, A.; Staeheli, P.; Stecher, B.; Amit, I.; Prinz, M. ; Host Microbiota Constantly Control Maturation and Function of Microglia in the CNS. Nat Neurosci 2015, 18, 965–977. [Google Scholar] [CrossRef]
- Ho, L.; Ono, K.; Tsuji, M.; Mazzola, P.; Singh, R.; Pasinetti, G.M. Protective Roles of Intestinal Microbiota Derived Short Chain Fatty Acids in Alzheimer’s Disease-Type Beta-Amyloid Neuropathological Mechanisms. Expert Rev Neurother 2018, 18, 83–90. [Google Scholar] [CrossRef]
- Zhou, Y.; Xie, L.; Schröder, J.; Schuster, I.S.; Nakai, M.; Sun, G.; Sun, Y.B.Y.; Mariño, E.; Degli-Esposti, M.A.; Marques, F.Z.; Grubman, A.; Polo, J.M.; Mackay, C.R. ; Dietary Fiber and Microbiota Metabolite Receptors Enhance Cognition and Alleviate Disease in the 5xFAD Mouse Model of Alzheimer’s Disease. Journal of Neuroscience 2023, 43, 6460–6475. [Google Scholar] [CrossRef]
- Wang, J.; Ji, H.; Wang, S.; Liu, H.; Zhang, W.; Zhang, D.; Wang, Y. Probiotic Lactobacillus Plantarum Promotes Intestinal Barrier Function by Strengthening the Epithelium and Modulating Gut Microbiota. Front Microbiol 2018, 9, 1–14. [Google Scholar] [CrossRef]
- Yi, H.; Wang, L.; Xiong, Y.; Wang, Z.; Qiu, Y.; Wen, X.; Jiang, Z.; Yang, X.; Ma, X. Lactobacillus Reuteri LR1 Improved Expression of Genes of Tight Junction Proteins via the MLCK Pathway in IPEC-1 Cells during Infection with Enterotoxigenic Escherichia Coli K88. Mediators Inflamm 2018, 2018. [Google Scholar] [CrossRef]
- Perdigón, G.; Maldonado Galdeano, C.; Valdez, J.C.; Medici, M. Interaction of Lactic Acid Bacteria with the Gut Immune System. Eur J Clin Nutr 2002, 56, S21–S26. [Google Scholar] [CrossRef]
- Sichetti, M.; De Marco, S.; Pagiotti, R.; Traina, G.; Pietrella, D.; Toscano, M.; De Grandi, R.; Stronati, L.; De Vecchi, E.; Drago, L.; et al. Effect of Lactobacillus Rhamnosus HN001 and Bifidobacterium Longum BB536 on the Healthy Gut Microbiota Composition at Phyla and Species Level: A Preliminary Study. Front Microbiol 2019, 10, S21–S26. [Google Scholar] [CrossRef]
- Marotta, A.; Sarno, E.; Casale, A. Del; Pane, M.; Mogna, L.; Amoruso, A.; Felis, G.E.; Fiorio, M. Effects of Probiotics on Cognitive Reactivity, Mood, and Sleep Quality. Front Psychiatry 2019, 10, 1–11. [Google Scholar] [CrossRef]
- Sharma, R.; Padwad, Y. Probiotic Bacteria as Modulators of Cellular Senescence: Emerging Concepts and Opportunities. Gut Microbes 2020, 11, 335–349. [Google Scholar] [CrossRef] [PubMed]
- Toscano, M.; De Grandi, R.; Stronati, L.; De Vecchi, E.; Drago, L. Effect of Lactobacillus Rhamnosus HN001 and Bifidobacterium Longum BB536 on the Healthy Gut Microbiota Composition at Phyla and Species Level: A Preliminary Study. World J Gastroenterol 2017, 23, 2696–2704. [Google Scholar] [CrossRef] [PubMed]
- Kechagia, M.; Basoulis, D.; Konstantopoulou, S.; Dimitriadi, D.; Gyftopoulou, K.; Skarmoutsou, N.; Fakiri, E.M. Health Benefits of Probiotics: A Review. ISRN Nutr 2013, 2013, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Azad, F.J.; Talaei, A.; Rafatpanah, H.; Yousefzadeh, H.; Jafari, R.; Jafari, M.; Talaei, A.; Hosseini, R.F.; Jabari, F. Copyright© Autumn; 2014; Vol. 13.
- Woo, J.Y.; Gu, W.; Kim, K.A.; Jang, S.E.; Han, M.J.; Kim, D.H. Lactobacillus Pentosus Var. Plantarum C29 Ameliorates Memory Impairment and Inflammaging in a d-Galactose-Induced Accelerated Aging Mouse Model. Anaerobe 2014, 27, 22–26. [Google Scholar] [CrossRef]
- Grompone, G.; Martorell, P.; Llopis, S.; González, N.; Genovés, S.; Mulet, A.P.; Fernández-Calero, T.; Tiscornia, I.; Bollati-Fogolín, M.; Chambaud, I.; Foligné, B.; Montserrat, A.; Ramón, D. ; Anti-Inflammatory Lactobacillus Rhamnosus CNCM I-3690 Strain Protects against Oxidative Stress and Increases Lifespan in Caenorhabditis Elegans. PLoS One 2012, 7. [Google Scholar] [CrossRef]
- Lin, X.; Xia, Y.; Wang, G.; Xiong, Z.; Zhang, H.; Lai, F.; Ai, L. Lactobacillus Plantarum AR501 Alleviates the Oxidative Stress of D-Galactose-Induced Aging Mice Liver by Upregulation of Nrf2-Mediated Antioxidant Enzyme Expression. J Food Sci 2018, 83, 1990–1998. [Google Scholar] [CrossRef]
- Eifler, N.; Vetsch, M.; Gregorini, M.; Ringler, P.; Chami, M.; Philippsen, A.; Fritz, A.; Müller, S.A.; Glockshuber, R.; Engel, A.; et al. Cytotoxin ClyA from Escherichia Coli Assembles to a 13-Meric Pore Independent of Its Redox-State. EMBO Journal 2006, 25, 2652–2661. [Google Scholar] [CrossRef]
- Gazerani, P. Probiotics for Parkinson’s Disease. Int J Mol Sci 2019, 20. [Google Scholar] [CrossRef]
- Magistrelli, L.; Amoruso, A.; Mogna, L.; Graziano, T.; Cantello, R.; Pane, M.; Comi, C. Probiotics May Have Beneficial Effects in Parkinson’s Disease: In vitro Evidence. Front Immunol 2019, 10, 1–9. [Google Scholar] [CrossRef]
- Castelli, V.; D’Angelo, M.; Lombardi, F.; Alfonsetti, M.; Antonosante, A.; Catanesi, M.; Benedetti, E.; Palumbo, P.; Cifone, M.G.; Giordano, A.; Desideri, G.; Cimini, A. ; Effects of the Probiotic Formulation SLAB51 in in vitro and in vivo Parkinson’s Disease Models. Aging 2020, 12, 4641–4659. [Google Scholar] [CrossRef]
- Liao, J.F.; Cheng, Y.F.; You, S.T.; Kuo, W.C.; Huang, C.W.; Chiou, J.J.; Hsu, C.C.; Hsieh-Li, H.M.; Wang, S.; Tsai, Y.C. Lactobacillus Plantarum PS128 Alleviates Neurodegenerative Progression in 1-Methyl-4-Phenyl-1,2,3,6-Tetrahydropyridine-Induced Mouse Models of Parkinson’s Disease. Brain Behav Immun 2020, 90, 26–46. [Google Scholar] [CrossRef] [PubMed]
- Srivastav, S.; Neupane, S.; Bhurtel, S.; Katila, N.; Maharjan, S.; Choi, H.; Hong, J.T.; Choi, D.Y. Probiotics Mixture Increases Butyrate, and Subsequently Rescues the Nigral Dopaminergic Neurons from MPTP and Rotenone-Induced Neurotoxicity. Journal of Nutritional Biochemistry 2019, 69, 73–86. [Google Scholar] [CrossRef] [PubMed]
- O’Hagan, C.; Li, J. V.; Marchesi, J.R.; Plummer, S.; Garaiova, I.; Good, M.A. Long-Term Multi-Species Lactobacillus and Bifidobacterium Dietary Supplement Enhances Memory and Changes Regional Brain Metabolites in Middle-Aged Rats. Neurobiol Learn Mem 2017, 144, 36–47. [Google Scholar] [CrossRef] [PubMed]
- Distrutti, E.; O’Reilly, J.A.; McDonald, C.; Cipriani, S.; Renga, B.; Lynch, M.A.; Fiorucci, S. Modulation of Intestinal Microbiota by the Probiotic VSL#3 Resets Brain Gene Expression and Ameliorates the Age-Related Deficit in LTP. PLoS One 2014, 9. [Google Scholar] [CrossRef]
- Jeong, J.J.; Woo, J.Y.; Kim, K.A.; Han, M.J.; Kim, D.H. Lactobacillus Pentosus Var. Plantarum C29 Ameliorates Age-Dependent Memory Impairment in Fischer 344 Rats. Lett Appl Microbiol 2015, 60, 307–314. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Sugahara, H.; Shimada, K.; Mitsuyama, E.; Kuhara, T.; Yasuoka, A.; Kondo, T.; Abe, K.; Xiao, J.Z. Therapeutic Potential of Bifidobacterium Breve Strain A1 for Preventing Cognitive Impairment in Alzheimer’s Disease. Sci Rep 2017, 7, 1–10. [Google Scholar] [CrossRef]
- Chandra, S.; Sisodia, S.S.; Vassar, R.J. The Gut Microbiome in Alzheimer’s Disease: What We Know and What Remains to Be Explored. Mol Neurodegener 2023, 18, 1–21. [Google Scholar] [CrossRef]
- Mossello, E.; Ballini, E.; Boncinelli, M.; Monami, M.; Lonetto, G.; Mello, A.M.; Tarantini, F.; Baldasseroni, S.; Mannucci, E.; Marchionni, N. Glucagon-like Peptide-1, Diabetes, and Cognitive Decline: Possible Pathophysiological Links and Therapeutic Opportunities. Exp Diabetes Res 2011, 2011. [Google Scholar] [CrossRef]
- Franco-Robles, E.; López, M.G. Implication of Fructans in Health: Immunomodulatory and Antioxidant Mechanisms. Scientific World Journal 2015, 2015. [Google Scholar] [CrossRef]
- Hall, D.A.; Voigt, R.M.; Cantu-Jungles, T.M.; Hamaker, B.; Engen, P.A.; Shaikh, M.; Raeisi, S.; Green, S.J.; Naqib, A.; Forsyth, C.B.; Chen, T.; Manfready, R.; Ouyang, B.; Rasmussen, H.E.; Sedghi, S.; Goetz, C.G.; Keshavarzian, A. ; An Open Label, Non-Randomized Study Assessing a Prebiotic Fiber Intervention in a Small Cohort of Parkinson’s Disease Participants. Nat Commun 2023, 14. [Google Scholar] [CrossRef]
- Becker, A.; Schmartz, G.P.; Gröger, L.; Grammes, N.; Galata, V.; Philippeit, H.; Weiland, J.; Ludwig, N.; Meese, E.; Tierling, S.; Walter, J.; Schwiertz, A.; Spiegel, J.; Wagenpfeil, G.; Faßbender, K.; Keller, A.; Unger, M.M. ; Effects of Resistant Starch on Symptoms, Fecal Markers, and Gut Microbiota in Parkinson’s Disease — The RESISTA-PD Trial. Genomics Proteomics Bioinformatics 2022, 20, 274–287. [Google Scholar] [CrossRef] [PubMed]
- Barichella, M.; Pacchetti, C.; Bolliri, C.; Cassani, E.; Iorio, L.; Pusani, C.; Pinelli, G.; Privitera, G.; Cesari, I.; Faierman, S.A.; Caccialanza, R.; Pezzoli, G.; Cereda, E. ; Probiotics and Prebiotic Fiber for Constipation Associated with Parkinson Disease. Neurology 2016, 87, 1274–1280. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, A.; Raja Ali, R.A.; Abdul Manaf, M.R.; Ahmad, N.; Tajurruddin, F.W.; Qin, W.Z.; Md Desa, S.H.; Ibrahim, N.M. Multi-Strain Probiotics (Hexbio) Containing MCP BCMC Strains Improved Constipation and Gut Motility in Parkinson’s Disease: A Randomised Controlled Trial. PLoS One 2020, 15, 1–17. [Google Scholar] [CrossRef]
- Chen, D.; Yang, X.; Yang, J.; Lai, G.; Yong, T.; Tang, X.; Shuai, O.; Zhou, G.; Xie, Y.; Wu, Q. Prebiotic Effect of Fructooligosaccharides from Morinda Officinalis on Alzheimer’s Disease in Rodent Models by Targeting the Microbiota-Gut-Brain Axis. Front Aging Neurosci 2017, 9, 1–28. [Google Scholar] [CrossRef]
- Sarparast, M.; Dattmore, D.; Alan, J.; Lee, K.S.S. Cytochrome P450 Metabolism of Polyunsaturated Fatty Acids and Neurodegeneration. Nutrients 2020, 12, 1–40. [Google Scholar] [CrossRef]
- Şimşek, H.; Uçar, A. Polyunsaturated Fatty Acids as a Nutraceutical for Age-Related Neurodegenerative Diseases: Current Knowledge and Future Directions. Clinical Nutrition Open Science 2024, 56, 65–73. [Google Scholar] [CrossRef]
- Luchtman, D.W.; Song, C. Cognitive Enhancement by Omega-3 Fatty Acids from Child-Hood to Old Age: Findings from Animal and Clinical Studies. Neuropharmacology 2013, 64, 550–565. [Google Scholar] [CrossRef]
- Youdim, K.A.; Martin, A.; Joseph, J.A. Essential Fatty Acids and the Brain: Possible Health Implications. International Journal of Developmental Neuroscience 2000, 18, 383–399. [Google Scholar] [CrossRef]
- Joffre, C.; Dinel, A.L.; Chataigner, M.; Pallet, V.; Layé, S. N-3 Polyunsaturated Fatty Acids and Their Derivates Reduce Neuroinflammation during Aging. Nutrients 2020, 12, 1–25. [Google Scholar] [CrossRef]
- Uauy, R.; Dangour, A.D. Nutrition in Brain Development and Aging: Role of Essential Fatty Acids. Nutr Rev 2006, 64. [Google Scholar] [CrossRef]
- Kerdiles, O.; Layé, S.; Calon, F. Omega-3 Polyunsaturated Fatty Acids and Brain Health: Preclinical Evidence for the Prevention of Neurodegenerative Diseases. Trends Food Sci Technol 2017, 69, 203–213. [Google Scholar] [CrossRef]
- Singh, P.K.; Gupta, M.K.; Nath, R. Omega-3 Fatty Acid as a Protectant in Lead-Induced Neurotoxicity; Elsevier Inc., 2023; ISBN 9780323900522.
- Bousquet, M.; Saint-Pierre, M.; Julien, C.; Salem, N.; Cicchetti, F.; Calon, F. Beneficial Effects of Dietary Omega-3 Polyunsaturated Fatty Acid on Toxin-induced Neuronal Degeneration in an Animal Model of Parkinson’s Disease. The FASEB Journal 2008, 22, 1213–1225. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Chen, H.; Fung, T.T.; Logroscino, G.; Schwarzschild, M.A.; Hu, F.B.; Ascherio, A. Prospective Study of Dietary Pattern and Risk of Parkinson Disease. American Journal of Clinical Nutrition 2007, 86, 1486–1494. [Google Scholar] [CrossRef] [PubMed]
- De Lau, L.M.L.; Bornebroek, M.; Witteman, J.C.M.; Hofman, A.; Koudstaal, P.J.; Breteler, M.M.B. Dietary Fatty Acids and the Risk of Parkinson Disease: The Rotterdam Study. Neurology 2005, 64, 2040–2045. [Google Scholar] [CrossRef]
- Kamel, F.; Goldman, S.M.; Umbach, D.M.; Chen, H.; Richardson, G.; Barber, M.R.; Meng, C.; Marras, C.; Korell, M.; Kasten, M.; et al. Dietary Fat Intake, Pesticide Use, and Parkinson’s Disease. Parkinsonism Relat Disord 2014, 20, 82–87. [Google Scholar] [CrossRef]
- Bousquet, M.; St-Amour, I.; Vandal, M.; Julien, P.; Cicchetti, F.; Calon, F. High-Fat Diet Exacerbates MPTP-Induced Dopaminergic Degeneration in Mice. Neurobiol Dis 2012, 45, 529–538. [Google Scholar] [CrossRef]
- Bousquet, M.; Gibrat, C.; Saint-Pierre, M.; Julien, C.; Calon, F.; Cicchetti, F. Modulation of Brain-Derived Neurotrophic Factor as a Potential Neuroprotective Mechanism of Action of Omega-3 Fatty Acids in a Parkinsonian Animal Model. Prog Neuropsychopharmacol Biol Psychiatry 2009, 33, 1401–1408. [Google Scholar] [CrossRef]
- Bousquet, M.; Calon, F.; Cicchetti, F. Impact of Omega-3 Fatty Acids in Parkinson’s Disease. Ageing Res Rev 2011, 10, 453–463. [Google Scholar] [CrossRef]
- Calon, F.; Cicchetti, F. Can We Prevent Parkinson’s Disease with n-3 Polyunsaturated Fatty Acids? Future Lipidol 2008, 3, 133–137. [Google Scholar] [CrossRef]
- Morgese, M.G.; Schiavone, S.; Bove, M.; Colia, A.L.; Dimonte, S.; Tucci, P.; Trabace, L. N-3 PUFA Prevent Oxidative Stress in a Rat Model of Beta-Amyloid-Induced Toxicity. Pharmaceuticals 2021, 14. [Google Scholar] [CrossRef]
- Olde Rikkert, M.G.M.; Verhey, F.R.; Blesa, R.; Von Arnim, C.A.F.; Bongers, A.; Harrison, J.; Sijben, J.; Scarpini, E.; Vandewoude, M.F.J.; Vellas, B.; et al. Tolerability and Safety of Souvenaid in Patients with Mild Alzheimer’s Disease: Results of Multi-Center, 24-Week, Open-Label Extension Study. Journal of Alzheimer’s Disease 2015, 44, 471–480. [Google Scholar] [CrossRef] [PubMed]
- Faxén-Irving, G.; Freund-Levi, Y.; Eriksdotter-Jönhagen, M.; Basun, H.; Hjorth, E.; Palmblad, J.; Vedin, I.; Cederholm, T.; Wahlund, L.O. Effects on Transthyretin in Plasma and Cerebrospinal Fluid by Dha-Rich n-3 Fatty Acid Supplementation in Patients with Alzheimer’s Disease: The Omegad Study. Journal of Alzheimer’s Disease 2013, 36, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Calon, F.; Lim, G.P.; Yang, F.; Morihara, T.; Teter, B.; Ubeda, O.; Rostaing, P.; Triller, A.; Salem, N.; Ashe, K.H.; et al. Docosahexaenoic Acid Protects from Dendritic Pathology in an Alzheimer’s Disease Mouse Model. Neuron 2004, 43, 633–645. [Google Scholar] [CrossRef] [PubMed]
- Calon, F.; Lim, G.P.; Morihara, T.; Yang, F.; Ubeda, O.; Salem, N.; Frautschy, S.A.; Cole, G.M. Dietary N-3 Polyunsaturated Fatty Acid Depletion Activates Caspases and Decreases NMDA Receptors in the Brain of a Transgenic Mouse Model of Alzheimer’s Disease. European Journal of Neuroscience 2005, 22, 617–626. [Google Scholar] [CrossRef]
- Arsenault, D.; Julien, C.; Tremblay, C.; Calon, F. DHA Improves Cognition and Prevents Dysfunction of Entorhinal Cortex Neurons in 3xTg-AD Mice. PLoS One 2011, 6. [Google Scholar] [CrossRef]
- Casali, B.T.; Corona, A.W.; Mariani, M.M.; Karlo, J.C.; Ghosal, K.; Landreth, G.E. Omega-3 Fatty Acids Augment the Actions of Nuclear Receptor Agonists in a Mouse Model of Alzheimer’s Disease. Journal of Neuroscience 2015, 35, 9173–9181. [Google Scholar] [CrossRef]
- Perez, S.E.; Berg, B.M.; Moore, K.A.; He, B.; Counts, S.E.; Fritz, J.J.; Hu, Y.S.; Lazarov, O.; Lah, J.J.; Mufson, E.J. DHA Diet Reduces AD Pathology in Young APPswe/PS1ΔE9 Transgenic Mice: Possible Gender Effects. J Neurosci Res 2010, 88, 1026–1040. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




