Submitted:
08 April 2025
Posted:
09 April 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Experimental Section
2.1. Experimental Samples
2.2. Preparation of Semi-Coke
2.3. Characterization of Combustion Residues
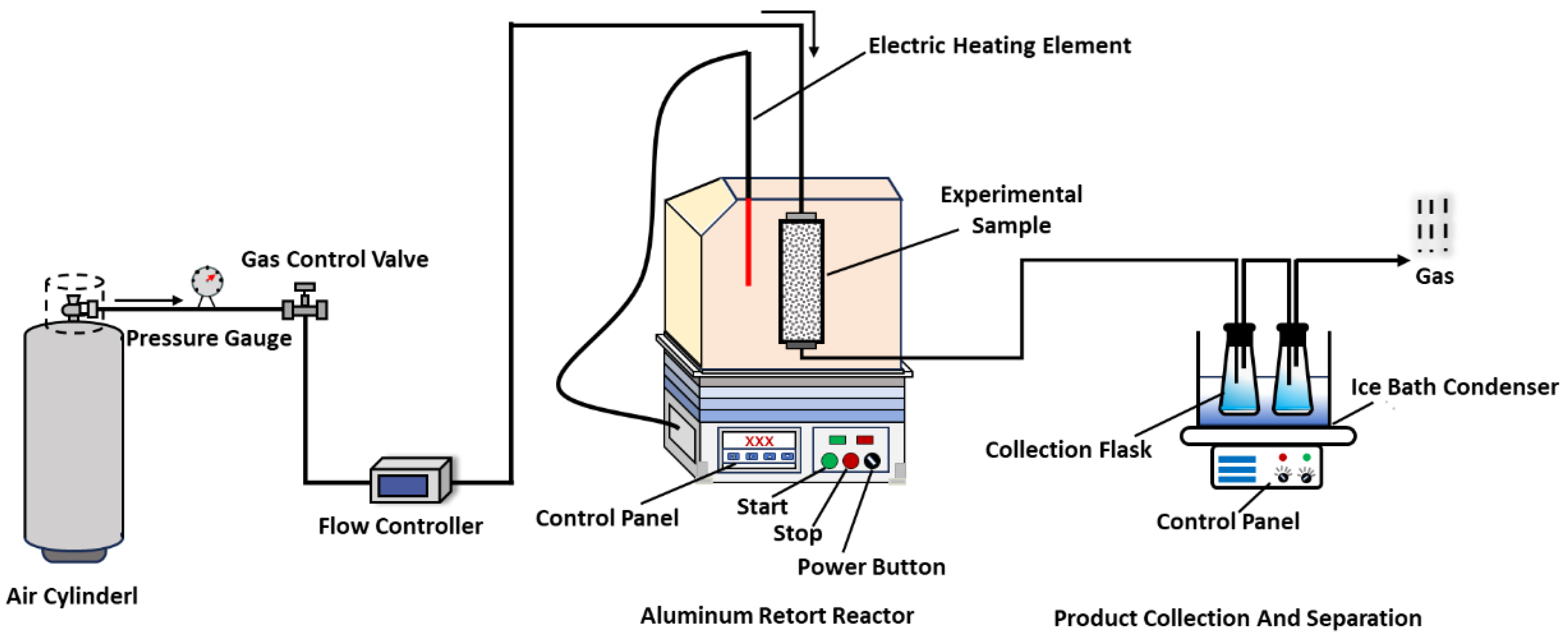
2.4. Thermogravimetric Analysis (TGA)
2.5. Kinetic Model Analysis
- (1)
- Model-Free Methods
- (2)
- Model-based Method
3. Results and Discussion
3.1. Combustion Characteristics of Oil Shale Semi-Coke
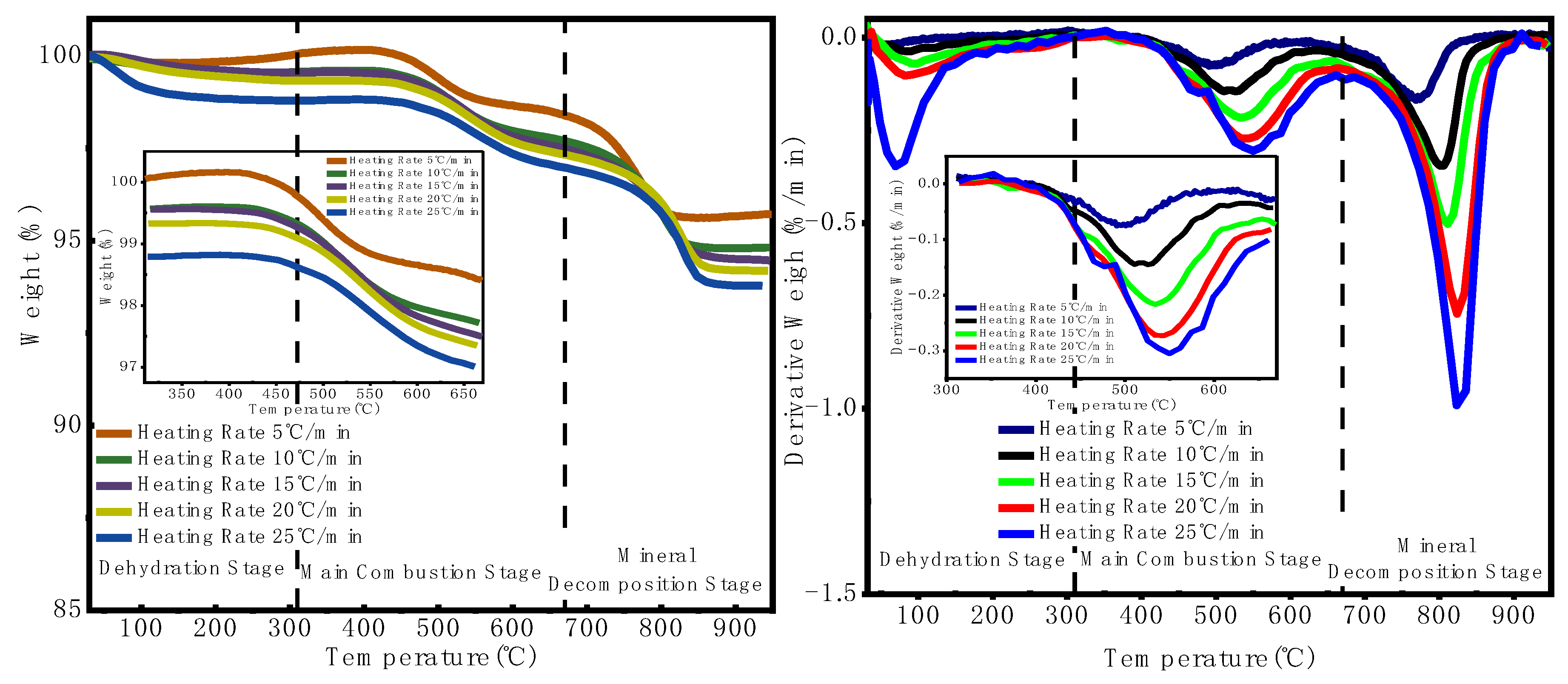
3.1.1. Thermogravimetric Analysis (TGA) Results
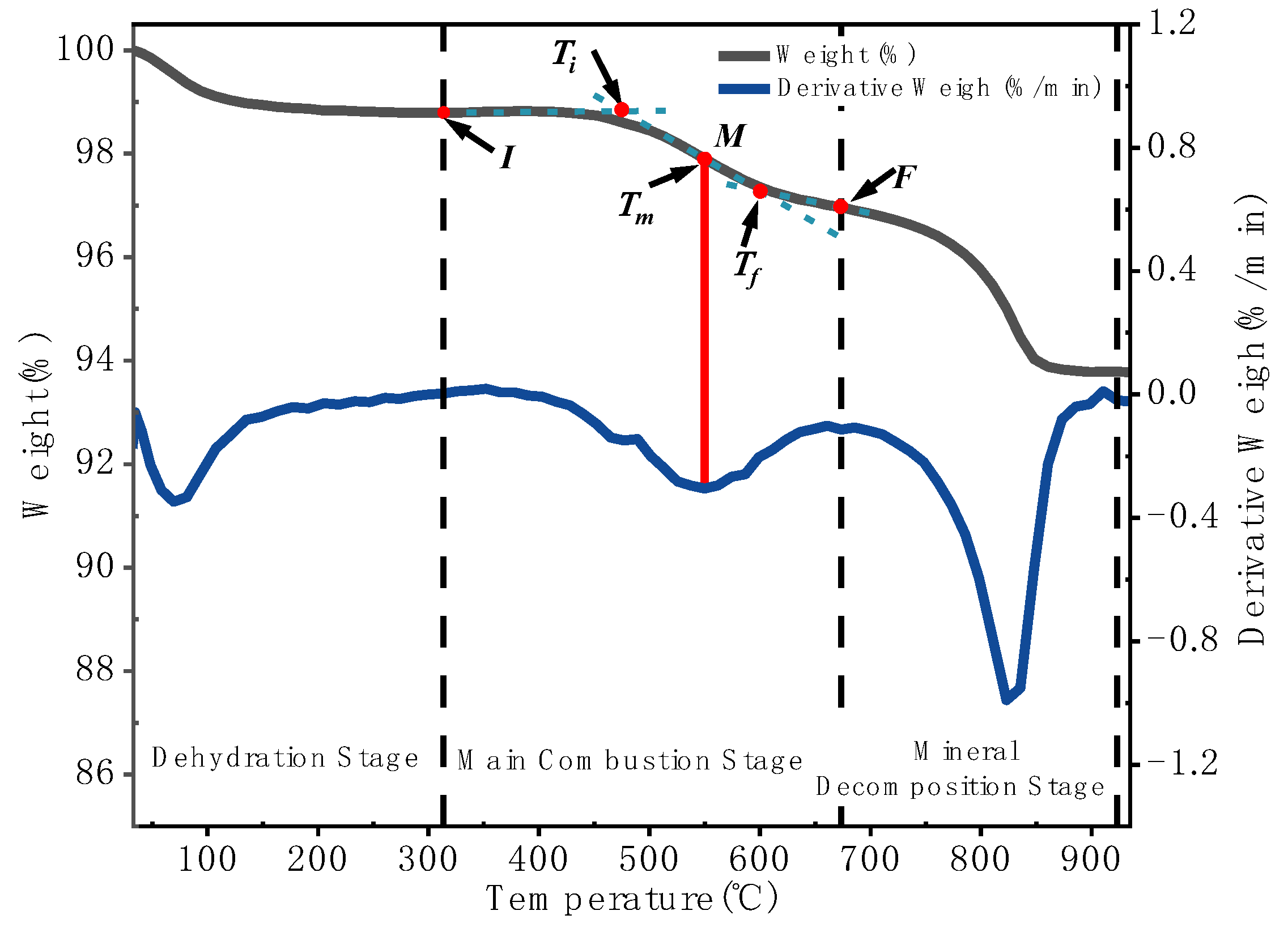
3.1.2. Extraction of Combustion Characteristic Parameters
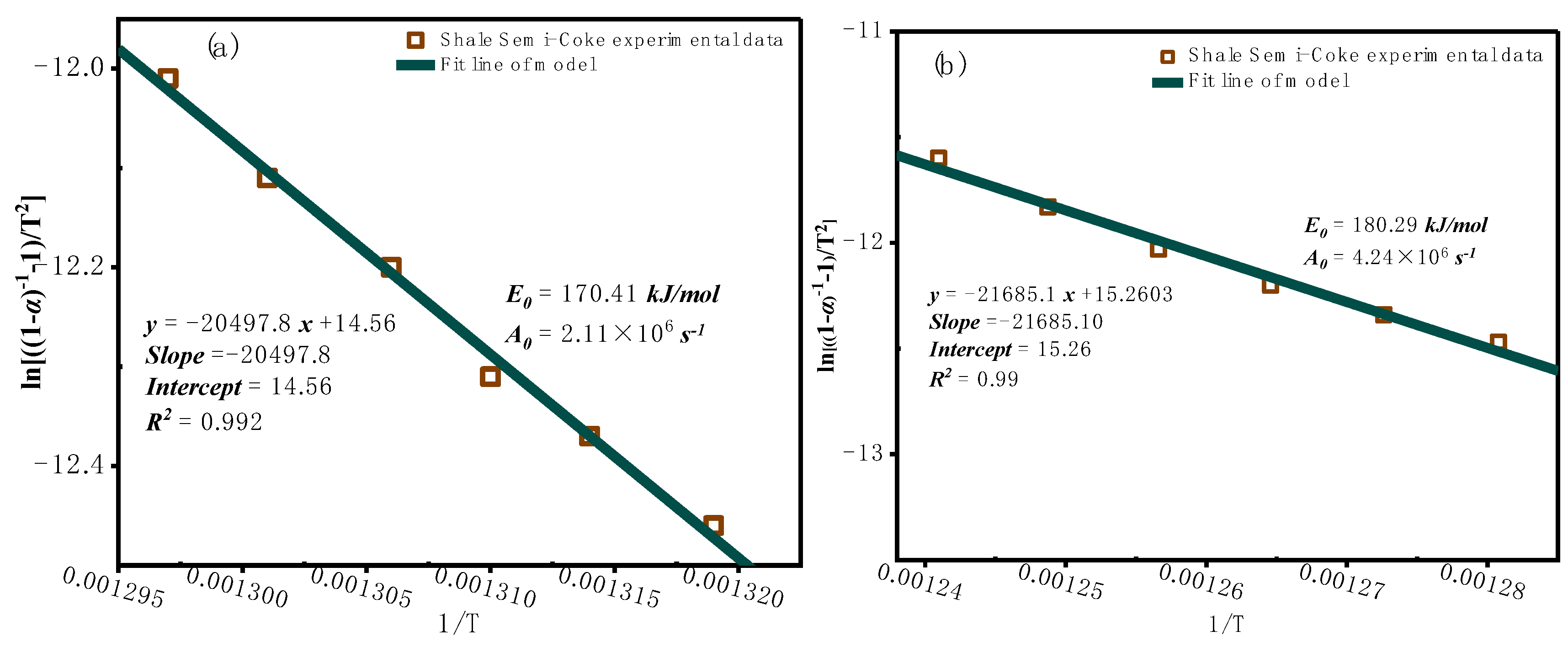
3.2. Kinetic Analysis
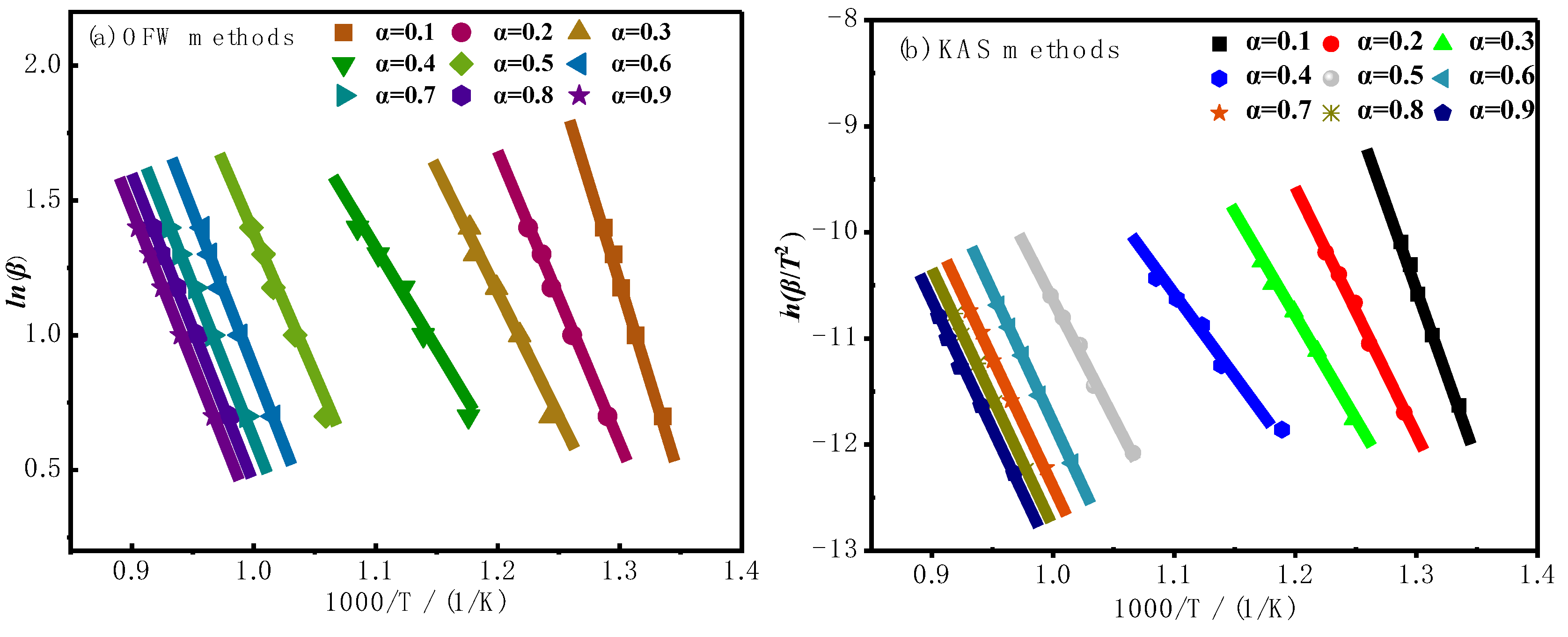
3.2.1. Model-Free Kinetic Analysis
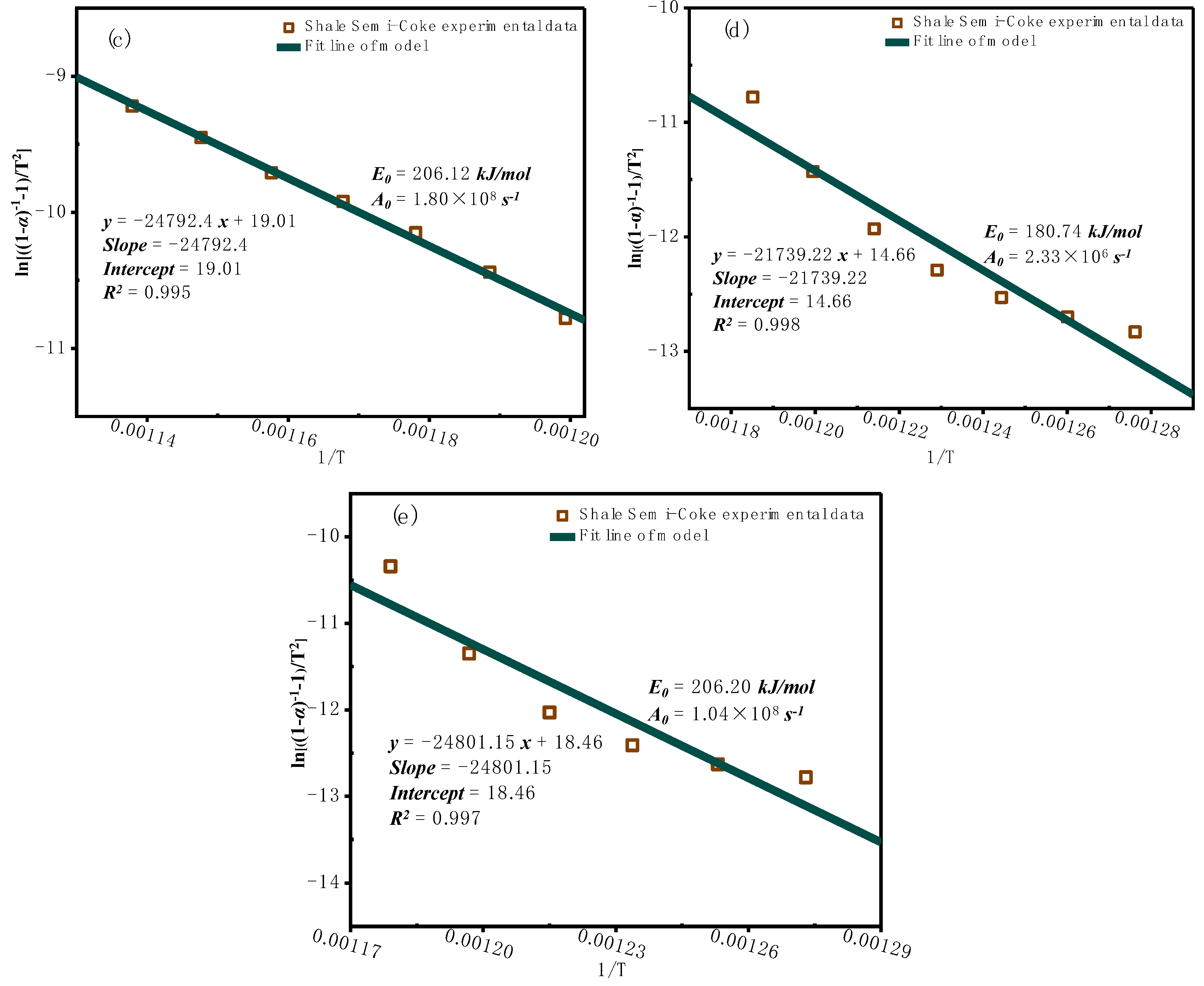
3.2.2. Model-Based Kinetic Function Analysis
3.3. Characterization of Combustion Residues
3.3.1. SEM Characterization
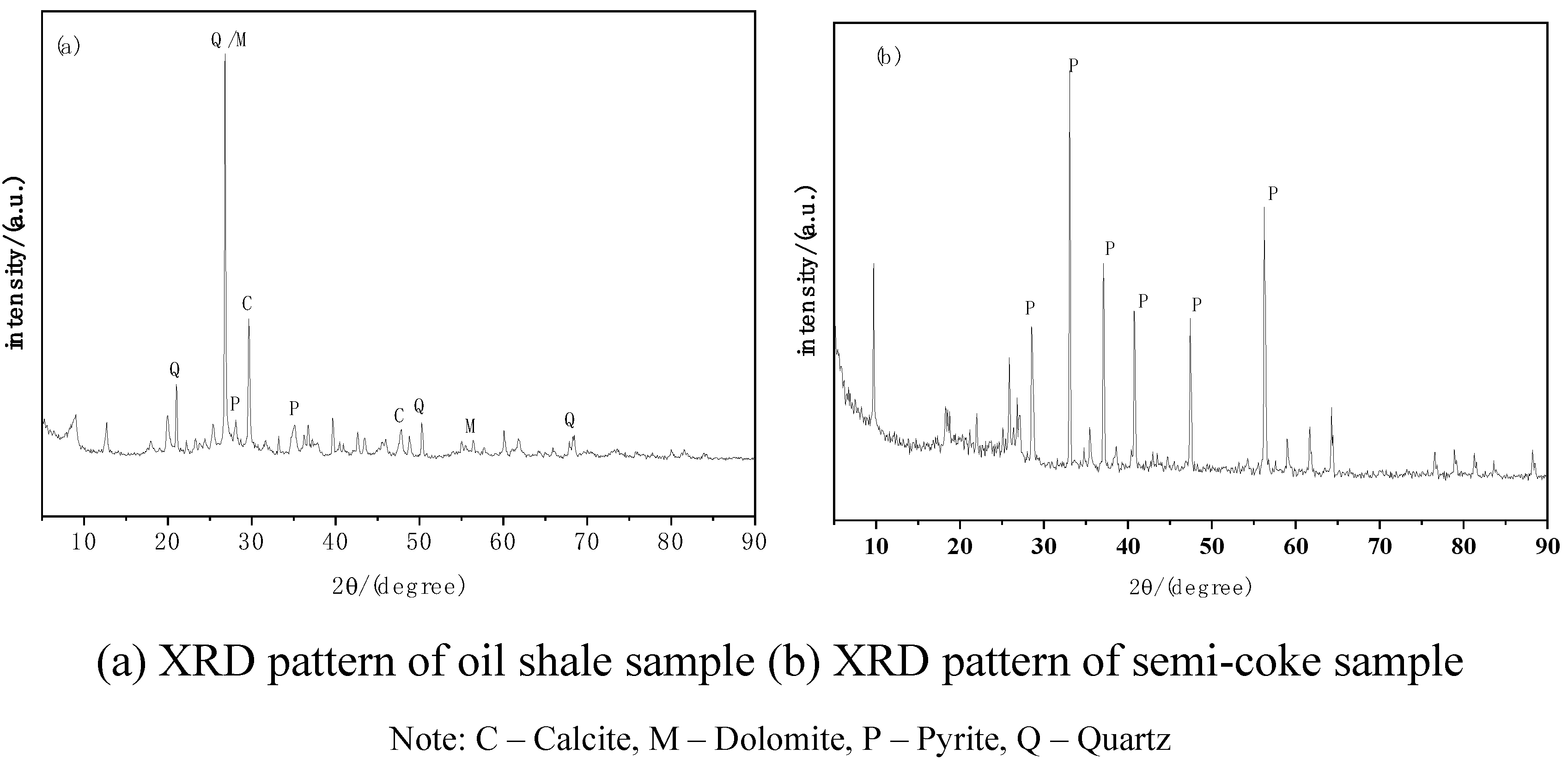
3.3.2. XRD Characterization
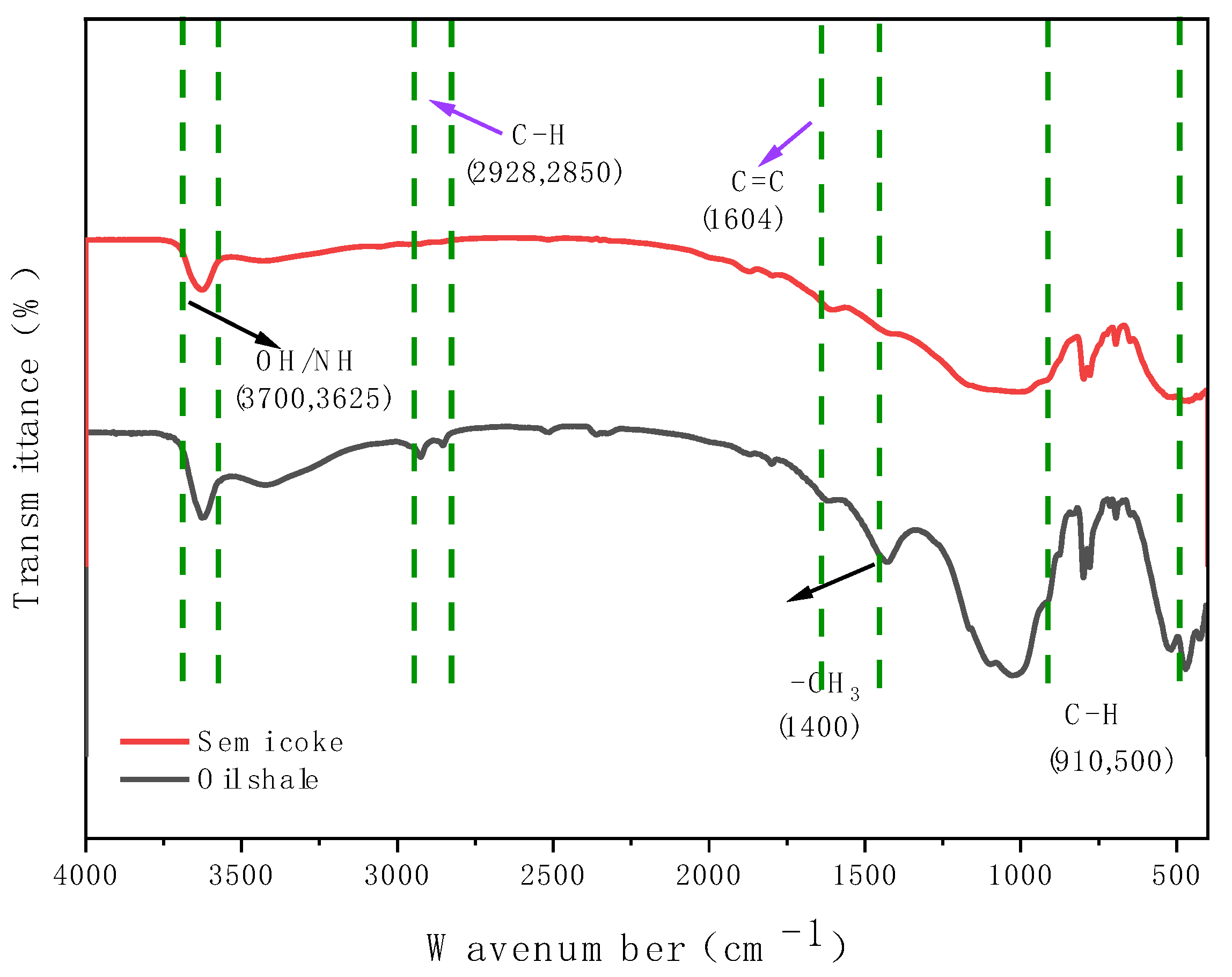
3.3.3. Infrared Spectroscopy (IR) Characterization
4. Conclusions
- (1)
- The combustion process of oil shale semi-coke can be divided into three stages: a low-temperature stage (50–310 °C, involving dehydration and release of volatiles), a medium-temperature stage (310–670 °C, the main combustion phase with oxidation of carbonaceous components), and a high-temperature stage (670–950 °C, involving mineral decomposition and oxidation of residual carbon). The medium-temperature stage is the core of the combustion process, accounting for approximately 28%–37% of the total mass loss, where energy release is concentrated and significant thermochemical activity is observed.
- (2)
- In the model-free analysis, the average activation energy calculated by the OFW method is 180.80 kJ/mol, and by the KAS method is 180.81 kJ/mol. Both methods achieved R² values above 0.996, indicating that the OFW and KAS methods are suitable for describing the combustion kinetics of oil shale semi-coke.
- (3)
- Kinetic analysis shows that the activation energy increases gradually with the conversion rate, indicating a distinct staged nature of the combustion process and reflecting its multi-step reaction characteristics. Although the activation energy calculated by the Coats-Redfern integral method is close to that of the model-free methods, the overall fit for the pyrolysis process is less ideal, with certain errors and limitations, and does not accurately capture the overall behavior of oil shale semi-coke combustion.
- (4)
- The model-free method is suitable for rapid analysis of complex reactions, especially when the reaction mechanism is unclear, providing reliable kinetic parameters. The model-fitting method can provide deeper insights into reaction mechanisms. The combustion of oil shale semi-coke shows clear multi-stage kinetic behavior, so model selection must balance mechanistic validity with agreement to experimental data. The F2-R3-F2 model, with its segmented mechanism (interface reaction + second-order reaction), better reflects the physicochemical changes during semi-coke combustion and more reasonably explains mineral phase transformations. Therefore, the F2-R3-F2 model is identified as the most appropriate.
- (5)
- SEM analysis of oil shale semi-coke before and after combustion shows that before combustion, the sample surface is smooth with small and evenly distributed pores, mainly formed by volatile release during pyrolysis. After combustion, the surface shows numerous irregular pores with increased pore size and a honeycomb-like structure. XRD analysis indicates that the characteristic peak intensities of quartz (Q) and dolomite (M) increase after combustion, suggesting enrichment of quartz during pyrolysis, while the peak intensities of calcite (C) and pyrite (P) decrease, indicating decomposition or transformation during combustion. IR spectroscopy shows a reduction in hydrocarbons and the presence of aromatic compounds and partially decomposed organics in the post-combustion semi-coke, further confirming the transformation of organic matter during pyrolysis.
Acknowledgments
References
- Sun, B.Z.; Wang, Q.; Shen, P.Y.; Qin, H.; Li, S.H. Kinetic analysis of co-combustion of oil shale semi-coke with bituminous coal. Oil Shale 2012, 29, 63–75. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, H.; Sun, B.; Bai, J.; Guan, X. Interactions between oil shale and its semi-coke during co-combustion. Fuel 2009, 88, 1520–1529. [Google Scholar] [CrossRef]
- Qin, H.; Yue, Y.; Zhang, L.; Liu, Y.; Chi, M.; Liu, H.; Liu, B. Study on co-combustion kinetics of oil shale sludge and semicoke. Energy & Fuels 2016, 30, 2373–2384. [Google Scholar]
- Qing, W.; Baizhong, S.; Xiahua, W.; Jingru, B.; Jian, S. Influence of retorting temperature on combustion characteristics and kinetic parameters of oil shale semicoke. Oil Shale 2006, 23, 328–339. [Google Scholar] [CrossRef]
- Qin, H.; Wang, W.; Liu, H.; Zhang, L.; Wang, Q.; Shi, C.; Yao, K. Thermal behavior research for co-combustion of furfural residue and oil shale semi-coke. Applied Thermal Engineering 2017, 120, 19–25. [Google Scholar] [CrossRef]
- Wang, P.; Wang, C.A.; Wang, C.; Du, Y.; Che, D. Experimental investigation on co-combustion characteristics of semi-coke and coal: Insight into synergy and blending method. Process Safety and Environmental Protection 2023, 175, 290–302. [Google Scholar] [CrossRef]
- Pan, N.; Li, D.; Lü, W.; Dai, F. Kinetic study on the pyrolysis behavior of Jimsar oil shale. Oil Shale 2019, 36, 462–482. [Google Scholar] [CrossRef]
- Chi, M.; Xu, X.; Cui, D.; Zhang, H.; Wang, Q. A TG-FTIR investigation and kinetic analysis of oil shale kerogen pyrolysis using the distributed activation energy model. Oil Shale 2016, 33, 228–247. [Google Scholar] [CrossRef]
- Li, S.; & Yue, C.; Yue, C. Study of pyrolysis kinetics of oil shale. Fuel 2003, 82, 337–342. [Google Scholar] [CrossRef]
- Han, X.; Jiang, X.; Cui, Z. Thermal analysis studies on combustion mechanism of oil shale. Journal of thermal analysis and calorimetry 2006, 84, 631–636. [Google Scholar] [CrossRef]
- Han, X.; Kulaots, I.; Jiang, X.; Suuberg, E.M. Review of oil shale semicoke and its combustion utilization. Fuel 2014, 126, 143–161. [Google Scholar] [CrossRef]
- Ozawa, T. A new method of analyzing thermogravimetric data. Bulletin of the chemical society of Japan 1965, 38, 1881–1886. [Google Scholar] [CrossRef]
- Kissinger, H.E. Reaction kinetics in differential thermal analysis. Analytical chemistry 1957, 29, 1702–1706. [Google Scholar] [CrossRef]
- Coats, A.W.; Redfern, J.P. Kinetic parameters from thermogravimetric data. Nature 1964, 201, 68–69. [Google Scholar] [CrossRef]
- Torrente, M.C.; Galan, M.A. Kinetics of the thermal decomposition of oil shale from Puertollano (Spain). Fuel 2001, 80, 327–334. [Google Scholar] [CrossRef]
- Zanoni, M.A. B.; Massard, H.; Martins, M.F. Formulating and optimizing a combustion pathways for oil shale and its semi-coke. Combustion and Flame 2012, 159, 3224–3234. [Google Scholar] [CrossRef]
- Bai, F.; Sun, Y.; Liu, Y. Thermogravimetric analysis of Huadian oil shale combustion at different oxygen concentrations. Energy & Fuels 2016, 30, 4450–4456. [Google Scholar]
- Yao, Z.; Ma, X.; Wang, Z.; Chen, L. Characteristics of co-combustion and kinetic study on hydrochar with oil shale: a thermogravimetric analysis. Applied Thermal Engineering 2017, 110, 1420–1427. [Google Scholar] [CrossRef]
- Han, X.; Kulaots, I.; Jiang, X.; Suuberg, E.M. Review of oil shale semicoke and its combustion utilization. Fuel 2014, 126, 143–161. [Google Scholar] [CrossRef]
- Bai, F.; Sun, Y.; Liu, Y.; Li, Q.; Guo, M. Thermal and kinetic characteristics of pyrolysis and combustion of three oil shales. Energy conversion and management 2015, 97, 374–381. [Google Scholar] [CrossRef]
- Kok, M.V.; Bal, B.; Varfolomeev, M.A.; Nurgaliev, D.K. Thermal characteristics and model-free kinetics of oil shale samples. Journal of Thermal Analysis and Calorimetry 2023, 148, 8933–8943. [Google Scholar] [CrossRef]

| Elemental Analysis (wt%) | Industrial Analytics (wt%) | Mineral Composition (wt%) | |||||
|---|---|---|---|---|---|---|---|
| C | 13.44 | Moisture content | 3.8 | Quartz | 28 | Dolomite | 7.6 |
| H | 0.46 | Volatile matter | 28.4 | Feldspar | 5.2 | Siderite | 0.5 |
| N | 0.38 | Ash content | 64.2 | Clay minerals | 23.2 | Pyrite | 1.7 |
| S | 0.58 | Fixed carbon | 3.6 | Calcite | 33.8 | ||
| Function | Mechanism | Differential form f(α) | Integral form G(α) |
|---|---|---|---|
| Reaction Order Models | |||
| First order | F1 | (1-α) | -ln(1-α) |
| Second order | Chemical reaction(F2) | (1-α)2 | (1-α)-1-1 |
| Diffusion Models | |||
| Jander equation | 2D, n=0.5 | 4(1-α)1/2[1-(1-α)1/2]1/2 | [1-(1-α)1/2]1/2 |
| Jander equation | 3D, n=0.5 | 6(1-α)2/3[1-(1-α)1/3]1/2 | [1-(1-α)1/3]1/2 |
| G-B equation | 3D, D4 (column symmetry) | 3/2[(1-α)-1/3-1]-1 | 1-2/3α-(1-α)2/3 |
| Geometrical Contraction Models | |||
| Contracting area | R2, n=2 | (1-α)1/2 | 2[1-(1-α)1/2] |
| Contracting volume | R3, n=3 | (1-α)2/3 | 3[1-(1-α)1/3] |
| Nucleation Models | |||
| Avrami−Erofeev | Random nucleation and nuclei growthA2, 2D, n=2 | 1/2(1-α)[-ln(1-α)]-1 | [-ln(1-α)]2 |
| Avrami−Erofeev | Random nucleation and nuclei growthA3, 3D, n=3 | 1/3(1-α)[-ln(1-α)]-2 | [-ln(1-α)]3 |
| Mample power | n=1/4 | 4α3/4 | α1/4 |
| Mample power | n=1/3 | 3α2/3 | α1/3 |
| Mample power | n=1/2 | 2α1/2 | α1/2 |
| Mample power | n=2 | 1/2α-1 | α2 |
| Heating rate/°C /min | Ti / °C | Tp /°C | Tf / °C | Δm / % |
|---|---|---|---|---|
| 5 | 427.94 | 490.83 | 546.53 | 4.29 |
| 10 | 453.42 | 510.80 | 564.52 | 5.18 |
| 15 | 461.19 | 533.64 | 584.39 | 5.54 |
| 20 | 465.38 | 543.61 | 589.62 | 5.82 |
| 25 | 475.56 | 549.78 | 599.80 | 6.22 |
| α | Eα, kJ/mol | A (1/s) | Eα,avg, kJ/mol | Aavg | Ravg2 | |
|---|---|---|---|---|---|---|
| OFW | 0.1 | 145.58 | 6.27×106 | 180.80 | 1.04×109 | 0.998 |
| 0.2 | 194.41 | 8.95×109 | ||||
| 0.3 | 161.96 | 3.72×107 | ||||
| 0.4 | 132.61 | 2.07×105 | ||||
| 0.5 | 183.26 | 2.57×107 | ||||
| 0.6 | 205.11 | 1.57×108 | ||||
| 0.7 | 201.73 | 7.67×107 | ||||
| 0.8 | 200.39 | 6.01×107 | ||||
| 0.9 | 202.20 | 7.93×107 | ||||
| KAS | 0.1 | 145.26 | 5.1×106 | 180.81 | 1.07×109 | 0.997 |
| 0.2 | 194.31 | 9.27×109 | ||||
| 0.3 | 161.82 | 3.35×107 | ||||
| 0.4 | 132.53 | 1.41×105 | ||||
| 0.5 | 183.02 | 2.23×107 | ||||
| 0.6 | 204.93 | 1.46×108 | ||||
| 0.7 | 201.54 | 6.93×107 | ||||
| 0.8 | 200.19 | 5.36×107 | ||||
| 0.9 | 201.99 | 7.06×107 |
| F2-F2-F2 | F2-F2 | ||||
| stage I | Stage Ⅱ | Stage Ⅲ | stage I | Stage Ⅱ | |
| Eα / KJ·mol-1 | 180.130 | 249.799 | 263.730 | 170.646 | 277.451 |
| A / S-1 | 3.93×109 | 4.58×1010 | 1.35×1011 | 8.04×108 | 4.73×1011 |
| Contribution | 0.395 | 0.278 | 0.355 | 0.431 | 0.596 |
| Eα,avg / KJ·mol-1 | 231.22 | 224.05 | |||
| R2 | 0.98 | 0.97 | |||
| f(α) | (1-α)2 | (1-α)2 | (1-α)2 | (1-α)2 | (1-α)2 |
| F2-D4-F2 | F2-D4 | ||||
| stage I | Stage Ⅱ | Stage Ⅲ | stage I | Stage Ⅱ | |
| Eα / KJ·mol-1 | 94.340 | 50.227 | 265.536 | 61.982 | 21.187 |
| A / S-1 | 3.08×103 | 2.19 | 5.83×1010 | 6.12 | 2.26×10-2 |
| Contribution | 0.431 | 0.157 | 0.443 | 0.542 | 0.410 |
| Eα,avg / KJ·mol-1 | 136.70 | 41.58 | |||
| R2 | 0.94 | 0.82 | |||
| f(α) | (1-α)2 | 3/2[(1-α)-1/3-1]-1 | (1-α)2 | (1-α)2 | 3/2[(1-α)-1/3-1]-1 |
| F2-R3-F2 | A2-F2 | ||||
| stage I | Stage Ⅱ | Stage Ⅲ | stage I | Stage Ⅱ | |
| Eα / KJ·mol-1 | 131.344 | 102.546 | 203.089 | 114.196 | 221.454 |
| A / S-1 | 2.21×106 | 9.08×103 | 2.95×107 | 1.17×105 | 7.11×108 |
| Contribution | 0.248 | 0.187 | 0.583 | 0.386 | 0.649 |
| Eα,avg / KJ·mol-1 | 145.66 | 167.83 | |||
| R2 | 0.97 | 0.95 | |||
| f(α) | (1-α)2 | (1-α)2/3 | (1-α)2 | 1/2(1-α)[-ln(1-α)]-1 | (1-α)2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





